Submitted:
28 July 2025
Posted:
29 July 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
3.1. Investigation of the Electronic Properties of Magnesium-Stabilized Cubic Zirconia
3.2. Investigation of Ionic Conductivity
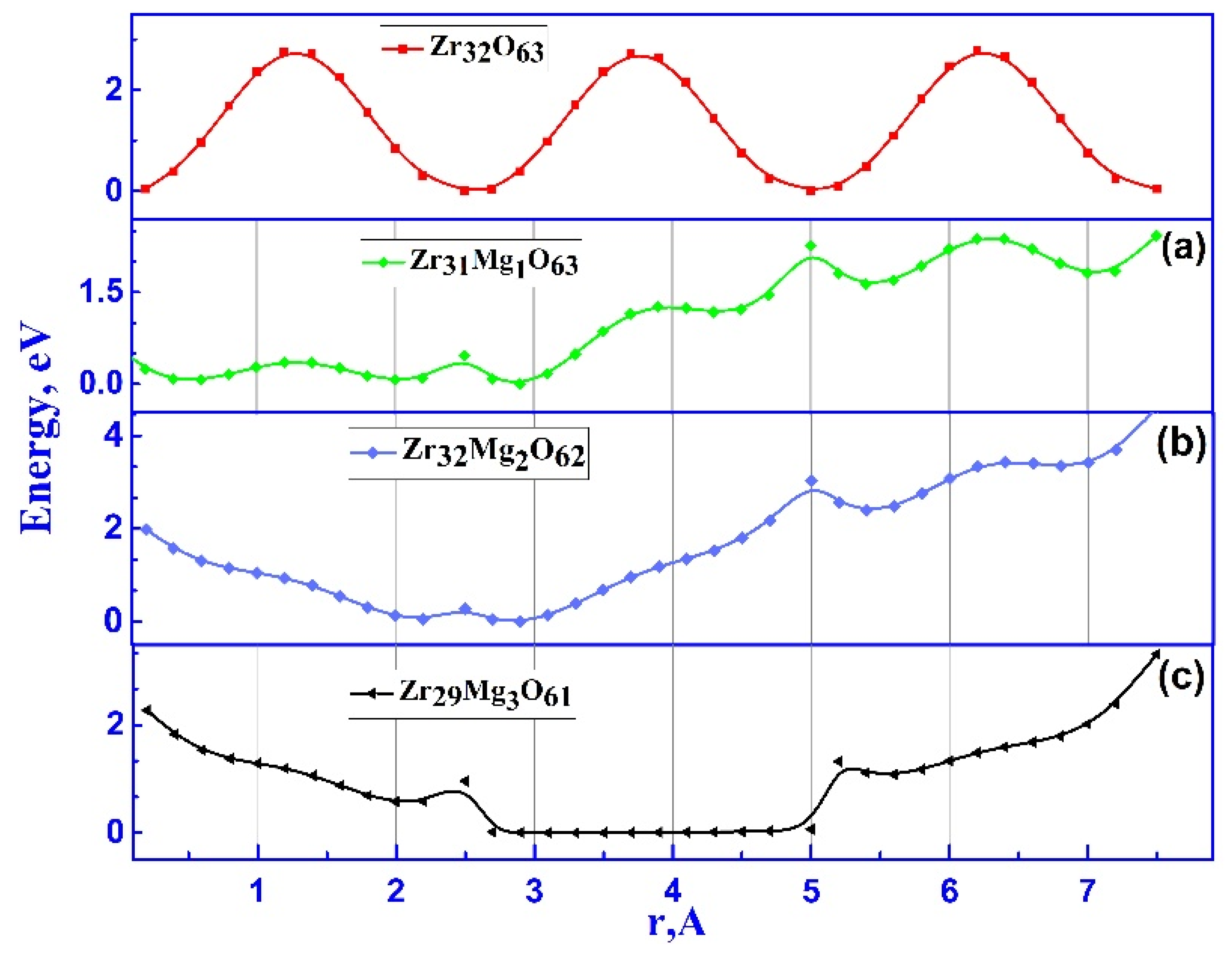
3.2.1. Ionic Conductivity via Oxygen Vacancy-Mediated Oxygen Ion Migration
3.2.2. Ionic Conductivity of ZrO₂ Stabilized with MgO Aligned Along the Migration Path
| Doping configuration | Number of Mg | Min. barrier, eV | Max. barrier, eV |
| Zr₃₁Mg₁O₆₃ | 1 | 0.4 | 2.4 |
| Zr₃₀Mg₂O₆₂ | 2 | 0.5 | 3.1 |
| Zr₂₉Mg₃O₆₁ | 3 | 0.8 | 1.32 |
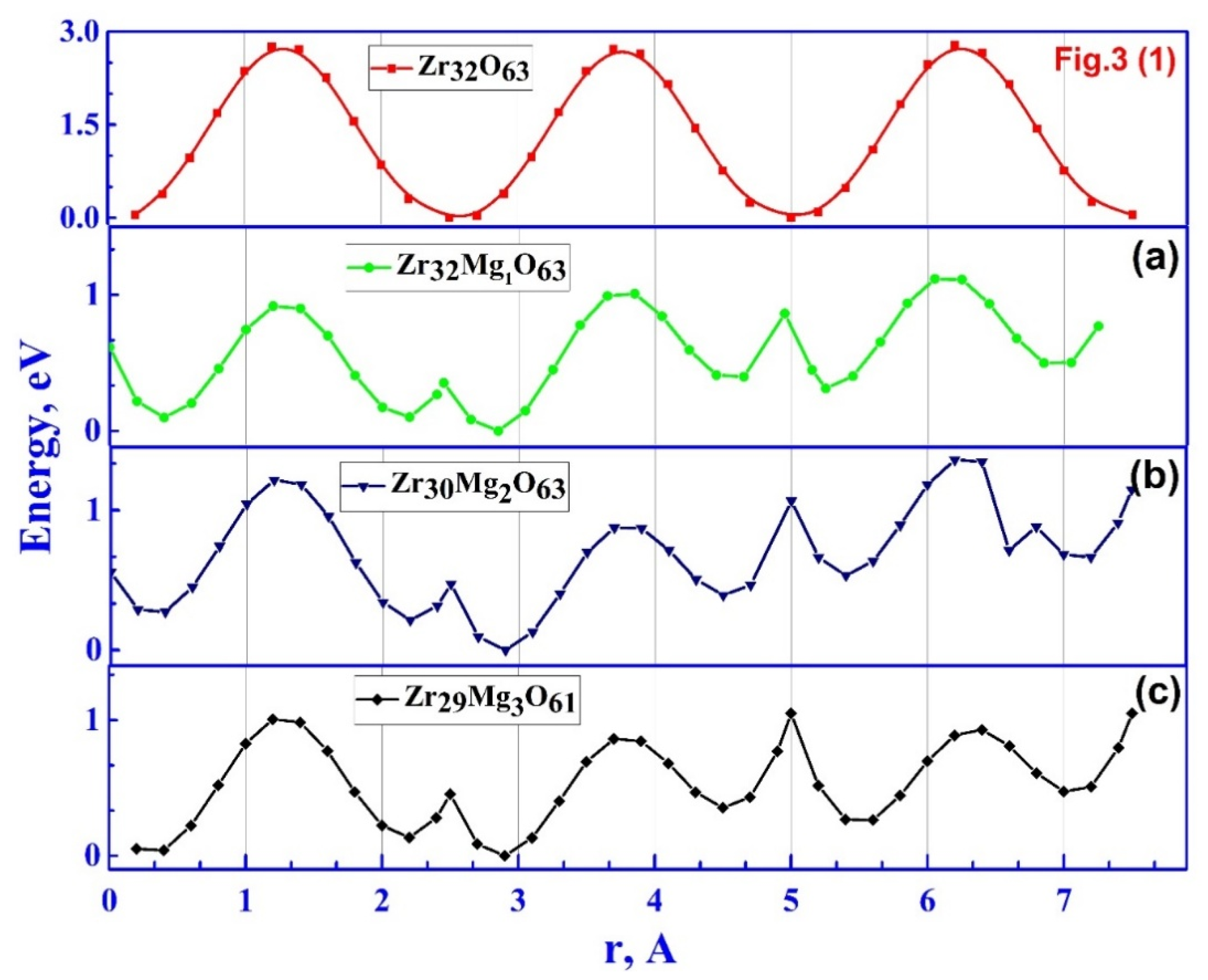
3.2.3. Ionic Conductivity of ZrO₂ Stabilized with MgO with Spatially Distributed Mg Ions
| Doping configuration | Number of Mg | Min. barrier, eV | Max. barrier, eV |
| Zr₃₁Mg₁O₆₃ | 1 | 0.4 | 1.12 |
| Zr₃₀Mg₂O₆₂ | 2 | 0.5 | 1.34 |
| Zr₂₉Mg₃O₆₁ | 3 | 0.5 | 1.05 |
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| DFT | Density functional theory |
| DOS | A function that describes the number of electronic states at each energy level that are available to be occupied in a system. |
| PDOS | A decomposition of the total DOS into contributions from specific atoms or orbitals helps to understand which atoms or orbitals contribute to particular energy levels. |
| B3LYP | A hybrid Density Functional Theory (DFT) functional that combines Becke’s three-parameter exchange functional with the Lee-Yang-Parr correlation functional. |
References
- Danilov, V. P.; Borisova, E. S.; Shukshin, V. E.; Runina, K. I.; Strekalov, P. V.; Mayakova, M. N.; & Petrova, O. B. (2025). Structure and optical properties of ZrO₂–Sc₂O₃ solid solution system obtained by the coprecipitation method. Glass and Ceramics, 81(9–10), 428–437. [CrossRef]
- Sathya, A.; Manikandan, K.; Praveen Kumar, P.; Kavitha, B.; & Boobalan, T. (2025). Hydrothermal synthesis of ZrO₂ nanoparticles: Study on structural, optical, morphology properties, and photocatalyst activity. Physics of the Solid State, 67(3), 196–206. [CrossRef]
- Fu, S.; Zhang, J.; Chen, X.; Wang, Z. & Zhao, Y. (2024). Oxygen defect-rich CeO₂₋ₓ@ZrO₂ Mott–Schottky electrocatalyst with tunable interfacial charge redistribution for accelerated sulfur redox kinetics in lithium–sulfur batteries. SSRN Electronic Journal. https://ssrn.com/abstract=5113016.
- Jin, H.-B.; Zhang, Z.-H.; Ma, P. & Li, H.-B. (2025). Synergically enhancing lithium-ion storage performance of silicon anode by designing shelled structure with reduced graphene oxide and ZrO₂. Rare Metals. [CrossRef]
- Jammee, R.; Wang, D.; Liu, X.; Xiao, D.; Xu, W.; & Yang, W. (2024). C–H bond activation by sulfated zirconium oxide is mediated by a sulfur-centered Lewis superacid. Angewandte Chemie International Edition, 63(22). [CrossRef]
- Florez, J.; Martinez-Monsalve, S.; Roldan, A.; Gutierrez, Y. & Martinez, F. (2025). Study of methylene blue removal and photocatalytic degradation on zirconia thin films modified with Mn-Anderson polyoxometalates. Dalton Transactions, 54(8), 2471–2482. [CrossRef]
- Han, W.; Tang, L.; Yang, C.; Li, Y. & Yu, M. (2025). Production of branched alkanes by upcycling of waste polyethylene over controlled acid sites of SO₄/ZrO₂–Al₂O₃ catalyst. Angewandte Chemie International Edition, 64, e202417923. [CrossRef]
- Huang, Q.-A.; Wei, Y. & Tang, H. (2024). Active and stable Au/ZrO₂ catalysts for isomerization of allylic esters. SSRN Electronic Journal. https://ssrn.com/abstract=5110250.
- Yang, Z.; Liu, Y.; Zhao, M.; Xie, Y. & Li, J. (2025). Synergistic adsorption-catalysis regulation effect for CO₂ over hierarchically structured Cu/ZrO₂ nanotubes. SSRN Electronic Journal. https://ssrn.com/abstract=5178857.
- Luo, J.; Chen, Y.; Tang, Y.; Yu, W. & Zhou, W. (2024). Facile oxygen functionalization strategy for mediating the activity–stability trade-off for metal encapsulated catalysts. SSRN Electronic Journal. https://ssrn.com/abstract=5124782.
- Li, M.; Hu, X.; Zhang, Y.; Yan, J. & Chen, W. (2025). Effect of metal-support interaction on catalytic performance of Pd/ZrOx in CO₂ hydrogenation to formate. Chemical Research in Chinese Universities. [CrossRef]
- Lee, S. H. & Kim, D.-H. (2025). Symmetry engineering in antiferroelectric ZrO₂ thin films via split-up behavior. ACS Applied Electronic Materials, 7(5), 2146–2152. [CrossRef]
- Wang, J.; Chen, H. & Liu, K. (2024). The influence of Ce element doping on the mechanical properties of ZrO₂ ceramic from first-principles calculations. SSRN Electronic Journal. https://ssrn.com/abstract=5171102.
- Yuan, J.; Li, F.; Zhao, R. & Tan, Z. (2025). First-principles study on thermodynamic stability and electronic structures of the ferroelectric binary HfO₂ and ZrO₂ (001) polar surfaces. Surfaces and Interfaces, 42, 105523. [CrossRef]
- Han, W.; Tang, L.; Yang, C.; Li, Y. & Yu, M. (2025). Production of branched alkanes from waste polyethylene using SO₄/ZrO₂–Al₂O₃ catalysts. Angewandte Chemie. [CrossRef]
- Huang, Q.-A.; Wei, Y. & Tang, H. (2024). Catalytic performance of Au/ZrO₂ in organic transformations. SSRN Electronic Journal. https://ssrn.com/abstract=5110250.
- Kadyrzhanov, K.K.; Kozlovskiy, A.A.; Konuhova, M.; Popov, A.I.; Shlimas, D.D.; Borgekov, D.B. Determination of gamma radiation shielding efficiency by radiation-resistant composite ZrO2-Al2O3-TiO2-WO3-Nb2O5 ceramics. Opt. Mater. 2024, 154, 115752. [Google Scholar] [CrossRef]
- Kozlovskiy, A.L.; Konuhova, M.; Shlimas, D.I.; Borgekov, D.B.; Zdorovets, M.V.; Shakirziyanov, R.I.; Popov, A.I. Study of the Effect of Nanostructured Grains on the Radiation Resistance of Zirconium Dioxide Ceramics During Gas Swelling under High-dose Irradiation with Helium Ions. ES Mater. Manuf. 2024, 24, 1165. [Google Scholar] [CrossRef]
- Kenzhina, I.E.; Kozlovskiy, A.L.; Begentayev, M.; Blynskiy, P.; Tolenova, A.; Popov, A.I. Study of Phase Transformations in ZrO2 Ceramics Stabilized by Y2O3 and Their Role in Changing Strength Characteristics and Heat Resistance. Sustainability 2025, 17, 4284. [Google Scholar] [CrossRef]
- Kozlovskiy, A.L.; Konuhova, M.; Borgekov, D.B. Study of irradiation temperature effect on radiation-induced polymorphic transformation mechanisms in ZrO2 ceramics. Opt. Mater. 2024, 156, 115994. [Google Scholar] [CrossRef]
- Imanova, G. (2024). Modeling defect formation in nano-ZrO2 under He and H+ Irradiation. Modern Physics Letters B, 38(22), 2450206.
- Costantini, J. M., Gutierrez, G., Lelong, G., Guillaumet, M., Rahman, M. M., & Yasuda, K. (2022). Raman spectroscopy study of damage in swift heavy ion-irradiated ceramics. Journal of Raman Spectroscopy, 53(9), 1614-1624.
- Liu, Y.; Zhu, Y.; Shen, T.; Chai, J.; Niu, L.; Li, S.; Jin, P.; Zheng, H.; Wang, Z. Irradiation response of Al2O3-ZrO2 ceramic composite under He ion irradiation. J. Eur. Ceram. Soc. 2021, 41, 2883–2891. [Google Scholar] [CrossRef]
- Dauletbekova, A.; Zvonarev, S.; Nikiforov, S.; Akilbekov, A.; Shtang, T.; Karavannova, N.; Akylbekova, A.; Ishchenko, A.; Akhmetova-Abdik, G.; Baymukhanov, Z.; et al. Luminescence Properties of ZrO2: Ti Ceramics Irradiated with Electrons and High-Energy Xe Ions. Materials 2024, 17, 1307. [Google Scholar] [CrossRef]
- Bandarenka, H.; Burko, A.; Laputsko, D.; Dronina, L.; Kovalchuk, N.; Podelinska, A.; Shapel, U.; Popov, A.I.; Bocharov, D. Ultraviolet Exposure Improves SERS Activity of Graphene-Coated Ag/ZrO2 Substrates. Crystals 2023, 13, 1570. [Google Scholar] [CrossRef]
- Nikiforov, S. V. , Kortov, V. S., Kiryakov, A. N., Konev, S. F., & Men’shenina, A. A. (2017). Increasing the luminescence yield of zirconia. Technical Physics Letters 2017, 43(12), 1074–1076. [Google Scholar]
- Swami, S.K.; Khan, J.I.; Dutta, V.; Lee, J.; Laquai, F.; Chaturvedi, N. Spray-Deposited Aluminum-Doped Zinc Oxide as an Efficient Electron Transport Layer for Inverted Organic Solar Cells // ACS Appl. Energy Mater. 2023. V. 6. P. 2906–2913.
- Kate, R.S.; Deokate, R.J. Effect of cobalt doping on electrochemical properties of sprayed nickel oxide thin films // Mater. Sci. Energy Technol. 2020. V. 3. P. 830–839.
- Ricca, C.; Ringuedé, A.; Cassir, M.; Adamo, C.; Labat, F. A comprehensive DFT investigation of bulk and low-index surfaces of ZrO₂ polymorphs // J. Comput. Chem. 2015. V. 36(1). P. 9–21. [CrossRef]
- Shin, H.; et al. Zirconia and hafnia polymorphs: ground-state structural properties from diffusion Monte Carlo // Phys. Rev. Mater. 2018. V. 2(7). Article 075001. [CrossRef]
- Rajesh, G.; Akilandeswari, S.; Govindarajan, D.; Thirumalai, K. Enhancement of photocatalytic activity of ZrO₂ nanoparticles by doping with Mg for UV light photocatalytic degradation of methyl violet and methyl blue dyes // J. Mater. Sci. Mater. Electron. 2020. V. 31(5). P. 4058–4072. [CrossRef]
- Marfin, A.Y.; Nikiforov, S.V.; Ananchenko, D.V.; Zyryanov, S.S.; Yakovlev, G.A.; Denisov, E.I. Thermoluminescence of monoclinic ZrO2 after electron irradiation. AIP Conf. Proc. 2022, 2466, 030012. [Google Scholar]
- Sredojević, D.; Lazić, V.; Pirković, A.; Periša, J.; Murafa, N.; Spremo-Potparević, B.; Živković, L.; Topalović, D.; Zarubica, A.; Jovanović Krivokuća, M.; et al. Toxicity of Silver Nanoparticles Supported by Surface-Modified Zirconium Dioxide with Dihydroquercetin. Nanomaterials 2022, 12, 3195. [Google Scholar] [CrossRef] [PubMed]
- Osinkin, D.A.; Antonova, E.P.; Lesnichyova, A.S.; Tropin, E.S.; Chernov, M.E.; Chernov, E.I.; Farlenkov, A.S.; Khodimchuk, A.V.; Eremin, V.A.; Kovrova, A.I.; et al. Application of Promising Electrode Materials in Contact with a Thin-Layer ZrO2-Based Supporting Electrolyte for Solid Oxide Fuel Cells. Energies 2020, 13, 1190. [Google Scholar] [CrossRef]
- Qi, S.; Porotnikova, N.M.; Ananyev, M.V.; Kuzmin, A.V.; Eremin, V.A.; Pankratov, A.A.; Molchanova, N.G.; Reznitskikh, O.G.; Farlenkov, A.S.; Vovkotrub, E.G.; et al. High-temperature glassy-ceramic sealants SiO2–Al2O3–BaO–MgO and SiO2–Al2O3–ZrO2–CaO–Na2O for solid oxide electrochemical devices. Trans. Nonferr. Met. Soc. China 2016, 26, 2916–2924. [Google Scholar] [CrossRef]
- Farlenkov, A.S.; Ananyev, M.V.; Eremin, V.A.; Porotnikova, N.M.; Kurumchin, E.K. Particle coarsening influence on oxygen reduction in LSM–YSZ composite materials. Fuel Cells 2015, 15, 131–139. [Google Scholar] [CrossRef]
- Nikiforov, S.; Dauletbekova, A.; Gerasimov, M.; Kasatkina, Y.; Denisova, O.; Lisitsyn, V.; Golkovski, M.; Akylbekova, A.; Bazarbek, A.-D.; Akilbekov, A.; et al. Thermoluminescent and Dosimetric Properties of Zirconium Dioxide Ceramics Irradiated with High Doses of Pulsed Electron Beam. Crystals 2023, 13, 1585. [Google Scholar] [CrossRef]
- Ananchenko, D.V.; Nikiforov, S.V.; Sobyanin, K.V.; Konev, S.F.; Dauletbekova, A.K.; Akhmetova-Abdik, G.; Akilbekov, A.T.; Popov, A.I. Paramagnetic Defects and Thermoluminescence in Irradiated Nanostructured Monoclinic Zirconium Dioxide. Materials 2022, 15, 8624. [Google Scholar] [CrossRef]
- Borik, M.A.; Bredikhin, S.I.; Bublik, V.T.; Kulebyakin, A.V.; Kuritsyna, I.E.; Lomonova, E.E.; Milovich, P.O.; Myzina, V.A.; Osiko, V.V.; Ryabochkina, P.A.; et al. Structure and Conductivity of Yttria and Scandia-Doped Zirconia Crystals Grown by Skull Melting. J. Am. Ceram. Soc. 2017, 100, 5536–5547. [Google Scholar] [CrossRef]
- Gayathri, P. , Balasubramani, V., Balraju, P., Sayed, M. A., & Shkir, M. (2025). Ultra-high photosensitivity response in MIS SBDs enabled by Zn-integrated ZrO2@ Zn interfacial layers for photovoltaic devices. Physica B: Condensed Matter.
- Aboraia, A. M. , Sharaf, I. M., Alradaddi, S., Trabelsi, A. B. G., & Alkallas, F. H. (2025). Advanced supercapacitors benefit from the electrode material Gd-Enhanced Cubic-ZrO2. Physica B: Condensed Matter.
- Cheng, Z.; Ren, H.; Wang, Y.; Ta, S.; Zhang, P.; Yang, Y.; Xu, S.; Goodman, B.A.; Deng, W. Cryst. Growth Des. 2022, 22, 5481. [CrossRef]
- Thammachart, Matina, et al. “Catalytic activity of CeO2–ZrO2 mixed oxide catalysts prepared via sol–gel technique: CO oxidation.” Catalysis Today 68.1-3 (2001): 53-61. [CrossRef]
- Li, Ping, I-Wei Chen, and James E. Penner-Hahn. “Effect of dopants on zirconia stabilization—an x-ray absorption study: II, tetravalent dopants.” Journal of the American Ceramic Society 77.5 (1994): 1281-1288. [CrossRef]
- Winczewski, J. P.; et al. “Additive manufacturing of 3D yttria-stabilized zirconia microarchitectures.” Materials and Design 238 (2024): 112701. [CrossRef]
- Feng, Y.; Wu, J.; Chi, Q.; Li, W.; Yu, Y.; Fei, W. Chem. Rev. 2020, 120, 1710. [CrossRef] [PubMed]
- Haering, C.; Roosen, A.; Schichl, H.; Schnöller, M. Solid State Ionics 2005, 176, 261. [CrossRef]
- Zeeshan, T.; Qureshi, M. T.; Kayani, Z. N.; Arshad, A.; Ullah, F.; Hameed, R. A.; Ragab, H.; Alam, N.; Rehman, W.; Saleem, M. Solid State Commun. 2022, 358, 115006. [CrossRef]
- King, A.; Singh, R.; Anand, R.; Behera, S.K.; Nayak, B.B. Optik (Stuttg). 2021, 242.
- Xie, Y.; Ma, Z.; Liu, L.; Su, Y.; Zhao, H.; Liu, Y., Zhang, Z.; Duan, H.; Li, J.; Xie, E. Appl. Phys. Lett. 2010, 97, 141916.
- Furasova, A.D.; Ivanovski, V.; Yakovlev, A.V.; Milichko, V.A.; Vinogradov, V.V.; Vinogradov, A.V. Nanoscale 2017, 9, 13069.
- Winczewski, J.; Herrera, M.; Cabriel, C.; Izeddin, I.; Gabel, S.; Merle, B.; Susarrey-Arce, A.; Gardeniers, H. ; Adv. Opt. Mater. 2022, 10, 2102758. [Google Scholar] [CrossRef]
- Winczewski, J.; Herrera, M.; Gardeniers, H.; Susarrey-Arce, A. Chem. Commun. 2023, 59, 3095. [CrossRef] [PubMed]
- Kurakhmedov, A.E.; Morzabayev, A.K.; Tleubay, I.; Berguzinov, A.; Kozlovskiy, A.L. Study of the Mechanisms of Polymorphic Transformations in Zirconium Dioxide upon Doping with Magnesium Oxide, as Well as Establishing the Relationship between Structural Changes and Strength Properties // Ceramics. 2023. V. 6(2). P. 1164–1178.
- Malyi, O.I.; Wu, P.; Kulish, V.V.; Bai, K.; Chen, Z. Formation and migration of oxygen and zirconium vacancies in cubic zirconia and zirconium oxysulfide //Solid State Ionics. 2012. Vol. 212. P. 117–122. [CrossRef]
- Kurakhmedov, A.E.; Alin, M.; Temir, A.M.; Ivanov, I.A.; Bikhert, Y.V.; Ungarbayev, Y.O.; ... & Kozlovskiy, A. L. (2021). Study of the Effect of Doping ZrO2 Ceramics with MgO to Increase the Resistance to Polymorphic Transformations under the Action of Irradiation. Nanomaterials, 11(12), 3172.
- Perevalov, T.V.; Islamov, D.R. “Oxygen Polyvacancies as Conductive Filament in Zirconia: First Principle Simulation” ECS Transactions, 2017, 80 (1), 357-362.
- Xue, Q.; Huang, X.; Wang, L.; Zhang, H. & Zhang, J. (2018). Computational and experimental investigations of defect interaction and ionic conductivity in doped zirconia. Physical Review Applied, 10(1), 014032.
- Dhingra, A.; Thakur, O. P. & Pandey, R. (2025). Structure–Property Relationship and Electronic Structure Calculation of Cubic YSZ Solid Electrolyte for Electrochemical Applications. Journal of Electronic Materials, 1-12.
- Erba, A.; Desmarais, J.K.; Casassa, S.; Civalleri, B.; Dona, L.; Bush, I.J.; Searle, B.; Maschio, L.; Edith-DaZr, L.; Cossard, A.; Ribaldone, C.; Ascrizzi, E.; Marana, N.L.; Flament J.-P. and Kirtman, B. J. Chem. Theory Comput., DOI: 10.1021/acs.jctc.2c00958 (2022) CRYSTAL23: A Program for Computational Solid-State Physics and Chemistry.
- Muhammad, I.D.; Awang, M. “Modelling the Interatomic Potential of Cubic Zirconia”, Journal of Advanced Research in Material Science and Mechanical Engineering, vol. 446-447, pp 151-157, Nov. 2014. [Google Scholar] [CrossRef]
- French, R.H.; Glass, S.J.; Ohuchi, F. S.; Xu, Y.-N.; Ching, W.Y. “Experimental and theoretical determination of the electronic structure and optical properties of three phases of ZrO2”, Journal of Physical Review, vol.49, pp 5133–5142, Mar. 1994. [Google Scholar] [CrossRef]
- Malyi, O.I.; et al. (2012). Formation energy and electronic structure of oxygen vacancies in ZrO₂. Phys. Rev. B, 86, 205112.
- Hinuma, Y.; Graciani, J.; Stacchiola, D.; et al. (2018). Density functional theory calculations of oxygen vacancy formation and subsequent molecular adsorption on zirconia surfaces. J. Phys. Chem. C, 122, 1687–1696.
- Han, D.; Zhao, J.; Chen, Z.; et al. (2025). Advancing the understanding of oxygen vacancies in ceria: insights into their formation, behavior, and catalytic role. Chem Catal.
- Emery, Antoine A. and Chris Wolverton. “High-throughput DFT calculations of formation energy, stability, and oxygen vacancy formation energy of ABO3 perovskites.” Scientific data 4.1 (2017): 1-10.
- Kaptagay, G.A.; Satanova, B.M.; Abuova, A.U.; Konuhova, M.; Zakiyeva, Zh.Ye.; Tolegen, U.Zh.; Koilyk, N.O.; Abuova, F.U. Effect of rhodium doping for photocatalytic activity of barium titanate // Optical Materials: X. 2025. — Vol. 25. — P.100382. [CrossRef]
- Inerbaev, T.M.; Abuova, A.U.; Zakiyeva, Z.Y.; Abuova, F.U.; Mastrikov, Y.A.; Sokolov, M.; Gryaznov, D.; Kotomin, E.A. Effect of Rh Doping on Optical Absorption and Oxygen Evolution Reaction Activity on BaTiO₃ (001) Surfaces // Molecules. — 2024. — Vol. 29. — Art. 11. [CrossRef]
- Abuova, A.U.; Mastrikov, Y.A.; Kotomin, E.A.; Kawazoe, Y.; Inerbaev, T.M.; Akilbekov, A.T. First principles modeling of Ag adsorption on the LaMnO₃ (001) surfaces // Solid State Ionics. — 2015. — Vol. 273. — P. 46–50. [CrossRef]
- Abuova, F.U.; Kotomin, E.A.; Lisitsyn, V.M.; Akilbekov, A.T.; Piskunov, S. Ab initio modeling of radiation damage in MgF₂ crystals // Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms. — 2014. — Vol. 326. — P. 314–317. [CrossRef]
- Dauletbekova, A.; Abuova, F.; Piskunov, S. First-principles modeling of the H color centers in MgF₂ crystals // Physica Status Solidi C: Current Topics in Solid State Physics. — 2013. — Vol. 10, No. 2. — P. 160–164. [CrossRef]
- Zhukovskii, Y. F. , Platonenko, A., Piskunov, S., & Kotomin, E. A. (2016). Ab initio simulations on migration paths of interstitial oxygen in corundum. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms, 374, 29–34.
- Kaewmeechai, C., Strand, J., & Shluger, A. L. (2025). Structure and Migration Mechanisms of Oxygen Interstitial Defects in β-Ga2O3. physica status solidi (b), 2400652.
- Usseinov, A.B.; Akilbekov, A.T.; Kotomin, E.A.; Karipbayev, Z.T. The first principles calculations of CO2 adsorption on (1010) ZnO surface. AIP Conf. Proc. 2019, 2174, 020181. [Google Scholar]
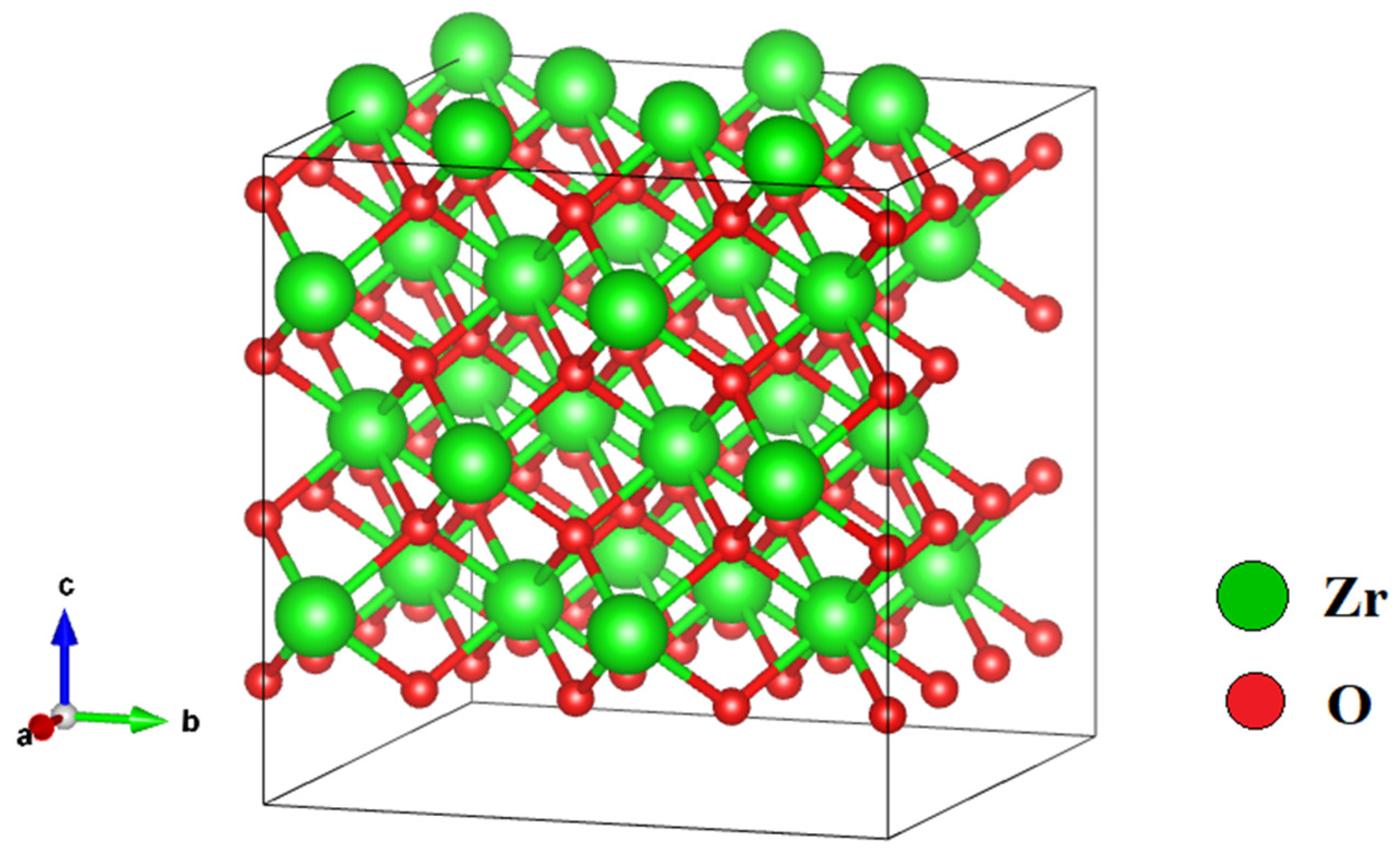
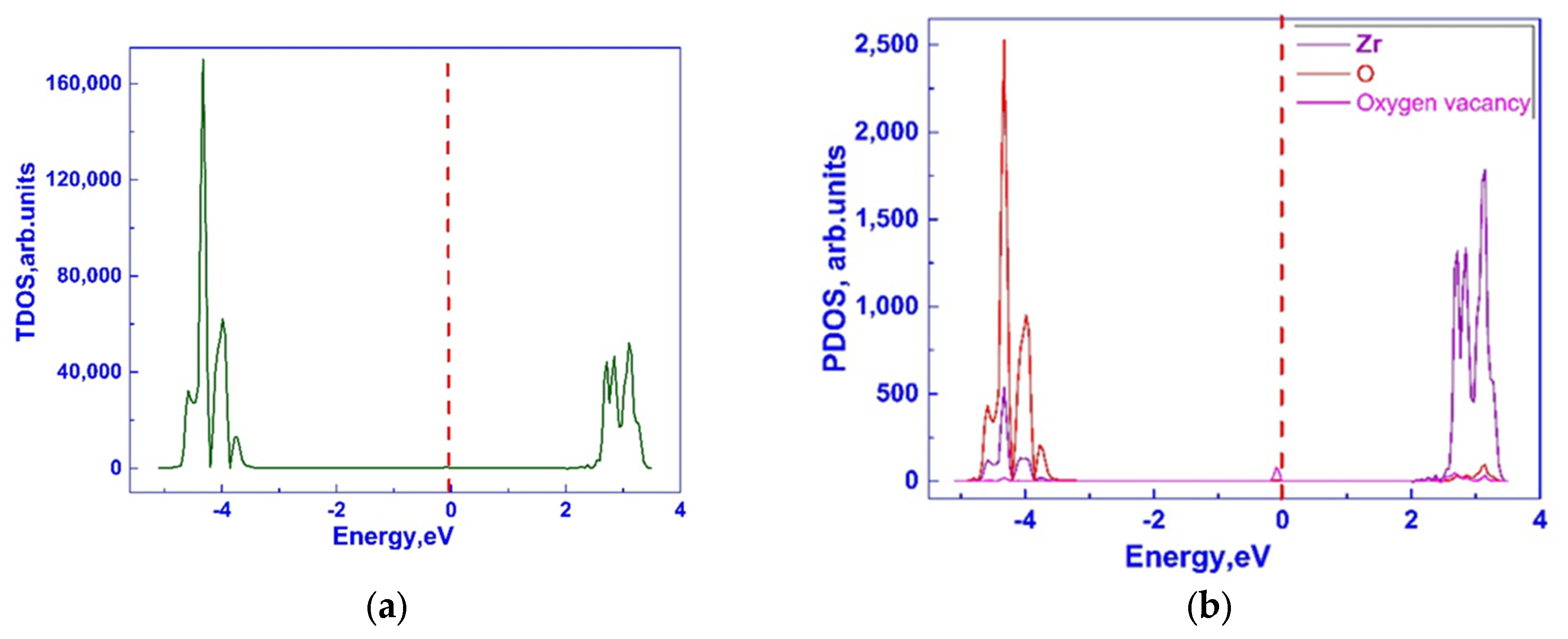
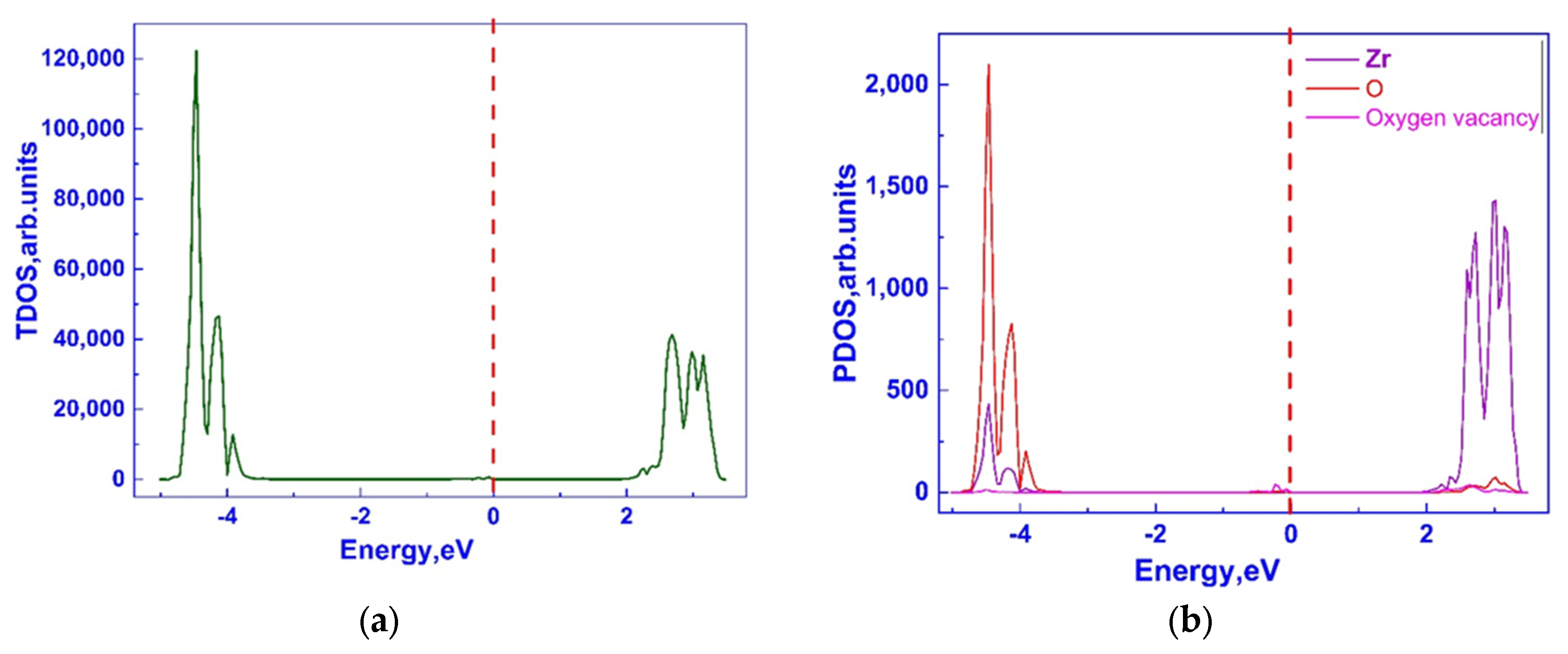
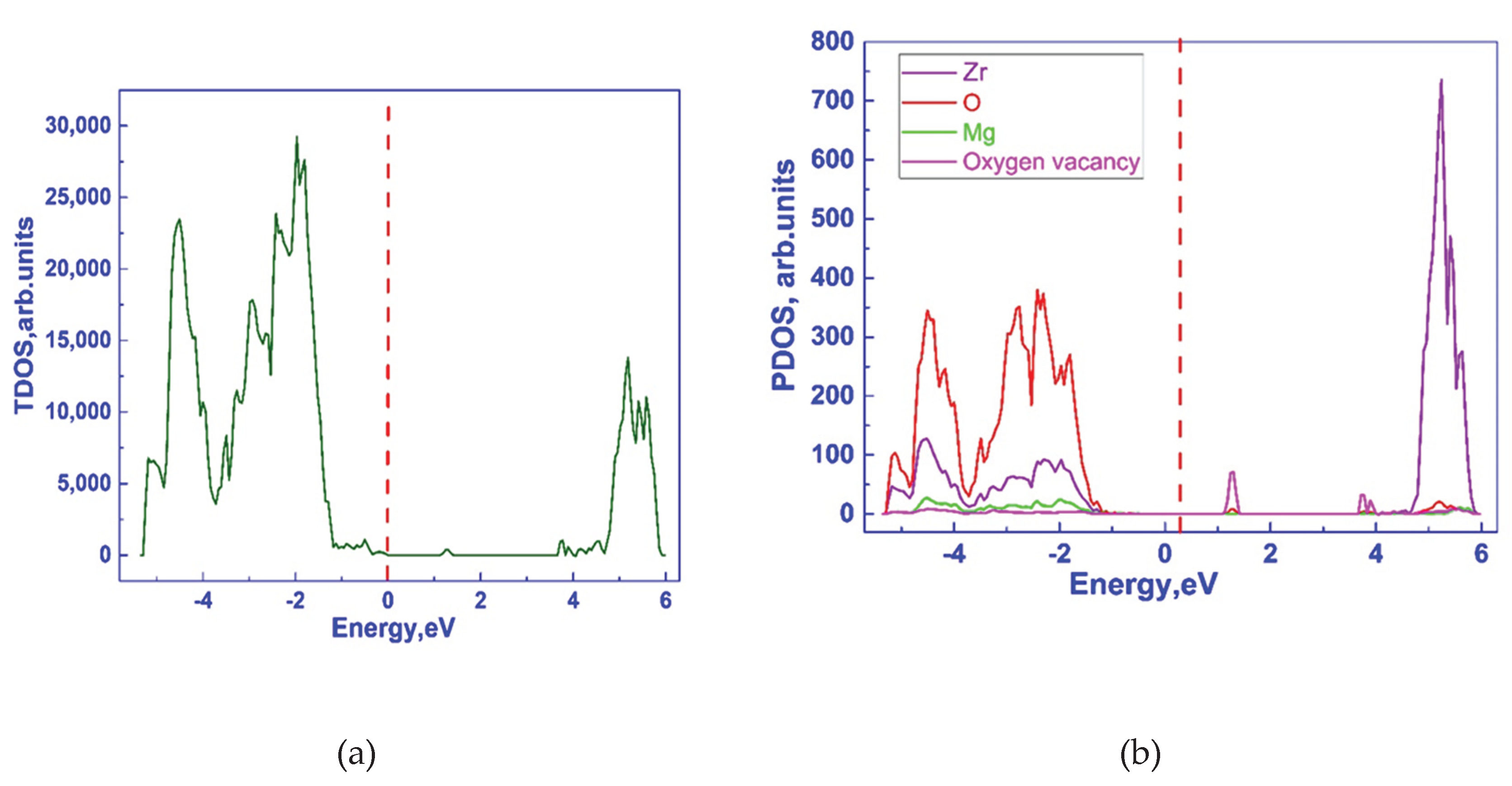
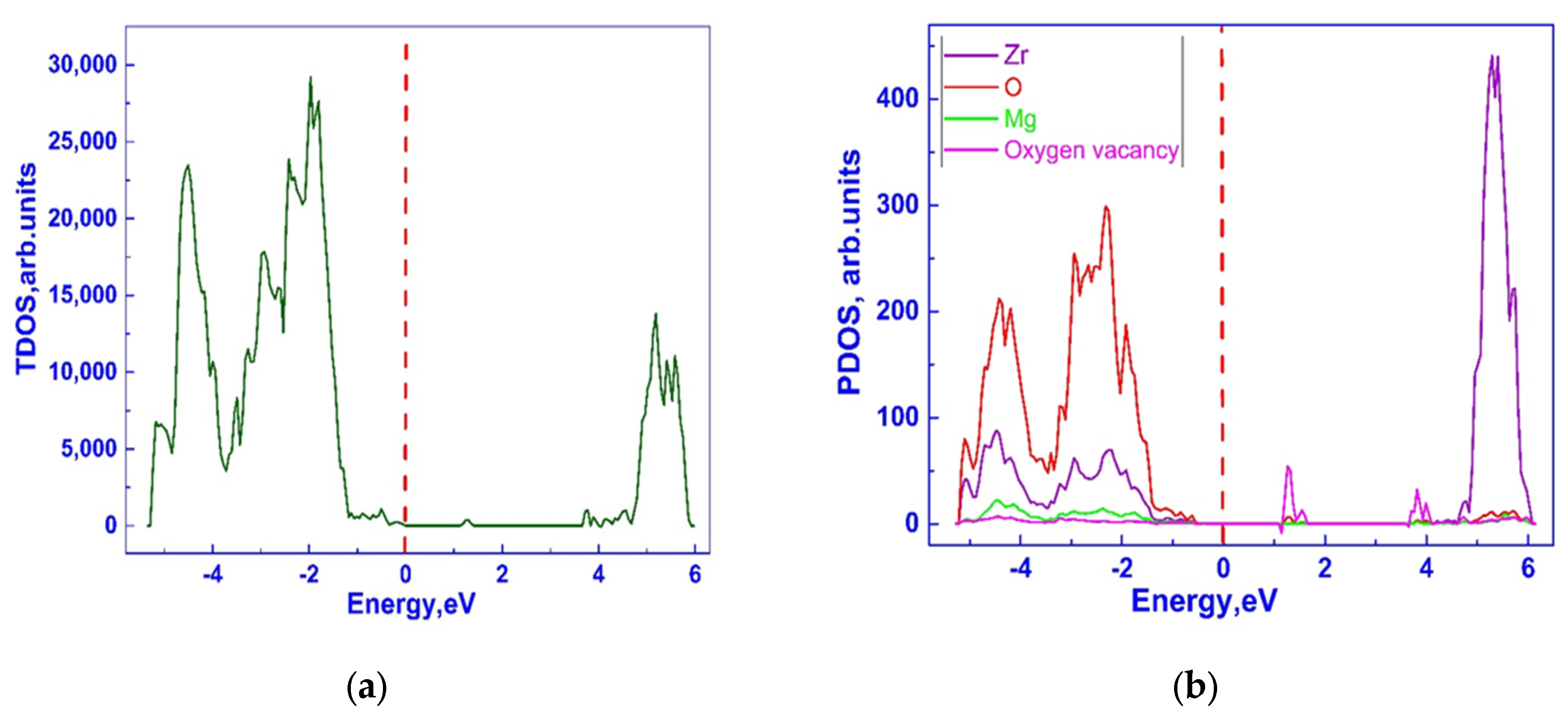
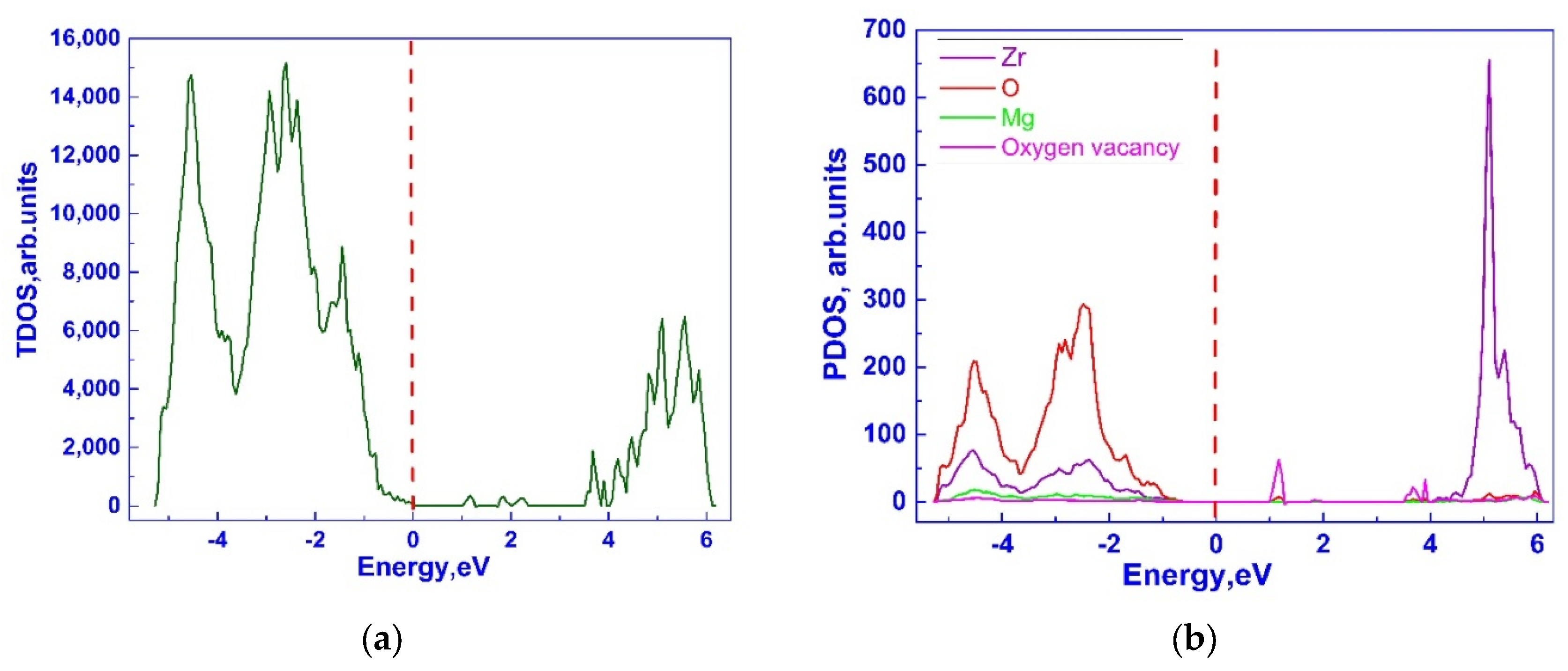
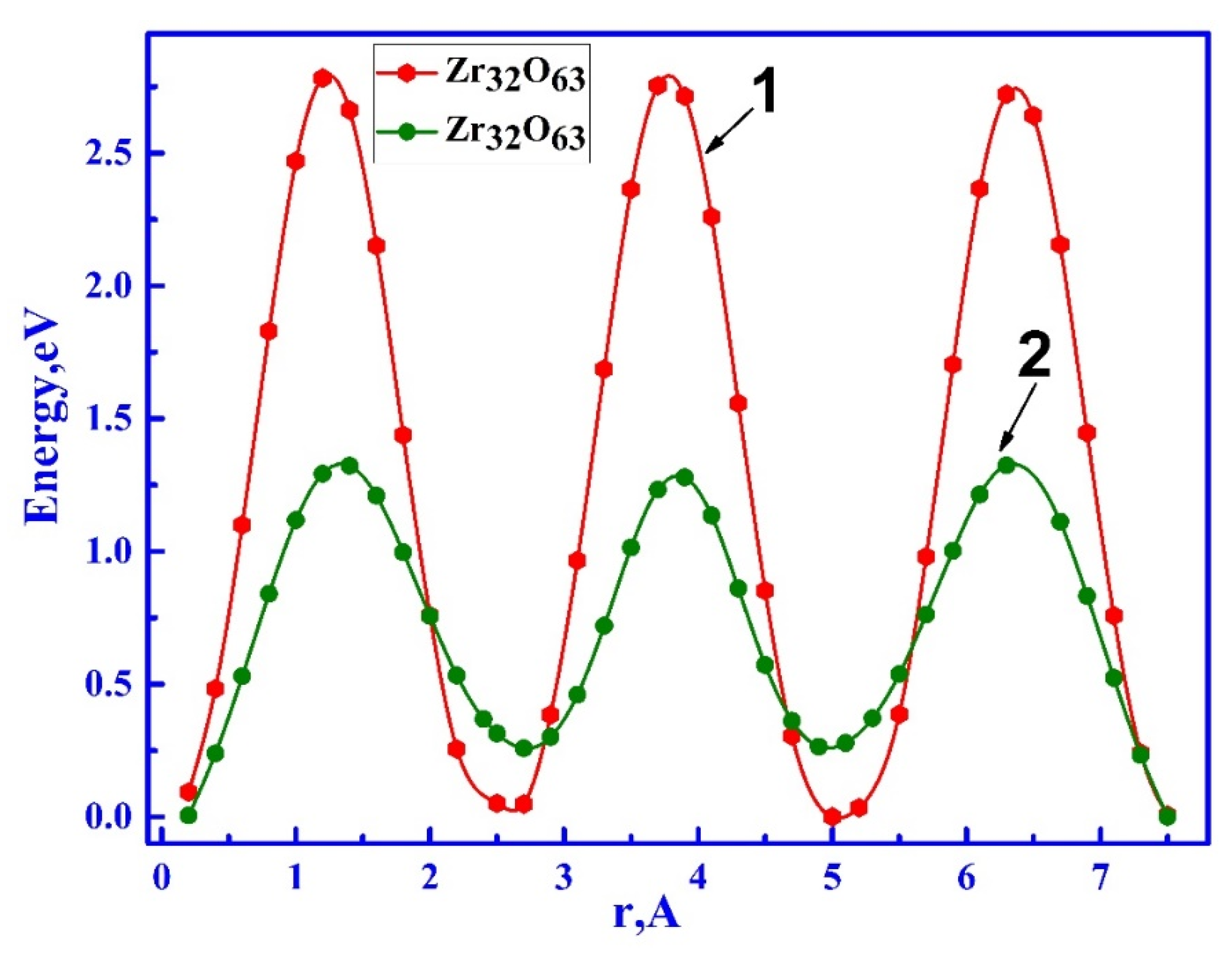
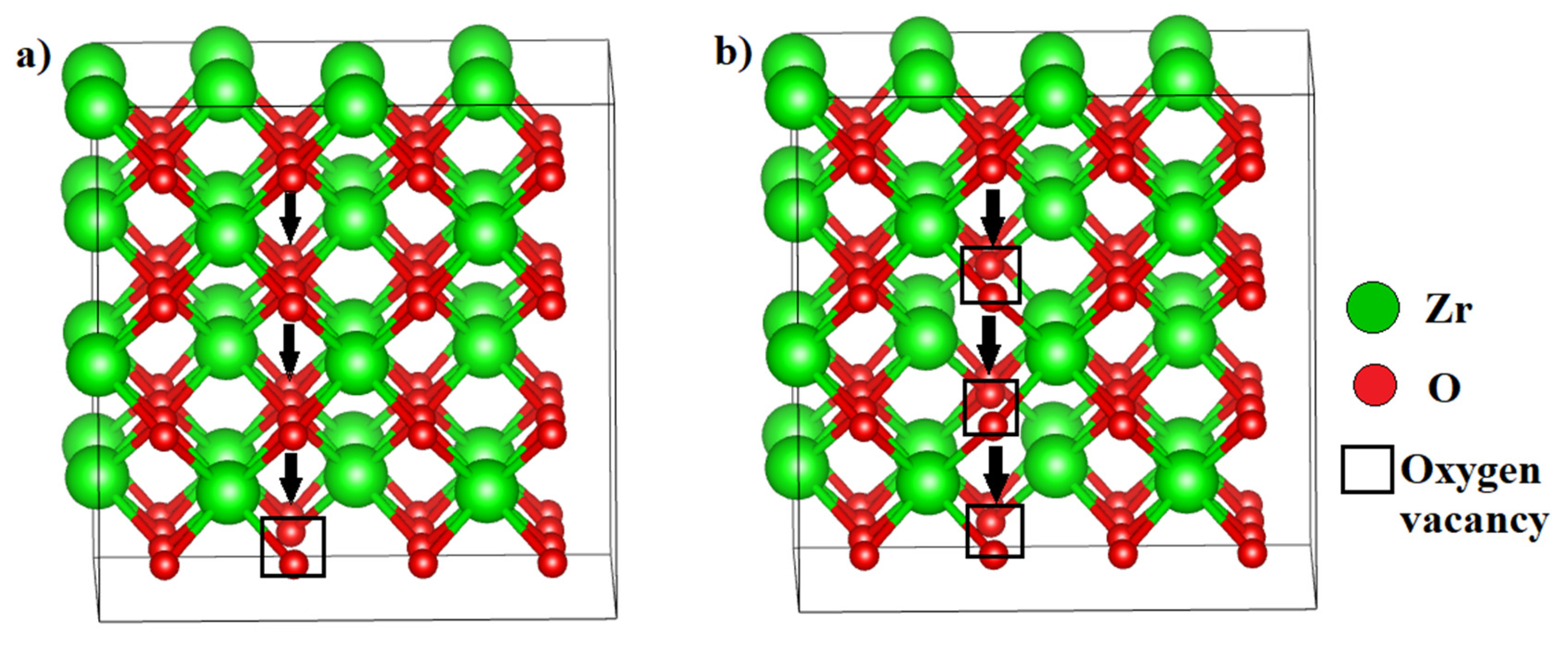
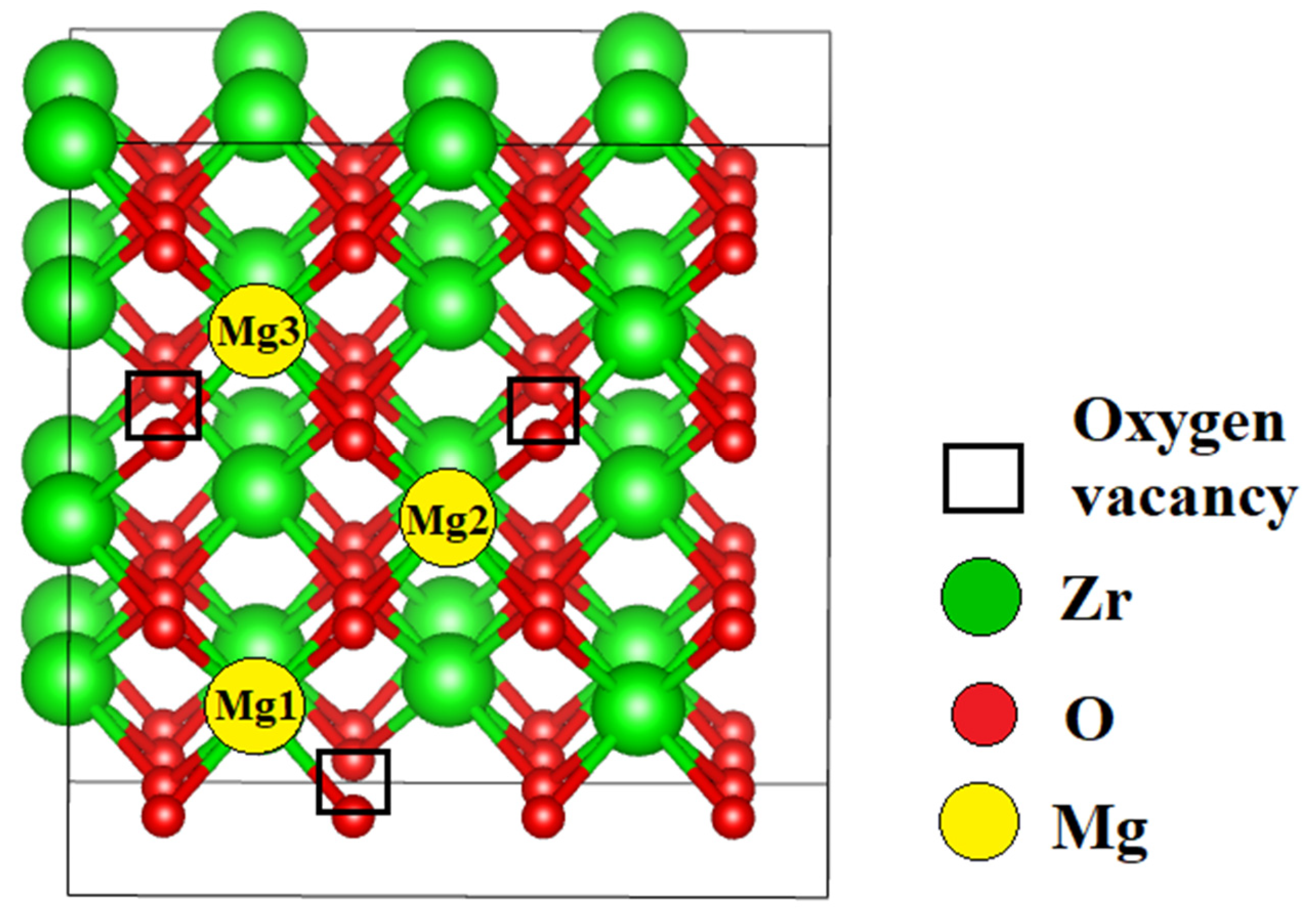
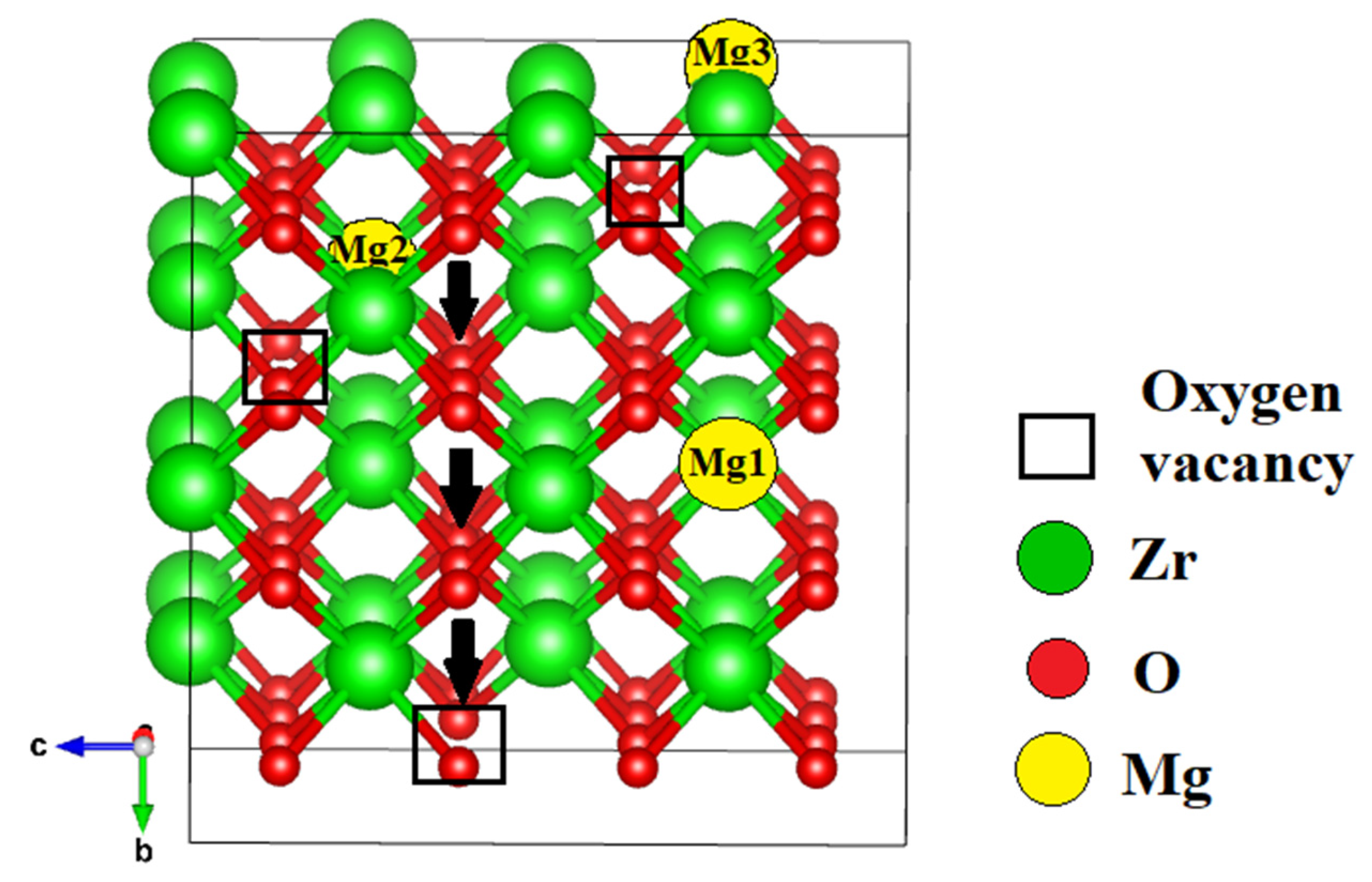
| Configuration | Mg Position | Total Energy (a.u) |
| Zr32O64 | −6311.78 | |
| Zr₃₂O₆₃ | - | −6237.92 |
| Zr₃₂O₆1 | + | −6085.65 |
| Zr₃₁Mg₁O₆₃ | along pathway | −6391.30 |
| Zr₃₀Mg₂O₆₂ | −6469.26 | |
| Zr₂₉Mg₃O₆₁ | −6547.24 | |
| Zr₃₁Mg₁O₆₃ | outside pathway | −6391.23 |
| Zr₃₀Mg₂O₆₂ | −6469.16 | |
| Zr₂₉Mg₃O₆₁ | −6547.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





