1. Introduction
In drug development, typical experiments for drug discovery often use animal experiments and cell-based assay tests. Animal experiments have various issues, such as ethical considerations and species differences. Cell-based assays have issues with cultured cells that do not retain the organ's function in vitro. In recent years, in medicine and life sciences, microphysiological systems (MPSs), including organ-on-a-chip (OoC), are attracting attention as promising technologies that can reproduce in vivo-like tissue structures and fluid flow on microfabricated platforms. MPSs are increasingly being utilized to evaluate drug pharmacokinetics, toxicity, absorption, metabolism, and interorgan interactions under controlled in vitro conditions [
1,
2,
3,
4]. Multiorgan microphysiological systems (MO-MPS) enable the co-culture of multiple organ-derived cells, allowing the study of complex organ-organ interactions in real time and contributing to more accurate drug evaluation systems [
5,
6,
7,
8].
A key element for reproducing physiological conditions in MPS is the application of dynamic fluid flow to simulate blood circulation. Conventionally, syringe and peristaltic pumps have been used to generate perfusion flow; however, these external pump systems require complex tubing connections, present contamination risks and air bubbles, and consume large volumes of culture medium, increasing experimental costs and reducing throughput [
9,
10]. Various integrated pumping methods, including pneumatic pumps, membrane pumps, and gravity-based systems, have been developed to address these issues. However, many still involve complicated operations or closed-channel configurations that reduce usability for long-term culture and co-culture setups.
To overcome these limitations, a stirrer-based kinetic pump system was previously developed, which generates fluid circulation by rotating miniature magnetic stir bars embedded inside the culture device [
11]. This technology led to the development of the Kinetic Pump Integrated Microfluidic Plate (KIM-Plate), which combines the stirrer-based kinetic pump with an open-access multiorgan microphysiological design [
12]. The KIM-Plate comprises six independent MO-MPS units conforming to ANSI/SBS microplate standards, allowing direct use with commercially available cell culture inserts and disks. Open-top culture chambers of the KIM-plate simplify handling and enable the co-culture of multiple organ models. However, the KIM-Plate was made of black polymer, which made it difficult to check the stirrer rotation. Smitomo Bakelite developed the BioStellar™ Plate as a redesigned next-generation system based on the KIM-Plate concept to improve usability. The BioStellar™ Plate retains the exact kinetic pump mechanism and open-access design but employs transparent polystyrene for full compatibility with standard microscopy. While the BioStellar™ Plate provides continuous perfusion, it cannot apply controlled fluidic shear stress (FSS), which is an important factor influencing cellular morphology, differentiation, and barrier function under physiological conditions, because of the open-top culture chambers.
To address this limitation, we developed FSS loading attachments capable of guiding fluid flow and introducing controlled FSS onto epithelial cells cultured within the BioStellar™ Plate in this study. We designed and fabricated fluidic shear stress attachments (FSSAs) for the BioStellar™ Plate, conducted flow simulations, and verified their hydrodynamic behaviour through experiments. We successfully introduced controlled FSS during dynamic perfusion by applying these attachments to epithelial cell cultures. As a result, cells exposed to FSS showed improved growth and organization compared to static cultures, demonstrating the effectiveness of this approach in enhancing the BioStellar™ Plate for future microphysiological applications.
2. Materials and Methods
2.1. BioStellar™ Plate
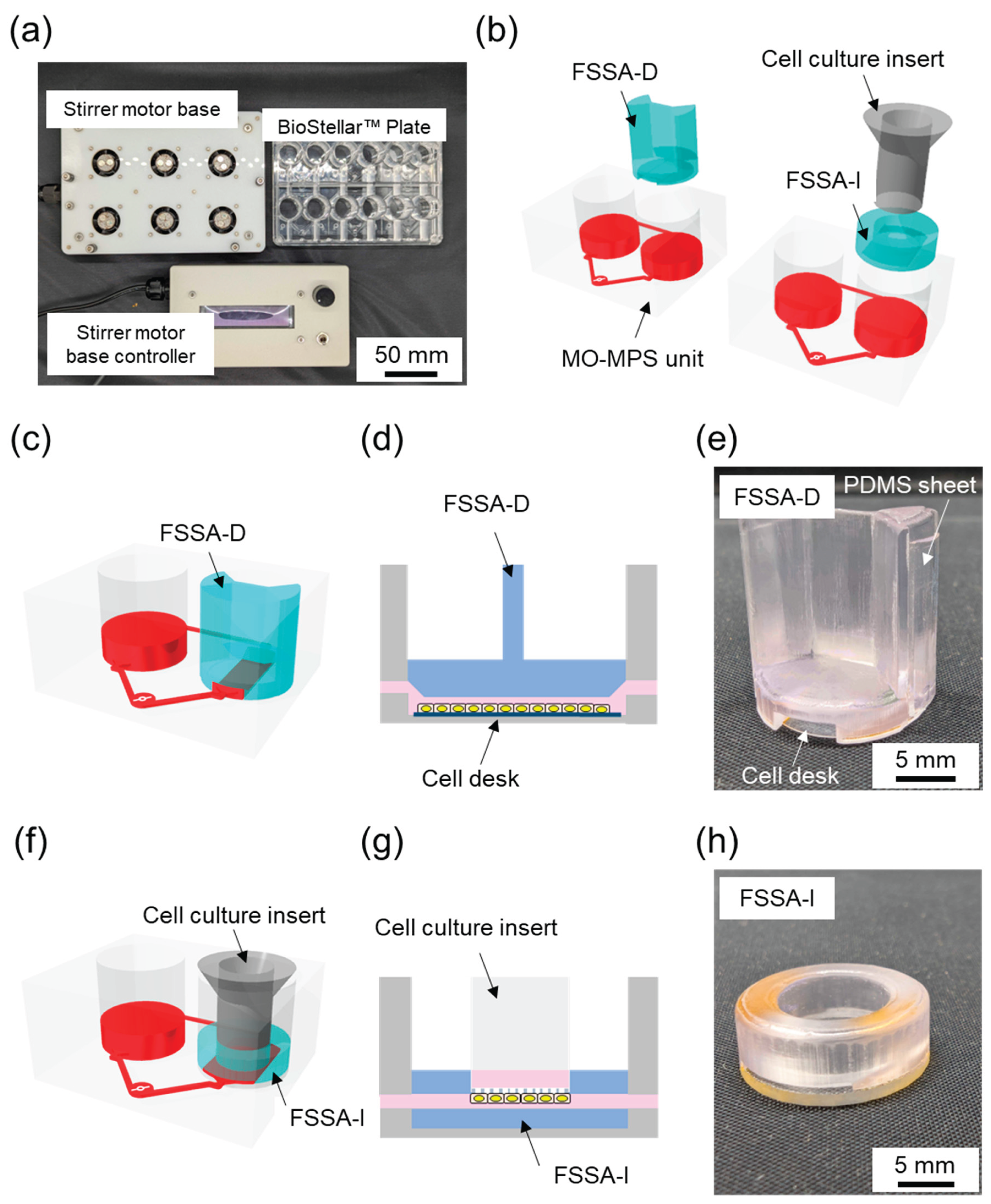
The BioStellar™ Plate (Sumitomo Bakelite, Tokyo, Japan) consists of six independent MO-MPS units that conform to ANSI/SBS microplate standards (128 mm × 85 mm × 40 mm). Each unit features a pair of open-top culture chambers with the same diameter and configuration as standard 24-well plates, making it fully compatible with conventional cell culture protocols. Commercially available cell culture inserts and disks can be used directly within the plate without any modifications (
Figure 1a). The entire plate body was fabricated from optically clear polystyrene (PS) using injection moulding. This enhances imaging compatibility and allows for seamless observation during culture. The microfluidic architecture connects each chamber pair by microchannels (1.0 mm wide × 0.3 mm high). These channels were positioned 1.2 mm above the bottom of the chamber. This construction limited direct fluid shear stress on cells.
Culture medium circulation was achieved through an embedded stirrer-based kinetic pump driven by a 3 mm × 0.2 mm stainless-steel stirrer bar. The clockwise rotation of a magnetic rotor beneath the plate generates forward flow through the main channel. In contrast, backflow through the secondary channel completes the loop, creating a continuous circulation system. The BioStellar™ Plate was designed to be used with a custom-built magnetic stirrer motor base that holds six magnetic rotor units, each driven by DC axial fans (108-AFB0412HHA-TA5F, Delta Electronics, Taipei, Taiwan). The base and its controller, produced by Microfluidic System Works, allow simultaneous control of rotor speeds, ensuring consistent and adjustable flow across all six MO-MPS units (
Figure 1a). The system could reach rotation speeds of up to 6,500 rpm in its current configuration, allowing for a broad operational range that generates various flow rates suitable for both low-shear and high-shear experimental conditions.
2.2. Fluidic Shear Stress Attachments
To specifically introduce regulated FSS to cell cultures within the BioStellar™ Plate, we developed two customised fluidic shear stress attachments (FSSAs), each designed to accommodate different cell culture formats while maintaining compatibility with the BioStellar™ Plate (
Figure 1b).
The Fluidic Shear Stress Attachment – Cell Desk (FSSA-D) was designed to support flat-surface cultures on the cell desk (
Figure 1c, d,
Figure S1a). The attachment features a centrally aligned straight microchannel that is 5.5 mm wide and 0.4 mm high after the installation of the cell desk. To ensure a consistent flow, the channel includes 35° slopes at both the inlet and outlet, effectively guiding fluid into the central region of interest. The entire FSSA-D body measures 15.9 mm in diameter and 17.0 mm in height, and it includes a concave slot (13.7 mm diameter, 0.3 mm depth) at the bottom to secure the cell desk (MS-92132, Sumitomo Bakelite, Tokyo, Japan) in place. Additionally, two grooves, each 0.5 mm deep and 7.6 mm wide, run along the sidewalls to accommodate PDMS sheets, creating a soft, watertight seal when interfaced with the BioStellar™ Plate. This channel configuration enables unidirectional laminar flow across the apical cell surface, ideal for mimicking physiological shear stimulation (
Figure 1e).
The Fluidic Shear Stress Attachment – Insert (FSSA-I) was specifically engineered for cell culture inserts used in 24-well plate formats (
Figure 1f, g,
Figure S1b). It comprises a circular body with a 16.0 mm diameter and 5.5 mm height, featuring a central insertion port that was 4.0 mm high and 9.4 mm in diameter, securely accommodating the insert from above. The flow-guiding region beneath the insert is structured as a rectangular channel with a 9.3 mm width and 0.5 mm height, allowing medium to perfuse evenly along the basal surface of the membrane. The bottom structure of the FSSA-I was made from 1 mm thick acrylic, providing rigidity while maintaining optical clarity for microscopy. By redirecting flow beneath the membrane, the FSSA-I enables localised FSS application to the basal side of epithelial layers, thereby replicating dynamic microenvironments similar to those found in intestinal, renal, or vascular systems (
Figure 1h).
2.3. Fabrication of the FSSAs
FSSAs were fabricated using a high-resolution 3D printer Formlabs Form 3B+ (PKG-F3B-COMPLETE-EW-1, Formlabs, Massachusetts, USA) with BioMed Clear resin (RS-F2-BMCL-01, Formlabs, Massachusetts, USA), a biocompatible photopolymer suitable for cell culture applications. The FSSAs’ geometries were designed using AutoCAD (Autodesk, California, USA) and exported as STL files, which were subsequently processed in PreForm software (Formlabs, Massachusetts, USA). The models were printed using default settings with a layer thickness of 50 µm to achieve high-dimensional precision and smooth surface quality suitable for microfluidic use. Following printing, the attachments were washed in isopropyl alcohol (IPA) using the Form Wash (FH-WA-02, Formlabs, Massachusetts, USA) for 15 minutes to remove uncured resin from channel structures and surfaces. The printed parts were then post-cured using Form Cure (FH-CU-01, Formlabs, Massachusetts, USA) at 60 °C for 30 minutes, ensuring complete crosslinking and mechanical stability of the material. The cured attachments underwent a detailed multi-step solvent-washing protocol designed to remove toxins from the printed material and prepare it for further biological use. Initially, each FSSA was submerged in acetone and cleaned with an ultrasonic washer for 60 minutes.-After the acetone wash, the attachments were rinsed in IPA for 30 minutes using an ultrasonic washer to ensure that any traces of acetone were thoroughly removed. The attachments underwent three consecutive washes, each lasting 30 minutes, with ion-exchanged water, again using an ultrasonic washer. Finally, the attachments were sterilized by autoclaving at 120 °C for 20 minutes. This high-temperature process eliminated any potential microbial contamination. After autoclaving, the FSSAs were allowed to cool overnight to minimize the risk of cracking caused by thermal stress, ensuring they were safe and ready for further applications.
The bottom of FSSA-D was installed using the cell desk and double-sided tape, forming a connected perfusion chamber. To reinforce the lateral fit and maintain structural alignment within the BioStellar™ Plate, a 0.5 mm thick PDMS sheet was applied along the sidewall’s grooves of the attachment (
Figure 1e). Conversely, the FSSA-I was assembled onto a custom-fabricated acrylic base to accommodate a cell culture insert (Transwell
®, 3450, Corning, New York, USA) (
Figure 1h). The components were joined using double-sided tape to ensure stable integration.
Following assembly, the FSSAs underwent gas sterilization to ensure sterility across all contact and interface surfaces. Before beginning biological experiments, the FSSAs were stored for at least one week to remove any potential cytotoxicity from residual sterilant gases.
2.4. Numerical Simulation in Flow
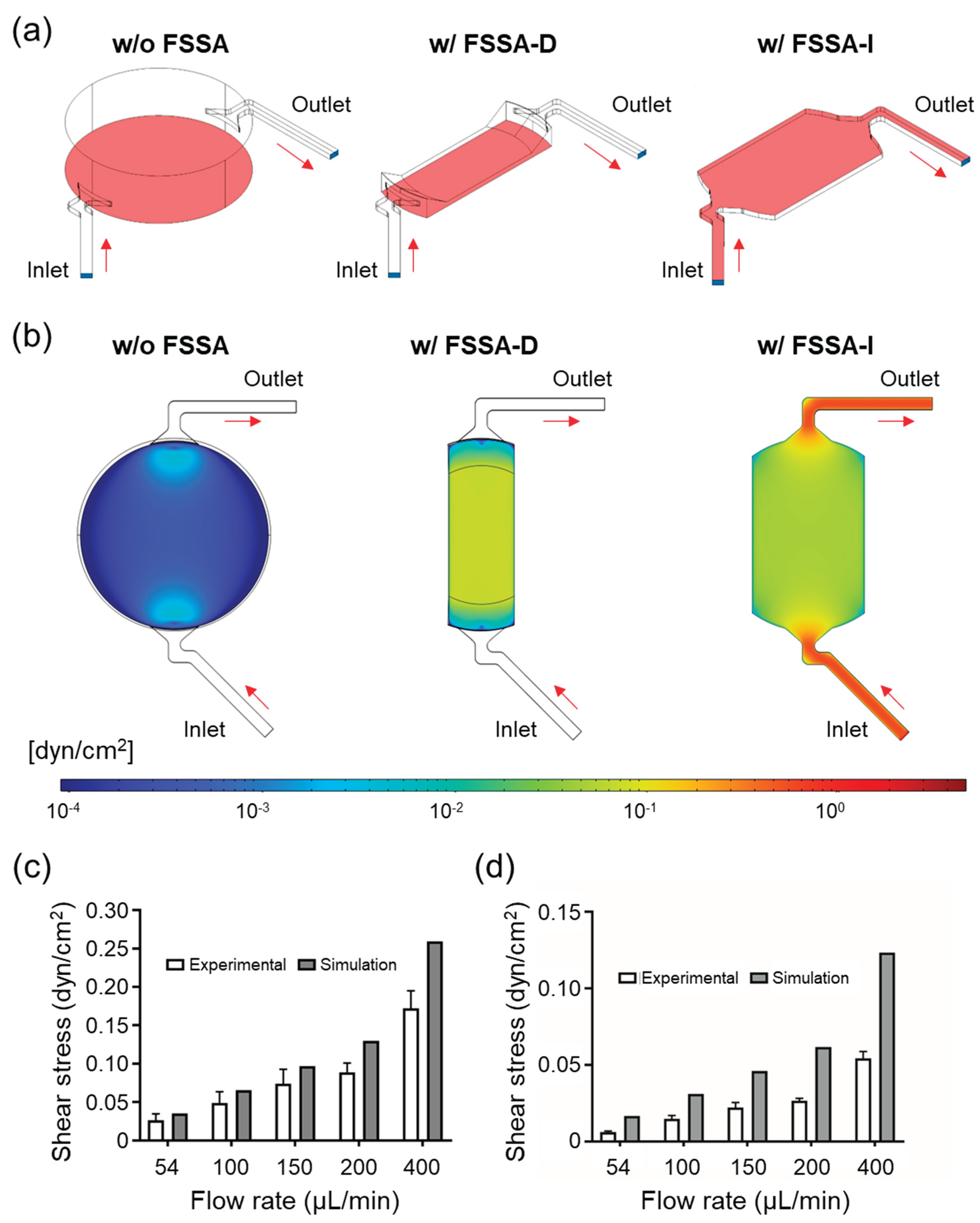
To evaluate the flow characteristics within the BioStellar™ Plate, 3D models of the cell culture chamber, FSSA-D, and FSSA-I were constructed using COMSOL Multiphysics (COMSOL, Stockholm, Sweden). The simulation domain included a cylindrical chamber measuring 16.4 mm in diameter and 4.7 mm in height, with two fluidic channels on opposite ends. Each channel measured 1.0 mm in width and 0.5 mm in height, functioning as the inlet and outlet. The working fluid was modelled using water properties at 37°C, with slight modifications to reflect physiological conditions accurately. Simulations were conducted under five different inlet flow velocities to analyse variations in velocity distribution across both FSSA designs.
2.5. Flow Observation
To experimentally validate flow simulations and characterise flow behaviour, we performed particle tracking velocimetry using fluorescent microbeads (10 μm in diameter, 1.1 × 10⁸ particles/mL, 18142, Polyscience, Illinois, USA) as flow tracers. Instead of using the full BioStellar™ Plate, we employed a custom-fabricated PDMS-based open-top culture chamber model that resembled the BioStellar™ Plate architecture. The chamber model retained the essential features of the BioStellar™ Plate, including inlet and outlet water channels and a single cell culture chamber, but also included a glass-bottom to facilitate high-resolution imaging (
Figure S2).
The FSSAs were mounted onto the chamber model to replicate the in-situ flow environment. Flow was driven by two syringe pumps programmed to mimic the pulsatile perfusion behaviour generated by the BioStellar™ Plate. We recorded bead movement in the central region of each attachment using an Olympus IX71 (Olympus Corporation, Tokyo, Japan) inverted fluorescence microscope paired with a video camera (FDR-AX700, Sony, Tokyo, Japan) operating in super slow-motion mode (240 fps).
The captured videos were analysed using FlowExpert64 software (Katokoken, Kanagawa, Japan), a particle image velocimetry (PIV) platform, to quantitatively extract flow velocity fields and estimate local FSS in the attachment channels. The
Supplementary Information includes a representative video of the microbead flow behaviour (
Figure S3, Video S1, S2).
2.6. Estimation of FSS
FSS (
τ) (Pa) was calculated from the velocity data using the following equation [
13,
14]:
where μ is the viscosity of water at 37 °C (0.6913 mPa·s), u is the average flow velocity (m/s), and h is the channel height (m).
It is worth noting that the flow velocity obtained through microscopy reflects the peak velocity at the centre (z-axis) of the microchannel rather than the average velocity across the entire cross-section, an important distinction when calculating FSS. Additionally, while FSS was calculated using standard SI units (Pascal, Pa) via the equation, the values reported throughout the results section are expressed in dyne per square centimetre (dyn/cm²) for consistency with the biological literature. For reference, 1 Pa is equivalent to 10 dyn/cm². In cylindrical channels, the average velocity is generally assumed to be half the maximum. However, this relationship does not carry over to rectangular channels like the FSSAs. To address this, we used ANSYS Fluent (Ansys, Pennsylvania, USA) to simulate the precise channel geometries of both FSSA-D and FSSA-I and to determine the ratio between maximum and average velocities. From these simulations, correction factors of 1.55 for FSSA-D and 1.56 for FSSA-I were obtained and applied to convert the measured peak velocities into more accurate average velocities (
Figure S4).
2.7. Cell Culture
Human epithelial colorectal adenocarcinoma cells (Caco-2, ATCC, Virginia, USA) were cultured in 100 mm culture dishes (3020-100, Iwaki, Shizuoka, Japan) using Dulbecco’s Modified Eagle Medium (DMEM, 11885092, Thermo Fisher Scientific, Massachusetts, USA). The medium contained 1 g/L glucose and 25 mM HEPES, supplemented with 10% fetal bovine serum (FBS, 10270-106, Thermo Fisher Scientific, Massachusetts, USA), 1% antibiotic-antimycotic (AA, 161-23181, Fujifilm Wako Pure Chemical Corporation, Osaka, Japan), and 1% non-essential amino acids (NEAA, 11140-050, Thermo Fisher Scientific, Massachusetts, USA). Cultures were maintained at 37°C in a humidified incubator with 5% CO₂. The culture medium was replaced every two days throughout the expansion and experimental culture periods to maintain nutrient balance and cell viability.
In the FSSA-D configuration, the FSS group was cultured using dynamic perfusion within the FSSA placed on the cell desk. The control group was cultured on cell desks in standard well plates without the FSSA under conventional static conditions. This configuration allowed for a direct comparison between conventional static culture and the effect of applied FSS generated by the FSSA-D. In the FSSA-I configuration, cells were cultured on the basal side of a microporous membrane of a cell culture insert (Transwell®, 3450, Corning, New York, USA) in both the control group and the FSS group. This configuration allowed access to the basal membrane surface for FSS exposure.
Two types of extracellular matrix (ECM) coatings were prepared. For FSSA-D, a Collagen Type I-P solution was prepared by mixing 1 mL of Collagen Type I-P (634-00663, Nitta Gelatin, Osaka, Japan) with 9 mL of ultrapure water, followed by the addition of 10 µL of hydrochloric acid to adjust the pH to approximately 3. The FSSA-D was submerged in this solution and incubated at 37°C for 1 hour to allow for uniform coating of the microchannel surfaces. For the FSSA-I, Matrigel (354230, Corning, New York, USA) was used at a 1:25 dilution in a cold medium and applied solely to the membrane surface of the cell culture insert. The inserts were incubated at 37°C for 1 hour to ensure a consistent ECM coating.
In case of cell seeding for FSSA-D, Caco-2 cells were seeded directly into the coated channels at 2 × 10⁵ cells/cm². Approximately 80 µL of the cell suspension was introduced into each channel, followed by a 5-hour pre-culture period in a 12-well plate containing the surrounding culture medium. After sufficient cell attachment, the FSSA-D were transferred into the BioStellar™ Plate to initiate perfusion culture.
In case of cell seeding for FSSA-I, inverted seeding facilitated basal membrane culture. The cell culture inserts were placed upside down in a 12-well plate, and 2 × 10⁵ cells/cm² of Caco-2 cells were seeded onto the basal membrane surface using a custom-fabricated PDMS cup-like holder to retain the seeding medium. Each insert received 100 µL of medium and was incubated inverted for 3 hours for cell attachment. Afterwards, the inserts were returned to their upright position and pre-cultured in a standard 24-well plate for 1 day, then transferred into the BioStellar™ Plate system for perfusion culture.
2.8. TEER Measurement
For the FSSA-I experiments, epithelial barrier formation was assessed using a transepithelial electrical resistance (TEER) measurement system (Millicell ERS-2, MERS00002, Merck Millipore, Massachusetts, USA). Chopstick electrodes were positioned with one probe in the apical compartment and the other in the basal compartment. TEER values were calculated by subtracting the background resistance of a blank insert from the resistance measured in the cell-seeded insert. TEER measurements were performed before each medium change to monitor barrier development under consistent conditions.
2.9. Fluorescence Staining and Imaging
To visualize cell morphology and tight junction formation, cultured cells were prepared for fluorescence staining. Cells were rinsed with PBS (+) (D8662-500mL, Sigma-Aldrich, Massachusetts, USA) and fixed using 4% paraformaldehyde (163-20145, Wako Pure Chemical Corporation, Osaka, Japan) for 15 minutes. After rinsing with PBS (-) (D1408-500mL, Sigma-Aldrich, Massachusetts, USA) the cells were permeabilized with 1% Triton X-100 (02081155, KISHIDA CHEMICAL, Osaka, Japan) for 10 minutes, followed by additional washes with PBS (-). Samples were blocked using 1% BSA/PBS for 1 hour at room temperature.
Tight junctions were stained using a primary ZO-1 antibody (21773-1-AP, Proteintech, Illinois, USA) diluted 1:500 in 1% BSA/PBS and incubated overnight at 4°C in the dark. The next day, cells were washed and stained with a combined solution of DAPI (340-07971, DOJINDO, Kumamoto, Japan) (1:1000), Alexa Fluor 488 Phalloidin (PHDG1, Cytoskelton.inc., Colorado, USA) (14 µM), and Alexa 568 secondary antibody (A10042, Thermo Fisher Scientific, Massachusetts, USA) (1:1000), all diluted in 1% BSA/PBS. The staining solution was applied to the inserts and incubated for 1 hour at room temperature. After the final washes with PBS (-), the samples were stored in PBS (-) and imaged using a confocal laser microscope at the Tokai University Imaging Center for Advanced Research.
2.10. Statistical Analysis
All data are presented as the mean ± standard deviation (SD) derived from a minimum of three independent experiments. Statistical analysis was conducted using Student's t-test, with statistical significance determined at a threshold of * p < 0.05, ** p < 0.01, *** p < 0.005.
4. Discussion
In this study, we developed and evaluated two FSSAs, FSSA-D and FSSA-I, for integration with the BioStellar™ Plate microphysiological system platform. These attachments enable the precise application of low-level FSS to epithelial cell cultures. Our combination of computational simulations and experimental flow validation demonstrated that both attachments effectively transformed the previously irregular recirculating flow within the BioStellar™ Plate chamber into stable, uniform laminar flow environments [
18]. The controlled hydrodynamic conditions produced by these devices facilitated reproducible FSS loading.
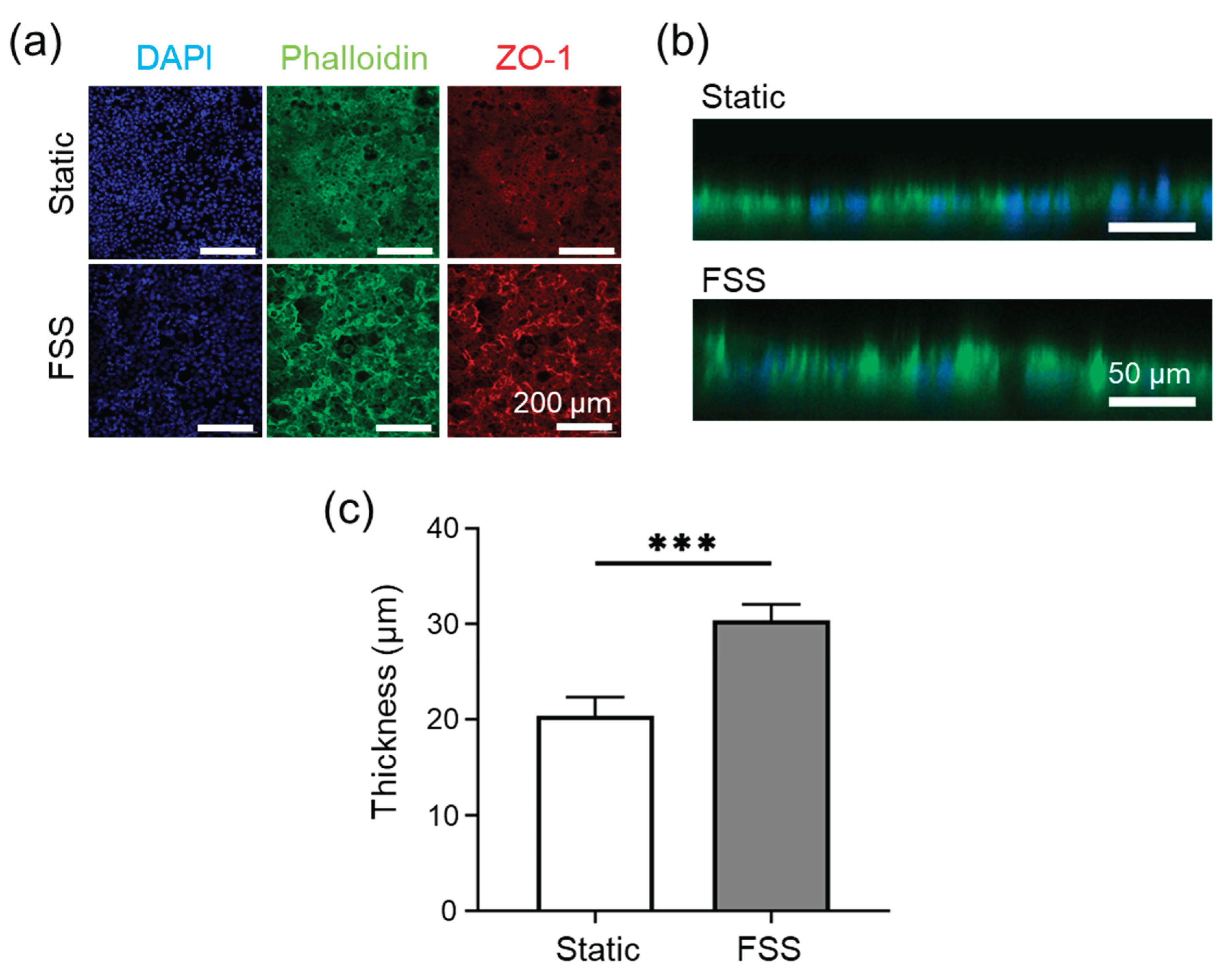
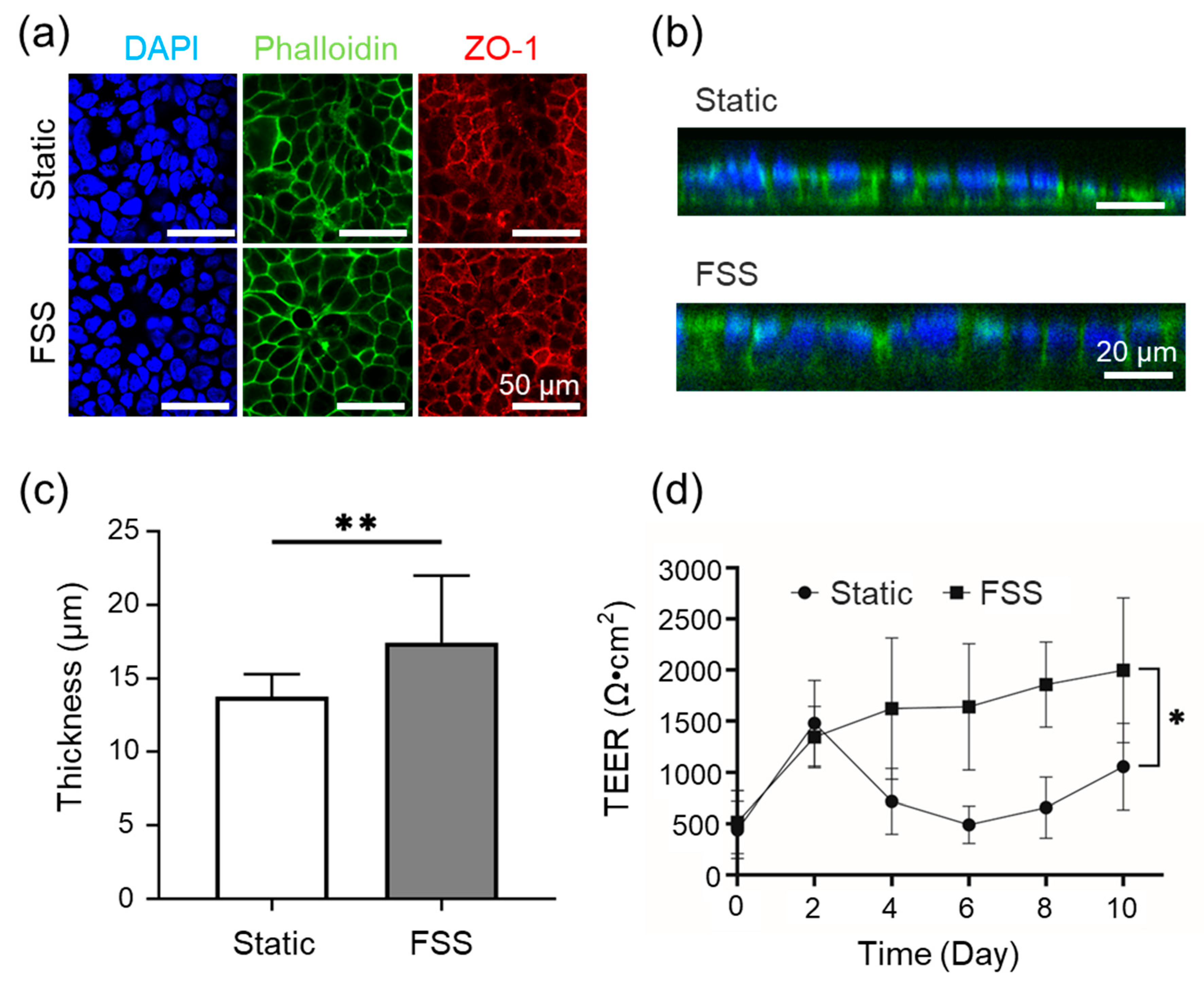
Biological assessments utilizing Caco-2 cells indicated that static and FSS conditions using both FSSA-D and FSSA-I supported the formation of a confluent monolayer. Cells exposed to controlled FSS exhibited enhanced transepithelial electrical resistance (TEER), increased epithelial layer thickness, and improved barrier function compared to static controls. The FSS of 0.02 dyn/cm² in the cell culture experiment is a physiologically relevant mechanical stimulus closely mimicking the in vivo environment for intestinal epithelial cells [
19,
20,
21,
22]. This value has been reported as optimal in prior studies: for example, Shin et al. demonstrated that flow-dependent physical cues control human intestinal morphogenesis in microengineered gut-on-chip systems, while other research has shown that physiological shear stress enhances differentiation, mucus formation, barrier integrity, and 3D tissue architecture at this stress level [
19,
20,
21,
22,
23,
24]. Most notably, quantitative functional assessment of epithelial barrier properties, as measured by TEER, was possible in the FSSA-I configuration [
25]. Here, application of FSS resulted in a progressive increase and stabilisation of TEER values, indicative of tighter junctions and improved barrier integrity [
26,
27]. These results corroborate extensive literature indicating that mechanical cues from physiologically relevant FSS accelerate epithelial maturation, enhance cytoskeletal and junctional organisation, and support the development of more predictive and robust in vitro intestinal models [
19,
20,
21,
22,
28]. These findings confirm that low-level FSS improves architecture and barrier function, demonstrating the effectiveness of the BioStellar™ Plate system with FSSAs and its reliability in modelling physiological epithelial responses [
24,
29,
30,
31].
In conclusion, incorporating these FSSAs significantly enhances the functional capabilities of the BioStellar™ Plate platform by introducing controllable mechanical stimulation while ensuring compatibility with standard culture formats. Our FSSAs provide a straightforward and accessible approach for mimicking physiologically relevant flow conditions in vitro. Our attachments help investigate FSS-mediated effects on epithelial cell function and intestinal barrier models within microphysiological systems.