1. Introduction
Pets are the second most common cause of indoor allergies, after dust mites [
1], also in Germany [
2]. Sensitization to pets is a risk factor for the development of allergic rhinitis/rhinoconjunctivitis (AR/ARC) and asthma [
3], with cat epithelial allergy being one of the most common allergic diseases [
4]. About 90% of cat allergy sufferers show an immunoglobulin E (IgE) - mediated reaction to Fel d 1, a protein produced by cats [
5]. Fel d 1 is mainly produced by sebaceous gland cells and is present on the surface of the epidermis and fur [
6]. Fel d 1 is found in saliva, from where it is transferred to the cat’s hair during grooming. The distribution of Fel d 1 in the environment is facilitated by cat hair, which acts as a vector for the dispersion of the allergen in the form of airborne particles [
6].
Three forms of therapy against IgE-mediated allergic reactions exist: 1) allergen avoidance; 2) symptomatic treatment (antihistamines, steroids, and bronchodilators); and 3) allergen-specific immunotherapy (AIT) [
7]. AIT treatments available so far are based on the use of native allergen extracts, administered subcutaneously (SCIT) or sublingually (SLIT). Clinical studies have demonstrated the efficacy of treatment with feline allergen extracts in patients suffering from cat allergy [
8], with clinical efficacy correlated to the proportion of Fel d 1 [
9]. Despite the proven clinical success with native extracts, there are reports of high incidences of adverse allergic reactions [
10]. There is also a sublingual tablet available in some European countries, which contains a monomeric cat allergoid; however, there is minimal clinical evidence of its effectiveness [
11].
Modified allergen extracts are a promising alternative to reduce adverse reactions of AIT with native allergen extracts. According to Carnes et al. 2018, there is a trend to apply allergoids as advanced products for allergy treatment because of new, beneficial mechanisms [
12].
During the manufacture of the depigmented allergoid from cat, a highly purified and concentrated allergen extract is produced from the native extract during depigmentation which is subsequently polymerized with glutaraldehyde [
13]. These chemical modifications reduce allergenicity while maintaining immunogenic effects, thereby increasing the safety of the AIT [
14].
In 2019, Mösges et al. conducted a meta-analysis evaluating the efficacy of AIT treatments containing depigmented-polymerized allergen extracts, in patients with pollen- or house dust mite-induced rhinoconjunctivitis with or without allergic asthma [
15]. Six double-blind placebo-controlled (DBPC) trials concerning pollen and two clinical trials referring to house dust mites were analyzed. It was shown that AIT was more efficient in patients with more severe symptoms of ARC, compared to patients with less severe symptoms of ARC, but even in the latter efficacy was demonstrated. Moreover, therapy with depigmented-polymerized allergen extracts did not result in an increased risk of local reactions (LR) (odds ratio (OR): 1.55, 95% confidence interval (CI): 0.86-2.79) or systemic reactions (SR) (OR: 1.94, 95% CI: 0.98-3.84) compared to placebo.
Taken together, AIT with depigmented-polymerized allergen extracts was shown to be effective, well tolerated and safe for patients with ARC with or without allergic asthma [
15].
In 2018, Dhami and Agarwal published a review evaluating the efficacy and safety of cat AIT treatments based on published studies [
16]. They focused on systematic reviews of EAACI (European Academy for Allergy and Clinical Immunotherapy) as evidence, including solely randomized DBPC trials. Regarding the efficacy and safety of SCIT, they evaluated 11 studies. Six trials showed mixed results between the active and placebo groups based on the bronchial provocation test. The number of studies reporting AEs was equal to those reporting no AEs. Two of the included studies investigated the efficacy and safety of AIT (SLIT) treatments; whereas one presented superiority of AIT compared to placebo, the second trial did not reveal differences between groups. No serious AEs were reported. One study investigating intra-lymphatic AIT (ILIT), revealed a positive response and a good safety profile. Overall, only three of the cited studies investigating subcutaneous cat AIT treatments reported AEs. No high-quality evidence regarding the cost-effectiveness of cat AIT could be demonstrated [
16].
Following this comprehensive review of the available evidence, it was determined that AIT may be of benefit to some patients, particularly those with moderate to severe disease. Dhami and Agarwal concluded that further evidence was required. This should take the form of extensive, high-quality, placebo-controlled and head-to-head trials of SCIT, SLIT and ILIT. It was also deemed necessary to conduct health economic evaluations of AIT [
16].
The current study aimed to collect real world data regarding safety of a depigmented allergoid from cat in patients with ARC.
2. Materials and Methods
2.1. Study Design
The current study was a non-interventional study (NIS) investigating the safety of a cat epithelia SCIT in everyday practice. The decision for the therapy had to be made before the inclusion in this safety study. For reasons of generalizability, it was planned to conduct the study in approximately 80 study centers in Germany.
Based on the sample size calculation, it was planned to include 400 patients (300 adults, 100 adolescents) to detect age-specific differences in safety or tolerability.
During the treatment period, participating patients documented the presence or absence of AEs on the days of injection and the following two days in an electronic patient diary. The investigators transferred the information on AEs into the eCRF (electronic Case Record Form) following discussion with the patient, if applicable. At the start and the end of the study, impact of AR/ARC symptoms on the quality of life (QoL) was documented via the validated SF-12 questionnaire [
17].
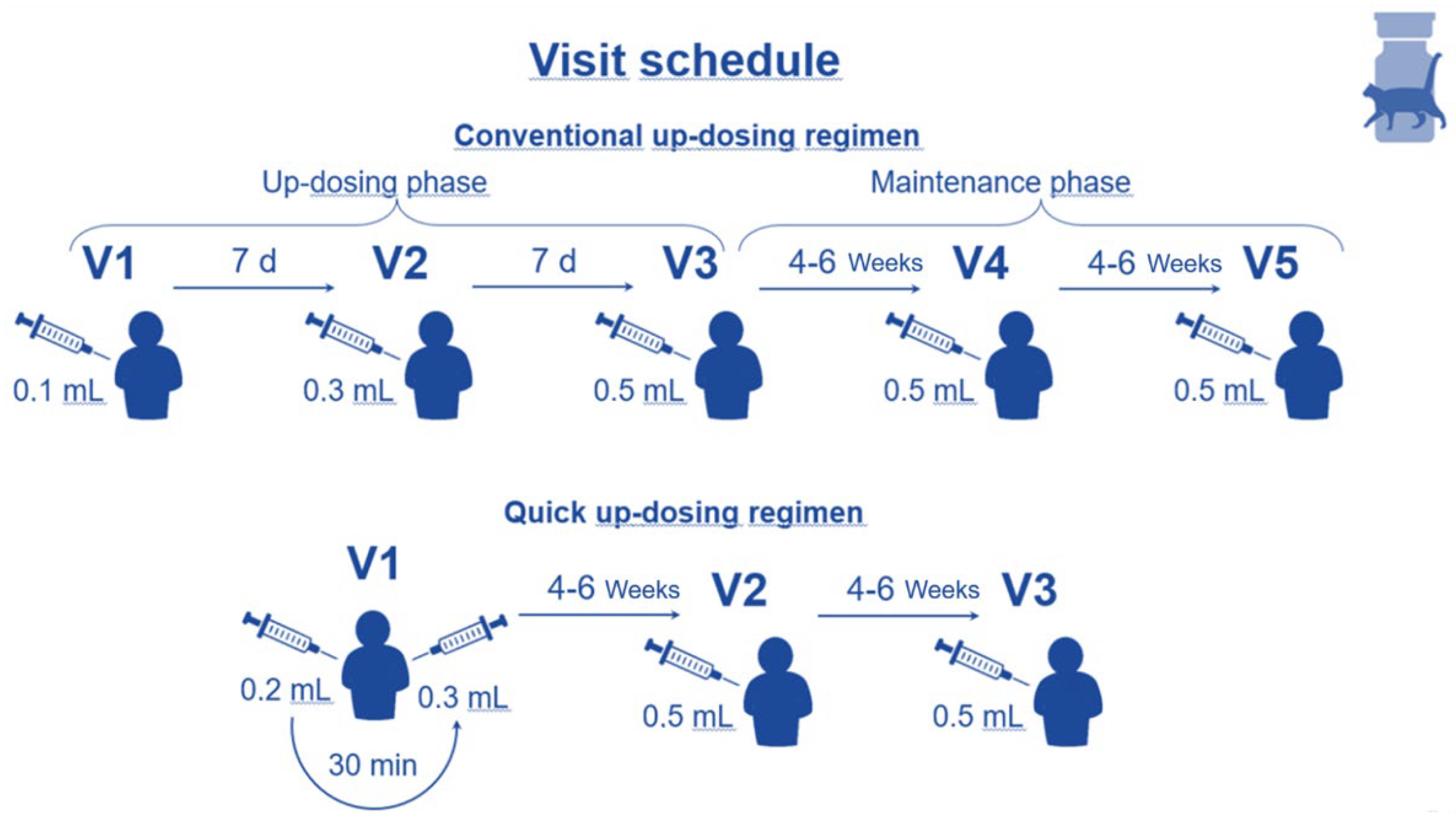
The depigmented, allergoid from cat was administered via subcutaneous injection. Different dosing schemes (conventional or quick) were used according to the Summary of Product Characteristics (SmPC) and later described in this paragraph and depicted in
Figure 1. This NIS-PASS observed patients during the initial 8-12 weeks of AIT, including the up-dosing phase. This time frame was chosen since higher rates of ADRs usually occur during the up-dosing compared to the maintenance phase.
2.2. Endpoints
The primary endpoints of the study were the number and severity of SRs categorized by the World Allergy Organisation (WAO) criteria [
17], the number and severity of LRs, and the onset of SR and/or LR (immediate or delayed).
The secondary endpoints of the study are listed below:
Comparison of two up-dosing regimens (Conventional Up-dosing Scheme (CUS) versus Quick Up-dosing Scheme (QUS)) regarding the primary variables
Comparison of CUS and QUS in terms of the proportion of patients reaching the maintenance treatment phase
Comparison of CUS and QUS regarding the proportion of patients with LR or SR and the level of severity
Determination of changes in the QoL determined using the SF-12 questionnaire (assessment period: 1 week)
2.3. Setting and Subjects
Patients aged ≥ 12 years suffering from persistent moderate to severe AR and/or ARC to cats with or without controlled asthma (no exacerbations within the past 3 months), with an indication for an AIT based on their symptoms and diagnostics, were eligible for a participation in this NIS-PASS.
Following standard clinical practice, patient and physician agreed to initiate an AIT with depigmented allergoid from cat. Thereafter, patients were informed about the study and the associated data collection. Patients had to be able to understand the content of the study and – prior to enrolment – had to sign a declaration of consent form for the use of their data collected in the study. For adolescent patients, the declaration of consent form had to be signed by both the adolescent and the parent(s)/legal guardian(s).
Patient’s clinically relevant sensitisation to cats had to be demonstrated by positive skin prick test (wheal diameter ≥ 3 mm) to Felis domesticus allergens.
Depending on the up-dosing regimen selected (conventional or quick), patients were observed for the initial 8-12 weeks of treatment with the depigmented allergoid from cat. Thereafter the AIT for cat allergy was continued according to the SmPC and clinical routine practice.
AEs occurring immediately following injection were documented by the investigators, whereas delayed AEs experienced between 30 minutes and 48 hours after injection were first documented by the patients in electronic patient diaries, then subsequently evaluated and transferred into the eCRF by the investigators.
2.4. Study Flow
Patients could be treated in outpatient centres by investigators specialized in allergology. The up-dosing regimen used – either conventional or quick – was individually selected for each patient. In consequence, 3 study visits (QUS, quick) or 5 study visits (CUS, conventional) were scheduled, with an overall study participation per patient of 8 – 12 weeks (see
Figure 1). In both cases the up-dosing phase finished upon reaching the maximum injection dose of 0.5 mL, which is the start of the maintenance phase (see
Figure 1).
Investigators were asked to adhere to the time schedules in accordance with the observational study plan. However, time adaptations in accordance with the SmPC were acceptable if medically justified. After the 3rd visit (QUS) or 5th visit (CUS), the patient’s study participation ended, and AIT was carried on following standard clinical practice.
2.5. Documentation of Adverse Events (AEs)
The following study-specific reporting and documentation of AEs had to be performed: via AE report form (paper-based), electronically via eCRF and via eDiary (electronic patient diary).
Investigators were obliged to document during the study:
On the days of injection plus 2 subsequent days, patients had to document any AE that occurred in an electronic diary.
With AITs, a major part of AEs observed during a study are indication specific ADRs classified as:
LRs were scored as immediate or delayed reactions and the symptoms redness and swelling were further evaluated as mild, moderate, or severe according to the following scoring scheme (see
Table 1). If only itching or pain at the injection site were observed as LR (without concurrent wheal and/or redness), this was documented as mild LR.
SRs also were scored as immediate or delayed reactions and their severity evaluated and ‘graded’ as Grade 1 to Grade 5 according to the WAO criteria (2010) [
17].
2.5. SF-12 Questionnaire
During visit 1 and the last visit (visit 3 or visit 5, depending on the up-dosing scheme) patients aged ≥14 years had to complete a paper-based SF-12 health-related QoL questionnaire during an interview with the investigator.
The SF-12 questionnaire was developed and validated as an instrument to measure health-related QoL in adults and adolescents aged 14 years and older [
19]. Based on 12 items, 8 aspects/dimensions are assessed: general health state/perception of health, physical capability, physical pain, physical ability to act, social capability, emotional ability to act, psychological well-being, and vitality. Adolescents aged 12-13 years did not complete a questionnaire.
2.6. Data Sources and Management
During the study, an electronic data capture system (EDC system) was used for data collection. Data was entered into the eCRF by the investigators or their study team.
The user concept of the secuTrial® software ensured that data access was only permitted for trained and authorized staff. The study-specific database stored in secuTrial® is cloud-based and stored in Germany on the servers of Noris network AG, hosted by the company iAS. This company is certified according to ISO/IEC 20000 and ISO-27001.
The CRO (Clinical Research Organization) was responsible for processing the pseudonymized study data for scientific purposes. With the publication of results in the form of scientific presentations or publications, the confidentiality of person-related patient data remains guaranteed.
While the CRO was processing the data, it was stored on servers hosted by the CRO and operated by the company Arwanet GmbH in Germany.
During the 1st visit of the patients for the study (Visit 1 (V1), investigators had to instruct participants about the usage of the eDiary and to hand out the respective login data. Only in justifiable exceptions (e.g., absence of internet), patients were allowed to document their symptoms in paper diaries.
Data were collected in a pseudonymous manner. For this purpose, the participants received a patient identification number.
2.7. Statistical Methods
All statistical analyses were performed using IBM SPSS Statistics for Windows, version 27.0 or older (Armonk, NY: IBM Corp.). The endpoints of the study were analyzed with descriptive and exploratory statistics. Subgroups were analyzed exploratively (e.g., subgroups in terms of gender, age, etc.).
Continuous data were analyzed by statistical ratios (mean, standard deviation, median, minimum, and maximum values). Categorical data were analyzed by absolute frequencies and the percentage of valid cases.
Confidence intervals were calculated using the Clopper-Pearson equation. The Student t-test or Mann-Whitney U-tests were used for continuous variables, and the Chi-square test or Fisher’s exact test for categorical variables in group comparisons for exploratory purposes. The two-sided p-value for significance was set at 0.05.
2.8. Monitoring
For monitoring of the study conduct at the investigational sites, 3 on-site visits of the CRA per centre were planned for this NIS-PASS: 1 site initiation visit and up to 2 regular monitor visits depending on the workload at the site (e.g. no. of patients). During the site initiation visit the CRA explained study procedures and aims, the use of the eCRF was trained and it was explained how the study team should instruct the patients for the use of the eDiary. During the regular monitor visits, correctness and completeness of the declaration of the consent forms, as well as the transfer of relevant data (especially AE) from the patient file into the eCRF, were checked. In addition, continuous remote monitoring of the data entered in the eCRF was performed, with special focus on completeness and plausibility. The entries from the specific AE report forms (source data), copies of which had been sent to the CRO and the sponsor, were compared (100%) with the data in the eCRF.
2.9. Ethical Supervision
All study documents were submitted to and approved by the responsible Ethics Committee of the University at Cologne (approval letter 21-1612_2-NIS dated 25.04.2022).
Where requested by the Ethics Committees of individual German federal states, specific patient information and declaration of consent forms were created.
3. Results
The total duration of the study was planned to be about 1.5 years, but due to slow recruitment, the study period was slightly prolonged, thereby lasting from May 2022 until December 2023. By end of the recruitment phase, 101 patients were enrolled in 22 outpatient allergy clinics in Germany.
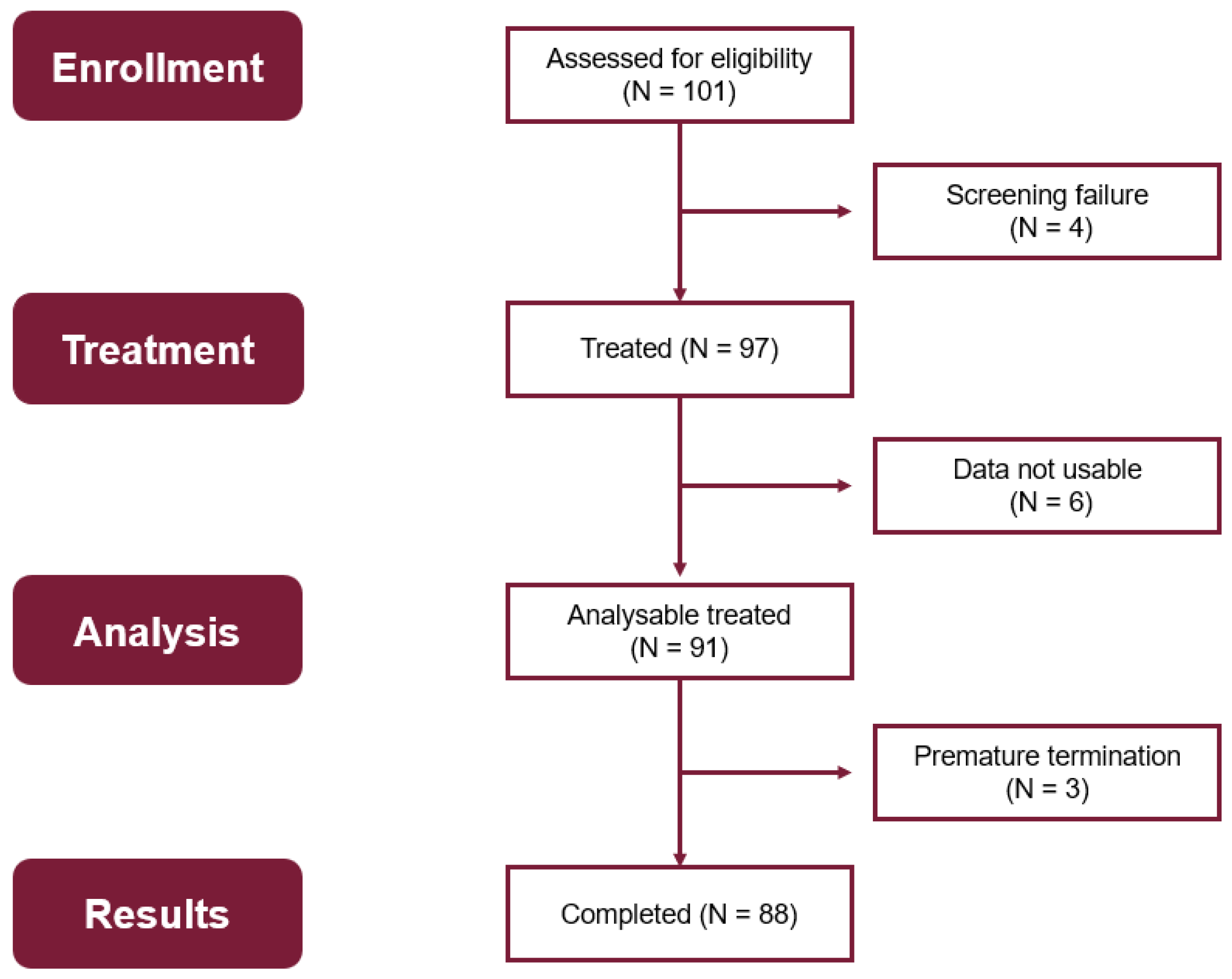
Figure 2 depicts an overview of the different patient populations for evaluation of the data. From 101 patients enrolled, 97 patients were treated with at least one injection, and 4 patients were screening failures. Data documentation of 6 patients was missing. Therefore, the Full Analysis Set comprises data of 91 patients who were treated with the study medication, including 59 women and 32 men. The baseline demographic characteristics are shown in
Table S2.
Three patients prematurely discontinued the study; they dropped out after V1 (1 patient), during V2 (1 patient), and after V4 (1 patient) for tolerability reasons.
88 patients completed the entire treatment course.
Overall, data of 9 adolescents (aged between 13 and 16 years) and 82 adults were analyzed, of which 88 participants completed the study. The overall mean age of the adult study population was 34 years, ranging from 18 to 67 years.
Patients had the option of being treated with one of the two up-dosing treatment regimens according to the SmPC. For 56 (62%) patients the CUS was selected and 35 (38%) received the QUS. 73.6% of the patients stated having a cat as a pet, with a maximum of four per household. These figures changed only marginally over the course of the study.
In addition to AR or ARC, 31.9% of the patients suffered from asthma. The majority of patients were polysensitized, with 63.9% also reacting to seasonal allergens, and 30% patients had other perennial allergies diagnosed.
During the study 54% of the patients reported AEs, being very similar between adolescents and adults (56% and 54%) as shown in
Table 2.
All but one participants reached the full maintenance dose, and 88 out of 91 treated participants completed the study as foreseen in the observational plan. The ratio of equal distribution (adolescents/adults) also corresponded to treatment-related AEs (= ADRs), with no significant differences in occurrence between adults and adolescents.
The incidence of ADRs did not differ significantly between the two up-dosing regimens, although more reactions were observed with the QUS.
The LRs were predominantly delayed, and the majority was mild (see
Table 3).
In total 41 related SRs were reported in 25 patients during the study – 1 in an adolescent, 40 in adults - being predominantly grade 2 for both - immediate (7 SRs) and delayed (8 SRs) - reactions (see
Table 4). The majority of SRs were delayed but did not lead to the use of adrenaline or emergency medical intervention at home. One SR was categorized as grade 2 by the investigator and documented as serious adverse reaction (SAR), since dyspnea occurred, but it resolved immediately. The narrative can be found in the supplementary material under S2. No SRs Grade 3 nor 4 occurred.
Most ADRs were local injection site reactions. Other ADRs occurred only occasionally and did not show any persistent impairment for the patients. They are listed in the supplementary material
Table S1.
The QoL data collected during the 8-12 weeks observation period did not reveal significant changes in any of the domains of the SF-12, as depicted in
Table 5. One adolescent 13 years old could not fill in the SF-12 for being underage. SF-12 of 1 adult was missing.
The comparison of the SF-12 values in the physical component score (PCS) and the mental component score (MCS) at baseline and at the end of the observation period – i.e. after 8 weeks or 12 weeks of AIT treatment - shows only marginal differences in both groups for the dimension of physical health. On the other hand, adolescents who received the quick regimen experienced an apparent increase of about 20% in their mental health status (i.e. 2 times the standard deviation), as shown in
Table 6.
4. Discussion
The German S2k-guideline on allergen specific immunotherapy [
20] states that allergen avoidance should be the primary approach in cat allergy and AIT should only be induced thereafter. This is, however, not a viable option for many cat owners (and lovers) or persons with frequent contact to cats. A safe and effective AIT for cat allergy would fill a gap for many of these often desperate patients.
This NIS was designed within the regulatory framework of a voluntary PASS, following the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP) Guide on Methodological Standards in Pharmacoepidemiology and thus meting high methodological standards. The study was initiated immediately after the market launch of the product (depigmented allergoid of cat) in Germany and encountered a challenging medical-economic environment, where the reimbursement of the therapy for cat-owning patients was particularly questioned. It was therefore not surprising that the recruitment period had to be extended, and only around a quarter of the originally planned 400 patients were included.
The effort required from the study participants was minimal: completing the SF-12 questionnaire twice (approximately 2 minutes per questionnaire) and maintaining an eDiary on the days of injection and the two subsequent days (approximately 5 minutes per day). Their participation in the study contributed to the improvement of the quality of safety data for the observed product. According to AMG
Section 4 (23) sentence 1, the format of a NIS was particularly suitable for collecting ‘real-world data’ in the context of routine clinical practice, that could not be detected in the defined and limited setting of a clinical trial.
Regardless of the age group, around 50% of patients reported ADRs, which were mainly delayed LRs (injection site reactions). Nevertheless, all but one of the 91 analyzed participants reached the full maintenance dose, and 88 out of 91 treated patients terminated the study regularly, while 3 participants dropped out for tolerability reasons. This is in clear contrast to other studies [
21] reporting dropout rates of 20% or more. The distribution of LRs and SRs did not reveal a difference between the CUS-group and the QUS-group, nor between adolescents and adults.
The number of reported SRs, all limited to grades 1 and 2, was low compared to results of other studies [
22]. These SRs primarily affected adults and were mostly delayed, thus supporting the good safety profile of the depigmented allergoid from cat.
In this study, in almost 100 patients, no emergency hospitalization or use of adrenaline was reported, confirming a significantly better safety profile than with native allergens for subcutaneous application [
22].
QoL did not improve significantly in the overall population during the relatively short observation period of up to three months. However, in the group of adolescents treated with the QUS, a clear improvement in mental health was observed. Given the small number of patients, this finding cannot be statistically confirmed. However, the observations made in this study correspond to those seen in a recently published real-world study in Spain [
23] performed in 62 cat allergic patients with the same AIT product. During 1 year of treatment significant improvements in rhinitis and asthma symptoms were found. Local reactions were reported in 12.9% of patients, while systemic reactions were limited to Grade 1 and occurred in 11.3% of the patients. This underlines the beneficial safety and tolerability profile as well as the effectiveness of this form of cat-AIT, which had undergone extensive in-vitro testing of efficacy and safety before the introduction into the market [
13].
The present study has some limitations. Firstly, the results may not be generalizable, since fewer than 100 patients completed the study. This applies particularly to the age group of adolescents, with just 9 persons (= 10%) in the subgroup of the total study population. Nevertheless, it is still one of the largest studies conducted with this allergen. A second limitation is the design of the study without a control group (e.g. placebo) and unblinded. However, since the focus of this investigation were the safety and tolerability aspects of this recently marketed SIT for cat-allergies, this type of study is generally accepted for the purpose. The strength of this study is the well-defined approach of a PASS within the pharma-epidemiologic framework of the ENCePP.
To summarize, SCIT with depigmented allergoid from cat – containing a chemically modified allergen extract – provides a well-tolerated and safe therapy option for patients with cat allergies.
5. Conclusions
This NIS, designed within the regulatory framework of a voluntary PASS, confirms the beneficial safety and tolerability profile of the AIT treatment containing a depigmented allergoid from cat newly introduced in the German market. Efficacy, especially regarding long-term application, still needs to be established.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Table S1. General AE symptoms distribution (n [%]) in the different study sub-groups (adolescents, adults plus subgroups for up-dosing schedules (conventional (CUS) and quick (QUS)). Table S2. Baseline demographic characteristics. S2 Narrative SAR.
Author Contributions
Conceptualization, R.M., E.C., S.A., A.D., C.A., A.R., A.S. and D.N.; methodology, C.A.; software, A.D.; validation, R.M., E.C., H.S., N.K., S.A., A.R. and E.T.; formal analysis, C.A., D.N.; investigation, E.T.; resources, E.C., A.R., A.D. and N.W., A.S.; data curation, A.D., H.S., N.K. and D.N.; writing—original draft preparation, I.L., R.M. and A.R.; writing—review and editing, R.M., C.A., S.A., A.R., H.S., N.K., E.T., N.W., A.S., D.N. and I.L.; visualization, R.M., A.R. and I.L.; supervision, R.M., S.A., A.R., E.C., E.T. and N.W.; project administration, R.M., S.A., E.C., A.S., N.W. and E.T.; funding acquisition, R.M, S.A. and A.S. All authors have read and agreed to the published version of the manuscript.” Please turn to the CRediT taxonomy for the term explanation. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by LETI Pharma GmbH, Ismaning, Germany.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki, and it was carried out in accordance with the German Medicinal Products Act (Arzneimittelgesetz, AMG), Section 67, Subsection 6. After counselling on professional regulations, it was approved by the competent ethics committee of the Cologne University, Cologne, Germany, and registered under the number 21-1612-NIS (approval date: 25 April 2022). This study protocol was submitted in the year 2022 to the competent regulatory agency Paul-Ehrlich-Institut under the number NIS656.
Informed Consent Statement
All patients and/or parents provided written informed consent before study inclusion.
Data Availability Statement
Upon request, data will be available from the corresponding author.
Acknowledgments
The authors are grateful to participating centres and their patients. We greatly appreciate the contribution of Dr. Stanislaus Wüst in implementing this study.
Conflicts of Interest
RM reports grants and personal fees from LETI during the conduct of the trial; personal fees from ALK, grants from ASIT biotech, personal fees from Allergopharma, personal fees from Allergy Therapeutics, grants and personal fees from Bencard, grants from Leti, grants, personal fees and non-financial support from Lofarma, non-financial support from Roxall, grants and personal fees from Stallergenes, grants from Optima, personal fees from Friulchem, personal fees from Hexal, personal fees from Servier, personal fees from Klosterfrau, non-financial support from Atmos, personal fees from Bayer, non-financial support from Bionorica, personal fees from FAES, personal fees from GSK, personal fees from MSD, personal fees from Johnson&Johnson, personal fees from Meda, personal fees and non-financial support from Novartis, non-financial support from Otonomy, personal fees from Stada, personal fees from UCB, non-financial support from Ferrero, grants from Hulka, personal fees from Nuvo, grants and personal fees from Ursapharm, personal fees from Menarini, personal fees from Mundipharma, personal fees from Pohl-Boskamp, grants from Cassella-med GmbH & Co. KG, personal fees from Laboratoire de la Mer, personal fees from Sidroga, grants and personal fees from HAL BV, personal fees from Lek, personal fees from PRO-AdWise, personal fees from Angelini Pharma, grants and non-financial support from JGL, grants and personal fees from bitop, grants from Sanofi, personal fees from Menarini, outside the submitted work; MC declares honoraria for presentations from ALK-Abelló, Allergopharma, AstraZeneca, Bencard Allergie/ Allergy Therapeutics, GalaxoSmithKline, HAL Allergy, Inmunotek, Novartis, Roxall, Sanofi-Aventis, Stallergenes outside the submitted work. Other non-financial interests: Member of German Society of Allergy (AeDA) and German Society of Oto-Rhino-Laryngology, Head and Neck Surgery DGHNO-KHC. Coordinating investigator of the present clinical trial;.D.N., E.T. and A.S. are employees of LETI, the sponsor of this study; A.R., E.C., C.A., A.D., H.S., N.K. and S.A. are employees of ClinCompetence Cologne GmbH; N.W. and I.L. have nothing to disclose.
Abbreviations
The following abbreviations are used in this manuscript:
| ADR |
Adverse Drug Reaction |
| AE |
Adverse Event |
| AIT |
Allergen-specific ImmunoTherapy |
| AMG |
German Medicines Act (Arzneimittelgesetz) |
| AR |
Allergic Rhinitis |
| ARC |
Allergic Rhinoconjunctivitis |
| CI |
Confidence Interval |
| CRO |
Clinical Research Organisation |
| CUS |
Conventional Up-dosing Scheme |
| DBPC |
Double-Blind Placebo-Controlled |
| EAACI |
European Association of Allergy and Clinical Immunology |
| eCRF |
Electronic Case Report Form |
| EDC System |
Electronic Data Capture System |
| eDiary |
Electronic patient Diary |
| ENCePP |
European Network of Centres for Pharmacoepidemiology and Pharmacovigilance |
| IgE |
Immunoglobulin E |
| ILIT |
Intralymphatic ImmunoTherapy |
| LR |
Local Reaction |
| MCS |
Mental Component Score |
| NIS |
Non-Interventional Study |
| OR |
Odd’s Ratio |
| p |
(p-value) value for significance |
| PASS |
Post-Authorisation Safety Study |
| PCS |
Physical Component Score |
| P25 |
Percentile 25 |
| P75 |
Percentile 75 |
| QoL |
Quality of Life |
| QUS |
Quick Up-dosing Scheme |
| SAE |
Serious Adverse Event |
| SAR |
Serious Adverse Reaction |
| SCIT |
Subcutaneous ImmunoTherapy |
| SF-12 |
(Health survey) Short Form 12 questions |
| SLIT |
SubLingual ImmunoTherapy |
| SmPC |
Summary of Product Characteristics |
| SR |
Systemic Reaction |
| V |
Visit |
| WAO |
World Allergy Organisation |
References
- Sheehan, W., & Phipatanakul, W. (2016, 12 28). Indoor allergen exposure and asthma outcomes. Curr Opin Pediatr., 772-777 . [CrossRef]
- Cacheiro-Llaguno C, Mösges R, Calzada D, González-de la Fuente S, Quintero E, Carnés J. Polysensitisation is associated with more severe symptoms: The reality of patients with allergy. Clin Exp Allergy. 2024 Aug;54(8):607-620. Epub 2024 Apr 27. PMID: 38676405. [CrossRef]
- Simpson, A., & Custovic, A. (2005, May 5). Pets and the development of allergic sensitization. Curr Allergy Asthma Rep, 212-20. [CrossRef]
- Heinzerling LM, Burbach GJ, Edenharter G, et al. GA(2)LEN skin test study I: GA(2)LEN harmonization of skin prick testing: novel sensitization patterns for inhalant allergens in Europe. Allergy. 2009;64(10):1498-1506. [CrossRef]
- van Ree, R., van Leeuwen, W., Bulder, Bond, J., & Aalberse, R. (1999, December). Purified natural and recombinant Fel d 1 and cat albumin in in vitro diagnostics for cat allergy. J Allergy Clin Immunol, 1223-30.
- Charpin, C., Mata, P., Charpin, D., Lavaut, M., Allasia, C., & Vervloet, D. (1991, July). Fel d I allergen distribution in cat fur and skin. J Allergy Clin Immunol, 77-82. [CrossRef]
- Satyaraj E, Sun P, Sherrill S. Fel d1 Blocking Antibodies: A Novel Method to Reduce IgE-Mediated Allergy to Cats. J Immunol Res. 2021 Jun 19;2021:5545173. [CrossRef]
- Alvarez-Cuesta, E., Berges-Gimeno, P., Goncàlez-Mancebo, E., Fernàndez-Caldas, E., Cuesta-Herranz, J., & Casanovas, M. (2007, July). Sublingual immunotherapy with a standardized cat dander extract: evaluation of efficacy in a double-blind placebo-controlled study. Allergy, 810-7. [CrossRef]
- Orengo JM, Radin AR, Kamat V, et al. Treating cat allergy with monoclonal IgG antibodies that bind allergen and prevent IgE engagement. Nat Commun. 2018;9(1):1421. [CrossRef]
- Borchers, A., Keen, C., & Gershwin, M. (2004, October). Fatalities following allergen immunotherapy. Clin Rev Allergy Immunol, 147-58. [CrossRef]
- Nguyen NT, Raskopf E, Shah-Hosseini K, Zadoyan G, Mösges R. A review of allergoid immunotherapy: is cat allergy a suitable target? Immunotherapy. 2016;8(3):331-49. [CrossRef]
- Carnes, Jeronimo, et al. “Allergoids for allergy treatment.” Recent patents on inflammation & allergy drug discovery 12.2 (2018): 110-119. [CrossRef]
- Morales, M., Gallego, M., Iraola, V., Taulés, M., de Oliveira, E., Moya R, R., & Carnés, J. (2017, February 24). In vitro evidence of efficacy and safety of a polymerized cat dander extract for allergen immunotherapy. BMC Immunol. [CrossRef]
- Ibarrola, I., Sanz, M., Gamboa, P., Mir, A., Benahmed, D., Ferrer, A., . . . Asturias, J. (2004, February). Biological characterization of glutaraldehyde-modified Parietaria judaica pollen extracts. Clin Exp Allergy, 303-9. [CrossRef]
- Mösges, R., Santiago , A., Allekotte, S., Jahed, N., Astvatsatourov, A., Sager, A., & Sánchez-López, J. (2019, June 5). Subcutaneous immunotherapy with depigmented-polymerized allergen extracts: a systematic review and meta-analysis. Clin Transl Allergy. [CrossRef]
- Dhami, S., & Agarwal, A. (2018, August). Does evidence support the use of cat allergen immunotherapy? Curr Opin Allergy Clin Immunol., 350-355. [CrossRef]
- Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996 Mar;34(3):220-33.
- Cox, L., Larenas-Linnemann, D., Lockey, R., & Passalacqua, G. (2010, March). Speaking the same language: The World Allergy Organization Subcutaneous Immunotherapy Systemic Reaction Grading System. J Allergy Clin Immunol. [CrossRef]
- Huo, T., Guo, Y., Shenkman, E., & Muller, K. (2018, Feb 13). Assessing the reliability of the short form 12 (SF-12) health survey in adults with mental health conditions: a report from the wellness incentive and navigation (WIN) study. . Health Qual Life Outcomes, 16(1). [CrossRef]
- Pfaar O, Ankermann T, Augustin M, et al. Guideline on allergen immunotherapy in IgE-mediated allergic diseases: S2K Guideline of the German Society of Allergology and Clinical Immunology (DGAKI), Society of Pediatric Allergology and Environmental Medicine (GPA), Medical Association of German Allergologists (AeDA), Austrian Society of Allergology and Immunology (ÖGAI), Swiss Society for Allergology and Immunology (SSAI), German Dermatological Society (DDG), German Society of Oto-Rhino-Laryngology, Head and Neck Surgery (DGHNO-KHC), German Society of Pediatrics and Adolescent Medicine (DGKJ), Society of Pediatric Pulmonology (GPP), German Respiratory Society (DGP), German Professional Association of Otolaryngologists (BVHNO), German Association of Paediatric and Adolescent Care Specialists (BVKJ), Federal Association of Pneumologists, Sleep and Respiratory Physicians (BdP), Professional Association of German Dermatologists (BVDD). Allergol Select. 2022; 6:167-232.
- Zemelka-Wiacek M., Agache I., Akdis C., Akdis M., Casale T., Dramburg S., Jahnz-Różyk K., Kosowska A., Matricardi P., Pfaar O., Shamji M., Jutel M. (2024, April). Hot topics in allergen immunotherapy, 2023: Current status and future perspective. J Allergy Clin Immunol. 830-831. [CrossRef]
- Lilja G., Sundin B., Graff-Lonnevig V., Hedlin G., Heilborn H., Norrlind K., Pegelow K.O., Løwenstein H. (1989, March). Immunotherapy with cat- and dog-dander extracts. IV. Effects of 2 years of treatment. J Allergy Clin Immunol. 42-44.
- Vázquez de la Torre M, López-González P, Haroun-Díaz E, Somoza ML, Cervera MD, Ruiz-García M, Ruano FJ. Depigmented, Polymerized Cat Epithelium Extract Is Safe and Improves Rhinitis and Asthma Symptoms in Cat-Allergic Patients: A Real-World Retrospective Study. Int Arch Allergy Immunol. 2025;186(6):532-542. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).