1. Introduction
H. pylori is still one of the most common human bacterial infections worldwide. Its eradication, due to its numerous negative effects and as the leading cause of infection-associated cancer, remains a critical public health goal.
Overview of current guidelines. Current consensus guidelines (the American College of Gastroenterology (ACG) 2024 Guideline and Maastricht VI/Florence consensus) recommends susceptibility-guided or local antibiotic resistance-guided treatments instead of empirical eradication regimens. Our short guideline overview is focused only on the two most popular clarithromycin-based triple and tetracycline-based quadruple regimens. In regions where primary clarithromycin resistance exceeds approximately 15%, and in cases where no previous antibiotic susceptibility testing is available, a 14-days tetracycline-based quadruple therapy (consisting of a PPI, bismuth, metronidazole and tetracycline) is advised generally as first line treatment. In this area, the empirical use of the classical clarithromycin based triple therapy (PPI + clarithromycin + amoxicillin or metronidazole) is not recommended in first line setting. In regions where estimated clarithromycin resistance is under 15%, as empirical treatment, both protocols, the amoxicillin-clarithromycin based triple therapy (amoxicillin +clarithromycin +PPI) or the above-mentioned tetracycline-based quadruple therapy is recommended as first line treatment. In countries where available, commercial preparations that contains antibiotics and bismuth can be given with PPI to achieve better patient compliance and success rates exceeding 90%. Eradication rates with PPI-clarithromycin triple therapy have been decreasing over time—due largely to the increasing prevalence of clarithromycin resistance related to the frequent use of macrolide antibiotics in clinical practice [
1,
2].
Clarithromycin resistance of H. pylori and routine eradication practice in Hungary. Estimating local
H. pylori clarithromycin resistance in Hungary lead to controversial data ranging from 5.5% (macrolide-naïve patients) to 17.3% in literature [
3,
4], therefore in routine clinical practice no equivocal data is available for guideline use purposes. However, heteroresistance —where resistant and susceptible strains coexist—may occur in ~7% of cases and contributes to treatment failures even below classical thresholds [
5]. Based this data and the continuous increase in the use of macrolide antibiotics, Hungary can be considered as a clarithromycin resistant area. Empirical eradication practice in Hungary has historically centered on amoxicillin-clarithromycin based triple therapy in treatment-naïve patients. However, with rising macrolide use over time, treatment failures have become more common [
6]. Recent trends in European practice drawn from the Hp-EuReg registry emphasize a shift toward single capsule bismuth quadruple therapy, achieving eradication rates around 90–95% as both first line and rescue therapy across resistant settings. These result can be obtained by only a minimum of 10 days duration of eradication treatments [
7]. Hungary-specific registry data remain limited, but overall Hungarian patterns likely mirror broader Central European trends about a general success rate around 80% [
8]. Based on literature data, in Hungary,
H. pylori success rates reaches international standards when tetracycline-based quadruple therapies were used (93.6%), however, in cases where the conventional clarithromycin-based regimens were given, only 75% efficacy was shown [
9].
Problems with drug availability: tetracycline, bismuth and combination drugs. Regarding pharmaceutical access, the availability of tetracycline and bismuth salts (as individual agents and in fixed dose combinations) are limited in Hungary. Tetracycline is only available by individualized drug import; bismuth drugs are only available in the form of bismuth oxide. Bismuth citrate, which is pharmacologically similar to the commercially available bismuth oxide, available as a magistral preparation as well. A combination drug that contains bismuth subcitrate, metronidazole and tetracycline approved in Europe since 2009, however in Hungary it is not yet available. Tetracycline and bismuth compounds are included in the national formulary and accessible via prescription, though historically under used due to entrenched triple therapy habits. National formulary gives the opportunity to prepare magistral bismuth-metronidazole, so by a very limited availability (it is prepared only in one pharmacy) we have the opportunity to use it in routine clinical setting.
Hungary can be generally considered as a tetracycline and bismuth naïve area, so a possible shift of using eradication protocols towards tetracycline containing regimens promising excellent outcomes that comparable to international success rates. As above mentioned, despite the limitation of its availability, the classic bismuth-containing quadruple therapy (PPI, bismuth, tetracycline, and metronidazole) has been recommended as a first-line option in areas with high and uncleared clarithromycin resistance.
Supplementations for increase treatment success. Tetracycline based regimens contain bismuth at baseline setting. It is well known that addition of bismuth to 14-day standard triple therapy with clarithromycin and amoxicillin eradicates
H. pylori infection in more than 90% of patients, resulting in a potential therapeutic gain (10%–20%) in populations with moderate to high clarithromycin resistance, with an acceptable safety profile and level of adherence [
8]. Although several data support bismuth addition to any eradication protocols [
10,
11,
12], comparison of the different available bismuth compounds (commercial bismuth oxide, magistral bismuth-citrate and bismuth-metronidazole) has not yet been examined to evaluate their effect on treatment outcome. Meta-analyses and clinical studies support the addition of probiotics to standard regimens, as these adjuvant probiotic supplements are reported to modestly increase eradication rates and reduce antibiotic related side effects particularly when combined with tetracycline based quadruple therapy [
13]. European registry data also indicate more frequent probiotic co-prescription is associated with improved tolerability and possibly adherence [
14,
15].
Limited data are available about the additional effect of different probiotic strains, but direct comparison about their effect on treatment efficacy hardly can be found. In many original articles, reviews and meta-analyses assessment of clinical symptoms was the trial outcome with a presumably positive result. As in this article we did not collect any patient reported outcomes, we overview only available data about treatment success here. A large meta-analysis of 25 randomized controlled trials (RCTs-3,769 patients) evaluating single strains as adjuncts to standard eradication therapy found that only
Saccharomyces boulardii CNCM I-745 that significantly improved eradication rates.
Lactobacillus rhamnosus GG, Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus reuteri, Clostridium butyricum showed no clear benefit on eradication success as well [
13]. Another high-quality systematic review (2025; 5,036 cases, 19 RCTs) confirmed that the addition of
Saccharomyces boulardii significantly increased eradication rates [
16]. Efficacy of Lactobacillus strains are investigated by a comprehensive RCT involving
Lactobacillus reuteri DSM17648 as adjunct to standard therapy achieved eradication rates exceeding 90% [
17]. However, pooled RCTs in meta-analyses failed to show a statistically significant improvement in eradication rates for Lactobacillus reuteri. As a head-to-head or combined probiotic comparison, a smaller RCT combined
Saccharomyces boulardii and
Lactobacillus reuteri in quadruple therapy were performed. While data about direct comparison was limited, the combined regimen suggested higher eradication rate and better tolerability, though sample size was small and no definitive strain efficacy separation was provided [
18].
Besides the lacking data of direct comparison of probiotic strains, their unique additional effect on different treatment protocols is still an opened question as well. Combination use of both supplements (bismuth and probiotics) has not yet examined regarding efficacy; however, a promising potential can be foreseen to further enhance eradication success.
2. Materials and Methods
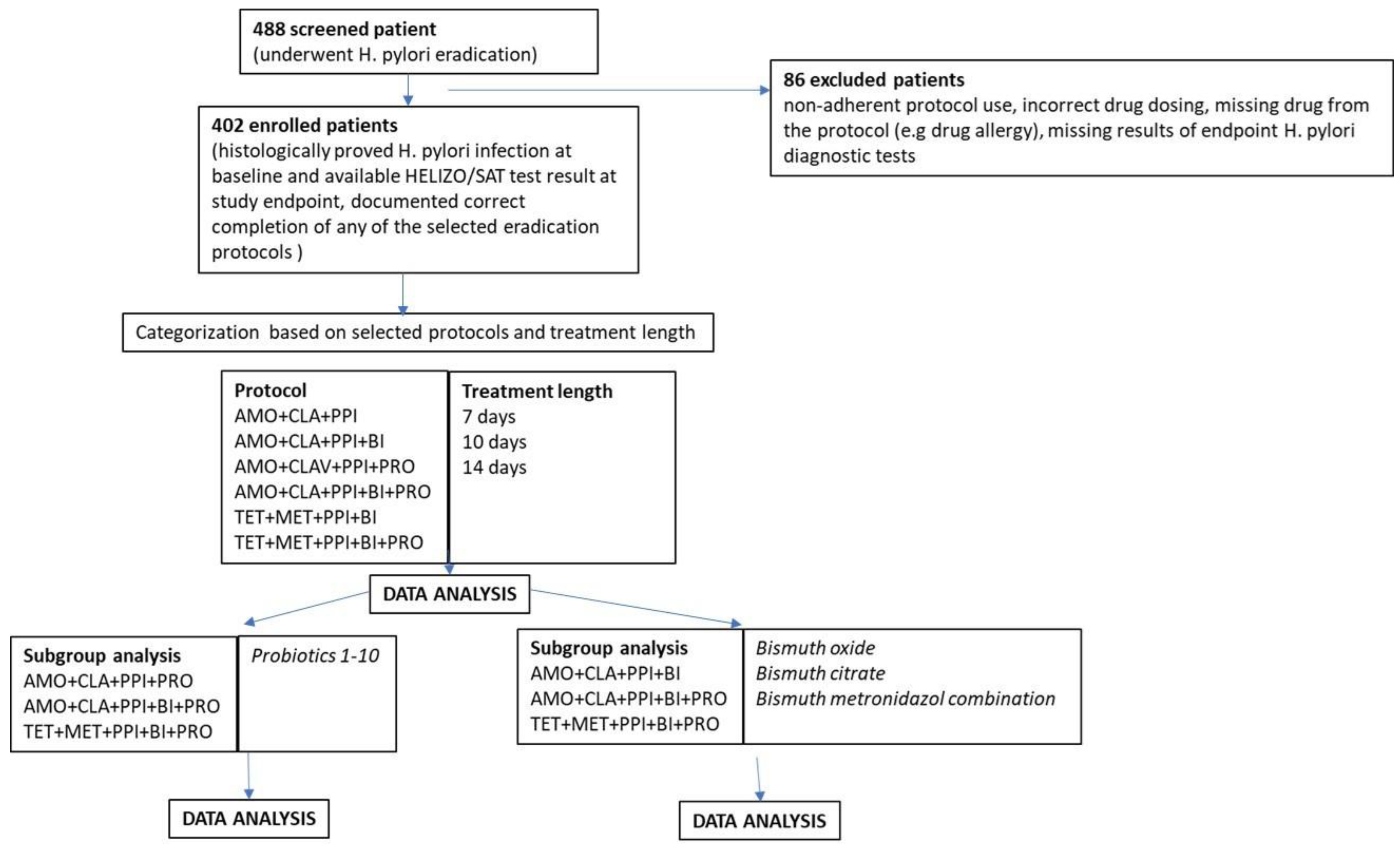
Patients. In this real-world retrospective trial, the case documentation of patients who underwent H. pylori eradication in our outpatient department were investigated in a nine year period (2016-2024). A total number of 486 eradication cases were screened and 402 cases were selected who met the inclusion criteria and enrolled to our trial after exclusion the not suitable cases (see exclusion criteria below) and underwent further data collection.
Diagnostic tools. The initial diagnosis of H. pylori infection was performed in histological samples that obtained by gastroscopy from antrum. Histological examination was performed by Giemsa staining; in doubtful cases a confirmation test was done by immunohistochemistry (H. pylori rabbit monoclonal antibody manufactured by Sigma-Aldrich). Envision Target Retrieval System was used for detection purposes. For the determination of treatment outcome, results of HELIZO™ exhalation tests (performed at the Nuclear Medicine Department of Buda Hospital of the Hospitaller Order of Saint John of God), or stool Helicobacter antigen (SAT) tests (provided by the Central Laboratory of Buda Hospital of the Hospitaller Order of Saint John of God) were used. HELIZO™ exhalation tests based on the 14C content of exhaled air formed from radioactively labelled carbamide. HELIZO™ exhalation test and HELIPROBE™ equipment for detection are manufactured by Isotope Institute Ltd. For stool antibody testing, DiaQuick H. pylori stool casette (sensitivity: 98.8%, specificity: 98.4%, manufactured by DiaLab Ltd.) were used.
Database search and case screening. Data collection was performed trough institutional database search (EMMA™). The basis of patient screening to our research was a histologically proved H. pylori infection at baseline and the availability of the result of either HELIZO™ exhalation tests or stool Helicobacter antigen (SAT) tests at study endpoint.
Inclusion criteria. We selected only patient documentations that met all inclusion criteria: (1) an initial diagnosis H. pylori infection, (2) a final result of eradication by each of the diagnostic tests (HELIZO™ or SAT) 6 to 8 weeks after completing eradication treatment (3) correct and prescription details of antibiotics used for eradication (agent name or drug trade name and dose) were available.
Exclusion criteria. Case documentations containing incomplete data (e.g. missing treatment length, exact type of medicines), as well as treatments deviating from the protocol (e.g. alternative combinations due to the patient's drug allergy, the patient's intolerance to one of the components, or any conditions that resulting in earlier termination of the prescribed treatment) were not included in the statistical calculation. All eradication cases were recorded regardless in which line the patient received the therapy (in first line or after a failure as second or more attempts).
Determination of investigated outcomes in database. „Baseline- H. pylori infection” meant an initial histologically proved H. pylori infection. Cases where the result of HELIZO™ or SAT test were negative after completing eradication treatment were determined as „Success”. However, a remaining positive value after eradication meant „Failure”.
Used antibiotics, PPI and supplementations. Commercially available amoxicillin, clarithromycin and PPIs were used in clinical practice therefore in our investigation as well. The used tetracycline was a commercial medicine that was available by an individual import process in one pharmacy. Several efforts were made to improve success rates in clinical routine by using bismuth compounds as a part of tetracycline-based regimes and as a supplement in clarithromycin-based protocols. The commercially available bismuth oxide is pharmacologically similar to the magistral prepared bismuth-citrate (in pharmacology nomenclature they are considered as synonyms), but due to their different preparation method they were examined as a separate entity in the trial. Bismuth-metronidazole capsule was prepared in a magistral way based on physician’s prescription as well. Regarding probiotics, commercially available probiotic strains were used by the choice of gastroenterologists.
Investigated protocols. According to the international recommendations, two standard types of eradication protocols, a clarithromycin-based (AMO+CLA+PPI), and an originally tetracycline based (TET+MET+PPI) were collected from database. As Hungary considered to a clarithromycin resistant area, and as above detailed, tetracycline and bismuth compound availability are limited, physicians made different efforts on improving success rates of the most frequently used clarithromycin based protocol by adding supplements, mainly probiotics, and in a some cases bismuth compounds (AMO+CLA+PPI±BI±PRO) Tetracycline based protocols originally contains bismuth and in clinical routine, supplemented with probiotics in many cases as well (TET+MET+BI±PRO). Therefore, eradication cases were collected and investigated in conventional settings of regimens or with supplementations: (1) standard triple clarithromycin-based protocol containing amoxicillin, clarithromycin, proton pump inhibitor, (AMO+CLAV+PPI); (2) a quadruple clarithromycin based combination containing amoxicillin, clarithromycin, proton pump inhibitor and bismuth (AMO + CLA+PPI+BIZ), (3) a quadruple clarithromycin based protocol containing amoxicillin, clarithromycin, proton pump inhibitor with probiotics (AMO+CLA+PPI+PRO), (4) a quintuple protocol of amoxicillin, clarithromycin, proton pump inhibitor, supplemented both bismuth and probiotics (AMO+CLA+PPI+BIZ+PRO). The other types of investigated regimens were the standard (5) tetracycline +metronidazole +PPI +bismuth regimen (TET + MET + PPI + BIZ), (6) supplemented with probiotics (TET+ MET + PPI + BIZ+PRO) (
Table 1 and
Table 2). All of the used protocols were investigated by different treatment length (7, 10 or 14 days) as well. The type of proton pump inhibitor is not examined in this trial, and therefore not detailed, all of the cases it was given in a standard prescription dose. Any other prescribed or given medication outside of eradication protocol was also not considered to participate in data collection.
Investigation of supplements. As a separate part of the trial, we examined the additional value of ten different probiotic strains in those three protocols where it was applicable (AMO+CLA+PPI+PRO, AMO+CLA+PPI+BIZ+PRO, TET+ MET + PPI + BIZ+PRO) (
Table 3). Additional bismuth was given is three different forms to protocols (AMO + CLA+PPI +BIZ, AMO+CLA+PPI+BIZ+PRO, TET + MET + PPI + BIZ, TET+ MET + PPI + BIZ+PRO) in their appropriate dosing: (1) commercially available bismuth oxide, (2) magistral prepared bismuth -citrate compound, (3) magistral prepared bismuth-metronidazole combination (
Table 4).
Database setup. Data collection was performed in a Microsoft Excel table. Physicians were anonymized and marked with randomly assigned numbers. Collected data were the date of birth and gender of the patients, the length of the treatment, the type of eradication protocol, the used agents (antibiotics, bismuth and proton pump inhibitor) with dosing, the additional probiotic or bismuth use (where applicable), the outcome of the therapy (success or failure) based on HELIZO™ or SAT tests. We did not collect any data based on patient reported outcomes (e.g. symptoms, compliance) regarding to the retrospective manner of our analysis and as the main goal of the investigation was the optimalization of society guideline use in daily clinical routine.
Statistical analysis. Statistical analysis was performed by TIBCO Statistica Software (version 14.1.0). The calculation is based on 2*2 or in some cases r*c contingence tables using chi-square test. The significance level was 5%.
Goals. The aim of our retrospective real world data analysis of 402 cases was to give a comprehensive view and an optimalization of the routine use of clinical guidelines in H. pylori eradication in a clarithromycin resistant and tetracycline-bismuth-naive area by answering the following questions: (1) description of trends regarding the number of eradication cases, used protocols, their length and success rate, frequency of used supplements throughout the years in our center (2) investigation of overall protocol success rates (3) determine the optimal duration of treatment, (4) find the protocol(s) that achieve the highest eradication rates (5) clear whether the addition of probiotic to treatment improves outcomes in different settings, (6) clarify the effect of bismuth addition to clarithromycin based protocols and investigate the possible difference between bismuth compounds regarding treatment success (7) find the most effective probiotic strain and determining future efforts to improve patient care.
3. Results
3.1. Patient and Study Characteristics
A total of 488 case documentation of patients who underwent
H. pylori eradication were screened initially. 402 patients, who met inclusion criteria were enrolled to the trial. 86 cases were excluded due to inadherent protocol use, incorrect drug dosing, missing drug from the protocol (e.g drug allergy), missing results of endpoint
H. pylori diagnostic tests (see
Table 5). After enrollment, patient data were categorized according to treatment protocol, treatment length, supplementation. Subgroup analyses were done in probiotic-containing cases to investigate the additional effect of different probiotic strains and in bismuth-containing cases to compare the effectivity of different bismuth compounds. (
Figure 1-Study flowchart).
3.2. Trends in H. pylori Eradication Between 2016-24
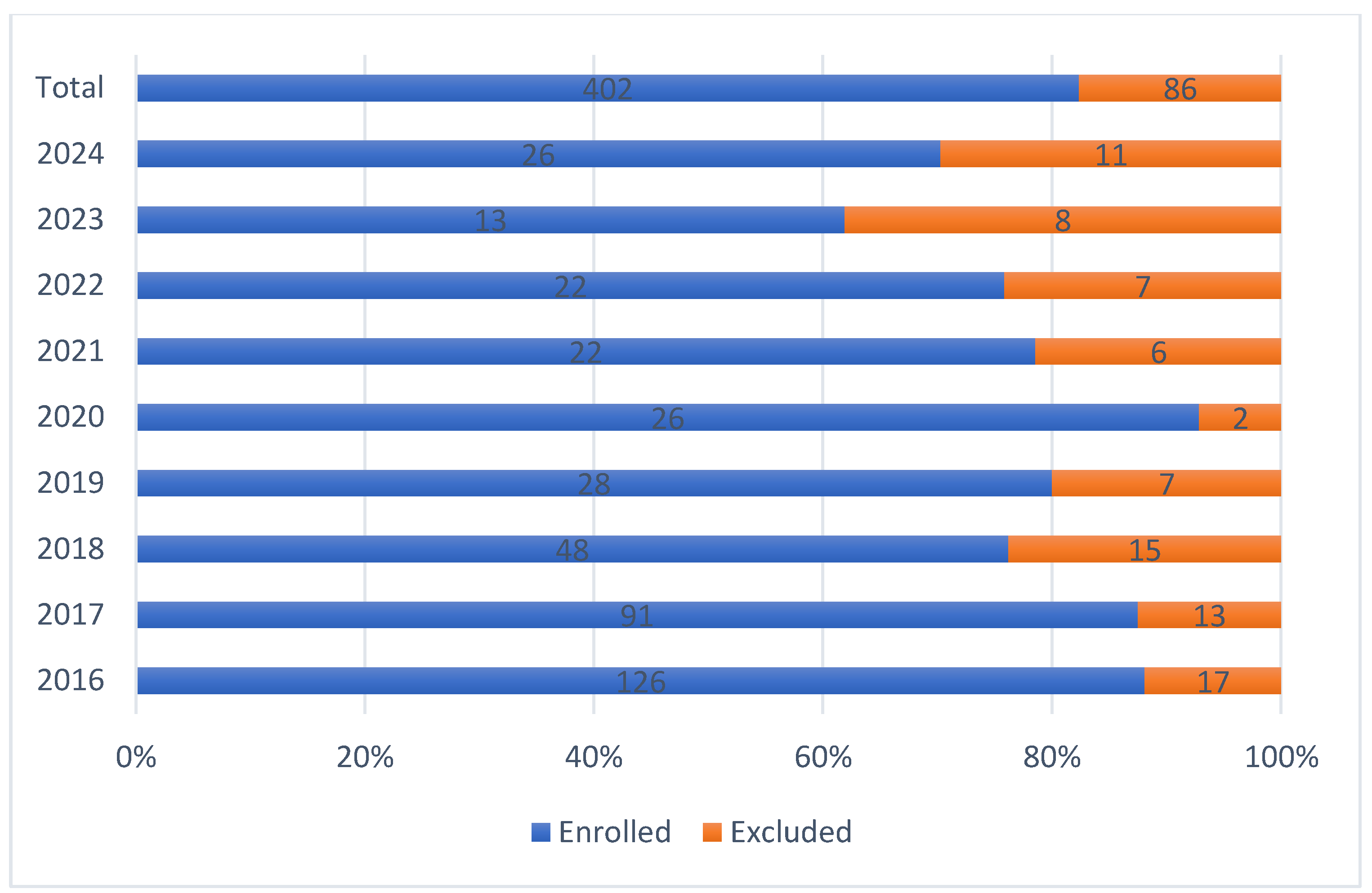
Although a continuous and sharp yearly decline was observed in the number of eradication cases, proportion of excluded cases and success rates remain the same (
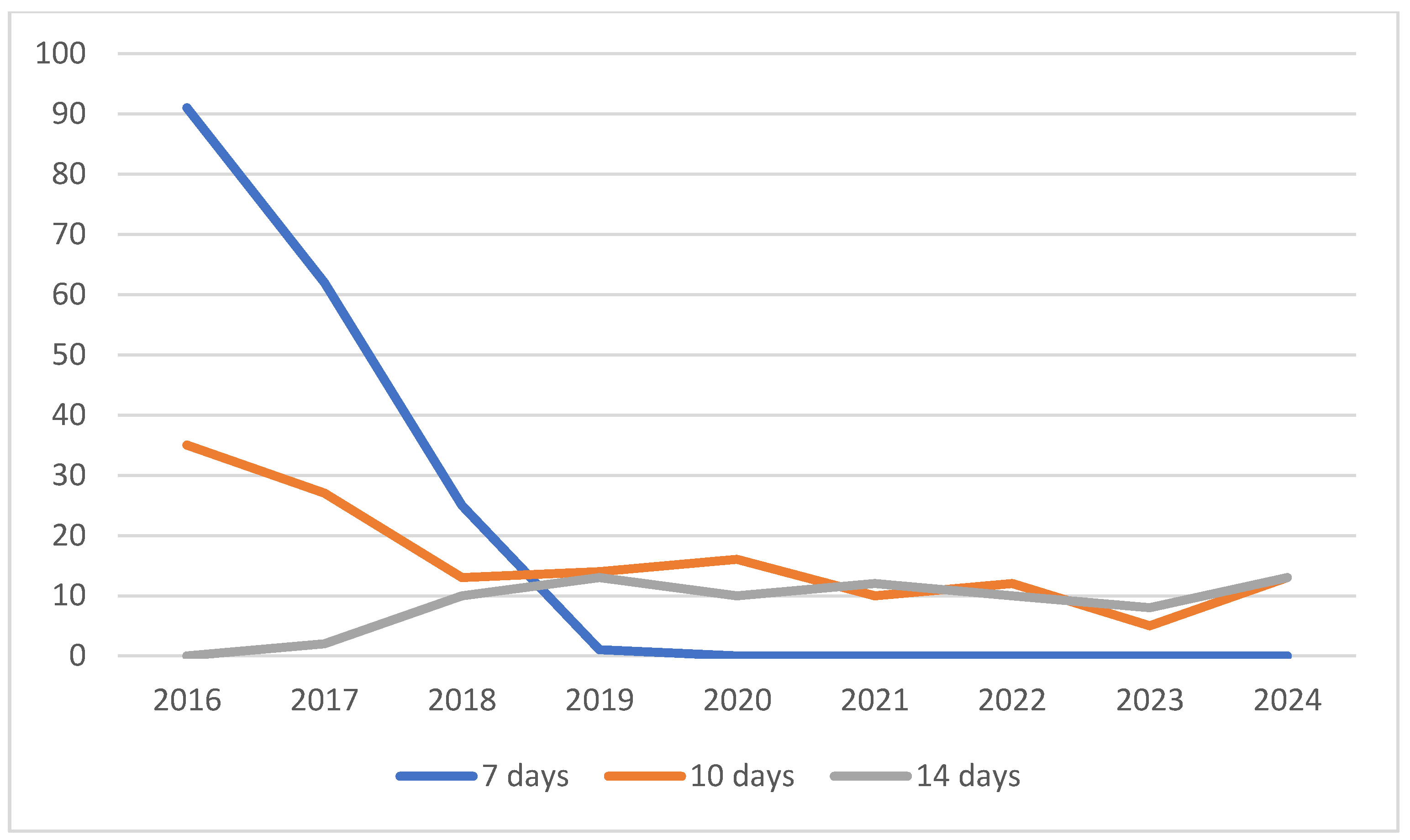
Figure 2). The incidence of clarithromycin-based protocols shows a continuous decline, tetracycline-based regimens were used in an increasing rate. A continuous decrease in the incidence of 7-days protocols that reached zero to 2019 were observed, the proportion of 10- and 14-days protocols remains stable since 2018 (
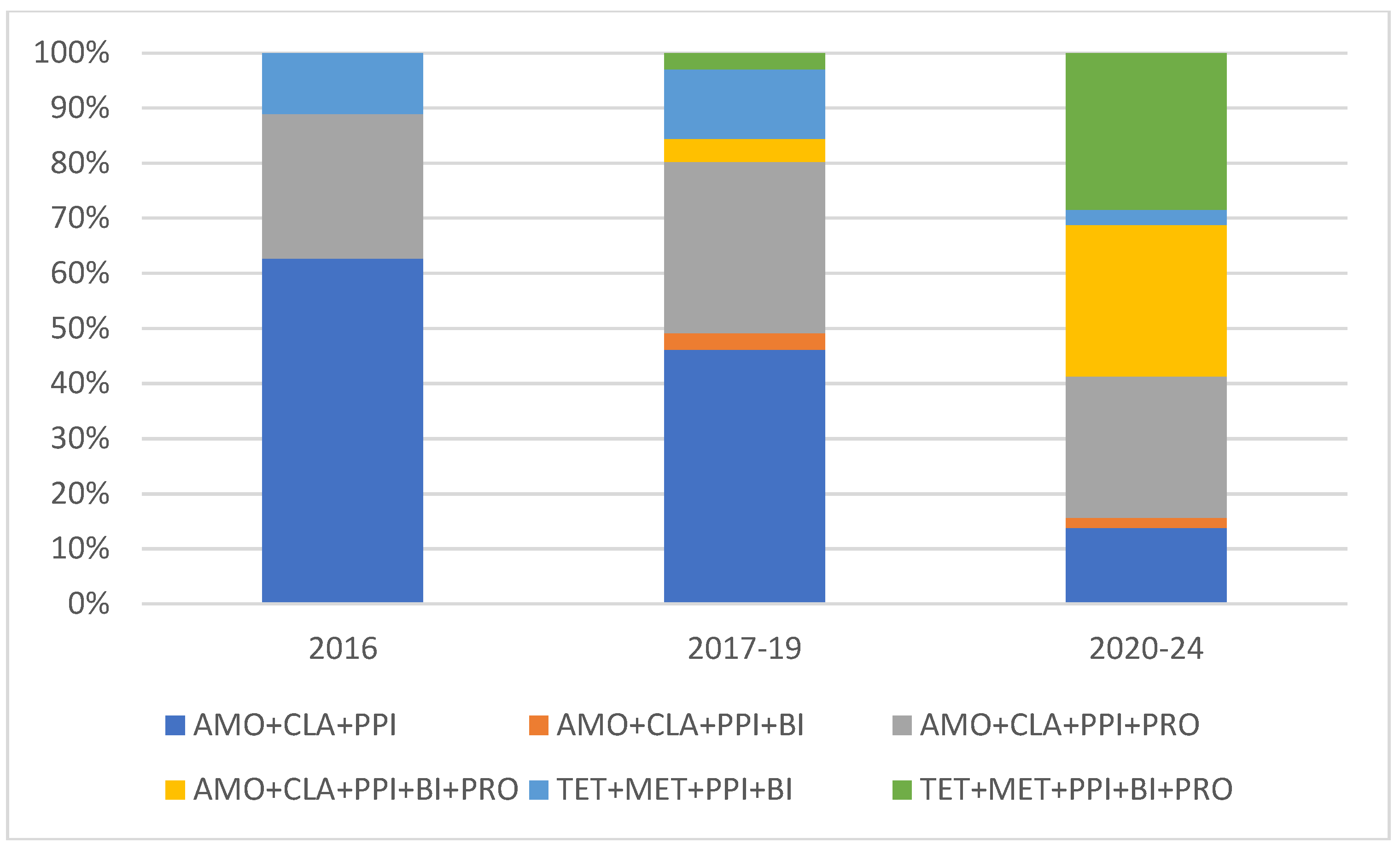
Figure 3). Despite local clarithromycin resistance rates, the most popular protocol was the basic AMO+CLA+PPI, although a continuous decline in yearly case numbers was observed. The proportion of tetracycline-based protocols, as well as supplemented AMO protocols continuously increased through our observational period. The use probiotic containing protocols were more popular from year to year, to the end of the trial, in 2023-2024 all eradication regimens were supplemented with them (
Figure 4). In
Figure 4., the division of the 10-years long study period was based on the proportion of probiotics containing cases to appropriately show their continuous elevation from year to year.
3.3. Investigation of Protocol Success Rates, Optimal Treatment Length, Determining the Best Eradication Protocol
Two basic protocols supplemented with bismuth and/or probiotics; therefore, the overall success rate of six different treatment types were examined. As it is seen in
Table 6, the most promising protocol is TET+MET+PPI+BI+PRO regarding success rates, followed by the two other supplemented clarithromycin-based protocols (AMO+CLA+PPI+PRO, AMO+CLA+PPI+BI+PRO) (
Table 6). Statistical analysis based on the used antibiotic type did not find any difference in overall success rates (
p=0.5902) by comparison of clarithromycin-based protocols and tetracycline-based regimens (
Table 7.). Generally, probiotics containing protocols are more successful (
p=0.0295) compared to those that not containing this supplement (
Table 8). The efficacy of protocols was investigated in all treatment length as well: for determination of optimal treatment duration, overall success rates of 7-, 10- and 14-days protocols were investigated: the success rates of 14 days protocols were significantly higher (
p=0.0056) compared to 7- and 10-days regimens (
Table 6.). Further investigations to determine the best eradication protocol type was performed within the most successful 14-days probiotic containing protocols: comparing success rates, probiotic containing 14 days protocols were significantly effective (
p=0.0002) than non-probiotic containing regimens in the same 14 days setting (
Table 8.), however, AMO+CLA+PPI+PRO (90.0%), AMO+CLAV+PPI+BI+PRO (95.0%) and TET+MET+PPI+BI+PRO (100.0%) have comparable results in treatment outcomes. (
Table 6.)
3.4. Investigation of Additional Probiotic and/or Bismuth Effect in Success Rates
To further investigate the general positive effect of probiotics on treatment outcome, we examined separately its effect on AMO and TET protocols. Based on the known ameliorating effect of bismuth supplementation in antibiotic treatments, we investigated the possible additional effect of bismuth supplementation of the two different regimens.
3.4.1. AMO Protocols
The success rate of clarithromycin based-probiotic containing protocol (AMO+CLA+PPI+PRO) and its bismuth supplemented version (AMO+CLA+PPI+BI+PRO) vs. the clarithromycin-based basic regimen (AMO+CLA+PPI) was examined: the addition of probiotics failed to show a significant difference in treatment success any of the treatment duration (7,10 and 14 days,
p-values are in
Table 9.), although success rates shows a marked increase in 14 days setting (83 vs 95%), when both supplement compounds were used. Despite the promising success rates in 14 days setting, a possible cause of our non-significant result is the low case number in this sub-category (
Table 9.). Addition of bismuth itself does not cause any marked elevation of treatment success in clarithromycin-based combinations (AMO+CLA+PPI+BI vs. AMO+CLA+PPI) (
Table 6.)
3.4.2. TET Protocols
In TET regimens, addition of probiotics to eradication protocol causes a significant increase in success rate of 14 days setting (p<0.0001), however, similarly to AMO protocols, this effect was not significant in 10 days treatment durations. This setting was not examined in 7 days treatments due to no available data (
Table 10.)
Generally, comparison of all probiotic containing protocols (186 cases) vs. all non-probiotic regimens (216 cases) showed significant increase in success rates (
Table 8.), but in subgroup analysis, this result was significant only in the case of TET protocols in 14 days setting.
3.5. Bismuth and Bismuth+ Probiotic Combination Effect on Success Rates
Investigation of bismuth effect itself is presumably difficult in our trial setting. In AMO protocols, bismuth addition has no significant effect on success rates. TET protocols containing bismuth in baseline setting (TET+MET+PPI+BI), therefore we examined a total effect of comparing all bismuth containing protocols (AMO+CLA+PPI+BI and TET+MET+PPI+BI vs. AMO+CLA+PPI) (
Table 11.). This comparison not shown any significant difference as well. However, interestingly, addition of probiotics to all bismuth containing regimens (comparison of AMO+CLA+PPI+BI+PRO and TET+MET+PPI+BI+PRO vs. AMO+CLA+PPI+BI and TET+MET+PPI+BI) showed a significant increase in treatment success. This phenomenon was observed in analysis of all cases (
p=0.0124) and only 14-days regimens (
p<0,0001) as well, therefore it can be concluded that additional probiotics significantly increase the efficacy of bismuth containing protocols (
Table 12.).
3.5. Investigation of Bismuth Compounds
In our database search, regimens containing different bismuth preparations (commercially available bismuth oxide, magistral prepared bismuth citrate and a magistral combination of bismuth and metronidazole) was collected and investigated. Comparing the effect of the three used preparation, better success rates were observed by using commercial bismuth oxide and magistral bismuth-metronidazole combination, however no significant effect (
p=0,0670) was observed in success rates based only on bismuth choice. (
Table 13).
3.6. Additional Value of Probiotic Choice by Investigating Different Preparations (Probiotic 1-10)
As in the previous parts of the trial a positive effect of probiotic use was observed in many settings, we have further investigated this phenomenon by comparing the effect of ten different probiotic preparations that were used in the observational period. Probiotic compounds were anonymized by using serial numbers in the order of occurrence in database (see above in
Table 3.). Statistical analysis was performed in settings where sample size allowed that. By AMO+CLA+PPI+PRO protocols (a total of 82 cases), no statistical difference (
p=0.8380) was found between the use of Probiotic 1, Probiotic 2, Probiotic 3 and Probiotic 5 preparations (
Table 14). Regarding AMO+CLA+PPI+BI+PRO protocol, 33 of the total 37 cases were supplemented with Probiotic 8 (27 cases) and Probiotic 6 (6 cases), with a success rate of 85.2% (23/27 cases) and 100% (6/6) respectively, no significant difference was observed (
p=0.3146). 27 of TET+MET+PPI+BI+PRO protocols (33 cases) received Probiotic 8 with a success rate of 100 % (27/27) the remaining 6 cases was supplemented with different strains, therefore no statistical comparison was possible. Subgroup analysis by dividing probiotics to “single strain” and “multiple strain” preparations not showed any statistical difference (
p=0.2042) regarding success rates as well. (
Table 15)
As a promising result, within the frames of our trial a case series of 27 patients receiving AMO+CLA+PPI+BI+PRO and 27 patients receiving TET+MET+PPI+BI+PRO all of them was supplemented with Probiotic 8 as probiotic compound of protocol with 85.2 and 100% success rate, respectively. No further statistical analysis was possible due to low sample size by the addition of other probiotic strains to these protocols. This observation in 54 cases requires further investigation in prospective randomized trial or in retrospective real world setting with higher case numbers and a possibility of statistical comparison to other strains. In 19 cases, probiotic type was not specified in patient documentation. However, in these cases a 94.7% (18/19) success rate was observed, data were not analyzed further and were not included in statistical analysis due to lack of relevant information and consequence. (
Table 14)
4. Discussion and Conclusions
Our retrospective real-world trial is a unique and comprehensive assessment of the routine use of H. pylori eradication protocols with focus on two basic regimens, an amoxicillin-clarithromycin based and a tetracycline-metronidazole based protocol in three different treatment length and with two possible supplementations. In literature, numerous trial data is available discussing and comparing original [
19,
20,
21], and different modified version of both protocols and many other treatment options [
22,
23,
24], but data from direct comparison of two already guideline-level protocols with promising supplementation (bismuth and probiotics) in real world clinical setting were missing.
The first main result of the trial is in concordance with literature and guideline data [
7,
8,
25] is the superiority of 14-days treatment duration comparing to 7- and 10-days eradications. Our analysis showed statistical significantly increased success rates of the three investigated probiotic containing regimens (AMO+CLA+PPI+PRO, AMO+CLA+PPI+BI+PRO, TET+MET+PPI+BI+PRO) comparing to non-probiotic settings. We proved that despite local clarithromycin resistance patterns of this region, comparable success rates could be achieved by supplementation of the amoxicillin-clarithromycin based protocols with bismuth and probiotics. Relevant study result is the most prominent effect of probiotics on success rates in both type of protocols when it is used together with bismuth as well. As a tetracycline-bismuth naive area, the best success rates were observed by the tetracycline-based protocols. In clinical guidelines this type of regimen recommended as first line setting, however in the case of tetracycline availability problems, non-inferior success rates can be achieved by supplemented amoxicillin-clarithromycin-based protocols.
As it was mentioned in introduction section, literature data are available about the effect of different bismuth compounds, however, results about their direct comparison were missing. In this trial direct comparison of different bismuth compounds (e.g. bismuth oxide, bismuth citrate and bismuth-metronidazole) has an equivalent success rate. Generally, we can conclude that the supplementation of any protocols by any type of bismuth and probiotic showed better overall success rates with a statistically significant level in the 14-days setting.
In our trial we conducted a direct comparison of those four probiotics where sample size allowed to investigate the effect of probiotic choice: results showed no statistical difference regarding success rates. The investigation includes Probiotic 1 (Lactobacillus acidophilus, Lactobacillus bulgaricus, Bacillus coagulans, Bifidobacterium animalis lactis, Streptococcus thermophilus), Probiotic 2 (Enterococcus faecium L3), Probiotic 3 (Lactobacillus acidophilus (LA-5), Bifidobacterium (BB-12), Lactobacillus paracasei (L. CASEI 431)) and Probiotic 5 (Saccharomyces boulardii CNCM I-745). Comparing our result to literature data (see introduction section) we can conclude that in our trial the non-inferiority of three probiotics was found in comparison to Saccharomyces boulardii, the only probiotic strain in literature that has a positive effect on eradication success rates in RCTs. The finding requires confirming clinical trials or database research to clarify the possible positive effect on eradication success of these Lactobacillus and other probiotic strains.
Important result of our investigation is the promising data of the two case series where AMO+CLA+PPI+BI+PRO protocol and and TET+MET+PPI+BI+PRO protocol were supplemented with Probiotic 8 (Lactobacillus reuteri) as the “PRO” part with a success rate of 85.2 and 100%, respectively, however these finding requires further comparison and clarification as well.
As a future direction, based on our promising data, randomized trials are needed to directly comparing probiotics added to amoxicillin- vs tetracycline-based bismuth regimens. Optimal dosage and strain selection for probiotic adjuncts (duration, timing relative to antibiotics) requires more investigation and standardization for optimizing routine clinical use.
Table 15.
Summary of results.
Table 15.
Summary of results.
| Goal |
Result |
| Determination of optimal treatment duration |
14 days regimens are significantly successful comparing to 7- and 10-days regimen |
| Find the protocols with the highest eradication rates |
AMO+CLA+PPI+PRO, AMO+CLA+PPI+BI+PRO, TET+MET+PPI+BI+PRO are the most effective protocols in a 14 days duration of treatment with comparable success rates |
| Clear whether the addition of probiotics to treatment improves outcomes in different setting |
Probiotic containing protocols are more successful comparing to non-probiotic containing regimen, their effect is more prominent when added to bismuth containing protocols |
| Clarify the effect of bismuth addition to clarithromycin-based protocols |
Bismuth addition itself has no effect on the success of clarithromycin-based protocols (AMO+CLA+PPI+BI vs. AMO+CLA+PPI) |
| Investigate the possible difference between bismuth compounds regarding treatment success |
No significant effect was observed in success rates based only on bismuth choice (commercial bismuth oxide, magistral bismuth citrate, magistral bismuth-metronidazole) |
| Find the most effective probiotic strain |
Based on treatment success, the most effective probiotic strain was the combination of Lactobacillus reuteri ATCC PTA 6475, Lactobacillus reuteri Protectis® DSM 17938 with a success rate of 85,2% when added to AMO+CLA+PPI+BI+PRO and 100% in combination with TET+MET+PPI+BI+PRO as the “PRO” part. |
| Determining future efforts to improve patient care (in a clarithromycin resistant and tetracycline/bismuth naïve area) |
Use a tetracycline-based bismuth and probiotic supplemented regimen (TET+MET+PPI+BI+PRO) as first line treatment to maximize treatment success.
Use of the bismuth and probiotic supplemented clarithromycin-based protocol (AMO+CLA+PPI+BI+PRO) protocol in 14 days setting to achieve comparable outcomes to TET+MET+PPI+BI+PRO protocol when the use of tetracycline-based regimens not possible due to limited access of tetracycline. |
Author Contributions
Conceptualization, András Gelley, Kinga Komka and Ibolya Czegle; Data curation, András Gelley, Noémi Kéri, Kinga Komka, Vajk Hardy, László Döngölő, Dóra Szeli and Ibolya Czegle; Formal analysis, Kinga Komka; Funding acquisition, András Gelley and Ibolya Czegle; Investigation, András Gelley and Ibolya Czegle; Methodology, András Gelley and Ibolya Czegle; Project administration, András Gelley and Ibolya Czegle; Resources, Péter Birinyi; Supervision, Ibolya Czegle; Visualization, Ibolya Czegle; Writing – original draft, Ibolya Czegle; Writing – review & editing, András Gelley, Péter Birinyi, Kinga Komka and Ibolya Czegle.
Funding
This research received no external funding. The APC was funded by András Gelley (50%) and Semmelweis University (50%).
Institutional and National Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, approved by the Institutional Review Board of Buda Hospital of the Hospitaller Order of Saint John of God and by the National Institute of Pharmacy and Nutrition (OGYÉI) under the title of „Investigation of H. pylori eradication success rates at Buda Hospital of the Hospitaller Order of Saint John of God, Gastroenterology Outpatient Unit in a retrospective real world data setting”. (OGYÉI/39499-5/2022). The magistral use of bismuth citrate was approved by the National Institute of Pharmacy and Nutrition and financed by the National Health Insurance Fund of Hungary.
Informed Consent Statement
As our trial was a non-investigational retrospective database search, regarding to ethical regulation, informed consent was not required from the patients.
Data Availability Statement
Data Availability Statement: For ethical and confidentiality reasons, the data presented in this study are available upon request from the corresponding author.
Acknowledgments
We would like to acknowledge Mária Nádai, Katalin Merényi, Aliz Németh for the consent of using the case documentations of their patients for research purposes.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
H. pylori: Helicobacter pylori
SAT: stool H. pylori antibody test
AMO: amoxicillin
CLA: clarithromycin
PPI: proton pump inhibitor
BI: bismuth
TET: tetracycline
MET: metronidazole
RCT: randomized controlled trial
AMO protocols: amoxicillin-clarithromycin-based regimens, traditionally these protocols called as, clarithromycin-based” in scientific literature
TET protocols: tetracycline-metronidazole based protocols
Abbreviations that were used in the probiotic strain description are a copy from summary of product characteristics of each compound.
References
- Chey, W.D.; Howden, C.W.; Moss, S.F.; Morgan, D.R.; Greer, K.B.; Grover, S.; Shah, S.C. ACG Clinical Guideline: Treatment of Helicobacter Pylori Infection. Am. J. Gastroenterol. 2024, 119, 1730–1753. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Megraud, F.; Rokkas, T.; Gisbert, J.P.; Liou, J.-M.; Schulz, C.; Gasbarrini, A.; Hunt, R.H.; Leja, M.; O’Morain, C.; et al. Management of Helicobacter Pylori Infection: The Maastricht VI/Florence Consensus Report. Gut 2022, 71, 1724. [Google Scholar] [CrossRef]
- Buzás, G.M.; Lotz, G.; Kiss, A. The Hungarian Epidemiology of Clarithromycin Resistance in Helicobacter Pylori Infection. Orv. Hetil. 2007, 148, 1461–1467. [Google Scholar] [CrossRef] [PubMed]
- Kocsmár, É.; Buzás, G.M.; Szirtes, I.; Kocsmár, I.; Kramer, Z.; Szijártó, A.; Fadgyas-Freyler, P.; Szénás, K.; Rugge, M.; Fassan, M.; Kiss, A.; et al. Primary and Secondary Clarithromycin Resistance in Helicobacter Pylori and Mathematical Modeling of the Role of Macrolides. Nat. Commun. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Kouhsari, E.; Sadeghifard, N.; Khadiv, A.; Sayadi, H.; Amiriani, T.; Ghafourian, S.; Valadbeigi, H.; Krutova, M. Heteroresistance to Clarithromycin and Metronidazole in Patients with a Helicobacter Pylori Infection: A Systematic Review and Meta-Analysis. Ann. Clin. Microbiol. Antimicrob. 2022, 21. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Park, M.I.; Park, S.J.; Moon, W.; Choi, Y.J.; Cheon, J.H.; Kwon, H.J.; Ku, K.H.; Yoo, C.H.; Kim, J.H.; et al. Trends in Helicobacter Pylori Eradication Rates by First-Line Triple Therapy and Related Factors in Eradication Therapy. Korean J. Intern. Med. 2015, 30, 801–807. [Google Scholar] [CrossRef]
- Nyssen, O.P.; Bordin, D.; Tepes, B.; Pérez-Aisa, Á.; Vaira, D.; Caldas, M.; Bujanda, L.; Castro-Fernandez, M.; Lerang, F.; Leja, M.; Rodrigo, L.; et al. European Registry on Helicobacter Pylori Management (Hp-EuReg): Patterns and Trends in First-Line Empirical Eradication Prescription and Outcomes of 5 Years and 21 533 Patients. Gut 2021, 70, 40–54. [Google Scholar] [CrossRef]
- Nyssen, O.P.; Moreira, L.; García-Morales, N.; Cano-Català, A.; Puig, I.; Mégraud, F.; O’Morain, C.; Gisbert, J.P. European Registry on Helicobacter Pylori Management (Hp-EuReg): Most Relevant Results for Clinical Practice. Front. Gastroenterol. 2022, 1. [Google Scholar] [CrossRef]
- Varga, M.; Drácz, L.; Kolbenheyer, E.; Varga, F.; Patai, Á. V.; Solymosi, N.; Patai, Á. A Helicobacter pylori -fertőzés első vonalbeli megszüntetésére alkalmazott hagyományos hármas és egy új, bizmuttartalmú négyes kezelés összehasonlítása. Orv. Hetil. 2019, 160, 1340–1345. [Google Scholar] [CrossRef]
- Van Zanten, S.V.; Farley, A.; Marcon, N.; Lahaie, R.; Archambault, A.; Hunt, R.; Bailey, R.; Owen, D.; Spénard, J.; Stiglick, A.; et al. Bismuth-Based Triple Therapy with Bismuth Subcitrate, Metronidazole and Tetracycline in the Eradication of Helicobacter Pylori: A Randomised, Placebo Controlled, Double-Blind Study. Can. J. Gastroenterol. 2000, 14, 599–602. [Google Scholar] [CrossRef]
- Oueslati, A.; Mohamed, G.; Bostani, S.; Boughoula, K.; Bizid, S.; Ben Abdallah, H.; Bouali, R.; Abdelli, M.N. Comparison of Bismuth and Concomitant Therapy for H. Pylori Eradication: A Prospective, Randomized Clinical Trial. Future Sci. OA 2025, 11. [Google Scholar] [CrossRef] [PubMed]
- Alkim, H.; Koksal, A.R.; Boga, S.; Sen, I.; Alkim, C. Role of Bismuth in the Eradication of Helicobacter Pylori. Am. J. Ther. 2017, 24, e751–e757. [Google Scholar] [CrossRef] [PubMed]
- McFarland, L.V. Meta-Analysis of Single Strain Probiotics for the Eradication ofHelicobacter Pyloriand Prevention of Adverse Events. World J. Meta-Anal. 2015, 3, 97. [Google Scholar] [CrossRef]
- Yao, G.; Fan, X.; Lu, D. Efficacy and Safety of Probiotic-Supplemented Bismuth Quadruple Therapy for the Treatment of Helicobacter Pylori Infection: A Systematic Review and Meta-Analysis. J. Int. Med. Res. 2023, 51. [Google Scholar] [CrossRef]
- Buzás, G.M.; Birinyi, P. Newer, Older, and Alternative Agents for the Eradication of Helicobacter Pylori Infection: A Narrative Review. Antibiotics 2023, 12, 946. [Google Scholar] [CrossRef]
- Li, M.; Xie, Y. Efficacy and Safety of Saccharomyces Boulardii as an Adjuvant Therapy for the Eradication of Helicobacter Pylori: A Meta-Analysis. Front. Cell. Infect. Microbiol. 2025, 15. [Google Scholar] [CrossRef]
- Ismail, N.I.; Nawawi, K.N.M.; Hsin, D.C.C.; Hao, K.W.; Mahmood, N.R.K.N.; Chearn, G.L.C.; Wong, Z.; Tamil, A.M.; Joseph, H.; Raja Ali, R.A. Probiotic Containing Lactobacillus Reuteri DSM 17648 as an Adjunct Treatment for Helicobacter Pylori Infection: A Randomized, Double-blind, Placebo-controlled Trial. Helicobacter 2023, 28. [Google Scholar] [CrossRef]
- Naghibzadeh, N.; Salmani, F.; Nomiri, S.; Tavakoli, T. Investigating the Effect of Quadruple Therapy with Saccharomyces Boulardii or Lactobacillus Reuteri Strain (DSMZ 17648) Supplements on Eradication of Helicobacter Pylori and Treatments Adverse Effects: A Double-Blind Placebo-Controlled Randomized Clinical Trial. BMC Gastroenterol. 2022, 22. [Google Scholar] [CrossRef]
- Shoosanglertwijit, R.; Kamrat, N.; Werawatganon, D.; Chatsuwan, T.; Chaithongrat, S.; Rerknimitr, R. Real-world Data of Helicobacter Pylori Prevalence, Eradication Regimens, and Antibiotic Resistance in Thailand, 2013–2018. JGH Open 2020, 4, 49–53. [Google Scholar] [CrossRef]
- Yan, T.-L.; Gao, J.-G.; Wang, J.-H.; Chen, D.; Lu, C.; Xu, C.-F. Current Status of Helicobacter Pylori Eradication and Risk Factors for Eradication Failure. World J. Gastroenterol. 2020, 26, 4846–4856. [Google Scholar] [CrossRef]
- Tai, W.-C.; Wu, I.-T.; Wang, H.-M.; Huang, P.-Y.; Yao, C.-C.; Wu, C.-K.; Yang, S.-C.; Liang, C.-M.; Hsu, P.-I.; Chuah, S.-K. The Multicenter Real-World Report of the Efficacies of 14-Day Esomeprazole-Based and Rabeprazole-Based High-Dose Dual Therapy in First-Line Helicobacter Pylori Eradication in Taiwan. J. Microbiol. Immunol. Infect. 2024, 57, 601–608. [Google Scholar] [CrossRef]
- Salmanroghani, H.; Mirvakili, M.; Baghbanian, M.; Salmanroghani, R.; Sanati, G.; Yazdian, P. Efficacy and Tolerability of Two Quadruple Regimens: Bismuth, Omeprazole, Metronidazole with Amoxicillin or Tetracycline as First-Line Treatment for Eradication of Helicobacter Pylori in Patients with Duodenal Ulcer: A Randomized Clinical Trial. PLOS ONE 2018, 13, e0197096. [Google Scholar] [CrossRef] [PubMed]
- Howden, C.W.; Sheldon, K.L.; Almenoff, J.S.; Chey, W.D. Pitfalls of Physician-Directed Treatment of Helicobacter Pylori: Results from Two Phase 3 Clinical Trials and Real-World Prescribing Data. Dig. Dis. Sci. 2022, 67, 4382–4386. [Google Scholar] [CrossRef]
- Zhao, Y.-R.; Wang, X.-J.; Zhu, M.-J.; Chen, A.-L.; Zhang, D.; Du, Q.; Kim, J.J.; Hu, W.-L. Efficacy and Safety of Low-Dose Tetracycline, Amoxicillin Quadruple Therapy in Helicobacter Pylori Infection: A Retrospective Single Center Study. World J. Gastroenterol. 2024, 30, 4295–4304. [Google Scholar] [CrossRef]
- Fallone, C.A.; Barkun, A.N.; Szilagyi, A.; Herba, K.M.; Sewitch, M.; Martel, M.; Fallone, S.S. Prolonged Treatment Duration Is Required for Successful Helicobacter Pylori Eradication with Proton Pump Inhibitor Triple Therapy in Canada. Can. J. Gastroenterol. 2013, 27, 397–402. [Google Scholar] [CrossRef]
Figure 1.
Study flowchart.
Figure 1.
Study flowchart.
Figure 2.
Proportion of enrolled and excluded cases.
Figure 2.
Proportion of enrolled and excluded cases.
Figure 3.
Trends in duration of eradication protocols.
Figure 3.
Trends in duration of eradication protocols.
Figure 4.
Proportion of different eradication protocols in study observational period.
Figure 4.
Proportion of different eradication protocols in study observational period.
Table 1.
Composition of study protocols: standard regimens and their supplementation.
Table 1.
Composition of study protocols: standard regimens and their supplementation.
| Standard |
Bismuth |
Probiotics |
| AMO+CLA+PPI |
|
|
| AMO+CLA+PPI+BI |
|
|
| AMO+CLA+PPI+PRO |
|
|
| AMO+CLA+PPI+BI+PRO |
|
|
| TET+MET+PPI+BI |
|
|
| TET+MET+PPI+BI+PRO |
|
|
Table 2.
Investigated protocols, used agents and doses.
Table 2.
Investigated protocols, used agents and doses.
| Protocol |
Used agents |
Dose |
| AMO+CLA+PPI |
Amoxicillin
Clarithromycin
PPI |
2x1000 mg
2x500 mg
standard dose |
| AMO+CLA+PPI+BIZ |
Amoxicillin
Clarithromycin
PPI
Bismuth oxide/bismuth citrate |
2x1000 mg
2x500 mg
standard dose
see Table 4. |
| AMO+CLA+PPI+PRO |
Amoxicillin
Clarithromycin
PPI
Probiotics |
2x1000 mg
2x500 mg
standard dose
any strain |
| AMO+CLAV+ PPI+BIZ+PRO |
Amoxicillin
Clarithromycin
PPI
Bismuth citrate/ Bismuth oxide
Probiotics |
2x1000 mg
2x500 mg
standard dose
see Table 4.
any strain |
| TET+MET++PPI+BIZ |
Tetracycline
Metronidazol
PPI
Bismuth oxide/bismuth citrate/bismuth metronidazole |
4x500 mg
2x500 mg
standard dose
see Table 4. (in cases when bismuth-metronidazole was given this combination substitutes both bismuth and metronidazole) |
| TET+MET+BIZ+PRO |
Tetracycline
Metronidazol
PPI
Bismuth
Probiotics |
4x500 mg
2x500 mg
standard dose
see Table 4. (in cases when bismuth-metronidazole was given this combination substitutes both bismuth and metronidazole)
any strain |
Table 3.
Investigated probiotic strains (serial Nr. for blinding commercial products was given in the timely occurrence order in database).
Table 3.
Investigated probiotic strains (serial Nr. for blinding commercial products was given in the timely occurrence order in database).
| Probiotic product |
Nr. of strains |
Strains |
| Probiotic 1 |
5 |
Lactobacillus acidophilus, Lactobacillus bulgaricus, Bacillus coagulans, Bifidobacterium animalis lactis, Streptococcus thermophilus |
| Probiotic 2 |
1 |
Enterococcus faecium L3 |
| Probiotic 3 |
3 |
Lactobacillus acidophilus (LA-5), Bifidobacterium (BB-12), Lactobacillus paracasei (L. CASEI 431) |
| Probiotic 4 |
7 |
Lactobacillus casei, Lactobacillus rhamnosus, Streptobacillus thermophilus, Lactobacillus acidophilus, Bifidobacterium breve, Bifidobacterium longum, Lactobacillus bulgaricus |
| Probiotic 5 |
1 |
Saccharomyces boulardii CNCM I-745 |
| Probiotic 6 |
9 |
Bifidobacterium bifidum W23, Bifidobacterium lactis W51, Bifidobacterium lactis W52, Lactobacillus acidophilus W22, Lactobacillus casei W56, Lactobacillus paracasei W20, Lactobacillus plantarum W62, Lactobacillus salivarius W24, Lactococcus lactis W19 |
| Probiotic 7 |
1 |
Lactobacillus rhamnosus GG/Lactobacillus acidophilus LA-5 |
| Probiotic 8 |
2 |
Lactobacillus reuteri ATCC PTA 6475, Lactobacillus reuteri Protectis® DSM 17938 |
| Probiotic 9 |
1 |
Bacillus clausii |
| Probiotic 10 |
14 |
Lactobacillus paracasei PXN® 37™, Lactobacillus plantarum PXN® 47™, Lactobacillus rhamnosus PXN® 54™, Bacillus subtilis PXN® 21®, Bifidobacterium bifidum PXN® 23™, Bifidobacterium breve PXN® 25™, Bifidobacterium longum PXN® 30™, Lactobacillus helveticus PXN® 35™, Lactococcus lactis ssp. lactis PXN® 63™, Streptococcus thermophilus PXN® 66™, Bifidobacterium infantis PXN® 27™, Lactobacillus delbrueckii ssp. bulgaricus PXN® 39™, Lactobacillus helveticus PXN® 45™, Lactobacillus salvarius PXN® 57™. |
Table 4.
Investigated bismuth compounds.
Table 4.
Investigated bismuth compounds.
| Drug or formulation |
Used dose |
| Bismuth-oxide (commercial drug) |
4x120 mg |
| Bismuth citrate (magistral) |
4x120 mg |
| Bismuth-metronidazol combination (magistral) |
Bismuth citrate: 4x120 mg, metronidazole: 4x375 mg (one capsule contains 120 mg bismuth citrate and 375 mg metronidazole) |
Table 5.
Patient characteristics.
Table 5.
Patient characteristics.
| Patients |
Nr. (%) |
| Total |
488 |
| Enrolled |
402 (82.4) |
| Excluded |
86 (17.6) |
| Success |
311 (77.3) |
| Failure |
91 |
| |
|
| Female |
256 (63.7) |
| Male |
146 (36.3) |
| |
|
| Age (±S.D.) |
57.85 (±15.83) |
| 0-39 |
60 (14.9) |
| 40-59 |
131 (32.6) |
| 60- |
211 (52.5) |
Table 6.
Summary of treatment success rates. * p=0.0056 for comparison of total success rate for 7-, 10- and 14-days regimens.
Table 6.
Summary of treatment success rates. * p=0.0056 for comparison of total success rate for 7-, 10- and 14-days regimens.
Protocol
|
Nr. of total success/total cases
(%)
|
Nr. of success/total (%) 7 days |
Nr. of success/total (%)
10 days |
Nr. of success/total (%) 14 days |
| AMO+CLA+PPI |
127/171 (74.3) |
65/93 (69.9) |
57/72 (79.2) |
5/6 (83.3) |
| AMO+CLA+PPI+BI |
5/7 (71.4) |
0/0 (0) |
2/3 (66.67) |
3/4 (75.0) |
| AMO+CLA+PPI+PRO |
89/113 (78.8) |
46/61 (75.4) |
25/32 (78.1) |
18/20 (90.0) |
| AMO+CLA+PPI+BI+PRO |
31/37 (83.8) |
0/1 (0) |
12/16 (75.0) |
19/20 (95.0) |
| TET+MET+PPI+BI |
26/38 (68.4) |
17/24 (70.8) |
9/11 (81.8) |
0/3 (0.0) |
| TET+MET+PPI+BI+PRO |
33/36 (91.7) |
0/0 (0) |
8/11 (72.7) |
25/25 (100.0) |
| Total |
311/402 (77.4) |
128/179 (71.5)* |
113/145 (77.9)* |
70/78 (89.7)* |
Table 7.
Comparison of AMO and TET based protocols. AMO based protocols: AMO+CLA+PPI, AMO+CLA+PPI+BI, AMO+CLA+PPI+BI+PRO, TET based protocols: TET+MET+PPI+BI TET+MET+PPI+BI+PRO *p=0.5902 for comparison of total success rate.
Table 7.
Comparison of AMO and TET based protocols. AMO based protocols: AMO+CLA+PPI, AMO+CLA+PPI+BI, AMO+CLA+PPI+BI+PRO, TET based protocols: TET+MET+PPI+BI TET+MET+PPI+BI+PRO *p=0.5902 for comparison of total success rate.
| Protocol |
Nr. of total success/total cases
(%) |
Nr. of success/total (%) 7 days |
Nr. of success/total (%)
10 days |
Nr. of success/total (%) 14 days |
| Total AMO |
252/328 (76.8)* |
111/155 (71.6) |
96/123 (78.0) |
45/50 (90.0) |
| Total TET |
59/74 (79.7)* |
17/24 (70.8) |
17/22 (77.3) |
25/28 (89.2) |
Table 8.
Comparison of probiotic containing and non-probiotic containing protocols. Total PRO: AMO+CLA+PPI+PRO, AMO+CLA+PPI+BI+PRO, Total non-PRO: AMO+CLA+PPI, AMO+CLA+PPI+BI, TET+MET+PPI+BI *p=0.0295 for comparison of total success rate; ▪p=0.0002 for comparison of success rate of 14 days regimens.
Table 8.
Comparison of probiotic containing and non-probiotic containing protocols. Total PRO: AMO+CLA+PPI+PRO, AMO+CLA+PPI+BI+PRO, Total non-PRO: AMO+CLA+PPI, AMO+CLA+PPI+BI, TET+MET+PPI+BI *p=0.0295 for comparison of total success rate; ▪p=0.0002 for comparison of success rate of 14 days regimens.
| Protocol |
Nr. of total success/total cases
(%)
|
Nr. of success/total (%) 7 days |
Nr. of success/total (%)
10 days |
Nr. of success/total (%) 14 days |
| Total PRO |
153/186 (82.3)* |
46/62 (74.2) |
45/59 (76.27) |
62/65 (95.4)▪ |
| Total non-PRO |
158/216 (73.1)* |
82/117 (70.1) |
68/86 (79.0) |
8/13 (61.5)▪ |
Table 9.
Addition of probiotics to AMO based protocols. comparison of success rate of AMO+CLA+PPI and AMO+CLA+PPI+PRO_. *p=0.4554 in 7 days regimens; **p=0.9044 in 10 days regimens; ***p=0.6539 in 14 days regimens; comparison of success rate AMO+CLA+PPI and AMO+CLA+PPI+BI+PRO. ▪▪p=0.7141 in 10 days regimens; ▪▪▪p=0.3469 in 14 days regimens
Table 9.
Addition of probiotics to AMO based protocols. comparison of success rate of AMO+CLA+PPI and AMO+CLA+PPI+PRO_. *p=0.4554 in 7 days regimens; **p=0.9044 in 10 days regimens; ***p=0.6539 in 14 days regimens; comparison of success rate AMO+CLA+PPI and AMO+CLA+PPI+BI+PRO. ▪▪p=0.7141 in 10 days regimens; ▪▪▪p=0.3469 in 14 days regimens
Protocol
|
Nr. of total success/total cases (%) |
Nr. of success/total (%) 7 days |
Nr. of success/total (%) 10 days |
Nr. of success/total (%) 14 days |
| AMO+CLA+PPI |
127/171 (74.3) |
65/93 (69.9)* |
57/72 (79.2)** |
5/6 (83.3)*** |
| AMO+CLA+PPI+PRO |
89/113 (78.8) |
46/61 (75.4)* |
25/32 (78.1)** |
18/20 (90.0)*** |
| AMO+CLA+PPI+BI+PRO |
31/37 (83.8) |
0/1 (0) |
12/16 (75.0) ▪▪ |
19/20 (95.0)▪▪▪ |
Table 10.
Probiotic supplementation to TET protocols. * p<0,0001 for comparison of success rate in 14 days regimens.
Table 10.
Probiotic supplementation to TET protocols. * p<0,0001 for comparison of success rate in 14 days regimens.
| Protocol |
Nr. of total success/total cases (%) |
Nr. of success/total (%) 7 days |
Nr. of success/total (%) 10 days |
Nr. of success/total (%) 14 days |
| TET+MET+PPI+BI |
26/38 (68.4) |
17/24 (70.8) |
9/11 (81.8) |
0/3 (0.0)* |
| TET+MET+PPI+BI+PRO |
33/36 (91.7) |
0/0 (0) |
8/11 (72.7) |
25/25 (100.0)* |
Table 11.
The effect of bismuth supplementation on treatment outcome. Total BI: AMO+CLA+PPI+BI and TET+MET+PPI+BI.
Table 11.
The effect of bismuth supplementation on treatment outcome. Total BI: AMO+CLA+PPI+BI and TET+MET+PPI+BI.
Protocol
|
Nr. of total success/total cases (%) |
Nr. of success/total (%) 7 days |
Nr. of success/total (%) 10 days |
Nr. of success/total (%) 14 days |
| AMO+CLA+PPI |
127/171 (74.3) |
65/93 (69.9) |
57/72 (79.2) |
5/6 (83.3) |
| Total BI |
31/45 (68.9) |
17/24 (70.8) |
11/14 (78.6) |
3/7 (42.9) |
Table 12.
Effect of probiotic to bismuth containing regimens. Total BI: AMO+CLA+PPI+BI and TET+MET+PPI+BI. Total BI+PRO: AMO+CLA+PPI+BI+PRO and TET+MET+PPI+BI+PRO. ▪p=0.0124 for total success rate. ▪p<0.0001 in 14 days regimens.
Table 12.
Effect of probiotic to bismuth containing regimens. Total BI: AMO+CLA+PPI+BI and TET+MET+PPI+BI. Total BI+PRO: AMO+CLA+PPI+BI+PRO and TET+MET+PPI+BI+PRO. ▪p=0.0124 for total success rate. ▪p<0.0001 in 14 days regimens.
Protocol
|
Nr. of total success/total cases (%) |
Nr. of success/total (%) 7 days |
Nr. of success/total (%) 10 days |
Nr. of success/total (%) 14 days |
| Total BI+PRO |
64/73 (87.6)* |
0/1 (0.0) |
20/27 (74.1) |
44/45 (97.8)▪ |
| Total BI |
31/45 (68.9)* |
17/24 (70.8) |
11/14 (78.6) |
3/7 (42.9) ▪ |
Table 13.
Comparison of bismuth containing agents. *p=0.0670.
Table 13.
Comparison of bismuth containing agents. *p=0.0670.
| Protocol |
Nr. of total success/total cases (%)
|
| Bismuth oxide (commercial) |
30/35 (85.7)* |
| Bismuth citrate (magistral) |
37/52 (71.2)* |
| Bismuth-metronidazol combination (magistral) |
28/31 (90.3)* |
Table 14.
The additional value of probiotic choice. *p=0.8380 for comparison of success rate for Probiotic 1, Probiotic 2, Probiotic 3 and Probiotic 5 preparations by AMO+CLA+PPI+PRO protocols. ▪p=0.3146 for comparison of success rate for Probiotic 6 and Probiotic 8 preparations by AMO+CLA+PPI+BI+PRO protocols.
Table 14.
The additional value of probiotic choice. *p=0.8380 for comparison of success rate for Probiotic 1, Probiotic 2, Probiotic 3 and Probiotic 5 preparations by AMO+CLA+PPI+PRO protocols. ▪p=0.3146 for comparison of success rate for Probiotic 6 and Probiotic 8 preparations by AMO+CLA+PPI+BI+PRO protocols.
| Protocol |
Probiotic not specified |
Probiotic 1 |
Probiotic 2 |
Probiotic 3 |
| AMO+CLA+PPI+PRO |
17/18 (94.4) |
7/11 (63.6)* |
20/26 (76.9)* |
14/19 (73.7)* |
| AMO+CLA+PPI+BI+PRO |
0/0 (0.0) |
0/0 (0.0) |
0/1 (0.0) |
0/0 (0.0) |
| TET+MET+PPI+BI+PRO |
1/1 (100.0) |
0/0 (0.0) |
0/0 (0.0) |
1/3 (33.33) |
| Total |
18/19 (94.7) |
7/11 (63.6) |
20/27 (74.1) |
15/22 (68.2) |
| Protocol |
Probiotic 4 |
Probiotic 5 |
Probiotic 6 |
Probiotic 7 |
| AMO+CLA+PPI+PRO |
0/2 (0.0) |
20/26 (76.9)* |
3/3 (100.0) |
3/3 (100.0) |
| AMO+CLA+PPI+BI+PRO |
0/0 (0.0) |
2/2 (100.0) |
6/6 (100.0)▪ |
0/1 (0.0) |
| TET+MET+PPI+BI+PRO |
2/2 (100.0) |
1/2 (50.0) |
1/1 (100.0) |
0/0 (0.0) |
| Total |
2/4 (50.0) |
23/30 (76.7) |
10/10 (100.0) |
3/4 (75.0) |
| Protocol |
Probiotic 8 |
Probiotic 9 |
Probiotic 10 |
| AMO+CLA+PPI+PRO |
3/3 (100.0) |
1/1 (100.0) |
1/1 (100.0) |
| AMO+CLA+PPI+BI+PRO |
23/27 (85.2)▪ |
0/0 (0.0) |
0/0 (0.0) |
| TET+MET+PPI+BI+PRO |
27/27 (100.0) |
0/0 (0.0) |
0/0 (0.0) |
| Total |
53/57 (93.0) |
1/1 (100.0) |
1/1 (100.0) |
Table 15.
Comparison of the efficacy of probiotics containing multiple vs. single strains. *p=0.2042 for comparison of success rate for multiple vs single strains.
Table 15.
Comparison of the efficacy of probiotics containing multiple vs. single strains. *p=0.2042 for comparison of success rate for multiple vs single strains.
| Probiotics |
Serial Nr. |
Nr. of success/total (%) |
| Multiple strain |
Probiotic 1 |
7/11 (63.6) |
| |
Probiotic 3 |
15/22 (68.2) |
| |
Probiotic 4 |
2/4 (50) |
| |
Probiotic 6 |
10/10 (100.0) |
| |
Probiotic 8 |
53/57 (93.0) |
| |
Probiotic 10 |
1/1 (100.0) |
| |
Total |
88/105 (83.8)* |
| Probiotics |
Serial Nr. |
Nr. of success/total (%) |
| Single strain |
Probiotic 2 |
20/27 (74.1) |
| |
Probiotic 5 |
23/30 (76.7) |
| |
Probiotic 7 |
3/4 (75.0) |
| |
Probiotic 9 |
1/1 (100.0) |
| |
Total |
47/62 (75.6)* |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).