1. Introduction
Breast cancer is the most common malignancy in women. Early diagnosis is a key factor in cure and survival rates. Fatal breast cancers mostly occur in young women, of median age 49 years [
1] who are excluded from regular screening in most countries. In addition, attendance of national screening programs remains remarkably low; less than 75% in Europe [
2]. 10% of breast cancer deaths are attributable to interval cancers [
1], with the interval cancer rates increasing with breast density and being elevated in women with a personal history of breast cancer [
3].
X-ray mammography has limited sensitivity for dense breasts [
3] which decreases drastically even further in cases of smaller lesions [
4]. Supplemental screening and diagnostic imaging methods are required to reduce interval cancer rates and support breast cancer diagnosis in young women and high-density breasts. Small increase in interval cancer detection comes with significant increase of recall rates for the state-of-the art imaging modalities though [
5]. The management of false positives brings substantial financial burden for the health system, delays in patients’ diagnoses and treatment.
Microwave Breast Imaging (MWBI) [
6,
7,
8,
9] is an emerging imaging modality which employs low-power electromagnetic waves to scan and image the human female breast, aiming to detect, localize and characterize breast lesions, which are dielectrically contrasted against the background healthy tissue [
10,
11,
12,
13,
14,
15], in the microwave frequency spectrum. The dielectric contrast, a priori increased in tissues with higher concentration of water [
16], is a physical property not exploited yet in state-of-the-art diagnostic breast im-aging. The diagnostic value of MWBI and its complementarity to the conventional breast imaging modalities is not fully understood [
17].
As it uses non-ionizing radiation, MWBI can be particularly useful for regular and safe scanning of women at high risk, due to family history or high breast density. In addition, MWBI holds interesting potential to outperform x-ray mammography’s low sensitivity in young and dense breasts [
18,
19], thus supporting a timelier detection of interval cancers, as a supplemental screening and diagnostic imaging method.
On the other hand, the specificity of the MWBI technology remains unknown. Management of false positives has not been systematically addressed in any earlier MWBI study, this representing a principal barrier to the clinical validation and adoption of MWBI [
20], after almost three decades of active research [
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29,
30,
31].
Going beyond the state-of-the art, Wavelia is the only MWBI system which generates clinically meaningful 3D images of the breast, for visual inspection by radiologists and morphological post-processing [
32]. Wavelia employs multi-static radar imaging technology enabling 3D volumetric breast imaging [
33,
34], semi-automated detection of breast lesions, based on intensity and persistence [
32,
35] and generation of radar signatures associated with the histological classification of the breast lesions [
36]. It is clearly differentiated from the active MWBI state-of-the art; the two main, currently active, competitor systems undergoing clinical trials [
19,
25,
26,
27,
28] scan the breast on a single coronal plane, to either provide a 2D representation of low spatial resolution for the full 3D breast volume being scanned, or employ machine learning methods directly on the measured scattering parameters to support differentiation between malignant and benign lesions without reconstructing an image of the breast.
In its First-In-Human (FiH) clinical investigation on 24 symptomatic patients (NCT03475992) [
37], Wavelia showed potential to detect and characterize breast lesions based on shape and texture descriptors of Regions-Of-Interest (ROIs) extracted from MWBI-based 3D volumetric breast images [
35,
36]. An upgraded prototype (Wavelia#2) [
38] was subsequently manufactured and recently tested on a larger and more diverse dataset of 62 symptomatic patients, collected at the Symptomatic Breast Unit of University Hospital of Galway, Ireland (NCT05757427) [
39].
The clinical investigation results presented in this article illustrate the performance of the Wavelia#2 MWBI system in terms of discrimination between malignant and benign lesions, and overall specificity. This article also focuses on the critical technological and methodological evolutions in Wavelia #2, as well as the remaining challenges, to handle the diversity of breast geometry and tissue consistency in larger patient datasets, towards standardization and clinical acceptance of the MWBI modality in the diagnostic setting. Multi-dimensional feature vector extraction by plugging the PyRadiomics [
40] open-source library to the ROI extraction module of the Wavelia Imaging Suite was implemented, to expand the diagnostic features analysis compared to the initial FiH study with Wavelia#1. Malignant-to-benign lesion separability analysis using ANOVA F-statistic was conducted and demonstrated significant diagnostic accuracy for a set of texture and intensity-based features of the ROIs extracted from the Wavelia#2 MWBI 3D volumetric study images.
The ambition of the Wavelia MWBI technology is to increase the sensitivity in dense breasts where x-ray mammography is of limited value, while contributing to reduction of the false positives and recall rates in breast cancer diagnosis and management, by developing: (a) MWBI image descriptors supporting malignant-to-benign lesion discrimination, and even beyond: (b) a MWBI-based BIRADS score associated with malignancy risk, thus potentially expanding the classic triple assessment at breast diagnosis clinic to a novel quadruple assessment process.
2. Materials and Methods
2.1. Clinical Investigation Dataset: The Diverse Study Population Profile
The Wavelia Microwave Breast Imaging (MWBI) investigational medical device was designed to scan and image the human female breast, aiming to detect, localize and characterize breast lesions, which are dielectrically contrasted against the background healthy tissue, in the microwave frequency spectrum. In the First-in-Human (FiH) study, conducted in 24 patients [
37], the Wavelia #1 prototype system demonstrated the ability to detect and discriminate between palpable breast lumps [
35,
36], the imaging procedure had no safety issues and patients reported a favorable experience of the MWBI scan. The promising findings from the study, which provided initial data to support a valid clinical association in accordance with Stage 1 of the Guidance on Clinical Evaluation (MDR) / Performance Evaluation (IVDR) of Medical Device Software (MDCG 2020-1), warranted the preparation of further clinical investigations with an upgraded prototype version of the Wavelia system (Wavelia #2) [
38]. The clinical data that was collected in this 2
nd study [
39] with Wavelia was intended to build upon the outcomes of the FiH study, as well as further address the current limitations of the state-of-the-art MWBI technology applied to clinical trials at this stage.
The intended purpose of the investigational device in this pilot clinical investigation was to assess the detectability and sizing of invasive breast cancers, the detectability of benign breast lesions, as well as the differentiation between malignant and benign breast lesions, using Wavelia #2. The study was conducted at the Symptomatic Breast Unit, University Hospital of Galway, Ireland. Female patients who presented to the symptomatic clinics with a discrete breast abnormality larger than >1cm were assessed for participation. Patients who had surgery on either breast within the past 12 months, who had any aesthetic breast implant, or any active or metallic implant in their breasts were excluded from the study. Patients with very small breasts of Cup-A, pregnant or breast-feeding patients were considered inappropriate for the imaging scanner setting and not accepted for inclusion in the study either. The full list of patient Inclusion and Exclusion criteria for this study is reported in [
39]. All enrolled subjects provided personal explicit written informed consent prior to any study related procedures
In this clinical investigation, the MWBI scan was not used for patient diagnosis and was conducted in addition to standard-of-care assessments. Standard-of-care assessments performed by the physician, as per normal practice in the symptomatic breast unit of University Hospital of Galway, included: conventional medical history, clinical assessment, breast examinations and standard-of-care reference imaging (e.g. x-ray mammogram and / or Ultrasound/and or breast MRI) and core biopsy (if applicable). The written radiology reports from standard-of-care reference imaging, as well as the core biopsy reports if applicable, were acquired and used to evaluate the performance of the MWBI system in terms of detecting and estimating size and consistency of the dominant discrete breast abnormality of each symptomatic breast. Relevant data from Multi-Disciplinary Team (MDT) meetings in relation to the therapeutic strategy for the patient and if surgery was planned were also obtained. In the case of patients who were scheduled for surgery, key reference data from their post-surgery histology report and post-surgery MDT meeting were also collected for the evaluation of the MWBI imaging results in relation to the estimated tumor size.
A total of 73 subjects were screened, 11 were excluded due to various reasons, thus the full analysis set was comprised of 62 subjects in total (84.93%). Among the 62 patients included in the study, 8 patients had bilateral radiologically reported lesions, resulting in a total of 70 lesions being analyzed for detectability and characterization by the Wavelia#2 MWBI system. 32 patients had a malignant primary lesion, 25 had solid benign lesions and 5 patients presented with simple cysts.
In this exploratory study, a wide range of patient breast sizes, breast density and patient age profiles were included [
39]. The mean age was 45.0 years (SD: 15.17) and ranged from 18 to 95 years old. 30.65% of the subjects had a breast density D, 25.81% had a breast density of C, 22.58% had a density of B, 3.23% had a density of A. The remaining 17.74% were of unknown breast density; no mammogram was performed as per standard-of-care in the hospital due to young age of the patient (<35 years). The mean radiological lesion size was 26.4 (mm) (SD: 10.0). 71.43% of the subjects had a biopsy clip marking the symptomatic dominant discrete lesion and 27.42% did not. The mean breast size was 756.7(mL) (SD: 374.43 mL).
Qualitative assessment of the breast density class, according to BIRADS Atlas 5
th Edition (2013) [
41] was reported by the study radiologist, as part of the patient reference data. The Volumetric Breast Density (VBD) was quantitatively assessed using x-ray mammography data, where relevant as reference imaging data for the study. ‘For processing’ DICOM datafiles were processed with the Volpara Lab software package [
42,
43] to compute VBD and the associated categorization of the breast per Volpara Density Grade (VDG) for the analyzed population. As part of this analysis, the volume of the breast was also computed by Volpara Lab, for the subset of the study population for whom ‘For processing’ DICOM datafiles of their x-ray mammogram were made available. If mammography-based computation of the breast volume was unavailable, the Wavelia MWBI scan-based reconstruction of the breast surface [
44,
45] was used to compute the breast volume. A notable overestimation of the volume was reported especially in cases of small breasts, because of no clear separation between the pendulous breast and the pectoral muscle being consistently and systematically defined, at this stage of the Wavelia MWBI technology development.
The study initiation took place between 16 February 2023 and 01 March 2023. First Patient First Visit (FPFV) and first MWBI scan was completed on 9 March 2023. The final participant was scanned on 30 May 2024, and the Last Patient Last Visit (LPLV) occurred on 13 June 2024. Database Lock was on 04 October 2024.
2.2. The Wavelia#2 Microwave Breast Imaging (MWBI) Examination
The patient is examined lying in a prone position, on the examination table of the Wavelia#2 MWBI scanner [
38]. One breast is scanned at a time; both breasts of the patient are scanned sequentially for the purpose of the study. The patient’s breast under examination is immersed in a transition liquid in a cylindrical container, through the circular opening of the examination table. The transition liquid is similar to a hydrating cream made of synthetic oil and water using appropriate emulsifiers and conservatives. It is a lossy propagation medium, thus attenuating the microwaves that propagate in the exterior of the breast. The transition liquid has dielectric constant close to the one of the human skin, to mitigate strong reflections on the external surface of the breast, before penetrating its interior tissues for imaging.
The scanner illuminates the breast using low-power electro-magnetic waves in the frequency range [0.8-4.1] GHz and measures the fields scattered inside the breast in order to image the interior breast tissues and detect lesions, based on their dielectric contrast with healthy tissues. The radiated power level by the scanner inside the cylindrical container where the breast is immersed is always lower than 60mW. Calculations have been performed to determine the localized Specific Absorption Rate (SAR) in the breast. The calculated maximum value of localized SAR, at 4 GHz for 10g average, amounts to 0.25W/kg and complies (safety factor 8) with the guidelines of the International Commission on Non-Ionizing Radiation Protection (ICNIRP) and the EU Council Recommendation 1999/519/EC on the limitation of exposure of the general public to electromagnetic fields. The MWBI scan is safe to be repeated as often as justified and needed, to support earlier breast cancer diagnosis, particularly in the populations at high-risk.
The microwave imaging scan is performed using an array of 21 wideband probes in a horizontal circular configuration. The probes are located outside of the cylindrical container that hosts the transition liquid. The probe array is piloted to perform a vertical motion, such that the full vertical extent of the breast is scanned. At each 2 mm vertical scan position, each antenna sequentially illuminates the breast, while the remaining antennas sequentially receive the electromagnetic scattering at various angles around the circumference of the circular probe array, in a multi-static radar imaging system configuration. In the Wavelia#2 implementation, the MWBI scan of each breast lasts between 15 and 30 minutes, depending on the vertical extent of the breast. This is a lengthy process; parallelization of the fully sequential probing mechanism is certainly required before clinical acceptance of such a MWBI system.
Wavelia#2 was upgraded to include the integration of an ‘aid-to-patient positioning’ module, based on a system of 6 endoscopic cameras integrated at the interior bottom of the cylindrical container of the scanner, to enable control of the positioning of the breast and ultimately enhance quality of the MWBI scan. A software toolkit guiding the patient breast positioning in the air, before automated filling of the cylindrical container with transition liquid from the bottom, has also been developed. The toolkit is intended to verify and track the breast centering, the breast verticality, the breast azimuthal orientation and the potentially off-centered location of the nipple on the pendulous breast, to guide the Wavelia device operator through optimization of the patient position for MWBI scanning.
In addition, in order to improve the control over the positioning of the patient’s breast in the MWBI scanner, a short 5-to-6-minute process was implemented in this clinical investigation for marking the patient’s breasts using easily applicable and removable, waterproof adhesive patches. The breast marking took place before installation of the breast in the MWBI scanner. The topology of the breast markings, defined while adopting the methodology in [
46] were used to annotate useful landmark lines on the endoscopic camera data of the patient’s breast, and better guide the operator through the positioning of the breast in the MWBI scanner.
Once the breast position in the scanner is fixed, images are recorded with the system of endoscopic cameras to support valid breast quadrant definition on the MWBI images
. As the MWBI images have limited spatial resolution, the data from the endoscopic cameras provide useful detail on the location of the nipple and any potential misalignment of the breast orientation to enable a more valid interpretation of the MWBI images. The optical data also provide additional information on persistent skin folds and any other identified zones of the breast, prone to generate imaging artefacts during the MWBI scan. The importance of the proper centering and alignment of the breast in the scanner has also been studied in detail for the breast-dedicated Magnetic Resonant Imaging (MRI) scan [
47]. MRI breast imaging is performed at prone position and the patient setting is similar to the one applicable to Wavelia MWBI, thus MRI ergonomic study outputs for image artefact mitigation and image quality enhancement provide useful inputs to the Wavelia development.
The total duration of the Wavelia#2 MWBI examination procedure is estimated to about 1.5 hour in total for both breasts, including marking of the breasts, positioning of the patient, filling/emptying of the transition liquid, the microwave scan, and the cleaning of the breasts.
Acceleration of the full procedure is required before acceptability in the clinical setting, for the scan itself as earlier mentioned, but also while managing more efficiently the breast positioning and the transition liquid. Emptying and refilling of the cylindrical container of the scanner with transition liquid in-between the MWBI scans of the two breasts of the patient is required, in the current MWBI examination procedure, because the transition liquid is creamy and of opaque color, hindering the function of the endoscopic cameras for positioning the 2nd breast.
2.3. MWBI 3D Image Formation
2.3.1. Sectorized Multi-Static Radar Imaging
Sectorization of the imaging scene is numerically performed in Wavelia MWBI methodology [
33,
38,
48], to more efficiently reconstruct the imaging scene, considering the lossy profile of the breast tissues and the transition liquid, but also the distinct physical meaning of the forward and backward scattering mechanisms of the microwaves. A subset of 8 out of the 21 probes of the Wavelia#2 system [
38] is used each time to numerically form a sub-array, the full multi-static data matrix of which is used to form a partial image of the breast per azimuthal sector of illumination. The TR-MUSIC [
49] imaging algorithm is applied to form the partial image of the breast at each azimuthal sector of illumination [
33]. The process is repeated 21 times; at each repetition, the center of the sub-array is numerically moved to the next, adjacent, probe. This way, a total of 21 partially overlapping sub-images of the breast are formed and subsequently stitched to reconstruct a coronal section of the pendulous breast, of thickness +/2mm around the horizontal symmetry plane of the probe at the given vertical scan position. The set of partially overlapping coronal breast sections are integrated to form the full 3D image of the breast.
2.3.2. Handling the Heterogeneity in the Dielectric Profile of the Breast Parenchyma
The speed of propagation of the electromagnetic waves through the breast tissues is determined by the dielectric properties (permittivity) of these tissues. The breast parenchyma is highly heterogeneous, especially in the cases of denser breasts, and its spatially-varying dielectric properties are a priori unknown. A parameter (pc_fib) has been earlier defined as part of the Wavelia imaging methodology [
32,
33,
34,
38]. This parameter is directly associated with the unknown permittivity of the background medium through which the electromagnetic waves propagate in each breast imaging setting. The partial image of the breast per azimuthal sector and per coronal section of each MWBI scan is generated under varying assumptions on the parameter pc_fib. The best-fitting assumption is selected independently per imaging sector, based on image focusing quality criteria [
50].
Multiple versions of the full 3D image of the breast are generated while varying the search range for the pc_fib parameter. Expanding on the methodology that was earlier employed in the FiH clinical investigation with Wavelia#1 [
32], where 5 search ranges for the pc_fib parameters were systematically employed (2 wide and 3 narrow ranges), in this clinical investigation, the 3D image of each breast is generated for a total of 11 pc_fib search ranges.
The following 3 MWBI images of each breast are systematically generated for visual inspection and interpretation by the clinical investigators and study radiologists:
Global Averaged image: averaging all the 11 pc_fib search-range images
Low permittivity image: averaging of the 4 pc_fib search-range images that involve values .
High permittivity image: averaging of the 4 pc_fib search-range images that involve values .
The concurrent review of the multiple representations serves to visually assess the persistence of a physically valid ROI, while modifying the breast dielectric content assumption. The few cases in which the Low permittivity or High permittivity image was clearly opted indicate cases of breasts lying towards either the lower or the upper border of the dielectric constant range, as considered in the Wavelia MWBI software. The representation that was considered the cleanest among the three, as per visual inspection, is the one that was used as input to the Region-Of-Interest (ROI) extraction module, for ROI extraction and diagnostic feature analysis.
2.3.3. Wavelia MWBI 3D Imaging Outputs Layout
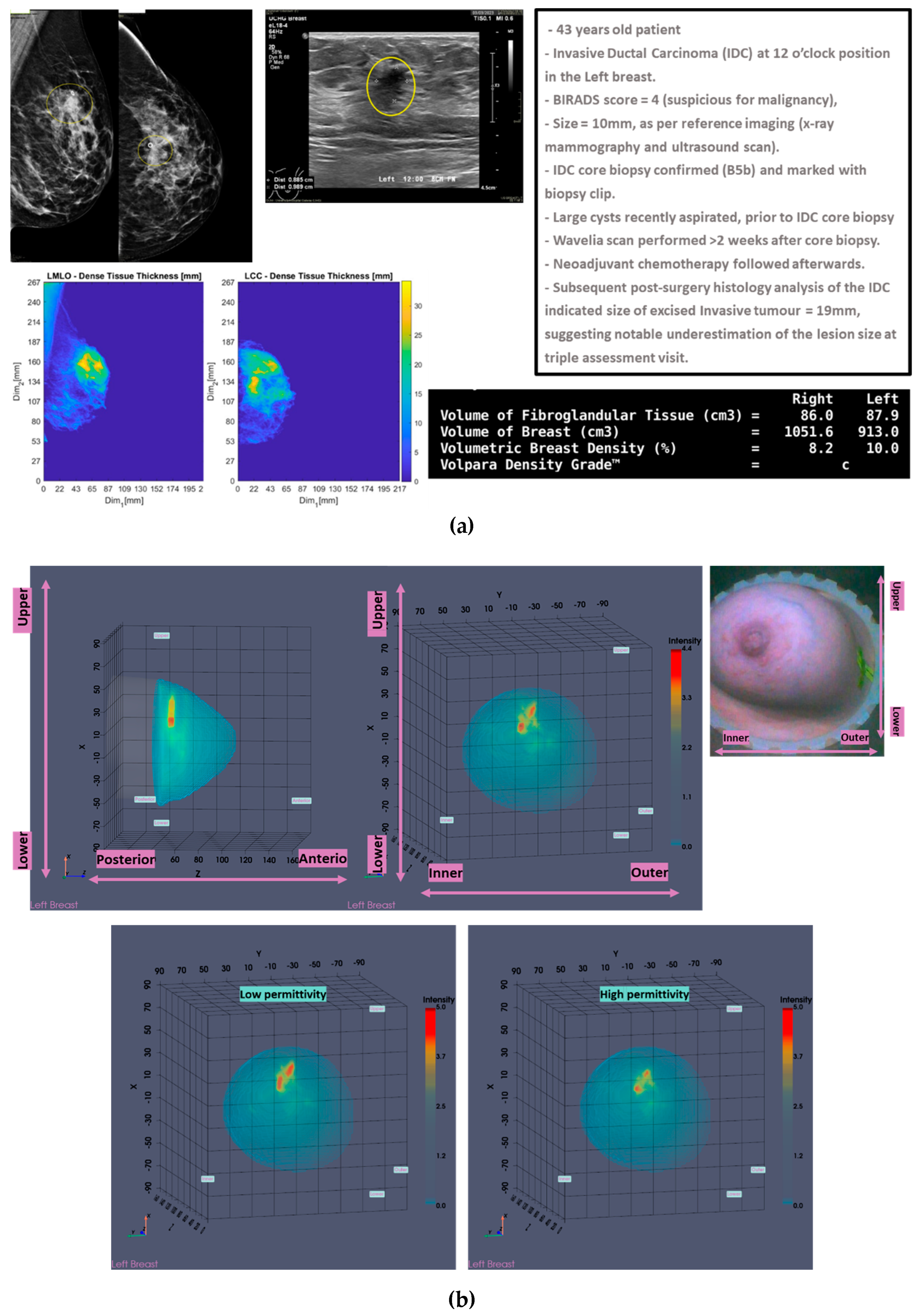
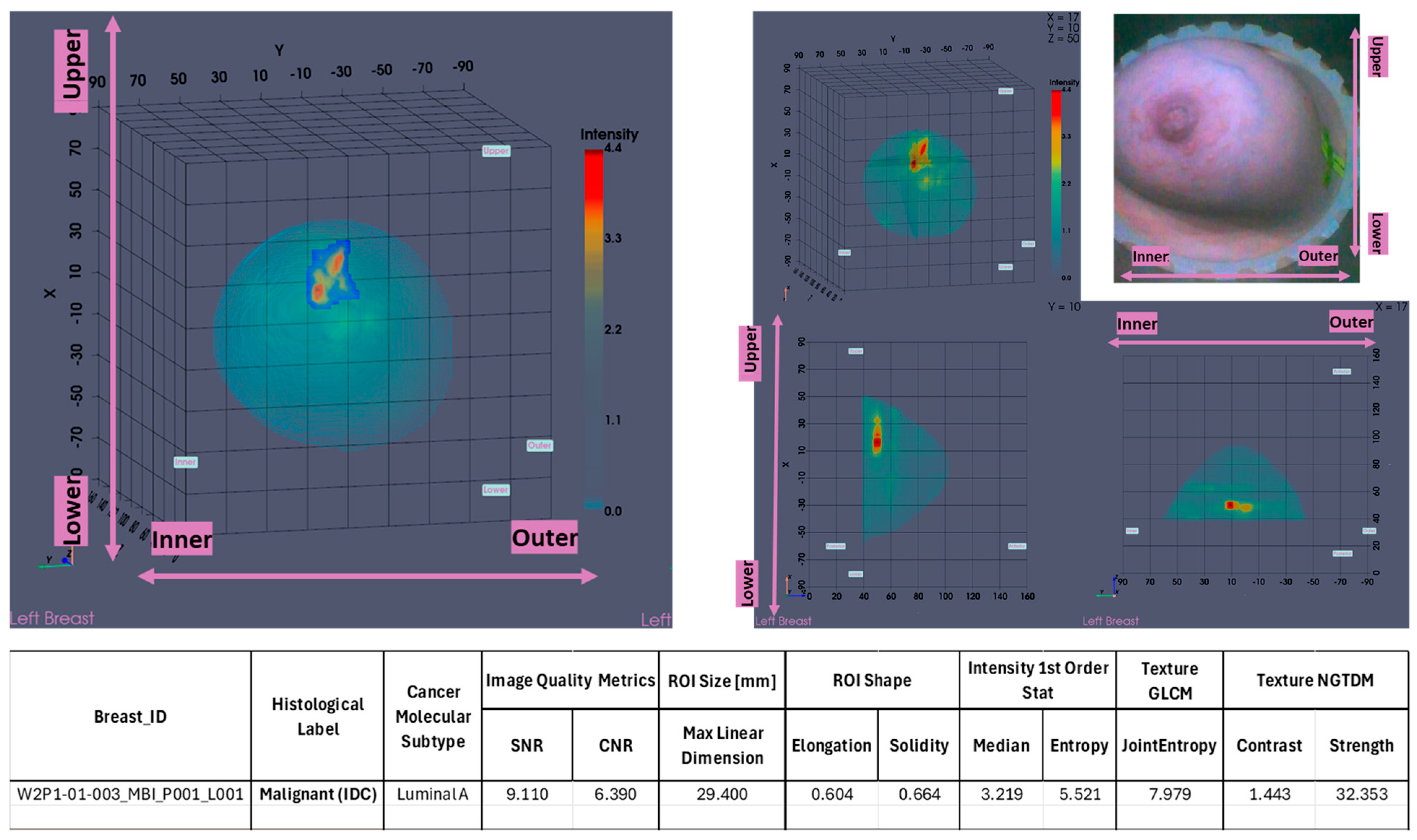
In
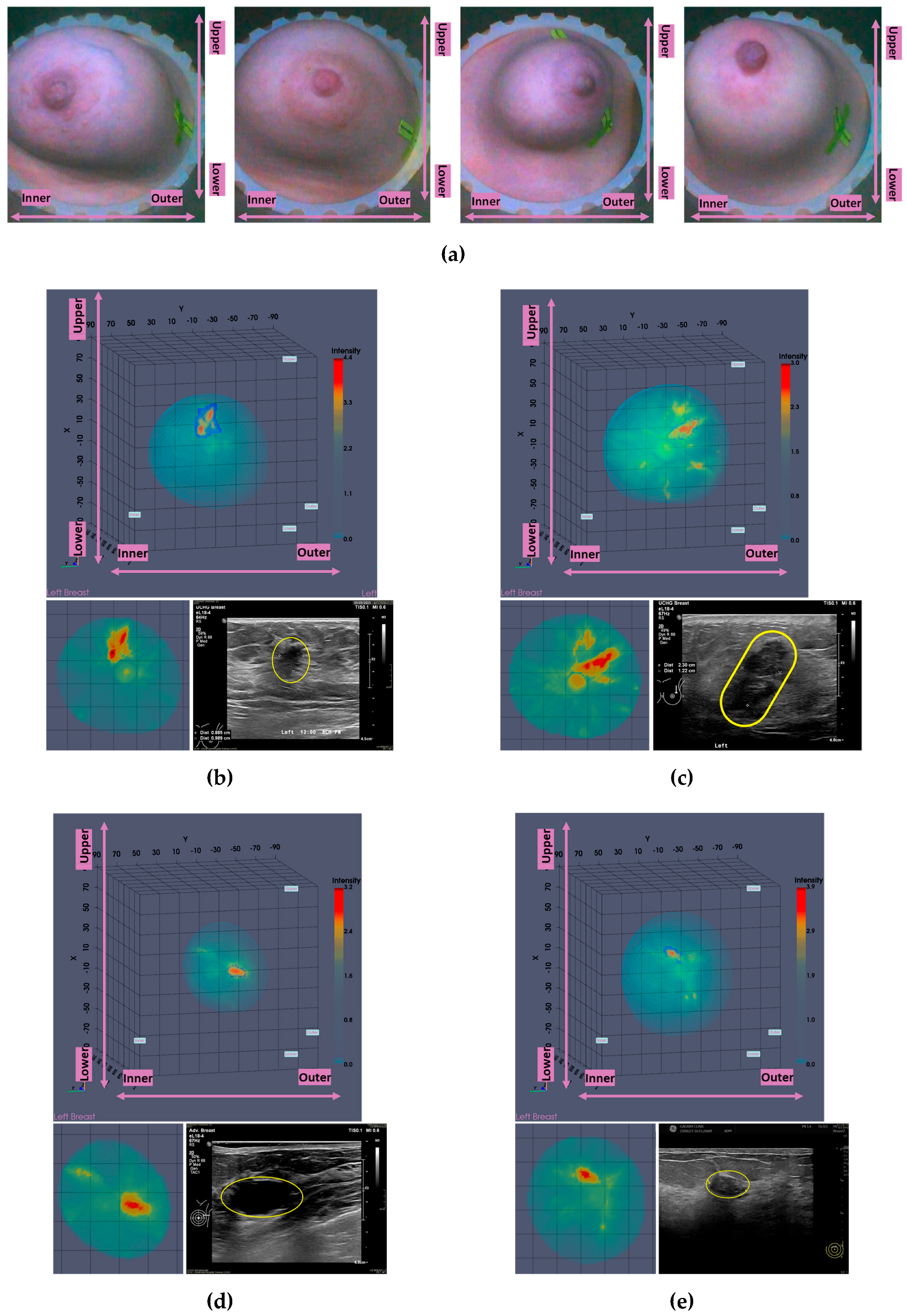
Figure 1, the layout of the Wavelia#2 MWBI 3D imaging results is depicted, as systematically presented for review aside the reference imaging and clinical data for an illustrative study case with an Invasive Ductal Carcinoma at 12 o’clock in the Left Breast (Patient 003, Left breast scan – P003-L).
The availability of the endoscopic camera image(s) provided as part of the Wavelia outputs was sufficient to explain the apparent misplacement of the lesion in the Wavelia MWBI images, compared to its radiological reference lesion location (as per x-ray mammography and/or ultrasound scan), in most patient cases where such ambiguity appeared. In
Appendix A, one of the few study cases with extremely misaligned position of the breast in the Wavelia MWBI scanner, resulting in difficult interpretation and explainability of the lesion apparent location in the Wavelia MWBI image for clinical association of the ROI with the reference, is presented. Few such cases appeared in the study and they were all fibroadenomas (soft-tissue, mobile lesions).
As part of the Wavelia MWBI scan data processing, the external envelope of the pendulous breast during the MWBI scan is first reconstructed and used to define the border of the two-propagation media: ‘in-breast’ and ‘out-of-breast’ in the MWBI imaging algorithm [
44]. The quality of the MWBI-based breast contour reconstruction is highly important for the quality of the MWBI images, while it also serves to define the shape and size of the breast, as inserted in the MWBI scanner. The methodological evolutions of the breast contour extraction module that were designed and implemented for the Wavelia#2 clinical investigation were recently presented in [
44]. In
Figure 1(b), the external surface of the estimated full scanned volume is depicted in light grey color and superimposed with the sagittal view of the 3D volumetric MWBI image, while applying partial transparency.
In addition, in this clinical investigation, a specific module for automatically identifying the uppermost (i.e. the closest to the examination table) scanned breast slice, for which adequate quality of scan was assured, has been employed, as an extension of the MWBI breast contour extraction module. Two main reasons for reduced quality of the MWBI scan at the posterior section of the pendulous breast have been identified during the clinical investigation and properly integrated in the module: (a) breast too large, potentially combined with sub-optimal positioning of the breast in the scanner, (b) part of the chest wall entering the scanning zone, mainly in cases of smaller breasts. The segment that was automatically ‘cut-out’ to define the partial breast scan data for imaging, if applicable, is systematically highlighted on all the side (sagittal) views of the Wavelia exported images, as depicted in
Figure 1(b).
The
Low permittivity and
High permittivity images for this breast are depicted at the bottom row of
Figure 1(b). While the Global Averaged image is systematically being used for image analysis in this clinical investigation, it is illustrated that the
Low permittivity representation contains a cleaner and more focused Region-Of-Interest (ROI) associated with the IDC in the case of this breast.
Automated, AI-driven, selection among the parametric MWBI imaging outputs available in the Wavelia scan results package, towards more efficient handling of the heterogeneity in the breast parenchyma, is pending to further enhance the achieved breast image quality in future MWBI developments.
2.4. MWBI Packaged Reporting per Detected Breast Lesion
2.4.1. ROI Extraction and Characterization: Multi-Dimensional Radiomics Features
The Wavelia#2 MWBI ROI extraction and validation module is based on the methodology earlier published in [
32] for breast lesion detection based on persistence, morphological image processing, structural (volume, solidity-based) and intensity contrast-based filtering, as introduced in the FiH clinical investigation with the Wavelia#1 MWBI prototype.
A series of operational evolutions in image post-processing [
51,
52] and thresholding [
53] were implemented to allow more standardized feature extraction on larger datasets in the Wavelia#2 clinical investigation. In addition, going beyond [
54] the limited scope of the Wavelia#1 FiH study involving analysis of only 3 specific features, the PyRadiomics Library [
40] was plugged to the Wavelia#2 ROI extraction module towards a more extended multi-dimensional radiomic feature analysis for the ROIs extracted from the Wavelia#2 MWBI images in this clinical investigation. PyRadiomics is an open-source comprehensive library, widely used and validated by the radiomics research community. It provides computation of a large set of first-order statistics, shape-based features (2D and 3D), and various texture features (GLCM, GLRLM, GLSZM, NGTDM, GLDM).
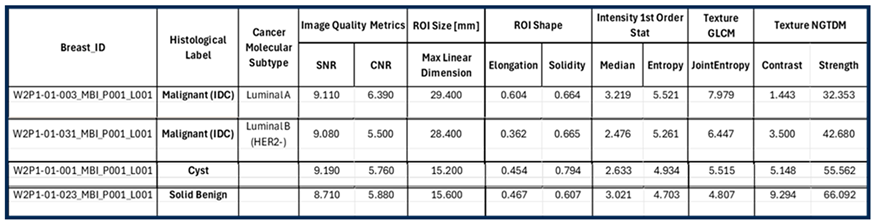
The maximal linear dimension [in mm] and the volume of the ROI, as well as the Signal-to-Noise Ratio (SNR) and Contrast-to-Noise Ratio (CNR) quality metrics are also systematically computed in the Wavelia#2 software for each extracted and validated ROI. All the information is exported in a .csv results file, available as input for multidimensional statistical analysis. A table including a selected subset of the exportable features for each ROI is shown at the bottom of
Figure 2.
2.4.2. Wavelia MWBI 3D Image Analysis Outputs Layout
In
Figure 2, the layout of the Wavelia MWBI 3D volumetric breast image analysis packaged output is illustrated for the same study case (P003, IDC at 12 o’clock position of the Left Breast).
The Wavelia MWBI scan image analysis output includes : (a) the set of extracted ROIs localized with reference to the reconstructed external surface of the breast, (b) Image Quality metrics assessment (Signal-to-Noise Ratio (SNR) and Contrast-to-Noise Ratio (CNR), (c) ROI size estimation: maximal linear dimension [mm], (d) Radiomic features computation for ROI characterization: shape descriptors, 1st order statistics of ROI intensity, Gray-Level Co-occurrence Matrix (GLCM) texture and Neighbor Grey-Tone Difference Matrix (NGTDM) texture metrics.
2.5. MWBI Multi-Modal Imaging: Parameterized Interaction Mechanisms Between Microwaves and the Imaged Breast at Varying Geometrical and Tissue Consistency Conditions
A highly diverse clinical study population in terms of: breast size, age, breast density, lesion size and depth of the lesion location in the breast, as defined in
Section 2.1 for the Wavelia#2 clinical investigation, is associated with a set of identified technical challenges for MWBI: (a) significantly varying distances between the probe array and the breast, (b) significantly varying levels of attenuation of the electromagnetic waves when propagating within a very dense breast or a fatty breast, (c) significantly varying speed of the waves propagating in the highly heterogeneous breasts. Tailored data preprocessing and filtering of the MWBI scan data before feeding the imaging algorithm is required, to ensure good quality MWBI images with reliable diagnostic content in all the distinct geometrical and breast tissue consistency configurations. Three modes of operation have been defined in the context of this clinical investigation for the Wavelia#2 MWBI system.
2.5.1. Wavelia#2 MWBI Scan Data Preprocessing Scheme Revisited
A block diagram with the main steps of the evolved MWBI scan data preprocessing scheme, as implemented in the Wavelia#2 scanner and earlier outlined in [
38,
44] is shown in
Figure 3.
Empirical Mode Decomposition (EMD) [
55] for MWBI scan data preprocessing was initially introduced to the Wavelia#2 MWBI scan data processing in [
38], then again in [
44], jointly focusing on the importance of the breast contour envelope for defining the border in/out of the breast for distance-based filtering. In Wavelia#2 MWBI, EMD is applied to the time-domain signal of each bistatic Tx/Rx channel, resulting after Inverse Discrete Fourier Transform (IDFT) of the measured S21 scattering parameters, as measured with a Vector Network Analyzer (VNA) in a stepped-frequency sweep configuration. The time-domain signal is an analytic signal, as there are physically no negative frequencies in the spectrum of the measured frequency-domain signal. This allows to apply the EMD on the real part of the time-domain signal and retrieve back the complex-valued analytic signal via Hilbert transform, without any loss of information [
55].
EMD is a powerful estimation tool allowing the decomposition of the signal to a number of Intrinsic Mode Functions (IMFs), each one associated with a distinct, physically meaningful, portion of the scattered wave. In Wavelia#2, EMD has been adopted for the first time to pre-process MWBI scan data, by identifying and filtering-out the residual unwanted interference of strong intensity, associated with the inter-probe coupling and/or reflections on the breast skin. These are identified as separate IMFs, after mapping either on the time-domain or the frequency-domain of the original signal space.
Distance-based filtering: (a) Filter-out IMF’s associated with residual antenna coupling: time-domain signal with maximum amplitude at very close distances to the antennas. (b) Filter-out IMF’s associated with radar-echoes from unrealistically long distances (multipath), to reduce signal complexity and stabilize the performance of the sectorized TR-MUSIC imaging algorithm [
32,
33,
34].
Propagation loss compensation: Required for the Time-Reversal principle to remain practically valid. Apply the classical term, originally defined for the transmission lines. It is a multiplicative term, applied to the frequency-domain signal associated with each IMF:
with
d being the distance from which the radar echo associated with each IMF originates,
f the operating frequency,
c0 the speed of light,
er the dielectric constant and
tanδ the loss-tangent of the propagation medium. The propagation loss compensation term is further computed as
, with
and approximation of
and
using the available estimate of the breast external surface and
d, as defined in [
44].
Amplitude-based filtering: The Power Spectral Density (PSD) integrated over the full length of the signal is expected to remain contained below a certain threshold (
PSDtot_threh < 5), at nominal behavior of the MWBI system, as benchmarked during testing and validation with experimental breast phantoms [
38], then confirmed with the majority of human breast scan datasets. This fixed threshold value does not apply optimally to all the breast configurations.
2.5.2. Custom Filters Defined During the Clinical Investigation
The Amplitude-based filtering is disabled to avoid unintentional over-filtering of useful portion of the signal, in case of smaller breasts and / or large lesions superficially located within the breast. In these two configurations, it has been observed and experimentally confirmed that the default PSDtot_threh value may be too strict, resulting in partial elimination of radar echoes contributing to the lesion imaging and detection.
As defined in [
38], the default sectorization applied for multi-static radar imaging with the Wavelia#2 MWBI scanner prototype employs in each azimuthal imaging sector Ns = 8 probes (out of the total of 21 probes covering the full circumference of the scanner). As depicted in the block diagram in
Figure 3, the Principal Component Analysis (PCA), which is performed before the EMD, employs by default
probes, thus extending the imaging sector by 2 probes bilaterally.
In case of very large breasts, or if skin folds are present generating strong artefacts in the scan, or in cases of breasts sub-optimally positioned in the scanner, thus approaching too much the probe array, a reduction of the arc length of the probe sub-array over which PCA is performed to define and subtract-out the common strong component of antenna coupling, tends to be more efficient, as more severe filtering is required. probes, thus extending the imaging sector by only 1 probe bilaterally (instead of 2 in the default setting) has been applied to define the Custom Filter#2 during this clinical investigation.
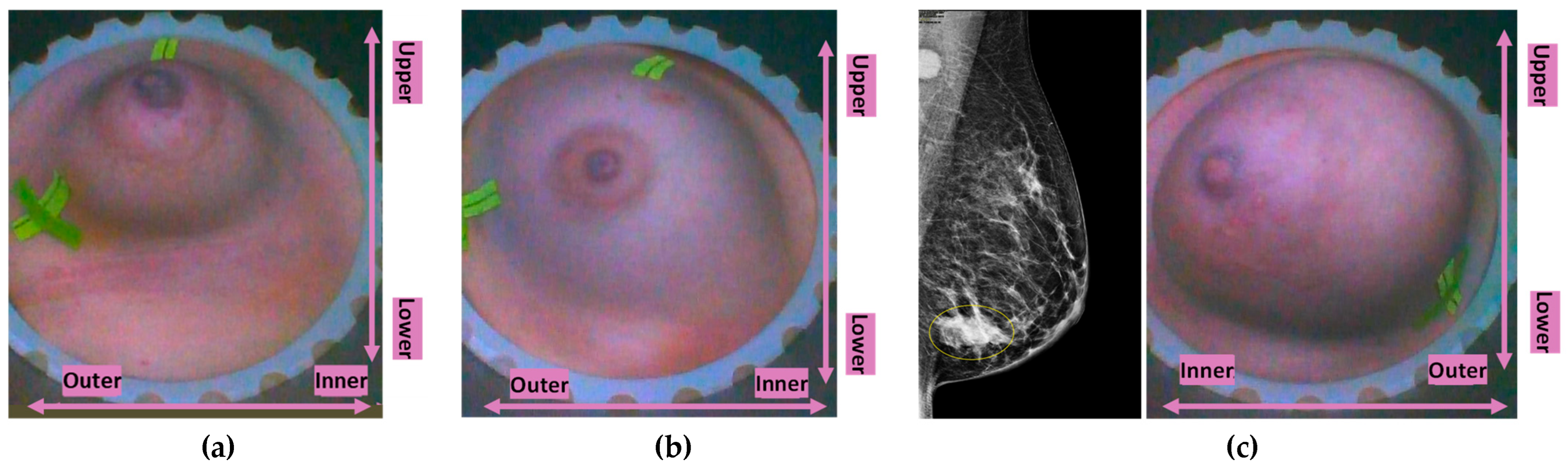
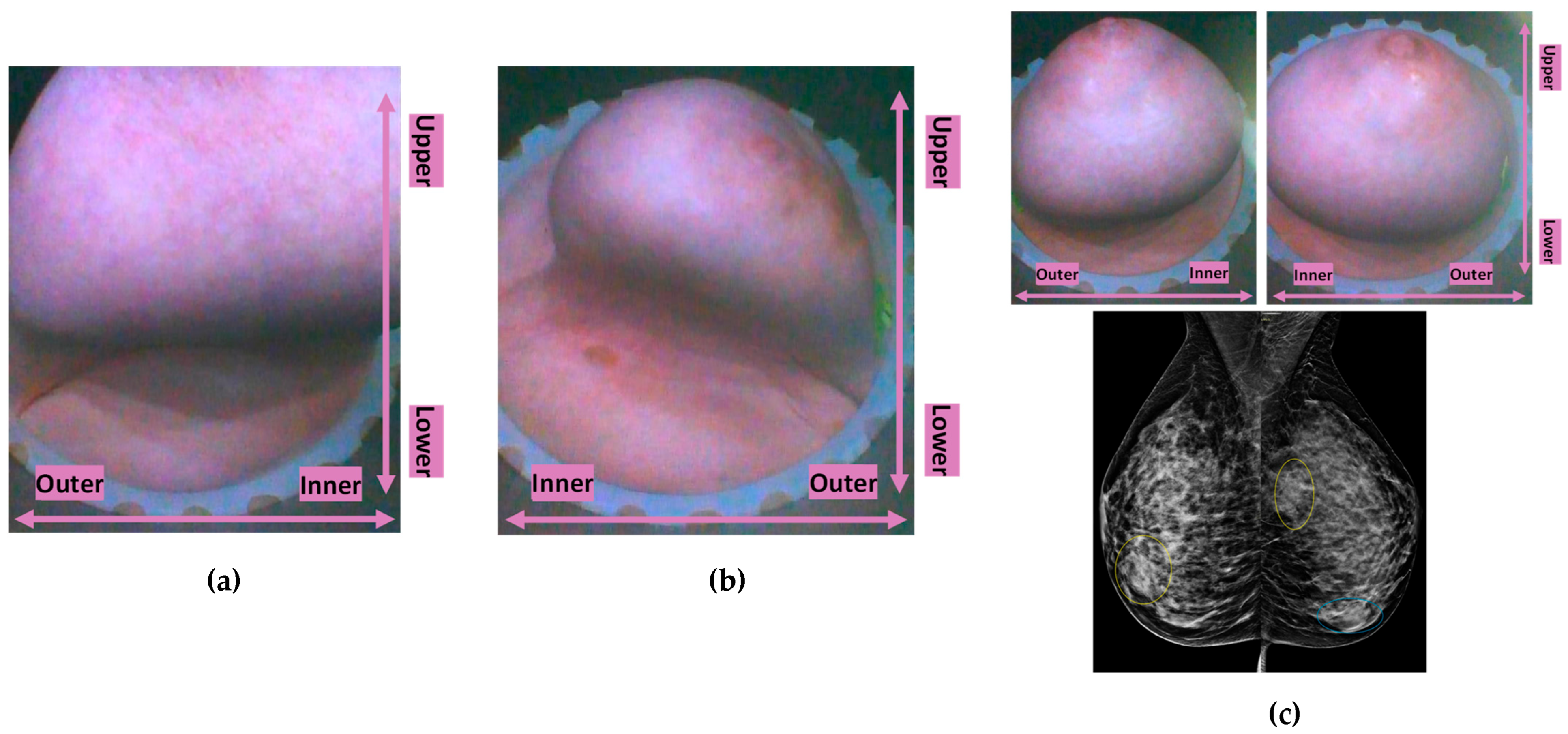
Typical breast imaging settings in which the Custom Filter#1 or the Custom Filter#2 was best-fitting are depicted in
Figure 4 and
Figure 5 correspondingly.
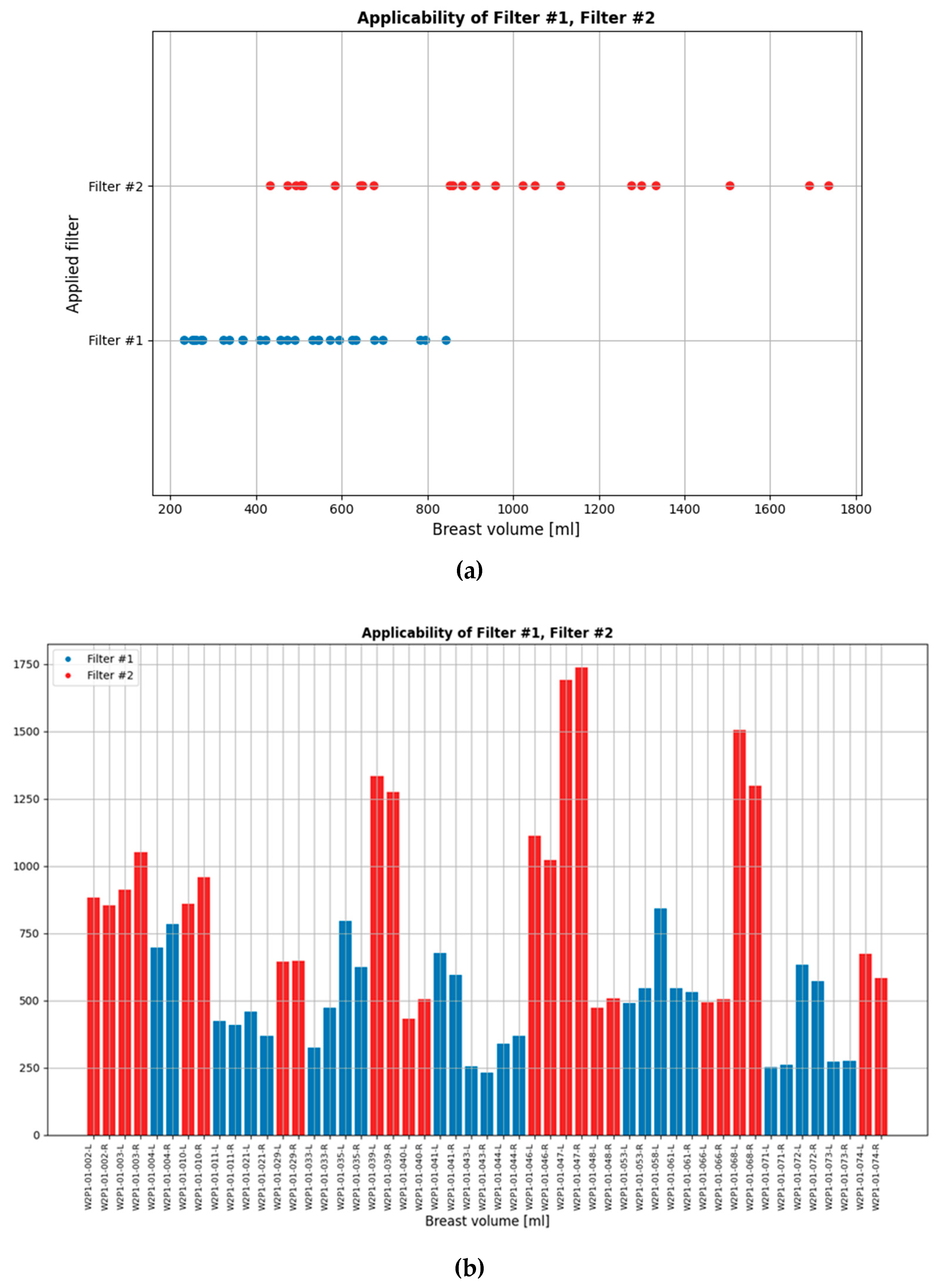
While the default setting of the data processing scheme was applied to most patient datasets in the clinical investigation, the two custom filters were applicable to non-negligible portions of the full analysis dataset. These are indicated in
Figure 6. The bilateral breast scan of the 62 evaluable patients that were included in the clinical investigation, i.e. a total of 124 breast scans, defines the full analysis dataset.
2.6. Malignant-to-Benign Lesion Separability Analysis
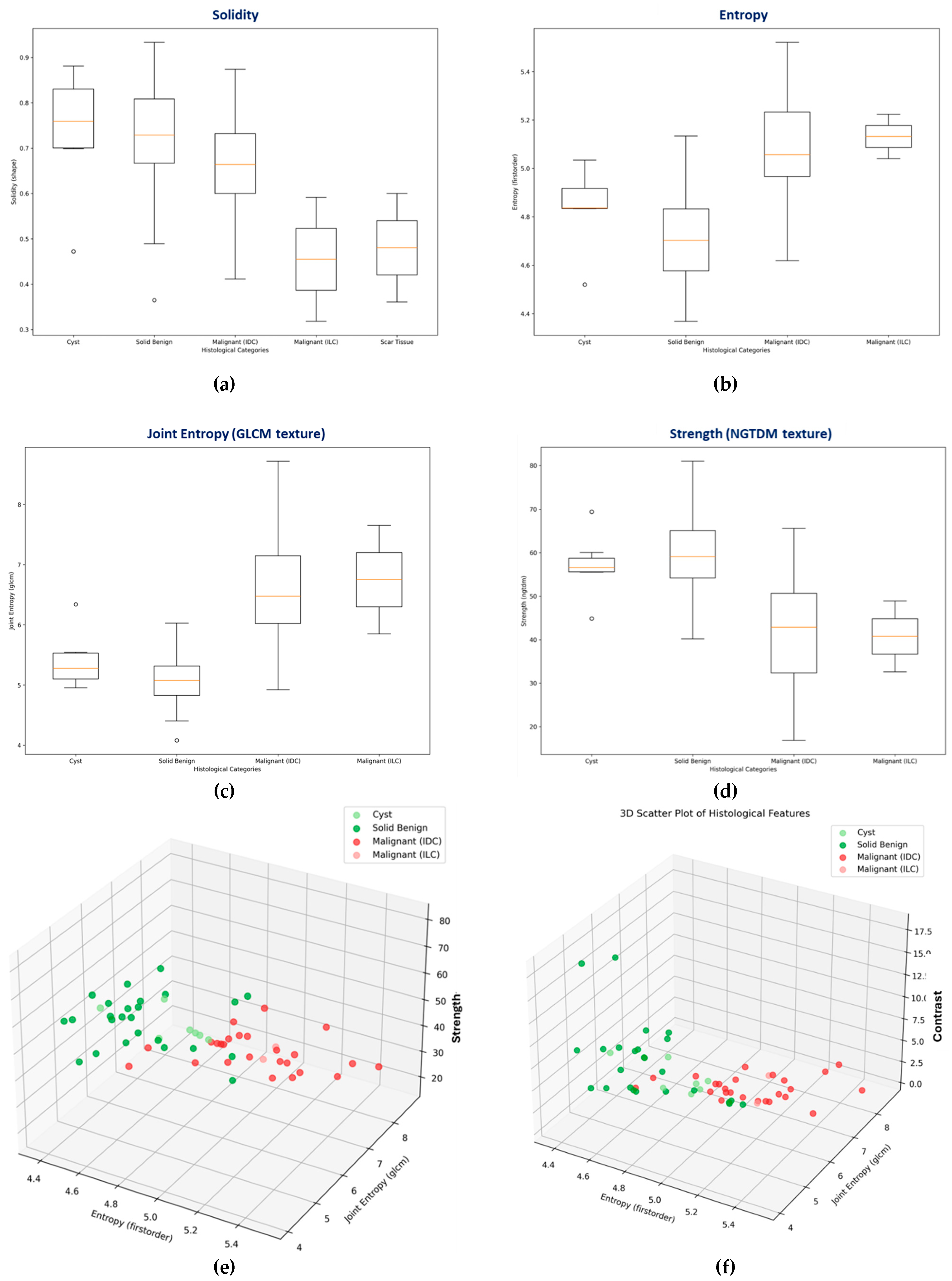
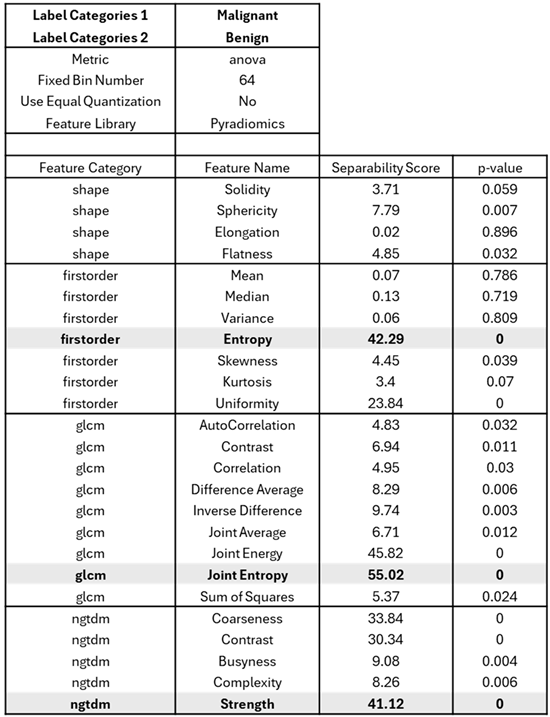
Traditional Analysis Of Variance (ANOVA) F-testing was applied for inter-class separability assessment, per analyzed feature, as computed in the PyRadiomics library for each extracted ROI from the MWBI 3D breast images in this clinical investigation. ANOVA calculates the F-statistic and corresponding p-value. A higher F-value indicates greater variance between the groups compared to the variance within the groups, suggesting better separability. The p-value indicates statistical significance. This analysis quantifies how well features distinguish between two user-defined groups of labels (classes).
The focus of the analysis was on the separability assessment between the malignant and benign lesion classes, for all the clinically relevant ROIs associated with the full set of malignant and benign lesions which were detectable with the Wavelia#2 MWBI prototype in this clinical investigation. The Results of this analysis are presented in
Section 3.1. The indicative set of radiomic features with higher diagnostic accuracy, i.e. higher F-statistic associated with low p-value, suggesting higher inter-class variability compared to the level of observed intra-class variability, thus also suggesting better separability between classes, are highlighted and discussed in the Results
Section 3.1. Interesting tendency for separability between the two classes, mainly while employing texture features in a multidimensional space, is demonstrated with the presented results.
2.7. Phenomenological Qualitative Analysis of Unspecified Findings
The malignant-to-benign lesion separability analysis was further expanded in this clinical investigation data analysis, to assess the separability between malignant lesions and unspecified findings (i.e. non clinically relevant ROIs extracted from the Wavelia MWBI images).
A separate class was defined in this analysis to include all the unspecified findings.
A new module was developed as part of the Wavelia#2 MWBI software to be used for the labeling of all the ROIs that were extracted from the MWBI images of the bilateral breast scan of the patients. The labelled data have been worked on and jointly reviewed by the clinical investigators, the R&D engineering team and an experienced radiologist / independent data reviewer.
Labelling of the ROIs was performed, to be associated with either: (a) the clinically relevant findings in the breast (as per reference data), (b) scar tissue in breasts known to have been operated in the past, (c) understood MWBI imaging artefacts.
All non-rationalized ROIs were left out in a separate class of ‘Unspecified findings’.
- 2.
MWBI imaging artefacts:
Four categories of recurrent and well recognizable imaging artefacts have been defined during the analysis of the Wavelia#2 MWBI study images. The corresponding labelled data have been tabulated, such that they can be reported and analyzed separately. Indicative cases of bad-quality MWBI scan cases generating such imaging artefacts are depicted in
Appendix B. One example for each of the four identified categories of imaging artefacts is shown.
- 3.
Unspecified findings analysis:
The rationale behind a preliminary phenomenological analysis towards assessment of the specificity of the MWBI technology in healthy breasts is outlined below:
First, to establish a notion of the level of outstanding / significant intensity of ROI for each subject, the Wavelia MWBI images for the bilateral breast scan of each symptomatic patient, for whom the dominant discrete lesion in their main symptomatic breast was detectable with Wavelia#2, were simultaneously reviewed.
The unspecified findings (i.e. non-clinically relevant ROIs) with intensity level comparable or superior to the intensity level of the detected dominant discrete lesion ROI of each symptomatic patient were only retained for analysis.
The unspecified findings of outstanding intensity and non-categorizable as imaging artefacts were mapped on the malignant-to-benign lesion separability feature space.
The number of unspecified findings being confused with the malignant lesions was quantified, to derive a preliminary indicator of specificity of the new imaging modality.
3. Results
3.1. Malignant-to-Benign Lesion Separability Assessment
The Wavelia#2 clinical investigation [
39] dataset, on which MWBI-based ROI characterization to assess the malignant-to-benign lesion separability was performed, included:
-
62 evaluable patients enrolled in the study: bilateral MWBI breast scan analyzed.
- ○
124 MWBI breast scans in total
-
8 patients with bilateral lesions, as per reference clinical/radiological data.
- ○
70 breast lesions targeted for detection and characterization with MWBI.
60 out of the 70 lesions detectable with MWBI.
-
59 detected lesions with clinically relevant and validated ROI for multi-dimensional radiomic feature analysis and characterization with MWBI.
- ○
1 study patient (P004-L) had advanced disease. Even though the MWBI imaging outputs were considered relevant by the clinical investigators, the breast zone that was affected by the disease was too extended, such that extraction of an associated, localized, ROI for feature analysis and characterization was not deemed meaningful in such a case.
The full analysis set was balanced in terms of representation of malignant and benign lesions, including:
The separability score (ANOVA F-statistic) and its significance level (p-value) is reported in
Table 1 for the set of features that were computed with PyRadiomics [
40] and included in the malignant to benign lesion separability analysis.
The subset of analyzed features that showed the highest and significant diagnostic accuracy, for discriminating between malignant and benign lesions in the Wavelia#2 clinical investigation dataset are highlighted in
Table 1 and listed below:
1st order statistics of image intensity:
Grey-Level Co-occurrence Matrix (GLCM) texture:
Neighbor Grey-Tone Difference Matrix (NGTDM) texture:
Box-plots representing the distributions of the most discriminative features, per lesion class (i.e. histological type) are shown in
Figure 7(b)-(d). A box-plot for the solidity shape descriptor, which was employed during the Wavelia#1 FiH study as main discriminator between malignant and benign lesions [
36] is also shown in
Figure 7(a). Cysts show a clear tendency for higher solidity values, while ILC’s and scar tissue (i.e. the non-mass-like ROIs) show a tendency for separability in terms of lower solidity. However, limited diagnostic accuracy for the discrimination between IDCs and solid benign lesions is reported, while analyzing the solidity feature on the larger and more diverse dataset of this 2
nd clinical investigation.
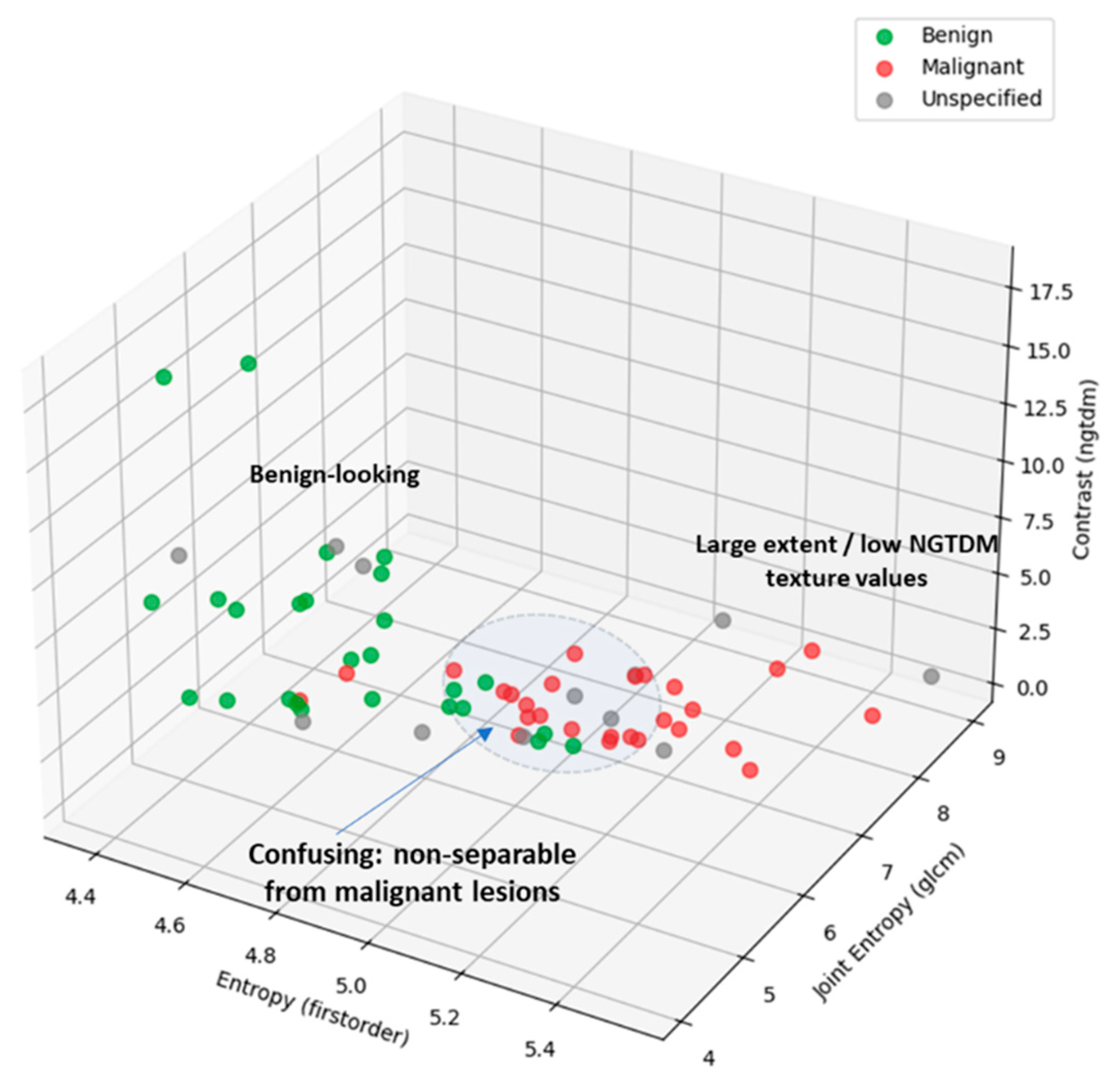
3D Scatter Plots, allowing to explore the relationship between three features simultaneously are depicted in
Figure 7(e)-(f), for two different combinations of features.
3.2. Typical Malignant and Benign Lesions: MWBI Images and Extracted Lesion Features
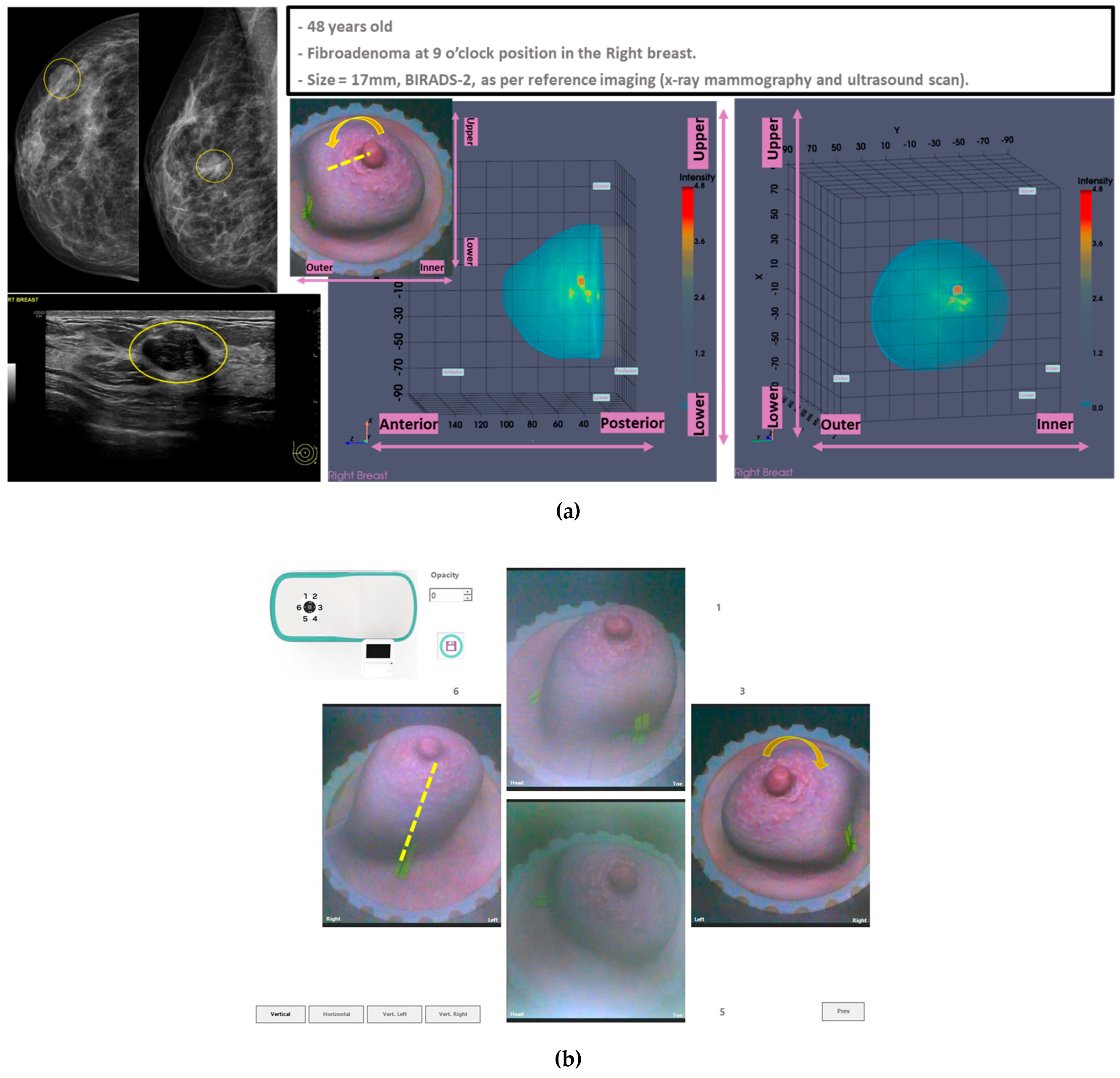
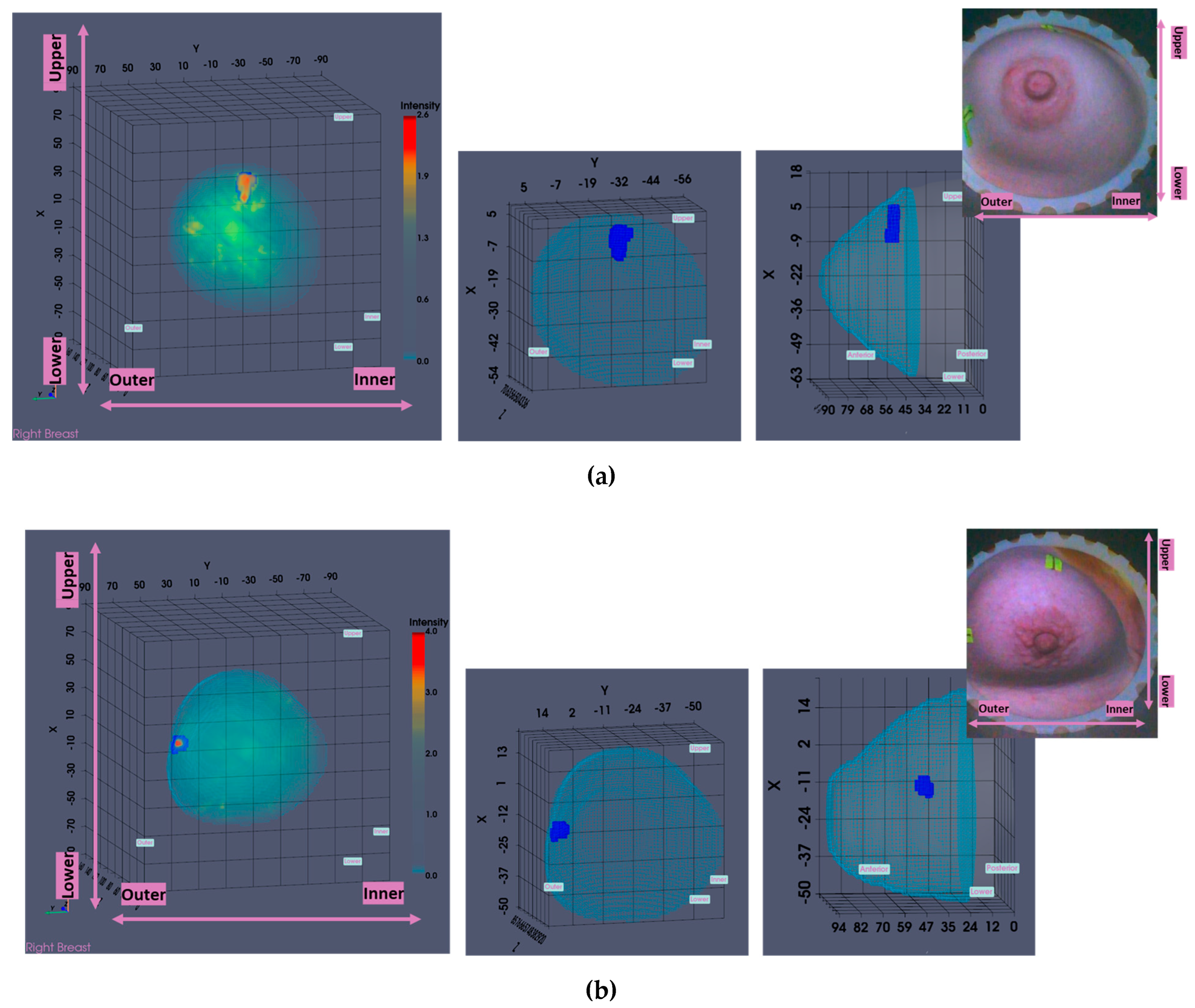
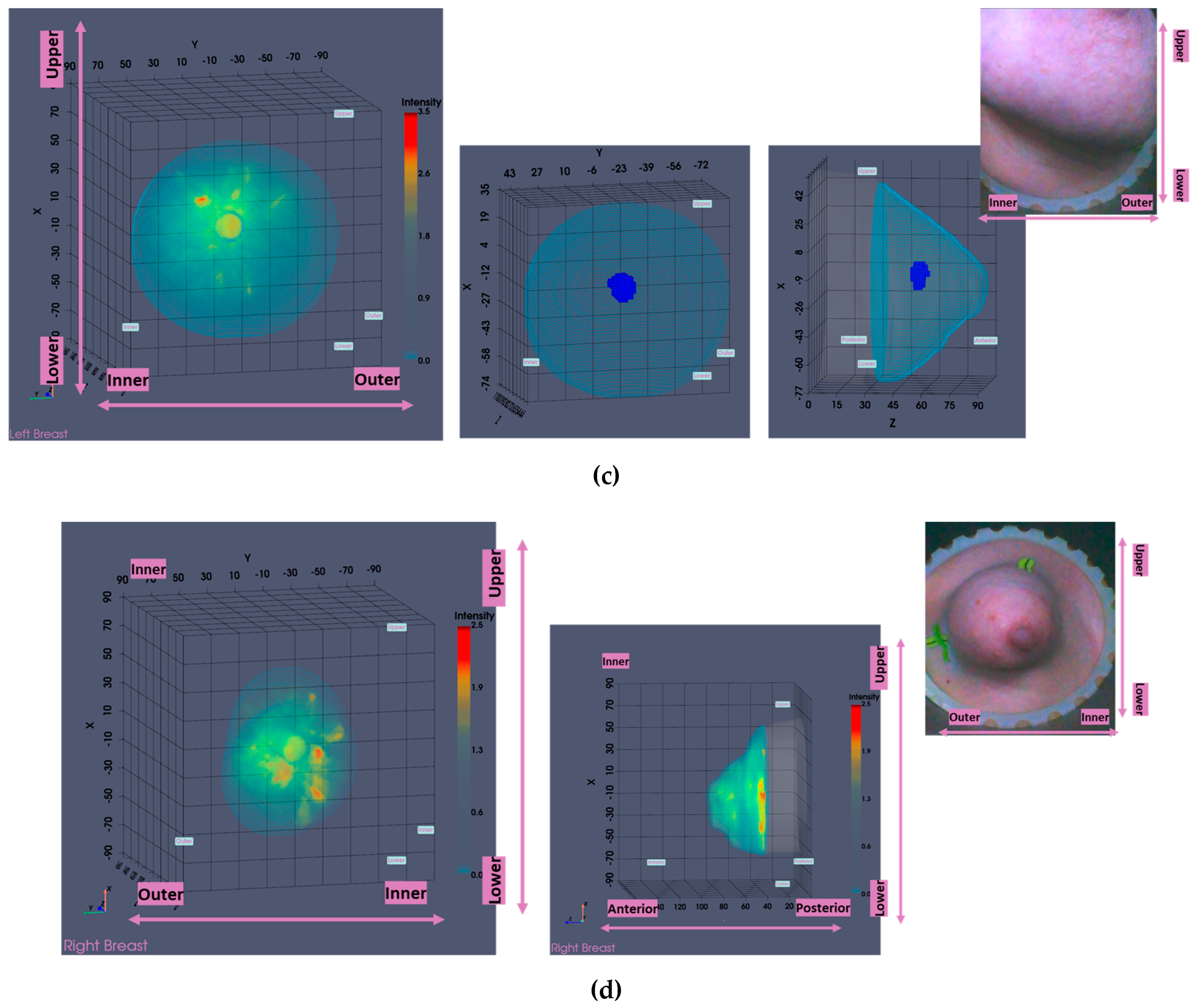
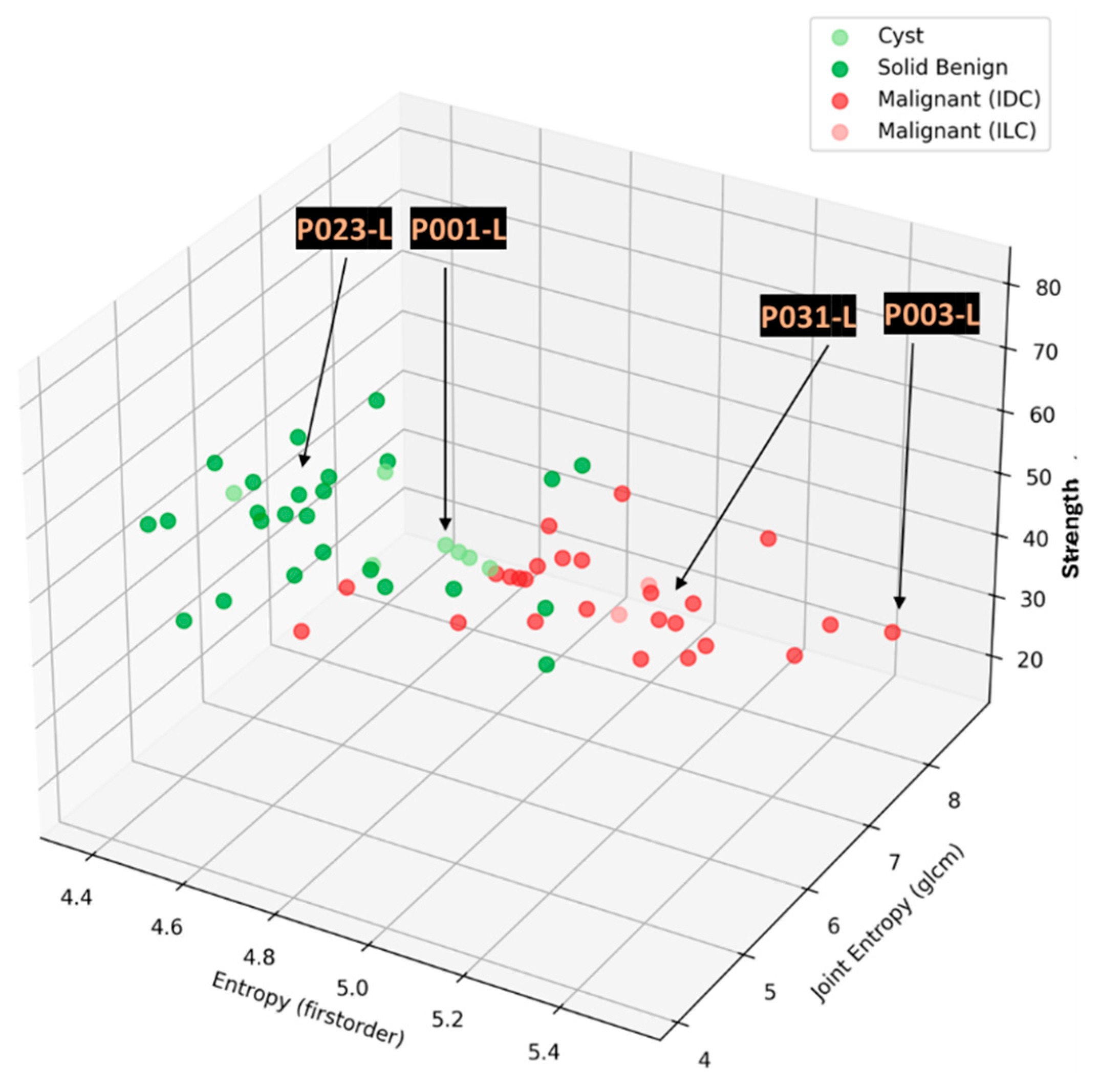
In this section, the characteristics of 4 typical cases of breast lesions -2 malignant and 2 benign- with MWBI scan of good quality, resulting in a clear representation of these lesions as malignant or benign in the Wavelia feature analysis space, are discussed.
The principal characteristics of these lesions and a representative subset of the features that were computed for the associated ROIs, as extracted from the MWBI images, are summarized in
Table 2. The points that represent the 4 lesions on the scatter plot of an indicative 3-dimensional representation of the Wavelia MWBI feature analysis space are highlighted and labelled in
Figure 8.
An illustrative subset of the Wavelia MWBI imaging outputs, together with the ultrasound reference scan, are depicted in
Figure 9 for each of the 4 cases. In
Figure 9(a), endoscopic camera views showing the position and orientation of the 4 breasts in the MWBI scanner are depicted.
A quite stable Signal-to-Noise Ratio (SNR) level, in conjunction with overall comparable Contrast-to-Noise Ratio (CNR) levels, slightly increased in the case of Patient 003 (IDC, molecular subtype Luminal A) is observed in
Table 2 for the 4 cases. The SNR measures the ratio of the mean signal intensity within the ROI to the standard deviation of the background noise. A higher SNR generally indicates a better image quality. The CNR measures the difference between the mean ROI intensity and the mean background intensity, relative to the background noise. CNR quantifies how well the ROI stands out from its surroundings. Insightful comparison of these levels to the ones of the images in confusing cases of lesions, which are not clearly identifiable as malignant or benign on the Wavelia MWBI feature analysis space, is provided in the following section.
Good correspondence between the morphology and maximal linear dimension of the extracted ROIs from the Wavelia MWBI volumetric images and the ultrasound reference representations of the lesions is notable by comparative visual inspection of the images in
Figure 9(c)-(e). The case in
Figure 9(b) is the same case used in
Section 2 to illustrate the layout of the Wavelia MWBI imaging and image analysis outputs in
Figure 1 and
Figure 2 accordingly. Based on the more detailed clinical reference information provided for this study case in
Figure 1(a), there is clear evidence that the ultrasound scan provided only partial visibility of this IDC, resulting in severe underestimation of its size in this case. The 19mm size of the excised tumor as per post-surgery histology report, also noting that the patient underwent neoadjuvant chemotherapy before surgery and after the Wavelia MWBI scan of the breast, renders the MWBI-based estimate of the lesion size relevant and worthwhile highlighting in this case.
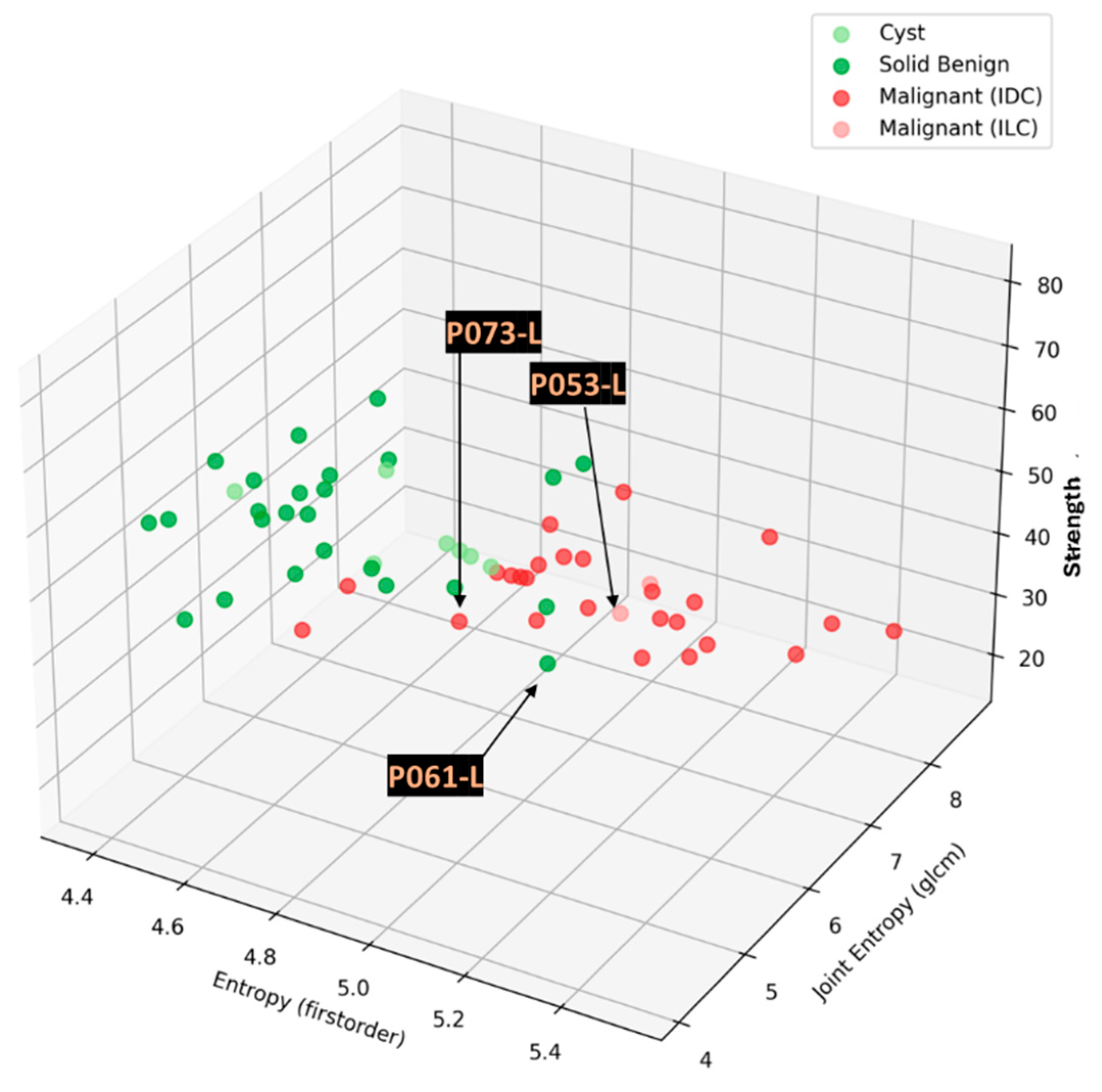
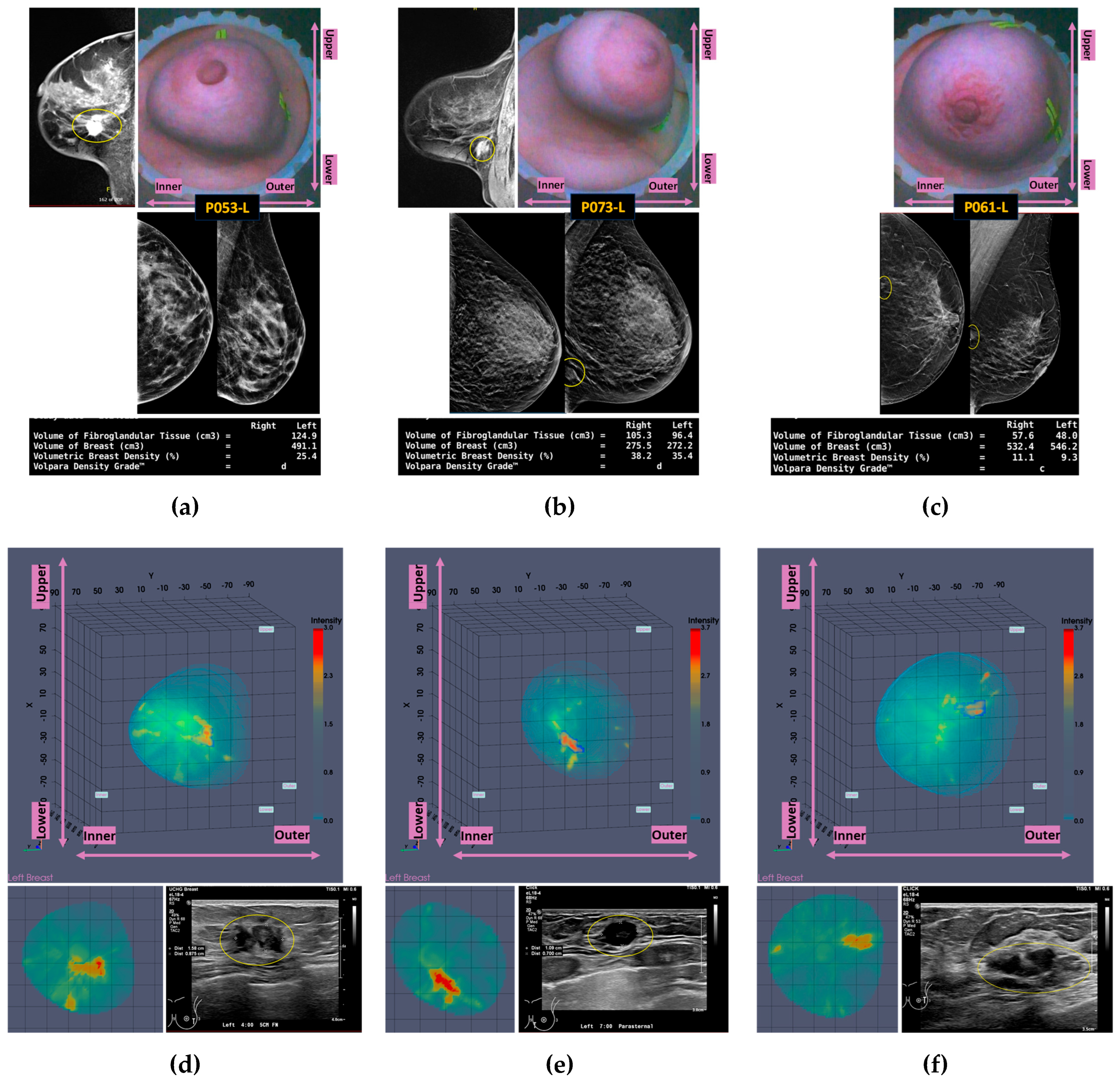
3.3. Confusing Cases Due to High Breast Density and/or Challenging Lesion Location in the Breast
In this section, the characteristics of 3 unclear cases of breast lesions -2 malignant and 1 benign- with MWBI scan of moderate quality, resulting in a confusing representation of these lesions as malignant or benign in the Wavelia feature analysis space, are presented. The principal characteristics of these lesions and a representative subset of the features that were computed for the associated ROIs, as extracted from the MWBI images, are summarized in
Table 3. The points that represent the 3 lesions on the scatter plot of the Wavelia MWBI feature analysis space are highlighted and labelled in
Figure 10.
An illustrative subset of the Wavelia MWBI imaging outputs, together with the ultrasound reference scan of the lesion, the x-ray mammogram, and the MRI scan where available, are depicted in
Figure 11 for each of the 3 cases. Endoscopic camera views showing the position and orientation of the 3 breasts in the MWBI scanner are also integrated in
Figure 11.
Lower SNR values are systematically reported for all the 3 cases in
Table 3, compared to the stable SNR values reported in
Table 2 for the four typical cases of good-quality MWBI scan in the previous section. The CNR values are also lower here, more notably for the Invasive Lobular Carcinoma (ILC) case of Patient 053.
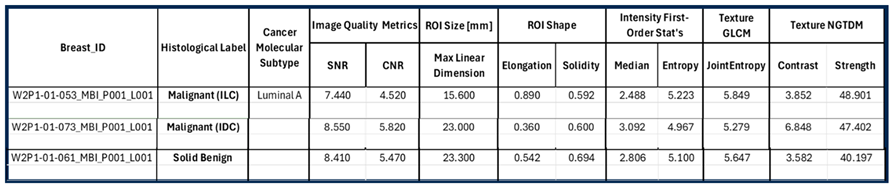
The mammographic images of the breasts provided as reference in
Figure 11, together with the associated computational outputs of Volpara Lab, reveal the highly challenging ROI extraction conditions due to very high breast density in the cases of Patients 053 and 073 (Volumetric Density Grade (VDG) score d for both P053 and P073, associated with VBD = 25.5% and VBD = 35.4%, correspondingly).
An additional complexity factor, valid in the cases of Patients 073 and 061 is the location of the lesion in high proximity to the chest wall. These are both cases of small / medium-sized breasts well inserted in the Wavelia MWBI scanner. The zone in proximity to the chest wall, where the lesions are located as per MRI and x-ray mammographic reference data, is expected to be well illuminated by the Wavelia scanner. However, the MWBI image in this zone is of limited contrast and diagnostic value due to the high dielectric constant and electromagnetic wave propagation losses, induced by the pectoral muscle and bones present in this zone [
10,
11].
The challenging conditions related to the expected low levels of dielectric contrast of the lesions in the aforementioned breast settings may explain, at least partially, the unclear representation of these lesions, and their confusion as either malignant or benign, when mapped on the Wavelia MWBI feature analysis space. Nevertheless, by comparative visual inspection of the Wavelia MWBI, the Ultrasound and the MRI scans in
Figure 11, it is worth noting that the morphology of the lesions on the Wavelia MWBI images is still quite well correlated with their morphology on the reference radiological images. In the case of Patient 073, the lesion morphology on the Wavelia image appears to better match the MRI reference image, rather than the Ultrasound one. Post-surgery histology data were made available as reference for this study case. The size of the invasive tumor in the excised tissue specimen was 16mm, well matching the 17mm MRI-based lesion size estimate. The ultrasound scan provided only partial visibility of this IDC resulting in severe underestimation of its size to 11mm. The Wavelia MWBI-based estimate of the maximal linear dimension of the lesion was 23mm. The total tumor size was 49mm, as per post-surgery histology report.
3.4. Phenomenological Qualitative Assessment of MWBI False Positives Rate in Healthy Breasts
The methodology that was described in
Section 2.7 was applied for a preliminary assessment of the Wavelia MWBI false positive rate (unspecified findings) in healthy breasts with no suspicious, clinically-reportable, finding. The healthy contralateral breasts of patients, for whom the dominant discrete lesion in the main symptomatic breast was detectable with Wavelia MWBI, were included in this analysis. All the patients with bilateral reported lesions, or other specific findings in the contralateral asymptomatic breast, such as scar tissue due to prior surgery in the breast, were excluded from this sub-analysis.
The analysis included in total 41 contralateral healthy breasts of symptomatic patients.
13 unspecified ROIs of non-negligible intensity in the contralateral breast, compared to the intensity of the symptomatic lesion ROI for the same patient, were extracted in total, out of the full analysis set. These could not be attributed to any of the 4 categories of identifiable MWBI imaging artefacts at the actual stage of development, as illustrated in
Appendix B.
The Unspecified ROIs are represented by points of grey color, when mapped on the Wavelia MWBI feature analysis space in
Figure 12. It is highlighted on the scatter plot that only 4 out of the 13 unspecified ROIs in a total of 41 healthy breasts appear to be confusing and non-separable from malignant lesions, when analyzed in terms of texture and 1
st order statistics of intensity, at this stage of development of the MWBI system.
This phenomenological analysis suggests a ~10% false positives rate for Wavelia MWBI, while assuming controlled levels of intensity in the MWBI images.
4. Discussion
To our knowledge, Wavelia is the only MWBI prototype system that generates 3D volumetric images of the breast for visual inspection and clinical interpretation, in conjunction with a comprehensive image analysis set per extracted ROI, to support semi-automated detection and characterization of breast lesions based on intensity, shape and texture features. In this study, the potential of the Wavelia#2 MWBI technology to support discrimination between malignant and benign lesions, further building on the preliminary findings reported for the Wavelia#1 FiH study in [
36], is demonstrated in the presented results. In this dataset, more information lies in the texture and intensity statistics–based features. Analysis in a multi-dimensional radiomic feature space, and not in a restricted 3-dimensional space combining a specific and well-defined set of shape and texture descriptors as optimistically suggested for Wavelia#1 [
36], was more appropriate.
A highly diverse clinical study population in terms of breast size, age, breast den-sity, lesion size and depth of the lesion location in the breast was included in the Wavelia#2 clinical investigation, thus being associated with a set of identified technical challenges for the MWBI system, to handle the geometrical diversity and breast tissue heterogeneity. A multimodal setting was designed for the MWBI scan data preprocessing scheme, towards standardization of the imaging outputs and controlled specificity, a main barrier for clinical validation and acceptance of the microwave breast imaging modality by now. At this stage of development of the Wavelia MWBI technology, the attribution of the best-fitting mode of operation to each breast scan dataset was performed by the R&D engineering and data analysis team. The process is not fully automated yet for stand-alone deployment of the system at the clinical investigation site. In future developments, supervised machine learning tools could be employed to train the mode selection process on much larger datasets and ultimately automate it, once the critical physical parameters mastering the biology-based interactions of the breast imaging scene with the microwave scanner, overall topology, frequency etc, have been fundamentally understood and modeled. The implemented multi-modal setting (including 3 modes) and the phenomenological analysis of the mode selection per category of breast imaging setting, as presented in this study, represents significant progress and a step ahead towards rationalization of the imaging outputs, modeling of the fundamental underlying physical mechanisms and ultimate plug-in with advanced AI tools supporting clinical diagnosis.
The challenging conditions related to the expected low levels of dielectric contrast of the lesions in cases of very dense breasts or challenging locations of the lesions in the breast (i.e. close to the chest wall, or in the retro-areolar zone) may explain, at least partially, the unclear representation of these lesions, and their confusion as either malignant or benign, when mapped on the Wavelia MWBI feature analysis space. Good correlation of the morphology of the lesions on the Wavelia MWBI images with the lesions’ morphology on Ultrasound and/or MRI reference radiological images was illustrated in the results. A meaningful estimate of the sizes of the lesions was also reported in cases with post-surgery histology reference data available for the tumor size.
In this study, preliminary understanding and classification of recurrent imaging artefacts, with rationalized and sufficiently identifiable patterns, was for the first time addressed and published for the MWBI technology.
Following separation of the recurrent and understood imaging artefacts, a preliminary sub-analysis of the MWBI specificity in asymptomatic contralateral breasts reported only 4 out of the 41 analyzed healthy breasts, with unspecified findings of significant intensity, non-characterizable nor separable from malignant lesions. At the actual Technology Readiness Level of Wavelia MWBI, the intensity level to define an outstanding ROI - for clinical reporting and analysis- was benchmarked by the symptomatic dominant discrete lesion-related ROI of each patient’s MWBI bilateral breast scan.
The Wavelia MWBI imaging procedure is lengthy and will require significant acceleration before clinical acceptance. The impact of the lengthy process for this study was that the MWBI scan of all the biopsy-proven lesions was performed post-biopsy -at least 2 weeks after biopsy- to allow for sufficient healing of the breast. The majority of the MWBI scans being analyzed included a biopsy clip marking the location of the pre-diagnosed lesion in the breast, as per standard-of-care at University Hospital of Galway. The metallic biopsy clip represents a potential cofounder, especially for lesion characterization, which will need separate attention and analysis in future developments before clinical validation of the here-reported findings, on a larger and more impactful scale.
5. Conclusions
Some of the most important findings of the Wavelia#2 prototype testing on human symptomatic breasts are first presented in this article. The potential of the Wavelia#2 MWBI technology in a larger and more diverse dataset compared to the one of the First-In-Human study with the Wavelia#1 prototype, mainly in terms of discrimination between malignant and benign lesions and overall specificity, is demonstrated.
The study focuses on the critical technological evolutions and remaining challenges to handle the diversity of breast geometry and consistency, lesion location in the breast and lesion histological type, before achieving standardization, objective interpretability of the MWBI images, full automation of the imaging suite with reliable outputs, and ultimate clinical acceptance of this new breast imaging modality in the diagnostic setting.
Author Contributions
Conceptualization, A.F., L.D., G.P. and P.A.; methodology, A.F., G.P., P.A. and J.D.G.C.; software, P.A., G.P and J.D.G.C.; validation, A.F., S.A.E., and M.K.; formal analysis, A.F., M.K., S.A.E.; investigation, S.A.E., J.O.D., M.K. and A.F.; resources, M.K., L.D. .; data curation, J.O.D., A.F.; writing—original draft preparation, A.F.; writing—review and editing, M.K. and L.D.; visualization, P.A., J.D.G.C. ; supervision, M.K., A.F. and L.D.; project administration, L.D. and A.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Irish National Office for Research Ethics Committees (application 22-NREC-MD-031, approved on the 21st October 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data are not publicly available due to Institutional (University Hospital of Galway, Ireland) regulations and non-violation of patients’ privacy.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A
Tolerance for association of Wavelia MWBI-based ROIs with the clinical and radiological reference lesion location in the breast
In the Wavelia#2 clinical investigation, there has been a set of patient study cases with detected ROIs in the MWBI 3D images being notably mislocated versus the theoretically expected lesion location in the breast (as per reference radiological data). The availability of the endoscopic camera views of the breast in the Wavelia MWBI scanner was critically important to support correlation of the Wavelia MWBI ROIs with the reference data, in most of these cases. Considerably twisted positioning of the breast in the Wavelia MWBI scanner rendered the association less straightforward, in several other cases. Such an ambiguous study case is depicted in
Figure A1.
Better control of the breast positioning in the MWBI scanner will be required in future MWBI developments, towards standardization, quality assurance for the breast scan and objective clinical association and validation of the MWBI imaging results with reference to the conventional multi-modality imaging data being available at the clinical investigation site.
Figure A1.
(a) P041-R, example of significant mis-location of fibroadenoma in the MWBI 3D images, when compared to the x-ray mammography and ultrasound reference images, (b) Inspection of the breast position in the scanner, as validated by the operator for MWBI scan. View by 4 endoscopic cameras. Significant misalignment of the breast with the scanner XYZ coordinates system. Notable non-verticality of insertion of the pendulous breast in the scanner, potentially explaining the lesion location in the Wavelia image.
Figure A1.
(a) P041-R, example of significant mis-location of fibroadenoma in the MWBI 3D images, when compared to the x-ray mammography and ultrasound reference images, (b) Inspection of the breast position in the scanner, as validated by the operator for MWBI scan. View by 4 endoscopic cameras. Significant misalignment of the breast with the scanner XYZ coordinates system. Notable non-verticality of insertion of the pendulous breast in the scanner, potentially explaining the lesion location in the Wavelia image.
Appendix B
Wavelia#2 MWBI scanner: recurrent imaging artefacts of identifiable pattern.
Four categories of imaging artefacts have been defined during the analysis of the Wavelia#2 MWBI clinical investigation dataset.
Tubular-type artefact: related to patient slight movement during the scan. Recognized based on elongation and its proximity to the breast skin contour.
Skin residual artefact: recognized based on its proximity to the breast skin contour.
‘Circular pattern’ artefact: related to residual (unfiltered) antenna-to-antenna direct coupling. Recognized based on its proximity to the center of the scanning zone and its circular shape on the x-y/coronal plane. The circularity of the pattern is directly associated with the circular symmetry of the probe array.
Residual of chest within the breast scan: artefact recognized based on its proximity to the examination table, or its proximity to the border of the selected portion of the scan for imaging, in case of partial scan mode being activated.
An illustrative example for each type of imaging artefacts is provided in
Figure A2.
Figure A2.
(a) P031-R: Tubular-type artefact (b) P061-R: Skin residual artefact, (c) P039-L: ‘Circular pattern’ artefact, d) P030-R: Residual of chest within the breast scan.
Figure A2.
(a) P031-R: Tubular-type artefact (b) P061-R: Skin residual artefact, (c) P039-L: ‘Circular pattern’ artefact, d) P030-R: Residual of chest within the breast scan.
References
- Webb ML, Kopans DB, Cady B. Reply to a failure analysis of invasive breast cancer: Most deaths from disease occur in women not regularly screened. Vol. 120, Cancer. 2014.
- Miller D, Livingstone V, Herbison P. Interventions for relieving the pain and discomfort of screening mammography. Cochrane Database of Systematic Reviews. 2008.
- Vourtsis A, Berg WA. Breast density implications and supplemental screening. Eur Radiol. 2019 Apr;29(4):1762–77.
- Strandberg R, Illipse M, Czene K, Hall P, Humphreys K. Influence of mammographic density and compressed breast thickness on true mammographic sensitivity: a cohort study. Sci Rep. 2023 Aug;13(1):14194.
- Berg WA, Rafferty EA, Friedewald SM, Hruska CB, Rahbar H. Screening Algorithms in Dense Breasts: AJR Expert Panel Narrative Review. Am J Roentgenol [Internet]. 2021;216(2):275–94. [CrossRef]
- Conceição RC, Mohr JJ, O’Halloran M. An introduction to microwave imaging for breast cancer detection. Biological and medical physics. 2016.
- O’Loughlin D, O’Halloran M, Moloney BM, Glavin M, Jones E, Elahi MA. Microwave breast imaging: Clinical advances and remaining challenges. IEEE Trans Biomed Eng. 2018;65(11):2580–90.
- Benny R, Anjit TA, Mythili P. An overview of microwave imaging for breast tumor detection. Prog Electromagn Res B. 2020;87(January):61–91.
- Origlia C, Rodriguez-Duarte DO, Tobon Vasquez JA, Bolomey JC, Vipiana F. Review of Microwave Near-Field Sensing and Imaging Devices in Medical Applications. Sensors [Internet]. 2024;24(14). Available from: https://www.mdpi.com/1424-8220/24/14/4515.
- Gabriel, C. Compilation of the Dielectric Properties of Body Tissues at RF and Microwave Frequencies. Environ Heal. 1996.
- Gabriel S, Lau RW, Gabriel C. The dielectric properties of biological tissues: III. Parametric models for the dielectric spectrum of tissues. Phys Med Biol. 1996;41(11):2271–93.
- Lazebnik M, Popovic D, McCartney L, Watkins CB, Lindstrom MJ, Harter J, et al. A large-scale study of the ultrawideband microwave dielectric properties of normal, benign and malignant breast tissues obtained from cancer surgeries. Phys Med Biol. 2007.
- Lazebnik M, McCartney L, Popovic D, Watkins CB, Lindstrom MJ, Harter J, et al. A large-scale study of the ultrawideband microwave dielectric properties of normal breast tissue obtained from reduction surgeries. Phys Med Biol. 2007.
- Sugitani T, Kubota SI, Kuroki SI, Sogo K, Arihiro K, Okada M, et al. Complex permittivities of breast tumor tissues obtained from cancer surgeries. Appl Phys Lett. 2014.
- Martellosio A, Pasian M, Bozzi M, Perregrini L, Mazzanti A, Svelto F, et al. Dielectric Properties Characterization From 0.5 to 50 GHz of Breast Cancer Tissues. IEEE Trans Microw Theory Tech. 2016.
- Campbell AM, Land D V. Dielectric properties of female human breast tissue measured in vitro at 3.2 GHz. Phys Med Biol. 1992;37(1):193–210.
- Moloney BM, O’Loughlin D, Elwahab SA, Kerin MJ. Breast cancer detection—a synopsis of conventional modalities and the potential role of microwave imaging. Vol. 10, Diagnostics. 2020.
- Shere M, Lyburn I, Sidebottom R, Massey H, Gillett C, Jones L. MARIA®M5: A multicentre clinical study to evaluate the ability of the Micrima radio-wave radar breast imaging system (MARIA®) to detect lesions in the symptomatic breast. Eur J Radiol [Internet]. 2019;116:61–7. [CrossRef]
- Sani L, Vispa A, Loretoni R, Duranti M, Ghavami N, Sánchez-Bayuela DA, et al. Breast lesion detection through MammoWave device: Empirical detection capability assessment of microwave images’ parameters. PLoS One. 2021;16(4 April).
- Sidebottom R, Webb D, Bishop B, Mohammed K, Allen S. Results for the London investigation into dielectric scanning of lesions study of the MARIA® M6 breast imaging system. Br J Radiol. 2024 Feb;97(1155):549–52.
- Nikolova, NK. Microwave imaging for breast cancer. IEEE Microw Mag. 2011.
- Porter E, Coates M, Popovic M. An Early Clinical Study of Time-Domain Microwave Radar for Breast Health Monitoring. IEEE Trans Biomed Eng. 2016.
- Fear EC, Bourqui J, Curtis C, Mew D, Docktor B, Romano C. Microwave breast imaging with a monostatic radar-based system: A study of application to patients. IEEE Trans Microw Theory Tech. 2013.
- Smith K, Bourqui J, Wang Z, Besler B, Lesiuk M, Roumeliotis M, et al. Microwave imaging for monitoring breast cancer treatment: A pilot study. Med Phys. 2023;50(11).
- Rana SP, Dey M, Loretoni R, Duranti M, Ghavami M, Dudley S, et al. Radiation-Free Microwave Technology for Breast Lesion Detection Using Supervised Machine Learning Model. Tomography. 2023;9(1):105–29.
- Sánchez-Bayuela DÁ, Ghavami N, Tiberi G, Sani L, Vispa A, Bigotti A, et al. A multicentric, single arm, prospective, stratified clinical investigation to evaluate MammoWave’s ability in breast lesions detection. PLoS One. 2023;18(7 July).
- Janjic A, Cayoren M, Akduman I, Yilmaz T, Onemli E, Bugdayci O, et al. Safe: A novel microwave imaging system design for breast cancer screening and early detection—clinical evaluation. Diagnostics. 2021;11(3).
- Yurtseven A, Janjic A, Cayoren M, Bugdayci O, Aribal ME, Akduman I. XGBoost Enhances the Performance of SAFE: A Novel Microwave Imaging System for Early Detection of Malignant Breast Cancer. Cancers (Basel) [Internet]. 2025;17(2). Available from: https://www.mdpi.com/2072-6694/17/2/214.
- Meaney PM, Fanning MW, Li D, Poplack SP, Paulsen KD. A clinical prototype for active microwave imaging of the breast. IEEE Trans Microw Theory Tech. 2000.
- Fear, E. C, Stuchly MA. Microwave detection of breast cancer. IEEE Trans Microw Theory Tech. 2000;48(1 PART 1):1854–63.
- Shere M, Preece A, Craddock I, Leendertz J, Klemm M. Multistatic radar: first trials of a new breast imaging modality. Breast Cancer Res. 2009.
- Fasoula A, Duchesne L, Cano JDG, Moloney BM, Elwahab SMA, Kerin MJ. Automated breast lesion detection and characterization with the wavelia microwave breast imaging system: Methodological proof-of-concept on first-in-human patient data. Appl Sci. 2021;11(21).
- Fasoula A, Moloney BM, Duchesne L, Cano JDG, Oliveira BL, Bernard JG, et al. Super-resolution radar imaging for breast cancer detection with microwaves: the integrated information selection criteria. Conf Proc. Annu Int Conf IEEE Eng Med Biol Soc IEEE Eng Med Biol Soc Annu Conf. 2019;2019:1868–74.
- Fasoula A, Duchesne L, Gil Cano J, Lawrence P, Robin G, Bernard JG. On-Site Validation of a Microwave Breast Imaging System, before First Patient Study. Diagnostics [Internet]. 2018;8(3):53. Available from: http://www.mdpi.com/2075-4418/8/3/53.
- Moloney BM, McAnena PF, Abd Elwahab SM, Fasoula A, Duchesne L, Gil Cano JD, et al. Microwave Imaging in Breast Cancer – Results from the First-In-Human Clinical Investigation of the Wavelia System. Acad Radiol. 2021.
- Moloney BM, McAnena PF, Elwahab SM, Fasoula A, Duchesne L, Gil Cano JD, et al. The Wavelia Microwave Breast Imaging System – Tumour discriminating features and their clinical usefulness. Br J Radiol. 2021;(Oct 5:20210907).
- MVG Industries. TN.32.1.17.SATF, First-In-Human Clinical Investigation Protocol [Internet]. 2018. Available from: https://clinicaltrials.gov/ct2/show/NCT03475992. 0347.
- Fasoula A, Arvanitis P, Duchesne L. Repeatability assessement of the Wavelia#2 Microwave Breast Imaging scan: experimental performance analysis prior to clinical investigation. In: Savci DHŞ, Arvas DE, editors. Microwave Technologies - Recent Advances and New Trends and Applications [Internet]. Rijeka: IntechOpen; 2023. [CrossRef]
- MVG Industries. TP.102.17.22.PAR, Wavelia#2, Pilot#1 Clinical Investigation Protocol [Internet]. 2023. Available from: https://clinicaltrials.gov/study/NCT05757427.
- Van Griethuysen JJM, Fedorov A, Parmar C, Hosny A, Aucoin N, Narayan V, et al. Computational radiomics system to decode the radiographic phenotype. Cancer Res. 2017;77(21).
- Sickles EA, D’Orsi CJ, Bassett LW, Appleton CM, Berg WA, Burnside ES, et al. Acr bi-rads®mammography. ACR BI-RADS®atlas, breast imaging Report data Syst. 2013;5.
- Highnam R, Brady M, Yaffe MJ, Karssemeijer N, Harvey J. Robust breast composition measurement - VolparaTM. In: Lecture Notes in Computer Science (including subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics). 2010.
- Teo I, Whelehan P, MacAskill JE, Vinnicombe S, Munnoch DA, Evans A. VolparaTM as a measurement tool for breast volume. Journal of Plastic, Reconstructive and Aesthetic Surgery. 2016.
- Papatrechas G, Fasoula A, Duchesne L. Wavelia #2: The Microwave Breast Surface Estimation Module. In: 2024 IEEE International Symposium on Biomedical Imaging (ISBI) [Internet]. IEEE; 2024. p. 1–5. Available from: https://ieeexplore.ieee.org/document/10635824/.
- Lawrence P, Fasoula A, Duchesne L. RF-based Breast Surface Estimation – Registration with Reference Imaging Modality. In: APS-URSI Proceedings. Boston, USA; 2018.
- Huang NS, Quan CL, Mo M, Chen JJ, Yang BL, Huang X, et al. A prospective study of breast anthropomorphic measurements, volume & ptosis in 605 Asian patients with breast cancer or benign breast disease. PLoS One. 2017;12(2).
- Yeh ED, Georgian-Smith D, Raza S, Bussolari L, Pawlisz-Hoff J, Birdwell RL. Positioning in breast MR imaging to optimize image quality. Radiographics. 2014.
- Fasoula A, Duchesne L, Abdoush Y, Baracco JM. Frequency-dependent, configurable, sensor fidelity zone for microwave breast imaging: system dimensioning and image quality enhancement. In: IEEE Conference on Antenna Measurements & Applications (CAMA). 2021. p. 487–92.
- Devaney, A. Super-resolution processing of multi-static data using time reversal and MUSIC. J Acoust Soc Am. 2000.
- Pertuz S, Puig D, Garcia MA. Analysis of focus measure operators for shape-from-focus. Pattern Recognit. 2013.
- van Timmeren JE, Cester D, Tanadini-Lang S, Alkadhi H, Baessler B. Radiomics in medical imaging—“how-to” guide and critical reflection. Vol. 11, Insights into Imaging. 2020.
- Jha AK, Mithun S, Jaiswar V, Sherkhane UB, Purandare NC, Prabhash K, et al. Repeatability and reproducibility study of radiomic features on a phantom and human cohort. Sci Rep. 2021;11(1).
- Goh TY, Basah SN, Yazid H, Aziz Safar MJ, Ahmad Saad FS. Performance analysis of image thresholding: Otsu technique. Meas J Int Meas Confed. 2018;114.
- Vallières M, Freeman CR, Skamene SR, El Naqa I. A radiomics model from joint FDG-PET and MRI texture features for the prediction of lung metastases in soft-tissue sarcomas of the extremities. Phys Med Biol. 2015.
- Tanaka T, Mandic DP. Complex empirical mode decomposition. IEEE Signal Process Lett. 2007;14(2):101–4.
Figure 1.
P003-L, Invasive Ductal Carcinoma: Layout of the imaging dataset: (a) Reference patient study data. Top row: annotated CC and MLO mammographic views of the Left breast performed post-biopsy of the IDC lesion, and snapshot of the annotated Ultrasound scan of the breast provided as reference data for the same lesion in the study. Bottom row: Spatial maps of the dense tissue thickness, provided as customized output of Volpara Lab on the CC and MLO mammographic planes, and Volpara Lab computational outputs to define Volumetric Breast Density (VBD). (b) Wavelia imaging outputs. Top row: Wavelia MWBI Global Averaged 3D volumetric image (Default View and Sagittal View), and endoscopic camera view of the breast after positioning in the MWBI scanner - depicted view aligned with the orientation of the MWBI 3D image Default View. Bottom row: Wavelia MWBI Low-permittivity and High-Permittivity 3D volumetric images presented at Default View.
Figure 1.
P003-L, Invasive Ductal Carcinoma: Layout of the imaging dataset: (a) Reference patient study data. Top row: annotated CC and MLO mammographic views of the Left breast performed post-biopsy of the IDC lesion, and snapshot of the annotated Ultrasound scan of the breast provided as reference data for the same lesion in the study. Bottom row: Spatial maps of the dense tissue thickness, provided as customized output of Volpara Lab on the CC and MLO mammographic planes, and Volpara Lab computational outputs to define Volumetric Breast Density (VBD). (b) Wavelia imaging outputs. Top row: Wavelia MWBI Global Averaged 3D volumetric image (Default View and Sagittal View), and endoscopic camera view of the breast after positioning in the MWBI scanner - depicted view aligned with the orientation of the MWBI 3D image Default View. Bottom row: Wavelia MWBI Low-permittivity and High-Permittivity 3D volumetric images presented at Default View.
Figure 2.
P003-L, Invasive Ductal Carcinoma: Illustrative subset of the Wavelia MWBI image analysis outputs. Packaged reporting per extracted ROI for clinical analysis. Top row: Wavelia MWBI Global Averaged 3D image Default View, with the extracted ROI delineated and superimposed in blue color. Slices of the MWBI image on the 3 planes, cut at the centroid of the clinically relevant ROI in the MWBI image. Endoscopic Camera view of the breast, aligned with the orientation of the MWBI 3D image Default View. Bottom Row: Tabulated ROI Characteristics: columns 1-3 contain reference data, columns 4-13 are part of the Wavelia MWBI scan analysis outputs – data structured per extracted ROI.
Figure 2.
P003-L, Invasive Ductal Carcinoma: Illustrative subset of the Wavelia MWBI image analysis outputs. Packaged reporting per extracted ROI for clinical analysis. Top row: Wavelia MWBI Global Averaged 3D image Default View, with the extracted ROI delineated and superimposed in blue color. Slices of the MWBI image on the 3 planes, cut at the centroid of the clinically relevant ROI in the MWBI image. Endoscopic Camera view of the breast, aligned with the orientation of the MWBI 3D image Default View. Bottom Row: Tabulated ROI Characteristics: columns 1-3 contain reference data, columns 4-13 are part of the Wavelia MWBI scan analysis outputs – data structured per extracted ROI.
Figure 3.
The data preprocessing and filtering scheme: Principal Component Analysis (PCA), followed by Empirical Mode Decomposition (EMD), applied to allow filtering per Intrinsic Mode Function (IMF), after mapping it either on the time- or on the frequency-domain signal space.
Figure 3.
The data preprocessing and filtering scheme: Principal Component Analysis (PCA), followed by Empirical Mode Decomposition (EMD), applied to allow filtering per Intrinsic Mode Function (IMF), after mapping it either on the time- or on the frequency-domain signal space.
Figure 4.
Custom Filter#1 applicability: very small breasts (a) P043-R, (b) P011-R, or (c) P035-L, large superficially located lesion in medium-sized breast.
Figure 4.
Custom Filter#1 applicability: very small breasts (a) P043-R, (b) P011-R, or (c) P035-L, large superficially located lesion in medium-sized breast.
Figure 5.
Custom Filter#2 applicability: (a) P039-R very large breast, (b) P048-L elder patient, breast badly positioned in the scanner with large inframammary fold being part of the scan and generating strong artefacts, (c) P010 extremely dense breasts of medium size, with bilateral cysts of low intensity being imaged.
Figure 5.
Custom Filter#2 applicability: (a) P039-R very large breast, (b) P048-L elder patient, breast badly positioned in the scanner with large inframammary fold being part of the scan and generating strong artefacts, (c) P010 extremely dense breasts of medium size, with bilateral cysts of low intensity being imaged.
Figure 6.
(a) Scanned breast volumes for which the two Custom Filters were selected to enhance the Wavelia MWBI scan outputs quality, (b) Patient ID’s and associated breast volumes for which each of the two filters was applied.
Figure 6.
(a) Scanned breast volumes for which the two Custom Filters were selected to enhance the Wavelia MWBI scan outputs quality, (b) Patient ID’s and associated breast volumes for which each of the two filters was applied.
Figure 7.
Graphical illustration of the malignant-to-benign lesion separability with Wavelia#2 MWBI:
(a)-
(d) Boxplots per analyzed feature, indicating the median, spread (IQR), range, and outliers points of each feature value distribution, for 4 classes of lesions: cysts, solid benign lesions, Invasive Ductal Carcinomas (IDC), Invasive Lobular Carcinomas (ILC).
(e)-
(f) Representation of the 4 classes of lesions on 3D scatter plots, for two different combinations of intensity 1
st order statistics and texture features. A different color is used to represent each class on the scatter plots. The features with the highest F-statistic (separability score), as highlighted with bold font type in
Table 1, have been selected for the graphical illustration. Higher-dimensional analysis is possible.
Figure 7.
Graphical illustration of the malignant-to-benign lesion separability with Wavelia#2 MWBI:
(a)-
(d) Boxplots per analyzed feature, indicating the median, spread (IQR), range, and outliers points of each feature value distribution, for 4 classes of lesions: cysts, solid benign lesions, Invasive Ductal Carcinomas (IDC), Invasive Lobular Carcinomas (ILC).
(e)-
(f) Representation of the 4 classes of lesions on 3D scatter plots, for two different combinations of intensity 1
st order statistics and texture features. A different color is used to represent each class on the scatter plots. The features with the highest F-statistic (separability score), as highlighted with bold font type in
Table 1, have been selected for the graphical illustration. Higher-dimensional analysis is possible.
Figure 8.
Highlighting the 4 lesions on the Wavelia MWBI feature analysis space: indicative 3D feature space depicted, considering three of the features with highest diagnostic accuracy in terms of discriminating malignant against benign lesions.
Figure 8.
Highlighting the 4 lesions on the Wavelia MWBI feature analysis space: indicative 3D feature space depicted, considering three of the features with highest diagnostic accuracy in terms of discriminating malignant against benign lesions.
Figure 9.
Reference and Wavelia MWBI images for the 4 typical cases of good-quality Wavelia MWBI images with clear characterization as malignant or benign in the Wavelia feature analysis space: (a) Endoscopic camera views of the 4 breasts after positioning in the MWBI scanner – Depicted camera views aligned with the orientation of the MWBI 3D image Default View, (b) P003-L IDC, Luminal A, (c) P031-L IDC, Luminal B, (d) P001-L Cyst, (e) P023-L Fibroadenoma. In (b)-(e): Wavelia MWBI 3D image Default View, with the extracted ROI delineated and superimposed in blue color, Coronal XY slice of the MWBI image cut at the centroid of the clinically relevant ROI in the MWBI image, snapshot of the Ultrasound scan of the breast provided as reference data for the same lesion in the study.
Figure 9.
Reference and Wavelia MWBI images for the 4 typical cases of good-quality Wavelia MWBI images with clear characterization as malignant or benign in the Wavelia feature analysis space: (a) Endoscopic camera views of the 4 breasts after positioning in the MWBI scanner – Depicted camera views aligned with the orientation of the MWBI 3D image Default View, (b) P003-L IDC, Luminal A, (c) P031-L IDC, Luminal B, (d) P001-L Cyst, (e) P023-L Fibroadenoma. In (b)-(e): Wavelia MWBI 3D image Default View, with the extracted ROI delineated and superimposed in blue color, Coronal XY slice of the MWBI image cut at the centroid of the clinically relevant ROI in the MWBI image, snapshot of the Ultrasound scan of the breast provided as reference data for the same lesion in the study.
Figure 10.
Highlighting the 3 lesions on the Wavelia MWBI feature analysis space: indicative 3D feature space depicted, considering three of the features with highest diagnostic accuracy in terms of discriminating malignant against benign lesions.
Figure 10.
Highlighting the 3 lesions on the Wavelia MWBI feature analysis space: indicative 3D feature space depicted, considering three of the features with highest diagnostic accuracy in terms of discriminating malignant against benign lesions.
Figure 11.
Reference and Wavelia MWBI images for the 3 confusing cases in the malignant-to-benign lesion separability analysis space: (a),(d) P053-L, (b),(e) P073-L, (c),(f) P061-L. Top row: endoscopic camera view of each breast after positioning in the MWBI scanner – Depicted view aligned with the orientation of the MWBI 3D image Default View, CC and MLO mammographic views of the breast, Volpara Lab - mammographic computational outputs (Volumetric Breast Density (VBD) and Breast Volume), Sagittal slice highlighting the lesion in the breast MRI scan (if available). Bottom Row: Wavelia MWBI 3D image Default View, with the extracted ROI delineated and superimposed in blue color , Coronal XY slice of the MWBI image cut at the centroid of the clinically relevant ROI in the MWBI image, snapshot of the Ultrasound scan of the breast provided as reference data for the same lesion in the study.
Figure 11.
Reference and Wavelia MWBI images for the 3 confusing cases in the malignant-to-benign lesion separability analysis space: (a),(d) P053-L, (b),(e) P073-L, (c),(f) P061-L. Top row: endoscopic camera view of each breast after positioning in the MWBI scanner – Depicted view aligned with the orientation of the MWBI 3D image Default View, CC and MLO mammographic views of the breast, Volpara Lab - mammographic computational outputs (Volumetric Breast Density (VBD) and Breast Volume), Sagittal slice highlighting the lesion in the breast MRI scan (if available). Bottom Row: Wavelia MWBI 3D image Default View, with the extracted ROI delineated and superimposed in blue color , Coronal XY slice of the MWBI image cut at the centroid of the clinically relevant ROI in the MWBI image, snapshot of the Ultrasound scan of the breast provided as reference data for the same lesion in the study.
Figure 12.
Unspecified findings mapped on the Wavelia MWBI feature analysis space: indicative 3D feature space depicted, considering three of the features with highest diagnostic accuracy in terms of discriminating malignant against benign lesions. Expanded feature space of higher dimension is available for exploitation and analysis.
Figure 12.
Unspecified findings mapped on the Wavelia MWBI feature analysis space: indicative 3D feature space depicted, considering three of the features with highest diagnostic accuracy in terms of discriminating malignant against benign lesions. Expanded feature space of higher dimension is available for exploitation and analysis.
Table 1.
Malignant-to-Benign lesion separability analysis, applying ANOVA on a set of 25 features computed with the PyRadiomics library. 59 clinically relevant ROIs extracted from the Wavelia MWBI 3D breast images and included in the analysis.
Table 1.
Malignant-to-Benign lesion separability analysis, applying ANOVA on a set of 25 features computed with the PyRadiomics library. 59 clinically relevant ROIs extracted from the Wavelia MWBI 3D breast images and included in the analysis.
Table 2.
Characteristics of typical good-quality ROIs of malignant and benign lesions, extracted from Wavelia MWBI 3D images: columns 1-3 contain reference data, columns 4-13 are part of the Wavelia MWBI image analysis outputs – data structured per extracted ROI.
Table 2.
Characteristics of typical good-quality ROIs of malignant and benign lesions, extracted from Wavelia MWBI 3D images: columns 1-3 contain reference data, columns 4-13 are part of the Wavelia MWBI image analysis outputs – data structured per extracted ROI.
Table 3.
Characteristics of confusing ROIs of malignant and benign lesions, as extracted from the Wavelia MWBI 3D images: columns 1-3 contain reference data, columns 4-13 are part of the Wavelia MWBI image analysis outputs – data structured per extracted ROI.
Table 3.
Characteristics of confusing ROIs of malignant and benign lesions, as extracted from the Wavelia MWBI 3D images: columns 1-3 contain reference data, columns 4-13 are part of the Wavelia MWBI image analysis outputs – data structured per extracted ROI.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).