Submitted:
04 August 2025
Posted:
06 August 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Obesity Model Induction Results
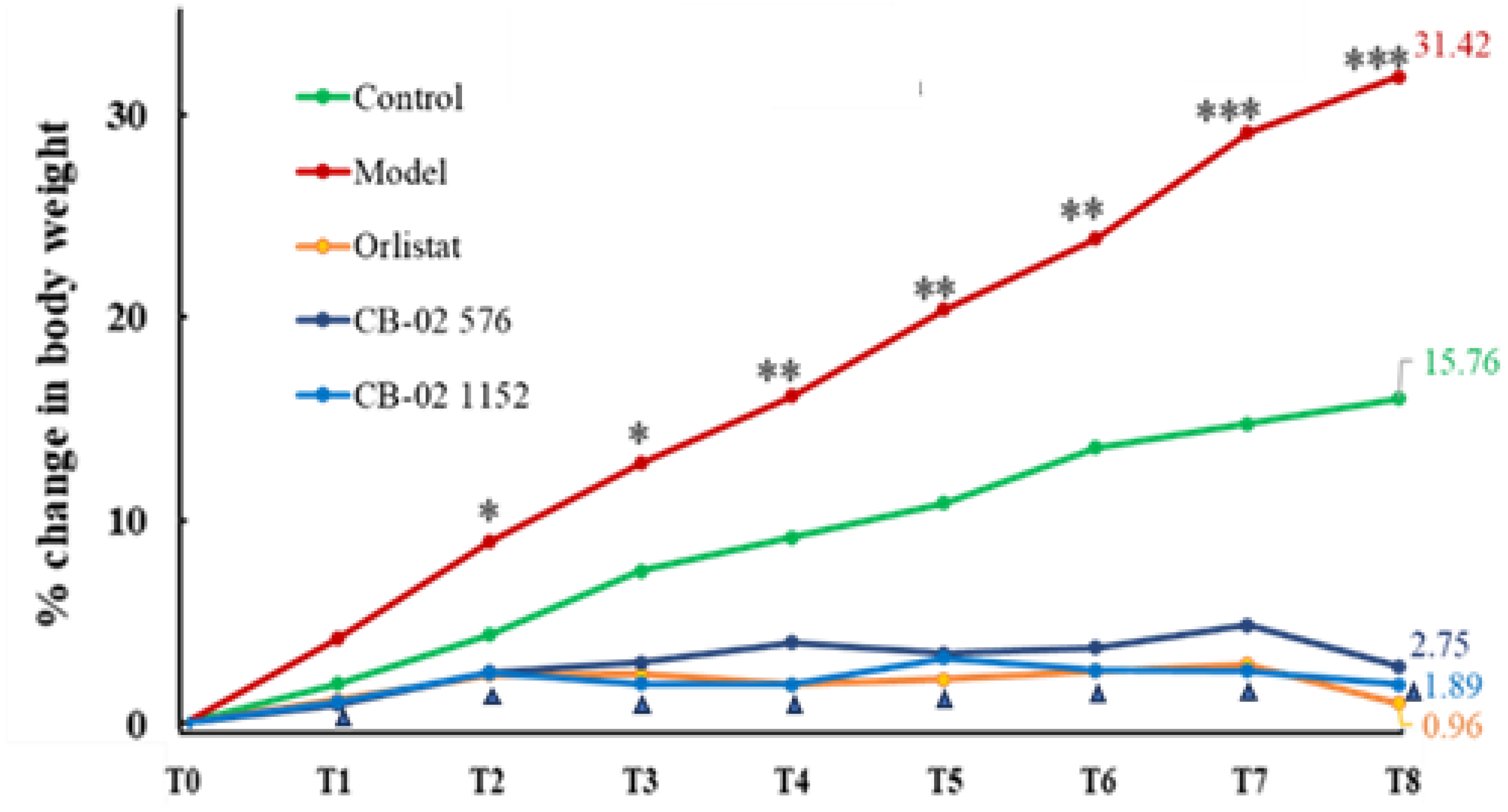
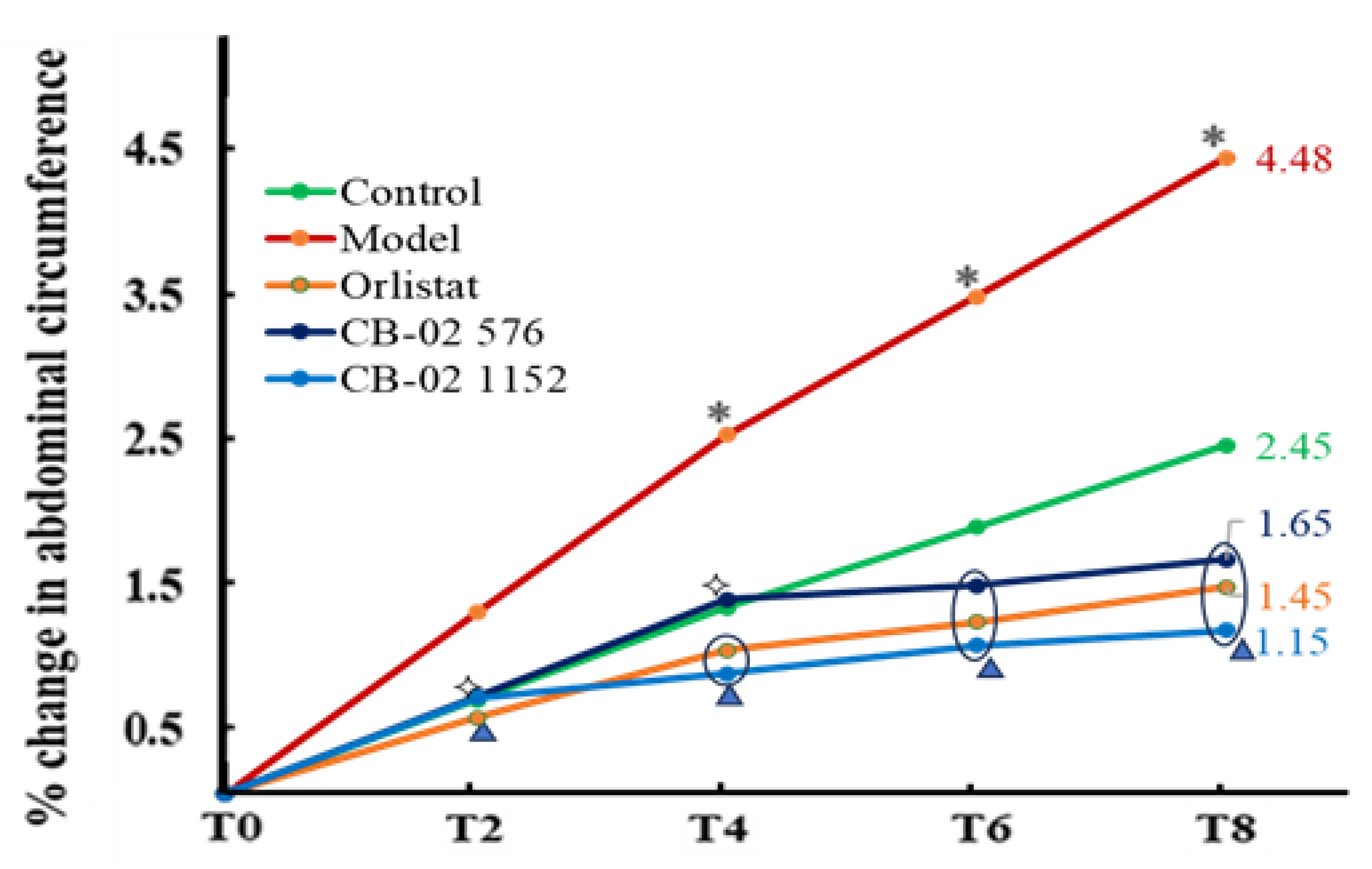
2.2. Effects of CB-02 on BW, AC, and Lee Obesity Index
2.3. Effects of CB-02 on Organ and Visceral Fat Weights
2.4. Effects of CB-02 on Blood Lipid Parameters
2.5. Effects of CB-02 on Blood Glucose Levels and Insulin Resistance
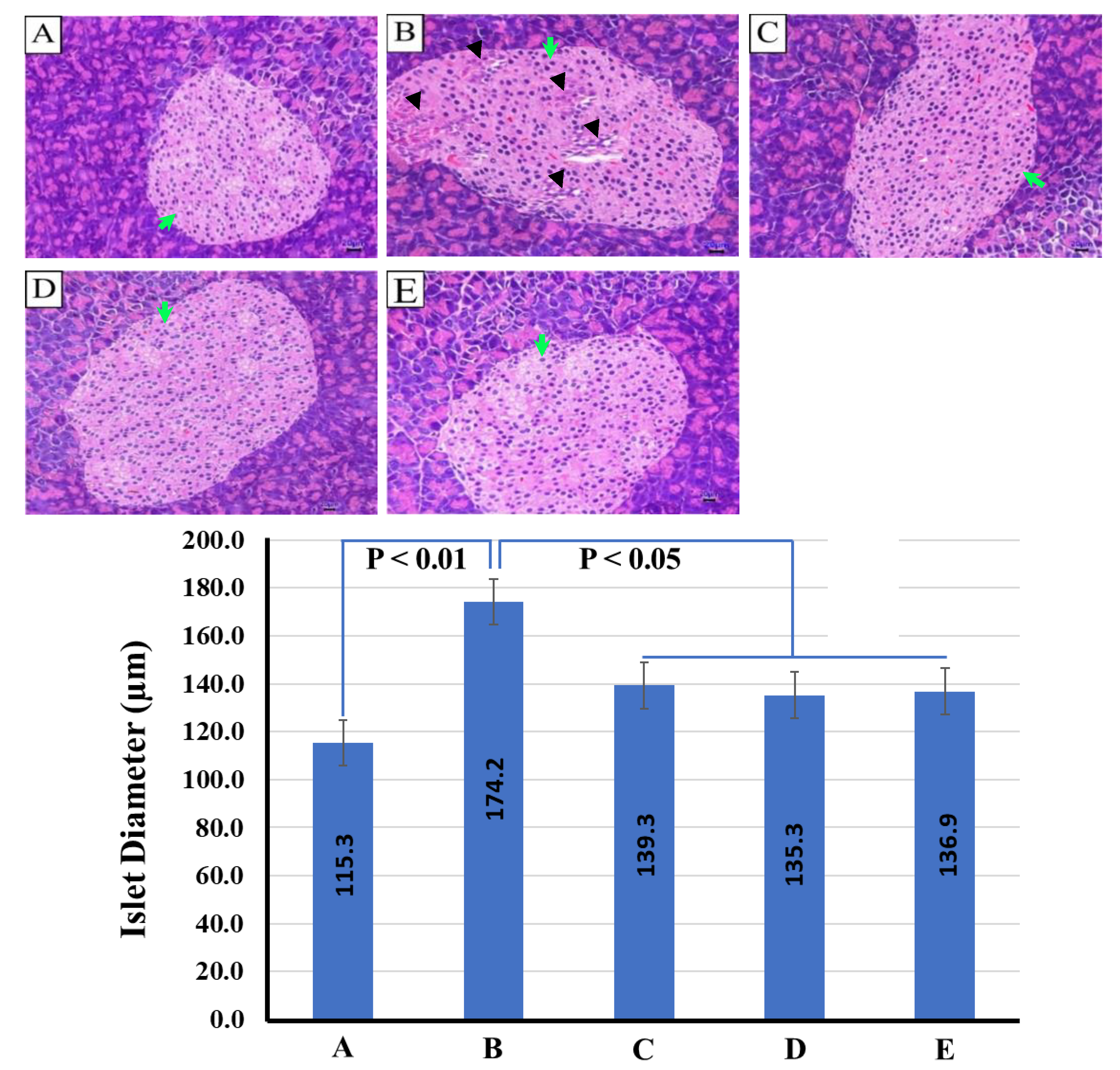
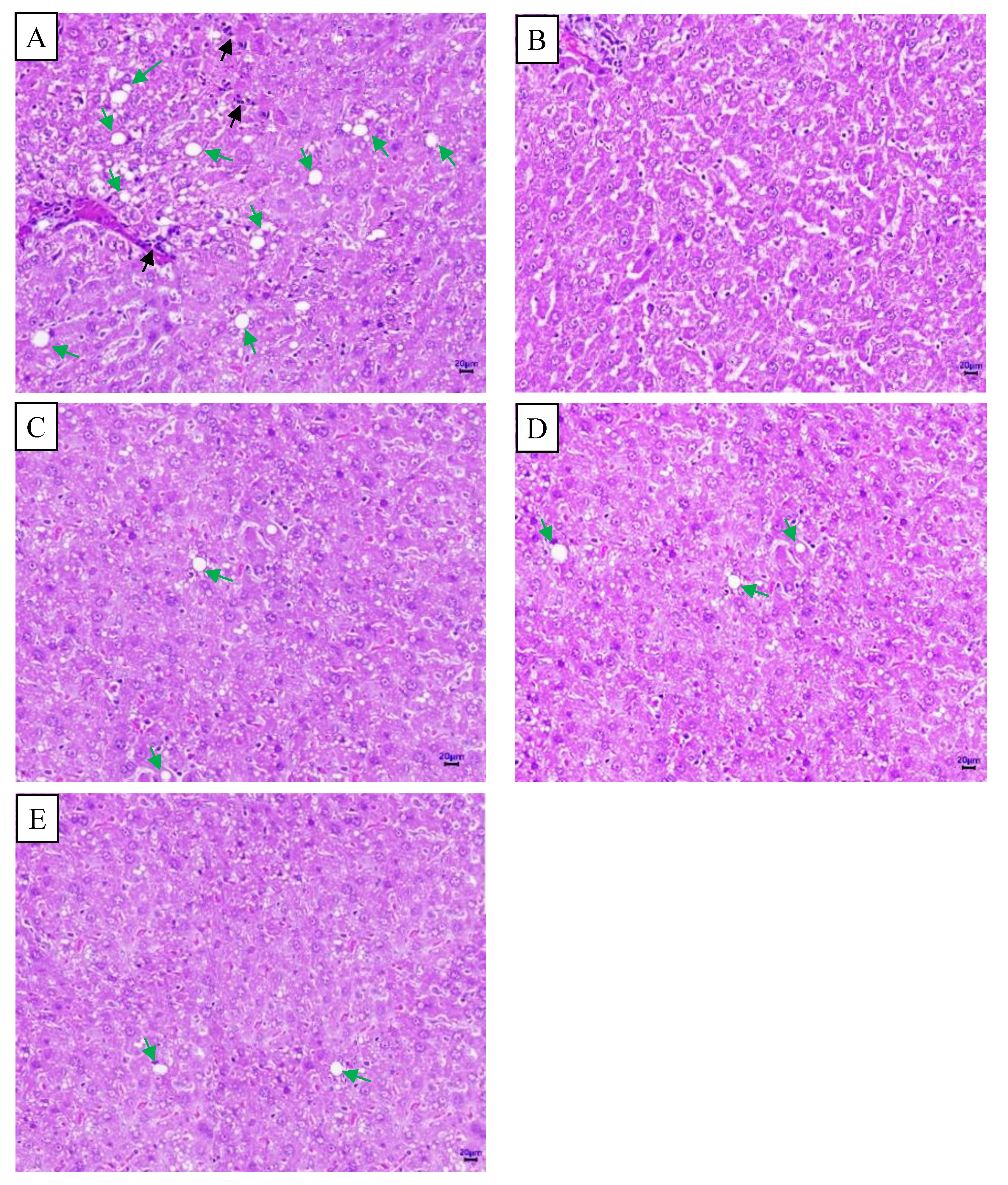
2.6. Histopathological Images of the Pancreas and Liver from Mice in Different Experimental Groups
3. Discussion
4. Materials and Methods
4.1. CB-02 Capsules
4.2. Animals
4.3. Equipment and Reagents
4.4. In Vivo Test
4.4.1. Phase 1 – Induction of Obesity Model
4.4.2. Phase 2 – Main Experiment
- + Physiological control group (G1): Healthy mice fed a standard diet and administered distilled water orally at a dose of 10 mL/kg.
- + Obese model group (G2): Obese mice continued the HFD and received distilled water at a dose of 10 mL/kg.
- + Positive control group (G3, reference drug): Obese mice continued the HFD and were treated with orlistat at 60 mg/kg/day.
- + CB-02 treatment group 1 (G4): Obese mice continued the HFD and were administered CB-02 suspension at 576 mg/kg/day.
- + CB-02 treatment group 2 (G5): Obese mice continued the HFD and were administered CB-02 suspension at 1152 mg/kg/day.
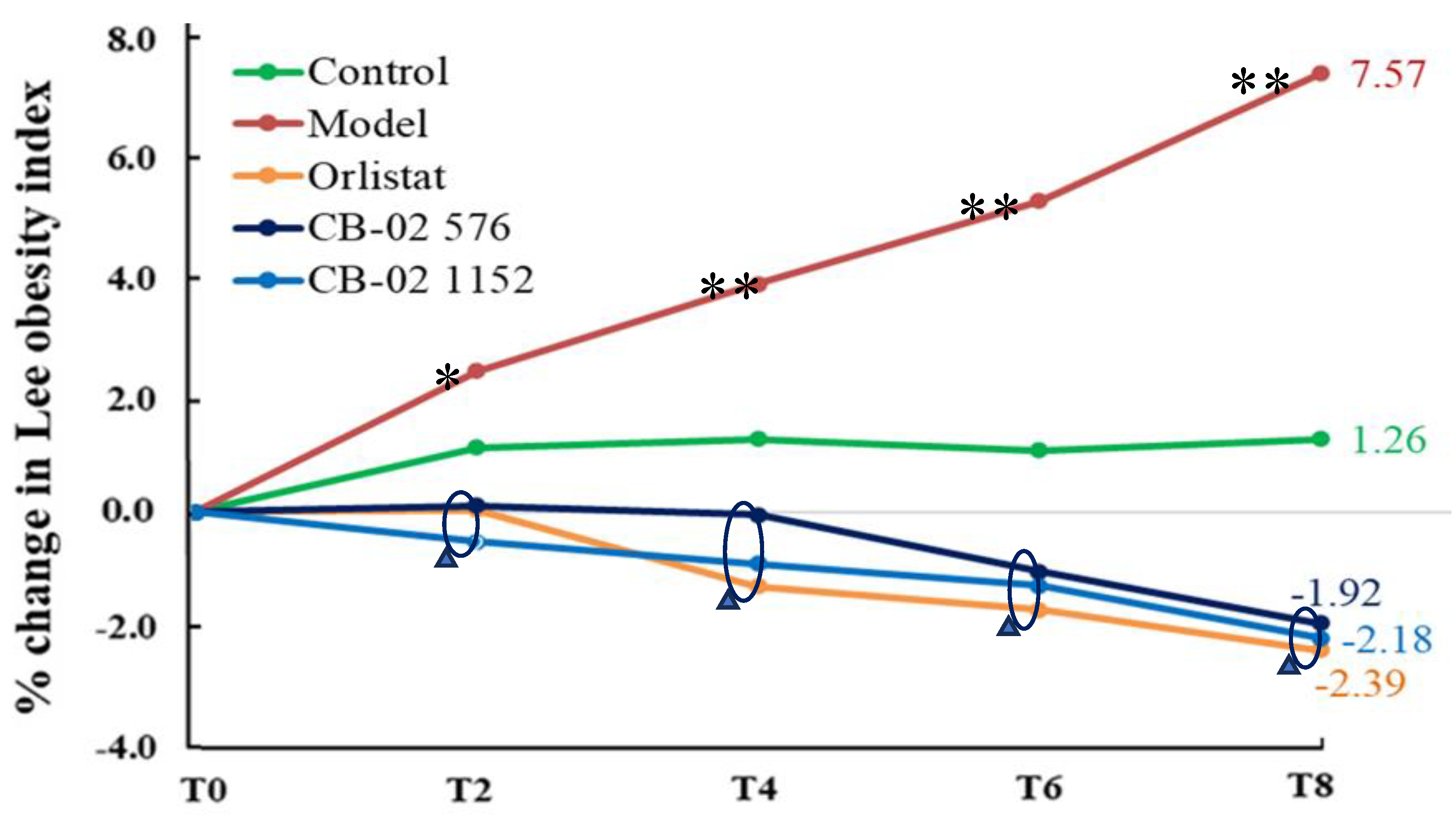
4.5. Statistical Analysis
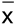 ± SD). One-way analysis of variance (ANOVA) was used to compare means across three or more groups. A post-hoc test was performed using Tukey's post-hoc test. A p-value of less than 0.05 was considered statistically significant.
± SD). One-way analysis of variance (ANOVA) was used to compare means across three or more groups. A post-hoc test was performed using Tukey's post-hoc test. A p-value of less than 0.05 was considered statistically significant.5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Abad-Jiménez, Z.; Vezza, T. Obesity: A global health challenge demanding urgent action. Biomedicines 2025, 13, 502. [Google Scholar] [CrossRef]
- Dhondge, R.H.; Agrawal, S.; Patil, R.; Kadu, A.; Kothari, M. A comprehensive review of metabolic syndrome and its role in cardiovascular disease and type 2 diabetes mellitus: Mechanisms, risk factors, and management. Cureus 2024, 16, e67428. [Google Scholar] [CrossRef]
- Bays, H.E.; Kirkpatrick, C.F.; Maki, K.C.; Toth, P.P.; Morgan, R.T.; Tondt, J.; Christensen, S.M.; Dixon, D.J.; Jacobson, T.A. . Obesity, dyslipidemia, and cardiovascular disease: A joint expert review from the Obesity Medicine Association and the National Lipid Association 2024. J. Clin. Lipidol. 2024, 18, e320–e350. [Google Scholar] [CrossRef] [PubMed]
- Shaik Mohamed Sayed, U,F. ; Moshawih, S.; Goh, H.P.; Kifli, N.; Gupta, G.; Singh, S.K.; Chellappan, D.K.; Dua, K.; Hermansyah, A.; Der, H.L.; Ming, L.C.; Goh, B.H. Natural products as novel anti-obesity agents: insights into mechanisms of action and potential for therapeutic management. Front. Pharmacol. 2023, 14, 1182937. [Google Scholar]
- Thaipitakwong, T.; Aramwit, P. A review of the efficacy, safety, and clinical implications of naturally derived dietary supplements for dyslipidemia. Am. J. Cardiovasc. Drugs 2017, 17, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.Y.; Chen, S.Y.; Lin, J.A.; Chen, Y.Y.; Chen, Y.Y.; Liu, Y.C.; Yen, G.C. Phyllanthus emblica fruit improves obesity by reducing appetite and enhancing mucosal homeostasis via the gut microbiota-brain-liver axis in HFD-induced leptin-resistant rats. J. Agric. Food Chem. 2024, 72, 10406–10419. [Google Scholar] [CrossRef]
- Chung, S.; Park, S.H.; Park, J.H.; Hwang, J.T. Anti-obesity effects of medicinal plants from Asian countries and related molecular mechanisms: a review. Rev. Cardiovasc. Med. 2021, 22, 1279–1293. [Google Scholar] [CrossRef]
- Hsu, Y.H.; Chen, S.Y.; Chenm, J.D.; Yen, G.C. Phyllanthus emblica L. polysaccharides mitigate obesity via modulation of lipid metabolism and gut microbiota in high-fat diet-fed mice. Food Funct. 2025, 16, 5008–5028. [Google Scholar] [CrossRef]
- Usharani, P.; Merugu, P.; Nutalapati, C. Evaluation of the effects of a standardized aqueous extract of Phyllanthus emblica fruits on endothelial dysfunction, oxidative stress, systemic inflammation and lipid profile in subjects with metabolic syndrome: a randomised, double blind, placebo controlled clinical study. BMC Complement. Altern. Med. 2019, 19, 97. [Google Scholar] [CrossRef]
- Lee, H.S.; Lim, S.M.; Jung, J.I.; Kim, S.M.; Lee, J.K.; Kim, Y.H.; Cha, K.M.; Oh, T.K.; Moon, J.M.; Kim, T.Y.; Kim, E.J. Gynostemma pentaphyllum extract ameliorates high-fat diet-induced obesity in C57BL/6N mice by upregulating SIRT1. Nutrients 2019, 11, 2475. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Xie, J.B.; Xiao, M.Y.; Guo, M.; Qi, Y.S.; Li, F.F.; Piao, X.L. Liver lipidomics analysis reveals the anti-obesity and lipid-lowering effects of gypnosides from heat-processed Gynostemma pentaphyllum in high-fat diet fed mice. Phytomedicine 2023, 115, 154834. [Google Scholar] [CrossRef]
- Qu, J.; Tan, S.; Xie, X.; Wu, W.; Zhu, H.; Li, H.; Liao, X.; Wang, J.; Zhou, Z.A.; Huang, S.; Lu, Q. Dendrobium officinale polysaccharide attenuates insulin resistance and abnormal lipid metabolism in obese mice. Front. Pharmacol. 2021, 12, 659626. [Google Scholar] [CrossRef]
- Zhou, R.; Wang, Y.; Chen, S.; Cheng, F.; Yi, Y.; Lv, C.; Qin, S. Anti-Inflammatory effect of Dendrobium officinale extract on high-fat diet-induced obesity in rats: Involvement of gut microbiota, liver transcriptomics, and NF-κB/IκB pathway. Antioxidants (Basel) 2025, 14, 432. [Google Scholar] [CrossRef]
- Martins, T.; Ferreira, T.; Nascimento-Gonçalves, E.; Castro-Ribeiro, C.; Lemos, S.; Rosa, E.; Antunes, L,M. ; Oliveira, P.A. Obesity rodent models applied to research with food products and natural compounds. Obesities 2022, 2, 171–204. [Google Scholar] [CrossRef]
- Johnston, S.L.; Souter, D.M.; Tolkamp, B.J.; Gordon, I.J.; Illius, A.W.; Kyriazakis, I.; Speakman, J.R. Intake compensates for resting metabolic rate variation in female C57BL/6J mice fed high-fat diets. Obesity (Silver Spring) 2007, 15, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Lutz, T.A.; Woods, S.C. Overview of animal models of obesity. Curr. Protoc. Pharmacol. 2012; 5.61.1–5.61.18. [Google Scholar]
- Preguiça, I.; Alves, A.; Nunes, S.; Fernades, R.; Gomes, P.; Viana, S.D.; Reis, F. Diet-induced rodent models of obesity-related metabolic disorders - A guide to a translational perspective. Obes. Rev. 2020, 21, e13081. [Google Scholar] [CrossRef] [PubMed]
- Bastías-Pérez, M.; Dolors, S.; Herrero, L. Dietary options for rodents in the study of obesity. Nutrients, 2020; 12, 3234. [Google Scholar]
- de Moura e Dias, M.; dos Reis, S.A.; da Conceição, L.L.; de Oliveira Sediyama, C.M.N.; Pereira, S.S.; de Oliveira, L.L.; do Carmo Gouveia Peluzio, M.; Martinez, J.A.; Milagro, F.I. Diet-induced obesity in animal models: points to consider and influence on metabolic markers, Diabetol. Metab. Syndr. 2021, 13, 32. [Google Scholar] [CrossRef] [PubMed]
- Department of Science, Technology and Training, Ministry of Health. Decision No. 2892/QD-BYT Decision on promulgating the professional document "Guidelines for diagnosis and treatment of obesity". [Online]. 2022 [cited 2025 Mar 14]. Available from: https://emohbackup.moh.gov.vn/publish/home?documentId=8975.
- Kamiji, M.M.; Inui, A. Neuropeptide Y receptor selective ligands in the treatment of obesity. Endocr. Rev. 2007, 28, 664–684. [Google Scholar] [CrossRef]
- Tak, Y.J.; Lee, S.Y. Long-term efficacy and safety of anti-obesity treatment: Where Do We Stand? Curr. Obes. Rep.
- Daneschvar, H.L.; Aronson, M.D.; Smetana, G.W. FDA-approved anti-obesity frugs in the United States. Am. J. Med. 2016, 129, 879.e1–879.e6. [Google Scholar] [CrossRef]
- Gou, X.; Ding, Y.; Wu, Y.; Tao, Y.; Wang, Y.; Wang, Y.; Liu, J.; Ma, M.; Zhou, X.; Nhamdriel, T.; Fan, G. Functional effects and mechanisms of Phyllanthus emblica fruit and gallic acid on metabolic diseases: Experimental evidence and clinical perspectives. Food Biosci. 2024, 59, 104039. [Google Scholar] [CrossRef]
- Lin, H.Y.; Lin, C.H.; Kuo, Y.H.; Shih, C.C. Antidiabetic and antihyperlipidemic activities and molecular mechanisms of Phyllanthus emblica L. extract in mice on a high-fat diet. Curr. Issues Mol. Biol. 2024, 46, 10492–10529. [Google Scholar] [CrossRef]
- Yeo, J.; Kang, Y.J.; Jeon, S.M.; Jung, U.J.; Lee, M.K.; Song, H.; Choi, M.S. Potential hypoglycemic effect of an ethanol extract of Gynostemma pentaphyllum in C57BL/KsJ-db/db mice. J. Med. Food. 2008, 11, 709–716. [Google Scholar] [CrossRef]
- Oskouei, Z.; Ghasemzadeh Rahbardar, M.; Hosseinzadeh, H. The effects of Dendrobium species on the metabolic syndrome: A review. Iran. J. Basic Med. Sci. 2023, 26, 738–752. [Google Scholar]
- Jiang, W.; Tan, J.; Zhang, J.; Deng, X.; He, X.; Zhang, J.; Liu, T.; Sun, R.; Sun, M.; Chen, K.; Xu, T.; Yan, Y.; Moazzami, A.; Wu, E.J.; Zhan, J.; Hu, B. Polysaccharides from Dendrobium officinale improve obesity-induced insulin resistance through the gut microbiota and the SOCS3-mediated insulin receptor substrate-1 signaling pathway. J. Sci. Food Agric. 2024, 104, 3437–3447. [Google Scholar] [CrossRef]
- Lempesis, I.G.; Tsilingiris, D.; Liu, J.; Dalamaga, M. Of mice and men: Considerations on adipose tissue physiology in animal models of obesity and human studies. Metabol. Open 2022, 15, 100208. [Google Scholar] [CrossRef]
- Kong, Y.; Yang, H.; Nie, R.; Zhang, X.; Zuo, F.; Zhang, H.; Nian, X. Obesity: pathophysiology and therapeutic interventions. Mol. Biomed. 2025, 6, 25. [Google Scholar] [CrossRef]
- Basil, B.; Myke-Mbata, B.K.; Eze, O.E.; Akubue, A.U. From adiposity to steatosis: metabolic dysfunction-associated steatotic liver disease, a hepatic expression of metabolic syndrome - current insights and future directions. Clin. Diabetes Endocrinol. 2024, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- Zaromytidou, M.; Savvatis, K. The weight of obesity in hypertrophic cardiomyopathy. Clin. Med. 2023, 23, 357–363. [Google Scholar] [CrossRef] [PubMed]
- León-Román, J.; López-Martínez, M.; Esteves, A.; Ciudin, A.; Núñez-Delgado, S.; Álvarez, T.; Lecube, A.; Rico-Fontalvo, J.; Soler, M.J. Obesity-related kidney disease: A growing threat to renal health. Int. J. Mol. Sci. 2025, 26, 6641. [Google Scholar] [CrossRef] [PubMed]
- Brummer, C.; Singer, K.; Renner, K.; Bruss, C.; Hellerbrand, C.; Dorn, C.; Reichelt-Wurm, S.; Gronwald, W.; Pukrop, T.; Herr, W.; Banas, M.; Kreutz, M. The spleen-liver axis supports obesity-induced systemic and fatty liver inflammation via MDSC and NKT cell enrichment. Mol. Cell Endocrinol. 2025, 601, 112518. [Google Scholar] [CrossRef]
- Dludla, P.V.; Mabhida, S.E.; Ziqubu, K.; Nkambule, B.B.; Mazibuko-Mbeje, S.E.; Hanser, S.; Basson, A.K.; Pheiffer, C.; Kengne, A.P. Pancreatic β-cell dysfunction in type 2 diabetes: Implications of inflammation and oxidative stress. World J. Diabetes 2023, 14, 130–146. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Hu, Y.; Sun, Y.A.; Shi, J.Q.; Xu, J. High-fat diet caused renal damage in ApoE-/- mice via the activation of RAGE-mediated inflammation. Toxicol. Res. 2025, 10, 1171–1176. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Luo, H.T.; Pei, W.J.; Xiao, M.Y.; Li, F.F.; Gu, Y.L.; Piao, X.L. Saponins derived from Gynostemma pentaphyllum regulate triglyceride and cholesterol metabolism and the mechanisms: A review. J. Ethnopharmacol. 2024, 319, 117186. [Google Scholar] [CrossRef]
- Zou, J.; Song, Q.; Shaw, P.C.; Zuo, Z. Dendrobium officinale regulate lipid metabolism in diabetic mouse liver via PPAR-RXR signaling pathway: Evidence from an integrated multi-omics analysis. Biomed. Pharmacother. 2024, 173, 116395. [Google Scholar] [CrossRef] [PubMed]
- Della Vedova, M.C.; Muñoz, M.D.; Santillan, L.D.; Plateo-Pignatari, M.G.; Germanó, M.J.; Rinaldi Tosi, M.E.; Garcia, S.; Gomez, N.N.; Fornes, M.W.; Gomez Mejiba, S.E.; Ramirez, D.C. A mouse model of diet-induced obesity resembling most features of human metabolic syndrome. Nutr. Metab. Insights 2016, 9, 93–102. [Google Scholar] [CrossRef]
- Wongwananuruk, T.; Prasongvej, P.; Chantrapanichkul, P.; Indhavivadhana, S.; Tanmahasamut, P.; Rattanachaiyanont, M.; Techatraisak, K.; Angsuwathana, S. Measures of serum markers HbA1c, HOMA-IR, HOMA-β, QUICKI and G/I ratio as predictors of abnormal glucose tolerance among Thai women with polycystic ovary syndrome. J. Clin. Med. 2025, 14, 1452. [Google Scholar] [CrossRef]
- Lin, H.Y.; Lin, C.H.; Kuo, Y.H.; Shih, C. C. Antidiabetic and antihyperlipidemic activities and molecular mechanisms of Phyllanthus emblica L. extract in mice on a high-fat diet. Curr. Issues Mol. Biol. 2024, 46, 10492–10529. [Google Scholar] [CrossRef]
- Sultana, Z.; Jami, S.; Ali, E.; Begum, M.; Haque, M. Investigation of antidiabetic effect of ethanolic extract of Phyllanthus emblica Linn. fruits in experimental animal models. Pharmacol. Phar. 2014, 5, 11–18. [Google Scholar] [CrossRef]
- Yeo, J.; Kang, Y.J.; Jeon, S.M.; Jung, U.J.; Lee, M.K.; Song, H.; Choi, M.S. Potential hypoglycemic effect of an ethanol extract of Gynostemma pentaphyllum in C57BL/KsJ-db/db mice. J. Med. Food 2008, 11, 709–716. [Google Scholar] [CrossRef]
- Gao, D.; Zhao, M.; Qi, X.; Liu, Y.; Li, N.; Liu, Z.; Bian, Y. Hypoglycemic effect of Gynostemma pentaphyllum saponins by enhancing the Nrf2 signaling pathway in STZ-inducing diabetic rats. Arch. Pharm. Res. 2016; 39, 221–230. [Google Scholar]
- Wang, Z.; Zhao, X.; Liu, X.; Lu, W.; Jia, S.; Hong, T.; Li, R.; Zhang, H.; Peng, L.; Zhan, X. Anti-diabetic activity evaluation of a polysaccharide extracted from Gynostemma pentaphyllum. Int. J. Biol. Macromol. 2019, 126, 209–214. [Google Scholar] [CrossRef]
- Huyen, V.T.T.; Phan, D.V.; Thang, P.; Ky, P.T. Hoa, N.K.; Ostenson, C.G. Antidiabetic effect of Gynostemma pentaphyllum tea in randomly assigned type 2 diabetic patients. Evid. Based Complement. Alternat. Med. 2012, 2012, 452313. [Google Scholar] [CrossRef]
- Hei, J.W.; Hung, W.; Yanbo, Z.; Zhang, Z.J. Dendrobium in diabetes: A comprehensive review of its phytochemistry, pharmacology, and safety. J. Transl. Sci. 2021, 7, 1–10. [Google Scholar] [CrossRef]
- Chen, W.; Lu, J.; Zhang, J.; Wu, J.; Yu, L.; Qin, L.; Zhu, B. Traditional uses, phytochemistry, pharmacology, and quality control of Dendrobium officinale Kimura et. Migo. Front. Pharmacol. 2011, 12, 726528. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.O. Determination of the surface area of the white rat with its application to the expression of metabolic results. Am. J. Physiol. 1929, 89, 24–33. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef] [PubMed]
| Group | n |
Research Indicators at T0 ( ± SD) ± SD)
|
|||
| BW (g) | Nose to anus length (cm) | AC (mm) | Lee obesity index | ||
| G1 (1) | 10 | 22.02 ± 2.10 | 9.69 ± 0.32 | 69.50 ± 1.15 | 292.07 ± 5.47 |
| G2 (2) | 10 | 33.93 ± 4.85 | 9.89 ± 0.34 | 81.35 ± 2.64 | 334.11 ± 8.98 |
| G3 (3) | 10 | 34.38 ± 4.49 | 9.85 ± 0.39 | 81.72 ± 2.68 | 337.10 ± 6.86 |
| G4 (4) | 10 | 32.67 ± 3.58 | 9.79 ± 0.33 | 82.02 ± 1.10 | 332.64 ± 3.61 |
| G5 (5) | 10 | 33.06 ± 4.46 | 9.82 ± 0.36 | 82.44 ± 1.19 | 331.97 ± 4.30 |
| p2,3,4,5-1 | < 0.001 | > 0.05 | < 0.001 | < 0.001 | |
| Group | n |
Research Indicators at T8( ± SD) ± SD)
|
||
| BW (g) | AC (mm) | Lee obesity index | ||
| G1 (1) | 10 | 25.79 ± 2.22 | 71.19 ± 0.67 | 292.66 ± 4.59 |
| G2 (2) | 10 | 44.49 ± 5.71 | 84.96 ± 1.77 | 351.36 ± 10.13 |
| G3 (3) | 10 | 34.58 ± 3.49 | 82.50 ± 2.55 | 321.70 ± 6.96 |
| G4 (4) | 10 | 33.58 ± 4.26 | 83.27 ± 1.16 | 319.89 ± 5.78 |
| G5 (5) | 10 | 33.67 ± 4.44 | 82.98 ± 1.29 | 319.12 ± 5.40 |
| p2,3,4,5-1 p3,4,5-2 p4,5-3 p5-4 |
< 0.001 | |||
| < 0.001 | < 0.05 | < 0.001 | ||
| > 0.05 | ||||
| Group | n |
Organweights(g)(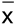 ± SD) ± SD)
|
||||
| Heart | Liver | Kidneys | Spleen | Pancreas | ||
| G1 (1) | 10 | 0.125 ± 0.020 | 1.119 ± 0.183 | 0.241 ± 0.027 | 0.083 ± 0.013 | 0.228 ± 0.027 |
| G2 (2) | 10 | 0.255 ± 0.024 | 2.495 ± 0.227 | 0.521 ± 0.054 | 0.193 ± 0.032 | 0.349 ± 0.035 |
| G3 (3) | 10 | 0.171 ± 0.018 | 1.647 ± 0.260 | 0.343 ± 0.048 | 0.119 ± 0.031 | 0.276 ± 0.039 |
| G4 (4) | 10 | 0.170 ± 0.021 | 1.626 ± 0.141 | 0.348 ± 0.050 | 0.123 ± 0.023 | 0.281 ± 0.041 |
| G5 (5) | 10 | 0.163 ± 0.022 | 1.584 ± 0.237 | 0.339 ± 0.056 | 0.116 ± 0.025 | 0.286 ± 0.036 |
| p2-1 | < 0.001 | |||||
| p3,4,5-2 | ||||||
| p3,4,5-1 | < 0.001 | < 0.01 | ||||
| p4,5-3 | > 0.05 | |||||
| p5-4 | ||||||
| Group | n |
Relative organto BWs (%)(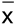 ± SD) ± SD)
|
|||||
| Heart | Liver | Kidneys | Spleen | Pancreas | |||
| G1 (1) | 10 | 0.484 ± 0.070 | 4.339 ± 0.624 | 0.943 ± 0.145 | 0.323 ± 0.062 | 0.885 ± 0.104 | |
| G2 (2) | 10 | 0.579 ± 0.073 | 5.712 ± 1.003 | 1.192 ± 0.211 | 0.445 ± 0.115 | 0.792 ± 0.101 | |
| G3 (3) | 10 | 0.498 ± 0.066 | 4.803 ± 0.862 | 1.002 ± 0.171 | 0.346 ± 0.097 | 0.799 ± 0.096 | |
| G4 (4) | 10 | 0.510 ± 0.058 | 4.891 ± 0.619 | 1.037 ± 0.088 | 0.368 ± 0.070 | 0.840 ± 0.097 | |
| G5 (5) | 10 | 0.491 ± 0.091 | 4.755 ± 0.792 | 1.013 ± 0.144 | 0.350 ± 0.090 | 0.855 ± 0.116 | |
| p2-1 | < 0.01 | > 0.05 | |||||
| p3,4,5-2 | < 0.05 | > 0.05 | |||||
| p3,4,5-1 | > 0.05 | ||||||
| p4,5-3 | |||||||
| p5-4 | |||||||
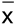 ± SD).
± SD).| Group | n | Visceral fat weights (mg) | Relative visceral fatto BWs (%) | ||||
| Mesenteric arteries fat | Retroperitoneal fat | Epididymal fat | Mesenteric arteries fat | Retroperitoneal fat | Epididymal fat | ||
| G1 (1) | 10 | 228.38 ± 46.39 | 192.29 ± 55.10 | 424.69 ± 70.21 | 0.890 ± 0.188 | 0.749 ± 0.208 | 1.650 ± 0.267 |
| G2 (2) | 10 | 766.22 ± 83.26 | 510.06 ± 92.23 | 1050.26 ± 104.47 | 1.743 ± 0.258 | 1.165 ± 0.264 | 2.403 ± 0.425 |
| G3 (3) | 10 | 410.44 ± 69.73 | 297.84 ± 79.29 | 619.53 ± 96.74 | 1.189 ± 0.178 | 0.876 ± 0.284 | 1.818 ± 0.381 |
| G4 (4) | 10 | 426.88 ± 81.83 | 309.01 ± 96.90 | 629.56 ± 83.15 | 1.278 ± 0.239 | 0.914 ± 0.249 | 1.886 ± 0.243 |
| G5 (5) | 10 | 393.38 ± 76.77 | 287.31 ± 81.85 | 616.11 ± 99.40 | 1.175 ± 0.221 | 0.863 ± 0.273 | 1.853 ± 0.335 |
| p2-1 | < 0.001 | < 0.01 | < 0.001 | ||||
| p3,4,5-2 | < 0.05 | < 0.01 | |||||
| p3,4,5-1 | < 0.001 | < 0.01 | < 0.001 | < 0.01 | > 0.05 | ||
| p4,5-3 | > 0.05 | ||||||
| p5-4 | |||||||
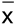 ± SD).
± SD).| Group | n | Blood lipid indices (mmol/L) | ||||
| TC | TG | HDL-C | LDL-C | Non-HDL-C | ||
| G1 (1) | 10 | 3.22 ± 0.50 | 0.76 ± 0.12 | 1.58 ± 0.19 | 1.29 ± 0.56 | 1.63 ± 0.61 |
| G2 (2) | 10 | 6.46 ± 0.86 | 1.79 ± 0.32 | 1.01 ± 0.16 | 4.63 ± 0.67 | 5.45 ± 0.81 |
| G3 (3) | 10 | 4.45 ± 0.85 | 1.12 ± 0.20 | 1.46 ± 0.21 | 2.49 ± 0.78 | 2.99 ± 0.86 |
| G4 (4) | 10 | 4.85 ± 0.69 | 1.27 ± 0.18 | 1.39 ± 0.25 | 2.88 ± 0.71 | 3.46 ± 0.79 |
| G5 (5) | 10 | 4.54 ± 0.63 | 1.16 ± 0.17 | 1.49 ± 0.23 | 2.52 ± 0.68 | 3.05 ± 0.75 |
| p2-1 | < 0.001 | < 0.01 | ||||
| p3,4,5-2 | < 0.001 | |||||
| p3,4,5-1 | < 0.001 | < 0.01 | < 0.001 | < 0.01 | > 0.05 | |
| p4,5-3 | > 0.05 | |||||
| p5-4 | ||||||
 ± SD).
± SD).| Group | n |
Blood glucose (mg/dL) |
Serum insulin (µIU/mL) |
Insulin resistance indices | |||
| QUICKI | HOMA-IR | HOMA-β | |||||
| G1 (1) | 10 | 96.40 ± 9.32 | 3.22 ± 0.67 | 0.404 ± 0.020 | 0.77 ± 0.22 | 35.91 ± 7.76 | |
| G2 (2) | 10 | 179.34 ± 24.68 | 7.54 ± 0.95 | 0.320 ± 0.009 | 3.35 ± 0.65 | 24.14 ± 4.89 | |
| G3 (3) | 10 | 127.76 ± 8.51 | 6.19 ± 0.87 | 0.346 ± 0.010 | 1.96 ± 0.37 | 34.64 ± 4.28 | |
| G4 (4) | 10 | 115.18 ± 10.60 | 6.23 ± 0.61 | 0.351 ± 0.008 | 1.77 ± 0.26 | 44.29 ± 8.37 | |
| G5 (5) | 10 | 111.06 ± 15.67 | 6.28 ± 0.89 | 0.353 ± 0.014 | 1.74 ± 0.42 | 50.81 ± 13.92 | |
| p2-1 | < 0.001 | ||||||
| p3,4,5-2 | < 0.001 | < 0.01 | < 0.001 | < 0.001 | < 0.001 | ||
| p3,4,5-1 | p3-1 < 0.001; p4-1 < 0.01; p5-1 < 0.05 | < 0.001 | p3-1 > 0.05; p4-1 < 0.05; p5-1 < 0.01 | ||||
| p4,5-3 | < 0.01 | > 0.05 | > 0.05 | > 0.05 | < 0.01 | ||
| p5-4 | > 0.05 | ||||||
| Nutrient | Energy (Kcal) | Percentage (%) |
| Protein | 641.0 | 14.26 |
| Carbohydrat | 1693.4 | 37.67 |
| Fat | 2161.4 | 48.08 |
| Total | 4495.8 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





