Submitted:
05 April 2024
Posted:
08 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
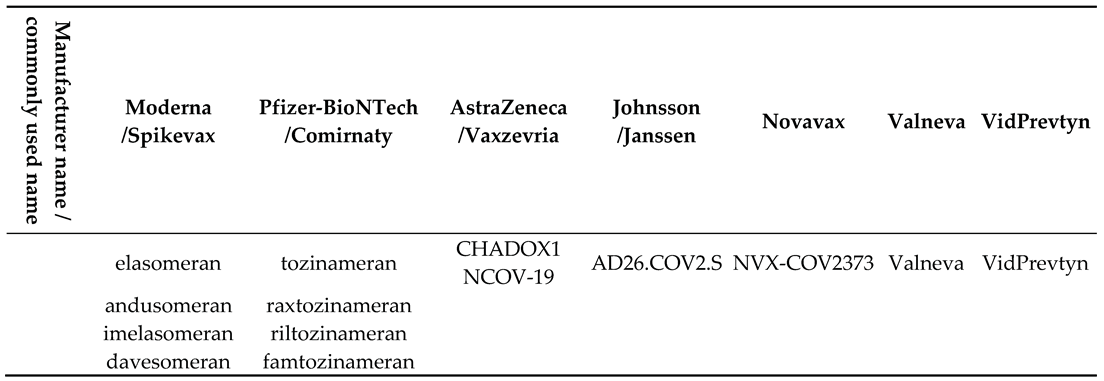
2. Materials and Methods
Statistical Analysis
3. Results
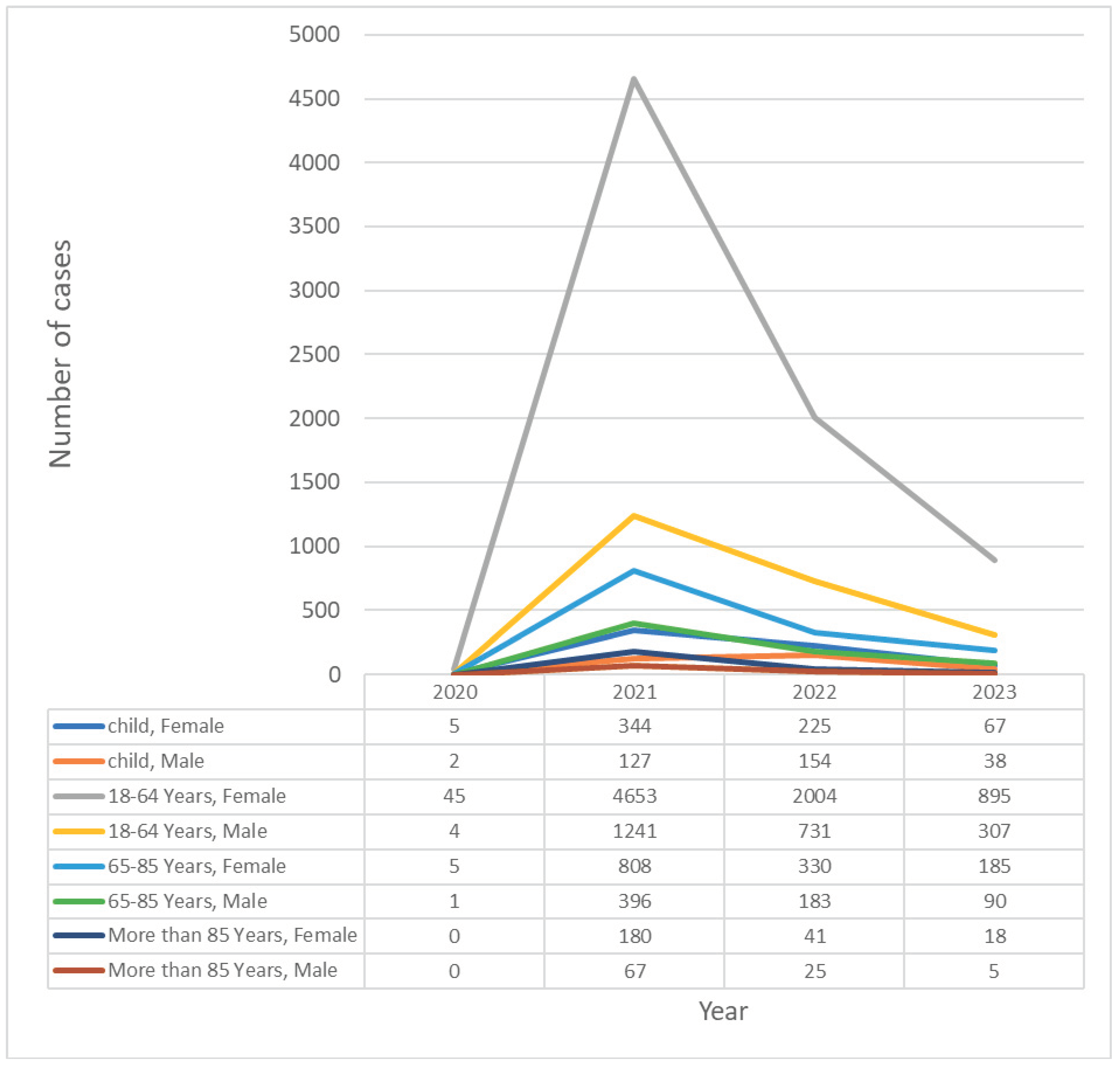
3.1. General Characteristics
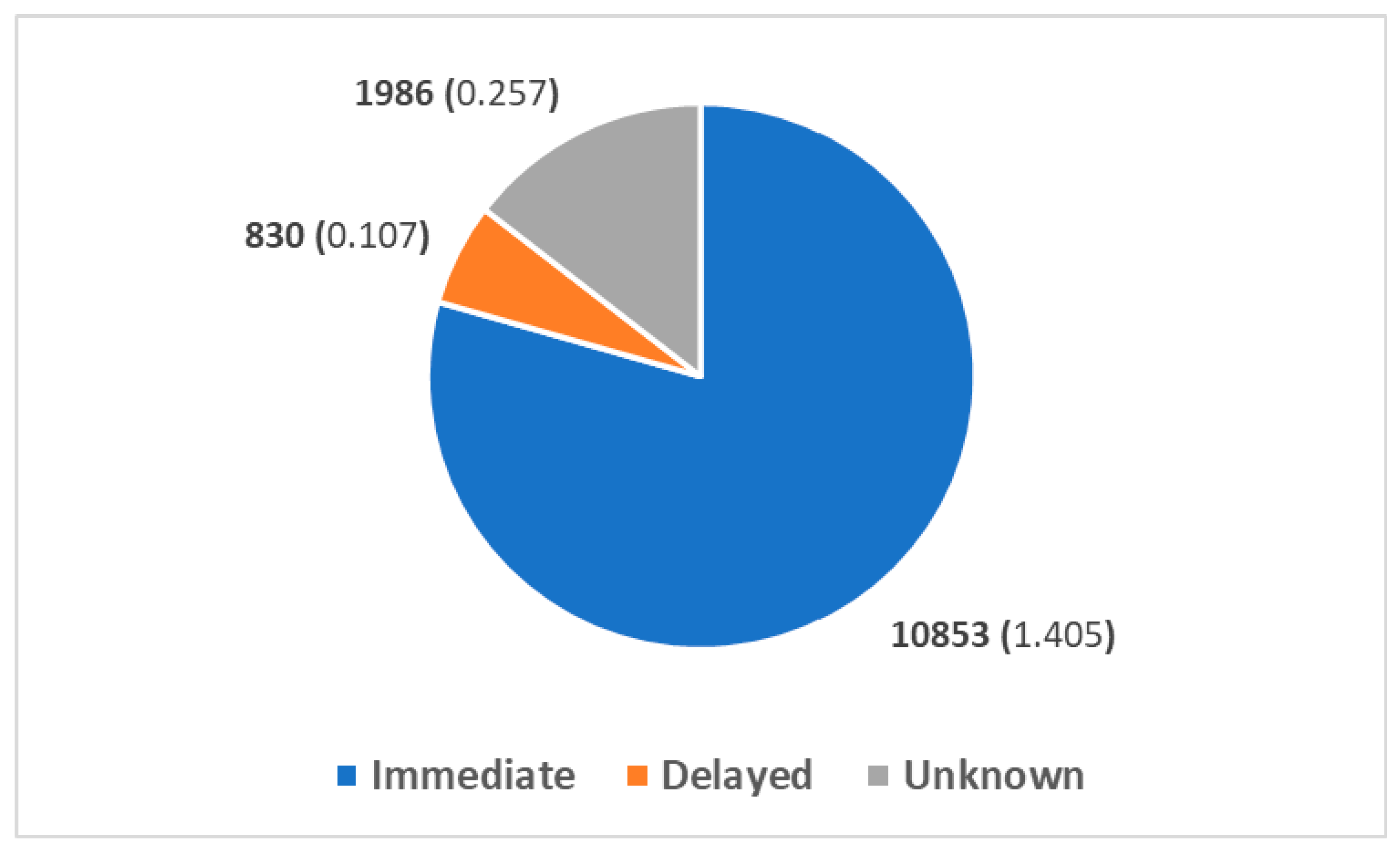
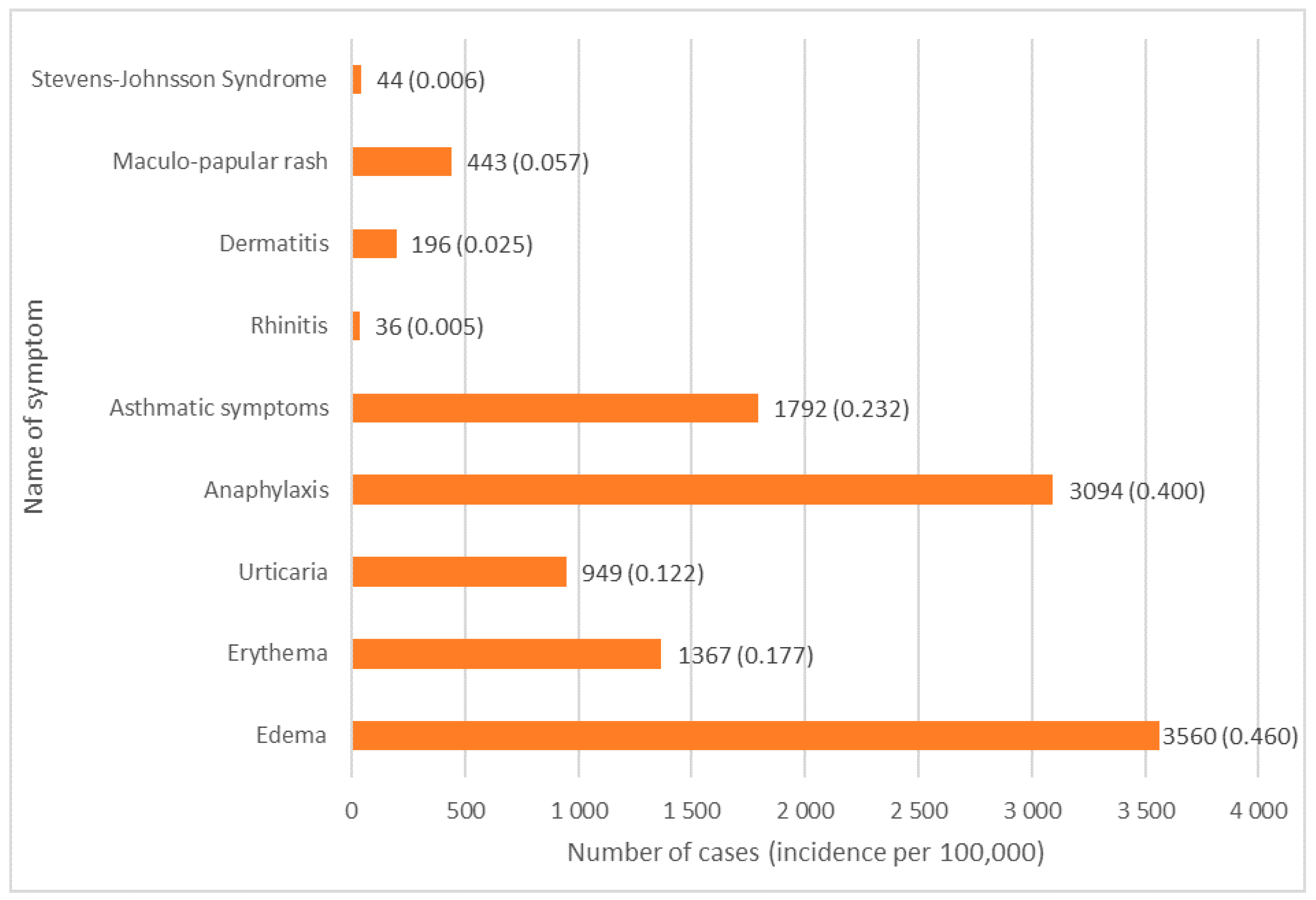
3.2. Type of Reaction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barbaud A, Garvey LH, Arcolaci A, Brockow K, Mori F, Mayorga C et al. Allergies and COVID-19 vaccines: An ENDA/EAACI Position paper. Allergy Published Online First: 22. 20 February. [CrossRef]
- Coroiu A, Moran C, Campbell T, Geller AC. Barriers and facilitators of adherence to social distancing recommendations during COVID- 19 among a large international sample of adults. PLoS One 2020;15. [CrossRef]
- Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N Engl J Med 2021;384:2187–2201.
- Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021;397:99–111.
- Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med 2021;384:403–416.
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. New England Journal of Medicine 2020;383:2603–2615.
- Smout, A. UK issues anaphylaxis warning on Pfizer vaccine after adverse reactions. Reuters. 2020.https://www.reuters.
- Kruszewski J, Cichocka-Jarosz E, Czarnobilska E, Jutel M, Kulus M, Kuna P et al. Rekomendacje Polskiego Towarzystwa Alergologicznego dotyczące kwalifikacji osób z alergią i anafilaksją do szczepienia przeciw COVID-19. Alergologia Polska - Polish Journal of Allergology 2021;8:1–8.
- Bogdanov G, Bogdanov I, Kazandjieva J, Tsankov N. Cutaneous adverse effects of the available COVID-19 vaccines: Effects of COVID-19 vaccines. Clin Dermatol 2021;39. [CrossRef]
- Gold MS, Amarasinghe A, Greenhawt M, Kelso JM, Kochhar S, Yu-Hor Thong B et al. Anaphylaxis: Revision of the Brighton collaboration case definition. Vaccine 2023;41. [CrossRef]
- Xu J, Vanijcharoenkarn K, Sexton ME, Martin L, Lee FEH, Kuruvilla ME. Delayed Hypersensitivity Reactions Following First Dose of the SARS-CoV2 mRNA Vaccines. J Gen Intern Med. 2021;36. [CrossRef]
- Romantowski J, Kruszewski J, Solarski O, Bant A, Chciałowski A, Pietrzyk I et al. Protocol of safe vaccination against COVID-19 in patients with high risk of allergic reactions. Clin Transl Allergy 2022;12:e12152.
- Mortz CG, Kjaer HF, Rasmussen TH, Rasmussen HM, Garvey LH, Bindslev-Jensen C. Allergy to polyethylene glycol and polysorbates in a patient cohort: Diagnostic work-up and decision points for vaccination during the COVID-19 pandemic. Clin Transl Allergy 2022;12:e12111.
- Nilsson L, Csuth Á, Storsaeter J, Garvey LH, Jenmalm MC. Vaccine allergy: evidence to consider for COVID-19 vaccines. Curr Opin Allergy Clin Immunol 2021;21:401–409.
- Romantowski J, Górska A, Zieliński M, Trzonkowski P, Rucka K, Niedoszytko M. Clinical Application of In Vitro Tests for COVID-19 Vaccine Delayed Hypersensitivity Diagnostics. Int J Mol Sci 2023;24. [CrossRef]
- European Medicines Agency 2017 Annual Report on EudraVigilance for the European Parliament, the Council and the Commission. https://www.ema.europa.eu/en/news/ema-annual-report-2022-published (accessed 29 Dec2023).
- European Centre for Disease Prevention and Control (ECDC). Covid19 Vaccine Tracker. 2023.https://vaccinetracker.ecdc.europa.eu/public/extensions/COVID-19/vaccine-tracker.html#uptake-tab (accessed 5 Oct2023).
- Takano T, Hirose M, Yamasaki Y, Hara M, Okada T, Kunishima H. Investigation of the incidence of immunisation stress-related response following COVID-19 vaccination in healthcare workers. Journal of Infection and Chemotherapy 2022;28. [CrossRef]
- Luxi N, Giovanazzi A, Arcolaci A, Bonadonna P, Crivellaro MA, Cutroneo PM et al. Allergic Reactions to COVID-19 Vaccines: Risk Factors, Frequency, Mechanisms and Management. BioDrugs. 2022;36. [CrossRef]
- Marković I, Božan M, Perković T, Paušek K, Nedeljković V, Perković M et al. Incidence of immediate allergic reactions to mRNA COVID-19 vaccines in adults with drug allergies and other allergic disorders. Medicine (United States) 2022;101. [CrossRef]
- Yuksel Bulut H, Ulusoy Severcan E, Ertugrul A. COVID-19 Vaccines Are Safely Tolerated in Adolescents with Cutaneous Mastocytosis. Int Arch Allergy Immunol 2023;184. [CrossRef]
- Ali SB, Perkins G, Ryoo D, Lee M, Tunbridge M, Yuson C et al. AstraZeneca ChAdOx1-S COVID-19 vaccine can be safely administered in patients with EDTA allergy. Allergy, Asthma and Clinical Immunology 2022;18. [CrossRef]
- Novembre E, Tosca M, Caffarelli C, Calvani M, Cardinale F, Castagnoli R et al. Management of BNT162b2 mRNA COVID-19 vaccine in children aged 5–11 years with allergies, asthma, and immunodeficiency: consensus of the Italian Society of Pediatric Allergy and Immunology (SIAIP). Ital J Pediatr. 2022;48. [CrossRef]
- Maltezou HC, Hatziantoniou S, Theodoridou K, Vasileiou K, Anastassopoulou C, Tsakris A. Anaphylaxis rates following mRNA COVID-19 vaccination in children and adolescents: Analysis of data reported to EudraVigilance. Vaccine 2023;41. [CrossRef]
- Yoon D, Jeon HL, Noh Y, Choe YJ, Choe SA, Jung J et al. A Nationwide Survey of mRNA COVID-19 Vaccinee’s Experiences on Adverse Events and Its Associated Factors. J Korean Med Sci 2023;38. [CrossRef]
- Jaggers J, Samarakoon U, Fu X, Gonzalez-Estrada A, Anvari S, Chong HJ et al. Anaphylaxis after COVID-19 vaccination: A registry-based study. Journal of Allergy and Clinical Immunology: In Practice 2022;10. [CrossRef]
- Toledo-Salinas C, Scheffler-Mendoza SC, Castano-Jaramillo LM, Ortega-Martell JA, Del Rio-Navarro BE, Santibáñez-Copado AM et al. Anaphylaxis to SARS-CoV-2 Vaccines in the Setting of a Nationwide Passive Epidemiological Surveillance Program. J Clin Immunol 2022;42. [CrossRef]
- Macy E, Pandya S, Sheikh J, Burnette A, Shi JM, Chung J et al. Population-Based Incidence, Severity, and Risk Factors Associated with Treated Acute-Onset COVID-19 mRNA Vaccination–Associated Hypersensitivity Reactions. Journal of Allergy and Clinical Immunology: In Practice 2022;10. [CrossRef]
- Hatziantoniou S, Anastassopoulou C, Lampropoulou V, Maltezou HC, Andreakos E, Poland GA et al. Comparative assessment of allergic reactions to COVID-19 vaccines in Europe and the United States. Allergy: European Journal of Allergy and Clinical Immunology. 2022;77. [CrossRef]
- Montano, D. Frequency and Associations of Adverse Reactions of COVID-19 Vaccines Reported to Pharmacovigilance Systems in the European Union and the United States. Front Public Health 2022;9. [CrossRef]
- Boufidou F, Hatziantoniou S, Theodoridou K, Maltezou HC, Vasileiou K, Anastassopoulou C et al. Anaphylactic Reactions to COVID-19 Vaccines: An Updated Assessment Based on Pharmacovigilance Data. Vaccines (Basel) 2023;11. [CrossRef]
- Popiołek I, Piotrowicz-Wójcik K, Porebski G. Hypersensitivity Reactions in Serious Adverse Events Reported for Paracetamol in the EudraVigilance Database, 2007–2018. Pharmacy 2019;7. [CrossRef]
- Jutel M, Agache I, Zemelka-Wiacek M, Akdis M, Chivato T, del Giacco S et al. Nomenclature of allergic diseases and hypersensitivity reactions: Adapted to modern needs: An EAACI position paper. Allergy 2023;78:2851–2874.
- EUDRA. Vigilance database. 2023.https://www.adrreports.eu/ (accessed 6 Dec2023).
- Gao Y, Ding M, Dong X, Zhang J, Kursat Azkur A, Azkur D et al. Risk factors for severe and critically ill COVID-19 patients: A review. Allergy 2021;76:428–455.
- Kricorian K, Civen R, Equils O. COVID-19 vaccine hesitancy: misinformation and perceptions of vaccine safety. Hum Vaccin Immunother 2022;18. [CrossRef]
- Chu DK, Abrams EM, Golden DBK, Blumenthal KG, Wolfson AR, Stone CA et al. Risk of Second Allergic Reaction to SARS-CoV-2 Vaccines A Systematic Review and Meta-analysis. JAMA Intern Med. 2022;182. [CrossRef]
- Shimabukuro T, Nair N. Allergic Reactions including Anaphylaxis after Receipt of the First Dose of Pfizer-BioNTech COVID-19 Vaccine. JAMA - Journal of the American Medical Association. 2021;325. [CrossRef]
- Shimabukuro TT, Cole M, Su JR. Reports of Anaphylaxis after Receipt of mRNA COVID-19 Vaccines in the US-, 2020-January 18, 2021. JAMA - Journal of the American Medical Association. 2021;325. 14 December. [CrossRef]
- Klein NP, Lewis N, Goddard K, Fireman B, Zerbo O, Hanson KE et al. Surveillance for Adverse Events after COVID-19 mRNA Vaccination. JAMA - Journal of the American Medical Association 2021;326. [CrossRef]
- McNeil MM, Weintraub ES, Duffy J, Sukumaran L, Jacobsen SJ, Klein NP et al. Risk of anaphylaxis after vaccination in children and adults. Journal of Allergy and Clinical Immunology 2016;137. [CrossRef]
- Hourihane JOB, Byrne AM, Blümchen K, Turner PJ, Greenhawt M. Ascertainment Bias in Anaphylaxis Safety Data of COVID-19 Vaccines. Journal of Allergy and Clinical Immunology: In Practice. 2021;9. [CrossRef]
- Lee K, Lee H, Kwon R, Shin YH, Yeo SG, Lee YJ et al. Global burden of vaccine-associated anaphylaxis and their related vaccines, 1967–2023: A comprehensive analysis of the international pharmacovigilance database. Allergy: European Journal of Allergy and Clinical Immunology Published Online First: 2023. [CrossRef]
- McNeil, MM. Vaccine-Associated Anaphylaxis. Curr Treat Options Allergy. 2019;6:297–308.
- Stone SF, Phillips EJ, Wiese MD, Heddle RJ, Brown SGA. Immediate-type hypersensitivity drug reactions. Br J Clin Pharmacol. 2014;78. [CrossRef]
- Macy E, Ho NJ. Multiple drug intolerance syndrome: prevalence, clinical characteristics, and management. Ann Allergy Asthma Immunol 2012;108:88–93.
- Eaddy Norton A, Broyles AD. Drug allergy in children and adults: Is it the double X chromosome? Annals of Allergy, Asthma & Immunology 2019;122:148–155.
- Zucker I, Prendergast BJ. Sex differences in pharmacokinetics predict adverse drug reactions in women. Biol Sex Differ 2020;11. [CrossRef]
- Tharpe, N. Adverse Drug Reactions in Women’s Health Care. J Midwifery Womens Health. 2011;56. [CrossRef]
- Watson S, Caster O, Rochon PA, den Ruijter H. Reported adverse drug reactions in women and men: Aggregated evidence from globally collected individual case reports during half a century. EClinicalMedicine 2019;17. [CrossRef]
- Torres MJ, Blanca M, Fernandez J, Romano A, De Weck A, Aberer W et al. Diagnosis of immediate allergic reactions to beta-lactam antibiotics. Allergy: European Journal of Allergy and Clinical Immunology 2003;58. [CrossRef]
- Pumphrey RSH. Lessons for management of anaphylaxis from a study of fatal reactions. Clinical and Experimental Allergy 2000;30. [CrossRef]
- Blumenthal KG, Peter JG, Trubiano JA, Phillips EJ. Antibiotic allergy. The Lancet. 2019;393. [CrossRef]
- Drug allergy: An updated practice parameter. Annals of Allergy, Asthma and Immunology 2010;105. [CrossRef]
- Zhou L, Dhopeshwarkar N, Blumenthal KG, Goss F, Topaz M, Slight SP et al. Drug allergies documented in electronic health records of a large healthcare system. Allergy: European Journal of Allergy and Clinical Immunology 2016;71. [CrossRef]
- International Rheumatic Fever Study Group. Allergic reactions to long-term benzathine penicillin prophylaxis for rheumatic fever. The Lancet 1991;337. [CrossRef]
- Apter AJ, Kinman JL, Bilker WB, Herlim M, Margolis DJ, Lautenbach E et al. Represcription of penicillin after allergic-like events. Journal of Allergy and Clinical Immunology 2004;113. [CrossRef]
- MacY E, Ho NJ. Multiple drug intolerance syndrome: Prevalence, clinical characteristics, and management. Annals of Allergy, Asthma and Immunology 2012;108. [CrossRef]
- Macy E, Poon K-Y T. Self-reported Antibiotic Allergy Incidence and Prevalence: Age and Sex Effects. American Journal of Medicine 2009;122. [CrossRef]
- Dhopeshwarkar N, Sheikh A, Doan R, Topaz M, Bates DW, Blumenthal KG et al. Drug-Induced Anaphylaxis Documented in Electronic Health Records. Journal of Allergy and Clinical Immunology: In Practice 2019;7. [CrossRef]
- Idsoe O, Guthe T, Willcox RR, de Weck AL. Nature and extent of penicillin side-reactions, with particular reference to fatalities from anaphylactic shock. Bull World Health Organ 1968;38.
- Ribeiro-Vaz I, Marques J, Demoly P, Polónia J, Gomes ER. Drug-induced anaphylaxis: A decade review of reporting to the Portuguese Pharmacovigilance Authority. Eur J Clin Pharmacol 2013;69. [CrossRef]
- Giles A, Foushee J, Lantz E, Gumina G. Sulfonamide Allergies. Pharmacy 2019;7. [CrossRef]
- Dorn JM, Alpern M, McNulty C, Volcheck GW. Sulfonamide Drug Allergy. Curr Allergy Asthma Rep. 2018;18. [CrossRef]
- Carr A, Swanson C, Penny R, Cooper DA. Clinical and laboratory markers of hypersensitivity to trimethoprim-sulfamethoxazole in patients with pneumocystis carinii pneumonia and AIDS. Journal of Infectious Diseases 1993;167. [CrossRef]
- Schönmann C, Brockow K. Adverse reactions during procedures: Hypersensitivity to contrast agents and dyes. Annals of Allergy, Asthma and Immunology. 2020;124. [CrossRef]
- Brockow K, Christiansen C, Kanny G, Clément O, Barbaud A, Bircher A et al. Management of hypersensitivity reactions to iodinated contrast media. Allergy: European Journal of Allergy and Clinical Immunology. 2005;60. [CrossRef]
- Cha MJ, Kang DY, Lee W, Yoon SH, Choi YH, Byun JS et al. Hypersensitivity reactions to iodinated contrast media: A multicenter study of 196 081 patients. Radiology 2019;293. [CrossRef]
- Motosugi U, Ichikawa T, Sano K, Onishi H. Acute adverse reactions to nonionic iodinated contrast media for CT: Prospective randomized evaluation of the effects of dehydration, oral rehydration, and patient risk factors. American Journal of Roentgenology 2016;207. [CrossRef]
- Wang CL, Cohan RH, Ellis JH, Caoili EM, Wang G, Francis IR. Frequency, outcome, and appropriateness of treatment of nonionic iodinated contrast media reactions. American Journal of Roentgenology 2008;191. [CrossRef]
- Bilò MB, Bignardi D. Iodinated contrast media hypersensitivity reactions: is it time to re-evaluate risk factors? Eur Ann Allergy Clin Immunol. 2022;54. [CrossRef]
- Meek IL, van de Laar MAFJ, Vonkeman HE. Non-steroidal anti-inflammatory drugs: An overview of cardiovascular risks. Pharmaceuticals. 2010;3. [CrossRef]
- Blanca-Lopez N, Soriano V, Garcia-Martin E, Canto G, Blanca M. Nsaid-induced reactions: Classification, prevalence, impact, and management strategies. J Asthma Allergy. 2019;12. [CrossRef]
- Kasper L, Sladek K, Duplaga M, Bochenek G, Liebhart J, Gladysz U et al. Prevalence of asthma with aspirin hypersensitivity in the adult population of Poland. Allergy: European Journal of Allergy and Clinical Immunology 2003;58. [CrossRef]
- Zembowicz A, Mastalerz L, Setkowicz M, Radziszewski W, Szczeklik A. Safety of Cyclooxygenase 2 Inhibitors and Increased Leukotriene Synthesis in Chronic Idiopathic Urticaria with Sensitivity to Nonsteroidal Anti-inflammatory Drugs. Arch Dermatol 2003;139. [CrossRef]
- McDonald JR, Mathison DA, Stevenson DD. Aspirin intolerance in asthma. Detection by oral challenge. J Allergy Clin Immunol 1972;50. [CrossRef]
- Jenkins C, Costello J, Hodge L. Systematic review of prevalence of aspirin induced asthma and its implications for clinical practice. Br Med J. 2004;328. [CrossRef]
- Caimmi S, Caimmi D, Bousquet PJ, Demoly P. How can we better classify NSAID hypersensitivity reactions? - Validation from a large database. Int Arch Allergy Immunol 2012;159. [CrossRef]
- Hernandez-Salazar A, de Leon-Rosales SP, Rangel-Frausto S, Criollo E, Archer-Dubon C, Orozco-Topete R. Epidemiology of Adverse Cutaneous Drug Reactions. A Prospective Study in Hospitalized Patients. Arch Med Res 2006;37. [CrossRef]
- Doña I, Blanca-López N, Torres MJ, García-Campos J, García-Núñez I, Gómez F et al. Drug hypersensitivity reactions: Response patterns, drug involved, and temporal variations in a large series of patients. J Investig Allergol Clin Immunol 2012;22.
- Harboe T, Guttormsen AB, Aarebrot S, Dybendal T, Irgens Å, Florvaag E. Suspected allergy to local anaesthetics: Follow-up in 135 cases. Acta Anaesthesiol Scand 2010;54. [CrossRef]
- Schatz M, Fung DL. Anaphylactic and anaphylactoid reactions due to anesthetic agents. Clin Rev Allergy 1986;4. [CrossRef]
- Kvisselgaard AD, Mosbech HF, Fransson S, Garvey LH. Risk of Immediate-Type Allergy to Local Anesthetics Is Overestimated—Results from 5 Years of Provocation Testing in a Danish Allergy Clinic. Journal of Allergy and Clinical Immunology: In Practice 2018;6. [CrossRef]
- Saff, RR. Immediate Local Anesthetic Reactions: Too Quick to Point the Finger? Journal of Allergy and Clinical Immunology: In Practice. 2018;6. [CrossRef]
- Kvisselgaard AD, Krøigaard M, Mosbech HF, Garvey LH. No cases of perioperative allergy to local anaesthetics in the Danish Anaesthesia Allergy Centre. Acta Anaesthesiol Scand 2017;61. [CrossRef]
- Zuo J, Gong R, Liu X, Zhao J. Risk of true allergy to local anesthetics: 10-year experience from an anesthesia allergy clinic in china. Ther Clin Risk Manag 2020;16. [CrossRef]
 |
| Number of cases | Spikevax | Comirnaty | Vaxzevria | Janssen | Novavax | Valneva | VidPrevtyn Beta | P value |
|---|---|---|---|---|---|---|---|---|
| General population | 2,857 | 8,734 | 1,717 | 340 | 18 | 2 | 1 | <0.001 |
| Female | 2,052 | 6,363 | 1,216 | 157 | 15 | 1 | 1 | <0.001 |
| Male | 753 | 2,064 | 409 | 141 | 3 | 1 | 0 | |
| Female, child | 89 | 479 | 65 | 6 | 1 | 0 | 1 | <0.001 |
| Male, child | 61 | 236 | 14 | 9 | 1 | 0 | 0 | |
| Male, Adult 18–64 years old | 572 | 1322 | 263 | 123 | 2 | 1 | 0 | <0.001 |
| Female, Adult 18–64 years old | 1,647 | 4,870 | 928 | 137 | 14 | 1 | 0 | |
| Female, elderly>85 | 27 | 202 | 10 | 0 | 0 | 0 | 0 | <0.001 |
| Male, elderly>85 | 14 | 78 | 3 | 2 | 0 | 0 | 0 | |
| Female Adult 65–85 years old | 289 | 812 | 213 | 14 | 0 | 0 | 0 | <0.001 |
| Male Adult 65–85 years old | 106 | 428 | 129 | 7 | 0 | 0 | 0 | |
| Incidence per 100,000 | Spikevax | Comirnaty | Vaxzevria | Janssen | Novavax | Valneva | VidPrevtyn Beta | |
| General population | 2,162 | 1,538 | 88,614 | 2,115 | 7,989 | 3,07 | 13,291 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





