1. Introduction
In our clinical setting, chronic rhinosinusitis with nasal polyps (CRSwNP) is an upper respiratory disease predominantly driven by type 2 inflammatory mechanisms managed with daily intranasal corticosteroids and saline rinses [
1]. The most burdensome patient-reported symptoms are nasal congestion, rhinorrhea and smell impairment, which severely impacts patient’s quality of life (QoL) to a similar extent than other devastating chronic diseases such as COPD or asthma [
2]. Most patients successfully control CRSwNP symptoms with the recommended appropriate medical therapy, whereas some fail to control inflammation and require recurrent courses of systemic corticosteroids (SCS) and the need of endoscopic sinus surgery (ESS) [
1]. However, overreliance on systemic corticosteroids comes with short and long term detrimental adverse effects for patients, and ESS fails to resolve type-2 inflammation, the underlying cause of the disease [
3]. Monoclonal antibodies (mAb) have recently emerged as an innovative, personalized treatment that allows refractory patients to restore disease control. These biologic therapies act on blocking key molecules involved in type-2 inflammation, such as IL-4/IL-13 (Dupilumab, anti IL-4r mAb) [
4], IgE (Omalizumab, anti IgE mAb) [
5], TSLP (Tezepelumab, anti TSLP mAb) or IL-5 (Mepolizumab [
6] and long-acting Depemokimab anti IL-5 mAb). Only dupilumab, omalizumab and mepolizumab have been approved for RSCwNP treatment.
In the SYNAPSE study, a 52-week, placebo controlled, phase III randomized clinical trial in severe CRSwNP patients [
6], mepolizumab demonstrated a reduction in endoscopic nasal polyp score (NPS), improvement in patient reported outcomes such as chronic rhinosinusitis quality of life related test Sinonasal Outcome Test-22 items (SNOT-22), nasal congestion and smell impairment in visual analogic scales (VAS), as well as a reduction in the need of ESS and SCS use. The wealth of evidence regarding the effectiveness of these therapies in addressing the most disturbing symptoms reported by patients in clinical settings is scarce and it is mainly focused on subjects with comorbid uncontrolled severe asthma [
7,
8,
9,
10,
11,
12,
13].
The present study aims to evaluate the effectiveness of Mepolizumab in real life settings in a diverse patient population, focusing on assessing the impact of this therapy on patient reported outcomes after six months of treatment.
2. Materials and Methods
2.1. Study Design and Population
This is a multicenter, observational study of CRSwNP patients treated with mepolizumab carried out in five hospitals located in Spain. The inclusion criteria were: adult patients with a diagnosis of uncontrolled CRSwNP despite appropriate medical treatment as per EPOS 2020 guidelines and treated with mepolizumab for the indication of CRSwNP according to disease management guidelines [
1,
14]. Subjects were excluded if they were receiving another concomitant biologic treatment for the same condition. Patients were required to have available clinical records at least 12 months before mepolizumab initiation and at least 6 months after the initiation of treatment. Patients were consecutively included in the study and data was retrospectively collected from electronic clinical records between April and November 2024. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was reviewed and approved by the ethics committee of the principal investigators' institution (Project identification code: MEPO-SINVAL-23) on 2024 april 24
th and was subsequently endorsed by the ethics committees of the collaborating centers before the start of data collection. The exemption from informed consent proposed for this study was approved by the ethics committee of our institutions in accordance with local legislation (Law 14/2007, of July 3, on Biomedical Research (Spanish Biomedical Research Act).
2.2. Clinical Endpoints
The primary endpoint was the change in NPS at six months of mepolizumab therapy compared with baseline values. Each nostril was scored on a scale of 0 to 4, with the total score being the sum of left and right nostril scores (range: 0-8) as evaluated by nasal endoscopy. Secondary endpoints included the change in SNOT-22 sinonasal related quality of life test score, nasal congestion VAS, smell impairment VAS, blood eosinophil count, and nasal polyp tissue eosinophil count, evaluated at six months Vs baseline values respectively. Smell impairment and nasal congestion severity were stratified as follows: mild (VAS ≤3), moderate (VAS >3-≤7) and severe (VAS >7). Prescribed courses of SCS and ESS events indicated for the control of CRSwNP were registered during the data collection period. In patients with concomitant diagnosis of asthma, the change in Asthma Control Test (ACT) score was also assessed. Mepolizumab-related adverse events as per investigator criteria were collected throughout the study. The spanish validated version of the SNOT-22 by De Los Santos et al. was used in this study [
15].
2.3. Statistical Analysis
Descriptive and inferential statistical analyses were performed using different libraries in RStudio software (version ‘2024.12.0.467’) [
16]. Qualitative variables were represented as percentages, and McNemar's Chi-squared test with Yates continuity correction was used to analyze paired nominal data. The quantitative variables were analyzed using a paired sample t-test, and when the assumptions of applicability of this technique were not met, the Wilcoxon signed rank test with continuity correction was used instead. Additionally, to compute the 95% confidence interval of the difference obtained before and after the intervention, the Hodges-Lehmann estimator was calculated.
Finally, to describe the magnitude of the difference or relationship between the means of two paired groups, the effect size was calculated through Cohen’s D with Hedges Correction. It quantifies the standardized difference between the means of two paired groups. It's calculated by dividing the mean difference between the paired observations by the standard deviation of those differences. It indicates the practical significance, or the strength of the relationship observed, independent of the sample size (0.2=Small, 0.5=Medium and 0.8=Large effect).
3. Results
3.1. Baseline Characteristics
A total of 47 patients with a mean age of 54 years were included in the study (
Table 1). Patients had a mean age of 38.4 years when CRSwNP was diagnosed and a mean elapsed time of approximately 15 years from diagnosis to initiation of biologic treatment. ESS was performed in 83% of the subjects. Mean time from the last surgery to the initiation of mepolizumab was around 5 years. Asthma was present in 91% of the cohort, with roughly a similar proportion of subjects classified as having severe and non-severe asthma. 81% required SCS prescription in the 12 months before mepolizumab treatment. The five most burdensome symptoms experienced by the studied cohort, ranked by order based on baseline SNOT-22 frequency of selection, were loss of taste/smell (11,9%), need to blow nose (11,4%), nasal congestion (10,8%), runny nose (8%) and thick nasal discharge (8%) (
Figure S1).
3.2. Nasal Polyp Score
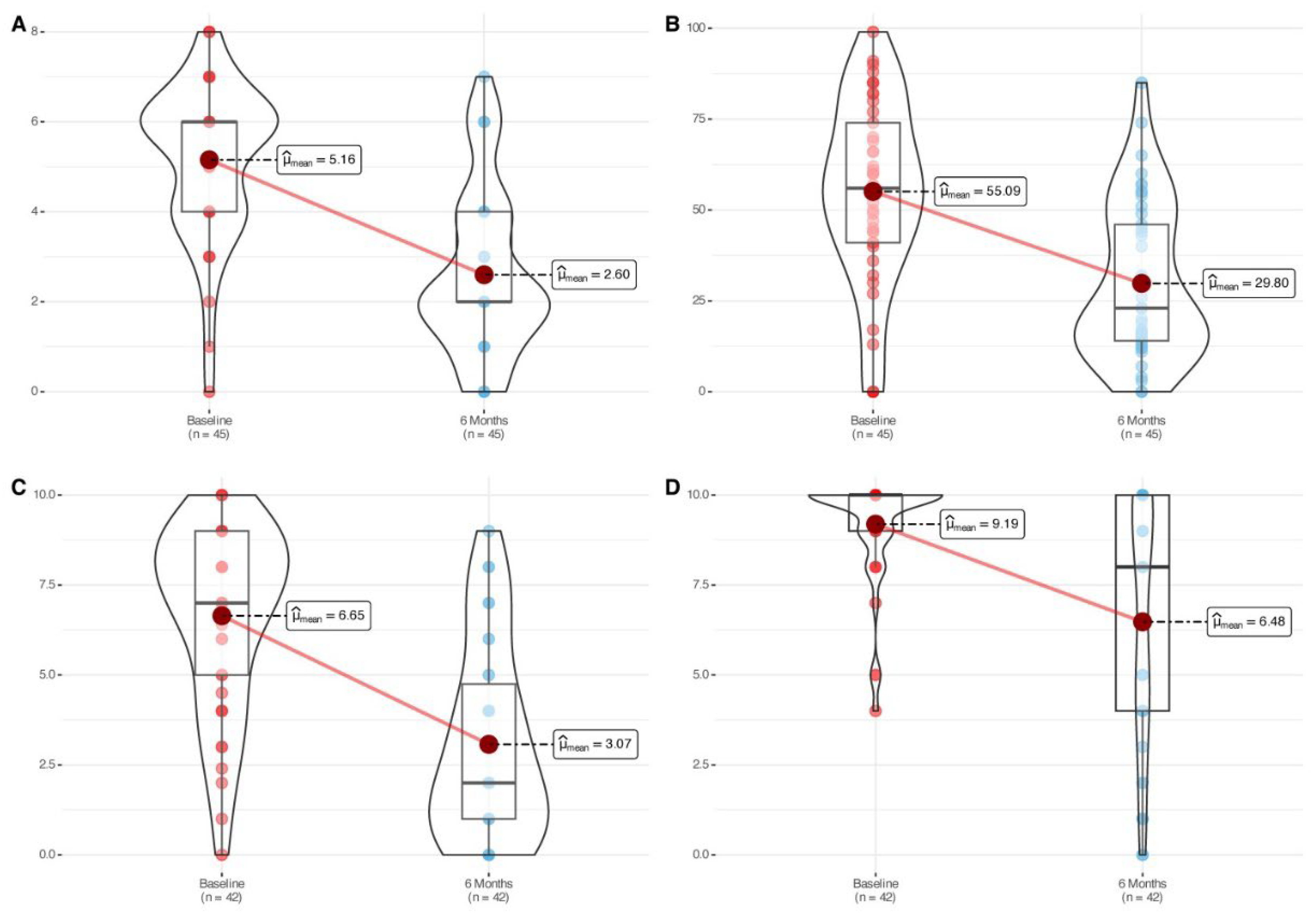
The cohort showed a statistically significant reduction in nasal polyp size (mean change: -2.56 [-3.24, -1.88], p<0.0001) after six months of mepolizumab treatment (
Table 2,
Figure 1). Significant and large-size effect improvements were symmetrically observed in both nostrils (
Figure S2). The proportion of patients achieving an improvement score of ≥1 or ≥2 in total NPS was 85% and 78% respectively, with 43% showing a remarkable improvement of ≥4 points after six months of therapy (
Figure S3). The proportion of patients that improved ≥1 point in nasal polyp score in the left nostril and ≥1 point in the right nostril simultaneously was 66%.
3.3. SNOT-22, Nasal Congestion VAS and Smell Impairment VAS
Mean SNOT-22 score improved 25.29 points after six months of therapy (p<0.0001) (
Table 2,
Figure 1). Around 76% of the patients achieved an improvement above the defined minimal clinically important difference (MCID) threshold for the questionnaire (MCID > 8.9). Assessment of the SNOT-22 by domains also led to significant improvements for each one of the domains (
Table S1,
Figure S4). Interestingly, significant improvements were observed for each one of the items displayed on the SNOT-22, with only one item of the twenty-two assessed (4%) showing a small effect size (
Table S2).
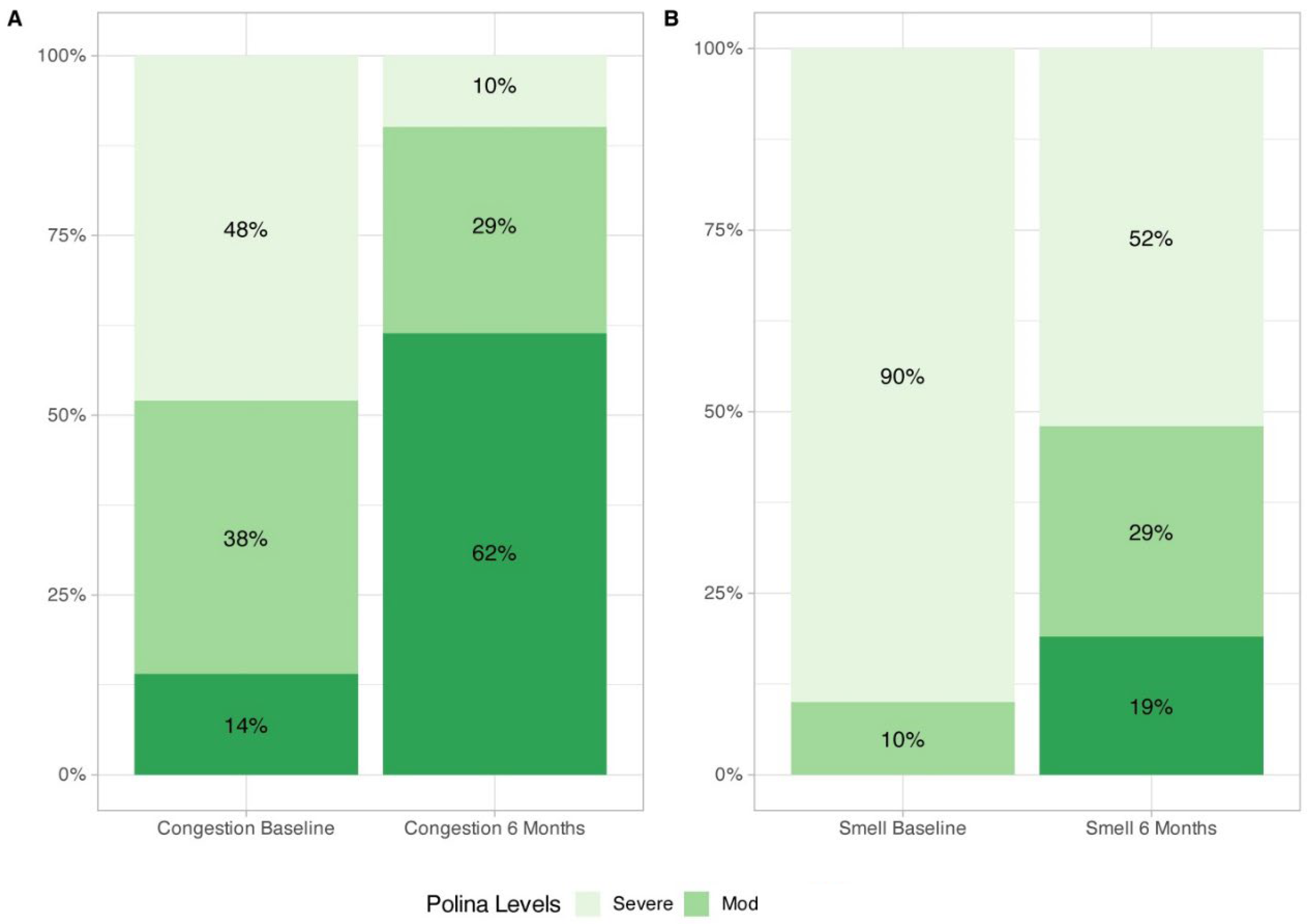
Nasal congestion (-3.57 [-4.50, -2.65], p<0.0001) and smell impairment (-4.0 [-5.50, -3.0], p<0.0001) VAS showed a significant improvement after six months of treatment (
Table 2,
Figure 1), with 90% and 48% of the patients displaying mild/moderate symptoms of congestion and smell impairment respectively after six months of mepolizumab treatment (
Figure 2).
3.4. ESS Events, SCS Intake and Inflammatory Biomarkers
Only one patient (2%) required ESS and 85% subjects did not require SCS prescription for the management of CRSwNP after mepolizumab initiation. Blood and tissue eosinophil median counts showed a significant reduction of 86% and 26% respectively versus baseline (
Table 2).
3.5. Asthma Control
Among those patients with a concomitant diagnosis of asthma, the ACT significantly improved by 8 points, achieving a median value of 24 points (
Table 2). An improvement of at least 3 points, the validated MCID for this test, was achieved by 65% of the subjects with CRSwNP and asthma. At baseline, only 35% of the subjects were classified as controlled (ACT≥20), whereas after six months of mepolizumab treatment this number increased to 84%. No statistically significant differences were observed in ACT improvements between severe versus non-severe asthma patients (
Table S3).
3.6. Safety and Treatment Discontinuation Events
Three patients experienced adverse effects that were resolved spontaneously: one patient reported arthralgia, one patient reported myalgia, and another patient reported mild rhinitis symptoms. Two patients abandoned treatment for reasons different to lack of efficacy or safety: one patient disliked using an injectable chronic treatment and opted out after four months, and another patient suspended treatment after five months as per suggestion by another healthcare professional.
4. Discussion
The findings from this study provide valuable insights into the real-world effectiveness of mepolizumab in a diverse population of patients with CRSwNP. The results demonstrate significant improvements in patient-reported outcomes, nasal polyp score, and asthma control after six months of treatment, as well as in the reduction of blood and tissue eosinophil counts, highlighting the potential of mepolizumab as a therapeutic option for this patient population and expanding on the existent data from randomized controlled studies and previous findings in clinical settings.
To the best of our knowledge this study evaluated, for the first time, every single item on the SNOT-22 as well as the improvement on the SNOT-22 domain scores in clinical settings, offering precise evidence regarding mepolizumab symptom management. A large proportion of patients achieved clinically relevant QoL improvements, and the most burdensome symptoms that worry to patients were among the items that showed the largest improvements after six months of mepolizumab treatment. Improvement of smell impairment and nasal congestion VAS were also consistent with data observed in SNOT-22 scores. It is worth highlighting the reduction on symptom burden according to VAS cut-offs, with 48% and 90% of patients classified as non-severe for smell impairment and congestion symptoms respectively. These data combined suggest that mepolizumab improves patient perception about the burden of the disease and quality of life status. Our results of improvement on total SNOT-22 and smell and congestion VAS are in line with previous evidence in clinical settings by other research groups such as Orlando’s and Cavaliere’s [
10,
11]. However, Dominguez et al. reported a numerically larger mean improvement of 63 points in SNOT-22 [
9]. In this study all patients had severe asthma, whereas in our study the proportion of patients with non-severe asthma was 56%. In some studies, such as that of Farhood et al. [
17], asthma has been reported as a comorbidity that increases SNOT-22 scores, supporting the single airway theory described by Krouse et al [
18]. For this reason, we believe that the coexistence of severe asthma with CRSwNP may have influenced the poorer baseline SNOT-22 score in the population of the study by Dominguez et al. and, at the same time, contributed to a numerically larger improvement following treatment compared to our findings.
The substantial reduction in total NPS after six months of therapy is particularly noteworthy. Data showed that more than 80% of patients experienced at least a 1-point improvement in NPS, considered in the literature as the MCID for this endpoint. As many as 50% of patients achieved even greater reductions beyond 3 points. This study reported also the evaluation of the simultaneous improvement in the left and in the right nostril. In fact, most of the patients that achieved a NPS improvement of at least two points was because of a concomitant, simultaneous reduction of one point in the left nostril and one point in the right nostril. Such simultaneous improvements in both nostrils are likely contributing to the observed large improvements in nasal congestion. Orlando et al showed that around 70% of the patients treated with mepolizumab achieved a NPS improvement of at least two points after one year, whereas the studies from Dominguez and Cavaliere displayed similar NPS reductions to what we have observed, highlighting the consistent benefits of mepolizumab in objectively reducing polyp size in clinical settings [9-11].
Improvements in ACT scores highlight the dual benefit of an anti IL-5 biologic therapy in patients with CRSwNP and comorbid type-2 asthma. Phase III SYNAPSE post-hoc studies evaluating the subgroup of patients with severe CRSwNP and asthma demonstrated a concomitant benefit of this therapy in lower airways [
19]. Those results are corroborated in real world settings, although most of the reports included a large proportion of patients with comorbid severe asthma [9-11]. Our study evaluated a more diverse patient population beyond those with comorbid severe asthma. After six months of mepolizumab initiation, the CRSwNP plus asthma study population group showed a statistically significant and clinically relevant improvement of asthma control, whereas no statistically significant differences were observed in asthma control improvements between both sub-groups, severe and non-severe asthmatics (
Table S3). The systemic and local reduction blood and tissue eosinophil counts are likely playing a key role for the observed benefits in this patient population. Actually, the reported findings may be explained due to the paramount importance of IL-5 in type-2 inflammation, a pleiotropic cytokine that not only plays a role in eosinophil activation and recruitment, but also in increasing mucus thickness, promoting immune disbalance, accelerating nasal polyp recurrence and contributing to tissue remodeling [
20]. Hence, anti IL-5 are suggested to be a suitable treatment to reduce the clinical manifestations derived from Type-2 inflammation in both upper and lower airways [
21].
This piece of research was designed as an observational multicenter retrospective study, yet it has several limitations such as missing data bias due to the nature of the recollection of data and lack of control arm. Also, the relatively short follow-up period of six months may not capture long-term outcomes and potential late-onset adverse events. On the contrary, its multicentric design reinforces the external validity of the data as bias from a monocentric observational design is likely avoided.
Certain specific characteristics of our study population warrant consideration. Notably, the proportion of individuals diagnosed with nonsteroidal anti-inflammatory drug–exacerbated respiratory disease (N-ERD) was relatively high. This can be attributed to the selection criteria for biologic therapy in our healthcare setting, which primarily targets patients with more severe disease phenotypes, specifically those presenting with CRSwNP, asthma, and nonsteroidal anti-inflammatory drugs intolerance.
A distinctive aspect of our national healthcare system is that it remains the only one within our regional context to mandate a minimum of two prior surgical interventions for nasal polyposis as a prerequisite for public reimbursement of biologic therapy. Patients who did not undergo surgery prior to the initiation of mepolizumab treatment were those for whom surgical intervention was contraindicated due to specific clinical considerations. In all such instances, the decision to proceed with biologic therapy without prior surgery was endorsed by the corresponding institutional multidisciplinary committees responsible for evaluating biologic therapies in the context of severe respiratory disease.
5. Conclusions
This study provides compelling evidence for the effectiveness of mepolizumab in managing CRSwNP in a real-world setting. The significant improvements in patient-reported outcomes, nasal polyp score, and asthma control, coupled with a favorable safety profile, support the use of mepolizumab as a valuable treatment option for patients with recalcitrant CRSwNP. Further research is warranted to confirm these findings in a larger population and to explore the long-term impact of mepolizumab on disease management.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Conceptualization, A.G-P. and M.A-C; methodology, A.G-P., M.A-C and J.P-A; software, J.P-A; validation, M.A-C and A.G-P.; investigation, A.G-P., M.A-C, T.P-C, MJ. G-G, E.D-C, F.M-E, N. M-F, J. C-G, C.G-N, L. F-M, F.F-B, A.G-LL, N.M-LL, CH.T, C.Z-R, C.L-V; data curation, A.G-P. and J.P-A; writing—original draft preparation, A.G-P. and M.A-C; writing—review and editing, A.G-P. and M.A-C; supervision A.G-P. and M.A-C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and the protocol was reviewed and approved by the ethics committee of the principal investigators' institution (Project identification code: MEPO-SINVAL-23) on 2024 april 24th and was subsequently endorsed by the ethics committees of the collaborating centers before the start of data collection.
Informed Consent Statement
The exemption from informed consent proposed for this study was approved by the ethics committee of our institutions in accordance with local legislation (Law 14/2007, of July 3, on Biomedical Research (Spanish Biomedical Research Act).
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.
Conflicts of Interest
A.G-P., M.A-C, T.P-C and A.G-LL have received lecture fees and participated in experts’ board meetings of GSK, Novartis, and Sanofi. MJ. G-G, E.D-C, F.M-E, N. M-F, J. C-G, C.G-N, L. F-M, F.F-B, N.M-LL, CH.T, C.Z-R, C.L-V declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CRSwNP |
chronic rhinosinusitis with nasal polyps |
| QoL |
quality of life |
| SCS |
systemic corticosteroids |
| ESS |
endoscopic sinus surgery |
| mAb |
monoclonal antibodies |
| NPS |
nasal polyp score |
| SNOT-22 |
Sinonasal Outcome Test-22 items |
| VAS |
visual analogic scales |
| ACT |
Asthma Control Test |
| MCID |
minimal clinically important difference |
| N-ERD |
nonsteroidal anti-inflammatory drug–exacerbated respiratory disease |
References
- Fokkens WJ, Lund VJ, Hopkins C et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology. 2020 Feb 20;58(Suppl S29):1-464.
- Bachert C, Bhattacharyya N, Desrosiers M, Khan AH. Burden of Disease in Chronic Rhinosinusitis with Nasal Polyps. J Asthma Allergy. 2021 Feb 11;14:127-134. [CrossRef]
- Hellings PW, Alobid I, Anselmo-Lima WT et al. EUFOREA/EPOS2020 statement on the clinical considerations for chronic rhinosinusitis with nasal polyps care. Allergy. 2024 May;79(5):1123-1133. [CrossRef]
- Bachert C, Han JK, Desrosiers M et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet 2019;394:1638-1650. [CrossRef]
- Gevaert P, Omachi TA, Corren J, Mullol J, Han J, Lee SE, et al. Efficacy and safety of omalizumab in nasal polyposis: 2 randomized phase 3 trials. J Allergy Clin Immunol 2020;146:595-605. [CrossRef]
- Han JK, Bachert C, Fokkens W et al. Mepolizumab for chronic rhinosinusitis with nasal polyps (SYNAPSE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med 2021;9:1141-1153. [CrossRef]
- Galletti C, Ciodaro F, Barbieri MA et al. Effectiveness and safety profile of mepolizumab in chronic rhinosinusitis with nasal polyps: Real life data in a tertiary care. Am J Otolaryngol. 2024 Jul-Aug;45(4):104329.
- Galletti C, Sireci F, Stilo G et al. Mepolizumab in chronic rhinosinusitis with nasal polyps: Real life data in a multicentric Sicilian experience. Am J Otolaryngol. 2025 Jan-Feb;46(1):104597.
- Domínguez-Sosa MS, Cabrera-Ramírez MS, Marrero-Ramos MDC et al. Real-Life Effectiveness of Mepolizumab in Refractory Chronic Rhinosinusitis with Nasal Polyps. Biomedicines. 2023 Feb 8;11(2):485. [CrossRef]
- Orlando P, Vivarelli E, Minzoni A et al. Effects of Mepolizumab in the treatment of type 2 CRSwNP: a real-life clinical study. Eur Arch Otorhinolaryngol. 2025 Jan;282(1):265-272. [CrossRef]
- Cavaliere C, Loperfido A, Ciofalo A et al. Real-Life Evidence of Mepolizumab Treatment in Chronic Rhinosinusitis with Nasal Polyps: A Multicentric Study. J Clin Med. 2024 Jun 18;13(12):3575. [CrossRef]
- Armengot-Carceller M, Gómez-Gómez MJ, García-Navalón C et al. Effects of Omalizumab Treatment in Patients With Recalcitrant Nasal Polyposis and Mild Asthma: A Multicenter Retrospective Study. Am J Rhinol Allergy. 2021 Jul;35(4):516-524. [CrossRef]
- De Corso E, Pasquini E, Trimarchi M et al. Dupireal Italian Study Group. Dupilumab in the treatment of severe uncontrolled chronic rhinosinusitis with nasal polyps (CRSwNP): A multicentric observational Phase IV real-life study (DUPIREAL). Allergy. 2023 Oct;78(10):2669-2683.
- Alobid I, Colás C, Castillo JA et al. POLINA group. Spanish Consensus on the Management of Chronic Rhinosinusitis With Nasal Polyps (POLIposis NAsal/POLINA 2.0). J Investig Allergol Clin Immunol. 2023 Oct 16;33(5):317-331.
- De los Santos G, Reyes P, del Castillo R et al. Cross-cultural adaptation and validation of the sino-nasal outcome test (SNOT-22) for Spanish-speaking patients. Eur Arch Otorhinolaryngol. 2015 Nov;272(11):3335-3340. [CrossRef]
- RStudio: Integrated Development Environment for R. Posit Software, PBC, Boston, MA. URL http://www.posit.co/.
- Farhood Z, Schlosser RJ, Pearse ME et al. Twenty-two-item Sino-Nasal Outcome Test in a control population: a cross-sectional study and systematic review. Int Forum Allergy Rhinol. 2016;6:271-277.
- Krouse JH, Brown RW, Fineman SM et al. Asthma and the unified airway. Otolaryngol Head Neck Surg. 2007;136(5 Suppl):S75-S106. [CrossRef]
- Mullol J, Backer V, Constantinidis J et al. Global airway disease: mepolizumab simultaneously improves outcomes in severe CRSwNP and asthma. Rhinology. 2025 Feb 1;63(1):113-115. [CrossRef]
- AbuJabal R, Ramakrishnan RK, Bajbouj K et al. Role of IL-5 in asthma and airway remodelling. Clin Exp Allergy. 2024 Aug;54(8):538-549. [CrossRef]
- Gevaert P, Han JK, Smith SG et al. The roles of eosinophils and interleukin-5 in the pathophysiology of chronic rhinosinusitis with nasal polyps. Int Forum Allergy Rhinol. 2022 Nov;12(11):1413-1423. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).