1. Introduction
In the 20th century, vitreous seeding (Reese–Ellsworth Group Vb and International Classification “D” and “E”) was the major reason retinoblastoma eyes were enucleated, because they were resistant to all conventional treatments (external beam irradiation [
1] and systemic chemotherapy [
2]). However, most of these eyes have been salvaged in the past 10–15 years with the introduction of intraarterial [
3] and intravitreal chemotherapy [
4,
5]. Using enhanced safety techniques when delivering intravitreal injections (as codified by Munier), extraocular extension has been prevented [
6,
7].
Although many chemotherapy drugs have been tried for intravitreal injections, most centers have been using melphalan at doses of 8–50 µG [
8,
9,
10] (usually 20–30 µG [
9,
10]). Researchers have reported high success rates in treating vitreous seeds (as high as 100%) without systemic side effects [
7,
11,
12]. With experience, however, disturbing toxicity was found in both the anterior and posterior segment, and this toxicity was found to be greater in heavily pigmented eyes [
12,
13,
14,
15].
Because of the toxicity of intravitreal melphalan, clinicians sought an alternative for intravitreal injection, and topotecan has been the chemotherapeutic agent most often used. Experimental work demonstrated its safety and predicted efficacy based on careful pharmacokinetics [
16,
17]. Numerous studies have confirmed its safety and efficacy in humans at doses of 8–90 µG [
18,
19,
20].
Nine years ago we reported on the use of melphalan for subretinal seeding [
21,
22,
23]. In that work, 98% of eyes responded (14.2% developed recurrences) without extraocular extension or death. Seven years ago, we reported on the use of intravitreal topotecan (at a dose of 90 µG) with or without intravitreal melphalan for subretinal tumors (combined with laser) [
22].
Our first report on the use of intravitreal topotecan for retinal tumors was in three patients [
24]. Those encouraging results have now been replicated in other centers [
25]. We now report on a larger cohort of eyes with recurrent retinal and subretinal retinoblastoma managed with intravitreal topotecan 90–180 µG with specific attention to efficacy and safety. This is considered a high dose because previous studies did not exceed 90 µG.
2. Materials and Methods
After Memorial Sloan Kettering Cancer Center institutional review board approval, we performed a prospective study of all retinoblastoma eyes with recurrent retinal or subretinal tumors treated with intravitreal topotecan 90–180 µG at the institution. All patients who received intravitreal topotecan 90–180 µG for retinal tumors between October 2022 and October 2024 were included. The technique used is now standard and has been reported elsewhere [
5].
Standard 30-Hz electroretinogram (ERG) was performed just prior to the injection (but before scleral indentation) and then a month later when the child returned for a subsequent exam under anesthesia. In all cases the pupils were dilated with our standard combination drop containing proparacaine, tropicamide, phenylephrine, and ketorolac [
26].
We graded efficacy as follows 1 month after each injection: complete response, tumor ophthalmoscopically gone; partial response, 50% reduction in size of tumor; stable disease, no change in tumor size; progressive disease, progressive growth of the tumor.
We assessed toxicity by repeated anterior segment exams, intraocular pressure measurements, retinal exams with the indirect ophthalmoscope and RetCam imaging, and 30-Hz flicker ERGs as previously reported from our center [
15].
Clinical and demographic information collected included age at diagnosis, laterality, age at treatment with topotecan injection, prior ophthalmic treatments, concurrent ocular treatments, ERG before and after each injection, and the length of follow-up; we also recorded both ocular and patient survival.
We used Student’s t-test to assess possible ERG toxicity of injections by comparing the 30-Hz flicker ERG immediately before the injection and 1 month later (Python’s SciPy stats package).
3. Results
We included 49 patients (17 girls and 32 boys; 18 unilateral and 31 bilateral; average age, 42 months) in our analysis who received a total of 114 intravitreal topotecan injections to 81 treated eyes; of these, 112 were with topotecan 90 µG and 2 with 180 µG (as two sequential 90-µG injections 5 minutes apart). For treatment history, 35 patients had previously received intraarterial chemotherapy, 5 had received multiagent systemic chemotherapy (given elsewhere), 5 had received both intravenous (given elsewhere) and intraarterial chemotherapy before receiving the injection, and 4 patients had previously received multiple combinations of these therapies. All the retinal and subretinal tumors treated represented recurrences in previously treated eyes, and no patient received any ophthalmic artery chemosurgery during the time they were receiving the topotecan injections. Overall, 75 injections were given at the same encounter with concurrent 810-nm diode laser with the indirect ophthalmoscope, and 39 received only intravitreal injection. The mean age at injection was 42.4 months (median, 37 months; range, 5–114 months). No patient developed metastasis or second cancer, and only one eye came to enucleation (for a vitreous hemorrhage likely from heavy cryotherapy delivered after continued growth of a treated tumor). Mean follow-up was 14 months, median was 13.5 months, and the range was 5–36 months.
At 1 month, 113 injections were associated with a response: 83 complete responses (CRs) and 30 partial responses (PRs). One injection was followed by progressive disease. Of the CR responses, 48 had received concurrent laser and 35 no laser. For the PR responses, 20 had concurrent laser and 10 received no laser. There were 3 recurrences (within months): 2 had been CRs and 1 PR at 1 month.
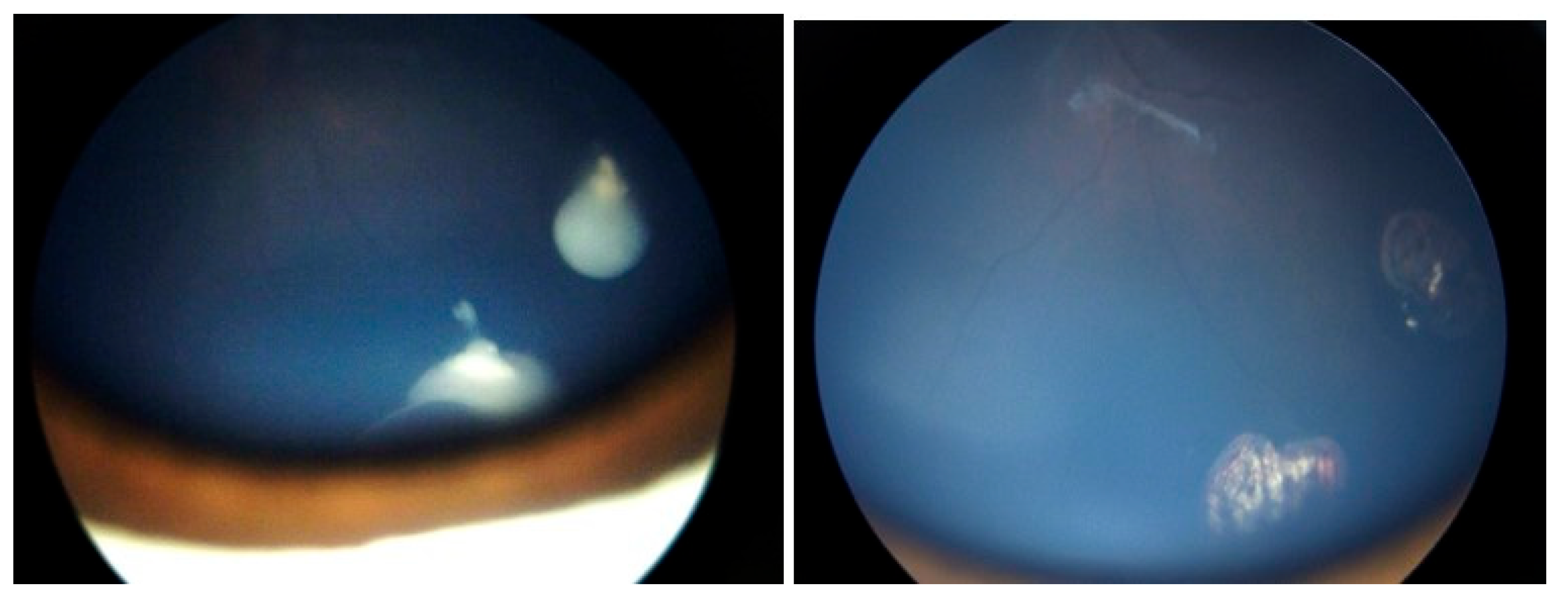
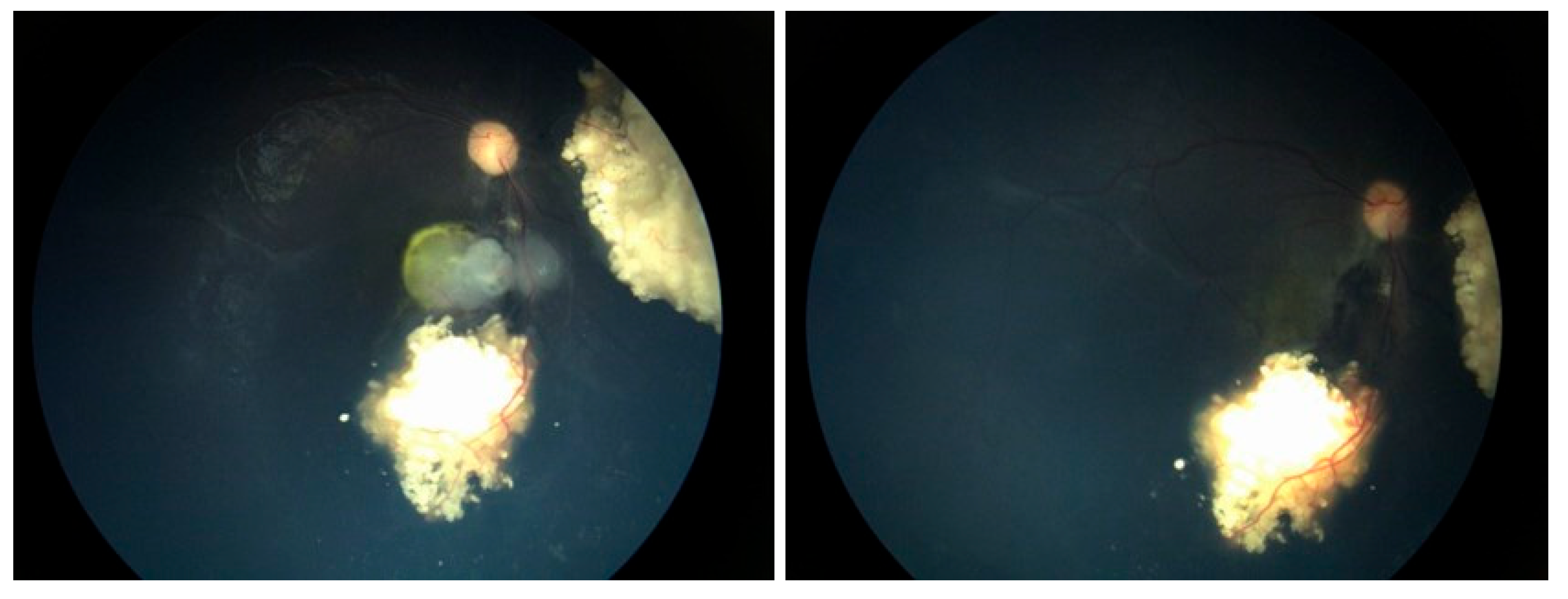
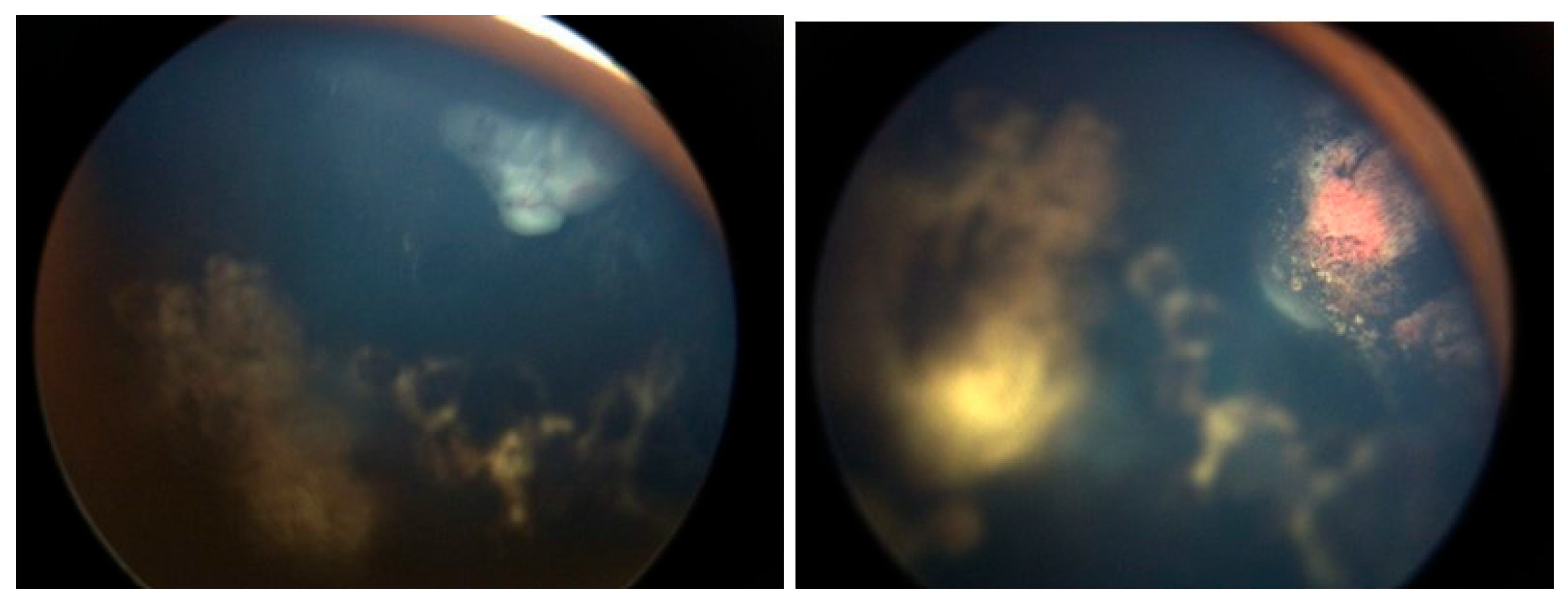
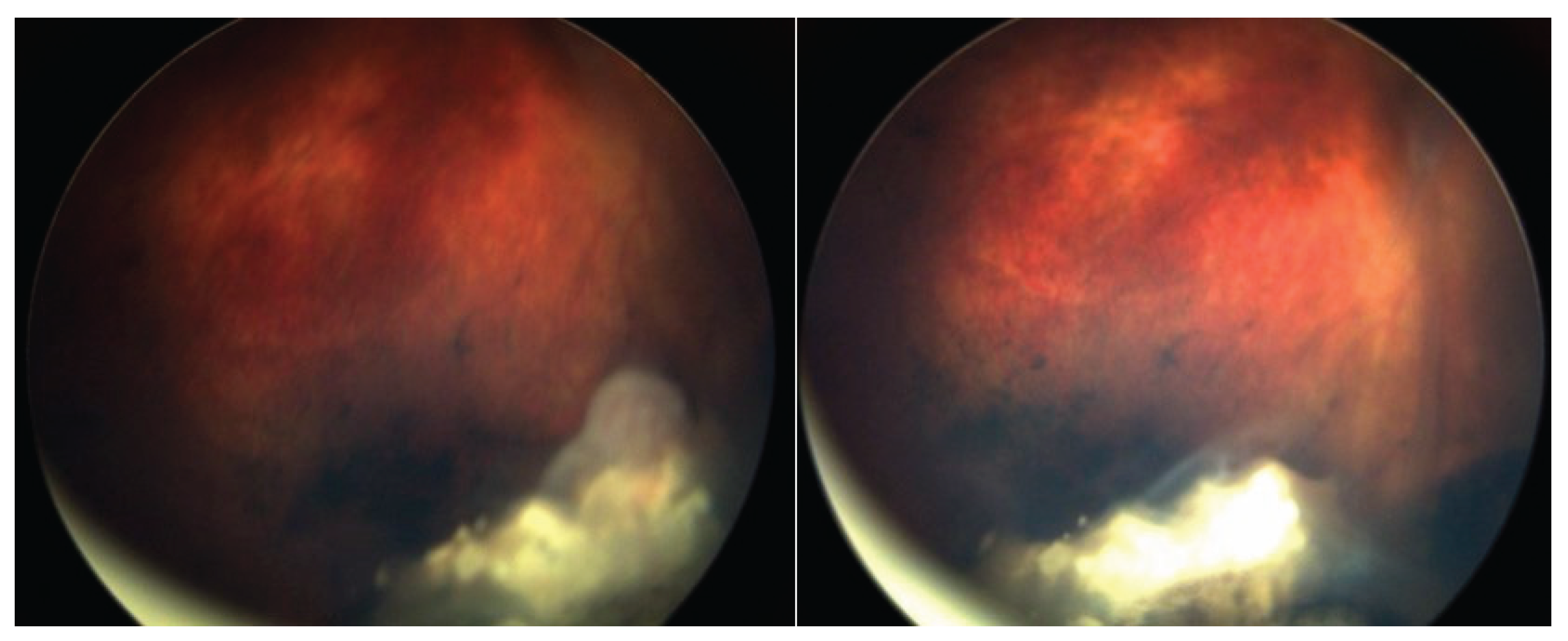
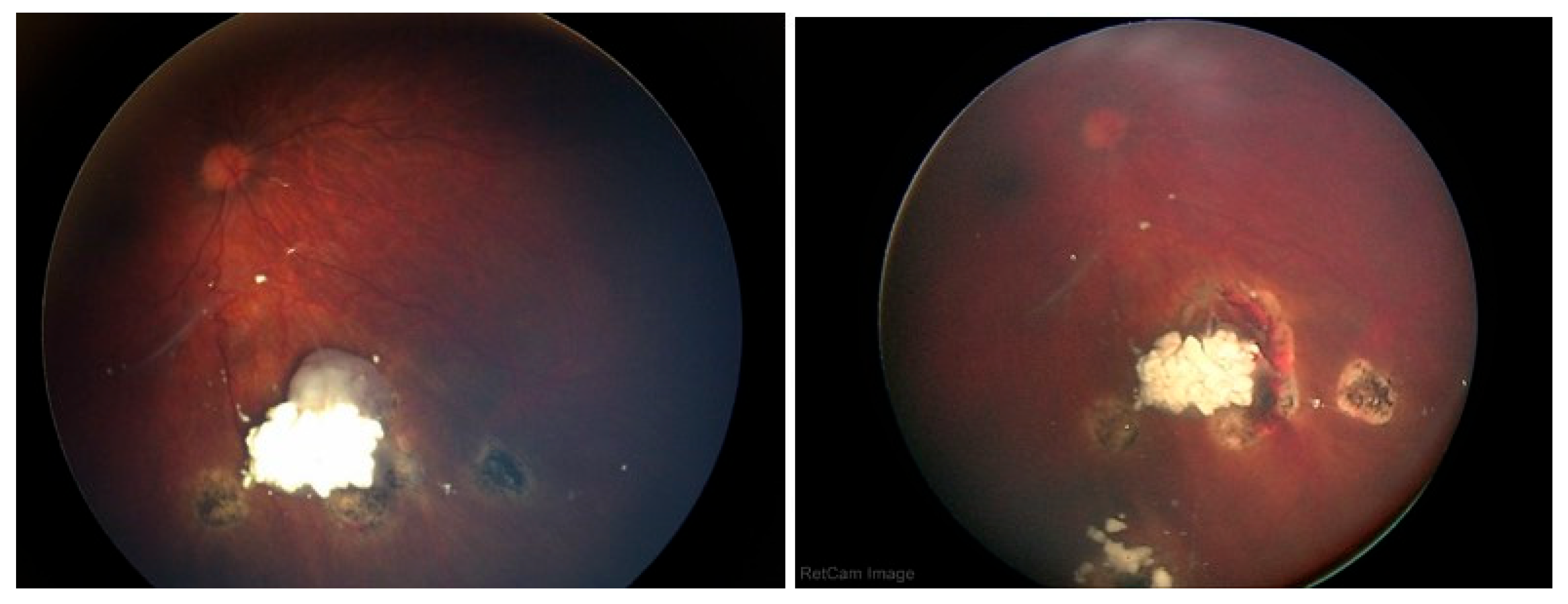
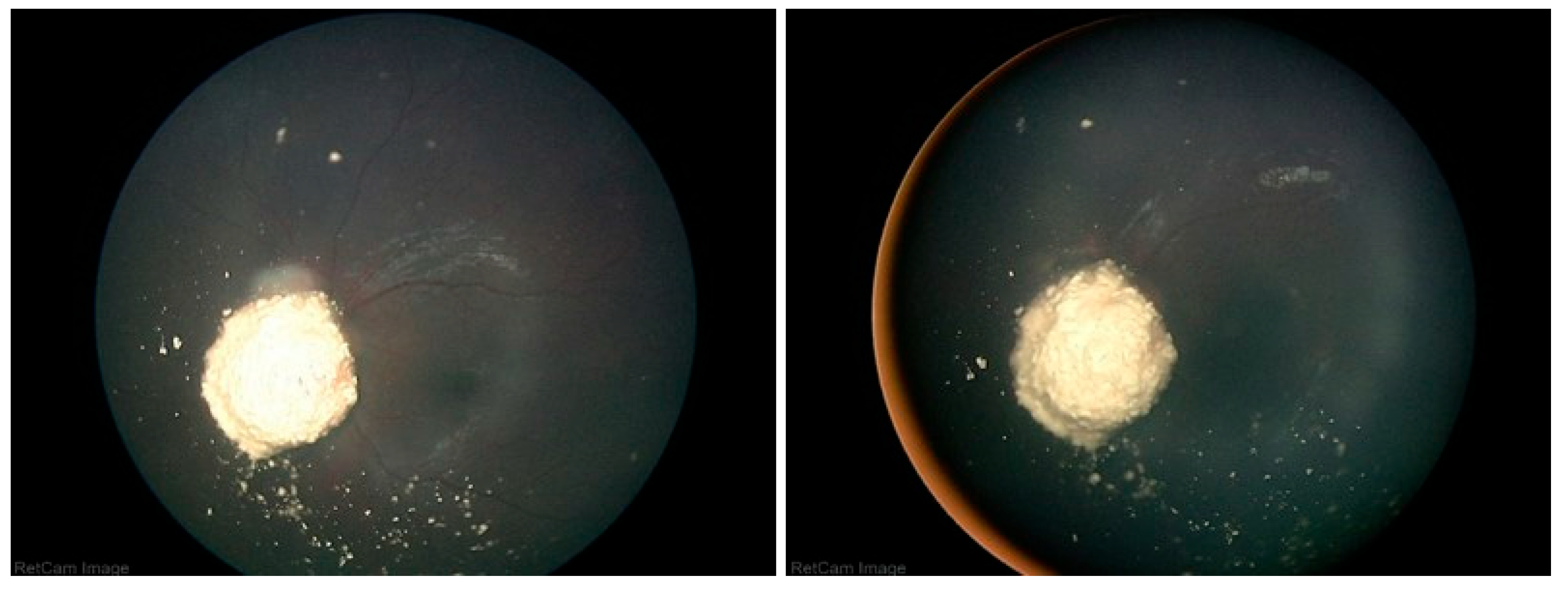
A Student’s t-test comparing the pre- and post-injection 30-Hz ERG had a P=0.76 (standard error of mean, 6.03 preinjection vs. 5.28 post-injection). There were no corneal abnormalities noted, no cataracts, and no elevated intraocular pressure in any patient. Images before and after are presented in
Figure 1,
Figure 2,
Figure 3,
Figure 4,
Figure 5 and
Figure 6.
4. Discussion
Both intravitreal melphalan [
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15] and topotecan [
7,
9,
14,
15,
16,
17,
18,
20] have been used in the past 13 years to treat
vitreous seeds with great success. Only recently have intravitreal injections been used to treat
retinal and subretinal tumors.
We reported on the use of intravitreal chemotherapy for retinal and subretinal disease (in 2018); using melphalan (often with concurrent laser) [
22,
23], we had a high success rate for recurrent subretinal seeding and local retinal recurrences. In 2023, we reported on the use of topotecan 90 µg for retinal disease in 3 patients with efficacy and no toxicity.
Recently, Shields and colleagues reported on their success using topotecan 90 and 100 µG [
25]. Of the 13 cases reported, 8 also received intraarterial chemotherapy and 1 intravenous chemotherapy. Of the three eyes that received intravitreal topotecan alone, local control was attained in all three initially but one subsequently recurred. No eye was lost.
In the cases we report here, there were no deaths, one eye was lost, and no eye suffered local complications; ERGs were not affected. In all of our cases, the retinal disease treated was recurrent and would have been difficult to control with other modalities—which certainly would have had more local toxicity. For example, in
Figure 1,
Figure 2,
Figure 3 and
Figure 4 the retinal recurrence also had overlying, new vitreous seeds, so laser alone would not have been curative. In
Figure 5 and
Figure 6, the recurrence was on a calcified tumor away from the retinal pigment epithelium, so laser alone was not an option.
Recurrent retinal and subretinal tumors are notoriously difficult to treat and often require enucleation to control disease. Rarely, these recurrences can be lasered, but often the recurrences are on white tumors (making laser impossible) and have overlying vitreous seeds (which cannot be destroyed with laser) [l].
As in the cases reported previously by us and by Shields and colleagues, many of these eyes received concurrent treatment with the diode laser. In the series reported here, 75 of the injections were associated with concurrent laser while 39 received only topotecan. Recurrence of retinal tumors after systemic or intraarterial chemotherapy and appearance and growth of subretinal seeds after treatment are notoriously difficult to treat, so the results presented here are encouraging. Future studies may allow us to appreciate the contribution of laser with the intravitreal injections, but the fact that 39 injections had measurable responses without laser is notable. To our knowledge, our current work is also the first instance of high-dose topotecan (90–180 µg) used in humans.
5. Conclusions
Additional experience will tell us when intravitreal chemotherapy can be used successfully and safely for retinal disease, but with high-dose intravitreal topotecan the retinoblastoma treatment algorithm is rapidly evolving.
Author Contributions
Conceptualization, D.H.A. and J.H.F.; methodology, D.H.A. and J.H.F.; validation, D.H.A. and J.H.F.; formal analysis, D.H.A. and J.H.F.; investigation, D.H.A. and J.H.F.; resources, D.H.A. and J.H.F.; data curation, D.H.A. and J.H.F.; writing—original draft preparation, D.H.A.; writing—review and editing, D.H.A. and J.H.F.; visualization, D.H.A. and J.H.F.; supervision, D.H.A.; project administration, J.H.F.; funding acquisition, D.H.A. and J.H.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Supported by the NIH P30 CA009748 and the Fund for Ophthalmic Knowledge Inc.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Memorial Sloan Kettering Cancer Center (protocol code #16-1470; approved 10/12/2016; progress report approved 8/22/2024).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to patient confidentiality.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CR |
Complete response |
| ERG |
Electroretinogram |
| PR |
Partial response |
References
- Abramson DH, Ellsworth RM, Tretter P, Adams K, Kitchin FD. Simultaneous bilateral radiation for advanced bilateral retinoblastoma. Arch Ophthalmol. Oct 1981;99(10):1763-6. [CrossRef]
- Dimaras H, Corson TW, Cobrinik D, et al. Retinoblastoma. Nat Rev Dis Primers. Aug 27 2015;1:15021. [CrossRef]
- Abramson DH. What have we learned about intraarterial chemotherapy (Ophthalmic Artery Chemosurgery) for retinoblastoma in the past 18 years? The third A. Linn Murphree Lecture. Ophthalmic Genet. Dec 2024;45(6):551-557. [CrossRef]
- Munier FL. Classification and management of seeds in retinoblastoma. Ellsworth Lecture Ghent August 24th 2013. Ophthalmic Genet. Dec 2014;35(4):193-207. [CrossRef]
- Munier FL, Soliman S, Moulin AP, Gaillard MC, Balmer A, Beck-Popovic M. Profiling safety of intravitreal injections for retinoblastoma using an anti-reflux procedure and sterilisation of the needle track. Br J Ophthalmol. Aug 2012;96(8):1084-7. [CrossRef]
- Francis JH, Abramson DH, Ji X, et al. Risk of Extraocular Extension in Eyes With Retinoblastoma Receiving Intravitreous Chemotherapy. JAMA Ophthalmol. Dec 1 2017;135(12):1426-1429. [CrossRef]
- Shields CL, Lally SE, Leahey AM, Jabbour PM, Caywood EH, Schwendeman R, Shields JA. Targeted retinoblastoma management: when to use intravenous, intra-arterial, periocular, and intravitreal chemotherapy. Curr Opin Ophthalmol. Sep 2014;25(5):374-85. [CrossRef]
- Ghassemi F, Shields CL. Intravitreal melphalan for refractory or recurrent vitreous seeding from retinoblastoma. Arch Ophthalmol. Oct 2012;130(10):1268-71. [CrossRef]
- Bogan CM, Pierce JM, Doss SD, et al. Intravitreal melphalan hydrochloride vs propylene glycol-free melphalan for retinoblastoma vitreous seeds: Efficacy, toxicity and stability in rabbits models and patients. Exp Eye Res. Mar 2021;204:108439. [CrossRef]
- Liao A, Hsieh T, Francis JH, Lavery JA, Mauguen A, Brodie SE, Abramson DH. TOXICITY AND EFFICACY OF INTRAVITREAL MELPHALAN FOR RETINOBLASTOMA: 25 microg Versus 30 microg. Retina. Jan 1 2021;41(1):208-212. [CrossRef]
- Francis JH, Iyer S, Gobin YP, Brodie SE, Abramson DH. Retinoblastoma Vitreous Seed Clouds (Class 3): A Comparison of Treatment with Ophthalmic Artery Chemosurgery with or without Intravitreous and Periocular Chemotherapy. Ophthalmology. Oct 2017;124(10):1548-1555. [CrossRef]
- Francis JH, Marr BP, Brodie SE, Abramson DH. Anterior Ocular Toxicity of Intravitreous Melphalan for Retinoblastoma. JAMA Ophthalmol. Dec 2015;133(12):1459-63. [CrossRef]
- Nadelmann J, Francis JH, Brodie SE, Muca E, Abramson DH. Is intravitreal topotecan toxic to retinal function? Br J Ophthalmol. Jul 2021;105(7):1016-1018. [CrossRef]
- Francis JH, Brodie SE, Marr B, Zabor EC, Mondesire-Crump I, Abramson DH. Efficacy and Toxicity of Intravitreous Chemotherapy for Retinoblastoma: Four-Year Experience. Ophthalmology. Apr 2017;124(4):488-495. [CrossRef]
- Francis JH, Schaiquevich P, Buitrago E, et al. Local and systemic toxicity of intravitreal melphalan for vitreous seeding in retinoblastoma: a preclinical and clinical study. Ophthalmology. Sep 2014;121(9):1810-7. [CrossRef]
- Del Sole MJ, Clausse M, Nejamkin P, et al. Ocular and systemic toxicity of high-dose intravitreal topotecan in rabbits: Implications for retinoblastoma treatment. Exp Eye Res. May 2022;218:109026. [CrossRef]
- Bogan CM, Kaczmarek JV, Pierce JM, et al. Evaluation of intravitreal topotecan dose levels, toxicity and efficacy for retinoblastoma vitreous seeds: a preclinical and clinical study. Br J Ophthalmol. Feb 2022;106(2):288-296. [CrossRef]
- Schaiquevich P, Fabius AW, Francis JH, Chantada GL, Abramson DH. Ocular Pharmacology of Chemotherapy for Retinoblastoma. Retina. Jan 2017;37(1):1-10. [CrossRef]
- Shields CL, Douglass AM, Beggache M, Say EA, Shields JA. INTRAVITREOUS CHEMOTHERAPY FOR ACTIVE VITREOUS SEEDING FROM RETINOBLASTOMA: Outcomes After 192 Consecutive Injections. The 2015 Howard Naquin Lecture. Retina. Jun 2016;36(6):1184-90. [CrossRef]
- Sen M, Rao R, Mulay K, Reddy VAP, Honavar SG. Intravitreal Topotecan for Vitreous Seeds in Retinoblastoma: A Long-term Review of 91 Eyes. Ophthalmology. Oct 2024;131(10):1215-1224. [CrossRef]
- Francis JH, Marr BP, Brodie SE, Gobin P, Dunkel IJ, Abramson DH. Intravitreal Melphalan as Salvage Therapy for Refractory Retinal and Subretinal Retinoblastoma. Retin Cases Brief Rep. Fall 2016;10(4):357-60. [CrossRef]
- Abramson DH, Catalanotti F, Brodie SE, Kellick MG, Francis JH. Intravitreal chemotherapy and laser for newly visible subretinal seeds in retinoblastoma. Ophthalmic Genet. Jun 2018;39(3):353-356. [CrossRef]
- Abramson DH, Ji X, Francis JH, Catalanotti F, Brodie SE, Habib L. Intravitreal chemotherapy in retinoblastoma: expanded use beyond intravitreal seeds. Br J Ophthalmol. Apr 2019;103(4):488-493. [CrossRef]
- Abramson DH, Francis JH. Intravitreal Topotecan 90 microg for Recurrent Solid Retinoblastoma Tumors Is Effective and Not Toxic. J Pediatr Ophthalmol Strabismus. Mar-Apr 2023;60(2):e16-e18. [CrossRef]
- Shields CL, Medina R, Evans H, et al. High-Dose Intravitreal Topotecan for Recurrent Retinoblastoma, Subretinal Seeds, and Vitreous Seeds. Retina. Jan 1 2025;45(1):1-6. [CrossRef]
- Abramson DH, Liu T, Guarini E, et al. Improved method of dilating pupils for ophthalmic exams under anesthesia (faster and easier). Asia Pac J Oncol Nurs. Aug 2024;11(8):100543. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).