1. Introduction
Carfilzomib (Kyprolis
®), a second-generation proteasome inhibitor, is a tetrapeptide ketoepoxide-based inhibitor that is specific for the chymotrypsin-like active site of the 20S proteasome. It is structurally and mechanistically distinct from the dipeptide boronic acid proteasome inhibitor bortezomib (Velcade
®).[
1,
2] In adults with multiple myeloma, a range of dosing strategies for carfilzomib have been used, and the most common adverse events include fatigue, neutropenia, nausea, diarrhea, thrombocytopenia, and dyspnea.[
3,
4] Carfilzomib has demonstrated significant
in vitro activity alone and in combination with other antineoplastic agents against a panel of pediatric leukemic and solid tumor cell lines, including neuroblastoma, Ewing sarcoma, osteosarcoma, rhabdomyosarcoma, and atypical teratoid rhabdoid tumor (ATRT).[
5,
6,
7,
8,
9]
In this study, carfilzomib was combined with a chemotherapy backbone of cyclophosphamide and etoposide, with all three agents administered daily for five days. The rationale for the use of this backbone include: 1)
In vitro studies demonstrated synergy of carfilzomib in combination with etoposide and, synergy between cyclophosphamide and proteasome inhibitors;[
5] 2) Both cyclophosphamide and etoposide are active agents in lymphoid and myeloid pediatric leukemias and solid tumors; 3) The combination of cyclophosphamide and etoposide has been used as a standard regimen to treat both solid tumors and leukemias; 4) Because of extensive experience with this chemotherapy backbone, the baseline toxicities are well known; 5) Due to the rapid clearance in adult trials, it was thought that giving carfilzomib sequentially with the standard chemotherapy would maximize synergy; 6) Since the cyclophosphamide and etoposide regimen is routinely administered daily for 5 days, it could be administered on the same days as carfilzomib, utilizing the 5-day dosing schedule of the initial phase 1 adult carfilzomib trial.[
10,
11]
Based on adult carfilzomib trial results, [
3,
12,
13] a phase 1 clinical trial of carfilzomib administered in combination with cyclophosphamide and etoposide was undertaken to: 1) determine the dose-limiting toxicities (DLTs) and the maximum tolerated dose (MTD) of carfilzomib administered in combination with cyclophosphamide and etoposide in pediatric patients with relapsed/refractory leukemia and solid tumors; 2) evaluate the toxicities of carfilzomib in the pediatric population when combined with conventional chemotherapy; and 3) gather preliminary efficacy data on the drug combination.
2. Methods
2.1. Eligibility Criteria
Patients age 6 months to < 30 years were eligible if they had relapsed/refractory leukemia, without central nervous system (CNS) 3 involvement, in second or greater relapse or who have failed at least one re-induction attempt after relapse or refractory disease (stratum A) or a relapsed/refractory non-CNS solid tumor including lymphoma (stratum B). Patients with CNS tumors were excluded due to lack of CNS penetration of carfilzomib.[
14] For patients with solid tumors, measurable disease was not required. Other eligibility criteria included: 1) life expectancy ≥ of 3 months; 2) Karnofsky/Lansky performance of ≥ 50; 3) adequate bone marrow function (absolute neutrophil count ≥ 750/µL, platelets ≥ 75,000/µL, and hemoglobin ≥ 10 g/dL with or without transfusion, unless cytopenias were secondary to leukemia or bone marrow infiltration with solid tumor); 4) adequate liver function (AST and ALT ≤ 3 × upper limit of normal (ULN) for age; total bilirubin ≤ 1.5 × ULN for age, direct bilirubin ≤ ULN for age unless elevation can be clearly attributed to liver leukemia or metastases); 5) adequate renal function (serum creatinine
< ULN for age or a glomerular filtration rate
> 70 ml/min/1.73 m
2); 6) echocardiography shortening fraction ≥ 27%; and 7) pulse oximetry measuring ≥ 95% saturation without supplemental oxygen. Specific eligibility and exclusion criteria are provided in detail in Supplement 2. Informed consent was obtained from the patients or their legal guardians before study entry in accordance with individual institutional policies.
2.2. Treatment and Dose Escalation
Treatment consisted of five consecutive days of cyclophosphamide 440 mg/m
2 IV over 60 minutes from hours 0-1, etoposide 100 mg/m
2 IV over 120 minutes from hours 1-3, then carfilzomib IV over 30 minutes from hours 3-3.5. Granulocyte colony-stimulating factor (G-CSF) was started on day 6, 24-36 hours after completion of the carfilzomib infusion. Cycles were at least 28 days long with no maximum number a cycles a patient could receive. For dose levels 4 and 5, patients were given carfilzomib at a dose of 20 mg/m
2 on days 1-2 followed by an escalation to 27 mg/m
2 or 36 mg/m
2, respectively, for days 3-5. This ramp up to full dose approach was to prevent reactions and improve tolerance. The full dose was given on days 1-5 for all subsequent cycles to achieve better proteasome inhibition beyond the primary subunit targets (proteasome subunits Beta 5, and Beta 1 and 2 at higher doses).[
10,
11] Patients with acute leukemia or non-Hodgkin lymphoma (NHL) received a single dose of intrathecal chemotherapy within 14 days of starting systemic therapy.
For cycle one, patients were pre-medicated with daily dexamethasone 0.1 mg/kg (max 4 mg). Dexamethasone premedication was optional for subsequent cycles.
Carfilzomib doses were escalated following a rolling-6 study design until the MTD or the highest dose level was reached, whichever came first.[
15] DLTs were based upon toxicities observed in cycle one only. Dose escalation was managed for each stratum independently. For stratum B, five additional patients were enrolled at the highest dose level to further assess safety of the regimen.
Toxicities were defined according to the Common Terminology Criteria for Adverse Events (CTCAE) of the National Cancer Institute (NCI) version 4.03.
2.3. Disease Assessment
For stratum A, bone marrow evaluation for disease assessment was performed after every cycle. For stratum B, disease evaluation by RECIST v1.1 criteria was performed after cycles 2, 4, and 6, and then a minimum of every 3 cycles. If a subject showed signs of disease progression, disease assessment was performed at that time. Specific response criteria for stratum B are included below.
2.4. Correlative Studies
Peripheral blood was collected for correlative studies, including evaluation of proteosome inhibition, at multiple time points on days 1, 2, 3, and 8 of cycle 1 and days 1 and 2 of cycle 2. Samples were banked, but not yet analyzed.
3. Results
3.1. Study Overview
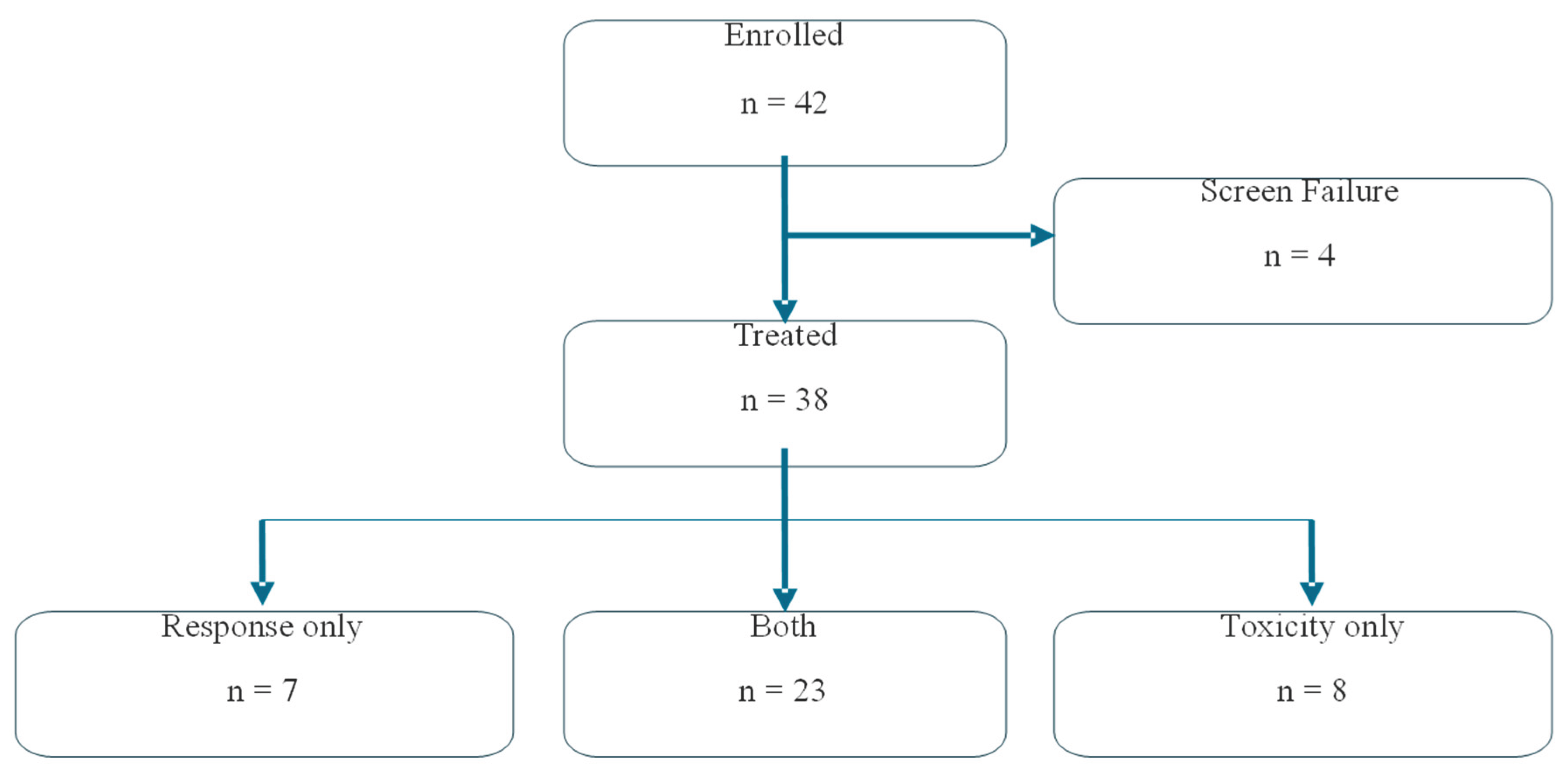
The study was opened to enrollment on 02/12/2016 and closed on 03/01/2023. Accruals were hampered by the COVID pandemic and the relocation of POETIC headquarters to Stanford in 2020. A total of 42 patients were screened, and 38 were treated (stratum A, 14; stratum B, 24) (
Figure 1).
Figure 1 Summarizes patient enrollment flow through treatment to evaluability for toxicity and/or disease response.The median age at consent for the treatment group was 13 years (range, 3 – 24 years). The study participants were equally divided between males and females. Race/ethnicity for the entire group was as follows: Non-Hispanic White, 20 (52.6%); Hispanic or Latino, 13 (34.2%); Black or African American, 3 (7.9%); Asian, 1 (2.6%); and other, 1 (2.6%). (
Table 1)
3.2. DLTs, MTD and Recommended Phase 2 Dose (RP2D)
For stratum A, three DLTs were observed at dose level 2: thrombocytopenia, pericarditis, and posterior reversible encephalopathy syndrome (PRES). Therefore, for patients with leukemia, the MTD and RP2D for carfilzomib given daily for 5 days along with with cyclophosphamide 440 mg/m2/day and etoposide 100 mg/m2/day, was 11 mg/m2/day (dose level 1).
Only a single DLT of PRES was observed in stratum B, at dose level 5. The MTD was not reached. With an additional dose expansion phase, a total of 11 patients were treated at dose level 5. No further toxicities meeting the DLT definition were seen. Therefore, the the RP2D was determined to be dose level 5, the same cyclophosphamide and etoposide dosing as above in combination with carfilzomib 20 mg/m
2/day on days 1-2 and 36 mg/m
2/day on days 3-5 for the first cycle, then 36 mg/m
2 for days 1-5 of all subsequent cycles.[
10] (
Table 2)
Table 2 Summarizes dose limiting toxicity for stratum A (leukemia) and B (solid tumors); dose level 4 and 5 had split dosing of carfilzomib days 1-2 at 20 mg/m
2, and days 3-5 at 27 or 36 mg/m
2.
Of the 38 patients treated on the study, 21 patients received two or more cycles of therapy (stratum A, 1; stratum B, 20 [range: 2 – 14 cycles]).
The most common severe adverse event (SAE) was febrile neutropenia, which was observed in over 50% of patients at all dose levels. See tables 3 and 4 for list of severe adverse events during cycle 1 by dose level and tumor type, respectively. AEs not categorized as SAEs during cycle 1 are presented by dose level and tumor type in tables 5 and 6, respectively. See data supplement for additional tables for adverse events during all treatment cycles.
Table 3 Number and percentage of toxicity-evaluable subjects (n = 31) who experienced Serious Adverse Events (SAEs). SAEs are categorized by dose level and reported from treatment start through the end of cycle 1. For toxicity evaluable subjects who discontinued treatment prior to the end of cycle 1 (subjects who experienced a DLT or died before completion of the cycle), all SAEs up to the time of discontinuation are included. Values in parentheses represent the proportion of subjects (%) experiencing each listed SAE for each dose level.
Table 4 Number and percentage of toxicity-evaluable subjects (n = 31) who experienced Serious Adverse Events (SAEs). SAEs are categorized by tumor type and reported from treatment start through the end of cycle 1. For toxicity evaluable subjects who discontinued treatment prior to the end of cycle 1 (subjects who experienced a DLT or died before completion of the cycle), all SAEs up to the time of discontinuation are included. Values in parentheses represent the proportion of subjects (%) experiencing each listed SAE for each tumor type.
Table 5 Number and percentage of toxicity-evaluable subjects (n = 31) who experienced Adverse Events (AEs) of grade 3 or higher. AEs are categorized by dose level and reported from treatment start through the end of cycle 1. For subjects who discontinued treatment prior to the end of cycle 1 (subjects who experienced a DLT or died before completion of the cycle), all AEs up to the time of discontinuation are included. Values in parentheses represent the proportion of subjects (%) experiencing each listed AE for each dose level. Only AEs reported in more than 5% of all subjects are included.
Table 6 Number and percentage of toxicity-evaluable subjects (n = 31) who experienced Adverse Events (AEs) of grade 3 or higher. AEs are categorized by tumor type and reported from treatment start through the end of cycle 1. For subjects who discontinued treatment prior to the end of cycle 1 (subjects who experienced a DLT or died before completion of the cycle), all AEs up to the time of discontinuation are included. Values in parentheses represent the proportion of subjects (%) experiencing each listed AE for each tumor type. Only AEs reported in more than 5% of all subjects are included.
3.3. Disease Response
Disease responses were observed in both strata at all dose levels. Three objective responses were observed in stratum A - one complete remission with incomplete platelet recovery (CRp) (mature B-cell leukemia) and two partial responses (PRs) (AML; pre-B ALL).
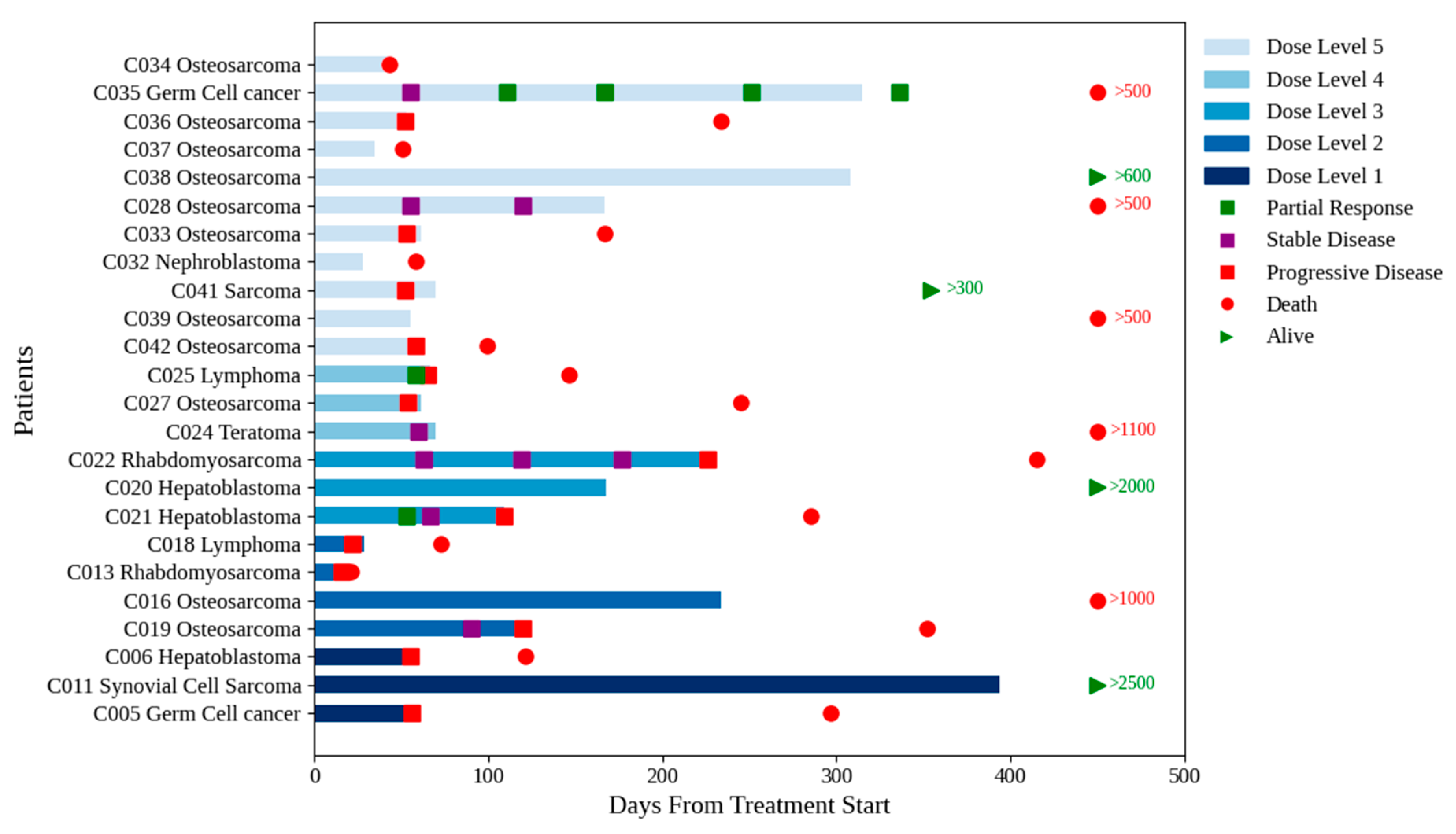
In stratum B, three PRs (germ cell tumor, non-Hodgkin lymphoma and hepatoblastoma) and four stable diseases (SDs) (rhabdomyosarcoma, teratoma, and two cases of osteosarcoma (OS)) were observed based upon the RECIST criteria (
Figure 2). Of note, the two patients with OS had no evidence of disease progression after four and five cycles, respectively.
Eight patients were enrolled in stratum B without measurable disease, primarily due to lung metastases not meeting the RECIST criteria. Of the six study patients who received
> six cycles of treatment, four did not have measurable disease. One patient with synovial sarcoma and lung metastases received fourteen cycles at dose level 1, achieving pathologically confirmed CR, and is still alive and well more than seven years after completion of protocol therapy.[
16] Two patients with OS received eight and nine cycles, respectively, without evidence of progressive disease when the protocol therapy was discontinued. In the first case, the patient refused to continue treatment, and in the second case, the treating physician elected to discontinue treatment to limit exposure to etoposide. One patient with hepatoblastoma received six cycles before the alpha-fetoprotein level started to rise.
Figure 2 Shows a swimmer plot for stratum B (solid tumors). Dose levels are coded by colors, from bottom level 1 to top level 5. Several patients at all dose levels transitioned to other therapies. Figure shows number of days until death in red, and alive in green. The numbers correspond to last study follow-up.
4. Discussion
Proteasome inhibition has been shown to effectively kill tumors in cells that have acquired resistance to other anti-cancer treatments. Carfilzomib is an irreversible epoxyketone and second-generation proteasome inhibitor. Its clinical development has focused primarily on hematologic malignancies in adults, particularly multiple myeloma.
In vitro studies of carfilzomib performed on solid tumor pediatric cancer cell lines provided data and the rationale for developing a clinical trial incorporating carfilzomib into a standard chemotherapy backbone of cyclophosphamide and etoposide in patients with relapse/refractory leukemia or non-CNS solid tumors. [
5]
This trial used a 5-day schedule, whereas the majority of previous trials of carfilzomib used a 2-day per week schedule for patient convenience.[
10,
12,
13] The 5-day dosing schedule of carfilzomib, cyclophosphamide, and etoposide, chosen for this trial to maximize synergy, was well tolerated with a toxicity profile similar to that observed with the chemotherapy backbone by itself as well as other multi-agent chemotherapy regimens. As expected, myelosuppression with febrile neutropenia was the main adverse event observed in the trial.
The MTD for leukemia patients (stratum A) was established at dose level 1 (carfilzomib dose 11 mg/m
2/day × 5 days), with thrombocytopenia, PRES, and pericarditis as the observed DLTs. It is unclear whether PRES should be attributed to the carfilzomib since dexamethasone, which was administered daily for 5 days prior to each carfilzomib dose to reduce infusion reactions during cycle 1, is known to cause hypertension and PRES. [
17] However, since PRES is also a known toxicity of carfilzomib, we labelled it as a DLT. It was also unclear if the percarditis was due to carfilzomib as a pericardiocentesis revealed T-cell leukemia cells in the patient’s pericardial fluid, leading to our decision to enroll additional patients until a third DLT occurred.
Compared with patients enrolled in stratum B (solid tumors), patients in stratum A (leukemia) were more heavily pre-treated at study entry. There was one CR
p seen in a patient with mature B-cell leukemia. This is noteworthy because combinations with proteasome inhibitors have been used successfully to treat patients with multiple myeloma, the most differentiated mature B-cell neoplasm.[
13] Response was also seen in patients with AML and pre-B ALL. The frontline COG study AAML1031 explored the use of bortezomib in AML during each chemotherapy phase, but this approach did not show improved outcomes.[
18] For ALL, a different schedule of carfilzomib was studied by incorporating it into a standard four-drug ALL induction, with responses in 50% of B- and 69% of T-ALL cases.[
19]
The MTD was not reached for stratum B with a single DLT of PRES observed at dose level 5. As discussed above, it was unclear if the PRES was due to the carfilzomib or the dexamethasone premedication. There were no cases of PRES reported during any second or later cycles, when patients were not required to receive dexamethasone premedication. No further toxicity concerns were evident in the dose expansion, bringing the total to 11 patients treated at dose level 5. Therefore, the RP2D for carfilzomib in combination with cyclophosphamide and etoposide was determined to be 20 mg/m2/day on days 1-2 and 36 mg/m2/day on days 3-5 for cycle 1, then 36 mg/m2/day for days 1-5 in all subsequent cycles.
There was evidence of activity in different disease groups at all dose levels. Since the trial was not designed to assess efficacy in specific disease groups, conclusions could not be drawn about the effective dosing and schedule of carfilzomib for each malignancy type. A future phase 2 trial of this regimen in specific diseases is warranted to further assess treatment efficacy.
Correlative studies looking at proteosome inhibition may help in determining optimal dosing with this regimen. When available, correlative study data from this trial will be reported in a future paper.
Of the twenty-four patients enrolled in stratum B, fifteen (63%) had a diagnosis of sarcoma, highlighting the need for novel therapies in this patient population. One patient with synovial sarcoma (SS) and non-measurable lung disease received fourteen cycles of treatment (at dose level 1), without disease progression. This patient underwent resection of her residual lung nodules and was found to have pathological CR. The patients is alive and well more than seven years after completing protocol therapy and has since successfully conceived and given birth to a healthy child. As the outcome for metastatic SS is typically dismal, the response to this regimen is noteworthy.[
20]
Eleven patients in stratum B (48%) had OS. Of those patients, five had lung metastases that were technically considered non-measurable disease according to the RECIST criteria and, therefore, were not eligible for studies requiring measurable disease. RECIST has long been shown to be suboptimal for response assessment in OS.[
21,
22,
23] Since this was a phase 1 trial, the study did not incorporate time to progression in the assessment of response as proposed by COG for OS patients.[
15] Throughout the trial, investigators observed that patients with OS significantly benefitted clinically from this combination. Incorporation of patient-reported outcome (PRO) measures may have provided additional objective insight into the impact of this treatment regimen on patients’ quality of life.
Ifosfamide in combination with etoposide, which is commonly used in patients with progressing/refractory OS, can produce significant neurotoxicity and nephrotoxicity along with low disease response rates.[
16] The use of cyclophosphamide and etoposide in combination with carfilzomib may be an alternative treatment for these patients. Two out of eleven patients (18%) with OS who showed response on this study had previously received ifosfamide in combination with etoposide or ifosfamide alone.
In conclusion, the 5-day combination of cyclophosphamide/etoposide/carfilzomib is well tolerated and easily administered in an outpatient setting, making it a viable alternative to salvage therapy for children, adolescents and young adults with multiple malignancy types. Moving forward with an efficacy trial of this promising combination in solid tumors, particularly sarcomas, is warranted.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Authors Contribution
Conception and design: Jessica Boklan, Aru Narendran. Administrative and study management support: Pediatric Oncology Experimental Therapeutics Investigators’ Consortium (POETIC) Research Development and Management Center (RDMC).Provision of study materials or patients: All authors and POETIC RDMC.Collection and assembly of data: All authors and POETIC RDMC.Data analysis and interpretation: Jessica Boklan, Jonathan Gelfond, Tamar Shlopobersky, Ativ Zomet, Aru Narendran, Anne-Marie Langevin, Norman Lacayo.Manuscript writing: Anne-Marie Langevin, Jessica Boklan, Steven G. DuBois, Ativ Zomet, Jonathan Gelfond, Tamar Shlapobersky, Aru Narendran, Norman Lacayo.Final approval of manuscript: All authors.Accountable for all aspects of the work: All authors and POETIC RDMC.
Funding Statement
The study was supported by Amgen Inc. Full financial sponsorship of this investigator-initiated trial with full publication rights by authors and the POETIC consortium. Amgen had no role in choice of research project, study design, data analysis or interpretation, writing of the manuscript, or decision to publish results.
Ethical Compliance
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Data Access Statement
Research data supporting this publication are available from the POETIC consortium upon e-mail request to:
Acknowledgments
The authors thank the patients who participated in the trial and their families. The authors also thank the physicians, nurses, research coordinators, and other staff at each site who assisted with this study. We also thank Amgen for providing carfilzomib and for funding this investigator-initiated study. .
Conflicts of Interest Declaration
The authors declare that they have no affiliations with or involvement in any organization or entity with any financial interest in the subject matter or materials discussed in this manuscript. The institutions of Drs. Boklan and Narendren received minor salary support for time spent on study conduct.
References
- Demo SD, Kirk CJ, Aujay MA, et al. Antitumor activity of PR-171, a novel irreversible inhibitor of the proteasome. Cancer Res. Jul 1 2007;67(13):6383–91. [CrossRef]
- Arastu-Kapur S SK, Parlati F and Bennet M. . Non-Proteosomal Targets of Proteasome Inhibitors Bortezomib and Carfilzomib. . Blood (ASH Annual Meeting Abstracts). 2008;112:#2657.
- Kortuem KM, Stewart AK. Carfilzomib. Blood. Feb 7 2013;121(6):893–7. [CrossRef]
- Niesvizky R, Martin TG, 3rd, Bensinger WI, et al. Phase Ib dose-escalation study (PX-171-006) of carfilzomib, lenalidomide, and low-dose dexamethasone in relapsed or progressive multiple myeloma. Clin Cancer Res. Apr 15 2013;19(8):2248–56. [CrossRef]
- Thakur S, Ruan Y, Jayanthan A, Boklan J, Narendran A. Cytotoxicity and Target Modulation in Pediatric Solid Tumors by the Proteasome Inhibitor Carfilzomib. Curr Cancer Drug Targets. 2021;21(9):804–811. [CrossRef]
- O’Connor OA, Smith EA, Toner LE, et al. The combination of the proteasome inhibitor bortezomib and the bcl-2 antisense molecule oblimersen sensitizes human B-cell lymphomas to cyclophosphamide. Clin Cancer Res. May 1 2006;12(9):2902–11. [CrossRef]
- Lew G, Chen Y, Lu X, et al. Outcomes after late bone marrow and very early central nervous system relapse of childhood B-acute lymphoblastic leukemia: a report from the Children’s Oncology Group phase III study AALL0433. Haematologica. Jan 1 2021;106(1):46–55. [CrossRef]
- Walczak BE, Irwin RB. Sarcoma chemotherapy. J Am Acad Orthop Surg. Aug 2013;21(8):480–91. [CrossRef]
- Owen A O’Connor 1 AKS, Marcy Vallone, Christopher J Molineaux, Lori A Kunkel, John F Gerecitano, Robert Z Orlowski. A phase 1 dose escalation study of the safety and pharmacokinetics of the novel proteasome inhibitor carfilzomib (PR-171) in patients with hematologic malignancies. Clin Cancer Res. 2009;15(22):7085–91. [CrossRef]
- Lendvai N, Hilden P, Devlin S, et al. A phase 2 single-center study of carfilzomib 56 mg/m2 with or without low-dose dexamethasone in relapsed multiple myeloma. Blood. Aug 7 2014;124(6):899–906. [CrossRef]
- Xiang Zhou AB, Jessica Peter, Maximilian Johannes Steinhardt, Cornelia Vogt, Silvia Nerreter, Eva Teufel, Emilia Stanojkovska, Xianghui Xiao, Hannah Hornburger, Larissa Haertle, Max Mendez Lopez, Umair Munawar, Angela Riedel, Seungbin Han, Elmer Maurits, Herman S Overkleeft Bogdan Florea, Hermann Einsele , K Martin Kortüm , Christoph Driessen, Lenka Besse , Leo Rasche High-dose carfilzomib achieves superior anti-tumor activity over low-dose and recaptures response in relapsed/refractory multiple myeloma resistant to lowdose carfilzomib by co-inhibiting the β2 and β1 subunits of the proteasome complex. Haematologica.2023 Jun 1 2023;108(6):1628-1639.. [CrossRef]
- Papadopoulos KP, Siegel DS, Vesole DH, et al. Phase I study of 30-minute infusion of carfilzomib as single agent or in combination with low-dose dexamethasone in patients with relapsed and/or refractory multiple myeloma. J Clin Oncol. Mar 1 2015;33(7):732–9. [CrossRef]
- Xie C, Wei M, Yang F, Liu Q, Wu F, Huang J. Efficacy and toxicity of carfilzomib- or bortezomib-based regimens for treatment of transplant-ineligible patients with newly diagnosed multiple myeloma: A meta-analysis. Medicine (Baltimore). Sep 30 2022;101(39):e30715. [CrossRef]
- Gozzetti A, Papini G, Candi V, Brambilla CZ, Sirianni S, Bocchia M. Second Generation Proteasome Inhibitors in Multiple Myeloma. Anticancer Agents Med Chem. 2017;17(7):920–926. [CrossRef]
- Skolnik JM, Barrett JS, Jayaraman B, Patel D, Adamson PC. Shortening the timeline of pediatric phase I trials: the rolling six design. J Clin Oncol. Jan 10 2008;26(2):190–5. [CrossRef]
- Kojima Y, Shimoi T, Seo T, et al. Poor Treatment Outcomes with Second-Line Chemotherapy in Advanced Synovial Sarcoma. Oncology. 2022;100(7):370–375. [CrossRef]
- Irvin W, MacDonald G, Smith JK, Kim WY. Dexamethasone-induced posterior reversible encephalopathy syndrome. J Clin Oncol. Jun 10 2007;25(17):2484–6. [CrossRef]
- Aplenc R, Meshinchi S, Sung L, et al. Bortezomib with standard chemotherapy for children with acute myeloid leukemia does not improve treatment outcomes: a report from the Children’s Oncology Group. Haematologica. Jul 2020;105(7):1879–1886. [CrossRef]
- Burke MJ, Ziegler DS, Bautista F, et al. Phase 1b study of carfilzomib with induction chemotherapy in pediatric relapsed/refractory acute lymphoblastic leukemia. Pediatr Blood Cancer. Dec 2022;69(12):e29999. [CrossRef]
- Moreau-Bachelard C, Campion L, Toulmonde M, et al. Patterns of care and outcomes of 417 patients with METAstatic SYNovial sarcoma (METASYN): real-life data from the French Sarcoma Group (FSG). ESMO Open. Apr 2022;7(2):100402. [CrossRef]
- Omer N, Le Deley MC, Piperno-Neumann S, et al. Phase-II trials in osteosarcoma recurrences: A systematic review of past experience. Eur J Cancer. Apr 2017;75:98–108. [CrossRef]
- Guenther LM, Rowe RG, Acharya PT, et al. Response Evaluation Criteria in Solid Tumors (RECIST) following neoadjuvant chemotherapy in osteosarcoma. Pediatr Blood Cancer. Apr 2018;65(4). [CrossRef]
- Lagmay JP, Krailo MD, Dang H, et al. Outcome of Patients With Recurrent Osteosarcoma Enrolled in Seven Phase II Trials Through Children’s Cancer Group, Pediatric Oncology Group, and Children’s Oncology Group: Learning From the Past to Move Forward. J Clin Oncol. Sep 1 2016;34(25):3031–8. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).