Introduction
Hypercalcemia is a clinical condition marked by elevated calcium levels in the bloodstream, which can lead to significant morbidity, including complications such as kidney stones, bone pain, and neurological disturbances.(1, 2) This condition not only affects the quality of life of patients but also imposes substantial healthcare costs.(3) Current diagnostic modalities primarily rely on serum calcium measurements. Studies have already suggested that electrocardiogram (ECG) changes could serve as potential indicators of electrolyte imbalances, including those seen in hypercalcemia. In the previous literatures, the effects of hypercalcemia on the ECG are primarily characterized by shortened QT intervals, elevated ST segments, and alterations in T waves(4). Shortened QT intervals represent the most common ECG manifestation of hypercalcemia, resulting from accelerated myocardial cell repolarization due to elevated serum calcium levels. Hypercalcemia may also lead to ST segment elevation, a finding that must be carefully differentiated from acute myocardial infarction in clinical settings. (5)In patients with mild hypercalcemia, ECG changes may be subtle or limited to a slight shortening of the QT interval. In contrast, patients with moderate to severe hypercalcemia often exhibit more pronounced abnormalities, including marked QT interval shortening, ST segment elevation, and T wave inversion.(6) The presence of these ECG changes underscores the necessity for prompt therapeutic intervention. Therefore, early recognition and management of hypercalcemia are critically important in clinical practice. Clinicians should be well-versed in the common etiologies of hypercalcemia and its associated ECG findings to enable timely diagnosis and appropriate treatment. However,those findings including QT shortening had poor predication for hypercalcemia or were not easy to be detected or calculated. Traditionally, the ECG diagnosis of hypercalcemia has traditionally relied on QT interval shortening as the key criterion. However, nearly in half of patients with elevated serum calcium levels, both the QT and corrected QT (QTc) intervals remained within normal ranges. Therefore,the ECG manifestations have not been fully explored, representing a significant gap in the literature.
Occasionally, Dr zhai found that specific alterations in T-wave morphology (characterized by V1-V3 ST segment disappearing and leftward shift of the T-wave peak) maybe have been associated with the increase in calcium levels, which may reflect underlying cardiac dysfunction. Such findings suggest that ECG could become an easier way to diagnose hypercalcemia, potentially aiding in the identification of patients at risk of complications due to elevated calcium levels.
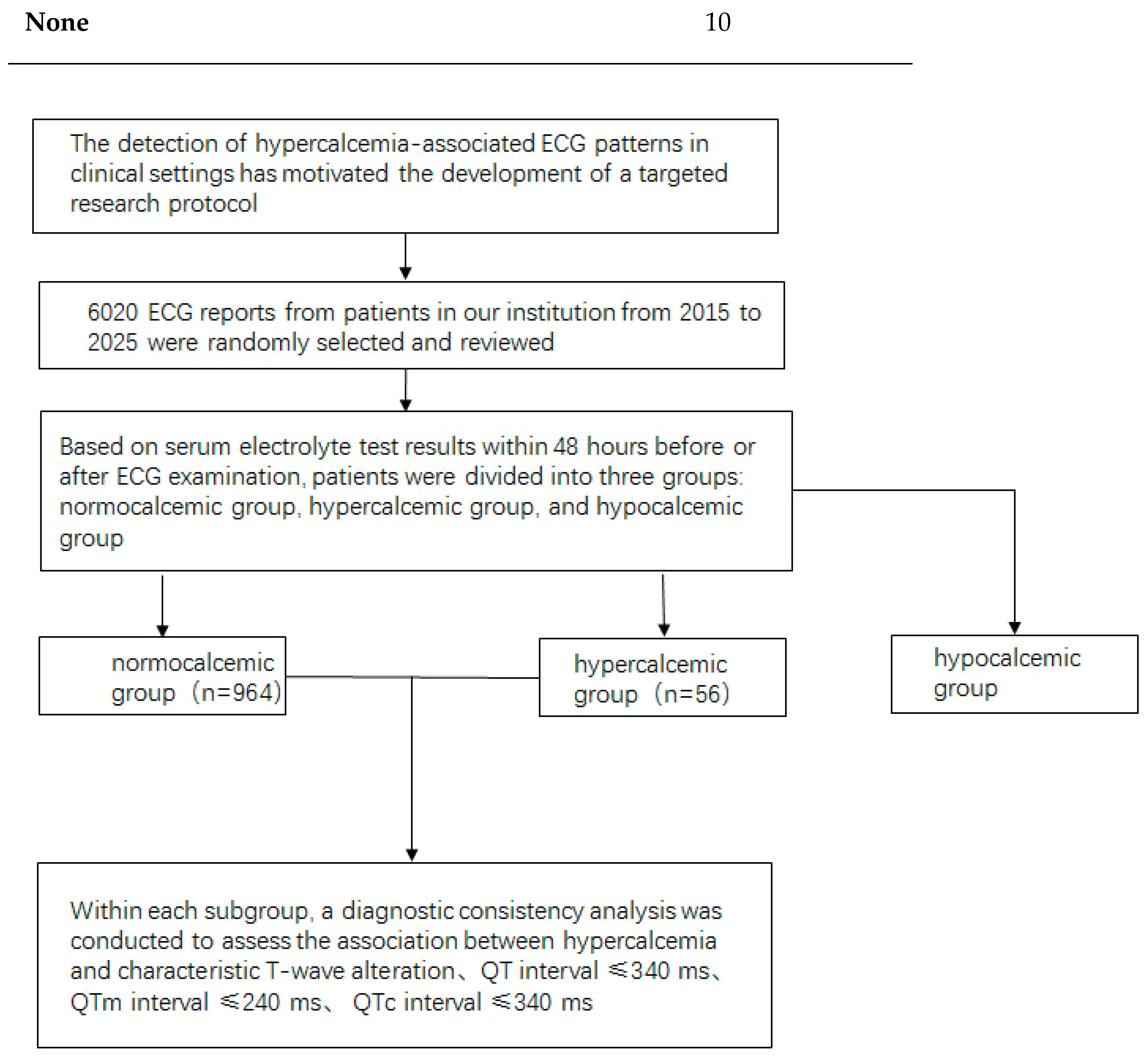
This research employs a retrospective observational study design to explore the relationship between ECG changes and hypercalcemia. 6020 patients were screened and then hypercalcemic patients and a control group of normocalcemic individuals were compared. The study aims to provide a comprehensive evaluation of ECG characteristics associated with elevated calcium levels. The focus is particularly on T-wave alterations, which have been previously identified as significant indicators of electrolyte imbalances. The primary objective of this research is to assess the sensitivity and specificity of these ECG changes in diagnosing hypercalcemia, thereby enhancing the diagnostic accuracy and clinical management of affected patients.
Methods:
Study Subjects: A retrospective random screening was conducted on 6,020 patients who visited the outpatient department or were hospitalized at our hospital between January 2020 and January 2025. The inclusion criteria were as follows: availability of electrocardiogram (ECG) records and serum calcium measurements within 48 hours before or after the ECG examination. Ultimately, 64 patients with hypercalcemia were identified and included in the experimental group based on their 12-lead ECGs. During the same period, 956 patients with normal serum calcium levels were randomly selected to the control group. Both groups were analyzed for characteristic T-wave alterations on ECG (notably, V1-V3 ST segment disappearing and leftward shift of the T-wave peak), as well as QT, QTc, and QTm interval shortening.
Characteristic T-wave alterations in hypercalcemic patients were primarily defined as leftward shift of the T-wave peak. It may be accompanied by disappearance of the ST segment in leads V1–V3, shortening of QTm (the interval from the onset of the QRS complex to the peak of the T wave), an increased angle between the ascending limb of the T wave and the horizontal baseline compared to the angle between the descending limb and the baseline, absence of tall or peaked T waves, and in some cases, flattened or reduced T wave amplitude. QTm ≤ 240 ms, QT ≤ 340 ms, and QTc ≤ 340 ms were considered as indicators of interval shortening.
Diagnostic criteria for hypercalcemia: Normal serum calcium concentration ranges from 2.25 to 2.58 mmol/L. Mild hypercalcemia is defined as 2.5–3.0 mmol/L, moderate hypercalcemia as 3.0–3.5 mmol/L, severe hypercalcemia as greater than 3.5 mmol/L, and hypercalcemic crisis as exceeding 3.75 mmol/L.
Statistical Analysis:
Categorical variables were presented as frequency rates and percentages and were compared by chi-square or Fisher exact test. Continuous variables were presented as Means ± SD. Means for continuous variables were compared using independent group t-test or ANOVA when the data were normally distributed; otherwise, the Mann-Whitney test was used. Two-sided p < 0.05 was considered statistically significant. All statistical analyses were performed with SPSS software (version 24.0, IBM). Sensitivity was calculated to evaluate the ability of the EKG changes (characteristic T-wave alterations) to correctly identify hypercalcemic cases. The formula used for sensitivity calculation was as follows:
Specificity was calculated to evaluate the ability of the EKG alterations to correctly identify true negative cases. The formula used for specificity calculation was as follows:
The formula used for accuracy calculation was as follows:
True Positives (TP) were defined as subjects correctly identified as positive by both the EKG alterations and the blood test.
False Negatives (FN) were defined as subjects incorrectly identified as negative by the EKG alterations but positive by the blood test.
True Negatives (TN) were defined as subjects correctly identified as negative by both the EKG alterations and the blood test.
False Positives (FP) were defined as subjects incorrectly identified as positive by the EKG alterations but negative by the blood test.
Results:
Among the 64 patients in the hypercalcemia group, there were 35 males and 29 females, with a mean age of 62 ± 17 years. In the control group of 956 patients, 458 were male and 498 were female, with a mean age of 58 ± 16 years. Among the 64 patients diagnosed with hypercalcemia, 54 had underlying conditions closely associated with elevated serum calcium levels. Hyperparathyroidism, renal failure, and malignant tumors collectively accounted for 76.6% of these cases. (
Table 1)
Figure 1 shows the schematic diagram of the study operation.
Table 1.
Clinical Profile of 64 Hypercalcemic Patients.
Table 1.
Clinical Profile of 64 Hypercalcemic Patients.
| Causes |
Cases |
| Hyperparathyroidism |
17 |
| History of Malignant Tumor |
16 |
| Renal Failure |
16 |
| Acidosis |
12 |
| Bone Metabolism Disorder |
6 |
| None |
10 |
Figure 1.
The schematic diagram of the study operation.
Figure 1.
The schematic diagram of the study operation.
Table 2.
Comparison of QT, QTc, and QTm Between Hypercalcemic and Normocalcemic groups.
Table 2.
Comparison of QT, QTc, and QTm Between Hypercalcemic and Normocalcemic groups.
| |
QT(ms) |
QTc(ms) |
QTm (ms)
|
| Normocalcemia |
380.6± 75.5 |
415.7±57.1 |
270.6±27.0 |
| Hypercalcemia |
340.5± 15.4 |
404.2±78.4 |
226.6±23.4 |
| P-value |
<0.01 |
<0.01 |
<0.01 |
Table 3.
Proportions of Characteristic T-Waves alteration, QTm ≤240 ms, QT ≤340 ms, and QTc ≤340 ms in Hypercalcemic vs. Normocalcemic groups.
Table 3.
Proportions of Characteristic T-Waves alteration, QTm ≤240 ms, QT ≤340 ms, and QTc ≤340 ms in Hypercalcemic vs. Normocalcemic groups.
| |
Characteristic T-Waves alteration |
QTm≤240ms |
QT≤340ms |
QTc≤340ms |
| Normocalcemia |
14.64% (14/956) |
2.72% (26/956) |
5.4.% (52/956) |
0.21% (2/956) |
| Hypercalcemia |
78.13% (50/64) |
68.75% (44/64) |
48.44% (31/64) |
10.94% (7/64) |
| P-value |
<0.001 |
<0.001 |
<0.001 |
<0.001 |
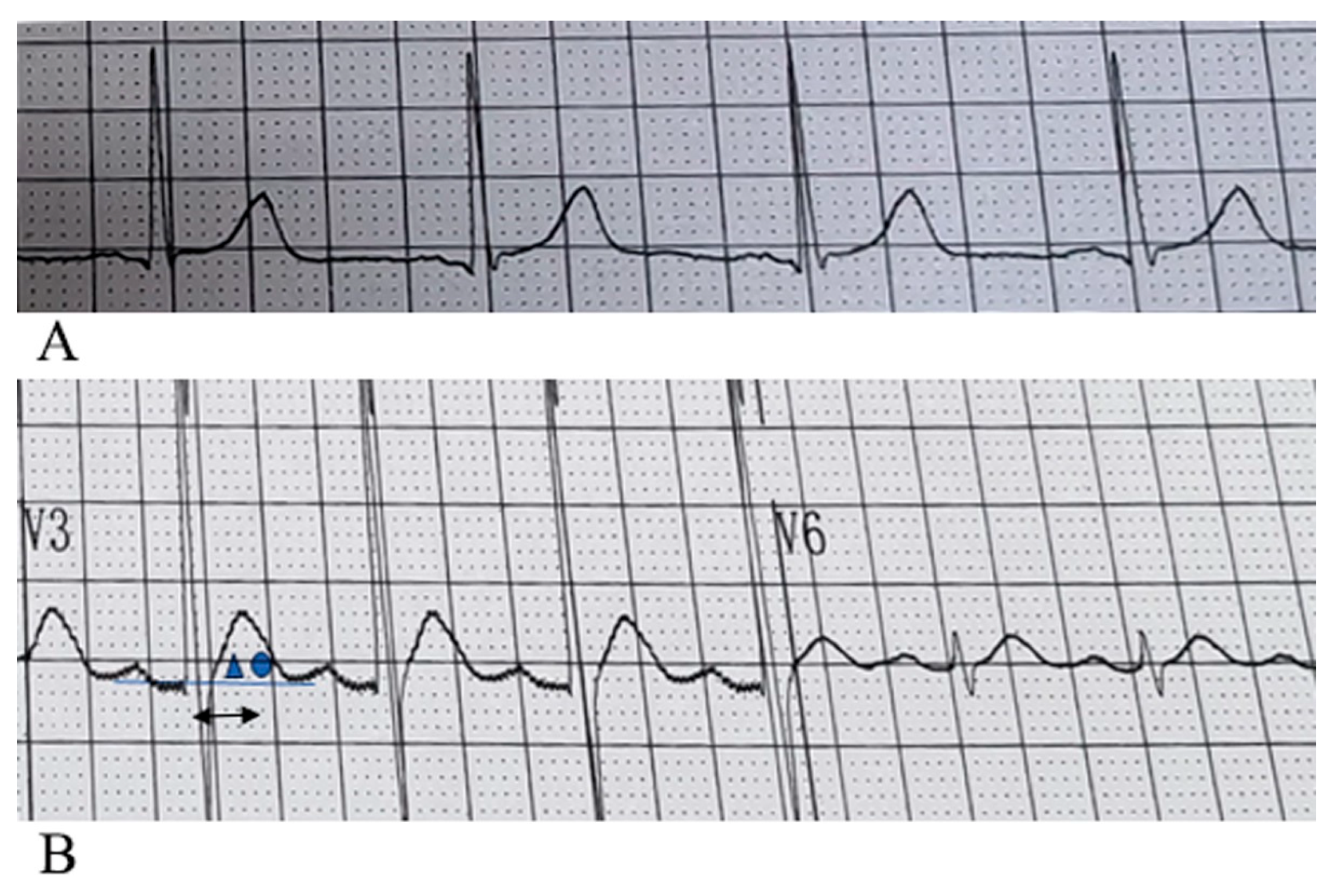
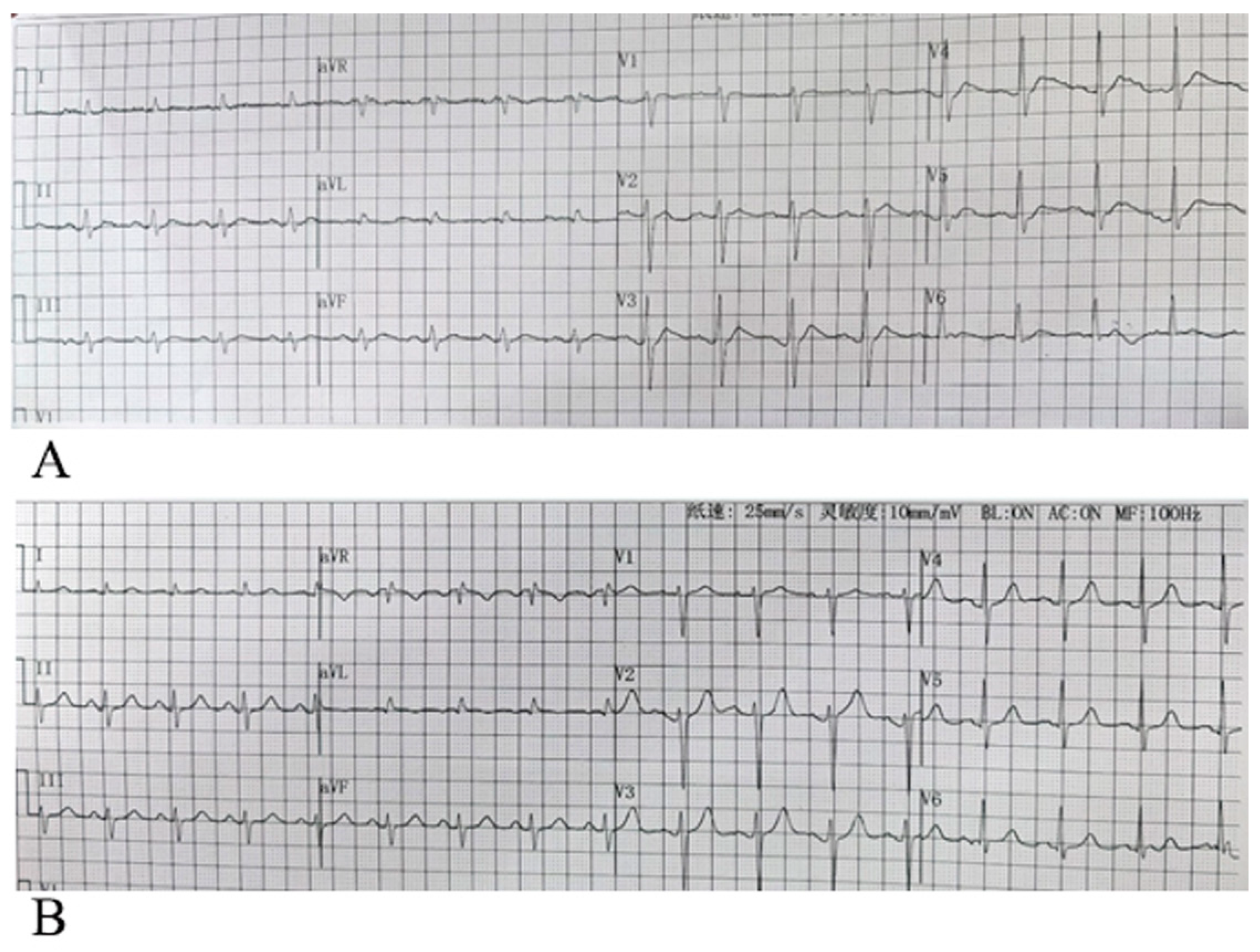
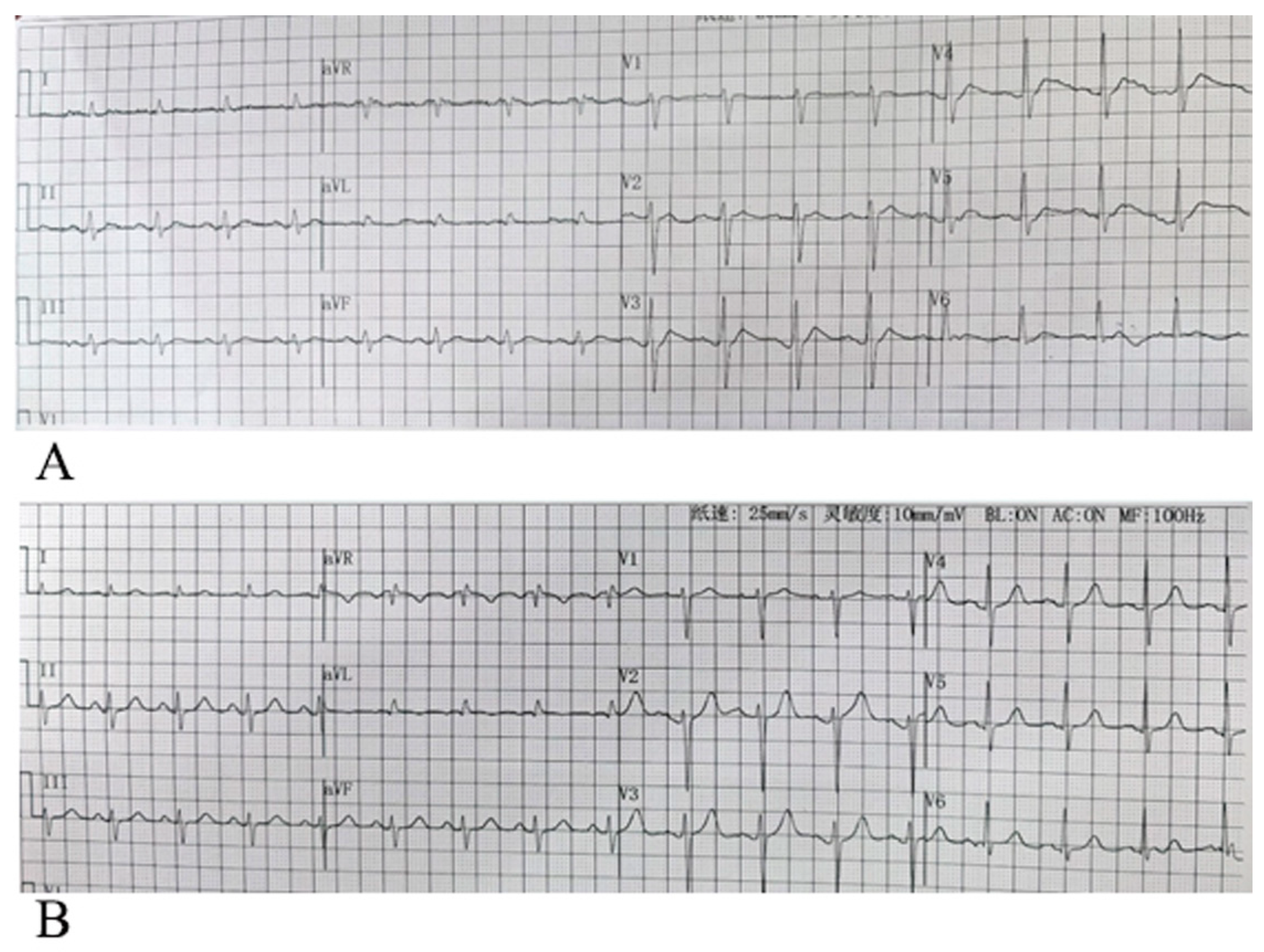
By analyzing the 12-lead surface electrocardiogram (ECG) patterns of 64 patients with hypercalcemia, we identified characteristic T-wave alterations in most cases: primarily characterized by leftward shift of the T-wave peak, sometimes manifested as disappearance of the ST segment in leads V1–V3, could companied by a shortened QTm (defined as the distance from QRS wave onset to T-wave peak), an anterior limb angle greater than the posterior limb angle relative to the horizontal baseline, and absence of tall or sharp T waves, with some cases even showing flattened or small T waves. The electrocardiograms of patients with normal blood calcium levels and those with hypercalcemia, showing typical T-wave alterations, are presented in
Figure 2 and
Figure 3, respectively. The electrocardiogram changes associated with hypercalcemia and following calcium correction in the same patient are illustrated in
Figure 4.
Discussion
In this article, Dr. Zhai identified and reported this specific electrocardiogram changes in patients with hypercalcemia, and compared these findings with those observed in patients with normal serum calcium levels. She summarized this change as “ leftward shift in the T-wave peak,” which may also be referred to as “Zhai’s change” within the context of this paper. This study employs a retrospective observational design, analyzing a cohort of 64 hypercalcemic patients in comparison to a control group comprising 956 normocalcemic individuals, with the aim of assessing the sensitivity and specificity of ECG changes “leftward shift in the T-wave peak” in diagnosing hypercalcemia. We found that Zhai’s change exhibit extremely high sensitivity and specificity in the diagnosis of hypercalcemia.
In the past, electrocardiogram diagnosis of hypercalcemia was based solely on QT shortening, that is, QT≤340ms. Later, QTc≤340ms was set as the QT shortening standard.(7-9) According to our research, it is not difficult to find that although QTc shows the highest specificity of 99.8% in diagnosing hypercalcemia and the specificity of QT shortening is 76.99%, the sensitivity of QT shortening is only 48.43%, and the sensitivity of QTc is even lower, at only 10.9%. Therefore, in the past, diagnosing hypercalcemia based on shortened QT and QTc would miss a considerable number of cases of hypercalcemia. Even with the QTm indicator, the sensitivity is only 64.06%, and the sensitivity based on the specific electrocardiogram morphology of hypercalcemia is 78.1%, with a specificity of 97.28%. In clinical practice, several factors can cause QT shortening, including fever, hyperkalemia, acidosis, acute myocardial injury, autonomic nervous system disorders, and ion channel diseases. As shown in
Table 4, the isolated use of QT shortening as an indicator lacks sufficient sensitivity for the diagnosis of hypercalcemia. Therefore, there is a pressing need in clinical settings for an ECG indicator that is both specific and highly sensitive for detecting hypercalcemia. The Zhai’s change has shown excellent reliability in diagnosing hypercalcemia. The hypercalcemia-specific ECG index we have summarized meets these criteria—it is easy for clinicians to learn, and demonstrates high sensitivity and specificity.
Hypercalcemia is a common clinical condition with diverse etiologies. According to recent studies, primary hyperparathyroidism (PHPT) is the leading cause of hypercalcemia among outpatients, accounting for the majority of cases.(10) In contrast, among hospitalized patients, hypercalcemia is most frequently associated with malignancies, comprising 54–65% of reported cases(2). Hypercalcemia can not only cause cardiovascular abnormalities but also lead to complications in multiple other organ systems. For example, hypercalcemia may result in neurological complications such as mental confusion, drowsiness, and even coma. Studies have demonstrated that the incidence of neurological complications is notably high among patients with severe hypercalcemia. In addition, hypercalcemia can cause gastrointestinal complications, including nausea, vomiting, constipation, and pancreatitis. Hypercalcemia-induced pancreatitis is relatively common in clinical practice and often presents with a severe clinical course.(11-13)
Normal myocardial cells depolarize from the endocardium to the epicardium and repolarize in the reverse direction, from the epicardium back to the endocardium. The T-wave duration typically ranges from 0.1 to 0.25 seconds. The limb leads are not symmetrical; the cardiac apex is closer to the left ventricle, resulting in faster return to baseline in those leads. The ascending limb of the positive T wave is longer than the descending limb, whereas the descending limb of the negative T wave is longer than the ascending limb (as shown in
Figure 1). In general, two-thirds of the T wave occurs before the peak and one-third after it. The reason is that the myocardium beneath the outer membrane begins to repolarize after excitation, making the repolarization process relatively slow. By the time the impulse reaches the subendocardium, the excitatory phase of the myocardium at this site has already passed, resulting in a relatively faster repolarization. (14)
Hypercalcemia is a common electrolyte disorder that can result in various abnormal electrocardiogram (ECG) findings. Its underlying mechanism is closely associated with the role of calcium ions in myocardial cells. Firstly, hypercalcemia shortens the action potential duration of myocardial cells, primarily by accelerating calcium ion influx, which leads to a reduction in the QT interval. These changes are typically reflected on the ECG as a shortened QT interval and alterations in the T wave morphology. Secondly, hypercalcemia may also influence the repolarization phase of the cardiac cycle. Elevated calcium levels can interfere with potassium ion flux, resulting in early repolarization and J-point elevation. (15)These effects are commonly observed as J-point elevation and features consistent with early repolarization syndrome on the ECG. In addition, hypercalcemia may increase the excitability of myocardial cells and enhance the activity of ectopic pacemakers, thereby predisposing to arrhythmias such as tachycardia and atrial fibrillation, which can be reflected in abnormal electrocardiogram findings.(16, 17)
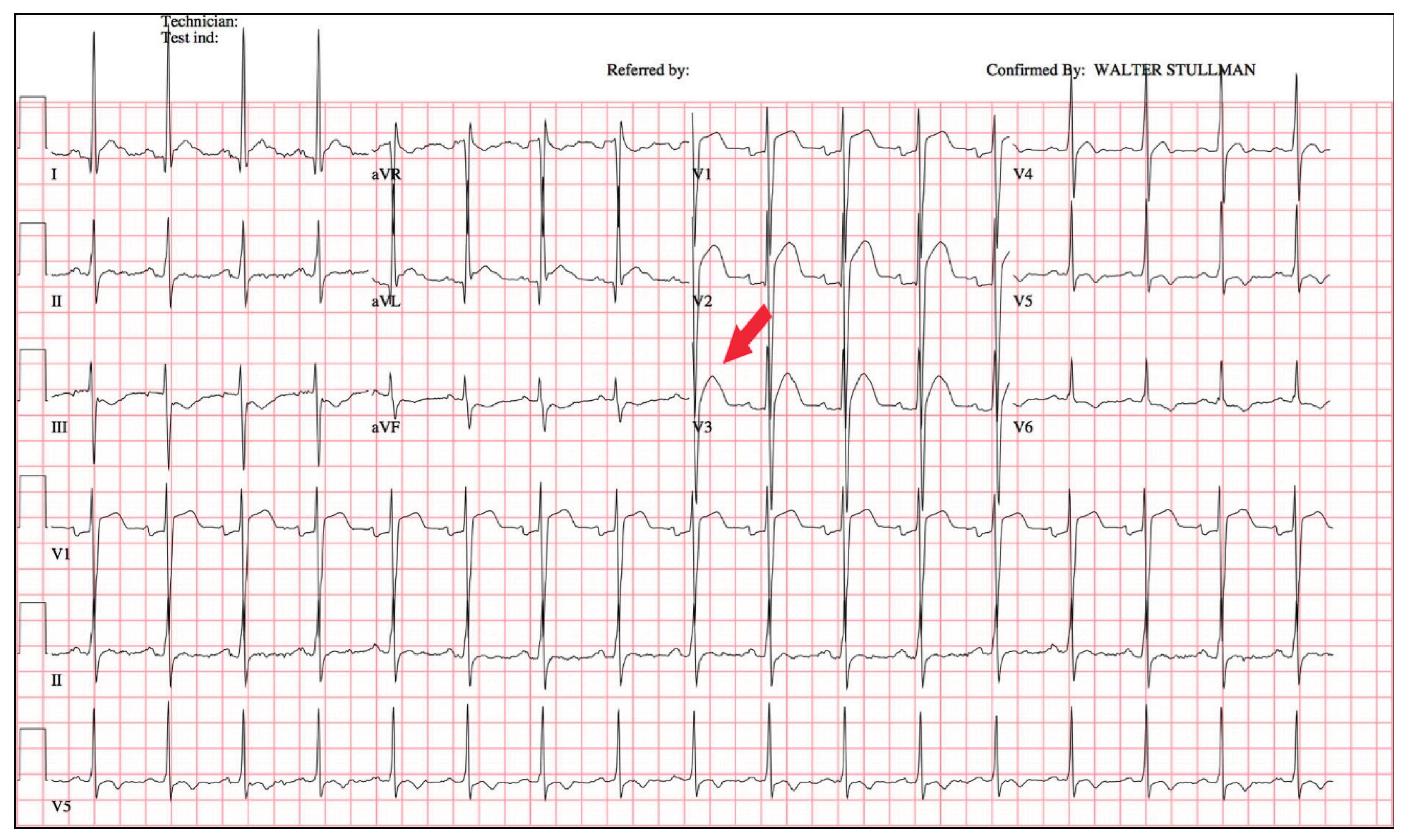
In many previously published articles on electrocardiogram (ECG) changes associated with hypercalcemia, although these changes have been described in various forms—such as patterns resembling myocardial infarction or Brugada syndrome—it is still evident that Zhai’s change can occur. However, this specific change was not formally recognized or described by previous researchers. For example, some reports have suggested that hypercalcemia may manifest with ST-segment elevation, mimicking either myocardial infarction , early repolarization or Brugada-Type, our findings indicate that the disappearance of the ST segment and a leftward shift of T waves may better characterize the potential electrocardiographic features associated with hypercalcemia. (14, 18, 19) As seen in
Figure 5, the electrocardiogram changes in lead V3 depicted in this document are highly consistent with the features described herein, namely “ST segment disappearing and a leftward shift in the T-wave peak.”(15)
We found that the characteristic manifestations of hypercalcemia on the electrocardiogram was leftward shift of the T-wave peak which was accompanied by disappearance of the ST segment in leads V1–V3. Specifically, the peak of the T-wave is located closer to the ascending limb of the preceding T-wave (also called a leftward shift in the T-wave peak), and the angle formed by the ascending limb is greater than that formed by the descending limb, which differs from the morphology of a normal T-wave (see
Figure 2). Since the heart depolarizes and repolarizes in a sequential manner, with the ventricular septum depolarizing and repolarizing first, elevated calcium levels most prominently affect the T-wave morphology in the right precordial leads V1–V3. When serum calcium levels are excessively high, the most characteristic features include the disappearance of the ST segment in leads V1–V3, an earlier T-wave peak, an apex approaching the anterior limb, and a larger angle between the ascending limb compared to the descending limb of the T-wave. Among the 64 cases, significant leftward shifts in the T-wave peak were observed in leads V1–V3 in 50 cases, with the angle of the ascending limb of the T-wave being greater than or equal to that of the descending limb. 22 cases exhibited a leftward shift of the T-wave peak across all 12 leads. In some cases, widened QRS complexes as well as flattened or biphasic T-waves were observed; however, these findings were considered less specific. Some patients may present with concurrent electrolyte disturbances, such as hypokalemia.
On the electrocardiogram, shortening of the QT interval is primarily observed in cases with significantly elevated blood calcium levels. Common causes of QT interval shortening in clinical practice include genetic factors, electrolyte imbalances, and drug effects, among others, involving multiple pathophysiological mechanisms.(9, 20) Genetic factors also represent a primary cause of QT interval shortening. This mechanism primarily operates through enhanced potassium ion efflux, which reduces the duration of the myocardial action potential.(21-24) However, in mild to moderate hypercalcemia, the QT interval typically does not shorten, which may be attributed to the fact that the phase 3 component represents a calcium-dependent transient increase in potassium ion conductance. During repolarization, potassium efflux continues for a certain duration and follows its intrinsic pattern. This phenomenon does not result in a shortening of the ventricular refractory period, a point that requires careful differentiation in clinical practice. When hypercalcemia coexists, potassium ions influence the amplitude of the T wave. The increase in potassium current during phase 3 is both dependent on calcium and transient in nature; therefore, the T wave amplitude is often not markedly elevated. In an observation of 64 cases of hypercalcemia, none exhibited T wave amplitude exceeding half the height of the R wave.
However, we acknowledge several limitations in our study that warrant discussion. The retrospective design and single-center approach may introduce selection bias, limiting the generalizability of our findings to broader populations. Additionally, the lack of longitudinal follow-up restricts our ability to assess the long-term outcomes of patients with hypercalcemia based on ECG findings. Future research should aim to include a larger, more diverse sample across multiple centers and employ prospective designs that allow for comprehensive assessments of the relationship between ECG changes and clinical outcomes over time. Overall, while our study lays a strong foundation for future investigations, addressing these limitations will be crucial for validating the clinical utility of ECG in managing hypercalcemia effectively.
In summary, the specific electrocardiogram (ECG) features of hypercalcemia—such as a leftward shift in the peak of the T wave, the disappearance of the ST segment in leads V1–V3, along with a larger angle between the anterior branch of the T wave and the horizontal baseline compared to that of the posterior branch across all 12 leads, and a shortened QTm—can serve as specific indicators for the ECG diagnosis of hypercalcemia for clinical medical staff, with a specificity of 78.1%. When combined with laboratory tests, these ECG changes can facilitate the timely detection of elevated blood calcium levels in patients.
References
- Bartkiewicz, P.; Kunachowicz, D.; Filipski, M.; Stebel, A.; Ligoda, J.; Rembialkowska, N. Hypercalcemia in Cancer: Causes, Effects, and Treatment Strategies. Cells. 2024, 13. [Google Scholar] [CrossRef] [PubMed]
- Izzedine, H.; Attieh, R.M.; Begum, F.; Cheung, Y.M.; Jhaveri, K.D. Hypercalcemia of malignancy: Cancer treatment can be a cause as well. Clin Nephrol. 2024, 102, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.D.; Shane, E. Hypercalcemia: A Review. JAMA. 2022, 328, 1624–1636. [Google Scholar] [CrossRef] [PubMed]
- Pepe, J.; Magna, A.; Sonato, C.; Sgreccia, A.; Colangelo, L.; Occhiuto, M.; et al. Twenty-four hour Holter ECG in normocalcemic and hypercalcemic patients with hyperparathyroidism. J Endocrinol Invest. 2024, 47, 1499–1504. [Google Scholar] [CrossRef]
- Sadeghian, G.; Ziaei, H.; Sadeghi, M. Electrocardiographic changes in patients with cutaneous leishmaniasis treated with systemic glucantime. Ann Acad Med Singap. 2008, 37, 916–918. [Google Scholar] [CrossRef]
- Kazama, I. High-calcium exposure to frog heart: a simple model representing hypercalcemia-induced ECG abnormalities. J Vet Med Sci. 2017, 79, 71–75. [Google Scholar] [CrossRef]
- Chorin, E.; Rosso, R.; Viskin, S. Electrocardiographic Manifestations of Calcium Abnormalities. Ann Noninvasive Electrocardiol. 2016, 21, 7–9. [Google Scholar] [CrossRef]
- Tse, G.; Chan, Y.W.; Keung, W.; Yan, B.P. Electrophysiological mechanisms of long and short QT syndromes. Int J Cardiol Heart Vasc. 2017, 14, 8–13. [Google Scholar] [CrossRef]
- Malik, M. Drug-Induced QT/QTc Interval Shortening: Lessons from Drug-Induced QT/QTc Prolongation. Drug Saf. 2016, 39, 647–659. [Google Scholar] [CrossRef]
- Tonon, C.R.; Silva, T.; Pereira, F.W.L.; Queiroz, D.A.R.; Junior, E.L.F.; Martins, D.; et al. A Review of Current Clinical Concepts in the Pathophysiology, Etiology, Diagnosis, and Management of Hypercalcemia. Med Sci Monit. 2022, 28, e935821. [Google Scholar] [CrossRef]
- Carsote, M.; Nistor, C.; Gheorghe, A.M.; Sima, O.C.; Trandafir, A.I.; Nistor, T.V.I.; et al. Turning Points in Cross-Disciplinary Perspective of Primary Hyperparathyroidism and Pancreas Involvements: Hypercalcemia-Induced Pancreatitis, MEN1 Gene-Related Tumors, and Insulin Resistance. Int J Mol Sci. 2024, 25. [Google Scholar] [CrossRef]
- Fayaz, A.; Shah, A.H.; Khan, M.A.; Parveen, S. Multiple Myeloma Presenting as Hypercalcemia-Induced Acute Severe Pancreatitis: A Case Report. Gastro Hep Adv. 2025, 4, 100549. [Google Scholar] [CrossRef]
- Nahass, M.; Sharma, R.; Penn, J. Hypercalcemia-induced pancreatitis presenting with acute ST-elevations mimicking a myocardial infarction. Am J Emerg Med. 2016, 34, 1187 e1-2. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, K.; Watanabe, H.; Hisamatsu, T.; Ashihara, T.; Ohno, S.; Hayashi, H.; et al. High Frequency of Early Repolarization and Brugada-Type Electrocardiograms in Hypercalcemia. Ann Noninvasive Electrocardiol. 2016, 21, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Durant, E.; Singh, A. ST elevation due to hypercalcemia. Am J Emerg Med. 2017, 35, 1033 e3-e6. [Google Scholar] [CrossRef]
- Bahadur, K.A.; Johnson, S.; Lentzner, B.; Gangat, M.; Carlson, J.; Balachandar, S. Hypercalcemia, hyperkalemia and supraventricular tachycardia in a patient with subcutaneous fat necrosis. J Pediatr Endocrinol Metab. 2018, 31, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Cheungpasitporn, W.; Jirajariyavej, T.; Chanprasert, S. Rate control medications for atrial fibrillation in the setting of hypercalcemia. Am J Emerg Med. 2011, 29, 830. [Google Scholar] [CrossRef]
- Schutt, R.C.; Bibawy, J.; Elnemr, M.; Lehnert, A.L.; Putney, D.; Thomas, A.S.; et al. Case report: Severe hypercalcemia mimicking ST-segment elevation myocardial infarction. Methodist Debakey Cardiovasc J. 2014, 10, 193–197. [Google Scholar] [CrossRef]
- Schmidt-Lauber, C.; Anneken, L.; Schodel, J. Hypercalcemia mimicking myocardial infarction. Kidney Int. 2019, 96, 1428. [Google Scholar] [CrossRef]
- Nachimuthu, S.; Assar, M.D.; Schussler, J.M. Drug-induced QT interval prolongation: mechanisms and clinical management. Ther Adv Drug Saf. 2012, 3, 241–253. [Google Scholar] [CrossRef]
- Cormier, C. Genetic hypercalcemia. Joint Bone Spine. 2019, 86, 459–466. [Google Scholar] [CrossRef]
- Dauber, A.; Nguyen, T.T.; Sochett, E.; Cole, D.E.; Horst, R.; Abrams, S.A.; et al. Genetic defect in CYP24A1, the vitamin D 24-hydroxylase gene, in a patient with severe infantile hypercalcemia. J Clin Endocrinol Metab. 2012, 97, E268–E274. [Google Scholar] [CrossRef]
- De Paolis, E.; Scaglione, G.L.; De Bonis, M.; Minucci, A.; Capoluongo, E. CYP24A1 and SLC34A1 genetic defects associated with idiopathic infantile hypercalcemia: from genotype to phenotype. Clin Chem Lab Med. 2019, 57, 1650–1667. [Google Scholar] [CrossRef]
- Lenherr-Taube, N.; Young, E.J.; Furman, M.; Elia, Y.; Assor, E.; Chitayat, D.; et al. Mild Idiopathic Infantile Hypercalcemia-Part 1: Biochemical and Genetic Findings. J Clin Endocrinol Metab. 2021, 106, 2915–2937. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
 is greater than that between the back branch and the horizontal baseline
is greater than that between the back branch and the horizontal baseline  . The peak of the T wave appears earlier. The arrow
. The peak of the T wave appears earlier. The arrow  in the figure represents QTm, which is 160ms (the distance from the starting point of QRS to the vertex of the T wave). The shortening is ≤240ms. The peak of the T wave moves leftward and approaches the QRS wave, which is a typical pattern change of hypercalcemia.
in the figure represents QTm, which is 160ms (the distance from the starting point of QRS to the vertex of the T wave). The shortening is ≤240ms. The peak of the T wave moves leftward and approaches the QRS wave, which is a typical pattern change of hypercalcemia.
 is greater than that between the back branch and the horizontal baseline
is greater than that between the back branch and the horizontal baseline  . The peak of the T wave appears earlier. The arrow
. The peak of the T wave appears earlier. The arrow  in the figure represents QTm, which is 160ms (the distance from the starting point of QRS to the vertex of the T wave). The shortening is ≤240ms. The peak of the T wave moves leftward and approaches the QRS wave, which is a typical pattern change of hypercalcemia.
in the figure represents QTm, which is 160ms (the distance from the starting point of QRS to the vertex of the T wave). The shortening is ≤240ms. The peak of the T wave moves leftward and approaches the QRS wave, which is a typical pattern change of hypercalcemia.