1. Introduction
Among neurodegenerative diseases, Alzheimer’s disease (AD) is the most common form of dementia, characterized by loss of memory, thinking, reasoning, and behavioral changes in the elderly population, and it is a gradually advancing condition inducing pathological alterations in the brain many years prior to the manifestation of clinical symptoms (1–3). AD stands as the prevailing neurodegenerative condition, characterized by an ongoing deterioration of cognitive functions and identifiable by pathological indicators such as the formation of senile plaques and neurofibrillary tangles within the brain (3). The World AD 2018 report highlights a concerning statistic, revealing that a new dementia patient is born every 3 seconds globally (4). AD dementia ranks as the sixth leading cause of death in the United States, claiming the fifth position among individuals aged 65 and older. The current count of individuals affected by AD dementia is 5.8 million, and without effective interventions, this figure is projected to surge to 13.8 million by 2050 (5). The collective occurrence of Alzheimer's dementia is projected to increase from approximately 5% at age 70 to 50% at age 90, signifying its prevalence as a highly common disease (6). Initially, there was an assumption that AD was a rare condition, and subsequently, it came to be regarded as an inevitable outcome of aging. The stigma associated with aging and various other factors hindered proactive research and treatment for individuals with AD. However, these misconceptions are gradually diminishing, and while initial treatments may have had modest efficacy, there is a growing availability of interventions (7).
The precise cause of AD remains undiscovered. Late-onset AD, the most prevalent form of the disease, typically manifests in individuals aged 65 and above. Although the exact etiology is unknown, various risk factors have been identified, including age, female gender, lower educational and occupational attainment, prior head injuries, sleep disorders, estrogen replacement therapy, and hypertension (8). The less common form of the disease, referred to as early-onset AD, emerges in individuals aged thirty to sixty-five years. This condition is attributed to genetic mutations in Amyloid Precursor Protein (APP), Presenilin-1 (PS1), and Presenilin-2 (PS2) proteins, which are subcomponents of γ-secretase (8,9). In addition to the factors mentioned earlier, other potential risk factors associated with the development of AD include cardiovascular diseases, cerebrovascular diseases, diabetes mellitus, overweight and obesity, elevated cholesterol levels, smoking, high alcohol intake, a diet rich in saturated fats, and depression (8,10). As of now, a cure for AD continues to elude us, and the pursuit of effective disease-modifying treatments proves to be a challenging endeavor. Scientists are faced with the intricate task of unraveling the complex mechanisms of neurodegeneration (11,12).
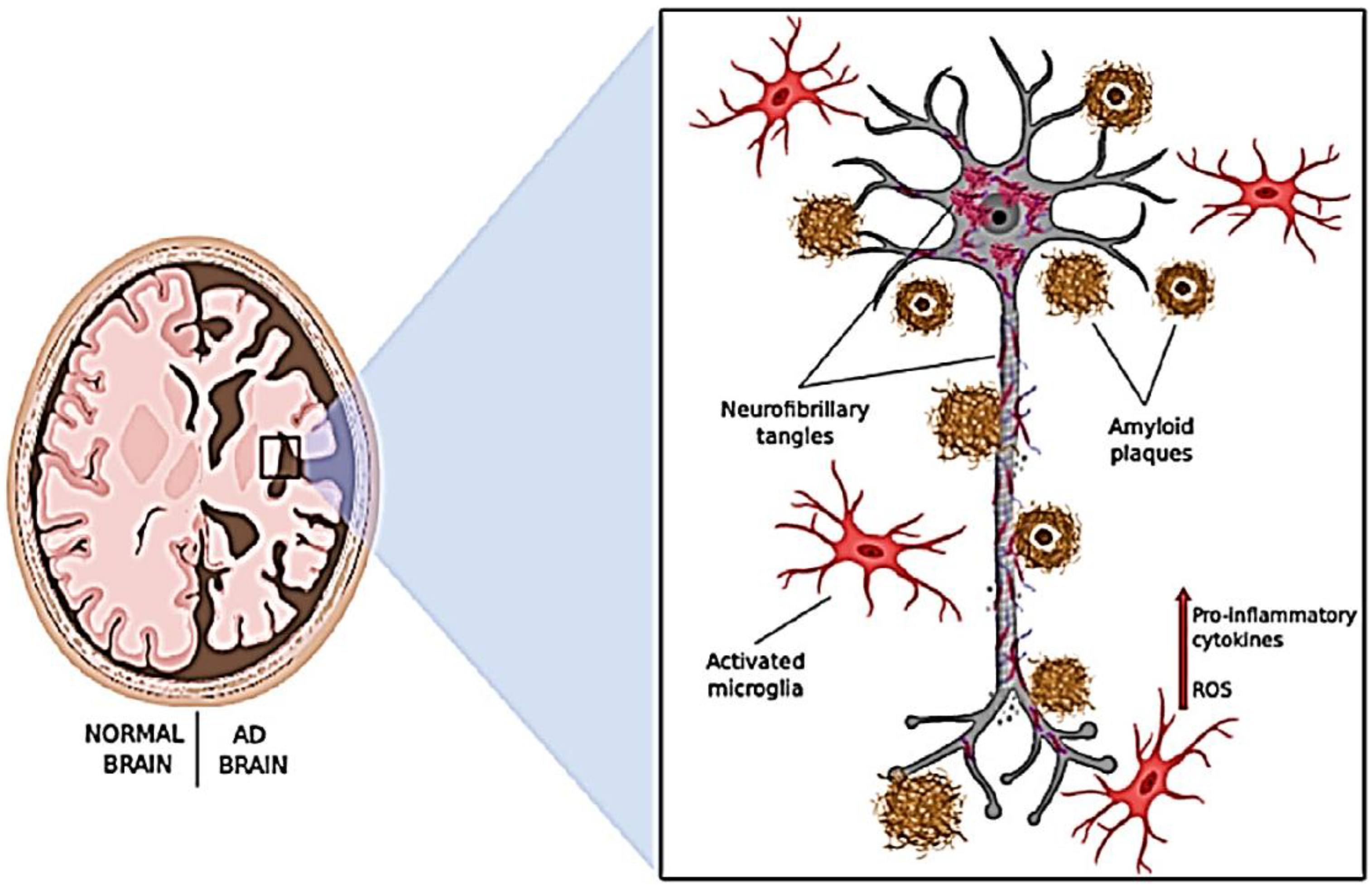
Histopathologically, AD is characterized by two common types of prominent hallmarks, one is substantial brain atrophy with the deposition of amyloid plaques containing amyloid beta (Aβ); and another is the presence of neurofibrillary tangles (NFTs) consisting of hyper phosphorylated tau protein associated with microtubules (14) (
Figure 1). In recent decades, the dominant "Amyloid Cascade Hypothesis" has been undergoing a gradual evolution. This hypothesis suggests that the accumulation of toxic β-amyloid plaques initiates a cascade of events, including the development of neurofibrillary tau tangles, cellular oxidative stress, vascular damage, neuro-inflammatory distress, and ultimately, neurodegeneration. However, the presence of β-amyloid plaques in individuals with normal cognitive profiles and the lack of success of anti-amyloid treatments in preventing AD-related dementia in clinical trials have raised doubts about the validity of the amyloid hypothesis (11,15). The tau hypothesis is closely interconnected with the amyloid hypothesis, where the latter proposes that the pathological sequences in AD involve Aβ deposition, tau phosphorylation, NFT formation, and neuronal death. However, the unsuccessful outcomes of anti-amyloid therapy have prompted numerous researchers to question whether Aβ is essential for tau neurotoxicity. This has raised inquiries about whether AD pathogenesis might be driven by tau independently of Aβ (16).
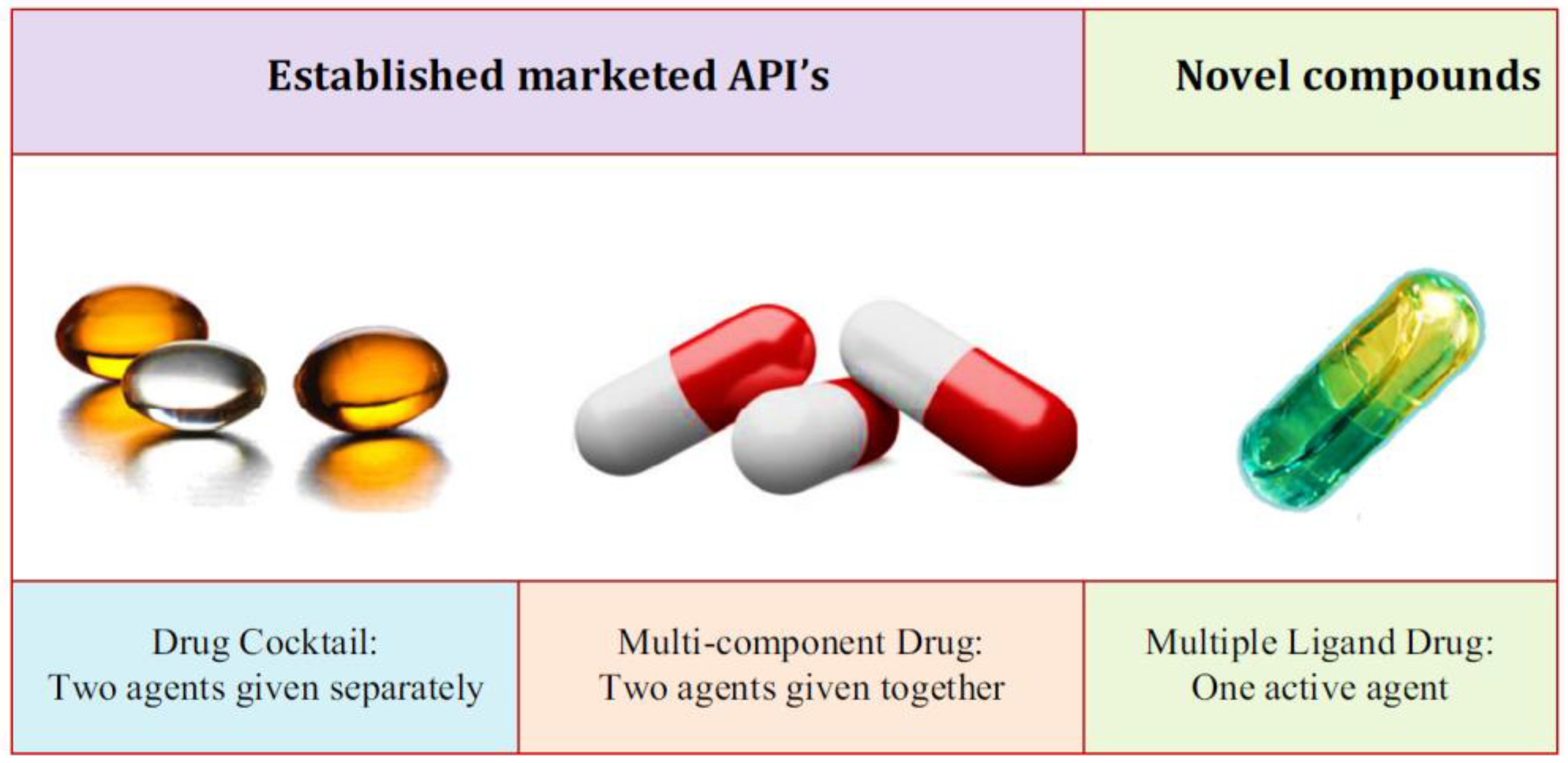
A prominent feature of AD is the accumulation of both Aß peptide and tau protein in the brain. These substances not only induce toxicity through self-aggregation but also elicit more potent toxicity through the synergistic interaction of Aß and tau (18). The hypothesis of one target, one ligand, falls short in offering a comprehensive solution to AD, given the multifactorial nature of the disease. Similarly, relying on one target for one drug proves inadequate in delivering an improved treatment for AD. Furthermore, the existing treatments are constrained, and many ongoing clinical trials focus on modulating a single target. Consequently, the contemporary research landscape in AD drug discovery is transitioning towards a novel approach. This approach aims to provide a more effective solution by simultaneously modulating multiple targets in the neurodegenerative cascade (19,20). There has been a shift towards an innovative approach utilizing the "multi-target-directed ligands" (MTDLs) strategy in the quest for potential anti-AD drugs (
Figure 2). This strategy involves the design of a singular compound capable of concurrently regulating various targets linked to AD (21,22).
The realm of in silico drug discovery, employing advanced computational methods, has evolved into a potent instrument for uncovering innovative drug candidates and fine-tuning their characteristics (23). This approach stands poised to expedite the drug discovery trajectory, significantly diminishing the time and resources conventionally demanded by experimental methodologies. Through in silico modeling and simulations, a profound understanding of the intricate interactions between potential drug compounds and their target proteins can be attained. This predictive capability not only allows for the assessment of therapeutic potential but also facilitates the optimization of efficacy and safety profiles, thereby contributing to the advancement of precision medicine (23,24). In light of the evolving landscape of AD research and the potential of in silico drug discovery, the current study aims to target both Aß peptide and tau protein (25,26).
In previous investigations, several compounds have emerged as promising candidates for their dual-targeting potential against amyloid beta and tau proteins, two hallmark features in the pathogenesis of AD (27–29). Notably, curcumin, glycitein, kaempferol, squalene, β-sitosterol and astaxanthin have been identified through rigorous in silico computational analyses (30–34). These compounds have exhibited the ability to interact with both amyloid beta and tau, as evident by their structural and molecular characteristics. The collective findings from these studies lay the groundwork for our research, providing a curated selection of compounds with demonstrated potential against key AD-associated proteins. In this study, we aim to contribute a novel dimension to the existing body of knowledge by employing a combinatorial approach. By combining these identified compounds using merging, fusion, and conjugation (linked) strategies, our investigation seeks to explore the synergistic effects and enhanced efficacy of these compound combinations against both tau and amyloid beta targets.
Innovative drug development strategies have transcended traditional single-target approaches, embracing the potential of Multi-Target Combination Compound (MTCC) design. This model shift encompasses diverse methodologies, including conjugation, fused hybrids, and merged hybrids. Conjugates involve linking pharmacophores through metabolically stable functional groups absent in the individual compounds. Fused hybrids employ shorter linkers, positioning pharmacophores in close proximity. Merged hybrids simplify molecular structures by integrating commonalities from parent molecules. Such hybrid concepts have demonstrated promise, particularly in antimalarial drug development, where researchers aim to synthesize effective yet safe compounds to combat resistant malaria parasites (16,23,35,36). This innovative approach seeks to maximize therapeutic efficacy while mitigating potential adverse effects. This section explores the versatility of these strategies, drawing inspiration from the progress made in antimalarial pharmacotherapy and multi-target drug discovery (35).
The evolution of drug development strategies from traditional, single-target approaches to the innovative realm of MTCC design reflects a dynamic shift in the pursuit of more effective and versatile therapeutics (
Figure 3). The exploration of linking, fused hybrids, and merged hybrids introduces a palette of creative techniques, each offering unique advantages in enhancing drug effectiveness. Our aim is to unravel new possibilities for AD treatment and detect which combination compounds offer the most promising efficacy against AD. By combining the best aspects of different compounds, we hope to contribute to finding effective solutions for the complex challenges posed by AD. Through this combinatorial model, we aspire to introduce innovative strategies in AD drug development, aiming for improved therapeutic outcomes in the pursuit of effective treatment modalities for this debilitating neurodegenerative disorder.
2. Significance
AD remains a devastating neurological disorder with no absolute cure. Current single-target therapies face limitations in efficacy and often target only one aspect of the complex pathology, leaving the other unchecked. This research, focusing on the development of synergistic Multi-Target Combination Compounds (MTCCs) through innovative merging, fusing, and linking strategies, addresses these challenges and holds significant promise for improving AD treatment. As AD continues to present a growing global challenges, this study seeks to extend the scope beyond conventional therapies. This innovative approach not only contributes to the progress of AD drug development but also aligns with precision medicine through the application of in silico methodologies. By addressing inherent challenges, validating candidates in preclinical models, and enhancing our understanding of AD holistically, our research positions itself at the forefront of transformative interventions. This work holds promise for the development of treatments that are not only more effective but also precisely targeted.
3. Hypothesis
The compounds curcumin, glycitein, kaempferol, squalene, astaxanthin, and β-sitosterol, previously recognized for their individual efficacy against single targets, amyloid beta, tau and other targets. We hypothesize that the combination of six distinct compounds through innovative merging, fusion, and linking strategies will lead to synergistic enhancement of therapeutic potential. By employing these strategies, we aim to combine all six compounds, subsequently evaluating and identifying the specific combination that demonstrates the highest efficacy against both amyloid beta and tau. This research seeks to unveil a novel and potent MTCC design, thereby advancing our understanding of effective strategies in AD therapeutics. Through the creation of a MTCC design, we also propose that the resulting synergy will not only surpass the efficacy of individual compounds but also present a novel avenue for addressing the intricate pathophysiology of AD. This hypothesis is underpinned by the understanding that a comprehensive, multi-target approach holds promise for achieving more substantial therapeutic outcomes than traditional single-target and single-compound interventions. The exploration of compound combinations aims to contribute to the advancement of innovative strategies in AD drug development and foster a nuanced understanding of the potential benefits derived from combined compound interventions.
4. Aims and Objectives
The aim of this research is to identify an optimal compound combination that demonstrates the highest efficacy against both amyloid beta and tau proteins, with the overarching goal of contributing to the development of targeted therapeutic interventions for neurodegenerative disorders. Through an in-depth investigation, this study aims to determine whether these combination strategies exhibit greater efficacy against amyloid beta and tau compared to single-target interventions, contributing to the refinement of AD therapeutics. To achieve this aim, following objectives have been determined,
Evaluate the effectiveness of curcumin, glycitein, kaempferol, squalene, astaxanthin, and β-sitosterol individually against amyloid beta and tau using in silico methods.
Investigate the synergistic potential of MTCC design by combining identified compounds using merging, fusion, and linking strategies.
Analyze the interactions between MTCC designs and amyloid beta, tau proteins from the Protein Data Bank (PDB), focusing on binding kinetics and structural modulations.
Prioritize the most promising MTCC candidates based on their predicted efficacy, safety, and pharmacokinetic profiles, using in silico pharmacodynamics modeling and simulation methods.
Compare the efficacy of MTCC designs against amyloid beta aggregation and tau pathology, benchmarking results with individual compound assessments.
Identify potential challenges and limitations of MTCC design and application in AD, paving the way for further advancements and refinements of these promising therapeutic strategies.
5. Methodology
5.1. Compound Selection: Collection and Preparation of Ligand Structures
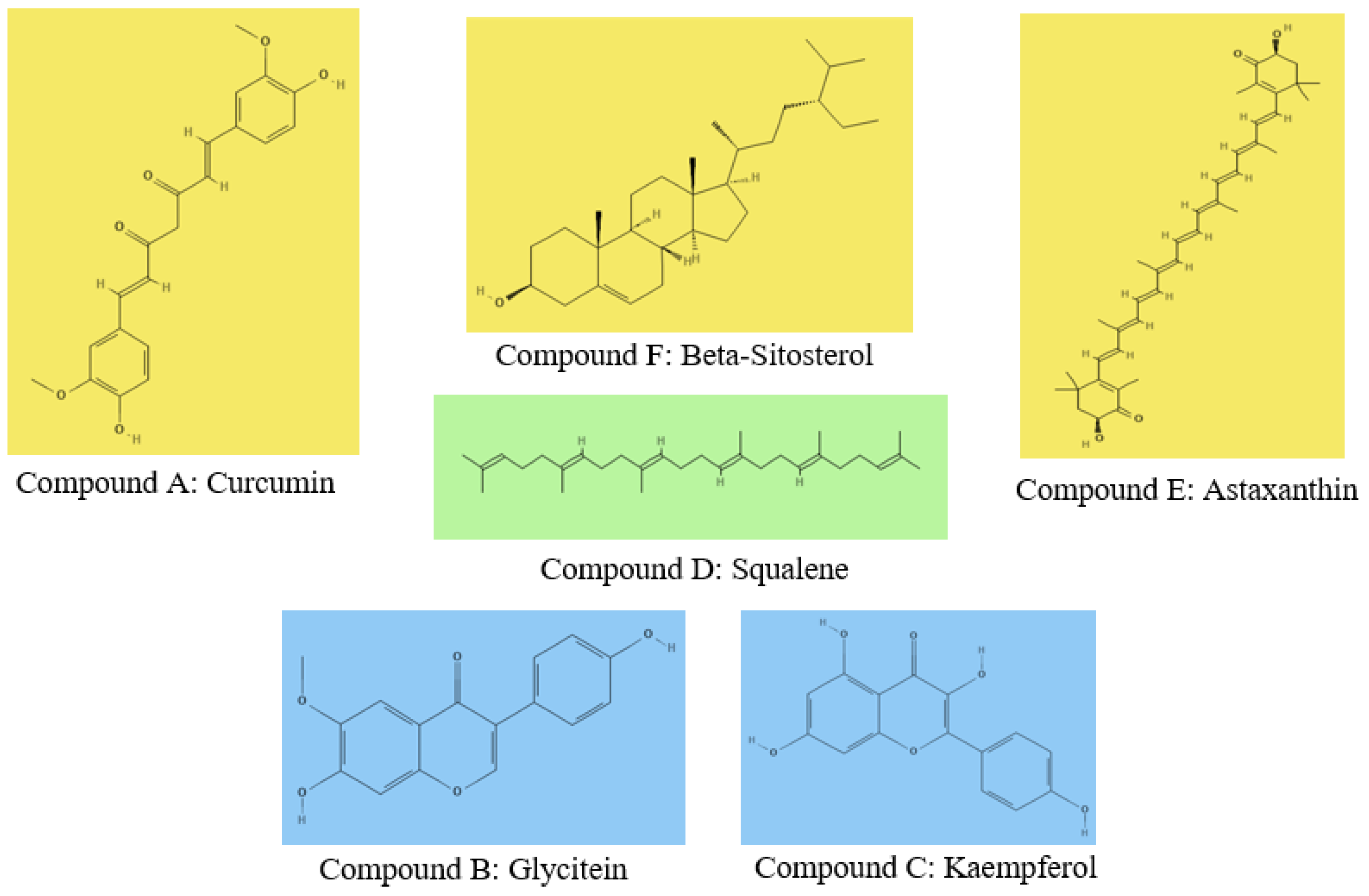
This study will focus on six distinct compounds known for their potential efficacy against AD. They are curcumin, glycitein, kaempferol, squalene, astaxanthin, and β-sitosterol (31–34,38,39). The selection of these compounds is grounded in their documented neuroprotective properties and their ability to target key mechanisms associated with AD pathology. Curcumin is derived from turmeric, has demonstrated anti-inflammatory and antioxidant properties while exhibiting potential against amyloid beta aggregation (33). Glycitein is a soy isoflavone, has shown promise in attenuating tau pathology and neuroinflammation (31). Kaempferol is a flavonoid found in various plants, and exhibits neuroprotective effects against oxidative stress and inflammation (32). Squalene is a natural compound found in shark liver oil and certain plants, and has been associated with neuroprotective benefits (38,39). Astaxanthin is a carotenoid with potent antioxidant properties, and has shown protective effects against neurodegeneration (31,34). β-sitosterol is a plant sterol, has demonstrated anti-inflammatory and antioxidant activities (32). The selection of these compounds is based on their diverse neuroprotective mechanisms which provide a comprehensive foundation for investigating multi-target compound designs against AD. The collection process involves accessing PubChem which is a reliable database to obtain the molecular structures of the selected compounds. PubChem ensures the accuracy and consistency of the structural data, providing a robust foundation for subsequent computational analyses. Ligands, representing the active components of these compounds, will be extracted and meticulously prepared for further in-depth investigations.
Figure 4.
Chemical Structures of Investigated Compounds: Curcumin (A), Glycitein (B), Kaempferol (C), Squalene (D), Astaxanthin (E), and β-Sitosterol (F).
Figure 4.
Chemical Structures of Investigated Compounds: Curcumin (A), Glycitein (B), Kaempferol (C), Squalene (D), Astaxanthin (E), and β-Sitosterol (F).
5.2. Target Identification: Collection and Preparation of 3D Structure
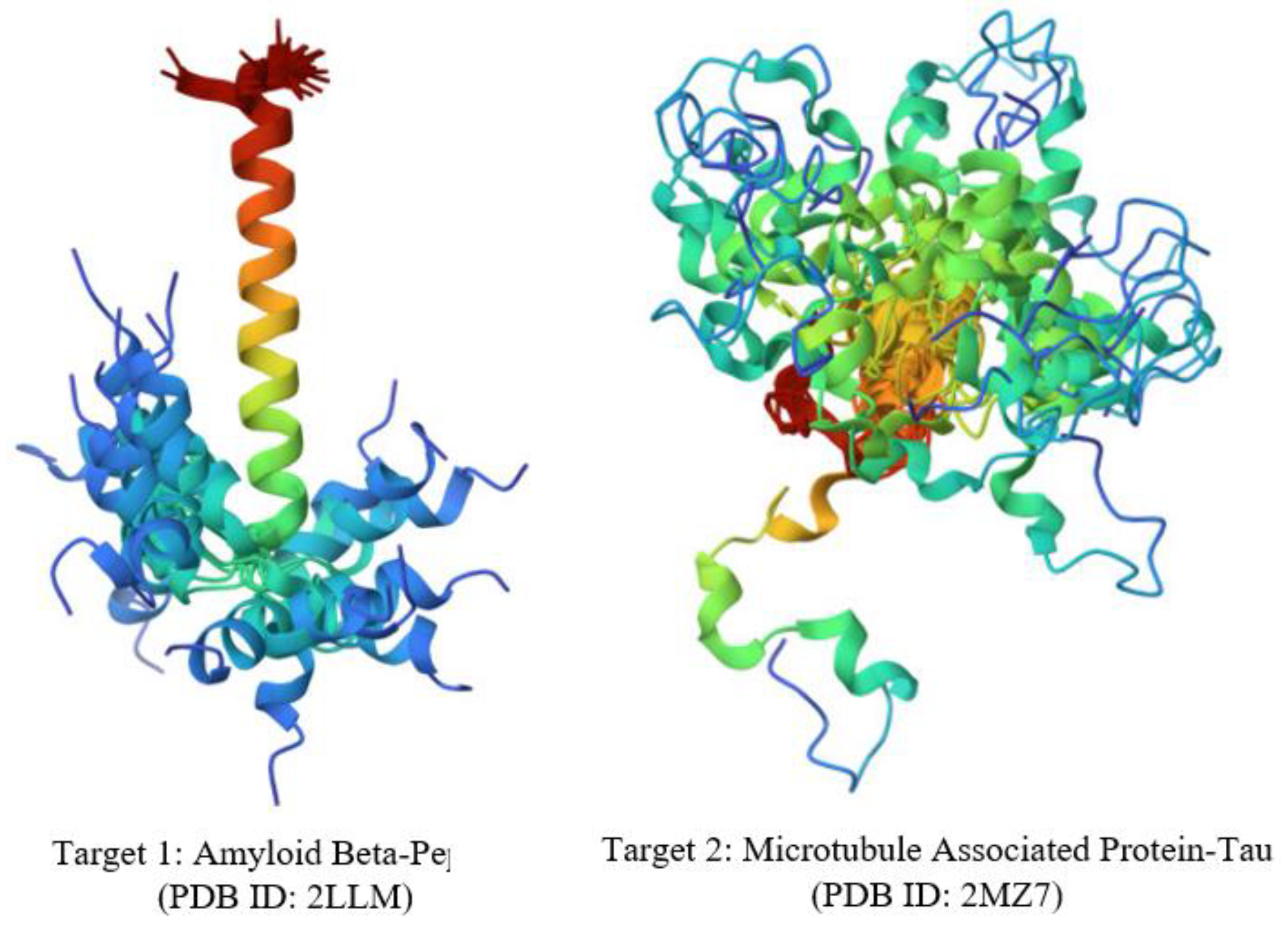
Several hypotheses have been proposed for AD pathogenesis, with the widely accepted theories being the Aβ cascade and tau hyper-phosphorylation (40). The primary targets selected for investigation were the amyloid beta and tau proteins, recognized as key players in the intricate pathogenesis of AD (41–43). The 3D structures of these targets were obtained from the PDB.
Figure 5.
3D Structures of Target Proteins.
Figure 5.
3D Structures of Target Proteins.
5.3. Approaches for Combination: Strategies and Formation
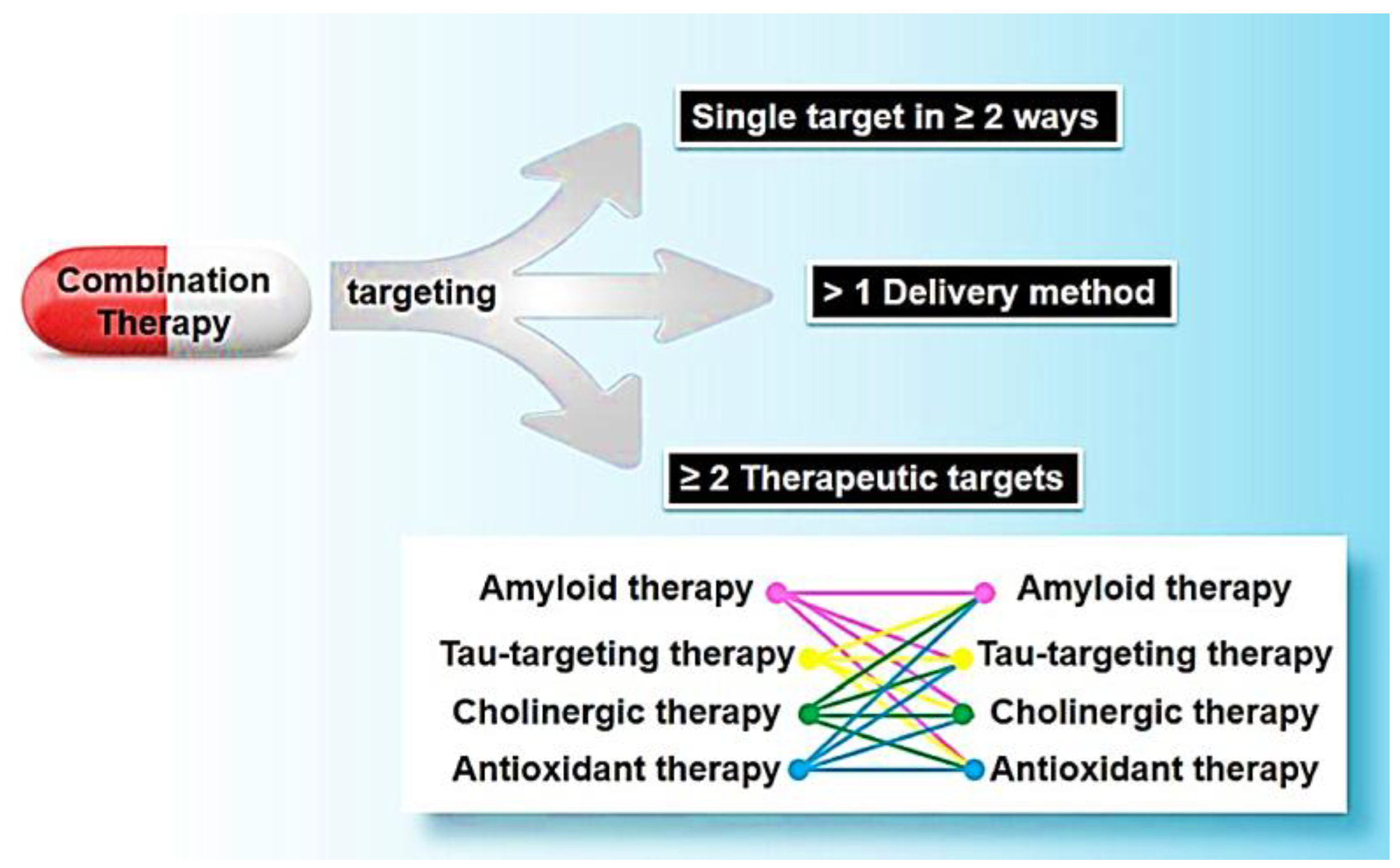
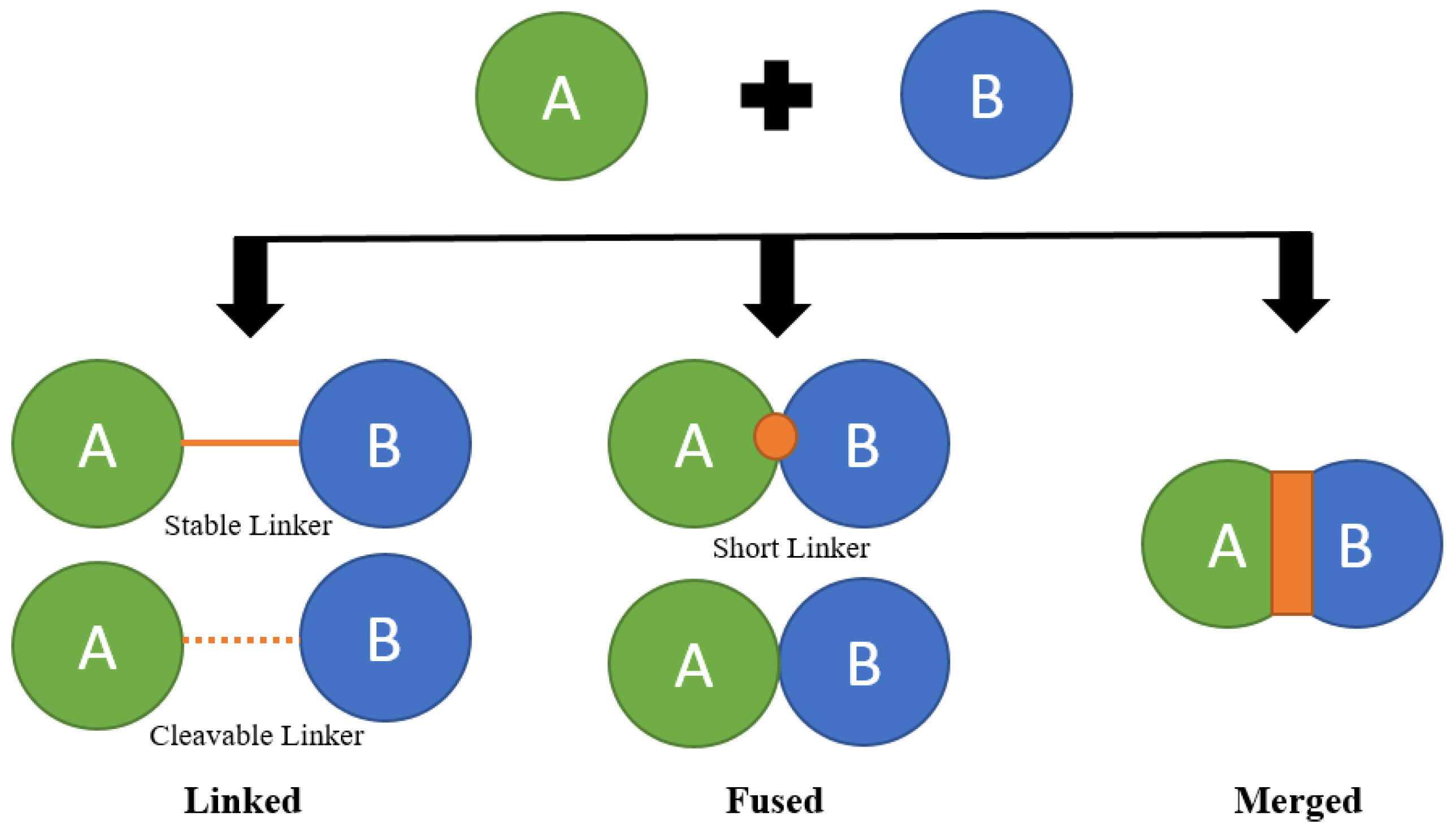
Combination therapy offers remarkable adaptability and importance, enabling the targeting of multiple entities, such as both tau and amyloid. As well as the option to address a singular target through different approaches, such as employing two distinct amyloid-targeting therapies. Additionally, it can be utilized with various administration methods, including both intravenous and oral delivery (37). For this research proposal, we have strategically identified three distinct approaches, which are Linked, Fused, and Merged. To explore the combination of six compounds. These carefully selected strategies aim to provide a comprehensive understanding of the synergistic possibilities and novel formations that can emerge from the interplay of different compounds. The Linked strategy involves establishing a strategic connection between two compounds, fostering a symbiotic relationship without losing the individuality of each component. The Linked strategy, often referred to as conjugation, encompasses two primary types: Conjugates and Cleavage Conjugates. In Conjugates, molecules are constructed with diverse pharmacophores connected by metabolically stable functional groups absent in the individual compounds.
On the other hand, Cleavage Conjugates involve the release of therapeutic moieties through linker metabolism, allowing the liberated components to act independently on specific targets. These variations within the Linked strategy enable a nuanced exploration of synergies between compounds, providing a foundation for uncovering novel and potent formations. In the fused approach, the compounds are directly attached to one another, and at times, the employed linkers are so shortened that the pharmacophores literally touch, fostering an intimate connection for enhanced synergy. The Merged strategy involves combining two compounds in a manner that integrates their features while preserving individual characteristics. This approach will involve careful consideration of optimal conditions, with the objective of making a novel compound that inherits beneficial traits from its parent components. These three chosen strategies linked, fused, and merged offer a holistic exploration of combination approaches (17,35,36,44).
By implementing these strategies, we aim to uncover unique formations, understand the underlying synergies, and contribute valuable insights to the field of compound combination. Schematic Showing the Three Possible Strategies to Design a Designed Multi-target Ligand (DML) Molecule Using a ‘Framework Combination’ Approach, exemplifying the combination of two compounds (36,44). Possible strategies to design MTDLs for AD are illustrated in the
Figure 6. MTDLs developed through structure-based drug design (SBDD) methods have demonstrated remarkable success, attributed to the growing accessibility of structural data for crucial targets in AD (36).
5.4. Molecular Docking:
Molecular docking is a computational approach that predicts the precise binding modes, affinities, and scores of small molecules or macromolecules interacting with protein receptors at the atomic level (16,23). In the pursuit of designing effective MTCC for AD, molecular docking serves as a pivotal computational tool. This technique facilitates the exploration of ligand-protein interactions at the atomic level, offering crucial insights into the binding modes, affinity, and stability of the designed compounds with their respective target proteins. The molecular docking approach will involve the utilization of advanced docking algorithms, specifically utilizing the Autodock software (45). To predict the spatial orientation and binding conformations of MTDLs within the binding pockets of target proteins associated with Alzheimer's pathology. This process will take into account the flexibility of both ligands and receptors, allowing for a dynamic and accurate representation of the ligand-protein interaction landscape (16,34).
5.5. Molecular Dynamics Simulation:
Molecular Dynamics Simulation (MDS) is a powerful computational technique employed to explore the dynamic behavior of biomolecular systems over time. MDS provides insights into the temporal evolution of molecular interactions which allows for a detailed examination of how ligands and proteins interact and influence each other's movements and bindings (16,45). In this study, MDS will be performed using the NAMD software (45). Through MDS, we aim to unravel the dynamic aspects of ligand-protein interactions, including conformational changes, flexibility, and the overall stability of the MTDL complexes. By analyzing dynamic behaviors, we can uncover insights into how MTDLs bind, fluctuate, and adapt structurally in the biological environment. MDS offers crucial insights into the dynamic nature of drug targets, complementing and enhancing the information obtained from docking studies. This further validates the results obtained by the latter, enhancing the robustness and reliability of our computational predictions and design strategies for multi-target ligands in the context of AD (16,19).
5.6. Pharmacokinetics Analysis:
This section of our study focuses on the comprehensive analysis of pharmacokinetic properties which surrounding Drug-Drug Interaction (DDI) Studies, Drug Likeness, and Absorption, Distribution, Metabolism, Excretion, and Toxicity (ADME/T) Analysis, along with Structure-Activity Relationship (SAR) Analysis (46). To conduct this analysis we will employ the admetSAR server, which is a web-based platform renowned for its efficacy in predicting and evaluating pharmacokinetic parameters (45). Through these analyses, we aim to enhance our understanding of the compounds' pharmacological profiles, laying the foundation for informed decision-making in the pursuit of effective multi-target compounds against Alzheimer's disease (47–49).
6. Data Analysis
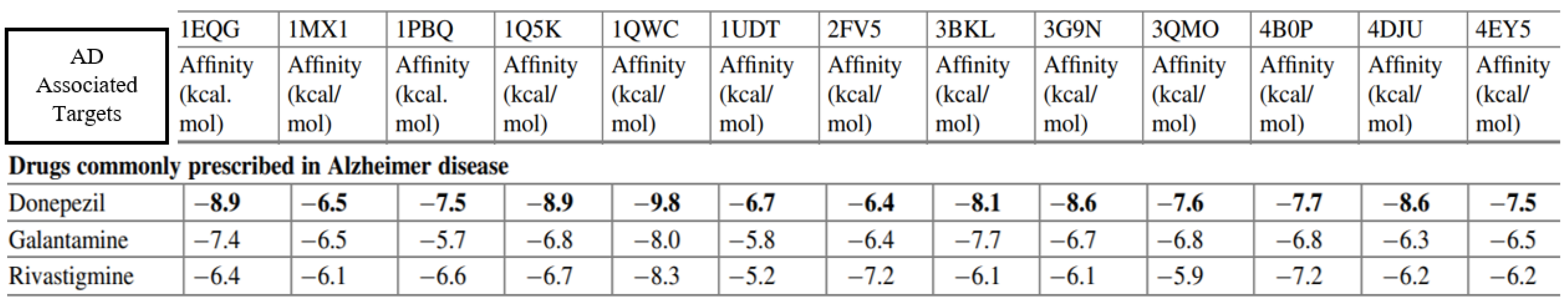
Data Analysis covers the exploration of Interactions and Affinities, Comparative Analysis, and the implementation of Validation Strategies. Leveraging advanced computational tools, we will delve into the intricate web of molecular interactions and affinities between our designed MTCCs and their respective targets. For a comprehensive validation process, we will utilize data from previous studies that include information about recently approved drugs such as Donepezil, Galantamine, and Rivastigmine. By comparing our new compound data with the established profiles of these approved drugs, we aim to strengthen the reliability of our MTCC designs, ensuring they align with known efficacies and standards in AD treatment. For analyzing the data, a combination of powerful tools will be employed, including AutoDock, PyRx for virtual screening, R-Studio for statistical and comparative analysis, and VMD for molecular dynamics visualization and validation strategies (18,35,45,50,51). This comprehensive approach aims to derive meaningful insights into the potential multi-target combination compounds designed for AD.
7. Expected Results
In the pursuit of discovering effective treatments for AD, this research anticipates that this innovative approach of designing MTCCs will yield promising outcomes. By employing merging, fusion, and linking strategies, we expect to identify specific combinations of curcumin, glycitein, kaempferol, squalene, astaxanthin, and β-sitosterol that demonstrate enhanced efficacy against both amyloid beta and tau proteins. The computational analyses using AutoDock Vina, PyRx, R Studio, and VMD will provide valuable insights into the interactions, affinities, and pharmacokinetics of the MTCCs. We anticipate that our MTCC design will surpass the efficacy of individual compounds, laying the foundation for a transformative approach in AD.
Figure 7.
Comparative binding scores of FDA-approved AD drugs (Donepezil, Galantamine, Rivastigmine) with various AD-associated targets (adapted from (31)).
Figure 7.
Comparative binding scores of FDA-approved AD drugs (Donepezil, Galantamine, Rivastigmine) with various AD-associated targets (adapted from (31)).
We also anticipate identifying the primary binding affinities of each compound within the MTCC against the target proteins. Precise binding modes will be explored to understand the molecular interactions at the binding site. We aim to predict the ADME/T profiles of the MTCC compounds, contributing to a comprehensive understanding of their pharmacokinetic behavior. We anticipate that the MTCC will demonstrate superior efficacy compared to FDA-approved drugs (31) (Table 1). Additionally, the validation strategy, involving the comparison of MTCCs with FDA-approved drugs like Donepezil, Galantamine, and Rivastigmine, which aims to establish the superiority of our proposed compounds in terms of efficacy. The comparison of binding affinities with AD-related proteins further enhances the robustness of our findings. These expected results collectively lay the foundation for evolving precision medicine and transformative interventions in AD therapeutics.
8. Conclusion
In conclusion, our research represents a significant step forward in addressing the complexities of AD. Through an integrative methodology involving in silico design, molecular docking, and pharmacokinetic analyses, we aim to contribute novel insights into the development of effective treatments. The potential success of our MTCC approach represents a significant advancement in Alzheimer's therapeutics. This approach offers hope to individuals affected by the condition by addressing its challenges. This research stands out for its transformative interventions, contributing to the foundation of precision medicine. This study aim not only to contribute to the continuous progress in AD therapeutics but also to significantly improve the lives of those facing the complexities of this condition. This commitment reflects a collective aspiration to redefine the landscape of Alzheimer's treatment, finally bringing an aim for positive change for individuals dealing with this intricate and challenging neurodegenerative disorder.
9. Future Prospects
The research holds promising implications beyond AD. The innovative methodologies utilized in this study could have a significant impact on drug development methodologies applicable to a spectrum of neurological disorders. The success of the MTCC design may encourage further investigation into combined approaches for drug development, potentially advancing the search for more efficient and precise therapeutics. Moreover, the findings could stimulate collaborations between computational and experimental research, potentially expediting the drug discovery process. Overall, this research holds potential implications for the field of precision medicine, offering avenues for advancements in treatments for neurodegenerative disorders and other related conditions.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest Statement
The author declares that there is no conflict of interest regarding the publication of this review.
References
- Salloway, S.; Farlow, M.; McDade, E.; Clifford, D.B.; Wang, G.; Llibre-Guerra, J.J.; Hitchcock, J.M.; Mills, S.L.; Santacruz, A.M.; Aschenbrenner, A.J.; et al. A trial of gantenerumab or solanezumab in dominantly inherited Alzheimer’s disease. Nat. Med. 2021, 27, 1187–1196. [Google Scholar] [CrossRef]
- Nguyen, T.T.D.; Vo, T.K.; Vo, V.G. Advances in developing therapeutic strategies for Alzheimer's disease. Biomed. Pharmacother. 2021, 139, 111623. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Li, R.; Sterling, K.; Song, W. Amyloid β-based therapy for Alzheimer’s disease: challenges, successes and future. Signal Transduct Target Ther. 2023, 8, 1–26. [Google Scholar]
- Liu, L.; Zhao, S.; Chen, H.; Wang, A. A new machine learning method for identifying Alzheimer's disease. Simul. Model. Pr. Theory 2020, 99. [Google Scholar] [CrossRef]
- Cummings, J.; Lee, G.; Zhong, K.; Fonseca, J.; Taghva, K. Alzheimer’s disease drug development pipeline: 2021. Alzheimer’s Dement Transl Res Clin Interv. 2021, 7, 1–24. [Google Scholar]
- Jahn, H. Memory loss in alzheimer’s disease. Dialogues Clin Neurosci. 2013, 15, 445–454. [Google Scholar]
- Schachter, A.S.; Davis, K.L. Alzheimer’s disease. 2022, 2000.
- Abeysinghe, A.A.D.T.; Deshapriya, R.D.U.S.; Udawatte, C. Alzheimer's disease; a review of the pathophysiological basis and therapeutic interventions. Life Sci. 2020, 256, 117996. [Google Scholar] [CrossRef]
- Bellenguez, C.; Grenier-Boley, B.; Lambert, J.C. Genetics of Alzheimer’s disease: where we are, and where we are going. Curr Opin Neurobiol. 2020, 61, 40–48. [Google Scholar]
- Santiago, J.A.; Potashkin, J.A. The Impact of Disease Comorbidities in Alzheimer’s Disease. Front Aging Neurosci. 2021, 13, 1–13. [Google Scholar]
- Das, N.; Raymick, J.; Sarkar, S. Role of metals in Alzheimer’s disease. Metab. Brain Dis. 2021, 36, 1627–1639. [Google Scholar] [CrossRef]
- Rabbito, A.; Dulewicz, M.; Kulczyńska-Przybik, A.; Mroczko, B. Biochemical Markers in Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 1989. [Google Scholar] [CrossRef]
- Blaikie, L.; Kay, G.; Maciel, P.; Lin, P.K.T. Experimental modelling of Alzheimer's disease for therapeutic screening. Eur. J. Med. Chem. Rep. 2022, 5. [Google Scholar] [CrossRef]
- Thoe, E.S.; Fauzi, A.; Tang, Y.Q.; Chamyuang, S.; Chia, A.Y.Y. A review on advances of treatment modalities for Alzheimer's disease. Life Sci. 2021, 276, 119129. [Google Scholar] [CrossRef]
- Fan, L.; Mao, C.; Hu, X.; Zhang, S.; Yang, Z.; Hu, Z.; et al. New Insights Into the Pathogenesis of Alzheimer’s Disease. Front Neurol. 2020, 10, 1–12. [Google Scholar]
- Martins, M.M.; Branco, P.S.; Ferreira, L.M. Enhancing the Therapeutic Effect in Alzheimer's Disease Drugs: The role of Polypharmacology and Cholinesterase inhibitors. ChemistrySelect 2023, 8. [Google Scholar] [CrossRef]
- Abdolmaleki, A.; Ghasemi, J.B. Dual-acting of Hybrid Compounds - A New Dawn in the Discovery of Multi-target Drugs: Lead Generation Approaches. Curr. Top. Med. Chem. 2016, 17, 1096–1114. [Google Scholar] [CrossRef]
- Kim, S.; Nam, Y.; Shin, S.J.; Prajapati, R.; Shin, S.M.; Kim, M.-J.; Kim, H.S.; Leem, S.H.; Kim, T.-J.; Park, Y.H.; et al. Dual modulators of aggregation and dissociation of amyloid beta and tau: In vitro, in vivo, and in silico studies of Uncaria rhynchophylla and its bioactive components. Biomed. Pharmacother. 2022, 156, 113865. [Google Scholar] [CrossRef]
- Kumar, A.; Tiwari, A.; Sharma, A. Changing Paradigm from one Target one Ligand Towards Multi-target Directed Ligand Design for Key Drug Targets of Alzheimer Disease: An Important Role of In Silico Methods in Multi-target Directed Ligands Design. Curr. Neuropharmacol. 2018, 16, 726–739. [Google Scholar] [CrossRef]
- Job, N.; Thimmakondu, V.S.; Thirumoorthy, K. In Silico Drug Design and Analysis of Dual Amyloid-Beta and Tau Protein-Aggregation Inhibitors for Alzheimer’s Disease Treatment. Molecules 2023, 28, 1388. [Google Scholar] [CrossRef]
- Kou, X.; Song, L.; Wang, Y.; Yu, Q.; Ju, H.; Yang, A.; Shen, R. Design, synthesis and anti-Alzheimer's disease activity study of xanthone derivatives based on multi-target strategy. Bioorganic Med. Chem. Lett. 2020, 30, 126927. [Google Scholar] [CrossRef]
- Sang, Z.; Wang, K.; Shi, J.; Liu, W.; Cheng, X.; Zhu, G.; Wang, Y.; Zhao, Y.; Qiao, Z.; Wu, A.; et al. The development of advanced structural framework as multi-target-directed ligands for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2020, 192, 112180. [Google Scholar] [CrossRef]
- Neto, L.R.d.S.; Moreira-Filho, J.T.; Neves, B.J.; Maidana, R.L.B.R.; Guimarães, A.C.R.; Furnham, N.; Andrade, C.H.; Silva, F.P. In silico Strategies to Support Fragment-to-Lead Optimization in Drug Discovery. Front. Chem. 2020, 8, 93. [Google Scholar] [CrossRef]
- Caldwell, G.W. In silicotools used for compound selection during target-based drug discovery and development. Expert Opin. Drug Discov. 2015, 10, 901–923. [Google Scholar] [CrossRef]
- Athar, T.; Al Balushi, K.; Alam Khan, S. Recent advances on drug development and emerging therapeutic agents for Alzheimer’s disease. Mol. Biol. Rep. 2021, 48, 5629–5645. [Google Scholar] [CrossRef]
- Gulisano, W.; Maugeri, D.; Baltrons, M.A.; Fà, M.; Amato, A.; Palmeri, A.; et al. Role of Amyloid-β and Tau Proteins in Alzheimer’s Disease: Confuting the Amyloid Cascade. J Alzheimer’s Dis. 2018, 64, S611–31. [Google Scholar]
- Makhaeva, G.F.; Kovaleva, N.V.; Boltneva, N.P.; Lushchekina, S.V.; Rudakova, E.V.; Stupina, T.S.; Terentiev, A.A.; Serkov, I.V.; Proshin, A.N.; Radchenko, E.V.; et al. Conjugates of tacrine and 1,2,4-thiadiazole derivatives as new potential multifunctional agents for Alzheimer’s disease treatment: Synthesis, quantum-chemical characterization, molecular docking, and biological evaluation. Bioorganic Chem. 2020, 94, 103387. [Google Scholar] [CrossRef]
- Sagar, S.R.; Singh, D.P.; Das, R.D.; Panchal, N.B.; Sudarsanam, V.; Nivsarkar, M.; Vasu, K.K. Pharmacological investigation of quinoxaline-bisthiazoles as multitarget-directed ligands for the treatment of Alzheimer’s disease. Bioorganic Chem. 2019, 89, 102992. [Google Scholar] [CrossRef]
- Najafi, Z.; Mahdavi, M.; Saeedi, M.; Karimpour-Razkenari, E.; Edraki, N.; Sharifzadeh, M.; Khanavi, M.; Akbarzadeh, T. Novel tacrine-coumarin hybrids linked to 1,2,3-triazole as anti-Alzheimer’s compounds: In vitro and in vivo biological evaluation and docking study. Bioorganic Chem. 2019, 83, 303–316. [Google Scholar] [CrossRef]
- Kareti, S.R.; P, S. In Silico Molecular Docking Analysis of Potential Anti-Alzheimer's Compounds Present in Chloroform Extract of Carissa carandas Leaf Using Gas Chromatography MS/MS. Curr. Ther. Res. 2020, 93, 100615. [Google Scholar] [CrossRef]
- Alam, A.; Tamkeen, N.; Imam, N.; Farooqui, A.; Ahmed, M.M.; Tazyeen, S.; et al. Pharmacokinetic and molecular docking studies of plant-derived natural compounds to exploring potential anti-Alzheimer activity. Silico Approach Sustain Agric. 2018, 217–38. Available from: https://link.springer.com/chapter/10.1007/978-981-13-0347-0_13.
- Vanaja, D.; Yellamma, K. Molecular Docking Studies on Evolvulus Alsinoides Compounds Against TAU Protein in Alzheimer’s Disease. Int. J. Sci. Res. 2012, 3, 21–24. [Google Scholar] [CrossRef]
- Sato, R.; Vohra, S.; Yamamoto, S.; Suzuki, K.; Pavel, K.; Shulga, S.; Blume, Y.; Kurita, N. Specific interactions between tau protein and curcumin derivatives: Molecular docking and ab initio molecular orbital simulations. J. Mol. Graph. Model. 2020, 98, 107611. [Google Scholar] [CrossRef]
- Praveen, K.; Yellamma, K. Insilco studies on astaxanthin derivatives against Tau protein - A novel approach to design anti-Alzheimers drug. 2017, 8, 226–31.
- Tibon, N.S.; Ng, C.H.; Cheong, S.L. Current progress in antimalarial pharmacotherapy and multi-target drug discovery. Eur. J. Med. Chem. 2020, 188, 111983. [Google Scholar] [CrossRef]
- Arrué, L.; Cigna-Méndez, A.; Barbosa, T.; Borrego-Muñoz, P.; Struve-Villalobos, S.; Oviedo, V.; Martínez-García, C.; Sepúlveda-Lara, A.; Millán, N.; Montesinos, J.C.E.M.; et al. New Drug Design Avenues Targeting Alzheimer’s Disease by Pharmacoinformatics-Aided Tools. Pharmaceutics 2022, 14, 1914. [Google Scholar] [CrossRef]
- Umar, T.; Meena, R.; Mustehasan; Kumar, P.; Khan, A.A. Recent updates in the development of small molecules as potential clinical candidates for Alzheimer's disease: A review. Chem. Biol. Drug Des. 2022, 100, 674–681. [Google Scholar] [CrossRef]
- Kukkarasapalli, P.; Kuna, Y. Docking Studies on Ache and Tau Proteins with Marine Bioactive Compound Squalene, A New Approach to Design Anti-Alzheimer’s Drug Targets. Int. J. Pharm. Sci. Rev. Res. 2021, 70. [Google Scholar] [CrossRef]
- Mantile, F.; Trovato, M.; Santoni, A.; Barba, P.; Ottonello, S.; De Berardinis, P.; et al. Alum and squalene-oil-in-water emulsion enhance the titer and avidity of anti-aβ antibodies induced by multimeric protein antigen (1-11)E2, preserving the Igg1-skewed isotype distribution. PLoS One 2014, 9. [Google Scholar]
- Yang, H-Q, Sun, Z-K, Chen, S-D. Current advances in the treatment of Alzheimer’s disease: focused on considerations targeting Aβ and tau. Transl Neurodegener. 2012, 1, 1–12.
- Lansdall, C.J. An effective treatment for Alzheimer's disease must consider both amyloid and tau. Biosci. Horizons 2014, 7, hzu002–hzu002. [Google Scholar] [CrossRef]
- Das, S.; Basu, S. Multi-targeting Strategies for Alzheimer's Disease Therapeutics: Pros and Cons. Curr. Top. Med. Chem. 2017, 17, 1–44. [Google Scholar] [CrossRef]
- Plotkin, S.S.; Cashman, N.R. Passive immunotherapies targeting Aβ and tau in Alzheimer's disease. Neurobiol. Dis. 2020, 144, 105010–105010. [Google Scholar] [CrossRef]
- Benek, O.; Korabecny, J.; Soukup, O. A Perspective on Multi-target Drugs for Alzheimer’s Disease. Trends Pharmacol Sci 2020, 41, 434–445. [Google Scholar] [CrossRef]
- Waqas, M.; Halim, S.A.; Alsalman, A.; Khan, A.; Elkord, E.; Al-Harrasi, A. Structure-based small inhibitors search combined with molecular dynamics driven energies for human programmed cell death-1 (PD-1) protein. J. Biomol. Struct. Dyn. 2023, 41, 14771–14785. [Google Scholar] [CrossRef]
- de Ruyck, J.; Brysbaert, G.; Blossey, R.; Lensink, M. Molecular docking as a popular tool in drug design, an in silico travel. Adv. Appl. Bioinform. Chem. 2016, ume 9, 1–11. [Google Scholar] [CrossRef]
- Shaker, B.; Ahmad, S.; Lee, J.; Jung, C.; Na, D. In silico methods and tools for drug discovery. Comput. Biol. Med. 2021, 137, 104851. [Google Scholar] [CrossRef]
- Bulusu, K.C.; Guha, R.; Mason, D.J.; Lewis, R.P.; Muratov, E.; Motamedi, Y.K.; Cokol, M.; Bender, A. Modelling of compound combination effects and applications to efficacy and toxicity: state-of-the-art, challenges and perspectives. Drug Discov. Today 2016, 21, 225–238. [Google Scholar] [CrossRef]
- Sabe, V.T.; Ntombela, T.; Jhamba, L.A.; Maguire, G.E.; Govender, T.; Naicker, T.; Kruger, H.G. Current trends in computer aided drug design and a highlight of drugs discovered via computational techniques: A review. Eur. J. Med. Chem. 2021, 224, 113705. [Google Scholar] [CrossRef]
- Prieto-Martínez, F.D.; López-López, E.; Eurídice Juárez-Mercado, K.; Medina-Franco, J.L. Computational Drug Design Methods—Current and Future Perspectives. Silico Drug Des Repurposing Tech Methodol. 2019, 19–44. [Google Scholar]
- Almihyawi, R.A.H.; Naman, Z.T.; Al-Hasani, H.M.H.; Muhseen, Z.T.; Zhang, S.; Chen, G. Integrated computer-aided drug design and biophysical simulation approaches to determine natural anti-bacterial compounds for Acinetobacter baumannii. Sci. Rep. 2022, 12, 1–14. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).