Submitted:
30 July 2025
Posted:
06 August 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Pre-Transport Phase: A Brief Overview of Melanogenesis
2.1. Melanin Synthesis
2.2. Melanosome Maturation
3. The Transport Phase
3.1. Intracellular Transport of Melanosomes
3.1.1. Microtubule-Based Long-Range Anterograde Transport
3.1.2. The Switch of Melanosome from Microtubule to Actin Filament Networks
3.1.3. Actin-Based Short-Range Transport
3.1.4. Microtubule-Based Long-Range Retrograde Transport
3.2. Intercellular Transfer of Melanocores or Melanosomes
4. Melanin Post-Transfer Processing in Keratinocytes
4.1. Melanin Uptake
4.2. Melanin Retention and degradation
5. Relevance to Cosmetic Dermatology and Therapeutics: Future Perspectives
Author Contributions
Conflicts of Interest
References
- Taylor, A. , Pawaskar M. , Taylor S. L., Balkrishnan R., Feldman S. R. “Prevalence of pigmentary disorders and their impact on quality of life: a prospective cohort study.” J Cosmet Dermatol 2008, 7, 164–168. [Google Scholar]
- Mpofana, N. , Paulse M. , Gqaleni N., Makgobole M. U., Pillay P., Hussein A., Dlova N. C. “The Effect of Melasma on the Quality of Life in People with Darker Skin Types Living in Durban, South Africa.” Int J Environ Res Public Health 2023, 20. [Google Scholar]
- Park H., Y. , Kosmadaki M. , Yaar M., Gilchrest B. A. “Cellular mechanisms regulating human melanogenesis.” Cell Mol Life Sci 2009, 66, 1493–1506. [Google Scholar]
- D’Mello S., A. , Finlay G. J., Baguley B. C., Askarian-Amiri M. E. “Signaling Pathways in Melanogenesis.” Int J Mol Sci 2016, 17. [Google Scholar]
- Bento-Lopes L C., L. , Charneca J, Neto MV, Seabra MC, Barral DC.. “Melanin’s Journey from Melanocytes to Keratinocytes Uncovering the Molecular Mechanisms of Melanin Transfer and Processing.” Int J Mol Sci. 2023.
- Wu, X. , Hammer J. A. “Melanosome transfer: it is best to give and receive.” Curr Opin Cell Biol 2014, 29, 1–7. [Google Scholar]
- Kobayashi, N. , Nakagawa A. , Muramatsu T., Yamashina Y., Shirai T., Hashimoto M. W., Ishigaki Y., Ohnishi T., Mori T. “Supranuclear melanin caps reduce ultraviolet induced DNA photoproducts in human epidermis.” J Invest Dermatol 1998, 110, 806–810. [Google Scholar]
- Lan, Y. , Zeng W. , Wang Y., Dong X., Shen X., Gu Y., Zhang W., Lu H. “Opsin 3 mediates UVA-induced keratinocyte supranuclear melanin cap formation.” Commun Biol 2023, 6, 238. [Google Scholar]
- Neto M., V. , Hall M. J., Charneca J., Escrevente C., Seabra M. C., Barral D. C. “Photoprotective Melanin Is Maintained within Keratinocytes in Storage Lysosomes.” J Invest Dermatol 2025, 145, 1155–1165. [Google Scholar]
- Correia M., S. , Moreiras H. , Pereira F. J. C., Neto M. V., Festas T. C., Tarafder A. K., Ramalho J. S., Seabra M. C., Barral D. C. “Melanin Transferred to Keratinocytes Resides in Nondegradative Endocytic Compartments.” J Invest Dermatol 2018, 138, 637–646. [Google Scholar]
- Murase, D. , Hachiya A. , Takano K., Hicks R., Visscher M. O., Kitahara T., Hase T., Takema Y., Yoshimori T. “Autophagy has a significant role in determining skin color by regulating melanosome degradation in keratinocytes.” J Invest Dermatol 2013, 133, 2416–2424. [Google Scholar]
- Homma, T. , Kageyama S. , Nishikawa A., Nagata K. “Melanosome degradation in epidermal keratinocytes related to lysosomal protease cathepsin V.” Biochem Biophys Res Commun 2018, 500, 339–343. [Google Scholar]
- Marubashi, S. , Fukuda M. “Rab7B/42 Is Functionally Involved in Protein Degradation on Melanosomes in Keratinocytes.” Cell Struct Funct 2020, 45, 45–55. [Google Scholar]
- Lee K., W. , Kim M., Lee S. H., Kim K. D. “The Function of Autophagy as a Regulator of Melanin Homeostasis. Cells.
- Ebanks J., P. , Koshoffer A. , Wickett R. R., Schwemberger S., Babcock G., Hakozaki T., Boissy R. E. “Epidermal keratinocytes from light vs. dark skin exhibit differential degradation of melanosomes.” J Invest Dermatol 2011, 131, 1226–1233. [Google Scholar]
- Kim J., Y. , Kim J. , Ahn Y., Lee E. J., Hwang S., Almurayshid A., Park K., Chung H. J., Kim H. J., Lee S. H., Lee M. S., Oh S. H. “Autophagy induction can regulate skin pigmentation by causing melanosome degradation in keratinocytes and melanocytes.” Pigment Cell Melanoma Res 2020, 33, 403–415. [Google Scholar]
- Yang, Z. , Zeng B. , Pan Y., Huang P., Wang C. “Autophagy participates in isoliquiritigenin-induced melanin degradation in human epidermal keratinocytes through PI3K/AKT/mTOR signaling.” Biomed Pharmacother 2018, 97, 248–254. [Google Scholar]
- Wang, F. , Ma W. , Fan D., Hu J., An X., Wang Z. “The biochemistry of melanogenesis: an insight into the function and mechanism of melanogenesis-related proteins.” Front Mol Biosci 2024, 11, 1440187. [Google Scholar]
- Liu, X. , Sun X. , Liu Y., Wang W., Yang H., Ge Y., Yang Y., Chen X., Lin T. “Metformin inhibits melanin synthesis and melanosome transfer through the cAMP pathway.” Sci Rep 2025, 15, 11442. [Google Scholar]
- Markiewicz, E. , Karaman-Jurukovska N. , Mammone T., Idowu O. C. “Post-Inflammatory Hyperpigmentation in Dark Skin: Molecular Mechanism and Skincare Implications.” Clin Cosmet Investig Dermatol 2022, 15, 2555–2565. [Google Scholar]
- Nakamura, H. , Fukuda M. “Establishment of a synchronized tyrosinase transport system revealed a role of Tyrp1 in efficient melanogenesis by promoting tyrosinase targeting to melanosomes.” Sci Rep 2024, 14, 2529. [Google Scholar]
- Wolf Horrell E., M. , Boulanger M. C., D’Orazio J. A. “Melanocortin 1 Receptor: Structure, Function, and Regulation.” Front Genet 2016, 7, 95. [Google Scholar]
- Kawakami, A. , Fisher D. E. “The master role of microphthalmia-associated transcription factor in melanocyte and melanoma biology.” Lab Invest 2017, 97, 649–656. [Google Scholar]
- Guo, Y. , Olle L., Proano-Perez E. , Aparicio C., Guerrero M., Munoz-Cano R., Martin M. “MRGPRX2 signaling involves the Lysyl-tRNA synthetase and MITF pathway.” Front Immunol 2023, 14, 1154108. [Google Scholar]
- Schepsky, A. , Bruser K. , Gunnarsson G. J., Goodall J., Hallsson J. H., Goding C. R., Steingrimsson E., Hecht A. “The microphthalmia-associated transcription factor Mitf interacts with beta-catenin to determine target gene expression.” Mol Cell Biol 2006, 26, 8914–8927. [Google Scholar]
- Yardman-Frank J., M. , Fisher D. E. “Skin pigmentation and its control: From ultraviolet radiation to stem cells.” Exp Dermatol 2021, 30, 560–571. [Google Scholar]
- Schallreuter K., U. , Kothari S. , Chavan B., Spencer J. D. “Regulation of melanogenesis--controversies and new concepts.” Exp Dermatol 2008, 17, 395–404. [Google Scholar]
- Waku, T. , Nakada S. , Masuda H., Sumi H., Wada A., Hirose S., Aketa I., Kobayashi A. “The CNC-family transcription factor Nrf3 coordinates the melanogenesis cascade through macropinocytosis and autophagy regulation.” Cell Rep 2023, 42, 111906. [Google Scholar]
- Berson J., F. , Harper D. C., Tenza D., Raposo G., Marks M. S. “Pmel17 initiates premelanosome morphogenesis within multivesicular bodies.” Mol Biol Cell 2001, 12, 3451–3464. [Google Scholar]
- Bissig, C. , Rochin L., van Niel G. “PMEL Amyloid Fibril Formation: The Bright Steps of Pigmentation. Int J Mol Sci, 2016 17. [Google Scholar]
- Hoashi, T. , Watabe H. , Muller J., Yamaguchi Y., Vieira W. D., Hearing V. J. “MART-1 is required for the function of the melanosomal matrix protein PMEL17/GP100 and the maturation of melanosomes.” J Biol Chem 2005, 280, 14006–14016. [Google Scholar]
- Kushimoto, T. , Basrur V. , Valencia J., Matsunaga J., Vieira W. D., Ferrans V. J., Muller J., Appella E., Hearing V. J. “A model for melanosome biogenesis based on the purification and analysis of early melanosomes.” Proc Natl Acad Sci U S A 2001, 98, 10698–10703. [Google Scholar]
- Giordano, F. , Bonetti C. , Surace E. M., Marigo V., Raposo G. “The ocular albinism type 1 (OA1) G-protein-coupled receptor functions with MART-1 at early stages of melanogenesis to control melanosome identity and composition.” Hum Mol Genet 2009, 18, 4530–4545. [Google Scholar]
- van Niel, G. , Charrin S. , Simoes S., Romao M., Rochin L., Saftig P., Marks M. S., Rubinstein E., Raposo G. “The tetraspanin CD63 regulates ESCRT-independent and -dependent endosomal sorting during melanogenesis.” Dev Cell 2011, 21, 708–721. [Google Scholar]
- Cortese, K. , Giordano F. , Surace E. M., Venturi C., Ballabio A., Tacchetti C., Marigo V. “The ocular albinism type 1 (OA1) gene controls melanosome maturation and size.” Invest Ophthalmol Vis Sci 2005, 46, 4358–4364. [Google Scholar]
- Giordano, F. , Simoes S. , Raposo G. “The ocular albinism type 1 (OA1) GPCR is ubiquitinated and its traffic requires endosomal sorting complex responsible for transport (ESCRT) function.” Proc Natl Acad Sci U S A 2011, 108, 11906–11911. [Google Scholar]
- Sitaram, A. , Marks M. S. “Mechanisms of protein delivery to melanosomes in pigment cells.” Physiology (Bethesda) 2012, 27, 85–99. [Google Scholar]
- Bellono N., W. , Escobar I. E., Lefkovith A. J., Marks M. S., Oancea E. “An intracellular anion channel critical for pigmentation.” Elife 2014, 3, e04543. [Google Scholar]
- Ancans, J. , Tobin D. J., Hoogduijn M. J., Smit N. P., Wakamatsu K., Thody A. J. “Melanosomal pH controls rate of melanogenesis, eumelanin/phaeomelanin ratio and melanosome maturation in melanocytes and melanoma cells.” Exp Cell Res 2001, 268, 26–35. [Google Scholar]
- Le, L. , Escobar I. E., Ho T., Lefkovith A. J., Latteri E., Haltaufderhyde K. D., Dennis M. K., Plowright L., Sviderskaya E. V., Bennett D. C., Oancea E., Marks M. S. “SLC45A2 protein stability and regulation of melanosome pH determine melanocyte pigmentation.” Mol Biol Cell 2020, 31, 2687–2702. [Google Scholar]
- Bajpai V., K. , Swigut T. , Mohammed J., Naqvi S., Arreola M., Tycko J., Kim T. C., Pritchard J. K., Bassik M. C., Wysocka J. “A genome-wide genetic screen uncovers determinants of human pigmentation.” Science 2023, 381, eade6289. [Google Scholar]
- Setty S., R. , Tenza D. , Sviderskaya E. V., Bennett D. C., Raposo G., Marks M. S. “Cell-specific ATP7A transport sustains copper-dependent tyrosinase activity in melanosomes.” Nature 2008, 454, 1142–1146. [Google Scholar]
- Ishida, M. , Ohbayashi N. , Maruta Y., Ebata Y., Fukuda M. “Functional involvement of Rab1A in microtubule-dependent anterograde melanosome transport in melanocytes.” J Cell Sci 2012, 125, 5177–5187. [Google Scholar]
- Moreiras, H. , Seabra M. C., Barral D. C. “Melanin Transfer in the Epidermis: The Pursuit of Skin Pigmentation Control Mechanisms. Int J Mol Sci.
- Ishida, M. , Ohbayashi N. , Fukuda M. “Rab1A regulates anterograde melanosome transport by recruiting kinesin-1 to melanosomes through interaction with SKIP.” Sci Rep 2015, 5, 8238. [Google Scholar]
- Robinson C., L. , Evans R. D., Briggs D. A., Ramalho J. S., Hume A. N. “Inefficient recruitment of kinesin-1 to melanosomes precludes it from facilitating their transport.” J Cell Sci 2017, 130, 2056–2065. [Google Scholar]
- Jiang, M. , Paniagua A. E., Volland S., Wang H., Balaji A., Li D. G., Lopes V. S., Burgess B. L., Williams D. S. “Microtubule motor transport in the delivery of melanosomes to the actin-rich apical domain of the retinal pigment epithelium. J Cell Sci, 2020; 133. [Google Scholar]
- Rogers S., L. , Gelfand V. I. “Myosin cooperates with microtubule motors during organelle transport in melanophores.” Curr Biol 1998, 8, 161–164. [Google Scholar]
- Provance D. W., Jr. , Wei M. , Ipe V., Mercer J. A. “Cultured melanocytes from dilute mutant mice exhibit dendritic morphology and altered melanosome distribution.” Proc Natl Acad Sci U S A 1996, 93, 14554–14558. [Google Scholar]
- Oberhofer, A. , Spieler P. , Rosenfeld Y., Stepp W. L., Cleetus A., Hume A. N., Mueller-Planitz F., Okten Z. “Myosin Va’s adaptor protein melanophilin enforces track selection on the microtubule and actin networks in vitro.” Proc Natl Acad Sci U S A 2017, 114, E4714–E4723. [Google Scholar]
- Wu, X. , Sakamoto T. , Zhang F., Sellers J. R., Hammer J. A., 3rd. “In vitro reconstitution of a transport complex containing Rab27a, melanophilin and myosin Va.” FEBS Lett 2006, 580, 5863–5868. [Google Scholar]
- Ramkumar, A. , Murthy D. , Raja D. A., Singh A., Krishnan A., Khanna S., Vats A., Thukral L., Sharma P., Sivasubbu S., Rani R., Natarajan V. T., Gokhale R. S. “Classical autophagy proteins LC3B and ATG4B facilitate melanosome movement on cytoskeletal tracks.” Autophagy 2017, 13, 1331–1347. [Google Scholar]
- Zhang, J. , Yue J. , Wu X. “Spectraplakin family proteins - cytoskeletal crosslinkers with versatile roles.” J Cell Sci 2017, 130, 2447–2457. [Google Scholar]
- Leung C., L. , Sun D. , Zheng M., Knowles D. R., Liem R. K. “Microtubule actin cross-linking factor (MACF): a hybrid of dystonin and dystrophin that can interact with the actin and microtubule cytoskeletons.” J Cell Biol 1999, 147, 1275–1286. [Google Scholar]
- Cusseddu, R. , Robert A. , Cote J. F. “Strength Through Unity: The Power of the Mega-Scaffold MACF1.” Front Cell Dev Biol 2021, 9, 641727. [Google Scholar]
- Li, X. , Goult B. T., Ballestrem C., Zacharchenko T. “The structural basis of the talin-KANK1 interaction that coordinates the actin and microtubule cytoskeletons at focal adhesions.” Open Biol 2023, 13, 230058. [Google Scholar]
- Bahadoran, P. , Aberdam E. , Mantoux F., Busca R., Bille K., Yalman N., de Saint-Basile G., Casaroli-Marano R., Ortonne J. P., Ballotti R. “Rab27a: A key to melanosome transport in human melanocytes.” J Cell Biol 2001, 152, 843–850. [Google Scholar]
- Kuroda T., S. , Ariga H. , Fukuda M. “The actin-binding domain of Slac2-a/melanophilin is required for melanosome distribution in melanocytes.” Mol Cell Biol 2003, 23, 5245–5255. [Google Scholar]
- Strom, M. , Hume A. N., Tarafder A. K., Barkagianni E., Seabra M. C. “A family of Rab27-binding proteins. Melanophilin links Rab27a and myosin Va function in melanosome transport.” J Biol Chem 2002, 277, 25423–25430. [Google Scholar]
- Lambert, J. , Onderwater J. , Vander Haeghen Y., Vancoillie G., Koerten H. K., Mommaas A. M., Naeyaert J. M. “Myosin V colocalizes with melanosomes and subcortical actin bundles not associated with stress fibers in human epidermal melanocytes.” J Invest Dermatol 1998, 111, 835–840. [Google Scholar]
- Jo C., S. , Park H. I., Jung H. J., Park J. I., Lee J. E., Myung C. H., Hwang J. S. “A novel function of Prohibitin on melanosome transport in melanocytes.” Theranostics 2020, 10, 3880–3891. [Google Scholar]
- Ohbayashi, N. , Maruta Y. , Ishida M., Fukuda M. “Melanoregulin regulates retrograde melanosome transport through interaction with the RILP-p150Glued complex in melanocytes.” J Cell Sci 2012, 125, 1508–1518. [Google Scholar]
- Aktary, Z. , Conde-Perez A. , Rambow F., Di Marco M., Amblard F., Hurbain I., Raposo G., Delevoye C., Coscoy S., Larue L. “A role for Dynlt3 in melanosome movement, distribution, acidity and transfer.” Commun Biol 2021, 4, 423. [Google Scholar]
- Maruta, Y. , Fukuda M. “Large Rab GTPase Rab44 regulates microtubule-dependent retrograde melanosome transport in melanocytes.” J Biol Chem 2022, 298, 102508. [Google Scholar]
- Matsui, T. , Ohbayashi N. , Fukuda M. “The Rab interacting lysosomal protein (RILP) homology domain functions as a novel effector domain for small GTPase Rab36: Rab36 regulates retrograde melanosome transport in melanocytes.” J Biol Chem 2012, 287, 28619–28631. [Google Scholar]
- Belleudi, F. , Purpura V. , Scrofani C., Persechino F., Leone L., Torrisi M. R. “Expression and signaling of the tyrosine kinase FGFR2b/KGFR regulates phagocytosis and melanosome uptake in human keratinocytes.” FASEB J 2011, 25, 170–181. [Google Scholar]
- Wolff, K. “Melanocyte-keratinocyte interactions in vivo: the fate of melanosomes. ” Yale J Biol Med 1973, 46, 384–396. [Google Scholar]
- Scott, G. , Leopardi S. , Printup S., Madden B. C. “Filopodia are conduits for melanosome transfer to keratinocytes.” J Cell Sci 2002, 115, 1441–1451. [Google Scholar]
- Domingues, L. , Hurbain I. , Gilles-Marsens F., Sires-Campos J., Andre N., Dewulf M., Romao M., Viaris de Lesegno C., Mace A. S., Blouin C., Guere C., Vie K., Raposo G., Lamaze C., Delevoye C. “Coupling of melanocyte signaling and mechanics by caveolae is required for human skin pigmentation.” Nat Commun 2020, 11, 2988. [Google Scholar]
- Tarafder A., K. , Bolasco G. , Correia M. S., Pereira F. J. C., Iannone L., Hume A. N., Kirkpatrick N., Picardo M., Torrisi M. R., Rodrigues I. P., Ramalho J. S., Futter C. E., Barral D. C., Seabra M. C. “Rab11b mediates melanin transfer between donor melanocytes and acceptor keratinocytes via coupled exo/endocytosis.” J Invest Dermatol 2014, 134, 1056–1066. [Google Scholar]
- Moreiras, H. , Pereira F. J. C., Neto M. V., Bento-Lopes L., Festas T. C., Seabra M. C., Barral D. C. “The exocyst is required for melanin exocytosis from melanocytes and transfer to keratinocytes.” Pigment Cell Melanoma Res 2020, 33, 366–371. [Google Scholar]
- Moreiras, H. , Bento-Lopes L., Neto M. V., Escrevente C., Cabaco L. C., Hall M. J., Ramalho J. S., Seabra M. C., Barral D. C. “Melanocore uptake by keratinocytes occurs through phagocytosis and involves protease-activated receptor-2 internalization.” Traffic 2022, 23, 331–345. [Google Scholar]
- Tadokoro, R. , Murai H. , Sakai K. I., Okui T., Yokota Y., Takahashi Y. “Melanosome transfer to keratinocyte in the chicken embryonic skin is mediated by vesicle release associated with Rho-regulated membrane blebbing.” Sci Rep 2016, 6, 38277. [Google Scholar]
- Halprin K., M. “Epidermal “turnover time”--a re-examination. ” Br J Dermatol 1972, 86, 14–19. [Google Scholar]
- Ebanks J., P. , Wickett R. R., Boissy R. E. “Mechanisms regulating skin pigmentation: the rise and fall of complexion coloration.” Int J Mol Sci 2009, 10, 4066–4087. [Google Scholar]
- Seiberg, M. , Paine C. , Sharlow E., Andrade-Gordon P., Costanzo M., Eisinger M., Shapiro S. S. “The protease-activated receptor 2 regulates pigmentation via keratinocyte-melanocyte interactions.” Exp Cell Res 2000, 254, 25–32. [Google Scholar]
- Cardinali, G. , Bolasco G. , Aspite N., Lucania G., Lotti L. V., Torrisi M. R., Picardo M. “Melanosome transfer promoted by keratinocyte growth factor in light and dark skin-derived keratinocytes.” J Invest Dermatol 2008, 128, 558–567. [Google Scholar]
- Nanni, M. , Ranieri D. , Raffa S., Torrisi M. R., Belleudi F. “Interplay between FGFR2b-induced autophagy and phagocytosis: role of PLCgamma-mediated signalling.” J Cell Mol Med 2018, 22, 668–683. [Google Scholar]
- Koike, S. , Yamasaki K. , Yamauchi T., Shimada-Omori R., Tsuchiyama K., Ando H., Aiba S. “TLR3 stimulation induces melanosome endo/phagocytosis through RHOA and CDC42 in human epidermal keratinocyte.” J Dermatol Sci 2019, 96, 168–177. [Google Scholar]
- Zhou B., K. , Boissy R. E., Pifko-Hirst S., Moran D. J., Orlow S. J. “Lysosome-associated membrane protein-1 (LAMP-1) is the melanocyte vesicular membrane glycoprotein band II.” J Invest Dermatol 1993, 100, 110–114. [Google Scholar]
- Yun C., Y. , Choi N., Lee J. U., Lee E. J., Kim J. Y., Choi W. J., Oh S. H., Sung J. H. “Marliolide Derivative Induces Melanosome Degradation via Nrf2/p62-Mediated Autophagy. Int J Mol Sci, 2021; 22. [Google Scholar]
- Jager, S. , Bucci C. , Tanida I., Ueno T., Kominami E., Saftig P., Eskelinen E. L. “Role for Rab7 in maturation of late autophagic vacuoles.” J Cell Sci 2004, 117, 4837–4848. [Google Scholar]
- Pillaiyar, T. , Manickam M. , Namasivayam V. “Skin whitening agents: medicinal chemistry perspective of tyrosinase inhibitors.” J Enzyme Inhib Med Chem 2017, 32, 403–425. [Google Scholar]
- Qian, W. , Liu W. , Zhu D., Cao Y., Tang A., Gong G., Su H. “Natural skin-whitening compounds for the treatment of melanogenesis (Review).” Exp Ther Med 2020, 20, 173–185. [Google Scholar]
- Zolghadri, S. , Bahrami A. , Hassan Khan M. T., Munoz-Munoz J., Garcia-Molina F., Garcia-Canovas F., Saboury A. A. “A comprehensive review on tyrosinase inhibitors.” J Enzyme Inhib Med Chem 2019, 34, 279–309. [Google Scholar]
- Saeedi, M. , Eslamifar M. , Khezri K. “Kojic acid applications in cosmetic and pharmaceutical preparations.” Biomed Pharmacother 2019, 110, 582–593. [Google Scholar]
- Maeda, K. , Fukuda M. “Arbutin: mechanism of its depigmenting action in human melanocyte culture.” J Pharmacol Exp Ther 1996, 276, 765–769. [Google Scholar]
- Saeedi, M. , Khezri K. , Seyed Zakaryaei A., Mohammadamini H. “A comprehensive review of the therapeutic potential of alpha-arbutin.” Phytother Res 2021, 35, 4136–4154. [Google Scholar]
- Fitton, A. , Goa K. L. “Azelaic acid. A review of its pharmacological properties and therapeutic efficacy in acne and hyperpigmentary skin disorders.” Drugs 1991, 41, 780–798. [Google Scholar]
- Nazzaro-Porro, M. , Passi S. “Identification of tyrosinase inhibitors in cultures of Pityrosporum.” J Invest Dermatol 1978, 71, 205–208. [Google Scholar]
- Pinnell S., R. “Cutaneous photodamage, oxidative stress, and topical antioxidant protection. ” J Am Acad Dermatol 2003, 48, 1–19; quiz 20–22. [Google Scholar]
- Hakozaki, T. , Minwalla L., Zhuang J. , Chhoa M., Matsubara A., Miyamoto K., Greatens A., Hillebrand G. G., Bissett D. L., Boissy R. E. “The effect of niacinamide on reducing cutaneous pigmentation and suppression of melanosome transfer.” Br J Dermatol 2002, 147, 20–31. [Google Scholar]
- Cabanes, J. , Chazarra S. , Garcia-Carmona F. “Kojic acid, a cosmetic skin whitening agent, is a slow-binding inhibitor of catecholase activity of tyrosinase.” J Pharm Pharmacol 1994, 46, 982–985. [Google Scholar]
- Kameyama, K. , Sakai C. , Kondoh S., Yonemoto K., Nishiyama S., Tagawa M., Murata T., Ohnuma T., Quigley J., Dorsky A., Bucks D., Blanock K. “Inhibitory effect of magnesium L-ascorbyl-2-phosphate (VC-PMG) on melanogenesis in vitro and in vivo.” J Am Acad Dermatol 1996, 34, 29–33. [Google Scholar]
- Tantanasrigul, P. , Sripha A. , Chongmelaxme B. “The Efficacy of Topical Cosmetic Containing Alpha-Arbutin 5% and Kojic Acid 2% Compared With Triple Combination Cream for the Treatment of Melasma: A Split-Face, Evaluator-Blinded Randomized Pilot Study.” J Cosmet Dermatol 2025, 24, e16562. [Google Scholar]
- Park J., I. , Lee H. Y., Lee J. E., Myung C. H., Hwang J. S. “Inhibitory effect of 2-methyl-naphtho [1,2,3-de]quinolin-8-one on melanosome transport and skin pigmentation.” Sci Rep 2016, 6, 29189. [Google Scholar]
- Ito, Y. , Kanamaru A. , Tada A. “Centaureidin promotes dendrite retraction of melanocytes by activating Rho.” Biochim Biophys Acta 2006, 1760, 487–494. [Google Scholar]
- Choi S., G. , Kim J. H., Hong S. H., Lee O. Y., Kang N. G. “Exogenous pyruvate alleviates UV-induced hyperpigmentation via restraining dendrite outgrowth and Rac1 GTPase activity.” J Dermatol Sci 2021, 101, 101–106. [Google Scholar]
- Seiberg, M. , Paine C. , Sharlow E., Andrade-Gordon P., Costanzo M., Eisinger M., Shapiro S. S. “Inhibition of melanosome transfer results in skin lightening.” J Invest Dermatol 2000, 115, 162–167. [Google Scholar]
- Kudo, M. , Kobayashi-Nakamura K. , Tsuji-Naito K. “Bifunctional effects of O-methylated flavones from Scutellaria baicalensis Georgi on melanocytes: Inhibition of melanin production and intracellular melanosome transport.” PLoS One 2017, 12, e0171513. [Google Scholar]
- Gillbro J., M. , Olsson M. J. “The melanogenesis and mechanisms of skin-lightening agents--existing and new approaches.” Int J Cosmet Sci 2011, 33, 210–221. [Google Scholar]
- Kumari, S. , Tien Guan Thng S. , Kumar Verma N., Gautam H. K. “Melanogenesis Inhibitors.” Acta Derm Venereol 2018, 98, 924–931. [Google Scholar]
- Greatens, A. , Hakozaki T. , Koshoffer A., Epstein H., Schwemberger S., Babcock G., Bissett D., Takiwaki H., Arase S., Wickett R. R., Boissy R. E. “Effective inhibition of melanosome transfer to keratinocytes by lectins and niacinamide is reversible.” Exp Dermatol 2005, 14, 498–508. [Google Scholar]
| a. Selected Molecular Biomarkers of Skin Pigmentation in Melanocytes | ||
| Process | Gene ID | Function |
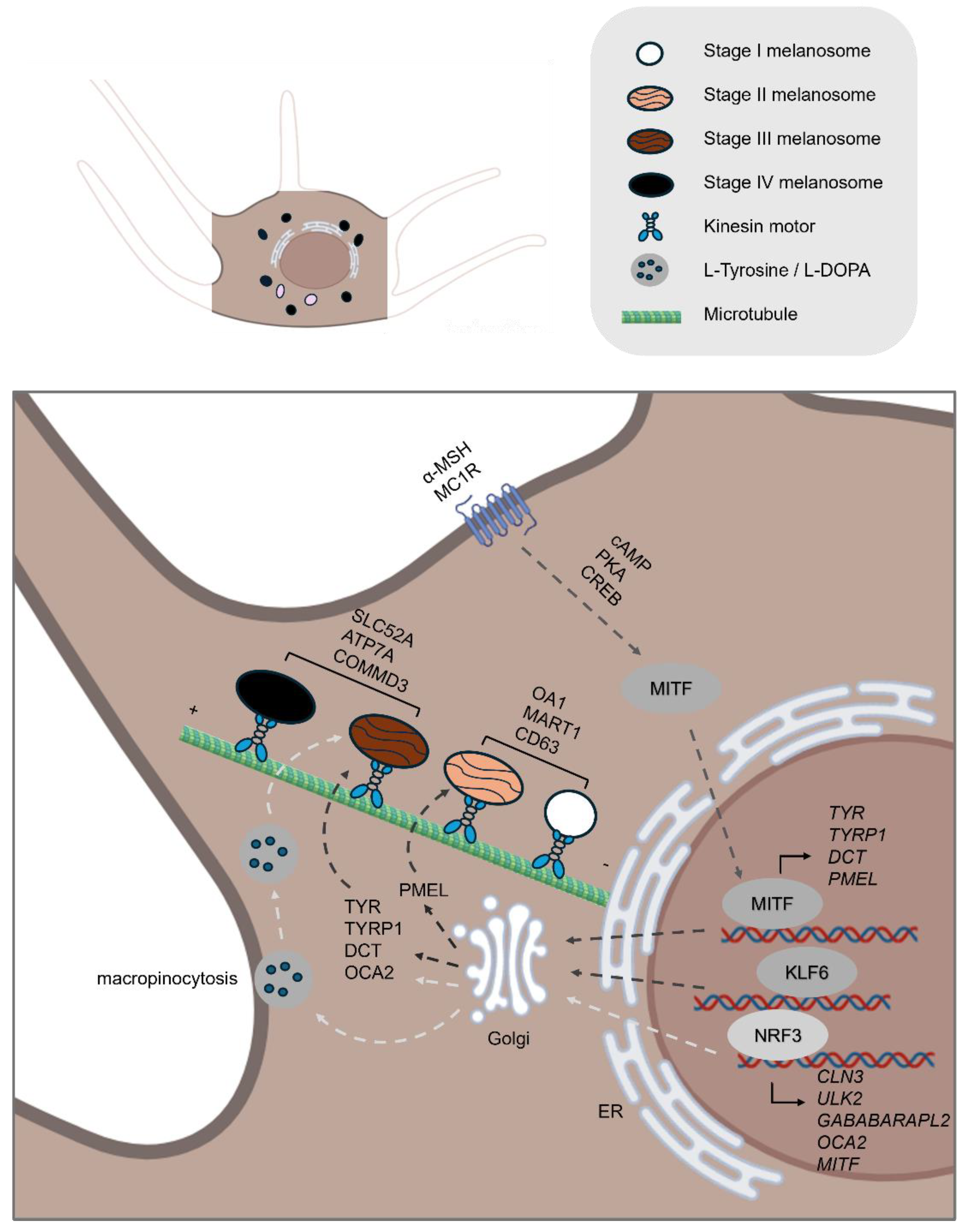
| Melanogenesis | TYR | Catalyzes the conversion of the amino acid tyrosine into melanin through a series of biochemical reactions [4]. |
| TYRP1 | Catalyzes the oxidation of 5,6-dihydroxyindole-2-carboxylic acid (DHICA) to indole-5,6-quinone-2-carboxylic acid in the melanin biosynthesis pathway [4]. | |
| DCT | Catalyzes the conversion of DHICA during melanin synthesis [4]. | |
| MITF | A transcription factor that controls the expression of numerous genes involved in melanin synthesis and pigmentation [23]. | |
| MC1R | A receptor activated by α-MSH that activates the cAMP signaling pathway, crucial for stimulating melanin production [22]. | |
| NRF3 | A transcription factor that regulates the uptake of melanin precursors, such as L-tyrosine and L-DOPA, through macropinocytosis and also controls the expression of autophagy-related genes involved in melanosome formation and degradation [28]. | |
| PMEL | Initiates the formation of melanosome [29]. | |
| MART1 | To form a complex with PMEL, thereby regulating PMEL’s expression, stability, trafficking, and proteolytic processing [31]. | |
| OA1 | Functions as a key regulator of melanosome maturation by controlling melanosome biogenesis and size at distinct stages [33,35]. | |
| OCA2 | A melanosomal membrane protein that contributes to a chloride ion current, which is essential for regulating melanosomal pH [38] | |
| SLC45A2 | A melanosomal membrane transporter that functions at the late stages of melanosome maturation to maintain a neutral pH within mature melanosomes [40]. | |
| ATP7A | A copper transporter that localizes to melanosomes in a BLOC-1–dependent manner, where it supplies copper directly to TYR [42]. | |
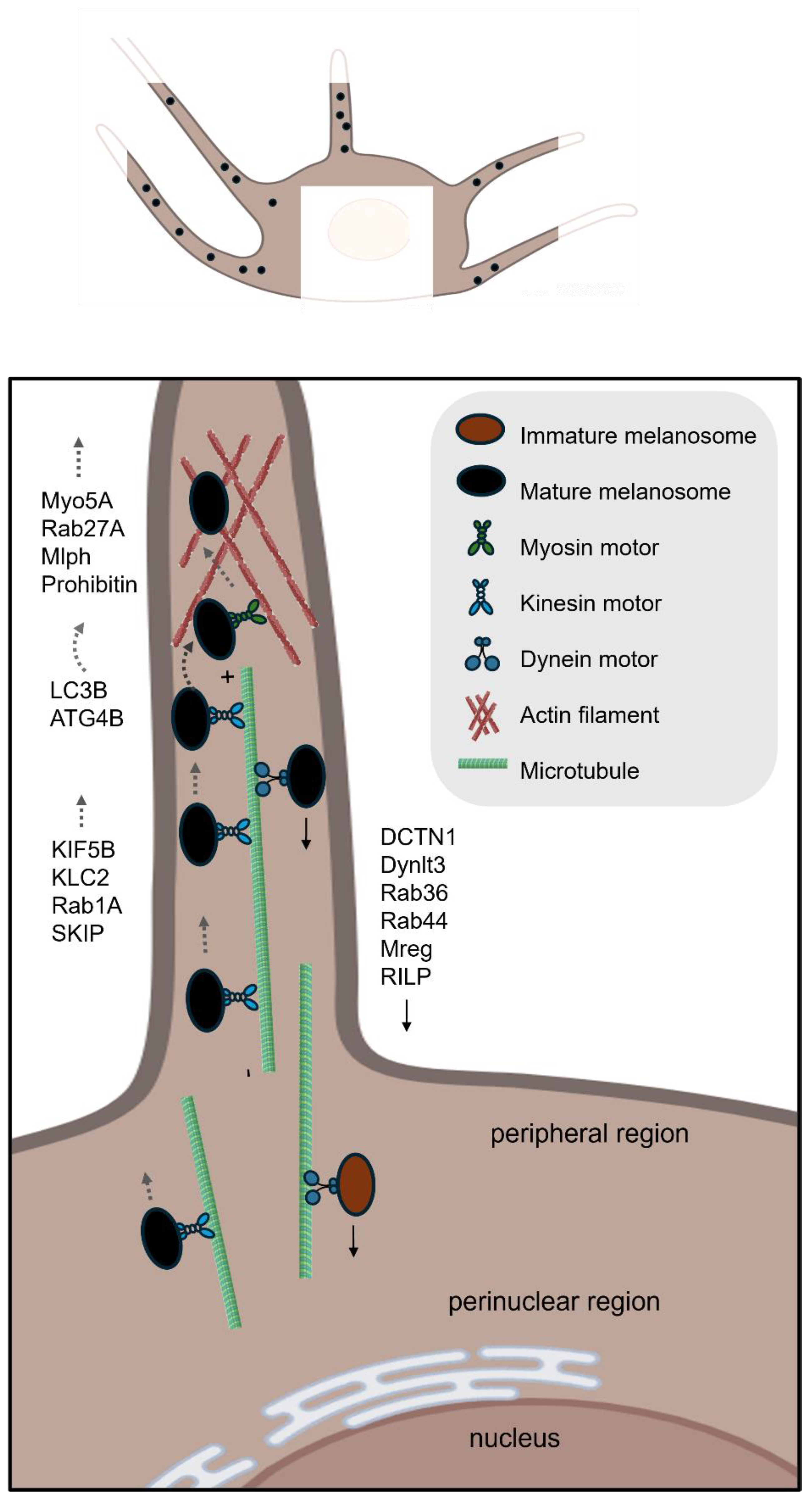
| Transport and transfer | RAB1A | A small GTPase that promotes melanosome microtubule anterograde transport [45]. |
| SKIP (PLEKHM2) | An adaptor protein that forms a transport complex with Rab1A and kinesin-1 to facilitate melanosome microtubule anterograde transport [45]. | |
| KIF5B | The kinesin-1 heavy chain that regulates melanosome microtubule anterograde transport [45]. | |
| KCL2 | The kinesin-1 light chain that regulates melanosome microtubule anterograde transport [45]. | |
| MAP1LC3B | Induces ERK dependent MITF expression, mediates melanosome-microtubule interactions to facilitate melanosome trafficking on microtubule and helps to translocate melanosome from microtubule to actin [14,52]. | |
| ATG4B | Removes LC3B from microtubule and further mediates melanosome trafficking on actin [14,52]. | |
| MACF1 | Functions as a cytoskeletal crosslinker that coordinates the interaction between microtubules and actin filaments [54,55]. | |
| RAB27A | A small GTPase that promotes melanosome actin transport [57,59]. | |
| Melanophilin | An adaptor protein that bridges Rab27A/Myo5A and promotes melanosome actin transport [58,59]. | |
| MYO5A | Functions as a processive actin-based motor protein that is essential for the short-range transport and peripheral capture of melanosomes in melanocytes [59,60]. | |
| RAB36 | A small GTPase that promotes melanosome microtubule retrograde transport [65]. | |
| RILP | Interacts with Rab36 and promotes melanosome microtubule retrograde transport [65]. | |
| Melanoregulin | Interacts with RILP and DCTN1 and mediates melanosome microtubule retrograde transport [62]. | |
| DYNLT3 | A critical regulatory subunit of the cytoplasmic dynein motor complex, specifically influencing melanosome retrograde transport in melanocytes [62,63]. | |
| RAB44 | Promotes melanosome microtubule retrograde transport [64]. | |
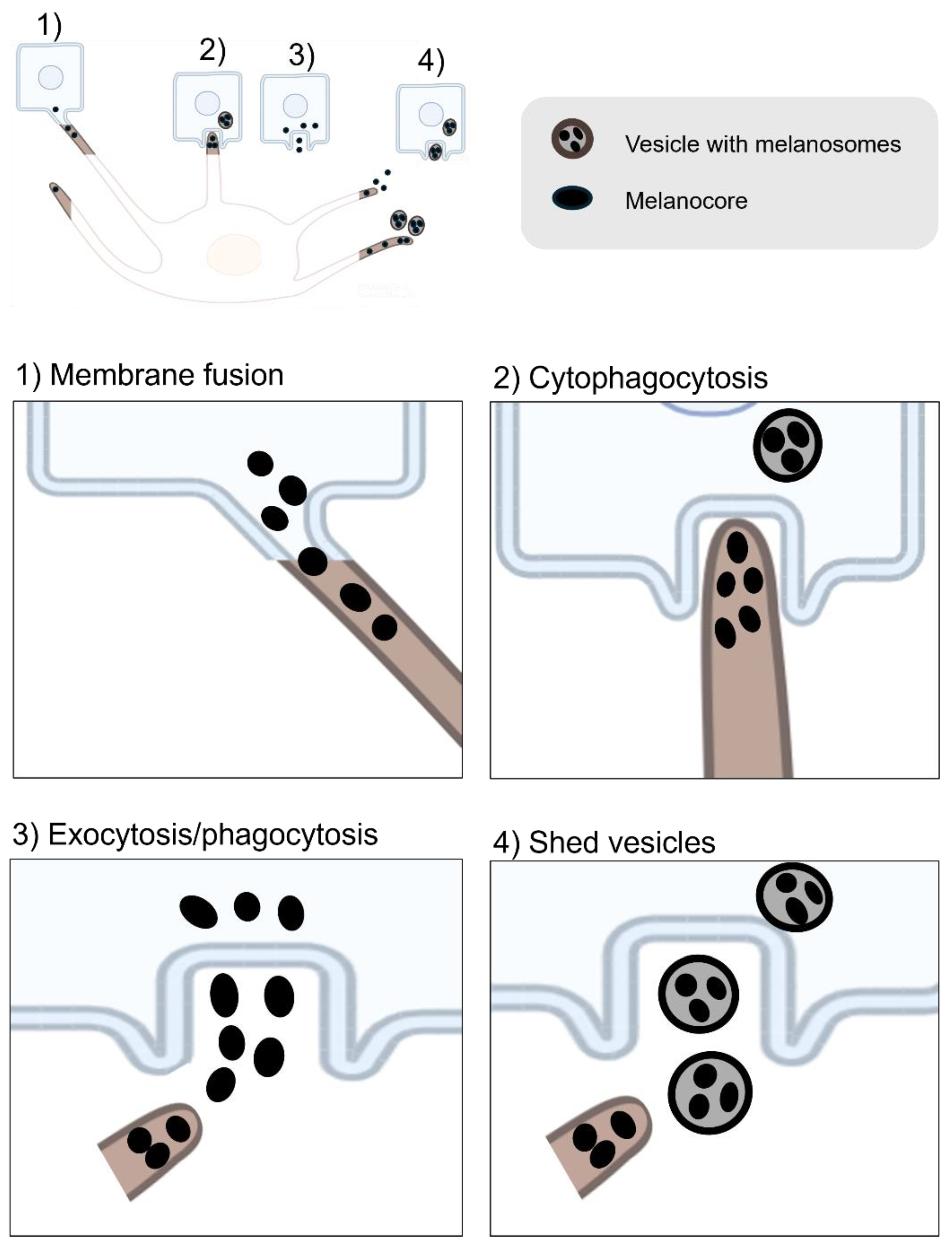
| RAB11B | A small GTPase that regulates keratinocytes induced melanin exocytosis and transfer [70,71]. | |
| EXOC7 | The subunits of the exocyst complex and is involved in melanin exocytosis and transfer [71]. | |
| EXOC4 | The subunits of the exocyst complex and is involved in melanin exocytosis and transfer [71]. | |
| CAV1 | Forms caveolae structures that facilitate melanocyte-keratinocyte interactions necessary for melanin transfer [69]. | |
| b. Selected Molecular Biomarkers of Skin Pigmentation in Keratinocytes | ||
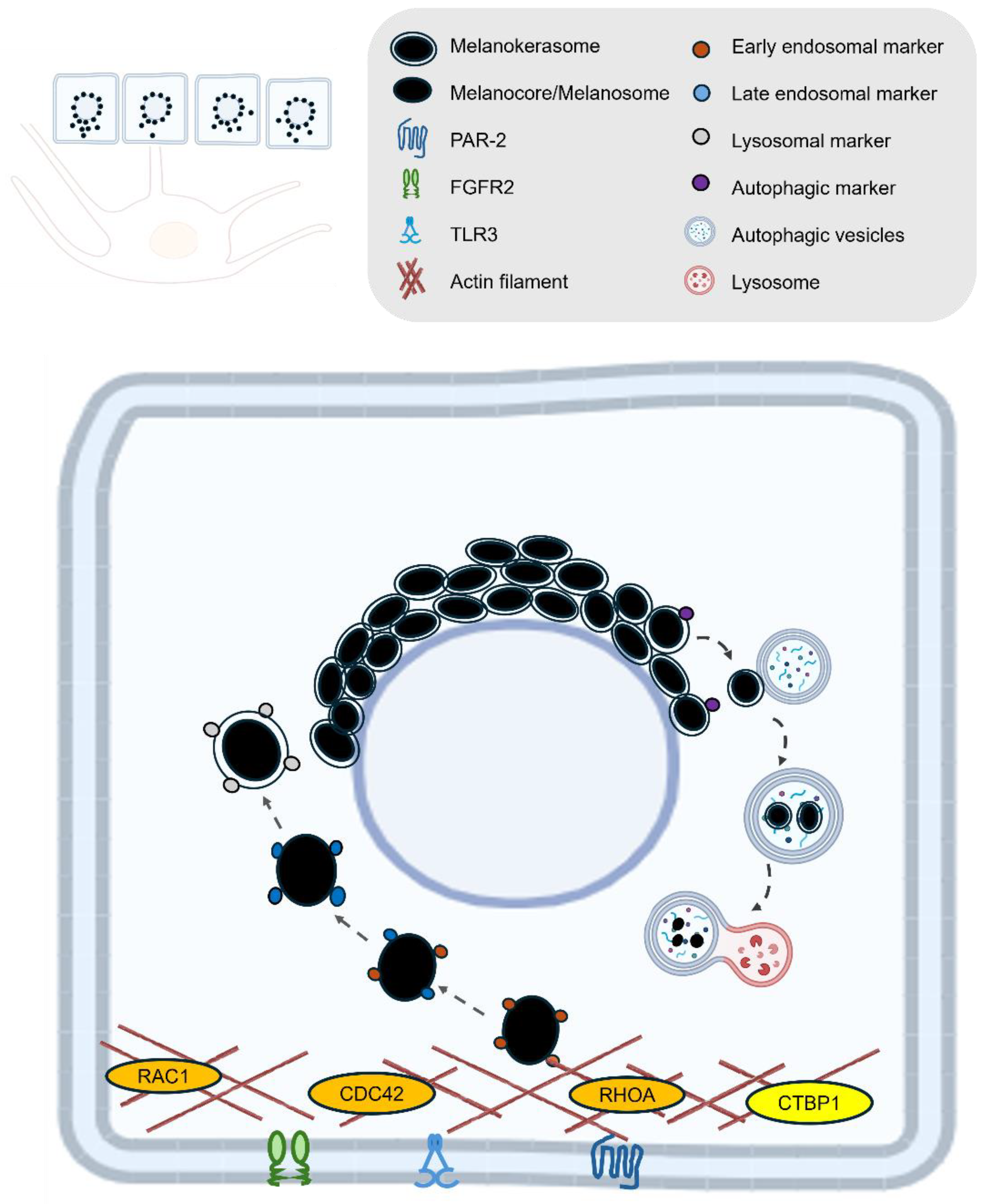
| Transfer and uptake | PAR-2 | Activates phagocytic capacity of keratinocytes, receptor, promotes melanocore uptake [10,72,76]. |
| TLR3 | UV-responsive regulator of melanin internalization. Enhances melanosome and melanocore uptake in keratinocytes via actin-dependent endocytosis, primarily by activating RHOA and CDC42 [79]. | |
| FGFR2 | Promotes melanosome uptake through phagocytosis and links this process to autophagy, controlling both the internalization and degradation of melanosomes in keratinocytes [66,78]. | |
| RAC1 | A Rho GTPase that mainly promotes melanocore uptake [72]. | |
| CDC42 | A Rho GTPase that mainly promotes melanocore uptake [72]. | |
| RHOA | A Rho GTPase that mainly promotes melanosome uptake [72]. | |
| CTBP1 | Encodes a protein involved in membrane fission events necessary for endocytosis, particularly affecting melanosome uptake [72]. | |
|
Processing |
LAMP1 | Regulates lysosomal exocytosis, a process critical for melanosome transport and integration into keratinocytes. Maintains lysosomal membrane integrity, protecting against enzymatic degradation and enabling melanin’s long-term photoprotective storage in keratinocytes [5,9,13,80]. |
| EEA1 | Early endosomal marker that surrounds melanocores in keratinocytes [5,10]. | |
| RAB5 | Early endosomal marker that surrounds melanocores in keratinocytes [5,10]. | |
| P62 | Functions as an autophagy adaptor protein in keratinocytes, mediating the selective degradation of melanosomes by linking them to the autophagy machinery and facilitating their clearance through the autophagy–lysosome pathway [11,81]. | |
| ATG7 | Essential for autophagy-dependent melanosome degradation in keratinocytes by enabling the formation of autophagosomes that engulf and facilitate the lysosomal breakdown of melanin-containing compartments [11]. | |
| MAP1LC3B | LC3 (specifically LC3-II, the lipidated form of MAP1LC3B) functions in melanosome degradation in keratinocytes by marking autophagosomes that engulf melanin-containing compartments, thereby facilitating their autophagic clearance through the lysosomal pathway [11]. | |
| RAB7B | Facilitates lysosomal fusion and protein degradation on melanosomes [13]. | |
| CTSV | Lysosomal protease plays a critical role in breaking down melanosome-associated proteins and melanosome integrity, indirectly influencing melanin persistence in keratinocytes [12]. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




