1. Introduction
Skin pigmentation is a common condition triggered by both internal (genetics) and external (sun exposure and medications) factors that impact on skin color [
1,
2]. Skin color primarily depends on the amount and localization of cutaneous pigments, with melanin being the most important in the epidermis, hemoglobin in the dermis, and carotenoids in adipocytes and the
stratum corneum. The perception of skin color is then determined by the complex interplay between absorption, scattering, and reflection of incident light along with the structural and optical properties of the skin [
3,
4].
Dysregulation of melanin production leads to hyperpigmentation, manifesting as skin imperfections commonly referred to as “dark spots”. This condition results from dermatological conditions (e.g., melasma, chloasma), sun exposure, age, genetics, epigenetics, and skin injuries (e.g., inflammation, acne) [
5,
6,
7]. Sun exposure is the most common cause of hyperpigmentation, as it significantly stimulates melanin production. Notably, several studies have demonstrated the efficacy of daily photoprotection in reducing and preventing the appearance of dark spots [
8,
9,
10]. Excess melanin accumulation and its abnormal distribution in the skin are also hallmark features of skin aging and post-inflammatory hyperpigmentation (PIH) [
7,
11].
Skin appearance plays a fundamental role in self-perception, social interactions, and cultural beauty ideals. Among various dermatological concerns, hyperpigmentation and dark spots are widely recognized as a primary aesthetic issue, particularly in Asian populations, where an even, luminous complexion is highly valued [
12]. The appearance of dark spots is not merely an aesthetic concern but also carries a psychological burden, affecting quality of life, overall well-being, and social functioning. It can evoke negative emotions such as shame and embarrassment, further impacting individuals’ mental health and confidence [
13,
14,
15].
Traditional treatments for skin lightening primarily rely on topical depigmenting ingredients (e.g., hydroquinone, kojic acid, vitamin C), dermatological procedures (e.g., chemical peels, laser therapy) and oral medications, however, these interventions may be associated with irritation, side effects, and limited long-term efficacy [
1]. In recent years, there has been a growing interest in oral food supplements as a complementary approach to skin lightening and pigmentation control [
16]. Certain bioactive compounds, including antioxidants, polyphenols, vitamins, and botanical extracts, have been shown to modulate melanogenesis, oxidative stress, and inflammation, thereby influencing skin pigmentation from within [
17,
18,
19,
20]. Among botanical extracts, licorice is widely used as a commercial skin lightening agent in modern cosmetic formulations [
21]. One of its key active compounds responsible for skin lightening is glabridin, a phytoconstituent derived from the
Glycyrrhiza glabra. Glabridin is the primary component of the hydrophobic fraction of licorice extract and is recognized for its depigmenting properties based on tyrosinase activity inhibition [
22,
23,
24]. However, a recent study by Nerya et al. demonstrated that the inhibitory effect of licorice extract on tyrosinase activity was greater than expected based on its glabridin content alone. Interestingly, the authors found that two polyphenols (glabrene and isoliquiritigenin) contained in the extract were effective in inhibiting both mono- and diphenolase tyrosinase activities [
25]. Two recent clinical trials have demonstrated the efficacy of olive extracts in improving skin hyperpigmentation [
26,
27]. Moreover, it was demonstrated a synergistic effect between glabridin and antioxidants in inhibiting tyrosinase activity [
28].
In this study, we aimed to evaluate the skin lightening efficacy and the safety of use of a commercially available active ingredient, SelectSIEVE
® Glitter (ROELMI HPC, Origgio, VA, Italy). This ingredient is composed of a synergistic blend of natural polyphenols derived from olive (
Olea europaea L.) and bioactive compounds extracted from licorice (
Glycyrrhiza glabra L.). Both olive and licorice are well-documented in the scientific literature for their antioxidant, anti-inflammatory, and depigmenting properties, making them promising candidates for hyperpigmentation and uneven skin tone [
29,
30,
31]. The skin lightening effect was assessed both in vitro and in vivo on humans. In vitro we evaluated the melanin synthesis by a human melanoma MNT-1 cell line, while in vivo we assessed the melanin staining within the dark spot and the surface area of dark spots. The effect of the test items on blood pressure was evaluated both in vitro and in vivo on humans. The in vitro assessment focused on the inhibition of the 11β-hydroxysteroid dehydrogenase type 2 (HSD11β2) enzyme. Inhibition of this enzyme may contribute to elevated blood pressure due to increased cortisol availability and activity at mineralocorticoid receptors [
33]. Blood pressure was also monitored in humans during the clinical trial.
2. Materials and Methods
2.1. In Vitro study
2.1.1. Inhibition of HSD11β Type 2 Enzyme
The inhibition of the HSD11β type 2 enzyme was measured indirectly by the cortisol level in the reaction mixture. Briefly, 0.04 mg SelectSIEVE® Glitter and 2 mg/ml of a benchmark titrated in 2% (w/w) glycyrrhizic acid (Nature Med, Castrovillari, CS, Italy) licorice extract were added to the reaction mixture containing 10 ng/ml HSD11β type 2 enzyme, 0.2 mM NADP+ and 1800 pg/ml cortisol (ThermoFisher Scientific, Monza, MB, Italy) in a PBS solution (ThermoFisher Scientific, Monza, MB, Italy) at pH 7.4 and at 37 °C. The level of cortisol was measured after 4 hours using an ELISA kit (ThermoFisher Scientific, Monza, MB, Italy), according to the manufacturer’s protocol. The reaction mixture without any test item served as the negative control.
2.1.2. Melanin Synthesis Inhibition
Human melanoma MNT-1 cells were purchased from ATCC (Manassas, VA, USA) and cultured in DMEM-high glucose containing 20% fetal bovine serum, 10% of AIM-V medium, 0.1 mM non-essential amino acids in the standard condition (37 °C and 5% CO
2) for 24 hours within a 6-well plate. After the incubation period, the culture medium was replaced with fresh medium containing non-cytotoxic concentrations of licorice extract (LC extr., 0.1 mg/mL), olive extract (OL extr., 0.1 mg/mL), and the test ingredient at two concentrations (SSG, 0.1 mg/mL and 0.5 mg/mL). The cells were then incubated for 72 hours. The test ingredient (SelectSIEVE
®Glitter, ROELMI HPC, Origgio, VA, Italy) was a commercially available blend of natural olive (
Olea Europaea L.) and licorice (
Glycyrrhiza glabra L.) extracts, standardized to contain at least 4% (w/w) polyphenols (≥ 4%, w/w). Hydroquinone (Hyd, 5 μg/ml) and DMEM culture medium served as the positive and negative controls (CTR-), respectively. After the incubation period, melanin production was assessed spectrophotometrically at 405 nm, while protein content was quantified using the Bradford assay [
34].
For both the melanin and protein content assay cells were washed once with PBS. Cells were then lysed with 0.1 N NaOH at 60°C for 1 hour for melanin and with sterile water at 4 °C for the protein content assay. Melanin and proteins were measured using a microplate reader (BioTek Synergy LX Multimode Reader, Agilent Technologies, Inc., Santa Clara, CA, USA) at 405 and 595 nm, respectively. Standard curves for the melanin and protein assay were created using melanin solutions ranging from 10 to 200 μg/ml melanin and albumin solutions ranging from 4 to 30 μg/ml albumin, respectively.
2.2. Clinical Study
2.2.1. Trial Design and Ethics
This was a multicentric, randomized (1:1), double-blind, placebo-controlled trial conducted at Complife Italia facilities in San Martino Siccomario (Pavia, Italy), Nutratech (Rende, Italy) and Complife Asia (Beijing, China), from April to September 2023. The trial consisted of a screening visit, a baseline visit (W0), and two follow-up visits after 4 (W4) and 8 (W8) weeks of product use. All the study outcomes were measured at each checkpoint.
This trial was conducted in compliance with the ethical principles outlined in the World Medical Association’s (WMA) Declaration of Helsinki and its amendments. The study protocol (H.E.HU.HV.NWH00.060.41.00_NT0000092/23 Rev.01 by March 7th, 2023) and all associated trial documents were reviewed and approved by the
Comitato Etico Indipendente per le Indagini Cliniche Non Farmacologiche (ref. No. 2023/02, approval date: March 9, 2023) and registered on ISRCTN registry (ISRCTN12882379) [
35].
Written informed consent (ICF) was obtained from all participants prior to the initiation of any study-related procedures.
2.2.2. Participants and Compliance with Treatment
The trial enrolled healthy Caucasian and Asian male and female subjects, aged 25 to 65, with dark spots due to aging, sun exposure, or post-inflammatory hyperpigmentation (PIH). Subject recruitment was conducted as follows: Caucasian subjects were enrolled by Complife Italia (San Martino Siccomario, PV, Italy); Asian subjects with darker skin phototypes (IV–V) were enrolled by Nutratech (Rende, CS, Italy); and Chinese Asian subjects with lighter skin phototypes (III–IV) were enrolled by Complife Asia (Xicheng District, Beijing, China). Exclusion criteria included acute or chronic conditions that could interfere with study outcomes, pose a risk to the participant, or be deemed incompatible with study requirements by the Investigator; dermatological skin conditions and medical histories that could interfere with study outcomes; pharmacological treatments interfering with the study outcomes; allergies or sensitivities to cosmetic products, medications, medical devices, or patches; breastfeeding; pregnancy, and unwillingness to adopt effective contraceptive measures (for women of childbearing potential).
Throughout the study period, participants were instructed to maintain a daily journal to record any significant changes in dietary habits, deviations from the prescribed product use, and the occurrence of any adverse events. They were also advised to avoid sun exposure (natural or artificial) and to refrain from using any facial cosmetic products or food supplements other than the base cream and the food supplement provided by the investigation center.
Compliance with treatment was determined by counting the number of capsule packs returned at weeks 4 and 8. A compliance rate of 80% or higher was required for participants to be considered adherent to the protocol.
2.2.3. Interventions and Randomization
The test item was a food supplement formulated with SelectSIEVE®Glitter. The composition (per capsule) of the active food supplement (SSG) was as follows: 200 mg SelectSIEVE®Glitter (Glycyrrhiza Glabra and Olea Europea L. extracts), 200 mg maltodextrin, 2 mg magnesium stearate and 2 mg silica dioxide. The placebo food supplement (PLA) composition per capsule contained: 2,25 mg charcoal, 435 mg maltodextrin, and 4 mg magnesium stearate. All the ingredients used in the food supplement formula are safe for their use. Participants in both the active and control arms received one capsule daily before breakfast.
To standardize the cosmetic routine, participants were supplied with a base day cream containing UV filters and formulated without any claimed efficacy. They were instructed to apply the product according to their usual skincare habits, using it
ad libitum. The estimated quantity for each application was 1.54 g/day [
36]. The ingredient list of the cream was as follows: aqua, ethylhexyl methoxycinnamate, peg-6 stearate, ethylhexyl salicylate, butyl methoxydibenzoyl methane, methylene bis-benzotriazolyl tetramethylbutylphenol (nano), octocrylene, triolein, glyceryl stearate, glycol stearate, peg-32 stearate, glyceryl dioleate, cetyl palmitate, decyl glucoside, xanthan gum, propylene glycol, hydroxyethyl acrylate/sodium acryloyldimethyl taurate copolymer, polyisobutene, peg-7 trimethylolpropane coconut ether, disodium EDTA, ethylhexylglycerin, caprylyl glycol, o-cymen-5-ol. All ingredients in the cosmetic products were included in the positive list of substances authorized for cosmetic use. The formulation did not contain any ingredients listed in Annex II (prohibited substances) of Regulation (EC) No 1223/2009 [
37]. Ingredients in Annex III (restricted substances), Annex V (preservatives), Annex V (preservatives), and Annex VI (UV filters) were used within the concentration limits specified by the Regulation and had been assessed for safety by the European Scientific Committee.
2.2.4. Outcomes
Both primary and secondary outcomes were assessed at baseline (W0) and after 4 (W4) and 8 (W8) weeks of product use. Clinical and instrumental measurements were taken under temperature and humidity-controlled conditions (temperature 22 ± 4 °C and relative humidity 50 ± 10%). Prior to each assessment, participants were allowed to acclimatize for 15–20 minutes.
2.2.4.1. Primary Outcomes
The primary outcomes of the trial included the evaluation of dark spot pigmentation and surface, skin radiance, skin complexion evenness, and overall lightening efficacy.
The dark spots pigmentation and the skin radiance were measured by the spectrophotometer/colorimeter CM 700D (Konica Minolta, Milan, Italy).
The pigmentation of the dark spots was evaluated by the calculation of the individual typology angle (ITA°) based on the L* (lightness) and b* (chroma) values measured inside the dark spot. ITA° was calculated according to the following equation:
Skin radiance was assessed on the cheek area by measuring the 8° gloss parameter, which quantifies the amount of light reflected from the skin surface, providing an objective evaluation of skin brightness/radiance.
The surface of dark spots was measured using morphometric image analysis performed on digital images captured with the VISIA
®-CR system (Canfield Scientific Europe BV, Utrecht, The Netherlands). The analysis employed a thresholding (segmentation) algorithm applied to RBX brown images [
38].
The improvement in skin complexion evenness and lightening efficacy was evaluated by a dermatologist using a 4-point clinical scale: 1 No variation, 2 Slight improvement, 3 Moderate improvement, and 4 Remarkable improvement. The assessment was performed on digital images captured with the VISIA®-CR system (Canfield Scientific Europe BV, Utrecht, The Netherlands).
2.2.4.2. Secondary Outcomes
The secondary endpoints of the trial were the assessment of the advanced glycation end products (AGEs) and the evaluation of product tolerability and self-assessment.
AGEs were measured by a non-invasive AGE Reader mu device (Diagnoptics Technologies B.V., Groningen, Netherlands) using ultraviolet light to excite autofluorescence correlated with AGEs level in the skin [
39,
40]. The measurement was taken by placing the bare forearm (below the elbow) on top of the AGE reader for 12 seconds. Evaluation of AGEs was carried out on 17 subjects, randomly selected from each group.
Product tolerability was evaluated by the investigator through the assessment of any local or systemic signs of intolerance (e.g., bloating, flatulence, nausea, mild abdominal discomforts, skin itching or tingling, mild rash, etc.) or adverse effects (e.g., diarrhea, constipation, vomiting, gastroesophageal reflux, urticaria, etc.). During all the study period, the subjects were asked to report any signs of intolerance or adverse effects. Blood pressure was monitored due to the well-known effects of licorice extract, particularly when it contains glycyrrhizin, on elevating blood pressure, which can lead to hypertension when consumed in high doses or over prolonged periods.
2.2.4.3. Self-Assessment Questionnaire
At both Week 4 (W4) and Week 8 (W8), participants completed a self-assessment questionnaire designed to evaluate their perception of the product’s efficacy and tolerability. The questionnaire included items related to improvements in skin complexion and texture, as well as overall product tolerability. Depending on the question, participants responded using either a four-point Likert scale (“completely agree,” “agree,” “disagree,” or “completely disagree”) or a binary choice (“Yes” or “No”). For analysis, the responses “completely agree” and “agree,” as well as “Yes,” were grouped and considered as positive feedback.
2.5. Randomization and Blinding
Participants were randomly assigned in a 1:1 ratio to receive either the active food supplement (SSG) or the placebo product (PLA). Randomization was performed using a computer-generated (PASS 11, version 11.0.8, PASS, LLC, Kaysville, UT, USA) randomization sequence using the “Efron’s biased coin” algorithm to ensure balanced allocation across groups.
The randomization sequence was concealed using sealed, opaque, and sequentially numbered envelopes to ensure allocation concealment. Each intervention (SSG or PLA) was pre-labeled according to the randomization sequence. To maintain blinding, both the investigational product and the placebo were identical in appearance, taste, packaging, and labeling. Study investigators, participants, and all personnel involved in outcome assessment remained blinded to treatment allocation throughout the trial. The randomization list was securely stored and remained inaccessible to study personnel until after database lock, unless emergency unblinding was warranted due to a serious adverse event.
2.6. Statistical Analysis
2.6.1. In Vitro Studies
The percentage of melanin synthesis and the percentage of the inhibition of the HSD11β type 2 enzyme were calculated according to equation (2) and (3), respectively.
The raw data used in the equation were the mean value of each triplicate measurement for both the melanin and protein content. Both intragroup and intergroup analysis were performed with the t test of Student using a Microsoft® Excel® (Microsoft, Redmond, WA, USA) datasheet (Microsoft 365 Apps for business, version 2403, build 17425.20176).
2.6.2. Clinical Study: Sample Size and Statistical Analysis
The sample size was determined based on prior similar studies, which provided the baseline average value, and the variability of the primary endpoint required for power analysis. Based on this information, a sample size of 25 subjects per group was determined to be sufficient to detect statistically significant differences with a power of 80% at a significance level of 0.05. The sample size analysis was conducted using PASS 11 statistical software (version 11.0.8; PASS, LLC, Kaysville, UT, USA). To account for potential dropouts or exclusions, an additional eight subjects were included.
All data were initially assessed for normality to determine the appropriate statistical approach. When data met the assumptions of normality, repeated measures analysis of variance (RM-ANOVA) was applied to evaluate differences between conditions or time points. For datasets that did not follow a normal distribution, the non-parametric Friedman test was used as an alternative to RM-ANOVA. Post hoc comparisons were conducted using the Tukey–Kramer test to account for multiple comparisons while controlling the family-wise error rate. Intragroup statistical analysis was performed on the raw data, whereas intergroup comparisons were conducted using percentage variation from baseline. All statistical analyses were performed using NCSS 10 software (version 10.0.7; NCSS, LLC, Kaysville, UT, USA), and statistical significance was set at p < 0.05.
3. Results
3.1. In Vitro Study
3.1.1. Inhibition of HSD11β Type 2 Enzyme
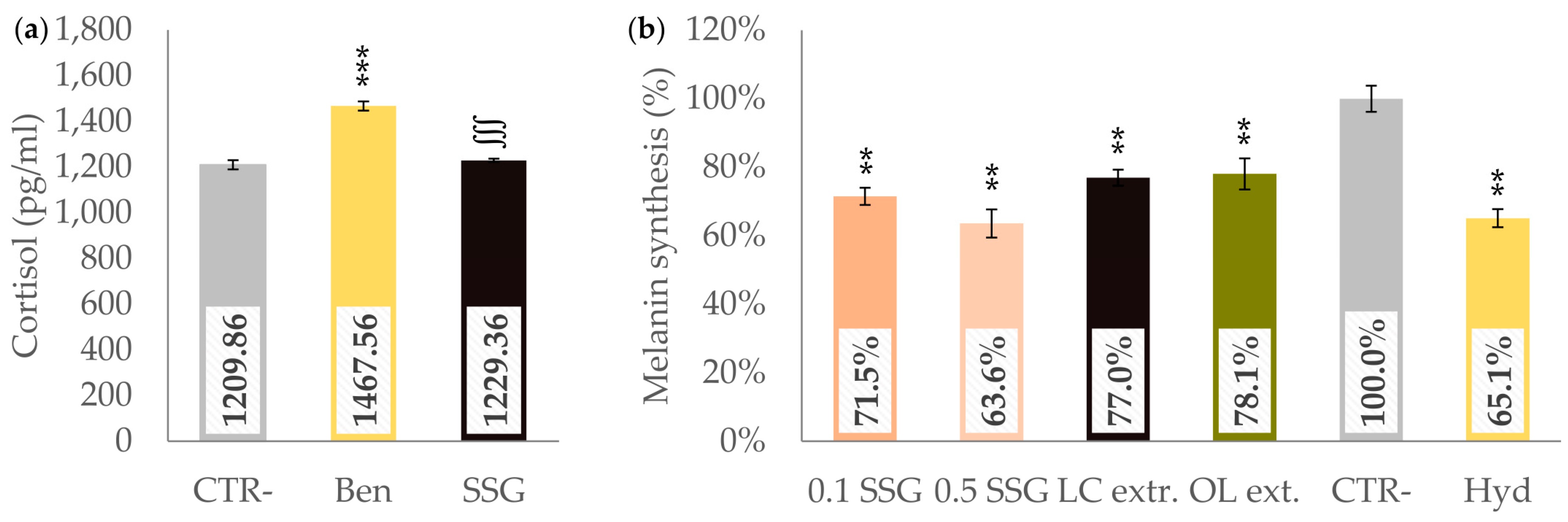
Four hours after the enzymatic reaction began, cortisol concentrations were as follows: 1209.86 ± 20.16 pg/ml in the negative control, 1467.56 ± 19.65 pg/ml in the benchmark sample, and 1229.36 ± 7.21 pg/ml in the SSG reaction mixture (
Figure 1a). The cortisol concentration in the SSG reaction mixture was not significantly different from that of the negative control (
p > 0.05), whereas the benchmark product significantly inhibited the enzymatic reaction by 21.3% (
p < 0.001 vs. both the negative control and SSG reaction mixture).
3.1.2. Melanin Synthesis
All the tested items decreased the melanin synthesis when compared to the negative control (
Figure 1b). SSG at 0.1 mg/ml concentration reduced (
p < 0.01) melanin synthesis to 71.5 ± 2.5%, corresponding to a 28.5% inhibition, while at 0.5 mg/ml concentration further decreased (
p < 0.01) melanin synthesis to 63.6 ± 4.1%, showing the highest inhibition among tested ingredients at 36.4%. Even if not statistically significant (
p > 0.05), these results indicate a dose-dependent inhibitory trend of SSG on melanogenesis. Treatment with 0.1 mg/ml LC extract resulted in 77.0 ± 2.3% melanin synthesis, achieving a 23.0% inhibition (
p < 0.01). The OL extract at 0.1 mg/ml showed a melanin synthesis level of 78.1 ± 4.5%, corresponding to a 21.9% inhibition (
p < 0.01). Its effect was comparable with the LC extract effect. Hydroquinone at 5 μg/ml exhibited inhibitory (
p < 0.01) activity with melanin synthesis at 65.1 ± 2.6%, translating to a 34.9% inhibition. Even if not statistically significant (
p > 0.05), a trend toward a synergistic effect was noted for the SSG when compared to each single extract. The inhibitory effect of all the tested ingredients was comparable with the hydroquinone effect.
3.2. Participant Characteristics, Tolerability, and Compliance with Treatment
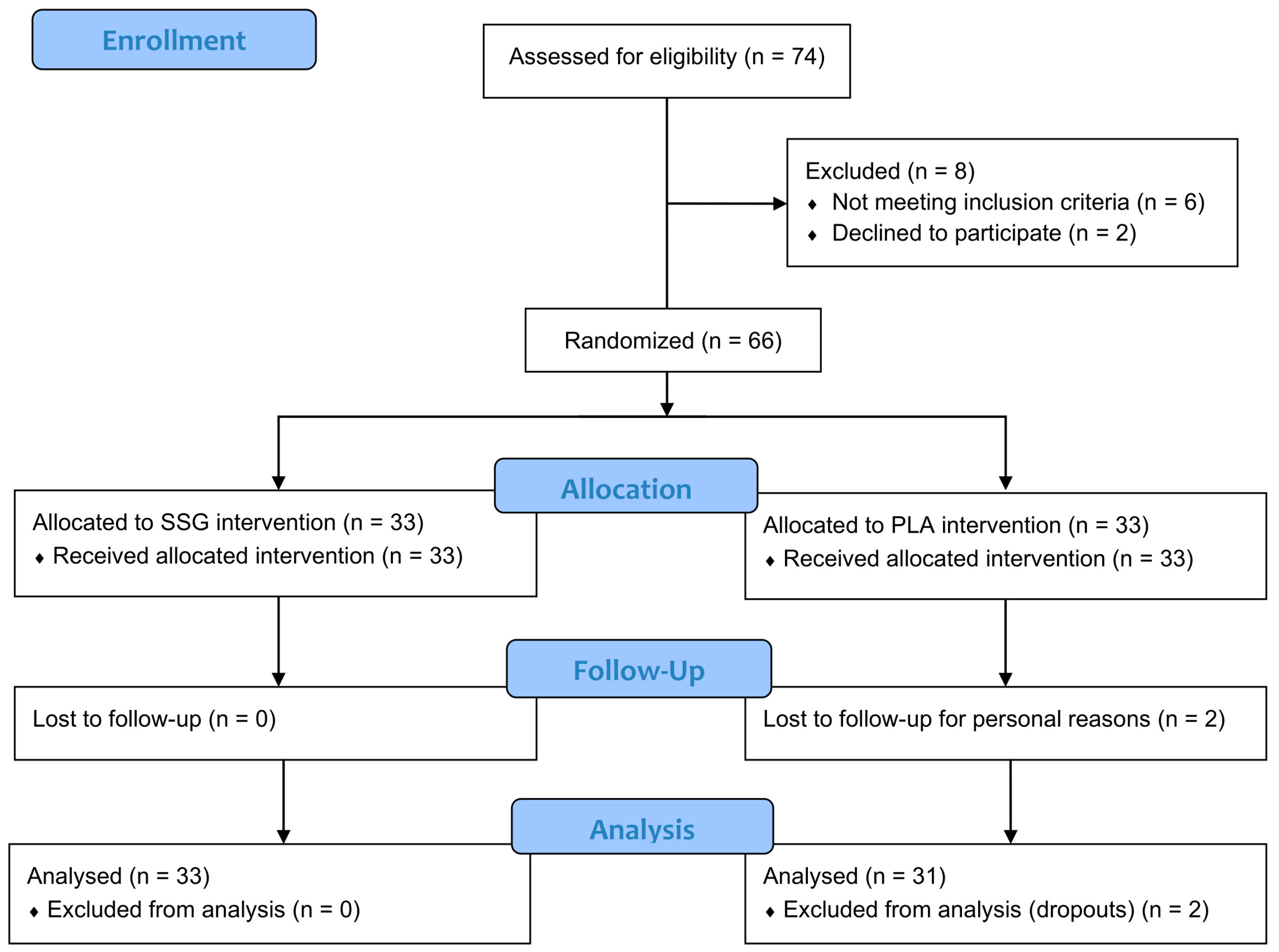
A total of 74 subjects were screened for eligibility. Of these, 6 did not meet the inclusion criteria and 2 declined to participate. As a result, 66 subjects (n = 66) were successfully randomized into two groups, with 33 participants allocated to each group. The study population included 22 Caucasian subjects enrolled by Complife Italia (San Martino Siccomario, PV, Italy), 12 Asian subjects with darker skin phototypes (IV–V) enrolled by Nutratech (Rende, CS, Italy), and 32 Chinese Asian subjects with lighter skin phototypes (III–IV) enrolled by Complife Asia (Xicheng District, Beijing, China). The study dataset (per-protocol population) included 64 subjects, as two participants in the PLA group withdrew at Day 28 due to personal reasons unrelated to product use. A detailed overview of participant flow is provided in
Figure 2.
No significant differences between groups were observed at baseline for any of the outcomes, confirming successful randomization and baseline homogeneity across the two groups (
Table 1).
Both the active and placebo products were well tolerated throughout the study period. No adverse events, side effects, or unexpected skin reactions were reported by participants or observed by clinical investigators at any point during the intervention. This absence of negative outcomes highlights the favorable safety profile of both formulations when used under the prescribed conditions. At both Week 4 (W4) and Week 8 (W8), 100% of participants in both groups confirmed overall tolerability of the products. These findings support the good safety profile of both the active and placebo formulations under the study conditions. In addition, no significant changes in blood pressure were observed in response to product intake (
Table S1).
Compliance with treatment was high in both groups (95.1% in the PLA group and 97.1% in the SSG group).
3.3. Primary Endpoints
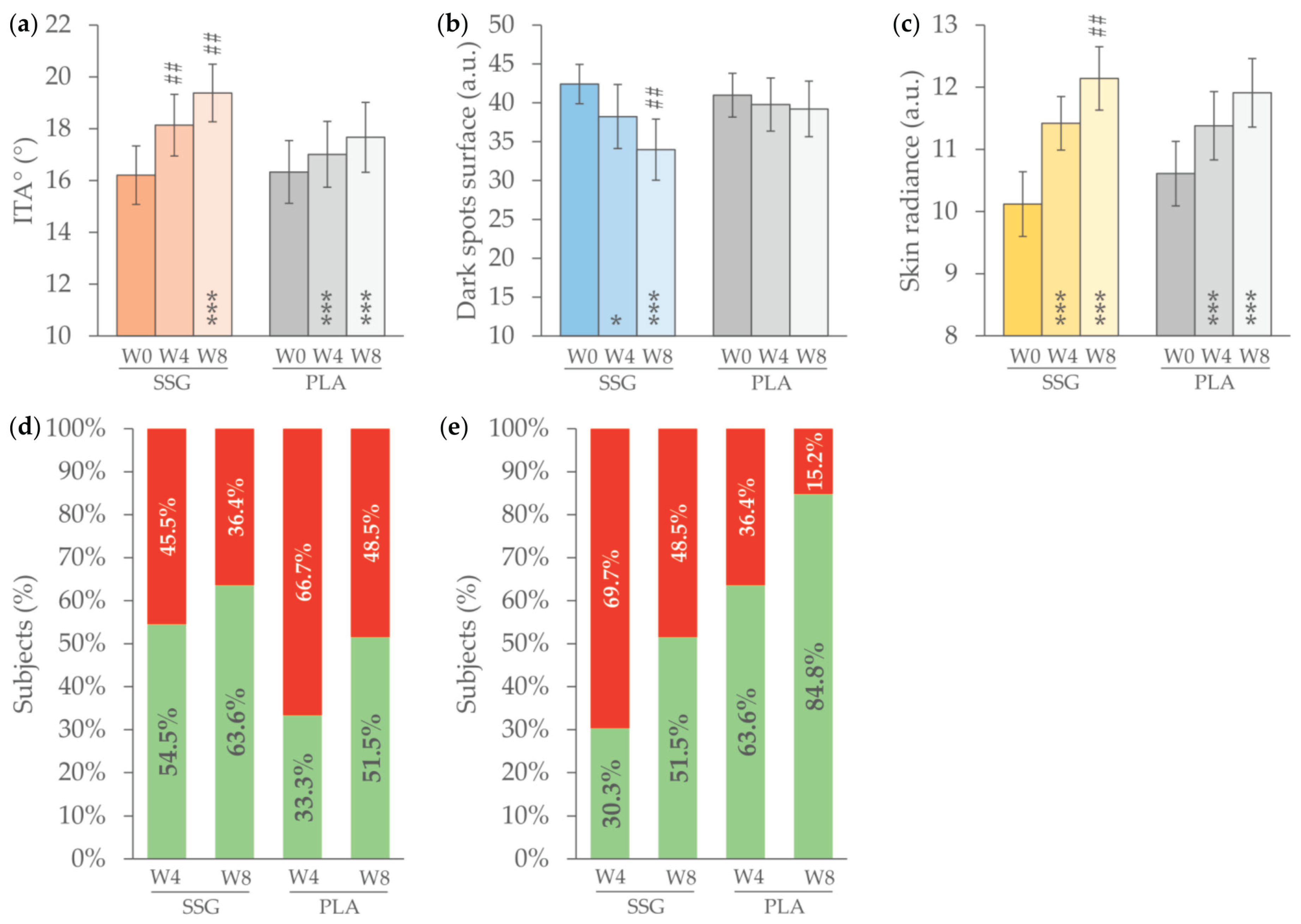
At baseline, the individual typology angle (ITA°), inside the dark spot, was 16.33 ± 1.21 in the PLA group and 16.21 ± 1.13 in the SSG group indicating a pigmented skin. In the SSG group, the ITA° value statistically significantly increased by 11.8% (18.14 ± 1.19;
p < 0.001) at W4 and 18.9% (19.38 ± 1.11;
p < 0.001) at W8 (
Figure 3a). The increase in ITA° is indicative of a lightening effect. In the PLA group, a statistically significant increase in ITA° of 8.1% (17.67 ± 1.35;
p < 0.001) was observed exclusively at W8. The ITA° increase in the SSG group was significantly higher than in the PLA group at both W4 and W8 (
p < 0.01). This instrumental variation, indicating a lightening effect on the dark spot, was corroborated by dermatologist assessments in the SSG group, with 54.5% of participants showing improvement at Week 4 and 63.6% at Week 8 (
Figure 3d).
In the SSG group, the surface area of dark spots decreased by 4.18 units (
p < 0.05) at W4 and 8.45 units (
p < 0.001) at W8, corresponding to reductions of 9.8% and 19.9%, respectively, compared to baseline (
Figure 3b). In the PLA group, the surface area of dark spots remained unchanged throughout the study period (
p > 0.05). At Week 8, the SSG group exhibited a significantly greater reduction compared to the PLA group (
p < 0.01). In the SSG group, the reduction in dark spot surface area was associated with a clinically observed improvement in skin complexion evenness in 63.6% of participants at W4 and 84.8% at W8 (
Figure 3e).
A statistically significant increase in skin radiance was observed in both the SSG and PLA groups at W4 and W8 (
Figure 3c). However, the magnitude of improvement was consistently greater in the SSG group. At Week 4, skin radiance increased by 14.0% in the SSG group compared to 8.0% in the PLA group (
p > 0.05), suggesting a trend toward enhanced efficacy, although the difference was not statistically significant at this time point. By W8, the difference became statistically significant, with the SSG group showing a 21.2% increase in radiance versus 12.7% in the PLA group (
p < 0.01).
3.4. Secondary Endpoints
The baseline AGEs levels were 1.89 ± 0.10 in the SSG group and 2.27 ± 0.18 in the PLA group. After 8 weeks of product use, the AGEs levels in the SSG group decreased by 10.6% (
p < 0.001) while they were unchanged in the PLA group (
Table 2). Differences between SSG and PLA groups were statistically significant (
p < 0.05).
4. Discussion
Nutraceuticals claiming skin health benefits and improving its appearance are becoming popular [
41,
42]. These products, often formulated with bioactive compounds such as antioxidants, vitamins, minerals, and plant extracts, are promoted for their potential to improve skin hydration, elasticity, brightness, and overall complexion. However, despite their growing presence in the market, there is a relative scarcity of robust scientific evidence supporting many of these claims [
41].
The skin lightening effect of topically applied licorice extract is well-documented and widely recognized in the literature, primarily due to its active compounds such as glabridin, which inhibit tyrosinase activity and melanin production [
20,
43,
44]. However, despite its long-standing use in topical formulations, there is currently a lack of scientific studies evaluating its efficacy when administered orally. The potential systemic effects of licorice-derived compounds on skin pigmentation, if any, remain unexplored. This gap in the literature highlights the need for well-designed clinical trials to assess the potential skin benefits of oral licorice extract supplementation.
The present study demonstrates the efficacy of the tested oral formulation (SSG) in improving multiple parameters related to skin pigmentation and overall appearance over an 8-week period. The in vitro data clearly demonstrated the efficacy of the test product inhibiting the melanin synthesis, giving insight into the mechanism of action. The significant increase in the Individual Typology Angle (ITA°) observed in the SSG group, both at W4 and W8, indicates a pronounced skin lightening effect within hyperpigmented areas. The decrease in skin pigmentation was further substantiated by dermatologist assessments, with more than half of the participants in the SSG group exhibiting noticeable clinical improvement as early as W4, increasing to nearly two-thirds by W8. This alignment between instrumental and clinical evaluations supports the reliability of the observed outcomes and suggests that the formulation’s effect is both measurable and perceivable. Furthermore, the reduction in dark spot surface area in the SSG group provides additional evidence of the product’s depigmenting properties. The absence of any improvement in the PLA group highlights the potential role of the active ingredients in modulating melanogenic activity or promoting skin turnover. Importantly, this reduction in hyperpigmented areas was paralleled by improved skin evenness, reported in more than 80% of subjects by the end of the study.
Skin radiance, a multifactorial attribute linked to skin smoothness, light reflectance, and hydration, also improved significantly in both groups. However, the greater and earlier enhancement in the SSG group suggests a cumulative or synergistic benefit that may involve multiple pathways. Although the initial difference between groups at Week 4 did not reach statistical significance, the trend became clear and significant by Week 8, reinforcing the formulation’s potential in enhancing overall skin luminosity.
Taken together, these findings indicate that the SelectSIEVE® Glitter is effective in improving skin tone uniformity, reducing hyperpigmented areas, and enhancing radiance, parameters that are often associated with a more youthful and healthier skin appearance. The consistency of in vitro and in vivo objective measurements and subjective assessments strengthens the validity of these results.
This study has several notable strengths but also some limitations. Firstly, it presents a well-controlled, randomized, placebo-controlled design, which enhances the reliability of the findings and reduces the risk of bias. The use of both instrumental measurements (e.g., ITA°, dark spot surface area, and skin radiance) and clinical assessments by dermatologists provides a comprehensive evaluation of treatment efficacy, combining objective data with perceptible, real-world outcomes. Additionally, the inclusion of multiple skin-related endpoints allowed for a multidimensional assessment of skin appearance and health. Another key strength is the multiethnic panel, allowing the results to extend to a broader population. Despite these strengths, some limitations should be acknowledged. First, the study sample size, while sufficient to detect statistically significant differences, may limit the generalizability of the findings to broader populations with diverse skin types or ethnic backgrounds. Second, although the study was multiethnic, black people were not included.
5. Conclusions
The findings of this study demonstrate that oral supplementation with SelectSIEVE® Glitter significantly improves key parameters associated with reduced dark spot appearance, enhanced skin radiance, and improved complexion evenness.
These effects were observed as early as four weeks and became more pronounced by Week 8, with results consistently superior to those of the placebo group. The combination of objective instrumental measurements and supportive clinical assessments provides strong evidence for the efficacy of this oral approach in addressing hyperpigmentation and promoting a more radiant, even-toned complexion. These results support the potential of oral nutraceuticals as a complementary strategy in cosmetic dermatology. Moreover, the efficacy of the product was not associated to increased blood pressure that is typical of licorice.
To the best of our knowledge, this is the first clinical study to demonstrate the skin-lightening efficacy of orally administered licorice and olive extract.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Table S1: Blood pressure.
Author Contributions
Conceptualization, V.N., E.C. and Z.S.; methodology, V.N. and X.Y.; formal analysis, V.N. and X.Y.; investigation, E.C., L.G. and Z.S.; resources, V.N.; data curation, V.N. and X.Y.; writing—original draft preparation, V.N.; writing—review and editing, V.N., E.C., L.G. and Z.S.; visualization, V.N.; supervision, V.N. and X.Y.; project administration, V.N.; funding acquisition, V.N. and X.Y.
Funding
This research was funded by ROELMI HPC S.R.L., grant number IT0001889/23. The APC was funded by ROELMI HPC S.R.L.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board “Comitato Etico Indipendente per le Indagini Cliniche Non Farmacologiche” (ref. No. 2023/02, approval date: March 9, 2023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
The data presented in this study is available on request from the corresponding author. The data are not publicly available since they are the property of the sponsor of the study (ROELMI HPC Srl, 21040 Origgio, VA, Italy).
Acknowledgments
The authors would like to express their gratitude to the Complife Italia, Nutratech and Complife Asia staff, who contributed to the study and recruited the subjects, for their professionalism and support during study development.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AGEs |
Advanced Glycation End products |
| ITA° |
Individual Typology Angle |
| PIH |
PostInflammatory Hyperpigmentation |
| PLA |
Placebo food supplement |
| RM-ANOVA |
Repeated Measures Analysis of Variance |
| SSG |
Active food supplement |
| W4 |
Week 4 |
| W8 |
Week 8 |
| AGEs |
Advanced Glycation End products |
References
- Thawabteh, A. M.; Jibreen, A.; Karaman, D.; Thawabteh, A.; Karaman, R. Skin Pigmentation Types, Causes and Treatment—A Review. Molecules 2023, 28(12), 4839. [Google Scholar] [CrossRef] [PubMed]
- Duperray, J.; Sergheraert, R.; Chalothorn, K.; Tachalerdmanee, P.; Perin, F. The Effects of the Oral Supplementation of L-Cystine Associated with Reduced L-Glutathione-GSH on Human Skin Pigmentation: A Randomized, Double-blinded, Benchmark- and Placebo-controlled Clinical Trial. J of Cosmetic Dermatology 2022, 21(2), 802–813. [Google Scholar] [CrossRef]
- Kollias, N.; Seo, I.; Bargo, P. R. Interpreting Diffuse Reflectance for in Vivo Skin Reactions in Terms of Chromophores. J Biophotonics 2010, 3(1–2), 15–24. [Google Scholar] [CrossRef]
- Tseng, S.-H.; Bargo, P.; Durkin, A.; Kollias, N. Chromophore Concentrations, Absorption and Scattering Properties of Human Skin in-Vivo. Opt Express 2009, 17(17), 14599–14617. [Google Scholar] [CrossRef]
- Liu, W.; Chen, Q.; Xia, Y. New Mechanistic Insights of Melasma. Clin Cosmet Investig Dermatol 2023, 16, 429–442. [Google Scholar] [CrossRef]
- Hushcha, Y.; Blo, I.; Oton-Gonzalez, L.; Mauro, G. D.; Martini, F.; Tognon, M.; Mattei, M. D. microRNAs in the Regulation of Melanogenesis. Int J Mol Sci 2021, 22(11), 6104. [Google Scholar] [CrossRef]
- Silpa-Archa, N.; Kohli, I.; Chaowattanapanit, S.; Lim, H. W.; Hamzavi, I. Postinflammatory Hyperpigmentation: A Comprehensive Overview: Epidemiology, Pathogenesis, Clinical Presentation, and Noninvasive Assessment Technique. J Am Acad Dermatol 2017, 77(4), 591–605. [Google Scholar] [CrossRef]
- Flament, F.; Mercurio, D. G.; Catalan, E.; Bouhadanna, E.; Delaunay, C.; Miranda, D. F.; Passeron, T. Impact on Facial Skin Aging Signs of a 1-Year Standardized Photoprotection over a Classical Skin Care Routine in Skin Phototypes II–VI Individuals: A Prospective Randomized Trial. Journal of the European Academy of Dermatology and Venereology 2023, 37(10), 2090–2097. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, R.; Garg, V. K.; Jain, A.; Agarwal, D.; Wagle, A.; Flament, F.; Verschoore, M. A Randomized Study to Evaluate the Efficacy and Effectiveness of Two Sunscreen Formulations on Indian Skin Types IV and V with Pigmentation Irregularities. Indian J Dermatol Venereol Leprol 2019, 85(2), 160–168. [Google Scholar] [CrossRef] [PubMed]
- Randhawa, M.; Wang, S.; Leyden, J. J.; Cula, G. O.; Pagnoni, A.; Southall, M. D. Daily Use of a Facial Broad Spectrum Sunscreen Over One-Year Significantly Improves Clinical Evaluation of Photoaging. Dermatol Surg 2016, 42(12), 1354–1361. [Google Scholar] [CrossRef]
- Skoczyńska, A.; Budzisz, E.; Trznadel-Grodzka, E.; Rotsztejn, H. Melanin and Lipofuscin as Hallmarks of Skin Aging. Postepy Dermatol Alergol 2017, 34(2), 97–103. [Google Scholar] [CrossRef]
- Poondru, S.; Gaurav, A.; Yang, L. J.; Kundu, R. V. Perceptions of Sun Protection, Skin Tone, Colorism, and Dermatologic Care Among South Asians in the USA. J Racial Ethn Health Disparities 2025, 12(2), 1021–1026. [Google Scholar] [CrossRef] [PubMed]
- Platsidaki, E.; Efstathiou, V.; Markantoni, V.; Kouris, A.; Kontochristopoulos, G.; Nikolaidou, E.; Rigopoulos, D.; Stratigos, A.; Gregoriou, S. Self-Esteem, Depression, Anxiety and Quality of Life in Patients with Melasma Living in a Sunny Mediterranean Area: Results from a Prospective Cross-Sectional Study. Dermatol Ther (Heidelb) 2023, 13(5), 1127–1136. [Google Scholar] [CrossRef]
- Simons, R. E.; Zevy, D. L.; Jafferany, M. Psychodermatology of Vitiligo: Psychological Impact and Consequences. Dermatologic Therapy 2020, 33(3), e13418. [Google Scholar] [CrossRef]
- Darji, K.; Varade, R.; West, D.; Armbrecht, E. S.; Guo, M. A. Psychosocial Impact of Postinflammatory Hyperpigmentation in Patients with Acne Vulgaris. J Clin Aesthet Dermatol 2017, 10(5), 18–23. [Google Scholar] [PubMed]
- Malathi, M.; Thappa, D. M. Systemic Skin Whitening/Lightening Agents: What Is the Evidence? Indian J Dermatol Venereol Leprol 2013, 79, 842. [Google Scholar] [CrossRef] [PubMed]
- Tursi, F.; Pourtau, L.; Roveda, G.; De Ponti, I.; Gaudout, D.; Moras, B.; Pouchieu, C.; Nobile, V. Clinical Efficacy of Belight3TM on Dark Spot Pigmentation in Caucasian Subjects. Cosmetics 2025, 12(1), 27. [Google Scholar] [CrossRef]
- Hanif, N.; Al-Shami, A. M. A.; Khalid, K. A.; Hadi, H. A. Plant-Based Skin Lightening Agents: A Review. J Phytopharmacol 2020, 9(1), 54–60. [Google Scholar] [CrossRef]
- Dilokthornsakul, W.; Dhippayom, T.; Dilokthornsakul, P. The Clinical Effect of Glutathione on Skin Color and Other Related Skin Conditions: A Systematic Review. J Cosmet Dermatol 2019, 18(3), 728–737. [Google Scholar] [CrossRef]
- Zaid, A. N.; Al Ramahi, R. Depigmentation and Anti-Aging Treatment by Natural Molecules. Curr Pharm Des 2019, 25(20), 2292–2312. [Google Scholar] [CrossRef]
- A Ali, S.; Choudhary, R. K.; Naaz, I. Melanogenesis: Key Role of Bioactive Compounds in the Treatment of Hyperpigmentory Disorders. Pigmentary Disorders 2015, 2(11). [Google Scholar] [CrossRef]
- Ribeiro, A. S.; Estanqueiro, M.; Oliveira, M. B.; Sousa Lobo, J. M. Main Benefits and Applicability of Plant Extracts in Skin Care Products. Cosmetics 2015, 2(2), 48–65. [Google Scholar] [CrossRef]
- Hanif, N.; Al-Shami, A. M. A.; Khalid, K. A.; Hadi, H. A. Plant-Based Skin Lightening Agents: A Review. J Phytopharmacol 2020, 9(1), 54–60. [Google Scholar] [CrossRef]
- Guo, Y.; Cariola, A.; Matera, R.; Gabbanini, S.; Valgimigli, L. Real-Time Oxygen Sensing as a Powerful Tool to Investigate Tyrosinase Kinetics Allows Revising Mechanism and Activity of Inhibition by Glabridin. Food Chem 2022, 393, 133423. [Google Scholar] [CrossRef]
- Nerya, O.; Vaya, J.; Musa, R.; Izrael, S.; Ben-Arie, R.; Tamir, S. Glabrene and Isoliquiritigenin as Tyrosinase Inhibitors from Licorice Roots. J. Agric. Food Chem. 2003, 51(5), 1201–1207. [Google Scholar] [CrossRef]
- de Toledo Bagatin J, Bagatin E, Campos PMBGM. A pilot clinical study to evaluate the effectiveness of olive extract containing hydroxytyrosol for oral and topical treatment of melasma. Biomed Biopharm Res. 2020, 17, 48–62. [CrossRef]
- D’Angelo Costa, G. M.; Maia Campos, P. M. B. G. Efficacy of Topical Antioxidants in the Skin Hyperpigmentation Control: A Clinical Study by Reflectance Confocal Microscopy. J of Cosmetic Dermatology 2021, 20(2), 538–545. [Google Scholar] [CrossRef]
- Wang, Y.; Hao, M.-M.; Sun, Y.; Wang, L.-F.; Wang, H.; Zhang, Y.-J.; Li, H.-Y.; Zhuang, P.-W.; Yang, Z. Synergistic Promotion on Tyrosinase Inhibition by Antioxidants. Molecules 2018, 23(1), 106. [Google Scholar] [CrossRef] [PubMed]
- Nunes, A.; Gonçalves, L.; Marto, J.; Martins, A. M.; Silva, A. N.; Pinto, P.; Martins, M.; Fraga, C.; Ribeiro, H. M. Investigations of Olive Oil Industry By-Products Extracts with Potential Skin Benefits in Topical Formulations. Pharmaceutics 2021, 13(4), 465. [Google Scholar] [CrossRef]
- Centrone, M.; D’Agostino, M.; Difonzo, G.; De Bruno, A.; Di Mise, A.; Ranieri, M.; Montemurro, C.; Valenti, G.; Poiana, M.; Caponio, F.; Tamma, G. Antioxidant Efficacy of Olive By-Product Extracts in Human Colon HCT8 Cells. Foods 2020, 10(1), 11. [Google Scholar] [CrossRef]
- El-Saber Batiha, G.; Magdy Beshbishy, A.; El-Mleeh, A.; Abdel-Daim, M. M.; Prasad Devkota, H. Traditional Uses, Bioactive Chemical Constituents, and Pharmacological and Toxicological Activities of Glycyrrhiza Glabra L. (Fabaceae). Biomolecules 2020, 10(3), 352. [Google Scholar] [CrossRef]
- Kwon, Y.-J.; Son, D.-H.; Chung, T.-H.; Lee, Y.-J. A Review of the Pharmacological Efficacy and Safety of Licorice Root from Corroborative Clinical Trial Findings. J Med Food 2020, 23(1), 12–20. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J. J.; Mangos, G.; Williamson, P. M.; Whitworth, J. A. Cortisol and Hypertension. Clin Exp Pharmacol Physiol Suppl 1998, 25, S51–56. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M. M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal Biochem 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Whitening efficacy of a food supplement. ISRCTN Registry. https://doi.org/10.1186/ISRCTN12882379 (accessed on 21 March 2025).
- SCCS Notes of Guidance for the Testing of Cosmetic Ingredients and Their Safety Evaluation—12th Revision—European Commission. Available online: https://health.ec.europa.eu/publications/sccs-notes-guidance-testing-cosmetic-ingredients-and-their-safety-evaluation-12th-revision_en (accessed on 21 March 2025).
- Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products (recast) (Text with EEA Relevance)—EU Law in Force—Publications Office of the EU. EU Law in Force. Available online: https://op.europa.eu/en/web/eu-law-in-force/bibliographic-details/-/elif-publication/4100396c-575c-4150-8d92-e7c23ab1ce97 (accessed on 21 March 2025).
- Demirli, R.; Otto, O.; Viswanathan, R.; Patwardhan, S.; Larkey, J. RBX® Technology Overview https://www.canfieldsci.com/FileLibrary/RBX%20tech%20overview-LoRz1.pdf (accessed on 21 March 2025).
- Meerwaldt, R.; Graaff, R.; Oomen, P. H. N.; Links, T. P.; Jager, J. J.; Alderson, N. L.; Thorpe, S. R.; Baynes, J. W.; Gans, R. O. B.; Smit, A. J. Simple Non-Invasive Assessment of Advanced Glycation Endproduct Accumulation. Diabetologia 2004, 47(7), 1324–1330. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, J.-Q.; Li, L.; Guo, M.; He, Y.; Dong, Y.; Meng, H.; Yi, F. Advanced Glycation End Products in the Skin: Molecular Mechanisms, Methods of Measurement, and Inhibitory Pathways. Front Med (Lausanne) 2022, 9, 837222. [Google Scholar] [CrossRef] [PubMed]
- Marcílio Cândido, T.; Bueno Ariede, M.; Vieira Lima, F.; de Souza Guedes, L.; Robles Velasco, M. V.; Rolim Baby, A.; Rosado, C. Dietary Supplements and the Skin: Focus on Photoprotection and Antioxidant Activity—A Review. Nutrients 2022, 14(6), 1248. [Google Scholar] [CrossRef]
- Januszewski, J.; Forma, A.; Zembala, J.; Flieger, M.; Tyczyńska, M.; Dring, J. C.; Dudek, I.; Świątek, K.; Baj, J. Nutritional Supplements for Skin Health-A Review of What Should Be Chosen and Why. Medicina (Kaunas) 2023, 60(1), 68. [Google Scholar] [CrossRef]
- Yokota, T.; Nishio, H.; Kubota, Y.; Mizoguchi, M. The Inhibitory Effect of Glabridin from Licorice Extracts on Melanogenesis and Inflammation. Pigment Cell Res 1998, 11(6), 355–361. [Google Scholar] [CrossRef]
- Chen, J.; Yu, X.; Huang, Y. Inhibitory Mechanisms of Glabridin on Tyrosinase. Spectrochim Acta A Mol Biomol Spectrosc 2016, 168, 111–117. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).