Introduction
Numerous studies have been devoted to the study of the mechanisms of erythrocyte aggregation in the form of rouleaux [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10]. However, to date, there is no single view on the causes leading to the union of red blood cells into aggregates.
It is known that the process of erythrocyte aggregation is determined by two types of biophysical and physicochemical factors: plasma properties and properties of erythrocytes themselves (mainly component membranes) [
2]. At the same time, there are only isolated works in the literature devoted to the study of the role of the erythrocyte shape in their aggregation [
11,
12].
We have previously shown that ATP - depleted erythrocytes leads to the formation of echinocytes and a sharp decrease in their aggregability in autologous plasma [
13].
The transformation of the erythrocyte shape from discocytes to echinocytes was previously observed on a suspension of washed erythrocytes placed between a slide and a cover glass [
14,
15]. It has also been shown that the blood protein albumin converts discocytes into stomatocytes [
16,
17].
In this work, using a light microscope at high magnification (1000x), we studied the effect of autologous plasma and 3% solutions of dextran 70 on the shape and aggregation of red blood cells placed between two glass surfaces. The effect of albumin and trypsin on the shape of red blood cells and their aggregation induced by dextran 70 was also studied.
Mfterials and Methods
Dextran 70 (MW= 70 kD), trypsin were purchased from Sigma, USA. Human serum albumin was used. All other reagents were analytically pure.
The blood of 10 healthy blood donors was used in the work. Venous blood was collected in vacuum tubes containing 3.8% sodium citrate (9:1 ratio). The blood was centrifuged for 20 min at 2000 g. Platelets and leukocytes were removed. Then the red blood cells were washed three times with an excess volume of NaCI saline solution. The washed erythrocytes were placed in buffered saline solution (10 mM Tris-HCI, 150 mM NaCI, pH 7.4) with 0.5% hematocrit.
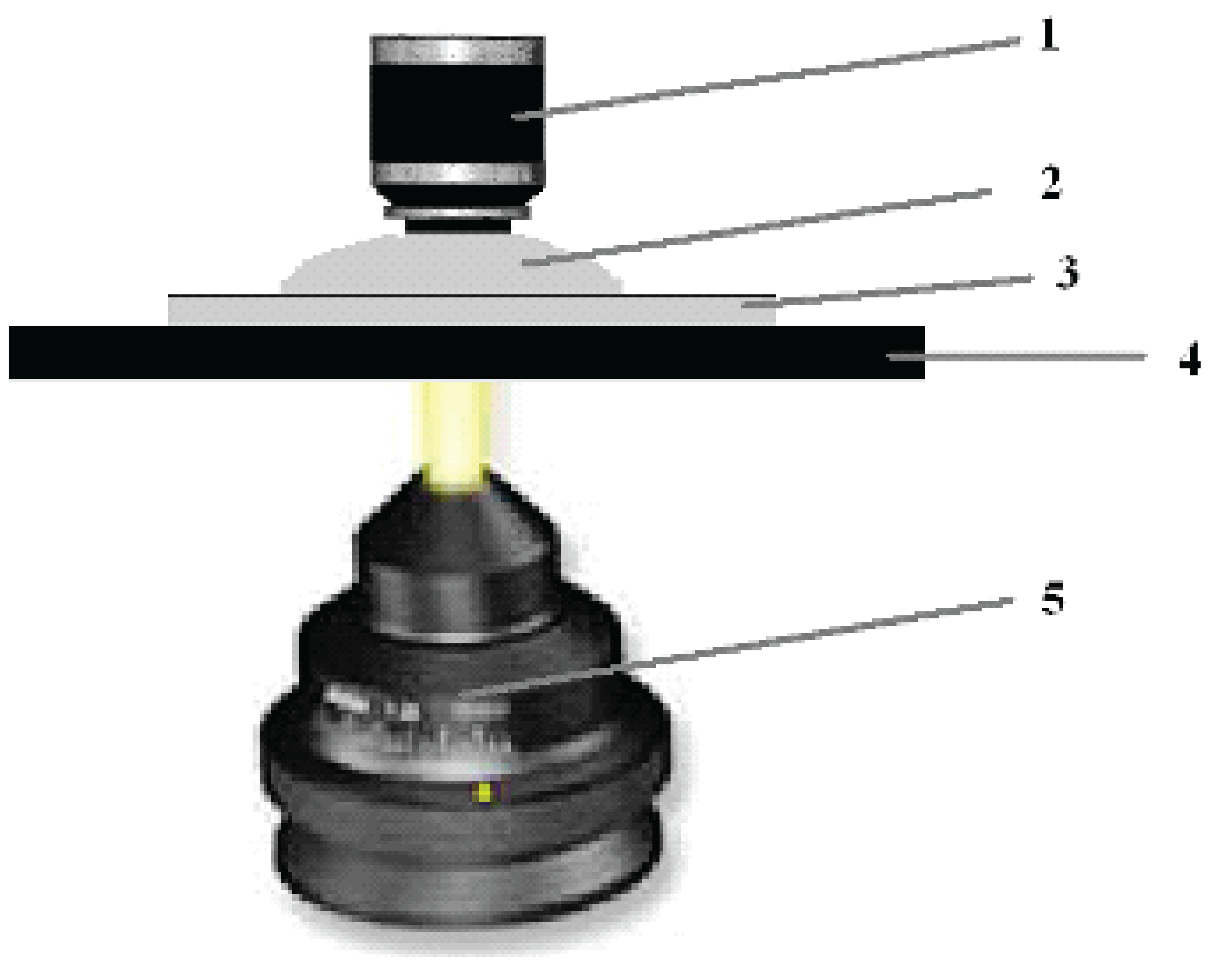
We used a light microscope (Primo Star Carl Zeiss, Germany) equipped with a megapixel digital color television camera. The morphology of erythrocyte aggregates was studied by our proposed method [
18,
19]. A suspension of erythrocytes was placed on a slide, and a 100
x microscope lens was lowered into a drop of suspension (
Figure 1). The liquid was sucked out with a pipette.
In experiments with the treatment of erythrocytes with trypsin, a 0.5% enzyme solution was added to the echinocytes and incubated for 10 min. After that, red blood cells were pipetted twice with saline solution pH 7.4. The liquid was removed, and dextran 70 solution was added to the echinocytes.
Results and Discussion
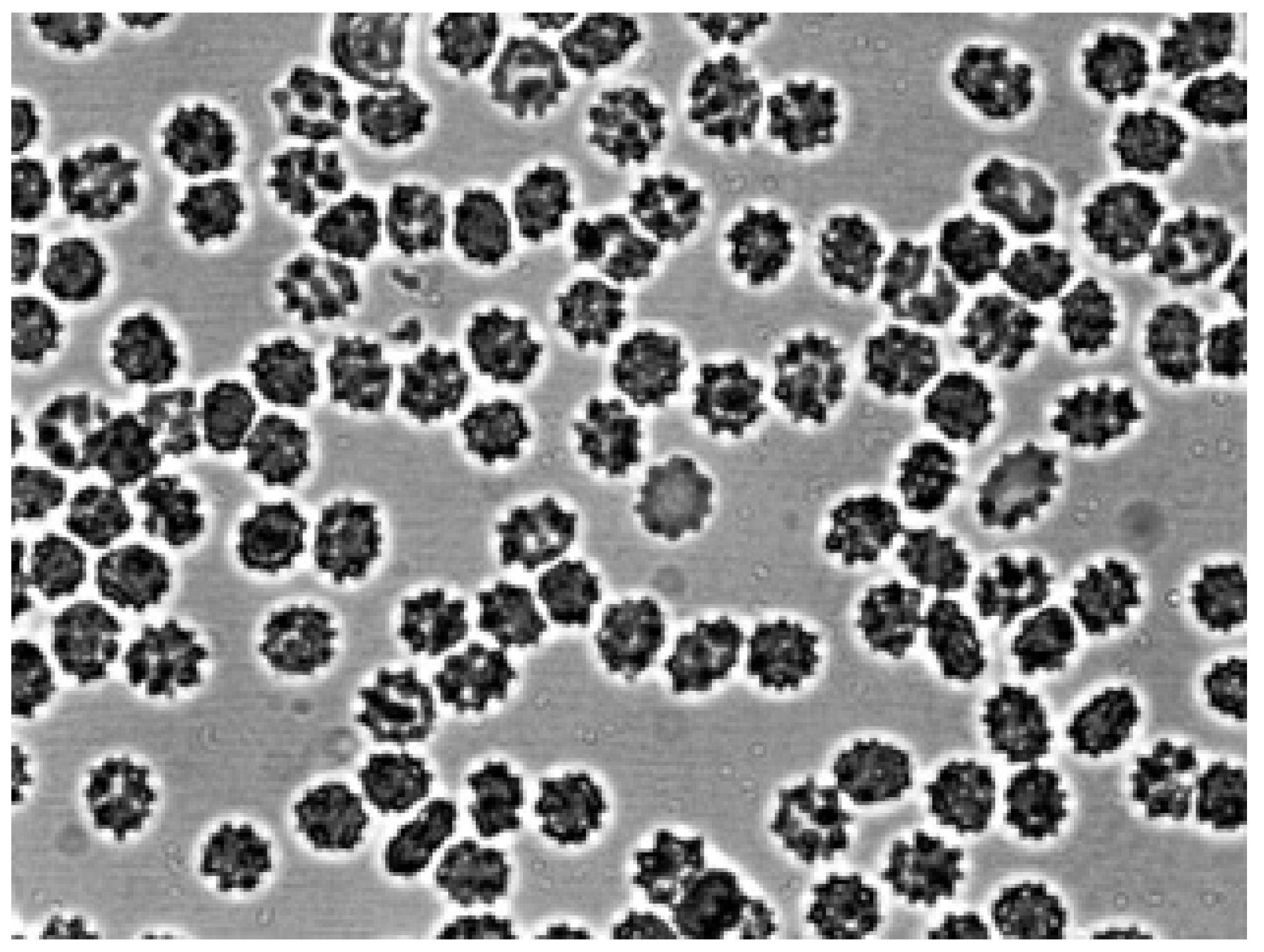
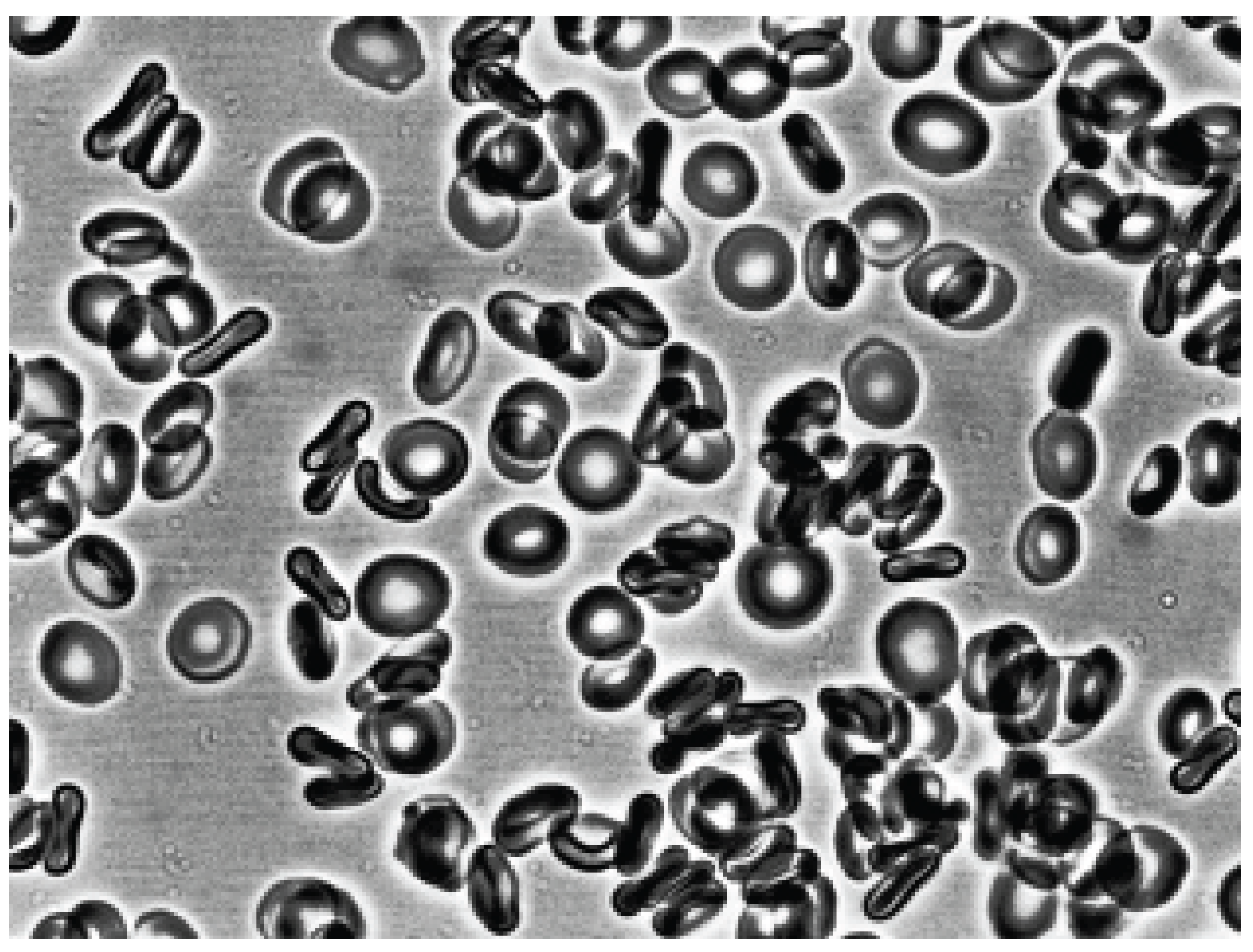
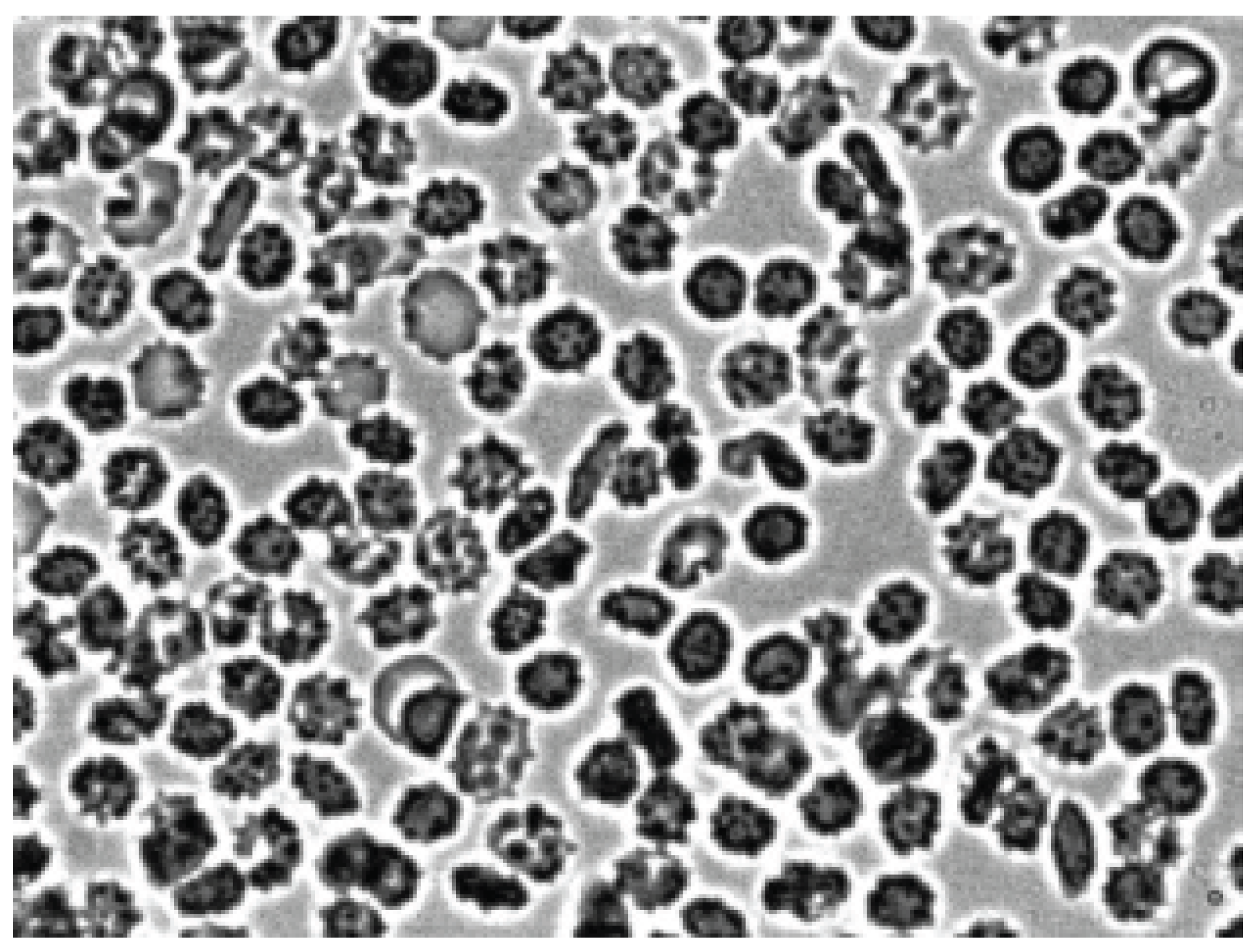
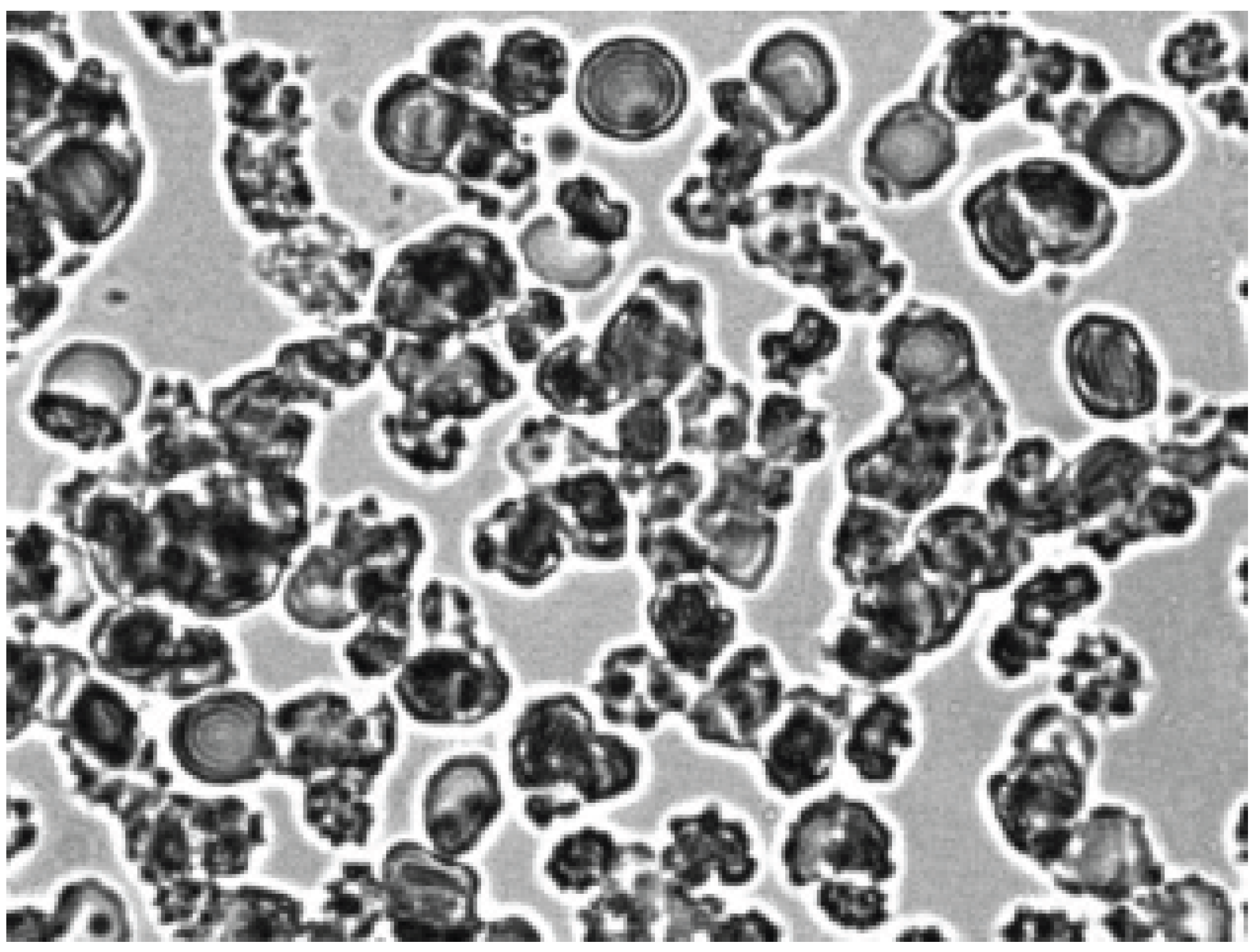
Figure 2 shows that after the contact of the two glass surfaces, the glass effect was observed: red blood cells from discocytes turned into echinocytes. Next, autologous plasma or 3% dextran 70 was added to the echinocytes and intensively agitation.
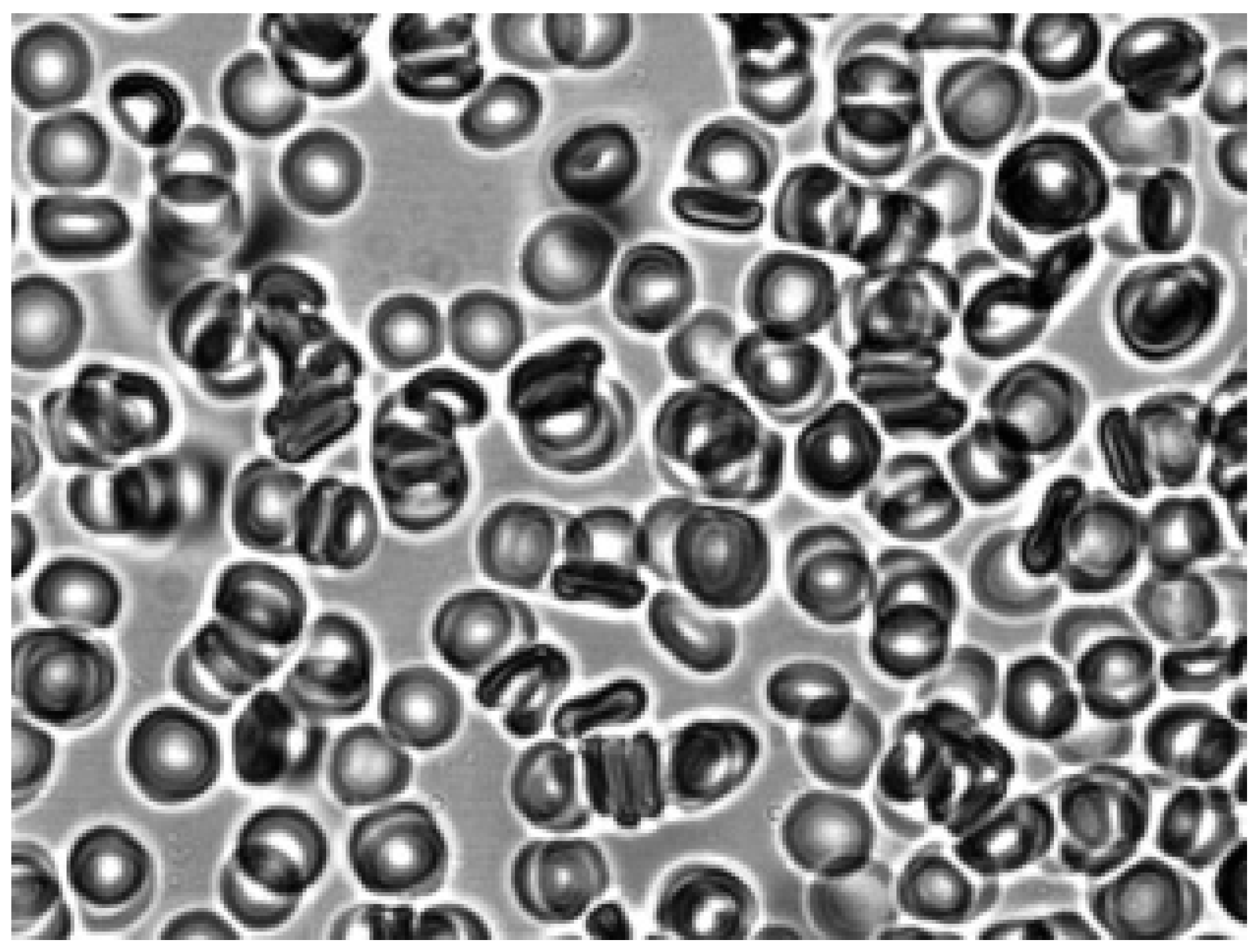
As can be seen in
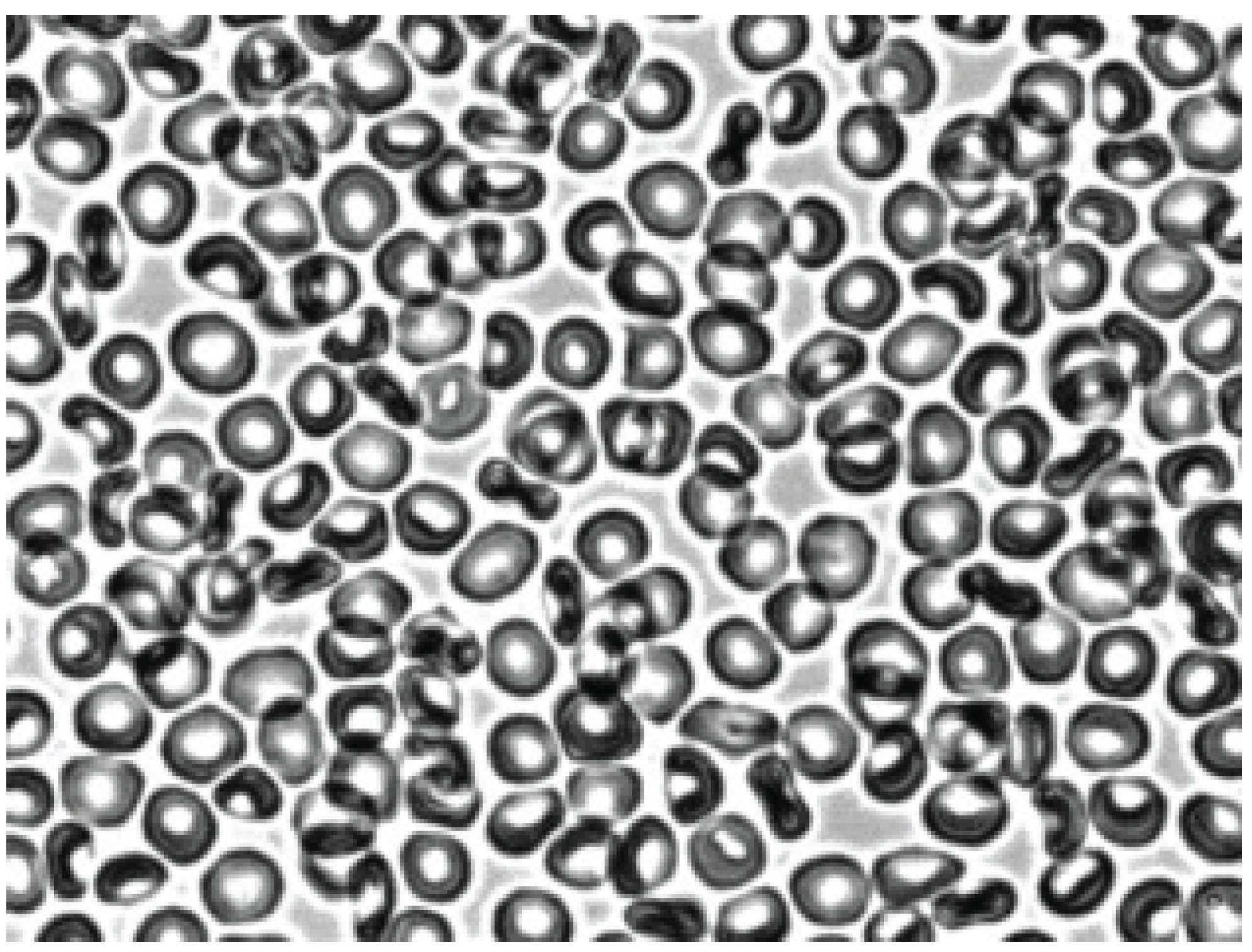
Figure 3, after adding autologous plasma to the echinocytes and agitation, the transformation of echinocytes into stomatocytes was observed. In this case, the cells first turn into discocytes, and then into stomatocytes. The stomatocytes forming in the autologous plasma combine into aggregates in the form of rouleaux (
Figure 4). The entire process of cell transformation from echinocytes to stomatocytes took place within 10 sec. The addition of saline solution to the aggregates of red blood cells in autologous plasma led to cell disaggregation. At the same time, the appearance of stomatocytes from aggregates was observed (
Figure 5).
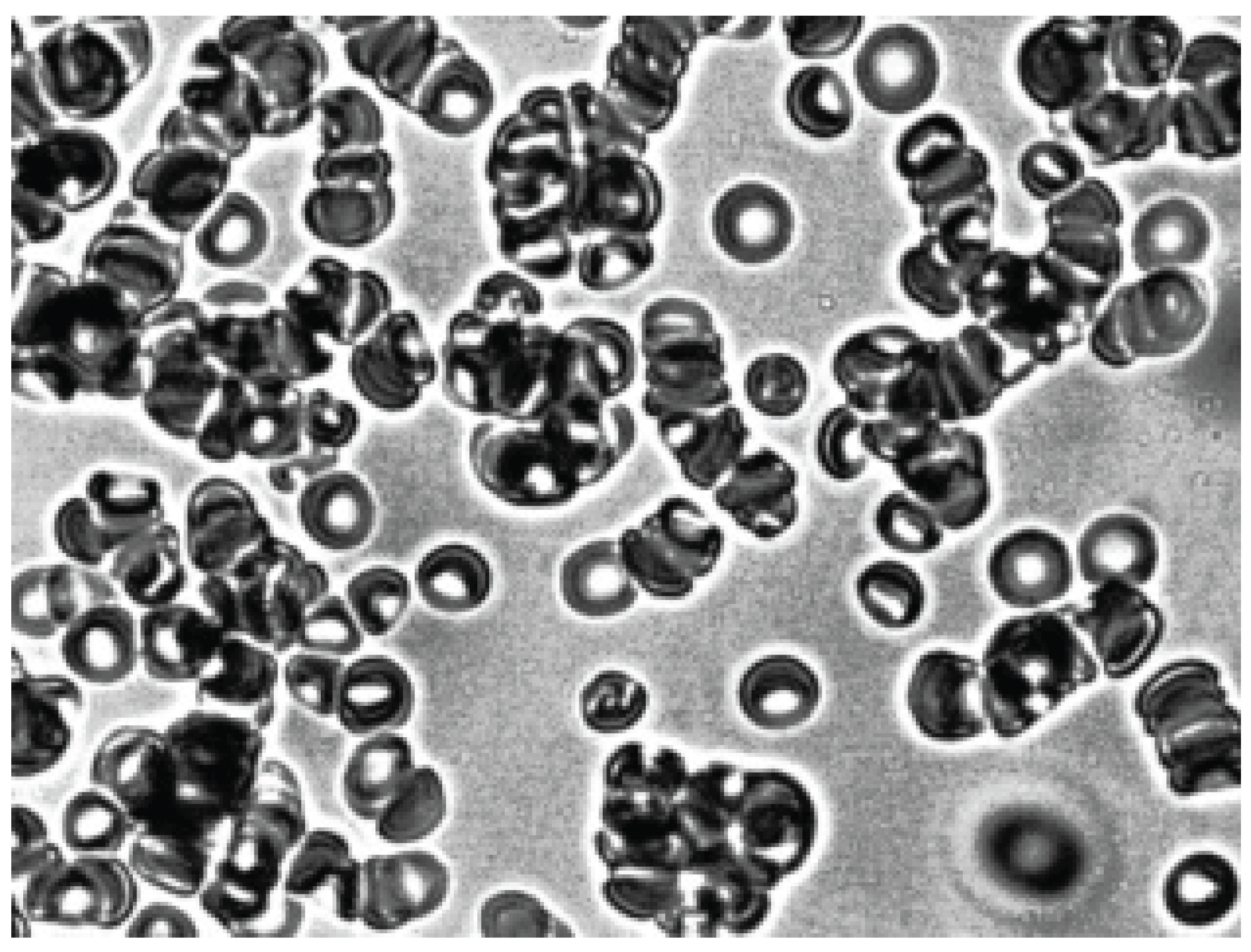
The addition of dextran 70 solution to echinocytes did not affect echinocytes (
Figure 6). However, if 3% human albumin was added to echinocytes, the echinocytes turned into discocytes and then into stomatocytes (
Figure 7). Such stomatocytes aggregated under the influence of dextran 70 (
Figure 8).
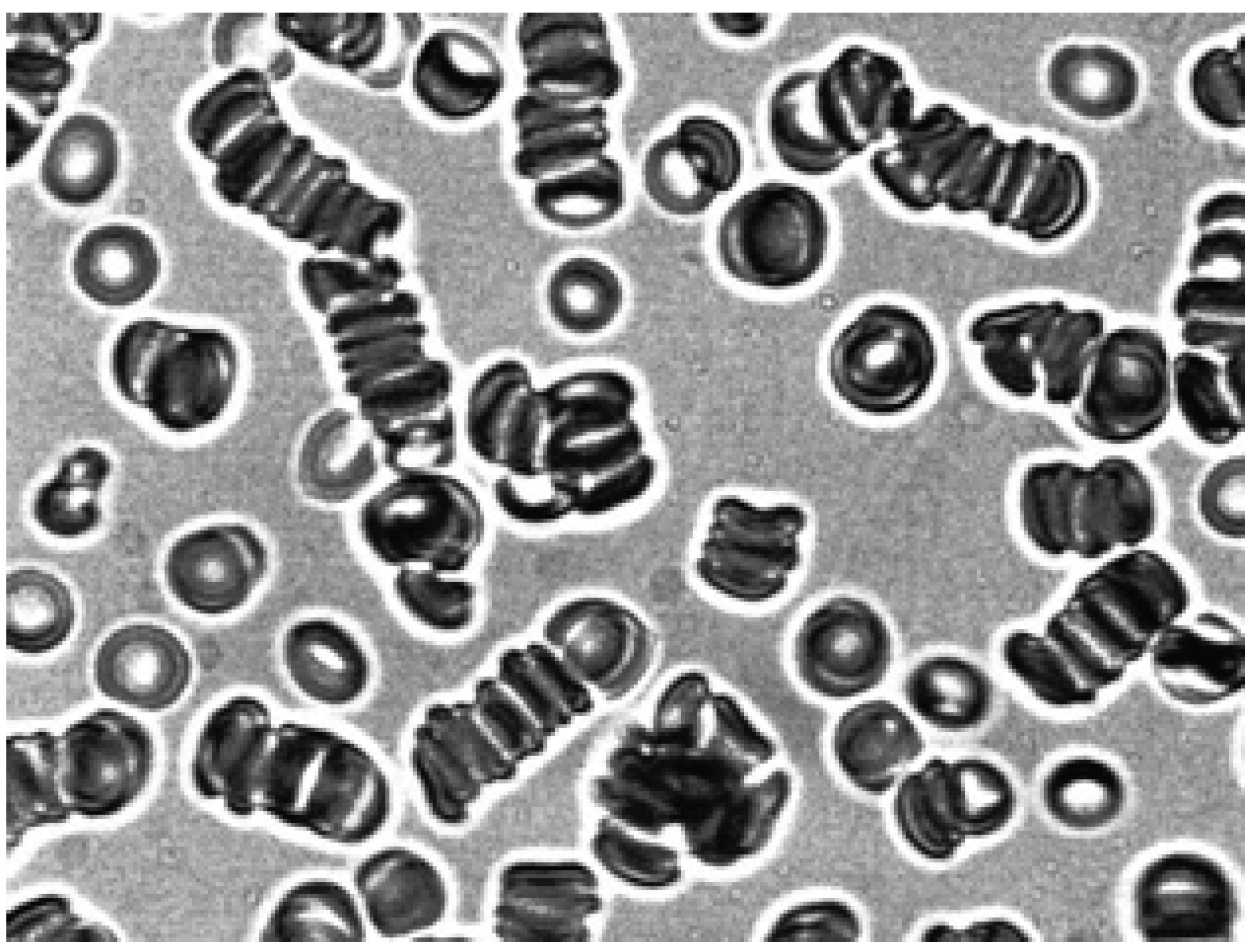
The effect of 0.5% trypsin solution on echinocytes was also studied. The results showed that treatment of echinocytes with trypsin had no effect on their shape. At the same time, dextran 70 induces a strong aggregation of echinocytes previously treated with trypsin (
Figure 9) Apparently, trypsin cleaves the integral protein glycophorin A of erythrocyte membrane which leads to the formation of discrete sites for binding to dextran 70. We have previously shown that treatment of erythrocytes with trypsin increases the degree of their aggregation in autologous plasma and blood serum [
20].
Thus, our results indicate that after intensive agitation (shear stress), red blood cells in the autologous plasma change their shape, turning into stomatocytes. After that, the stomatocytes begin to interact with each other in the autologous plasma to form rouleaux. Dextran 70 had no effect on echinocytes and did not induce their aggregation. However, after adding albumin to echinocytes, red blood cells turned into stomatocytes, which combined into aggregates under the influence of dextran 70. Aggregation of echinocytes was observed only after trypsin treatment.
There are two models explaining the mechanism of aggregation of erythrocytes consisting of rouleaux: bridging [
1] and depletion [
6]. However, none of these models is still generally accepted.
Previously, we hypothesized about the mechanism of red blood cells in the form rouleaux, based on a change in the spontaneous curvature of red blood cell [
18]. According to this hypothesis, the formation of a negative curvature of the erythrocyte membranes leads to the appearance of stomatocytes, followed by their unification into rouleaux.
It is assumed that under the influence of shear stress (in our case, intensive agitation), discocytes are transformed into stomatocytes. Previously, light microscopy showed the reversible transformation of discocytes into stomatocytes after mechanical mixing of a suspension of erythrocytes [
16]. An important point in the formation rouleaux is the normal shape of red blood cells. Red blood cells should have a biconcave discoid shape. Changes in the spontaneous curvature of erythrocyte membranes, which determines the shape of cells, will lead to an increase or decrease in their aggregability. A decrease in red blood cell aggregation is associated with the formation of echinocytes. Apparently, shear stress can not induce negative curvature in echinocyte-shaped erythrocytes and, as a result, the formation of rouleaux. A change in the spontaneous curvature of the membrane can lead to a change in its binding capacity to blood proteins such as fibrinogen and a
2 - macroglobulin. It is known that stomatocytes bind immunoglobulins 3 to 8 times more than normal cells [
21]. As a result of the formation of stomatocytes, discrete sites are formed on their membranes for binding blood proteins. The formation of protein bridges with discrete regions of neighboring cells leads to their aggregation.
Declaration of competing interest
The authors declare that they have no know competing financial interest or personal relationships that could have appeared to influence the work reported in this paper.
Statement of ethics
Ten healthy blood donors have given written informed consent. The study protocol was approved by Local Human Subjects Research Ethics Committee of the Privolzhsky Research Medical University (Russia) (number of investigation 103).
Funding
The research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Chien, S.; Jan, K-M. Red cell aggregation by macromolecules: role of surface adsorption and electrostatic repulsion, J. Supramol. Struct. 1973, 1, 385–409. [Google Scholar] [CrossRef] [PubMed]
- Baskurt, O.K.; Meiselman, H.J. Cellular determinants of low-shear blood viscosity. Biorheology 1997, 34, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Baskurt, O.K.; Meiselman, H.J. Erythrocyte aggregation: basic aspects and clinical importance, Clin. Hemorheol. Microcirc. 2013, 53, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.; Steffen, P.; Svetina, S. Aggregation of red blood cells: from rouleaux to clot formation. Comptes Rendus Phys 2013, 14, 459–469. [Google Scholar] [CrossRef]
- Barshtein, G.; Tamir, I.; Yedgar, S. Red blood cell rouleaux formation in dextran solution: dependence on polymer conformation, Eur. J. Biophys. 1998, 27, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Neu, B.; Meiselman, H.J. Depletion mediated red blood cell aggregation in polymer solutions. Biophysical J 2002, 83, 24–82. [Google Scholar] [CrossRef] [PubMed]
- Rampling, M.W.; Meiselman, H.J.; Neu, B.; Baskurt, O.K. Influence of cell specific factors on red blood cell aggregation. Biorheology 2014, 41, 91–112. [Google Scholar] [CrossRef]
- Semenov, A.N.; Lugovtsov, A.E.; Shirshin, E.A.; Yakimov, B.P.; Muravyov, A.V.; Wagner, C.; Shin, S.; Priezzhev, A.V. Assesment of fibrinogen macromolecules interaction with red blood cells membrane by means of laser aggregometry, flow cytometry; optical tweezers combined with microfluidics. Biomolecules 2020, 10, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Melpattu, M.P.; Maitrejean, G.; Wagner, C.; Podgorski, T. Influence of erythrocyte density on aggregability as a marker of cell age: dissociation dynamics in extensional flow, J. Biomechanics 2025, 183, 112603. [Google Scholar] [CrossRef]
- Moreno, N.; Korneev, K.; Semenov, A.N.; Ellero, M.; Wagner, C.; Fedosov, D.A. Aggregation and disaggregation of red blood cells: depletion versus bridging. Biophysical J. 2025, 124, 1285–1297. [Google Scholar] [CrossRef] [PubMed]
- Reinhart, W.H.; Singh, A.; Straub, P.W. Red blood aggregation and sedimentaion : the role of the cell shape, Br. J. Haematol. 1989, 73, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Reinhart, W.H.; Baerloher, G.; Cerny, T.; Owen, G.R.; Meiselman, H.J.; Beer, J.H. Ifosfamide - induced stomatocytosis and mesna - induced echinocytosis influence on biorheological properties of blood, Eur. J. Haematol. 1999, 62, 223–230. [Google Scholar] [CrossRef]
- Sheremet’ev, Yu.A. , Popovicheva, A. N.; Egorihina, M.N.; Levin, G.Y. Study of the relationship between shape and aggregation change in human erythrocytes, Biophysics 2013, 58, 193–196. [Google Scholar] [CrossRef]
- Ponder, E. Hemolysis and related phenomena; Grune and Stratton: New York, 1948. [Google Scholar]
- Eriksson, L.E.G. On the shape of human red blood cells interacting with flat artifical surfaces - the glass effect, Biochim. Biophys. Acta 1990, 1036, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Jay, A.W.L. Geometry of the human erythrocyte. 1. Effect of albumin on cell geometry, Biophysical J. 1975, 15, 205–222. [Google Scholar]
- Mehta, N.G. Role of membrane integral proteins in the modulation of red cell shape by albumin, dinitrophenol and glass effect, Biochim. Biophys. Acta 1983, 762, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Sheremet’ev, Yu.A. , Popovicheva, A. N.; Levin G.Y. Lysophosphatidic acid and human erythrocyte aggregation, Cell and Tissue Biology. 2014, 8, 237–243. [Google Scholar] [CrossRef]
- Sheremet'ev, Yu.A. , Popovicheva, A.N.; Rogozin, M.M.; Levin, G.Y. Red blood cell aggregation, disaggregation and aggregate morphology in autologous plasma and serum in diabetic foot disease, Clin. Hemorheol. Microcirc. 2019, 72, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Sheremet'ev, Y.A. Effect of trypsin on aggregation, disaggregation and aggregate morphology of red blood cells in autologous plasma and serum. bioRxiv 2021. [Google Scholar] [CrossRef]
- Cosgrove, P.; Sheetz, M.P. Effect of cell shape on extravascular hemolysis. Blood 1982, 59, 421–427. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).