Submitted:
01 August 2025
Posted:
04 August 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Medical Products
- N-acetylcysteine 300 mg/ 3ml(Fluimucil®, Zambon)
- Budesonide 0.5mg/ 2 ml, (Aircort®, Italchimici)
- Sobrerol 40 mg/ 3 ml (Sobrefluid®, Bayer)
- Beclomethasone dipropionate 0.8 mg / 2 ml (Clenil Aerosol®, Chiesi Farmaceutici)
- Thiamphenicol glycinate acetylcysteinate 0.5 mg/4 ml(Fluimucil antibiotico®, Zambon)
- Ipratropium bromide 0.5 mg / 2 ml (Atem®, Chiesi Farmaeutici)
- Rinubes, ADL Farmaceutici
- Mad Nasal, Sakura
- Spray-sol, Buona
- Rinowash, Air Liquide Medical Systems
2.2. Particles Size Assessments
2.3. Patients
2.4. Treatments
2.5. Statistical Analyses
3. Results
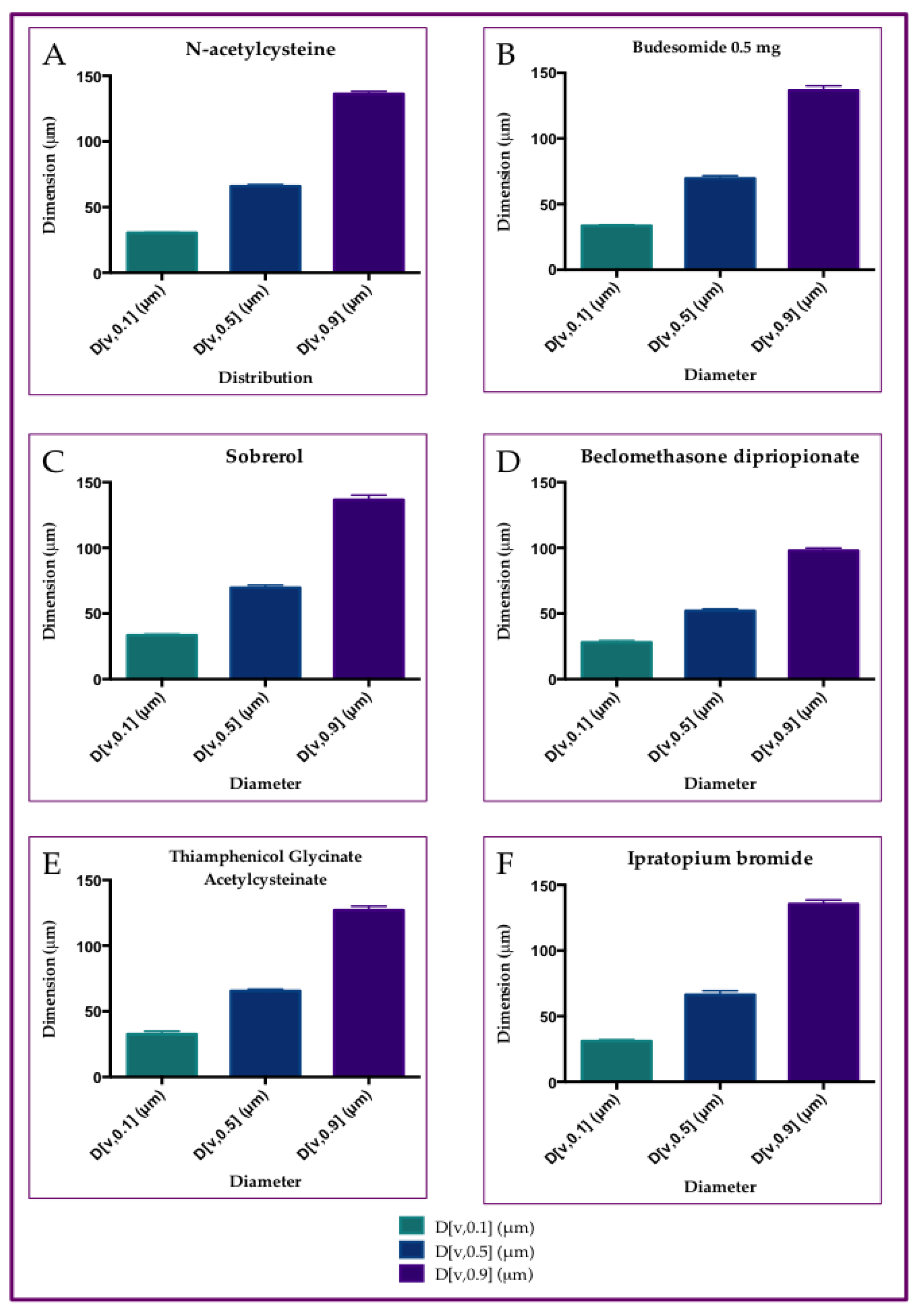
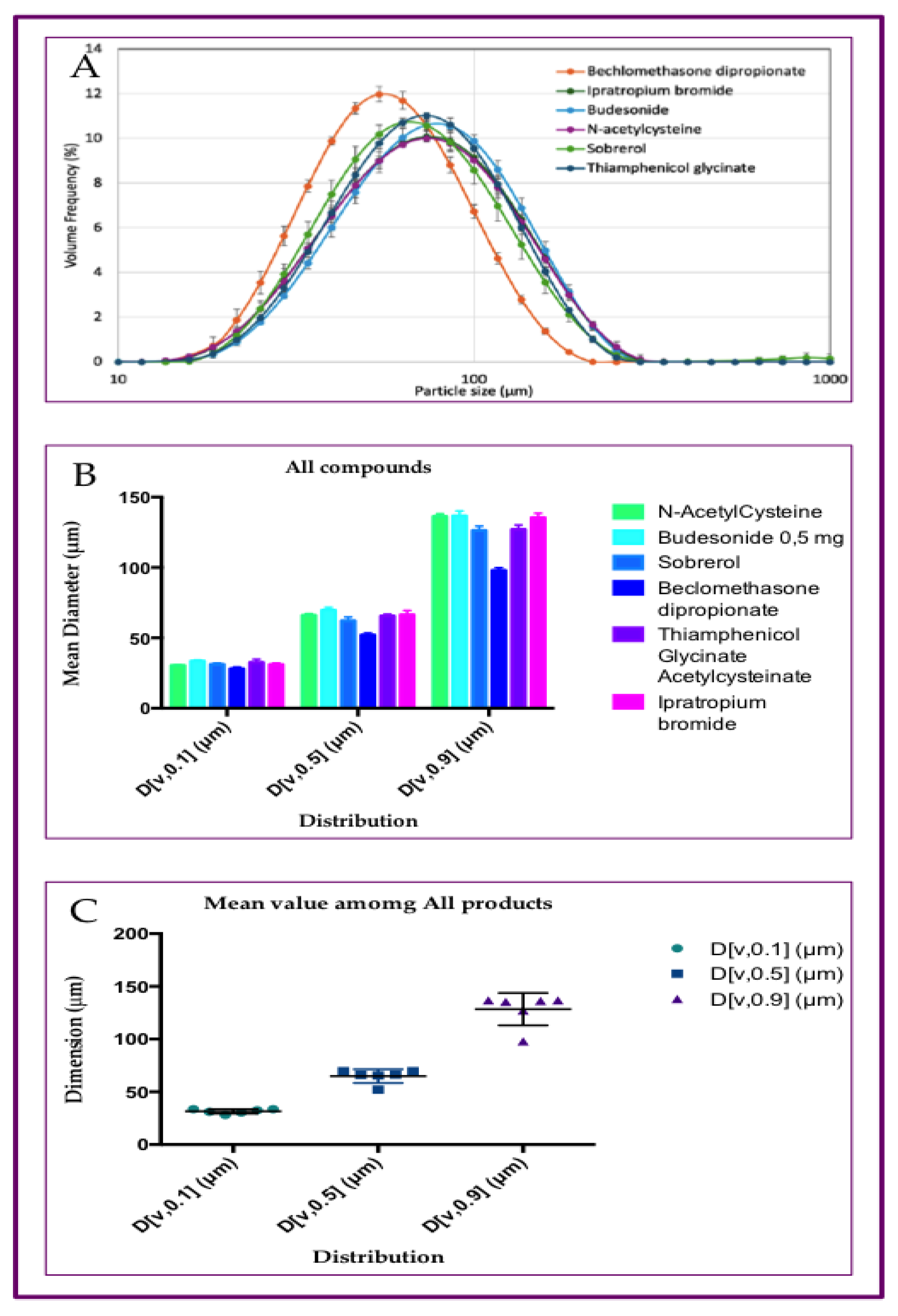
3.1. Particle Size Distribution of Aerosol Produced by Six Different Drugs, with Rinubes Device
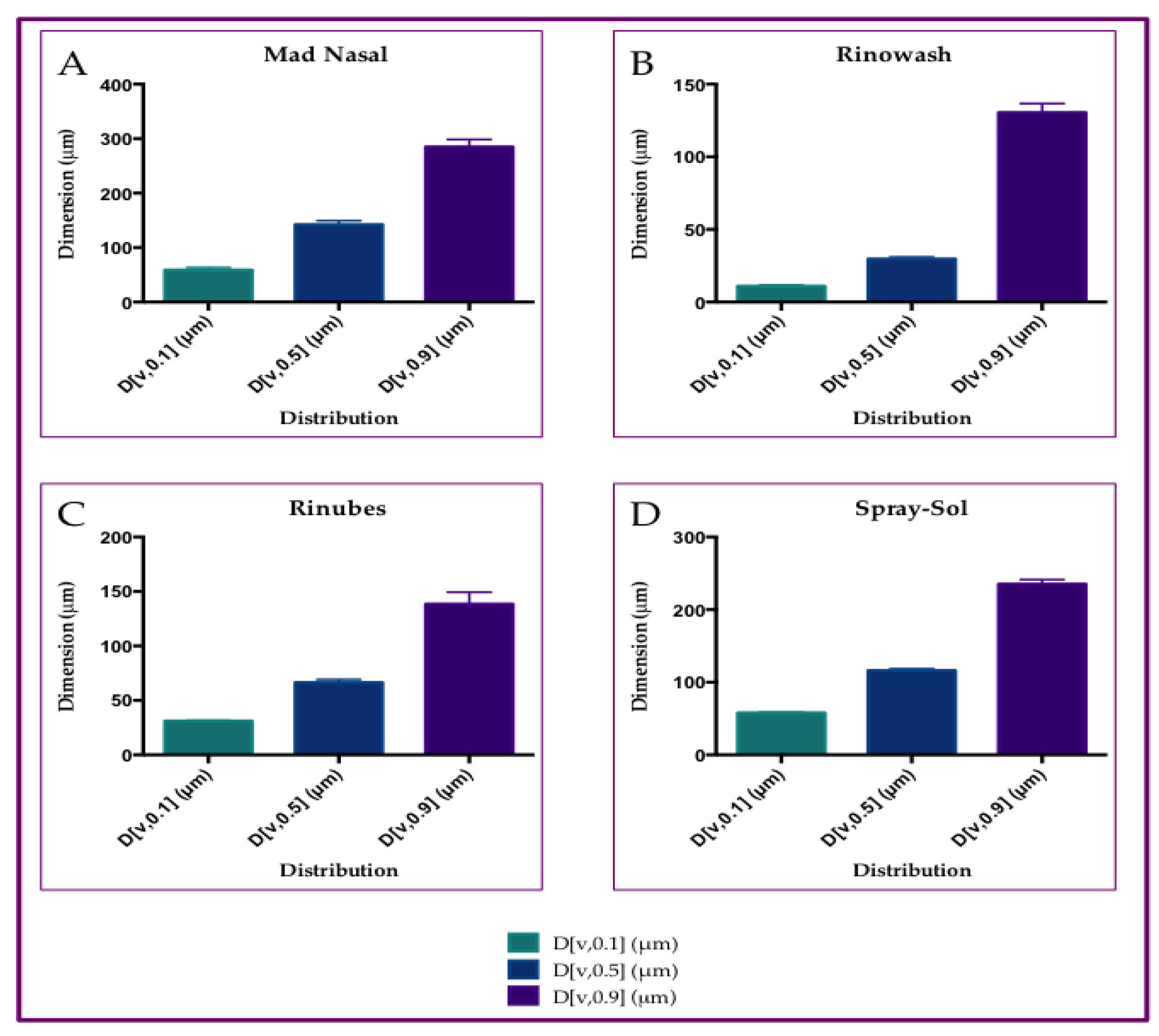
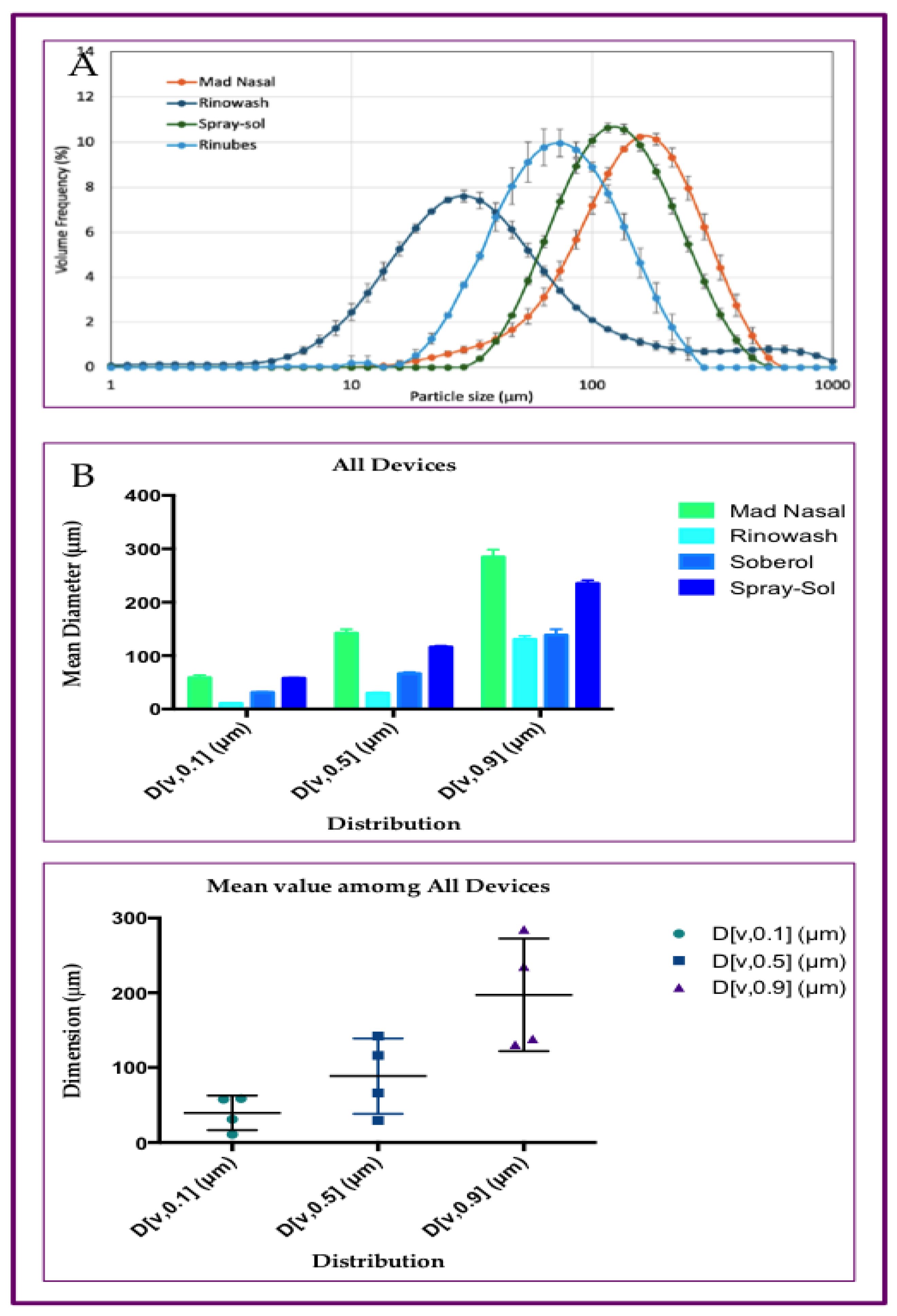
3.2. Particle Size Distribution of Aerosol Produced by Saline Solution, with Three Different Nasal Devices
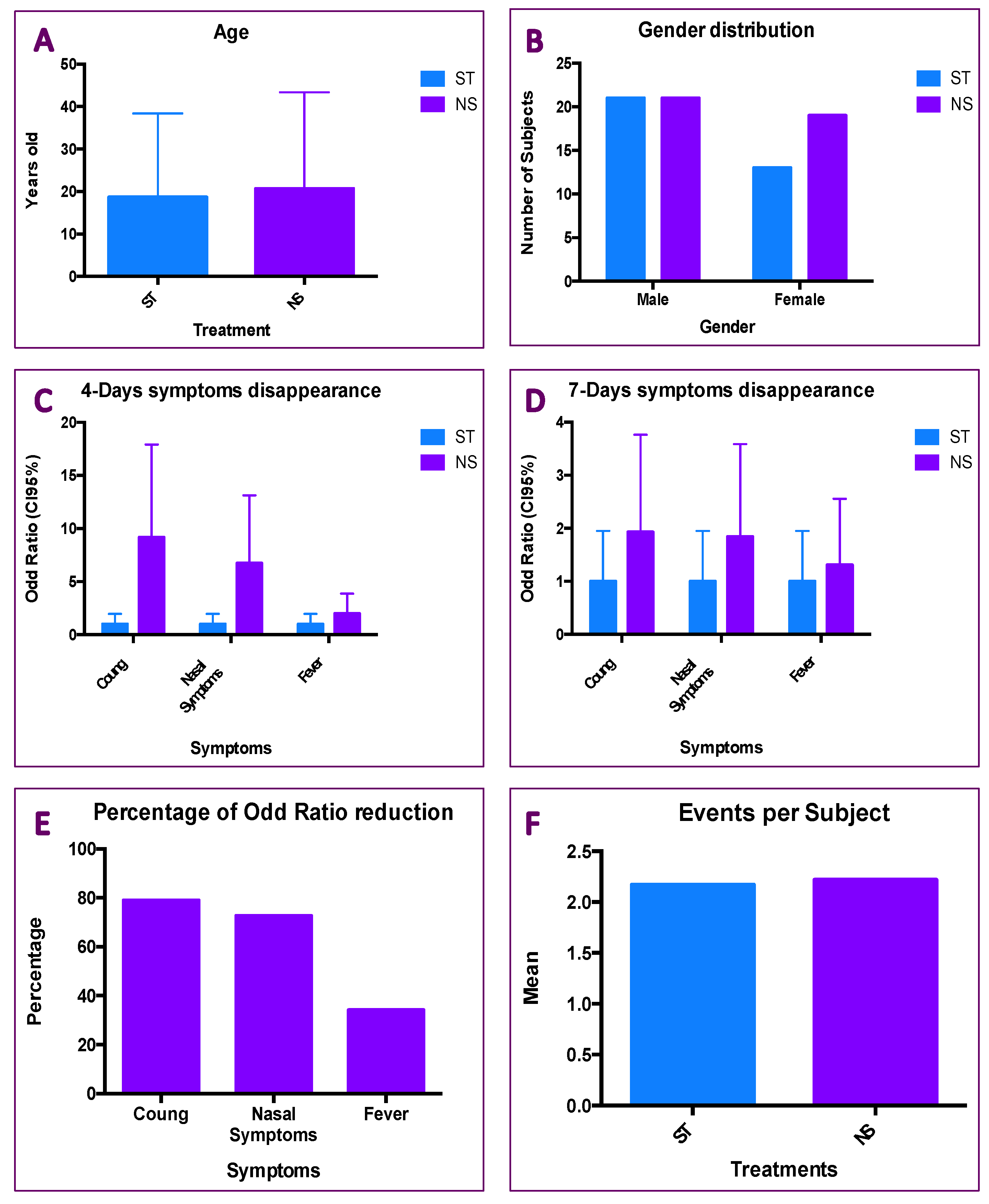
3.3. Results of Patients Treated with ST and NS
4. Discussion
5. Conclusions
6. Patents
- (1)
- Utility Model No. 202020000003820 IMPROVED NASAL NEBULIZER
- (2)
- Italian Patent for Industrial Invention No. 102020000015844 – USE OF SALT-BROMINE-IODINE WATERS FOR THE NASAL TREATMENT OF INFLAMMATION IN THE THREE AREAS OF THE UPPER AIRWAYS AND MEDICAL DEVICES ADAPTED FOR SUCH TREATMENT.
- (3)
- European Patent Application for Industrial Invention No. 21748658.8 – USE OF SALT-BROMINE-IODINE WATERS FOR THE NASAL TREATMENT OF THE INFLAMMATION OF THE THREE AREAS OF THE UPPER AIRWAYS AND MEDICAL DEVICES ADAPTED TO SUCH A TREATMENT.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Momtazmanesh, S.; Moghaddam, S.S.; Ghamari, S.-H.; Rad, E.M.; Rezaei, N.; Shobeiri, P.; Aali, A.; Abbasi-Kangevari, M.; Abbasi-Kangevari, Z.; Abdelmasseh, M.; et al. Global Burden of Chronic Respiratory Diseases and Risk Factors, 1990–2019: An Update from the Global Burden of Disease Study 2019. eClinicalMedicine 2023, 59, 101936. [Google Scholar] [CrossRef]
- Wise, S.K.; Damask, C.; Greenhawt, M.; Oppenheimer, J.; Roland, L.T.; Shaker, M.S.; Wallace, D.V.; Lang, D.M. A Synopsis of Guidance for Allergic Rhinitis Diagnosis and Management From ICAR 2023. J. Allergy Clin. Immunol. Pract. 2023, 11, 773–796. [Google Scholar] [CrossRef]
- Ciprandi, G.; Tosca, M.A. Turbinate Hypertrophy, Allergic Rhinitis, and Otitis Media. Curr. Allergy Asthma Rep. 2021, 21, 44. [Google Scholar] [CrossRef]
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J.; Alobid, I.; et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinol. J. 2020, 0, 1–464. [Google Scholar] [CrossRef]
- Varricchio, A.; Presutti, L.; La Mantia, I.; Ciprandi, G. Inter-Societal Delphi Consensus on the Topical Nasal Treatments in Italy. Multidiscip. Respir. Med. 2024, 19, 2. [Google Scholar] [CrossRef]
- Eccles, R. Common Cold. Front. Allergy 2023, 4, 1224988. [Google Scholar] [CrossRef]
- Bansil, R.; Turner, B.S. The Biology of Mucus: Composition, Synthesis and Organization. Adv. Drug Deliv. Rev. 2018, 124, 3–15. [Google Scholar] [CrossRef]
- Karkouli, G.; Douros, K.; Moriki, D.; Moutsatsou, P.; Giannopoulou, I.; Maratou, E.; Koumpagioti, D. Dysfunctional Breathing in Children: A Literature Review. Children 2024, 11, 556. [Google Scholar] [CrossRef]
- Taherali, F.; Varum, F.; Basit, A.W. A Slippery Slope: On the Origin, Role and Physiology of Mucus. Adv. Drug Deliv. Rev. 2018, 124, 16–33. [Google Scholar] [CrossRef]
- Keicho, N.; Hijikata, M.; Miyabayashi, A.; Wakabayashi, K.; Yamada, H.; Ito, M.; Morimoto, K. Impact of Primary Ciliary Dyskinesia: Beyond Sinobronchial Syndrome in Japan. Respir. Investig. 2024, 62, 179–186. [Google Scholar] [CrossRef]
- Ciprandi, G.; Cirillo, I. The Lower Airway Pathology of Rhinitis. J. Allergy Clin. Immunol. 2006, 118, 1105–1109. [Google Scholar] [CrossRef]
- Ciprandi, G.; Gelardi, M. Open and Clean: The Healthy Nose. Acta Bio Medica Atenei Parm. 2019, 90. [Google Scholar] [CrossRef]
- Https://Www.Nobelprize.Org/Prizes/Chemistry/1925 /Summary/.
- Cojocaru, E.; Petriș, O.R.; Cojocaru, C. Nanoparticle-Based Drug Delivery Systems in Inhaled Therapy: Improving Respiratory Medicine. Pharmaceuticals 2024, 17, 1059. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Serrano, A.; Martínez-Campos, P.; Seijoso-González, L.; Ruiz-Rojo, H. Revisión narrativa de la técnica de los lavados nasales en pediatría. Enferm. Clínica 2021, 31, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Forest, V.; Pourchez, J. Nano-Delivery to the Lung - by Inhalation or Other Routes and Why Nano When Micro Is Largely Sufficient? Adv. Drug Deliv. Rev. 2022, 183, 114173. [Google Scholar] [CrossRef] [PubMed]
- Hami, Z. A Brief Review on Advantages of Nano-Based Drug Delivery Systems. Ann. Mil. Health Sci. Res. 2021, 19. [Google Scholar] [CrossRef]
- Jin, Z.; Gao, Q.; Wu, K.; Ouyang, J.; Guo, W.; Liang, X.-J. Harnessing Inhaled Nanoparticles to Overcome the Pulmonary Barrier for Respiratory Disease Therapy. Adv. Drug Deliv. Rev. 2023, 202, 115111. [Google Scholar] [CrossRef]
- Dinakar, Y.H.; Karole, A.; Parvez, S.; Jain, V.; Mudavath, S.L. Organ-Restricted Delivery through Stimuli-Responsive Nanocarriers for Lung Cancer Therapy. Life Sci. 2022, 310, 121133. [Google Scholar] [CrossRef]
- Leong, E.W.X.; Ge, R. Lipid Nanoparticles as Delivery Vehicles for Inhaled Therapeutics. Biomedicines 2022, 10, 2179. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, S.; Wu, J.; Xi, Y.; Ma, J.; Wu, C.; Zhang, S.; Chen, X.D.; Wu, W.D. Enhancing Blending Efficiency and in Vitro Aerosol Performance of Low-Dose Inhalable Dry Powders with Spray Freeze Dried Microparticles. Eur. J. Pharm. Biopharm. 2025, 212, 114740. [Google Scholar] [CrossRef]
- Ullah, F.; Sabei, F.Y.; Shah, K.U.; Safhi, A.Y.; Bakkari, M.A.; Madkhali, O.A.; Albariqi, A.H.; Saeed, M.D.; Ramzan, M. Inhalable Microparticles Embedding Hyaluronic Acid–Coated Chitosan Nanoparticles: Fabrication and Evaluation for Preferential Accumulation of Montelukast in the Lung. J. Microencapsul. 2025, 1–12. [Google Scholar] [CrossRef]
- Kuntic, M.; Kuntic, I.; Cleppien, D.; Pozzer, A.; Nußbaum, D.; Oelze, M.; Junglas, T.; Strohm, L.; Ubbens, H.; Daub, S.; et al. Differential Inflammation, Oxidative Stress and Cardiovascular Damage Markers of Nano- and Micro-Particle Exposure in Mice: Implications for Human Disease Burden. Redox Biol. 2025, 83, 103644. [Google Scholar] [CrossRef]
- Liu, H.; Sun, Y.; Cai, S.; Zhao, C.; Xu, X.; Xu, A.; Zhou, H.; Yang, C.; Gu, X.; Ai, X. Formononetin-Loaded PLGA Large Porous Microparticles via Intratracheal Instillation for Bleomycin-Induced Pulmonary Fibrosis Treatment. AAPS PharmSciTech 2025, 26, 112. [Google Scholar] [CrossRef]
- Song, L.; Zhai, Z.; Ouyang, W.; Ding, J.; Wang, S.; Li, S.; Liang, M.; Xu, F.; Gao, C. Inhalation of Macrophage Membrane-Coated Hydrogel Microparticles for Inflammation Alleviation of Acute Lung Injury in Vivo. Acta Biomater. 2025, 192, 409–418. [Google Scholar] [CrossRef]
- Farnoud, A.; Tofighian, H.; Baumann, I.; Ahookhosh, K.; Pourmehran, O.; Cui, X.; Heuveline, V.; Song, C.; Vreugde, S.; Wormald, P.-J.; et al. Numerical and Machine Learning Analysis of the Parameters Affecting the Regionally Delivered Nasal Dose of Nano- and Micro-Sized Aerosolized Drugs. Pharmaceuticals 2023, 16, 81. [Google Scholar] [CrossRef]
- Biglarian, M.; MomeniLarimi, M.; Firoozabadi, B.; Inthavong, K.; Farnoud, A. Targeted Drug Delivery with Polydisperse Particle Transport and Deposition in Patient-Specific Upper Airway during Inhalation and Exhalation. Respir. Physiol. Neurobiol. 2023, 308, 103986. [Google Scholar] [CrossRef]
- Shang, Y.; Dong, J.; Inthavong, K.; Tu, J. Comparative Numerical Modeling of Inhaled Micron-Sized Particle Deposition in Human and Rat Nasal Cavities. Inhal. Toxicol. 2015, 27, 694–705. [Google Scholar] [CrossRef]
- Schroeter, J.D.; Tewksbury, E.W.; Wong, B.A.; Kimbell, J.S. Experimental Measurements and Computational Predictions of Regional Particle Deposition in a Sectional Nasal Model. J. Aerosol Med. Pulm. Drug Deliv. 2015, 28, 20–29. [Google Scholar] [CrossRef]
- Farhadi Ghalati, P.; Keshavarzian, E.; Abouali, O.; Faramarzi, A.; Tu, J.; Shakibafard, A. Numerical Analysis of Micro- and Nano-Particle Deposition in a Realistic Human Upper Airway. Comput. Biol. Med. 2012, 42, 39–49. [Google Scholar] [CrossRef]
- Heritage; Brook; Underdown; McDERMOTT Intranasal Immunization with Polymer-grafted Microparticles Activates the Nasal-associated Lymphoid Tissue and Draining Lymph Nodes. Immunology 1998, 93, 249–256. [CrossRef] [PubMed]
- Huang, Z.; Wu, H.; Yang, B.; Chen, L.; Huang, Y.; Quan, G.; Zhu, C.; Li, X.; Pan, X.; Wu, C. Anhydrous Reverse Micelle Nanoparticles: New Strategy to Overcome Sedimentation Instability of Peptide-Containing Pressurized Metered-Dose Inhalers. Drug Deliv. 2017, 24, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Clementino, A.R.; Pellegrini, G.; Banella, S.; Colombo, G.; Cantù, L.; Sonvico, F.; Del Favero, E. Structure and Fate of Nanoparticles Designed for the Nasal Delivery of Poorly Soluble Drugs. Mol. Pharm. 2021, 18, 3132–3146. [Google Scholar] [CrossRef] [PubMed]
- Clementino, A.; Climani, G.; Bianchera, A.; Buttini, F.; Sonvico, F. Polysaccharides: New Frontiers for Nasal Administration of Medicines. Polysaccharides 2025, 6, 6. [Google Scholar] [CrossRef]
- Shim, S.; Yoo, H.S. The Application of Mucoadhesive Chitosan Nanoparticles in Nasal Drug Delivery. Mar. Drugs 2020, 18, 605. [Google Scholar] [CrossRef]
- Canelli, E.; Ferrari, L.; Borghetti, P.; Candela, F.; Abiakam, N.S.; Bianchera, A.; Buttini, F.; Magi, G.E.; Sonvico, F.; Martelli, P.; et al. Nano-Adjuvanted Dry Powder Vaccine for the Mucosal Immunization against Airways Pathogens. Front. Vet. Sci. 2023, 10, 1116722. [Google Scholar] [CrossRef]
- Komalla, V.; Wong, C.Y.J.; Sibum, I.; Muellinger, B.; Nijdam, W.; Chaugule, V.; Soria, J.; Ong, H.X.; Buchmann, N.A.; Traini, D. Advances in Soft Mist Inhalers. Expert Opin. Drug Deliv. 2023, 20, 1055–1070. [Google Scholar] [CrossRef]
- Reddy, K.P.; Naraharisetti, L.T.; Prasanna, V.S.; Babu, S.S.; Ehsan, I.; Godugu, C.; Datta, P. Excipient-Free Inhalable Combination Shell-Core Microparticles with Clofazimine as Shell for Extended Pulmonary Retention of Isoniazid in Core. Int. J. Pharm. 2025, 672, 125310. [Google Scholar] [CrossRef]
- Varricchio, A.; Ciprandi, G. Sobrerol in Managing Acute Respiratory Infections in Clinical Practice During the “Cold” Season: An Italian Primary Care Experience. Int. J. Gen. Med. 2024, Volume 17, 5471–5477. [Google Scholar] [CrossRef]
- Sidell, D.; Shapiro, N.L.; Bhattacharyya, N. Obesity and the Risk of Chronic Rhinosinusitis, Allergic Rhinitis, and Acute Otitis Media in School-age Children. The Laryngoscope 2013, 123, 2360–2363. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, M.J.; Chiu, E.J.; Landgraf, J.M.; Gliklich, R.E. The Health Impact of Chronic Recurrent Rhinosinusitis in Children. Arch. Otolaryngol. Neck Surg. 2000, 126, 1363. [Google Scholar] [CrossRef]
- Haruna, S.; Sawada, K.; Nakajima, T.; Moriyama, H. Relationship between Pediatric Sinusitis and Middle Turbinate Pneumatization—Ethmoidal Sinus Pyocele Thought to Be Caused by Middle Turbinate Pneumatization. Int. J. Pediatr. Otorhinolaryngol. 2005, 69, 375–379. [Google Scholar] [CrossRef]
- Lovie-Toon, Y.G.; Chang, A.B.; Newcombe, P.A.; Vagenas, D.; Anderson-James, S.; Drescher, B.J.; Otim, M.E.; O’Grady, K.-A.F. Longitudinal Study of Quality of Life among Children with Acute Respiratory Infection and Cough. Qual. Life Res. 2018, 27, 891–903. [Google Scholar] [CrossRef]
- Hill, D.B.; Button, B.; Rubinstein, M.; Boucher, R.C. Physiology and Pathophysiology of Human Airway Mucus. Physiol. Rev. 2022, 102, 1757–1836. [Google Scholar] [CrossRef] [PubMed]
- Volsko, T.A. Airway Clearance Therapy: Finding the Evidence. Respir. Care 2013, 58, 1669–1678. [Google Scholar] [CrossRef] [PubMed]
- Ciprandi, G.; Varriccchio, A. Sobrerol: New Perspectives to Manage Patients with Frequent Respiratory Infections. Children 2023, 10, 1210. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




