Introduction
Nasal myiasis is a rare parasitic infestation of the nasal cavity and sinuses by fly larvae, primarily
Oestrus ovis. This zoonosis is commonly associated with sheep and goats, with humans becoming accidental hosts. Although this condition is generally uncommon [
1], its initial clinical presentation can be insidious, mimicking common nasal conditions, particularly allergic rhinitis. This symptomatic resemblance can lead to significant diagnostic delays and ineffective treatments, posing a major clinical challenge for otorhinolaryngologists.
The diagnosis of nasal myiasis relies on the direct visualization of live larvae within the nasal cavity, supplemented by a parasitological examination for precise species identification. Cases of infestation in otherwise immunocompetent individuals are considered rare and atypical [
2,
3], making the documentation of such series noteworthy for clinical and epidemiological interest.
In this study, we report a series of 14 cases of nasal myiasis in immunocompetent patients managed at the Military Hospital of Tunis. The aim of our work is to detail the clinical and paraclinical features of this disease, emphasizing the elements that allow us to suspect it and differentiate it from allergic rhinitis, thus ensuring early and appropriate management.
Methods
We conducted a retrospective study on cases of nasal myiasis diagnosed and managed at the ENT department of the Military Hospital of Tunis. The Parasitological diagnosis was made in the laboratory of parasitology of the same hospital by macroscopic and microscopic study according to Zumpt’s criteria. The study included all patients with confirmed nasal myiasis by endoscopic and/or parasitological examination over a 18-year period, from January 2007 to December 2025.
For each included patient, the following data were extracted from medical records: age, sex, geographical origin (rural/urban), socio-economic status, presenting symptoms, symptom duration before consultation, initial treatments received, results of clinical and endoscopic examination, results of complementary examinations (allergic skin prick tests, facial CT scans), parasitological identification of the larva, administered treatment, and clinical outcome.
The study complied with the ethical guidelines of the Declaration of Helsinki, ensuring strict confidentiality for all patients.
Results
We collected fourteen cases of nasal myiasis during the study period. The
Table 1 summarizes epidemiological, clinical, diagnosis, and therapeutic data of the 14 cases.
Demographic and Epidemiological Characteristics
The mean age of our patients was 43.2 years (range: 18 to 70 years), with a sex ratio of 4 males to 10 females. Six patients (43%) were from rural areas. Most patients were of medium to high socio-economic status.
Clinical Presentation and Initial Diagnosis
All patients presented with sudden-onset rhinological symptoms, initially suggesting allergic rhinitis. These symptoms included intense nasal pruritus, sneezing, nasal obstruction, rhinorrhea, and headaches. Otalgia was reported in three cases (21%). The mean time to consultation was 7.8 days (ranging from 3 to 15 days).
Initially, all patients
were treated empirically with antihistamines and local corticosteroids, with only partial improvement of symptoms. In one particular case (case No. 4 in
Table 1), a patient spontaneously expelled a whitish larva during a sneezing effort, prompting him to consult again and guiding the diagnosis.
ENT Examination and Nasal Endoscopy
Upon physical examination, congestion of the vestibule and nasal tip was observed in three patients (21%). All patients presented with mucopurulent secretions and congested nasal mucosa. Nasal endoscopy proved to be the key diagnostic tool, systematically revealing the presence of one or more motile, whitish, live larvae circulating within the nasal cavity and reaching the nasopharynx in twelve cases (85.7%), even in the patient who experienced spontaneous expulsion. Two patients had an expulsion of the larvae. Acute otitis was diagnosed in two patients.
3.4. Complementary Examinations and Parasite Identification
Allergic skin prick tests performed on all patients were negative, thus excluding an underlying allergic rhinitis diagnosis. Facial computed tomography (CT) scans were performed in five patients (36%) and revealed no structural abnormalities or signs of infestation extension.
Parasitological examination of the extracted larvae allowed for the identification of Oestrus ovis in 10 cases (71%).
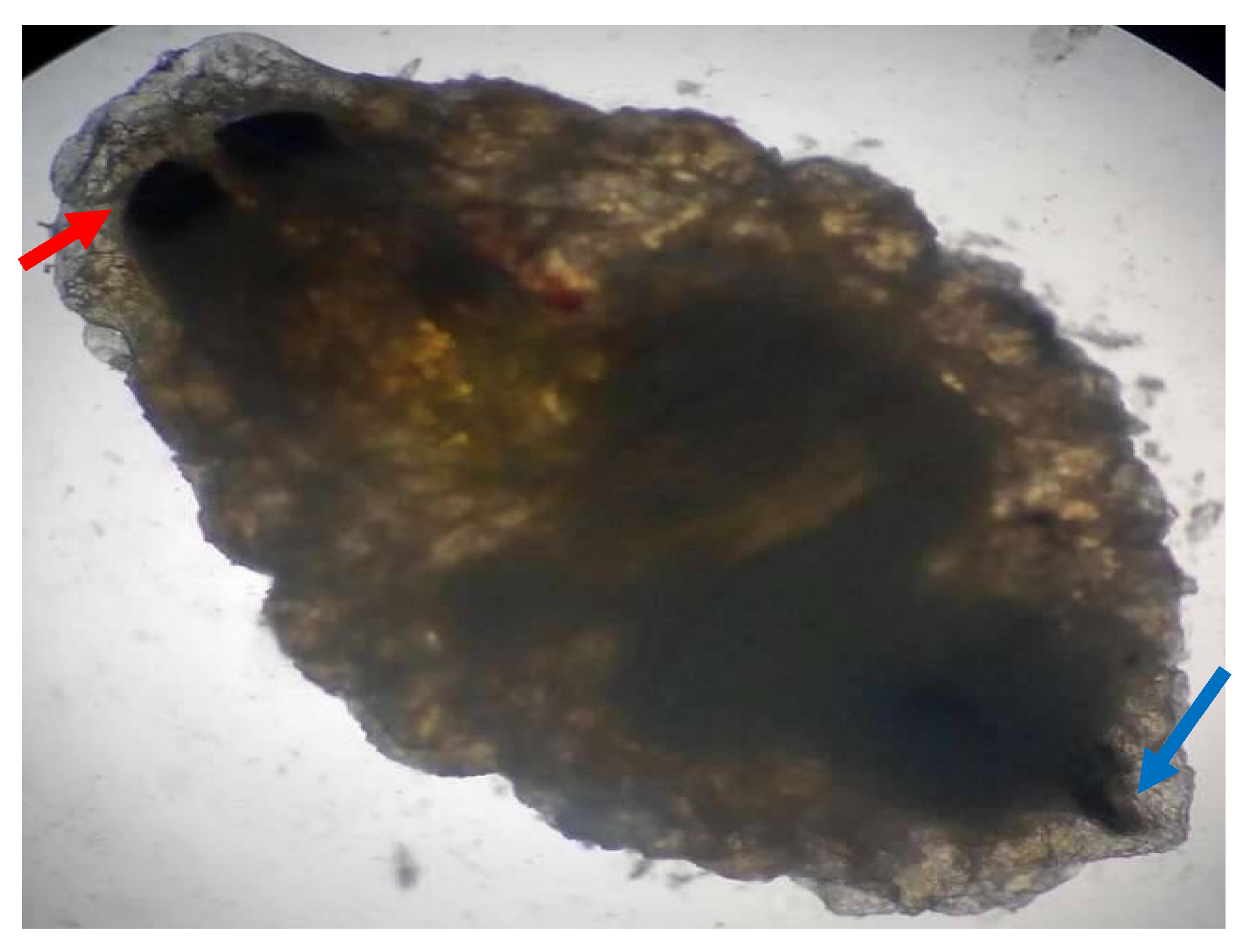
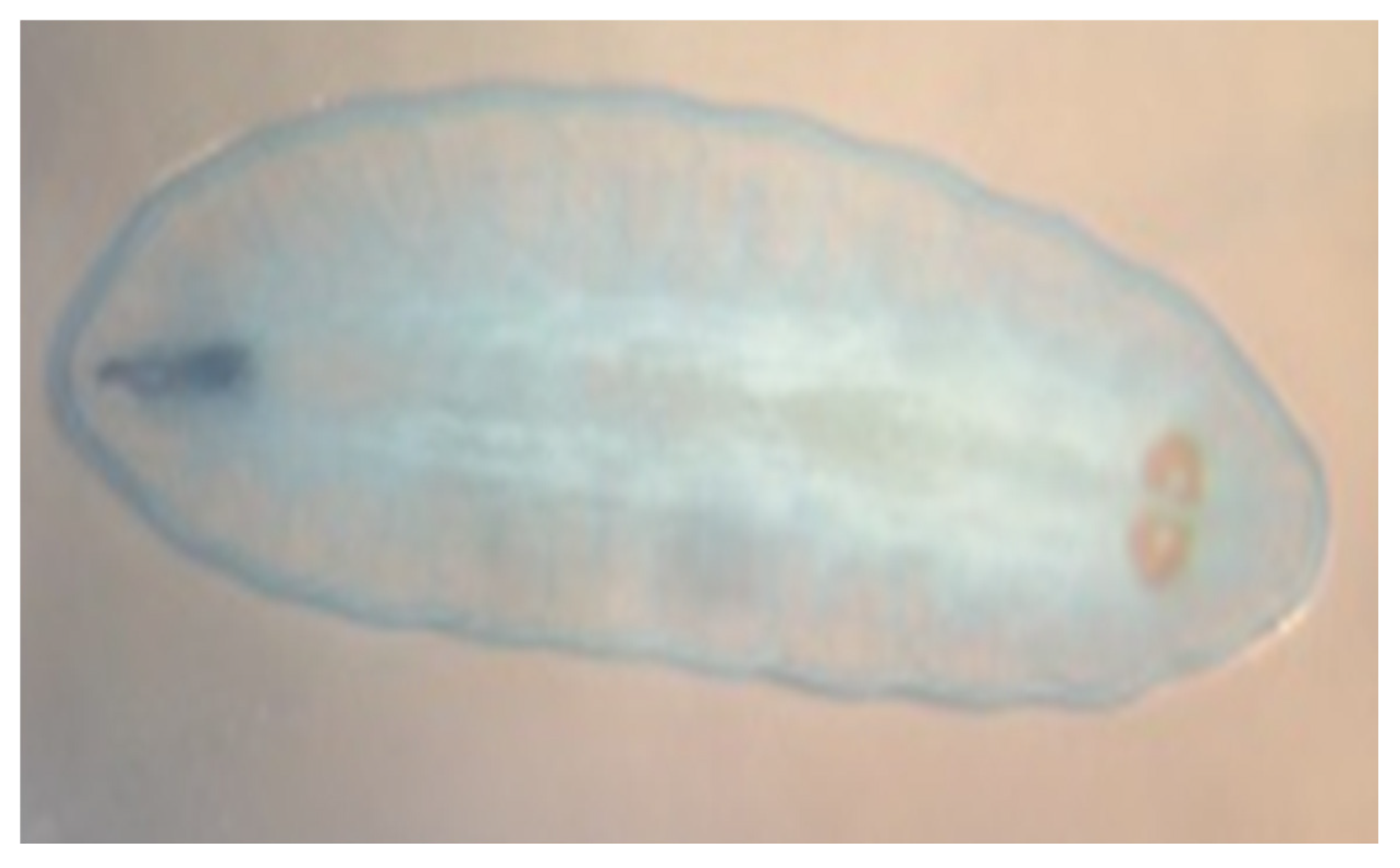
Macroscopic and microscopic examination of the larvae enabled us to identify the species
Oestrus ovis according to Zumpt’s criteria. They were L2 stage larvae (between 5 and 6 mm in size) (
Figure 1 and
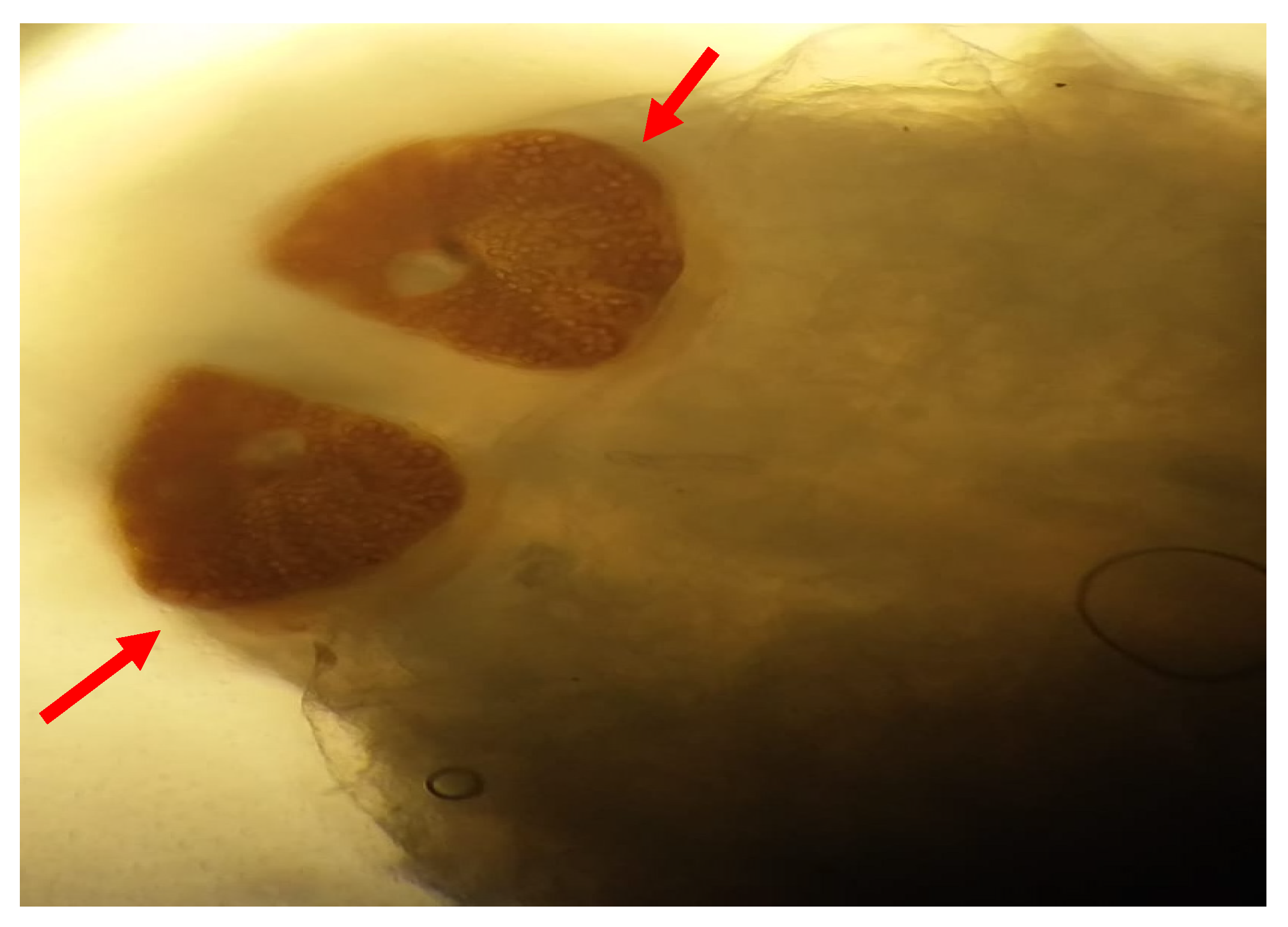
Figure 2). The larvae are semi-cylindrical in shape. The posterior respiratory stigmata are subcircular with a central knob and pierced by numerous pores (
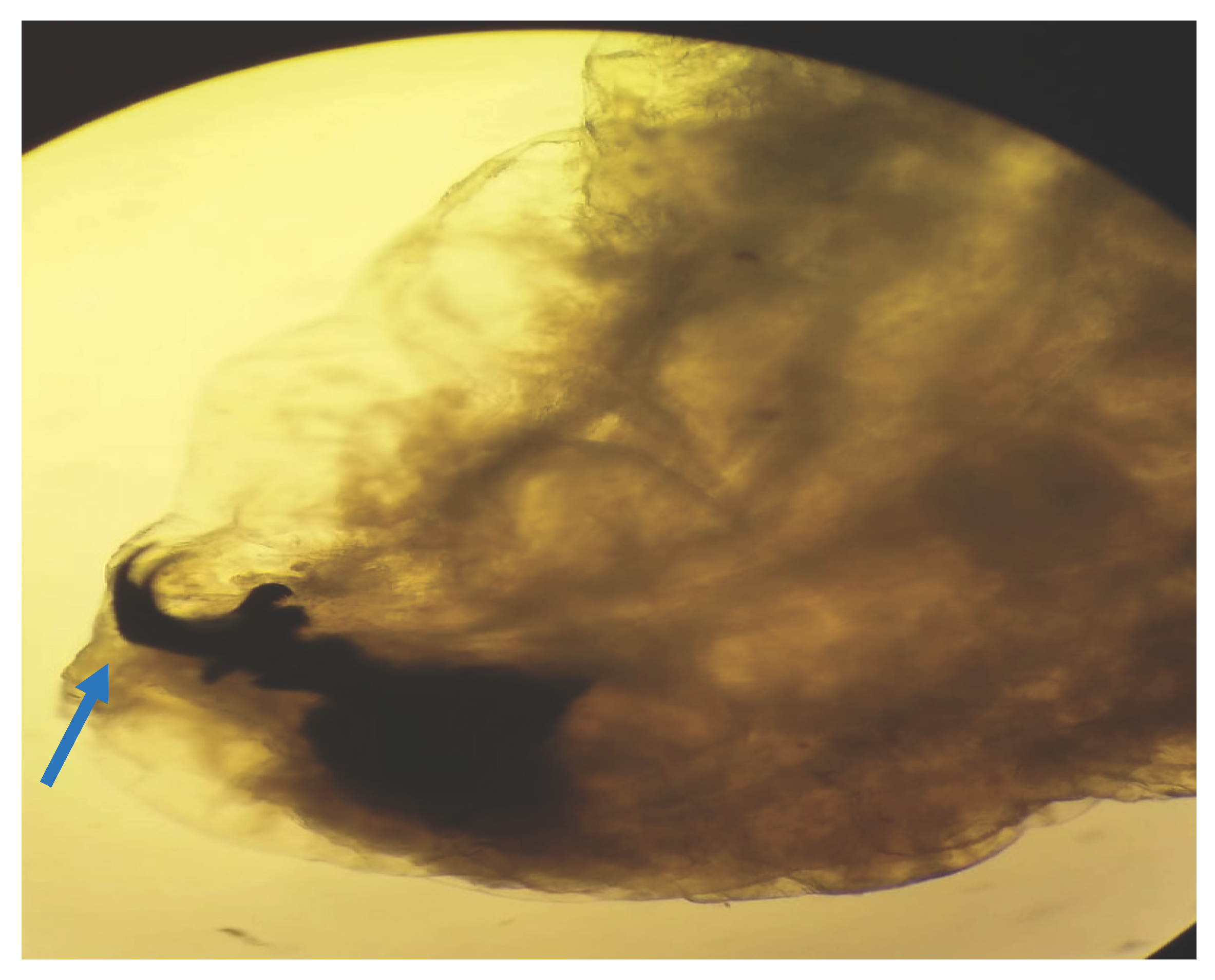
Figure 3).The pseudocephalon has two buccal hooks (
Figure 4).
In four cases, the identification was not possible.
Treatment and Outcome
The therapeutic management consisted of nasal irrigations with physiological saline mixed with an anthelminthic (albendazole), combined with systemic corticosteroid therapy and antihistamines. Clinical improvement was rapidly achieved, with symptom resolution in an average of 4 days. No severe complications or recurrences were observed during follow-up.
Discussion
Our series of fourteen cases of human nasal myiasis, mainly caused by Oestrus ovis, provides valuable insights into the clinical presentation, diagnostic challenges and management of this rare parasitic infestation, particularly in immunocompetent individuals. The most significant finding of our study, and key to its clinical relevance, is the initial misdiagnosis of allergic rhinitis in all our patients. This highlights a crucial diagnostic error that clinicians, particularly in endemic regions, need to be vigilant about.
4.1. The Mimicry of Allergic Rhinitis: A Diagnostic Challenge
The initial symptoms reported by our patients: intense nasal pruritus, sneezing, sudden onset, nasal obstruction, rhinorrhea and headache, are very consistent with those of acute allergic rhinitis. This symptomatic overlap, combined with the rarity of nasal myiasis, probably contributed to the average 6-day delay in diagnosis and ineffective initial treatment with antihistamines and local corticosteroids. Although allergic rhinitis is a common diagnosis worldwide, our findings highlight the need for a higher level of suspicion when such “allergic” symptoms are atypical or refractory to standard treatment.
The literature confirms this diagnostic dilemma. Many case reports describe nasal myiasis presenting with non-specific rhinitis-like symptoms, such as nasal discharge, congestion and irritation, leading to an initial misdiagnosis of sinusitis, common cold or even a foreign body [
4]. The sensation of something moving in the nose, severe pain, or epistaxis are often described as “red flags” that eventually differentiate myiasis [
5]. In our series, the spontaneous expulsion of a larva by a patient during a sneeze was a spectacular and immediate clue, which meant that the diagnosis was not further delayed.
4.2. Clinical Clues and Diagnostic Modalities
Our study highlights that, while initial symptoms may mimic allergic rhinitis, certain features should prompt further investigation. The sudden onset of severe symptoms, the lack of response to conventional anti-allergic treatments and the possible presence of mucopurulent secretions were consistent findings. Although otalgia, observed in three of our cases, is not a typical symptom of allergic rhinitis, it is a known though less frequent manifestation of myiasis, perhaps due to larval migration or inflammatory extension.
The crucial role of nasal endoscopy cannot be overestimated. In all our cases, endoscopy immediately revealed the characteristic whitish, motile larvae, even when the initially suspected symptoms were mild. This is in line with current recommendations, which call for a rapid endoscopic examination of any patient presenting with atypical or persistent nasal symptoms, particularly in the presence of predisposing factors or in the event of failure of initial treatments [
6]. Negative allergological skin tests in all our patients reinforced the exclusion of allergic rhinitis and confirmed the parasitic etiology.
Also of interest were the results of facial CT scans, which were normal in all five patients in whom they were performed. This suggests that in the early stages, or in cases of Oestrus ovis infestation (which generally remains superficial unless complicated), significant bone erosion or deep tissue invasion may not be immediately apparent. This reinforces the primacy of direct visualization by endoscopy for diagnosis.
4.3. Epidemiology and Predisposing Factors
Our series, with 43% of patients originating from rural areas, aligns with the known epidemiology of
Oestrus ovis myiasis, which is primarily a disease of sheep and goats, with humans becoming accidental hosts, often through direct contact or proximity to infested animals [
7]. The presence of myiasis in individuals with a “medium to high socio-economic level” and who are immunocompetent is particularly interesting. While myiasis is often associated with poor hygiene, debility, or immunocompromised states [
8,
9], our cases suggest that even otherwise healthy individuals in appropriate epidemiological settings are at risk. This underscores that myiasis should not be dismissed solely based on patient demographics or general health status, as highlighted by Ravichandran et al. [
2].
4.4. Treatment and Outcomes
The successful treatment protocol involving nasal lavages with saline mixed with an anthelminthic (albendazole), along with systemic corticosteroids and antihistamines, resulted in rapid clinical improvement within an average of 4 days. This aggressive approach, combining mechanical removal or expulsion inducement with antiparasitic agents and anti-inflammatory measures, is consistent with best practices reported in the literature [
10]. Manual extraction of larvae via endoscopy is often difficult to practice due to the mobility of the larvae in the nasal cavity, the use of substances that irritate or asphyxiate the larvae (like anthelminthics) can aid in their expulsion. The favorable outcomes in all our cases, without reported recurrences or severe complications, highlight the effectiveness of prompt and appropriate management.
4.5. Limitations
Our study’s main limitation is its retrospective nature and the relatively small sample size of thirteen cases. Although invaluable for a rare disease, this limits the scope for generalization. Furthermore, precise larval staging and complete species identification were not possible in three cases.
5. Conclusions
Our series of fourteen immunocompetent patients with nasal myiasis highlights the crucial importance of including this rare disease in the differential diagnosis of atypical or treatment-resistant rhinitis-like symptoms. The initial presentation may show a striking resemblance to allergic rhinitis, underscoring the need for a thorough clinical examination, including prompt nasal endoscopy. A high index of suspicion, particularly in patients with epidemiologically relevant exposure or symptoms that deviate from typical allergic patterns, is crucial for early diagnosis and effective management, preventing potential complications and ensuring a favorable prognosis.
Funding
No external funding was received for this study.
Institutional Review Board Statement
The local ethical committee of military hospital of Tunis approved the publication of this study (81/2024/CLPP/Military Hospital of Tunis, approved on 22 July 2024). The research was conducted ethically, with all study procedures being performed in accordance with the requirements of the World Medical Association’s Declaration of Helsinki.
Informed Consent Statement
informed written consent was obtained from each patient for publication.
Data Availability Statement
The data are available and verifiable upon request by the publisher and/or reviewers.
Conflict of interest statement
All other authors declare no conflict of interest
References
- Delhaes L, Bourel B, Pinatel F, Cailliez JC, Gosset D, Camus D, et al. Myiase nasale humaine à Œstrus ovis. Parasite. 1 déc 2001;8(4):289-96.
- Ravichandran K, Padmanabhan K, Thomas SK, S DP, Liji N. Unusual Presentation of Nasal Myiasis in Immunocompetent Young Individual: Case Report. Indian J Otolaryngol Head Neck Surg. févr 2025;77(2):1147-51. [CrossRef]
- Einer H, Ellegård E. Nasal myiasis by Oestrus ovis second stage larva in an immunocompetent man: case report and literature review. J Laryngol Otol. juill 2011;125(7):745-6. [CrossRef]
- Sante Fernández L, Hernández-Porto M, Tinguaro V, Lecuona Fernández M. Ophthalmomyiasis and nasal myiasis by Oestrus ovis in a patient from the Canary Islands with uncommon epidemiological characteristics. Enferm Infecc Microbiol Clin. 2017;35(7):461-2.
- Gupta SK, Nema HV. Rhino-orbital-myiasis. The Journal of Laryngology & Otology. avr 1970;84(4):453-5.
- Soni NK. Endoscopy in nasal myiasis. Trop Doct. oct 2000;30(4):225-7. [CrossRef]
- Tyagi AK, Suji PS, Kumar A, Varshney S, Mohanty A, Gupta P. First Report on Concomitant Infection of Nasal Myiasis and Trichosporonosis in an Uncontrolled Diabetic Patient: Case Report. Indian J Otolaryngol Head Neck Surg. oct 2022;74(Suppl 2):810-2. [CrossRef]
- Savaş N, Aykur M. Oral and Nasal Myiasis in Two Patients Hospitalized in the Intensive Care Unit: Diagnosis and Clinical Significance of Cases. Indian J Otolaryngol Head Neck Surg. oct 2024;76(5):4677-81. [CrossRef]
- Francesconi F, Lupi O. Myiasis. Clin Microbiol Rev. janv 2012;25(1):79-105.
- Sayeed A, Ahmed A, Sharma SC, Hasan SA. Ivermectin: A Novel Method of Treatment of Nasal and Nasopharyngeal Myiasis. Indian J Otolaryngol Head Neck Surg. nov 2019;71(Suppl 3):2019-24. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).