Submitted:
07 August 2025
Posted:
07 August 2025
You are already at the latest version
Abstract
Keywords:
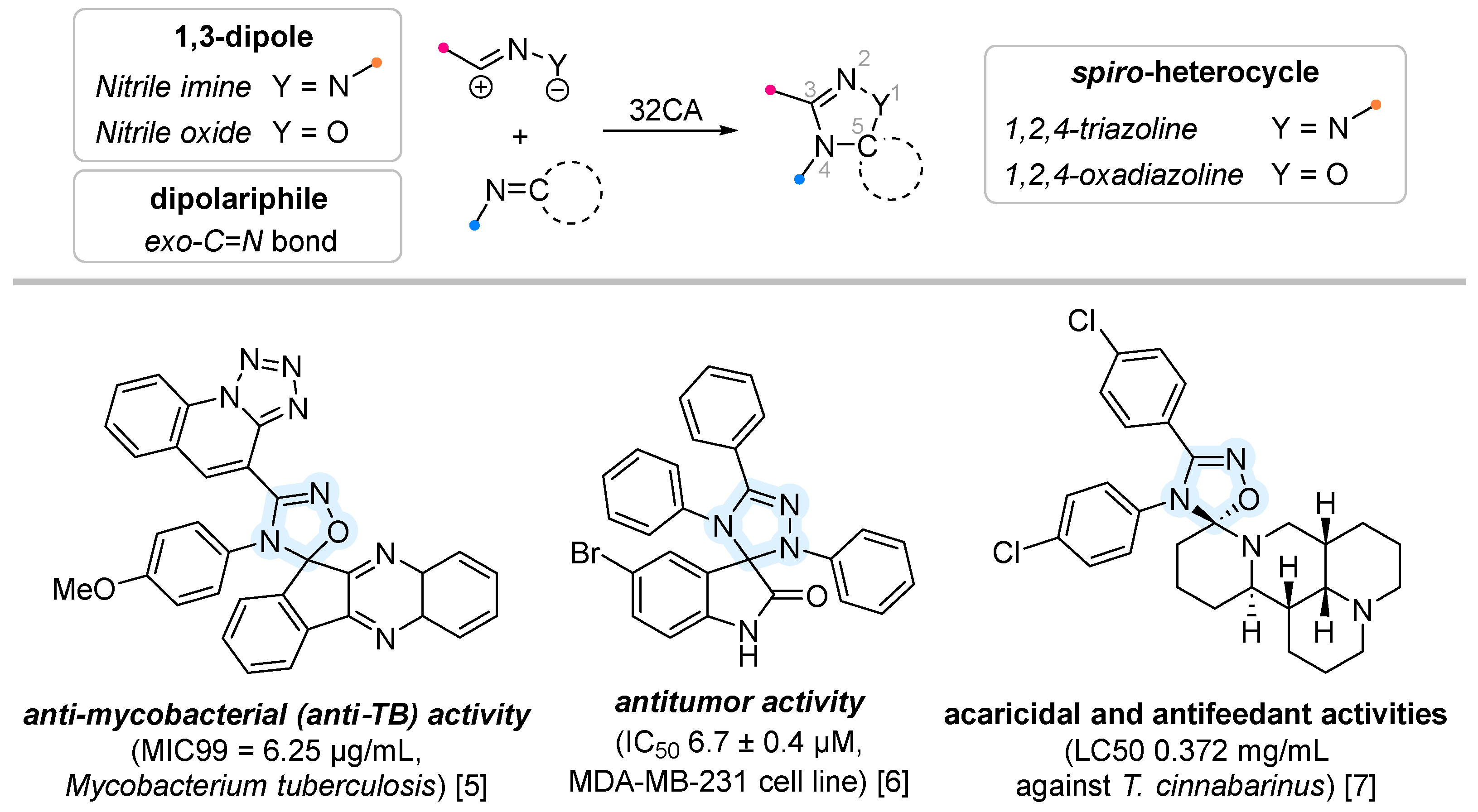
1. Introduction
2. Cycloaddition to Exocyclic Bonds C=N
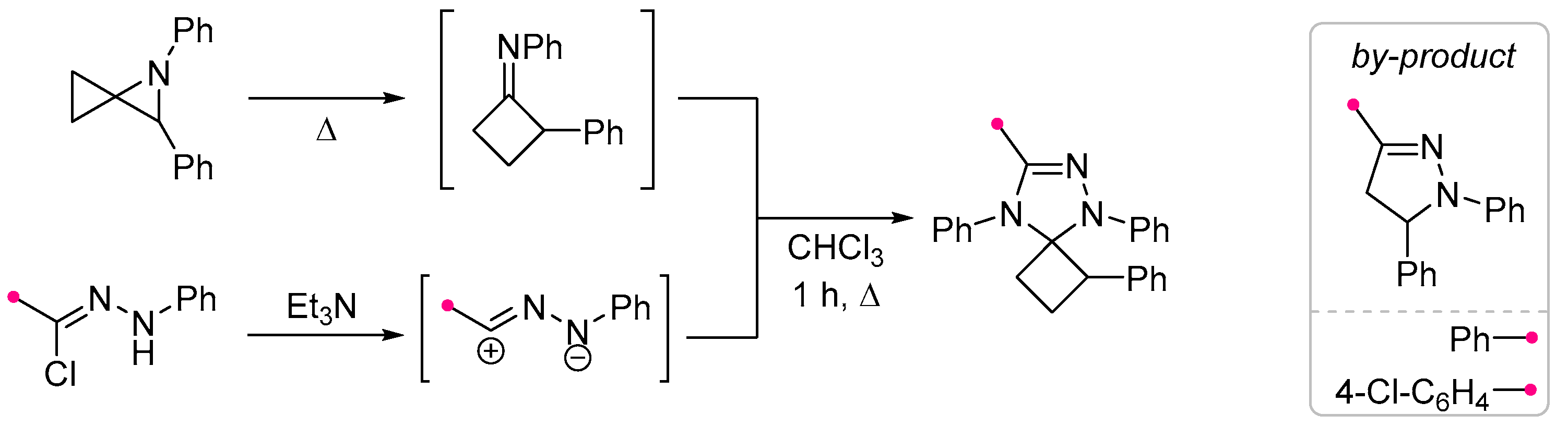
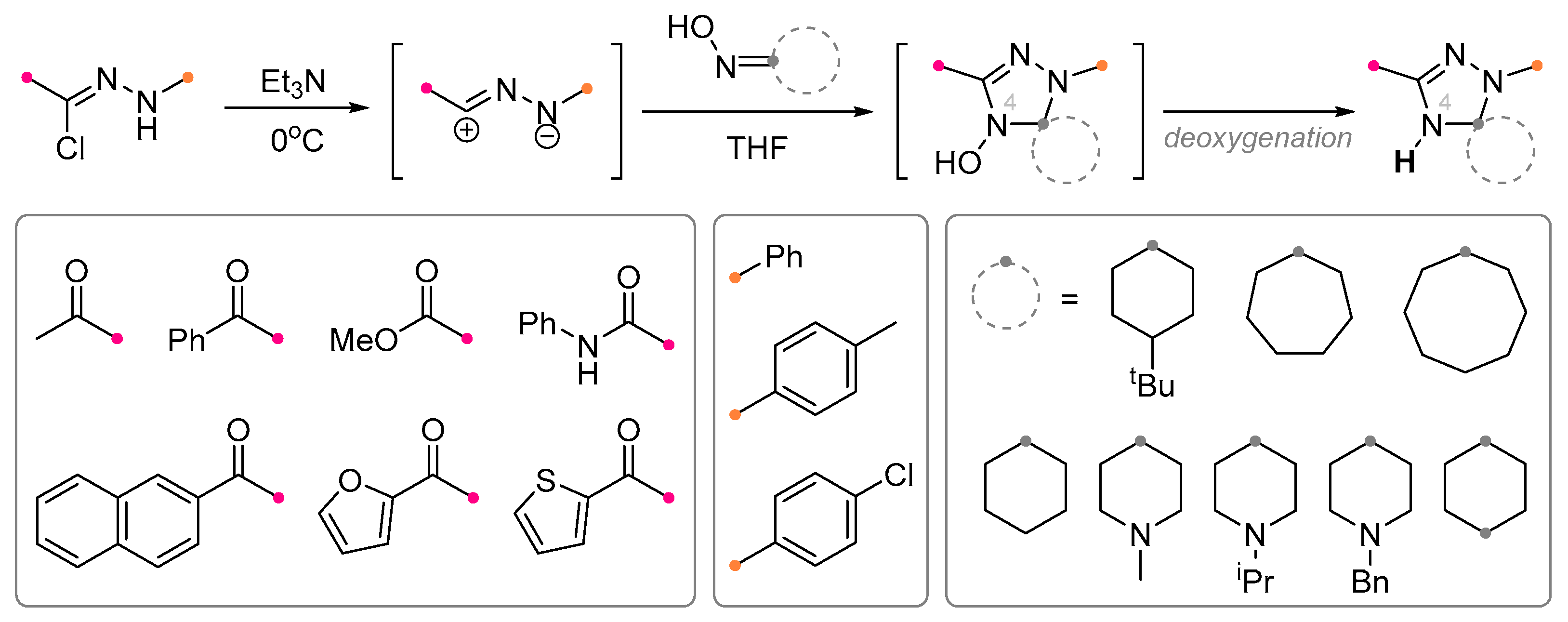
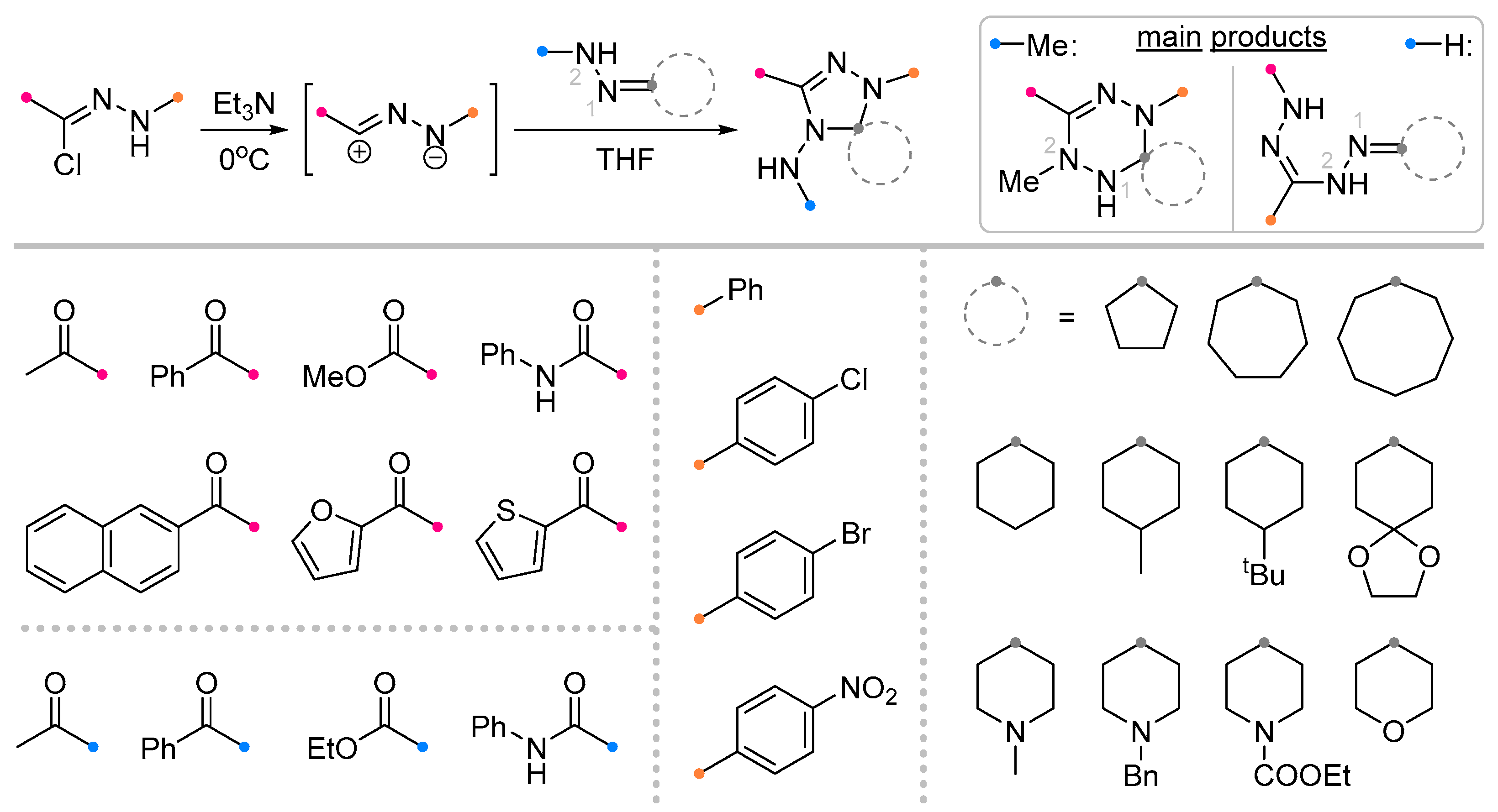
2.1. Saturated Carbocycles and Piperidine Derivatives with Exo-C=N-Bonds in Reactions with Nitrile Imines and Nitrile Oxides
2.2. Unsaturated Cycles with an Exocyclic C=N Bond as Dipolarophiles in Reactions with Nitrile Imines and Nitrile Oxides
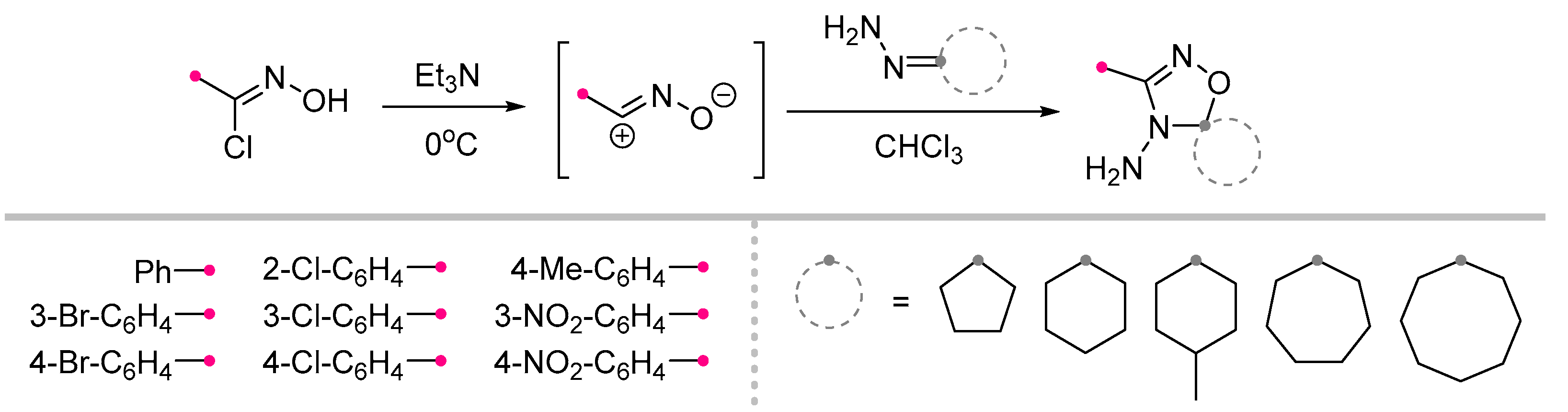
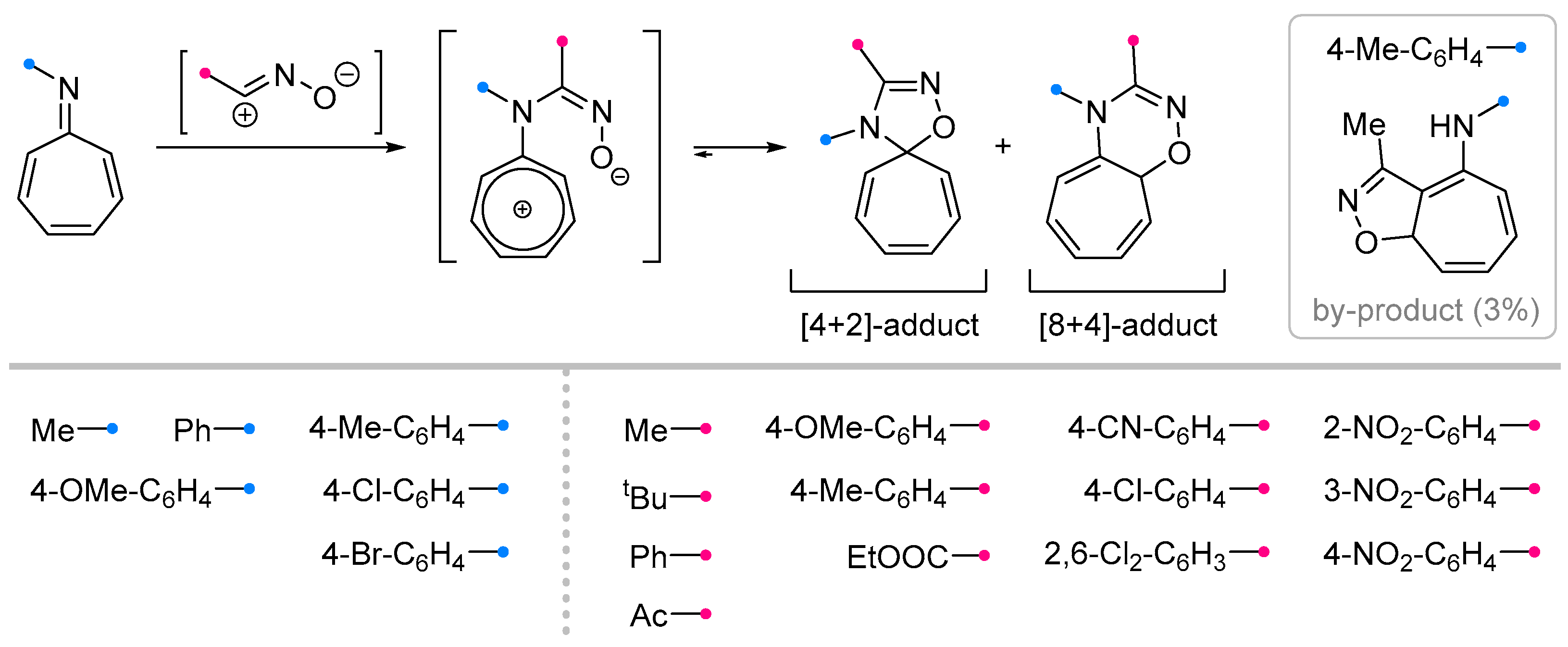
2.2.1. Reactions of Nitrile Imines and Nitrile Oxides with Exo-C=N-Bonds of Monocyclic Compounds
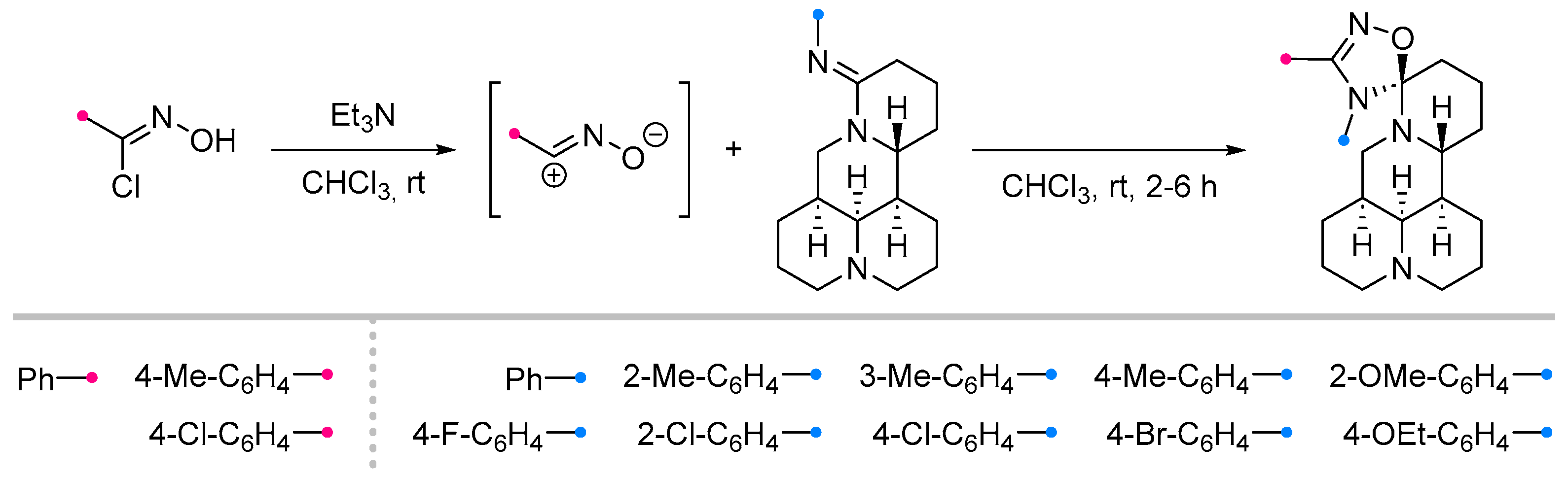
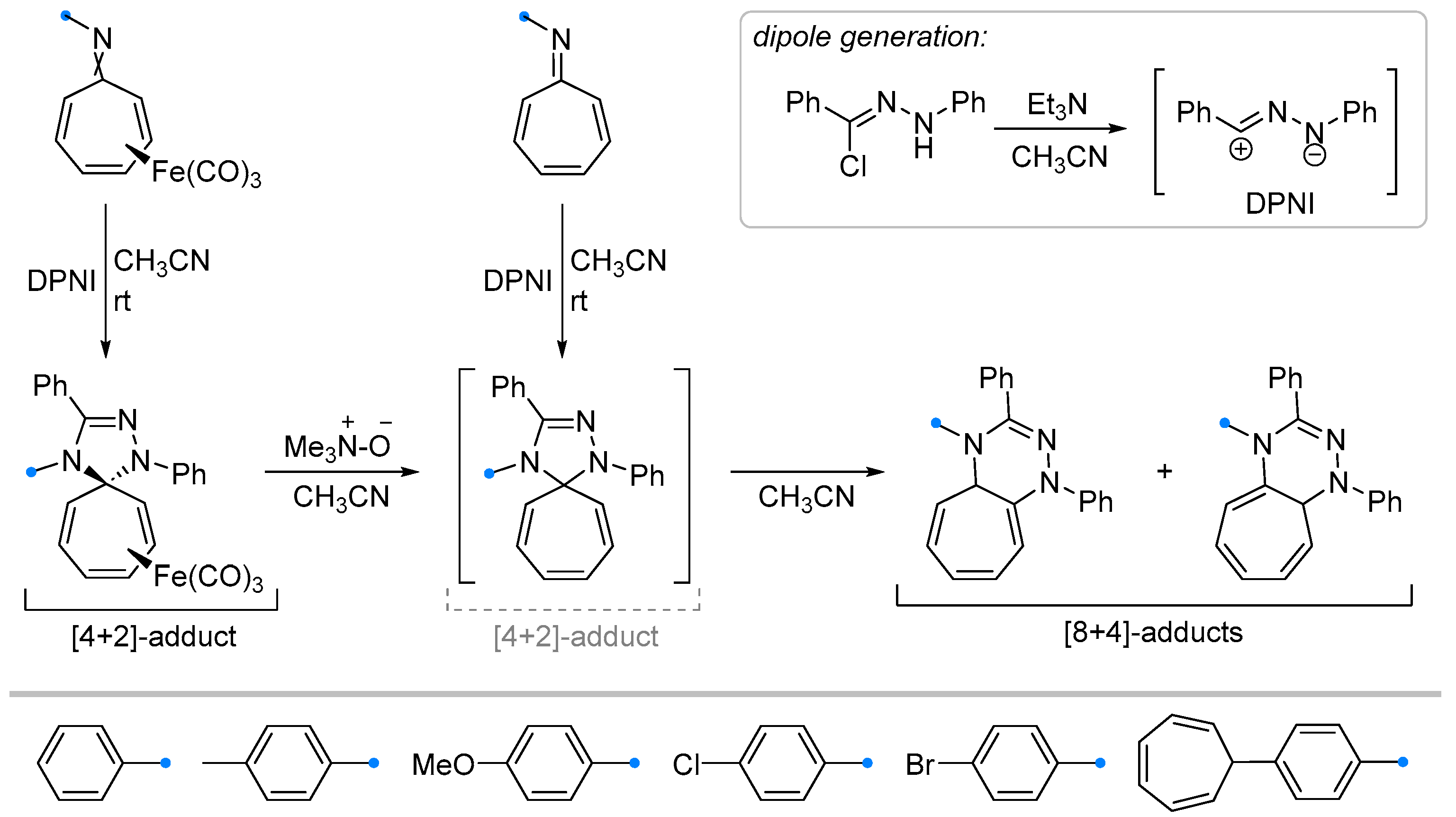
2.2.2. Addition of Nitrile Oxides and Nitrile Imines at the Exocyclic C=N Bond of Fused Carbocycles
2.2.3. Reactions of Nitrile Oxides and Nitrile Imines at Exocyclic C=N Bonds of Fused Heterocycles
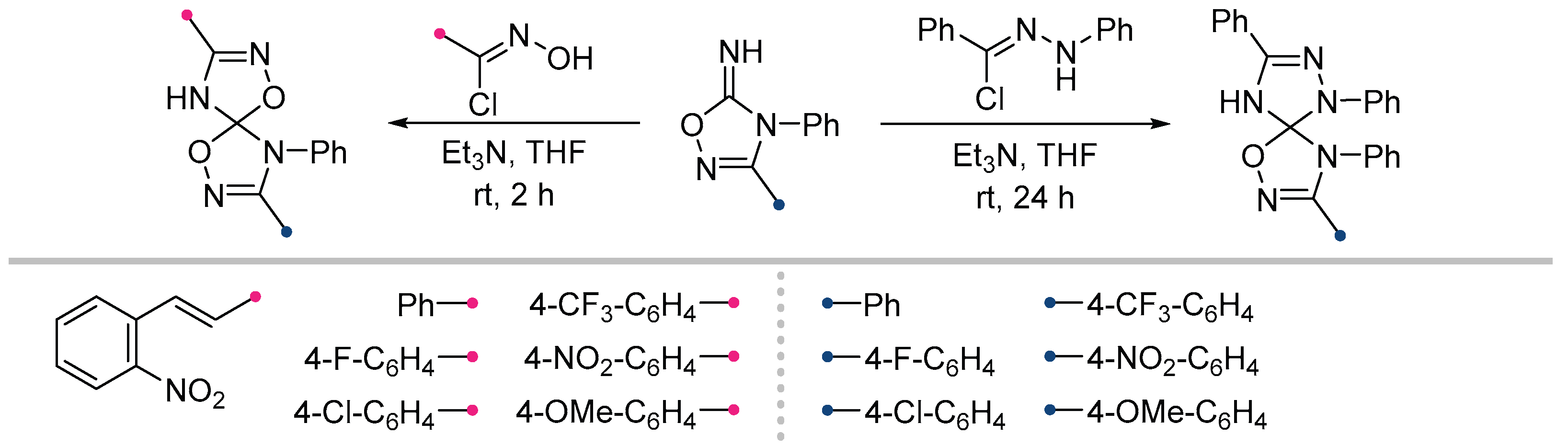
2.3. Addition Reactions of NO and NI at the Exocyclic Bond of C=N Cycles with Two Heteroatoms
2.4. Nitrile Oxide and Nitrile Imine Reactions at Exocyclic C=N Bonds of Heterocycles with Three Heteroatoms
3. Conclusions
Funding
Conflicts of Interest
Abbreviations
| 32CA | (3+2)-Cycloaddition |
| DPNI | Diphenyl nitrile imines |
References
- Huisgen, R. 1,3-Dipolar Cycloadditions. Past and Future. Angew. Chem. Int. Ed. Engl. 1963, 2, 565–598. [Google Scholar] [CrossRef]
- Jamieson, C.; Livingstone, K. The Nitrile Imine 1,3-Dipole; Springer International Publishing: Cham, Switzerland, 2020; ISBN 978-3-030-43480-9. [Google Scholar]
- Esipenko, A.A.; Samarai, L.I. Cycloaddition of Nitrile Oxides to Multiple Bonds Containing a Heteroatom. Russ. Chem. Rev. 1993, 62, 1097–1105. [Google Scholar] [CrossRef]
- Belen’kii, L.I. Nitrile Oxides. In Nitrile Oxides. In Nitrile Oxides, Nitrones, and Nitronates in Organic Synthesis; John Wiley & Sons, Inc.: New Jersey, USA, 2007; pp. 1–127. [Google Scholar]
- Kanchrana, M.; Gamidi, R.K.; Kumari, J.; Sriram, D.; Basavoju, S. Design, Synthesis, Anti-Mycobacterial Activity, Molecular Docking and ADME Analysis of Spiroquinoxaline-1,2,4-Oxadiazoles via [3 + 2] Cycloaddition Reaction under Ultrasound Irradiation. Mol. Divers. 2024, 28, 3979–3991. [Google Scholar] [CrossRef]
- Ribeiro, C.J.A.; Nunes, R.C.; Amaral, J.D.; Gonçalves, L.M.; Rodrigues, C.M.P.; Moreira, R.; Santos, M.M.M. Spirotriazoline Oxindoles: A Novel Chemical Scaffold with in Vitro Anticancer Properties. Eur J Med Chem 2017, 140, 494–509. [Google Scholar] [CrossRef]
- Lv, M.; Ma, Q.; Zhang, S.; Xu, H. Construction of Spiro-1,2,4-Oxadiazoline-Fused Matrine-Type Alkaloids as Pesticidal Agents. Bioorg. Med. Chem. Lett. 2021, 51, 128356. [Google Scholar] [CrossRef]
- Proceedings of the Chemical Society. October 1961. Proceedings of the Chemical Society 1961, 357. [Google Scholar] [CrossRef]
- Sharp, J.T. Nitrile Ylides and Nitrile Imines. In Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry Toward Heterocycles and Natural Products; Padwa, A., Pearson, W., Eds.; John Wiley & Sons, Inc.: New Jersey, USA, 2002; Volume 59, pp. 473–537. [Google Scholar] [CrossRef]
- Jäger, V.; Colinas, P.A. Nitrile Oxides. In Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry Toward Heterocycles and Natural Products; Padwa, A., Pearson, W., Eds.; John Wiley & Sons, Inc.: New Jersey, USA, 2002; Volume 59, pp. 361–472. [Google Scholar] [CrossRef]
- Grundmann, Ch. The Chemistry of Nitrile Oxides. In Organische Chemie; Springer: Berlin, Heidelberg, 2006; pp. 62–127. [Google Scholar]
- Paton, M.R. 3.20 - N-Substituted Nitriles and Other Heteroanalogues of Nitriles of the Type RCZ. In Comprehensive Organic Functional Group Transformations; Katritzky, A.R., Meth-Cohn, O., Rees, C.W., Eds.; Elsevier Science: Amsterdam, Netherlands, 1995; pp. 677–692. [Google Scholar] [CrossRef]
- Kumar, A.K.; Kumar, K.A.; Govindaraju, M.; Kumar, V.G. Nitrile Imines: Versatile Intermediates in the Synthesis of Five Membered Heterocycles. Int. J. Res. Pharm. Chem. 2013, 3, 140–152. [Google Scholar]
- Quilico, A. Advances in Nitrile Oxides Chemistry. Experientia 1970, 26, 1169–1183. [Google Scholar] [CrossRef]
- Grundmann, C. Synthesis of Heterocyclic Compounds with the Aid of Nitrile Oxides. Synthesis (Stuttg) 1970, 1970, 344–359. [Google Scholar] [CrossRef]
- Grundmann, C.; Grünanger, P. Addition Reactions Leading to Cyclic Structures: 1,3-Dipolar Cycloadditions. In The Nitrile Oxides. Organische Chemie in Einzeldarstellungen, Springer: Berlin, Heidelberg, 1971, Volume 59; pp. 85–139. [CrossRef]
- Deepthi, A.; Acharjee, N.; Sruthi, S.L.; Meenakshy, C.B. An Overview of Nitrile Imine Based [3+2] Cycloadditions over Half a Decade. Tetrahedron 2022, 116, 132812. [Google Scholar] [CrossRef]
- Molteni, G.; Silvani, A. Spiro-2-oxindoles via 1,3-dipolar Cycloadditions. A Decade Update. Eur. J. Org. Chem. 2021, 2021, 1653–1675. [Google Scholar] [CrossRef]
- Kadaba, P.K. 1,2,4-Triazolines. Adv. Heterocycl. Chem. 1989, 46, 169–281. [Google Scholar] [CrossRef]
- S. Shawali, A. Chemoselectivity in 1,3-Dipolar Cycloaddition Reactions of Nitrilimines with Multifunctionalized Dipolarophiles. Curr Org Chem 2014, 18, 598–614. [Google Scholar] [CrossRef]
- Ferwanah, A.-R.S.; Awadallah, A.M. Reaction of Nitrilimines and Nitrile Oxides with Hydrazines, Hydrazones and Oximes. Molecules 2005, 10, 492–507. [Google Scholar] [CrossRef] [PubMed]
- Tsuge, O.; Watanabe, H.; Kiryu, Y. The Reactions of 1,2-Diphenyl-1-Azaspiro[2,2]Pentane and 2-Phenyl-1-Azaspiro[2.2]Pent-1-Ene with C, N -Diarylnitrilimines. Bull. Chem. Soc. Jpn. 1979, 52, 3654–3658. [Google Scholar] [CrossRef]
- Dalloul, H.M. Heterocyclic synthesis using Nitrile imines. 4. synthesis of 3-substituted 1-aryl-1,2,4-triazaspiroalk-2-enes. Chem. Heterocycl. Compd. 2004, 40, 1402–1407. [Google Scholar] [CrossRef]
- Dalloul, H.M.; Boyle, P.H. HETEROCYCLIC SYNTHESIS USING NITRILIMINES: PART 7. SYNTHESIS OF SOME NEW SUBSTITUTED 1,2,4,8-TETRAAZASPIRO[4.5]DEC-2-ENES. Heterocycl. Comm. 2007, 13, 155–160. [Google Scholar] [CrossRef]
- Dalloul, H.M.; Samaha, A.S.A. Synthesis of Nitrogen-Containing Dispiroheterocycles Using Nitrilimines (II). J. Serb. Chem. Soc. 2010, 75, 1473–1479. [Google Scholar] [CrossRef]
- Dalloul Peter H Boyle, H.M.; Dalloul, H.M.; Boyle, P.H. Heterocyclic Synthesis Using Nitrilimines: Part 5. Synthesis of Some Novel Spiro Heterocycles. Turk. J. Chem. 2006, 30, 119–124. [Google Scholar]
- Awadallah, A.M. Synthesis of Schiff Bases, Oximes and Hydrazones of 1,2,4-Oxadiazole and 1,2,4-Triazoles, Asian J. Chem. 2006, 18, 2151–2158. [Google Scholar]
- Liu, B.; Li, X.F.; Liu, H.C.; Yu, X.Y. Unexpected Nitrilimine Cycloaddition of Thiazolo[3,2-a]Pyrimidine Derivatives. Tetrahedron Lett. 2013, 54, 6952–6954. [Google Scholar] [CrossRef]
- Li, X.; Zheng, A.; Liu, B.; Yu, X.; Yi, P. Synthesis of [1,2,4]Oxadiazolo[4,5- a ]Thiazolo[2,3- b ]Pyrimidin-9(10 H )-ones via 1,3-Dipolar Cycloaddition of Nitrile Oxide to Thiazolo[3,2- a ]Pyrimidin-3-one Derivatives. Chin. J. Chem. 2010, 28, 977–980. [Google Scholar] [CrossRef]
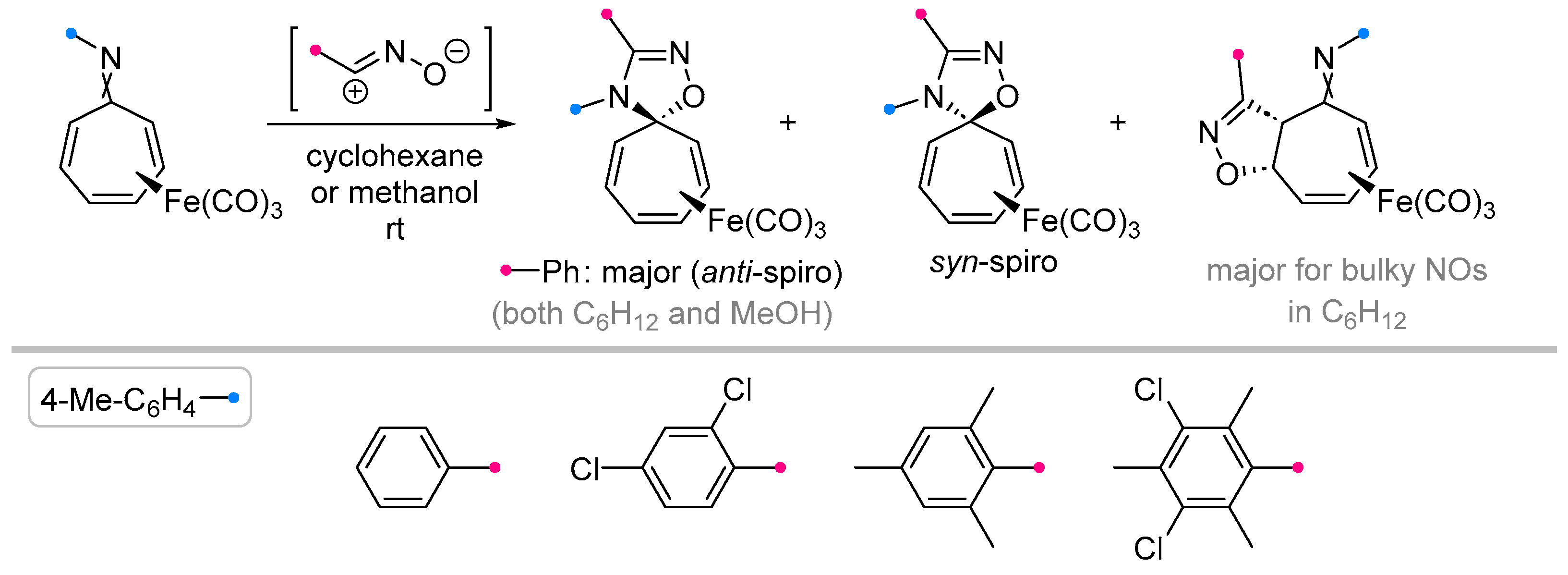
- Freccero, M.; Gandolfi, R. Reactions of O,O'-Disubstituted Benzonitrile Oxides with 8-Azaheptafulvenes. Heterocycles 1998, 47, 453–466. [Google Scholar] [CrossRef]
- Gandolfi, R.; Gamba, A.; Grünanger. P. 1,3-DIPOLAR CYCLOADDITION OF NITRILE OXIDES TO 8-AZAHEPTAFULVENES. Heterocycles 1995, 40, 619–638. [Google Scholar] [CrossRef]
- Saito, K.; Ito, K.; Takahashi, K. 1,3-Dipolar Cycloaddition Reactions of N-Aryl-2,4,6-Cycloheptarien-1-Imines with p-Substituted Benzonitrile Oxides: Formations of 1,2,4-Oxadiazaspiro[4.6]Undeca-6,8,10-Trienes. Heterocycles 1993, 36, 21–24. [Google Scholar] [CrossRef]
- Ito, K.; Saito, K. 1,3-Dipolar Cycloaddition Reactions of 2,4,6-Cycloheptatrien-1-Imines with Benzonitrile Oxides Leading to 1,2,4-Oxadiazaspiro[4.6]Undeca-2,6,8,10-Tetraenes. Bull. Chem. Soc. Jpn. 1995, 68, 3539–3547. [Google Scholar] [CrossRef]
- Freccero, M.; Gandolfi, R.; Sarzi-Amade’, M.; Bovio, B. Reactions between Triazolinediones and Equilibrating Forms of Cycloheptatriene Derivatives Featuring 7,7-Spiro and 1,7-Fused Heterocyclic Rings. Eur. J. Org. Chem. 2002, 569–579. [Google Scholar] [CrossRef]
- Burdisso, M.; Gandolfi, R.; Toma, L.; Oberti, R. Hexafluoroisopropanol as a Suitable Solvent for Rearrangements via Zwitterionic Intermediates. Tetrahedron 1991, 41, 6725–6736. [Google Scholar] [CrossRef]
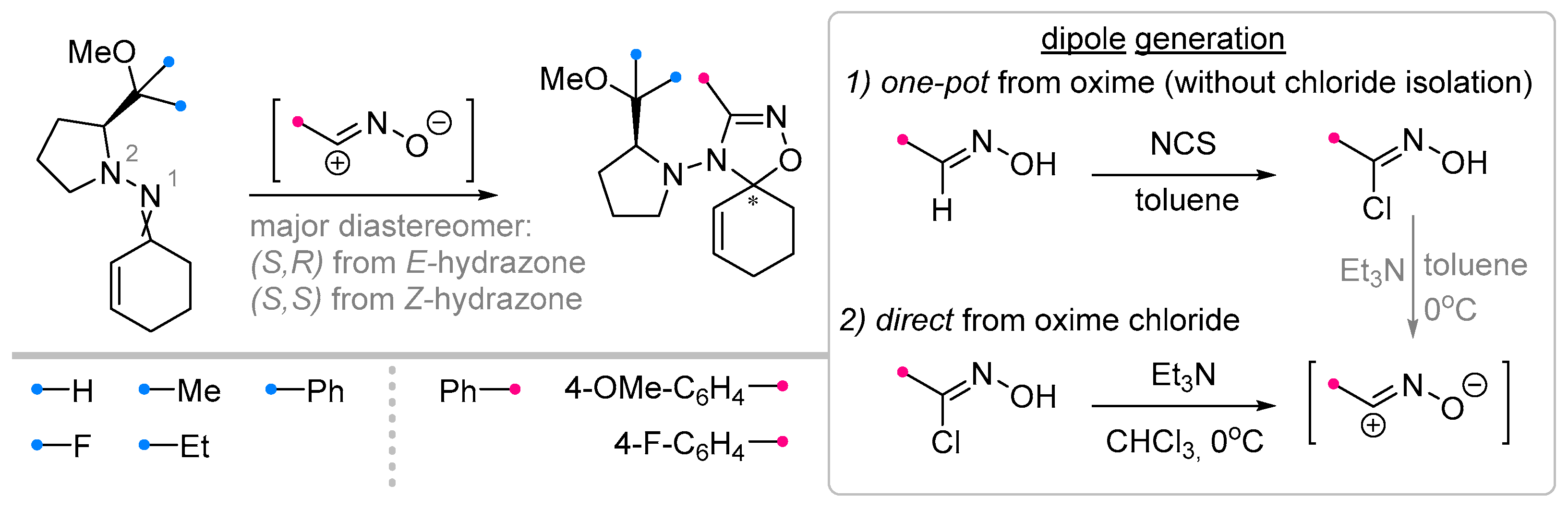
- Enders, D.; Meyer, I.; Runsink, J.; Raabe, G. Diastereo- and Enantioselective Synthesis of ∆2-1,2,4-Oxadiazolines by 1,3-Dipolar Cycloaddition of Nitrile Oxides with Chiral Hydrazones. Heterocycles 1999, 50, 995–1024. [Google Scholar] [CrossRef] [PubMed]
- Beltrame, P.; Cadoni, E.; Floris, C.; Gelli, G.; Lai, A. Reactions of a Stable Benzonitrile Oxide with Aminopyridines. Heterocycles 2000, 53, 1915–1938. [Google Scholar] [CrossRef]
- Awad, W.I.; Sobhy, M. 1,3-Dipolar Cycloaddition of Nitrile Oxides. II. Reactions with o -Quinoid Structures. Can. J. Chem. 1969, 47, 1473–1477. [Google Scholar] [CrossRef]
- Awad, W.I.; Mohayed, S.; Rahman Omran, A. Studies of Quinoid Structures. VII.1 Reaction of Benzonitrile Oxide with o-Quinones. J. Org. Chem. 1966, 31, 331–333. [Google Scholar] [CrossRef]
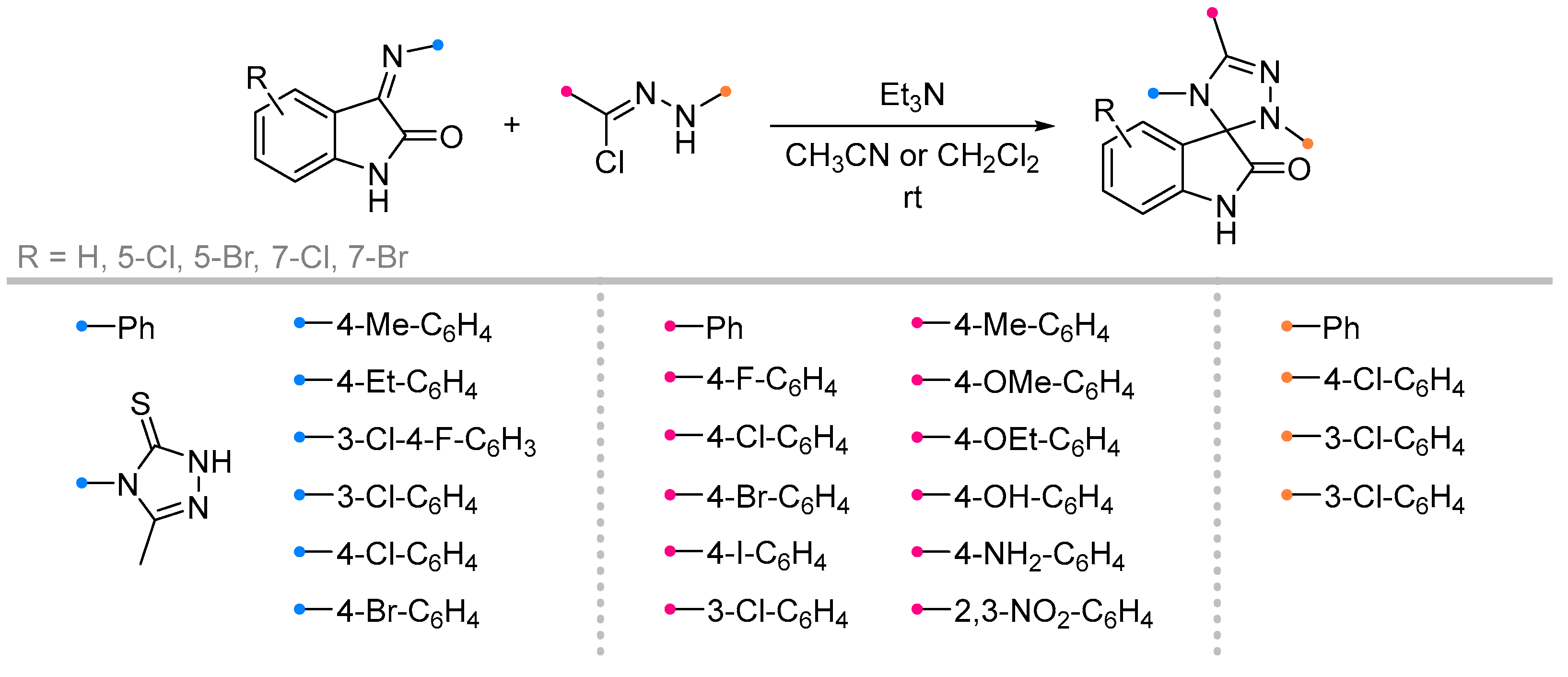
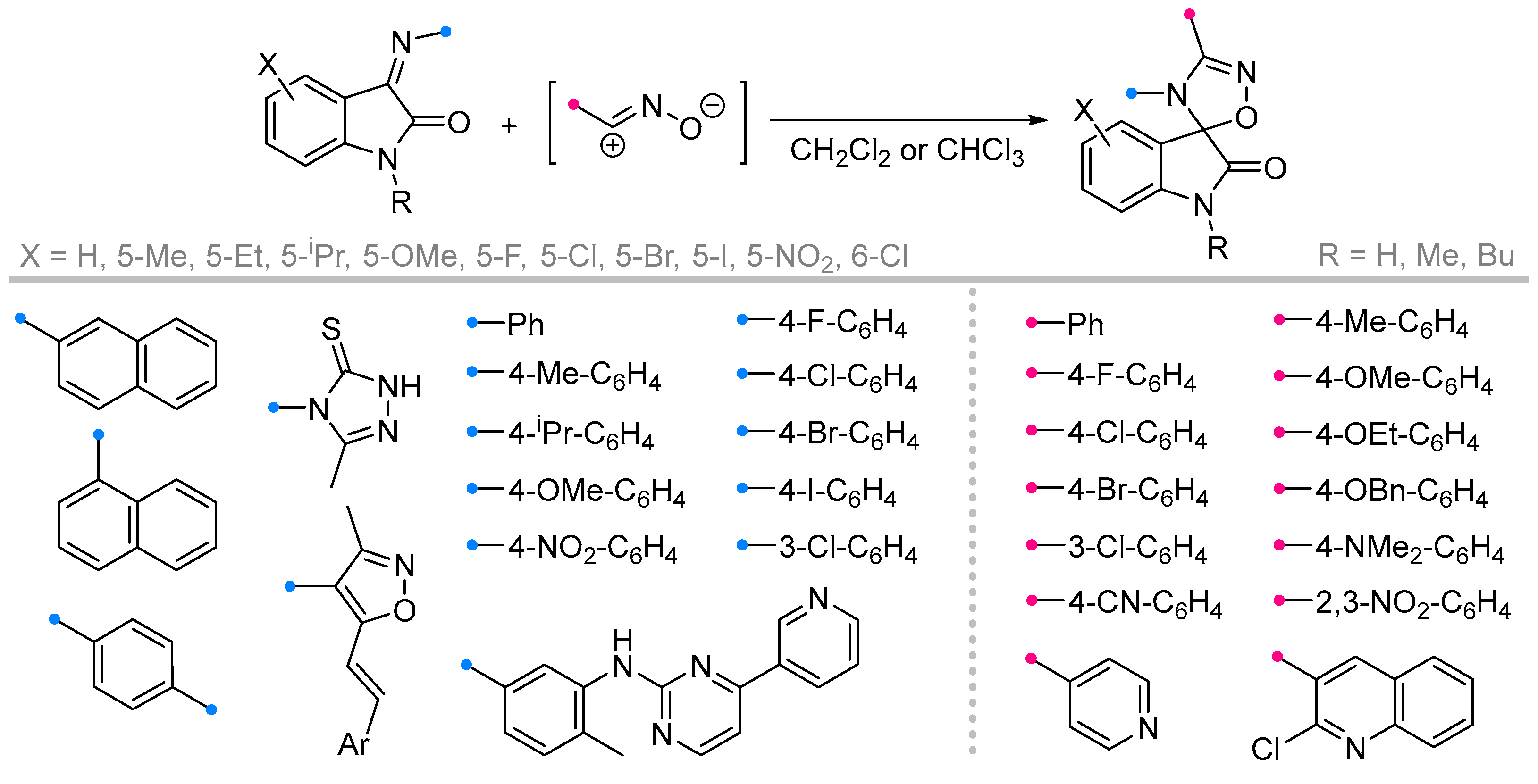
- Bazian, A.; Taheri, M.; Alavi, H. Synthesis of 4′-[3-Methyl-5-Thioxo-1H-1,2,4-Triazol-4(5H)-Yl]- 2′,5′-Diphenyl-2′,4′-Dihydro Spiro[Indolin-3,3′[1, 2,4]Triazol]-2-One Derivatives. Russ. J. Gen. Chem. 2014, 84, 586–592. [Google Scholar] [CrossRef]
- Souzangarzadeh, S.; Bazian, A.; Anaraki-Ardakani, H. A Facile Synthesis of Novel Spiro Indoline-Based Heterocycles through 1,3-Dipolar Cycloaddition Reactions. J. Chem. Res. 2012, 36, 94–95. [Google Scholar] [CrossRef]
- Azizian, J.; Soozangarzadeh, S.; Jadidi, K. Microwave-Induced One-Pot Synthesis of Some New Spiro[3H-Indole-3,5′(4′H)-[1,2,4]Triazoline]-2-Ones. Synth. Commun. 2001, 31, 1069–1073. [Google Scholar] [CrossRef]
- Franke, A. Spirocyclische 2-Indolinone Durch 1,3-dipolare Cycloaddition. Liebigs Ann. Chem. 1978, 1978, 717–725. [Google Scholar] [CrossRef]
- Rajanarendar, E.; Mohan, G.; Ramesh, P.; Rao, E. SYNTHESIS OF SOME NOVEL ISOXAZOLYL-SPIRO-[3H-INDOLE-3,2'-THIAZOLIDINE]-2,4'-(1H)-DIONES, [3H-INDOLE-3,4'-AZETIDINE]-2,2'-(1H)- DIONES AND [3H-INDOLE-3,5'-3'-PHENYL-1',2',4'-OXADIAZOLINE]-2(1H)-ONES. Heterocycl. Commun. 2006, 12, 431–436. [Google Scholar] [CrossRef]
- Ribeiro, C.J.A.; Amaral, J.D.; Rodrigues, C.M.P.; Moreira, R.; Santos, M.M.M. Spirooxadiazoline Oxindoles with Promising in Vitro Antitumor Activities. MedChemComm 2016, 7, 420–425. [Google Scholar] [CrossRef]
- de Azevedo, L.D.; Leite, D.I.; de Oliveira, A.P.; Junior, F.P.S.; Dantas, R.F.; Bastos, M.M.; Boechat, N.; Pimentel, L.C.F. Spirooxadiazoline-oxindoles Derived from Imatinib Show Antimyeloproliferative Potential in K562 Cells. Arch. Pharm. (Weinheim) 2024, 357, 2400029. [Google Scholar] [CrossRef]
- Azizian, J.; Sarrafi, Y.; Mehrdad, M. Synthesis of Some New I-Methyl-3’, 3’- Dichlorospiro[Indol-3, 4’-Azetidine]-2(3H), 2’- Diones and Bis[1-Methyl-3’, 3’- Dichlorospiro(Indole-3, 4’ -Azetidine)-2(3H), 2’-Diones]. Ind. J. Chem. 2000, 39B, 304–307. [Google Scholar]
- Souzangarzadeh, S. 1,3-Dipolar Cycloaddition Reaction of Nitrile Oxides to Isatin Imines. Iran. J. Chem. Chem. Eng. 2016, 35, 31–35. [Google Scholar]
- Shi, G.; He, X.; Shang, Y.; Xiang, L.; Yang, C.; Han, G.; Du, B. Synthesis of 3′,4′-Diaryl-4′H-Spiro[Indoline-3,5′-[1′,2′,4′]Oxadiazol]-2-Ones via DMAP-Catalyzed Domino Reactions and Their Antibacterial Activity. Chin. J. Chem. 2016, 34, 901–909. [Google Scholar] [CrossRef]
- Kanchrana, M.; Allaka, B.S.; Krishna, G.R.; Basavoju, S. An Ultrasound Assisted Synthesis of Spirooxindolo-1,2,4-Oxadiazoles via [3+2] Cycloaddition Reaction and Their Anti-Cancer Activity. Arkivoc 2023, 2023, 202211940. [Google Scholar] [CrossRef]
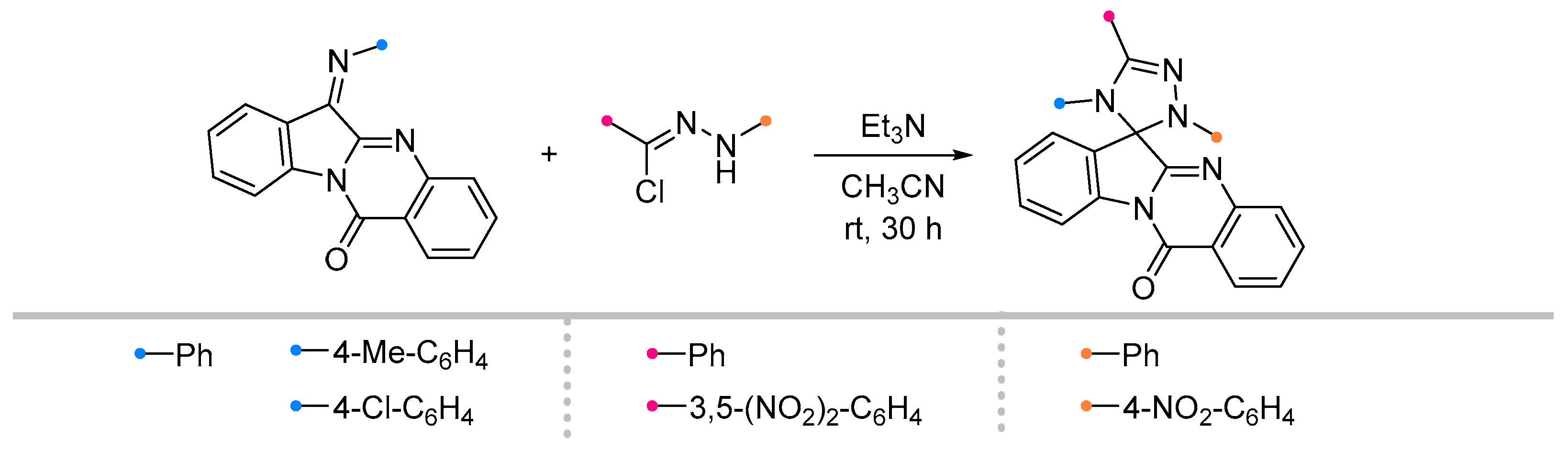
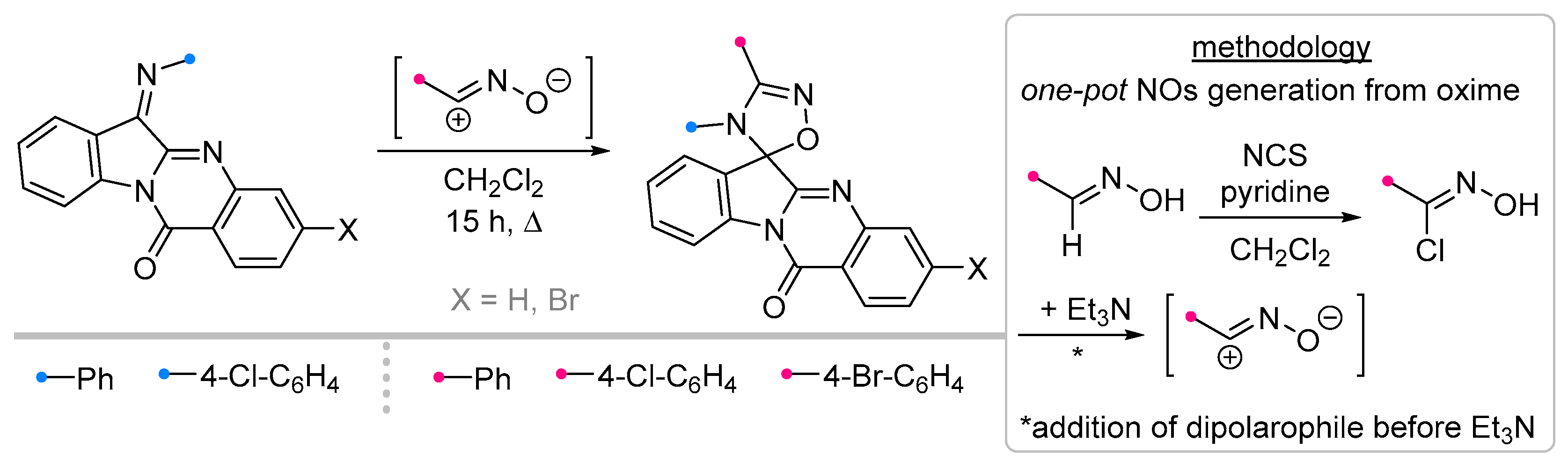
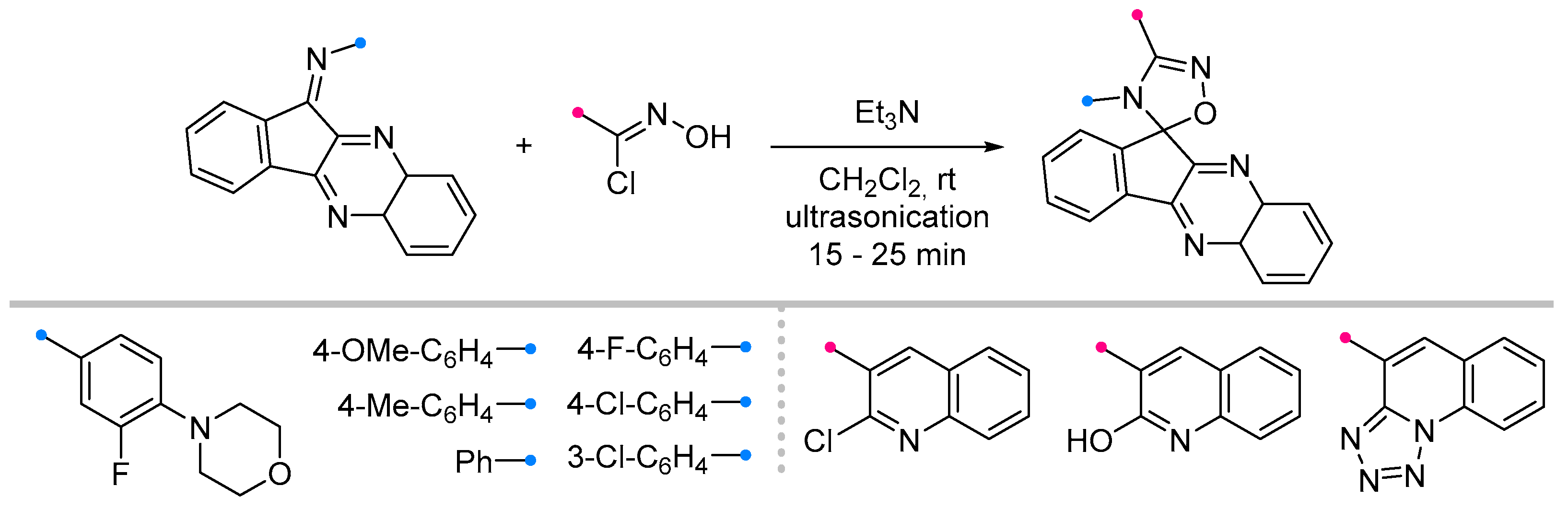
- Azizian, J.; Mohammadizadeh, M.R.; Javadi, M.; Mohammadi, A.A.; Karimi, A.R. One-Pot Synthesis of Some New Spiro 6H-Indolo[b-1,2]Quinazoline-12-One-[1,2,4]Triazolines. J. Chem. Res. 2004, 2004, 125–126. [Google Scholar] [CrossRef]
- Azizian, J.; Madani, M.; Souzangarzadeh, S. One-Pot Synthesis of Novel Derivatives of Spiro-[6H-Indolo [2,1-b]Quinazoline-6,3′-[1,2,4]Oxadiazoline]. Synth. Commun. 2005, 35, 765–768. [Google Scholar] [CrossRef]
- Dalloul, H.M. Synthesis of Spiro-Heterocycles with the Thiazolidinone Moiety from Nitrilimines. J. Chin. Chem. Soc. 2009, 56, 196–201. [Google Scholar] [CrossRef]
- Rajanarendar, E.; Raju, S.; Reddy, A.S.R. Synthesis of Novel Isoxazolyl 1,6-Dithia-4,9-Diazaspiro[4,4]Nonane-3,8- Diones and 1-Oxa-6-Thia-2,4,9-Triazaspiro[4,4]Non-2-Ene-8-Ones. Phosphorus Sulfur Silicon Relat. Elem. 2009, 184, 3139–3148. [Google Scholar] [CrossRef]
- Rajanarendar, E.; Rao, E.K.; Shaik, F.P.; Reddy, M.N.; Srinivas, M. Design, Synthesis, Antibacterial and Antifungal Activity of Novel Spiro-Isoxazolyl Bis-[5,5’]Thiazolidin-4-Ones and Spiro-Isoxazolyl Thiazolidin-4-One-[5,5’]-1,2-4 Oxdiazolines. J. Sulfur Chem. 2010, 31, 263–274. [Google Scholar] [CrossRef]
- Rajanarendar, E.; Reddy, A.S.R.; Reddy, K.G.; Raju, S. Synthesis and Antimicrobial Activity of Novel Spiro-isoxazolyl Oxazolidin-4-one-[5,5’]-1,2,4-oxadiazolines and Thiazolidin-4-one-[5,5’]-1,2,4-oxadiazolines. Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem. 2011, 12, 1800–1806. [Google Scholar]
- Vodela, S.; Reddy Mekala, R.; Gadegoni, H. Synthesis, Characterization and Biological Evaluation of Some Novel 9-Benzooxazol-4-Yl-3,4-Diphenyl-1-Oxa-6-Thia-2,4,9-Triaza-Spiro [4.4]Non-2-En-8-Ones. Int. J. Biomed. Res. 2013, 5, 5–12. [Google Scholar] [CrossRef]
- Vodela, S.; Reddy Mekala, R.; Reddy Modugu, N.; Vannada, J. SYNTHESIS AND ANTIMICROBIAL EVALUATION OF SOME NOVEL SPIROTHIAZOLIDIN-BASED DERIVATIVES. World J. Pharm. Pharm. Sci. 2014, 3, 800–810. [Google Scholar]
- Kishore, B; Brahmeshwari, G. SYNTHESIS AND ANTIMICROBIAL SCREENING OF NOVEL 4-(1H-BENZO[d] IMIDAZOL-2-YL)-8,9-DIARYL-1,6-DIOXA-4,7,9-TRIAZASPIRO[4,5]DEC-7-EN-3-ONES. Heterocycl. Lett. 2018, 8, 631–639. [Google Scholar]
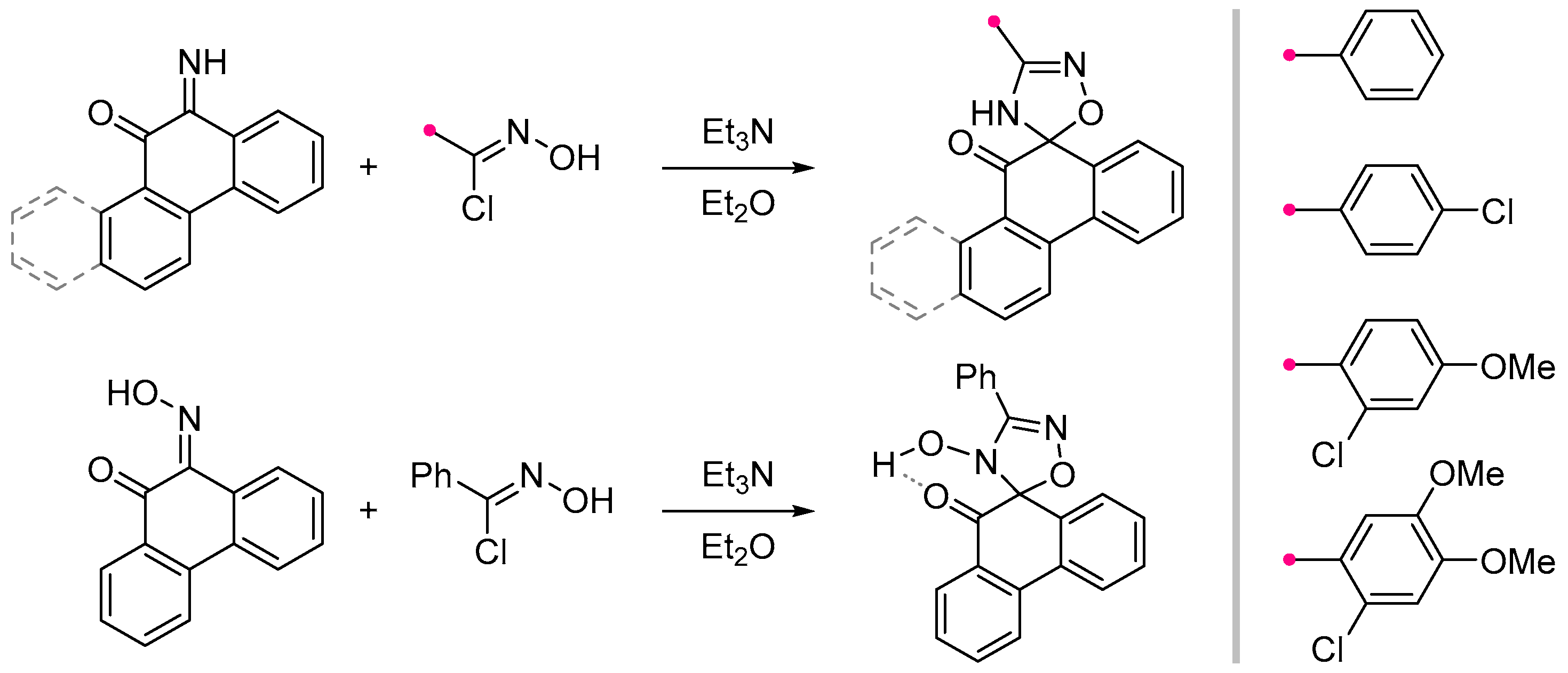
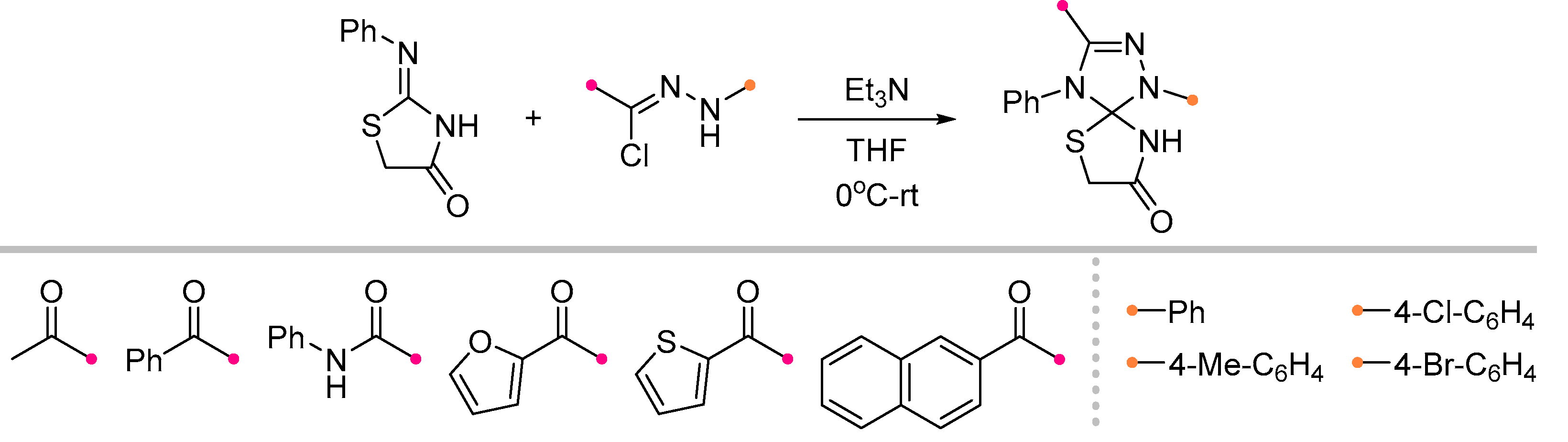
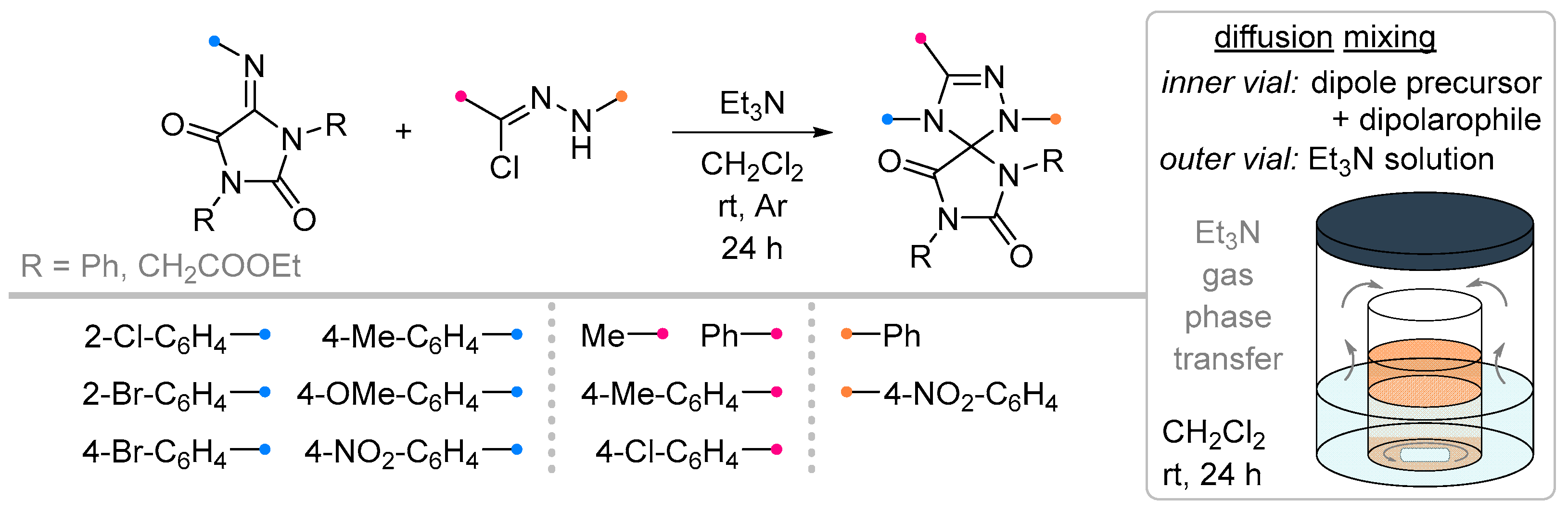
- Kuznetsova, J. V.; Tkachenko, V.T.; Petrovskaya, L.M.; Filkina, M.E.; Shybanov, D.E.; Grishin, Y.K.; Roznyatovsky, V.A.; Tafeenko, V.A.; Pestretsova, A.S.; Yakovleva, V.A.; et al. [3+2]-Cycloaddition of Nitrile Imines to Parabanic Acid Derivatives—An Approach to Novel Spiroimidazolidinediones. Int. J. Mol. Sci. 2023, 25, 18. [Google Scholar] [CrossRef]
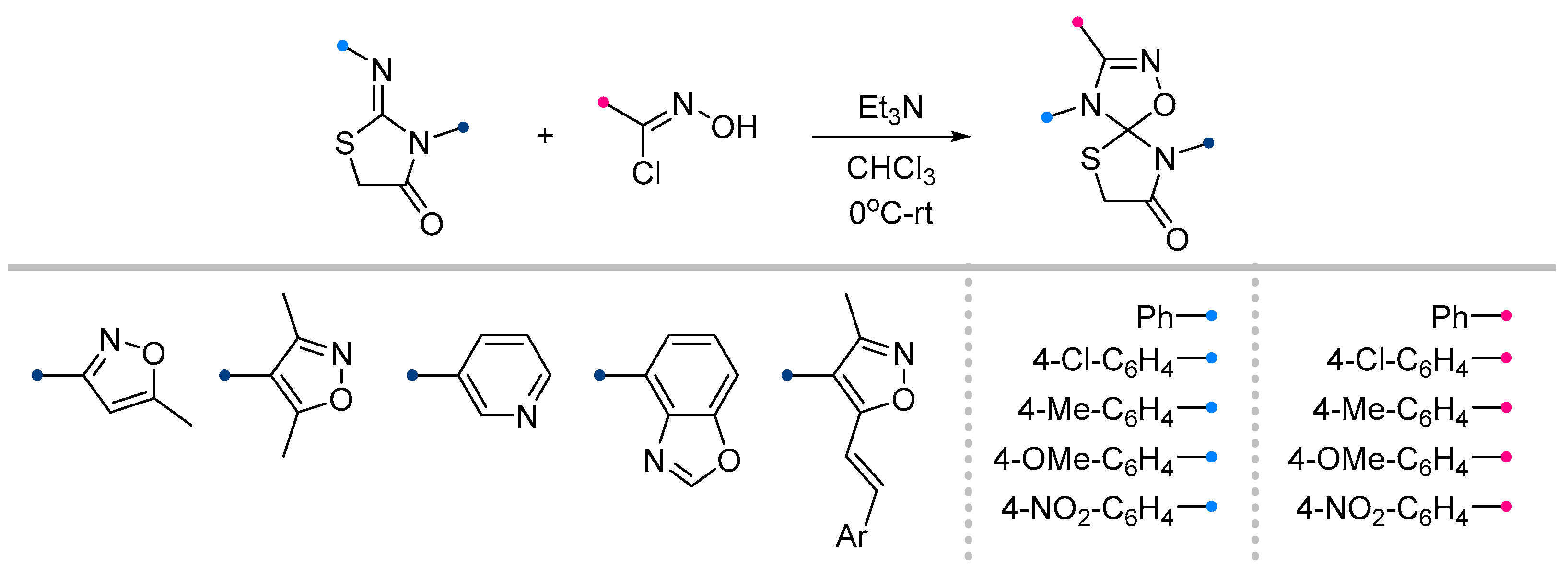
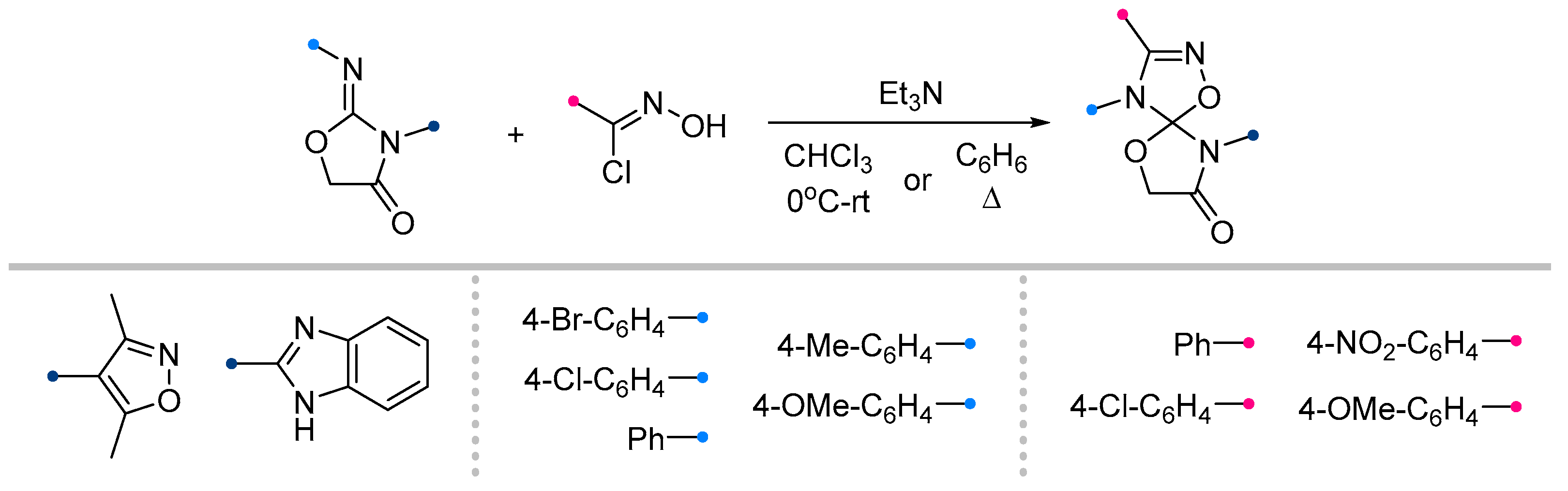
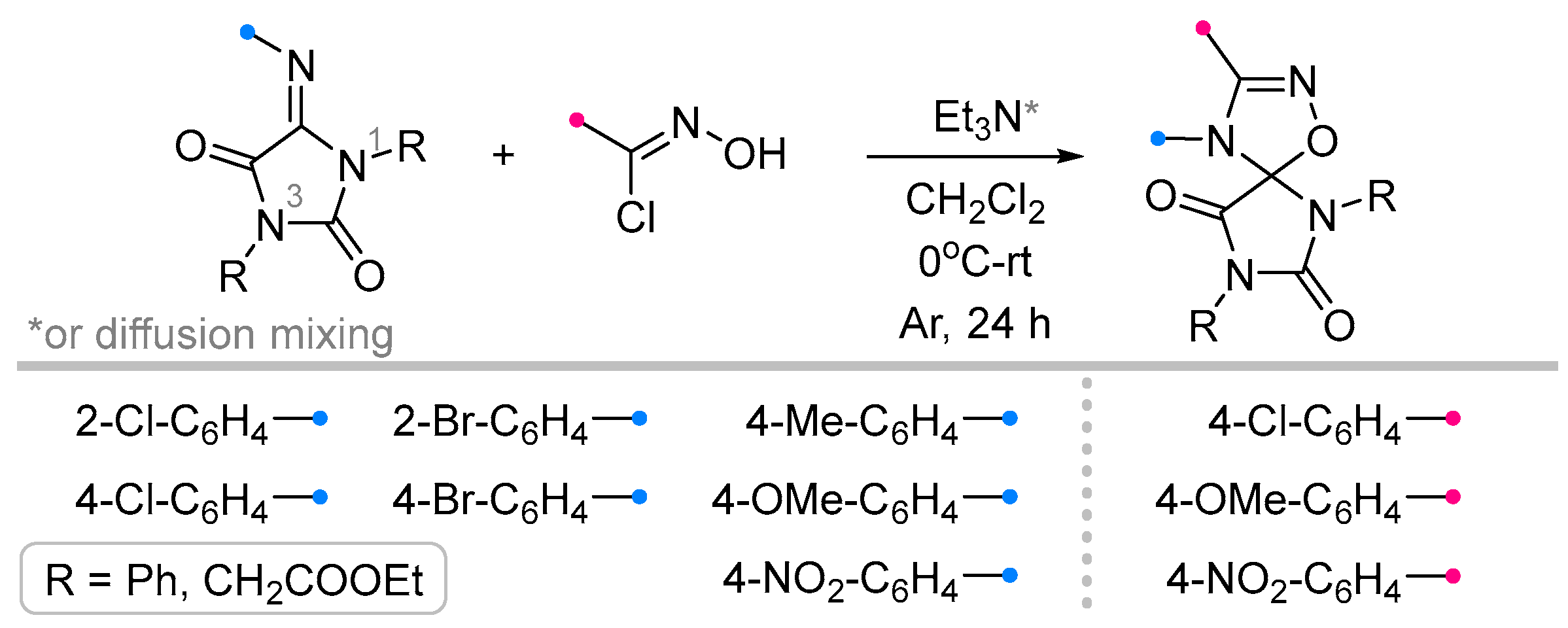
- Petrova, J. V; Tkachenko, V.T.; Tafeenko, V.A.; Pestretsova, A.S.; Pokrovsky, V.S.; Kukushkin, M.E.; Beloglazkina, E.K. Facile Synthesis of Hydantoin/1,2,4-Oxadiazoline Spiro-Compounds via 1,3-Dipolar Cycloaddition of Nitrile Oxides to 5-Iminohydantoins. Beilstein J. Org. Chem. 2025, 21, 1552–1560. [Google Scholar] [CrossRef]
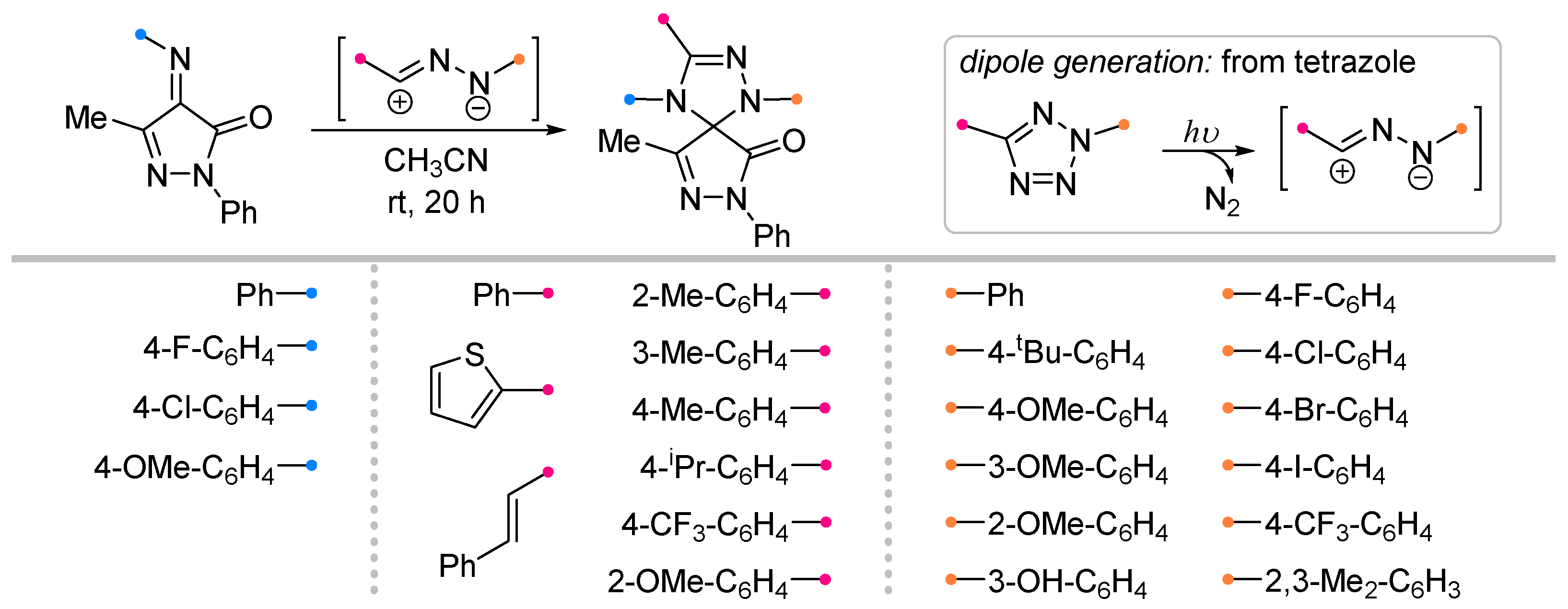
- Jia, P.; Lin, Z.; Luo, C.; Liang, J.; Lai, R.; Guo, L.; Yao, Y.; Wu, Y. Synthesis of Spirotriazolines and Spirooxadiazolines via Light-Induced 1,3-Dipolar [3+2] Cycloadditions. New J. Chem. 2023, 47, 20248–20252. [Google Scholar] [CrossRef]
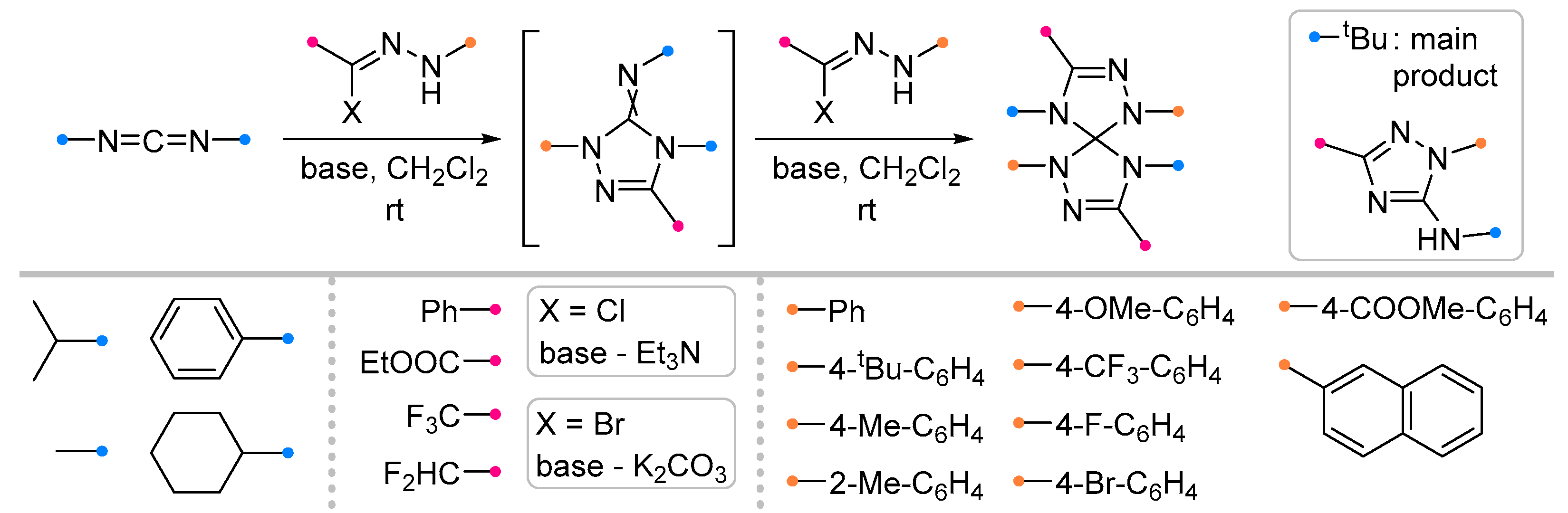
- Huisgen, R.; Grashey, R.; Kunz, R.; Wallbillich, G.; Aufderhaar, E. 1.3-Dipolare Cycloadditionen, XVII: Bisaddukte Aus Nitriliminen Und Carbodiimiden. Chem. Ber. 1965, 98, 2174–2184. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, X.; Liu, X.; Huang, D.; Wang, K.-H.; Wang, J.; Hu, Y. Synthesis of Di/Trifluoromethyl Bis(1,2,4-Triazoline)Spiranes and 1,2,4-Triazoles via 1,3-Dipolar Cycloaddition of Nitrilimines and Carbodiimides. Org. Biomol. Chem. 2025, 23, 2662–2671. [Google Scholar] [CrossRef]
- Huisgen, R.; Aufderhaar, E. 1.3-Dipolare Cycloadditionen, XVIII: Die Umsetzung Des Phenyl-cyanamids Mit C-Äthoxycarbonyl-N-phenyl-nitrilimin. Chem. Ber. 1965, 98, 2185–2192. [Google Scholar] [CrossRef]
- Yen, W.; Kung, F.; Wong, F.F. 1,3-Dipolar Cycloaddition of Carbodiimides and Nitrilimines: Synthesis and Mechanistic Study of 5-Amino-1,2,4-triazoles. Eur. J. Org. Chem. 2016, 2016, 2328–2335. [Google Scholar] [CrossRef]
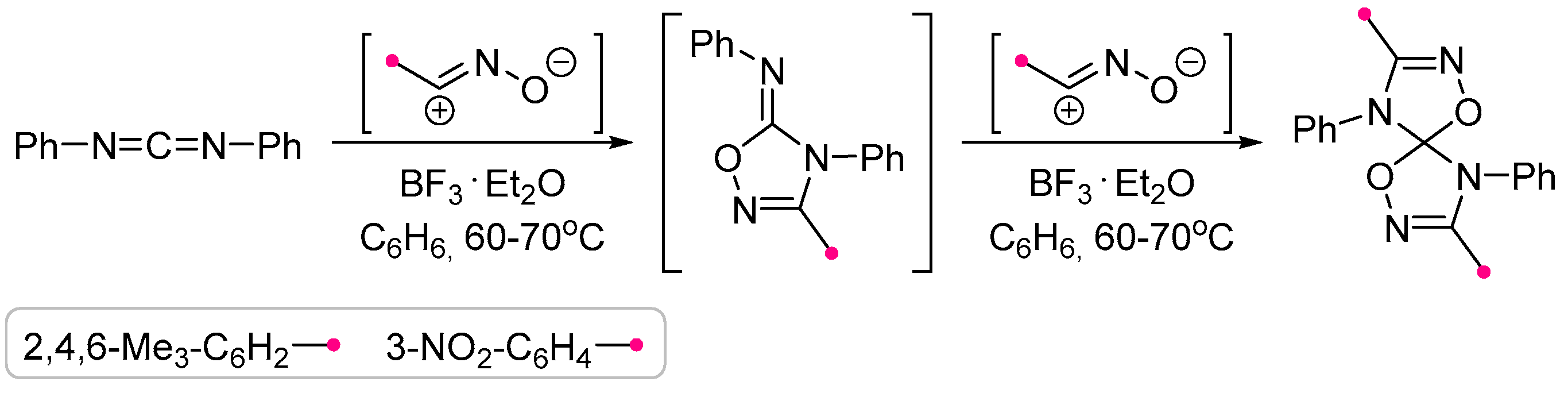
- Grundmann, C.; Richter, R. Die Bortrifluorid Katalysierte Addition von Nitriloxiden an Cn-Doppelbindungssysteme. Tetrahedron Lett. 1968, 9, 963–966. [Google Scholar] [CrossRef]
- Sharma, P.; Ranga Prabhath, M.R.; Wong, D.; Ampem-Lassen, M.A.; Bhat, S. V.; Williams, L.; Carvalho, T.G. Synthesis of Biologically Active Heterospirocycles through Iterative 1,3-Dipolar Cycloaddition Pathways. J. Org. Chem. 2021, 86, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




