1. Introduction
Skin aging is a multifactorial process influenced by internal and external factors, causing structural and physiological changes that can lead to loss of skin brightness, uneven complexion tone and skin pigmentation disorders. [
1] Cutaneous hyperpigmentation is a common dermatological condition characterized by localized or diffuse darkening of the skin due to the overproduction of melanin. This condition can result from various factors, including inflammation, sun damage, hormonal imbalances, and certain systemic diseases. Post-inflammatory hyperpigmentation (PIH) is particularly prevalent in individuals with skin of color and represents a therapeutic challenge due to a higher tendency to develop persistent discoloration and adverse reactions to certain depigmenting treatments. [
2,
3] Particularly, managing hyperpigmentation in higher Fitzpatrick phototypes or skin of color (SOC) requires personalized strategies to avoid adverse effects such as secondary hypopigmentation or irritation. [
4,
5]
In this context, current treatments include photoprotection as a core element in dermatological therapy. Broad-spectrum sunscreens should be recommended as they provide protection against both UVA and UVB radiation, mitigating the complete range of solar radiation-induced skin damage. In addition, innovative topical and even oral formulations that include new active ingredients (e.g., antioxidants) improve photoprotection. [
6] In addition to photoprotection, the therapeutic approach to pigmentation disorders includes both topical and systemic options, each with different mechanisms of action and varying degrees of efficacy. Topical agents remain the first-line treatment and encompass tyrosinase inhibitors, antioxidants, and modulators of cellular renewal. Among the most commonly used ingredients are hydroquinone, kojic acid, niacinamide, azelaic acid, arbutin, cysteamine, glutathione, tranexamic acid, retinoids, and corticosteroids. Additionally, natural compounds like Polypodium leucotomos extract (PLE), silymarin, and bakuchiol have shown promise due to their antioxidative and photoprotective properties. Among newer agents, thiamidol has demonstrated superior tyrosinase inhibition with minimal side effects. Combinations of these compounds have demonstrated superior efficacy in reducing skin pigmentation. [
3,
7,
8]
Oral treatments have also gained interest in the dermatological field, offering a complementary strategy for cases of hyperpigmentation refractory to topical treatments. Many of these compounds act by modulating the tyrosinase pathway or exert direct and indirect anti-inflammatory properties. [
9] Available options include antioxidant supplements such as vitamin C, glutathione, and polyphenols, which act by reducing oxidative stress and modulating melanocyte activity. Furthermore, certain oral drugs such as tranexamic acid have shown significant benefits in reducing hyperpigmentation, particularly in melasma. TXA inhibits the plasminogen/plasmin pathway by blocking plasminogen’s lysine-binding sites, reducing plasmin formation. This downregulates inflammatory mediators that promote melanogenesis, thus decreasing melanocyte activity and melanin production. TXA also lowers VEGF expression and may disrupt keratinocyte–melanocyte interactions, contributing to its depigmenting effects. [
10] Additionally, natural compounds like PLE, silymarin, and phlorotannins offer photoprotective and antioxidative benefits, complementing existing therapies. While these treatments provide effective options, patient-specific factors such as skin type, severity, and risk of recurrence must be considered to optimize therapeutic outcomes and minimize potential side effects. [
3,
7,
9] In this context, Juhasz et al. published a review on systemic treatments for pigmentary conditions concluding that carotenoids, glutathione, melatonin, PLE, procyanidins, and tranexamic acid (TA) have shown varying degrees of efficacy in reducing pigmentation through antioxidant effects, tyrosinase inhibition, and photoprotection. [
2] Oral glutathione and carotenoids provide photoprotective benefits, while TA and procyanidins have demonstrated effectiveness in melasma management. Though generally safe, further research is needed to determine long-term efficacy, recurrence rates, and standardized treatment protocols. Dermatologists should be well-informed to provide appropriate patient counseling and ensure optimal therapeutic outcomes. [
2]
Despite advances in hyperpigmentation treatment, significant challenges remain, especially in populations with skin of color, where recurrence rates and adverse effects may be higher. Continued research is essential for developing new therapeutic strategies that are both effective and safe for these populations. [
2]
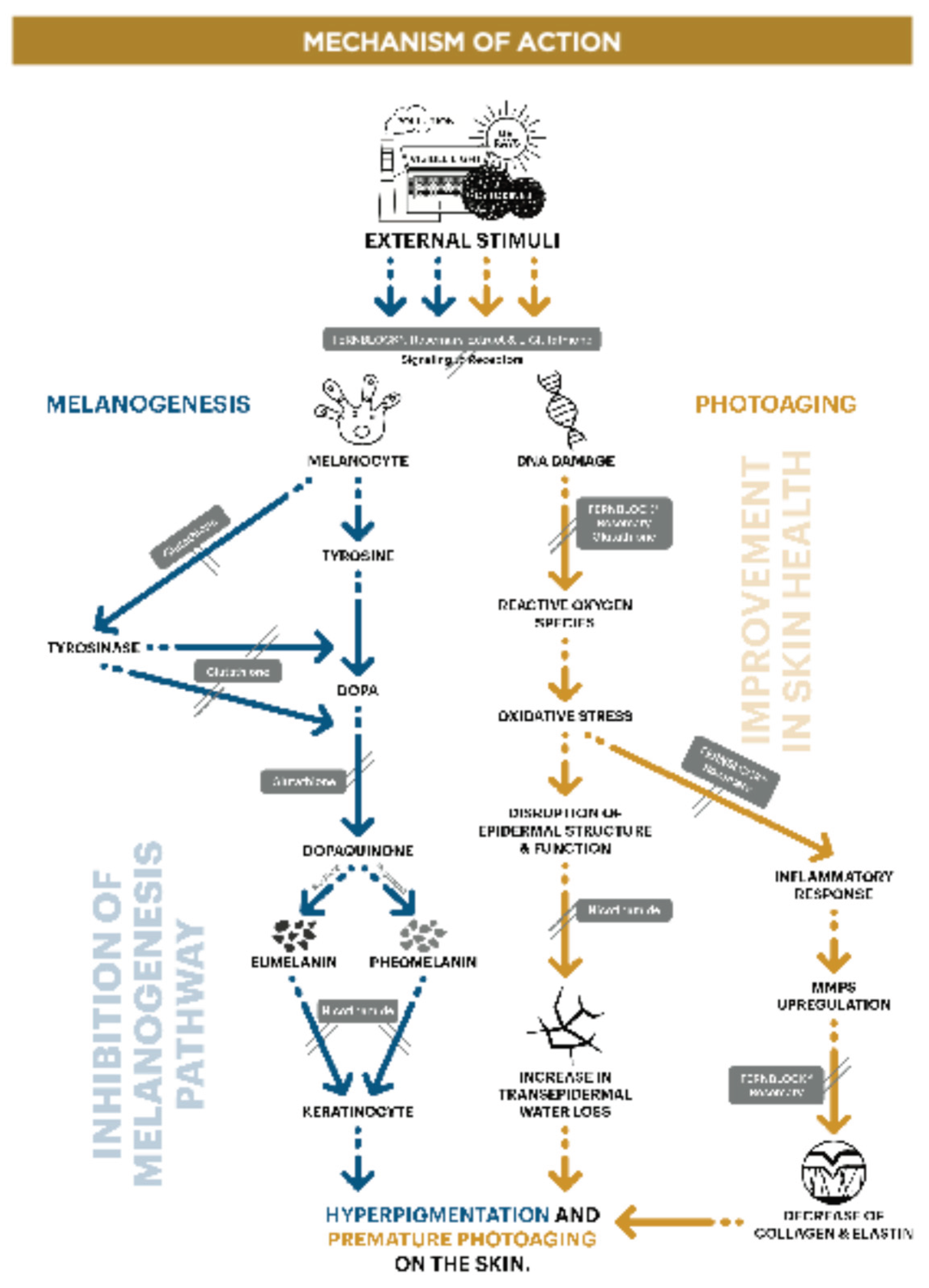
The purpose of this work was to evaluate the efficacy of the combination of different active ingredients, (that had previously shown to have benefits in different alterations observed in skin aging, improving hyperpigmentation and skin appearance) vs placebo. Specifically, the study product Heliocare
® Luminance capsules, are an oral supplement for photoprotection, formulated to reduce oxidative stress and decrease UV-triggered pigmentation. The capsules contain Fernblock
® 240 mg, Niacinamide 8 mg, Glutathione 125mg y Rosemary extract 20 mg. These are key ingredients that focus on the entire melanogenesis pathway, working internally to target deeper hyperpigmentation that topical treatments may not effectively reach. In addition, the actives can also work improving photoaging. (Graph 1) [
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28].
In this manuscript, we will explore the various therapeutic approaches available for managing hyperpigmentation in Asian skin and we evaluate their efficacy, safety, and clinical applicability.
Graph 1.
Mechanisms of action of the various active ingredients in the formulation under investigation, highlighting their effects on melanogenesis and photoaging pathways.
Graph 1.
Mechanisms of action of the various active ingredients in the formulation under investigation, highlighting their effects on melanogenesis and photoaging pathways.
2. Materials and Methods
2.1. Aim
The purpose of this study was to assess the efficacy of Heliocare® Luminance to prevent hyperpigmentation and skin damages induced by solar radiation and to improve area, length and depth of the crow’s feet, skin firmness and elasticity, skin barrier function and skin hydration in female subjects.
2.2. Study Product
The study product combined PLE, L-Glutathione reduced, Maltodextrine, Rosemary extract, Niacinamide and Magnesium stearate.
2.3. Study Population
40 female subjects with spots, uneven complexion tone and aging signs were included according to the inclusion criteria. Subjects who voluntarily signed the written consent form were enrolled. Inclusion criteria included healthy female adults aged 25-60, with skin blotches and uneven skin tone who do not have acute or chronic diseases including skin diseases. Exclusion criteria included pregnancy or plans to become pregnant within 6 months, breastfeeding women, infectious skin diseases or having used topical steroids or dermatological procedures within the last 6 months. In addition, subjects with food allergies or anaphylaxis history were excluded.
2.4. Study Design
A randomized and placebo-controlled double-blind study was designed. Subjects were randomly assigned to active group (26 subjects) or Placebo group (14 subjects) and were provided with the test product to take 2 capsules a day for 12 weeks, combined with current sunscreen. Participants were allowed to maintain their skincare regimen, consisting of facial cleansing in the morning and evening, and the use of photoprotection in the morning. Eighty percent used a sunscreen with SPF 50+ (Randomization plans for assigning subjects and test site were generated by randomization plan generator (
http://randomization.com). Placebo capsules contained corn starch and maltodextrins.
2.5. Evaluations
Assessment of pigmentation intensity, skin brightness, skin elasticity and firmness, deep wrinkles, skin hydration and transepidermal water loss (TEWL) were performed at Week 0, Week 4, Week 8 and Week 12. Subject questionnaire about efficacy of the test product and skin safety assessment were conducted at Week 4, Week 8 and Week 12. (
Table 1) To assess these different variables, the following instrumental evaluations were performed (
Table 2).
Deep wrinkles were assessed with the skin replica made of Silflo
® that were illuminated in the Visioline
® with the light at an angle of 35°, and the mountains in the replica representing the wrinkles of the skin produce measurable shadows. Area, length and depth of wrinkles can be calculated in numbers. (
Table 3)
The demographic information and general skin characteristics are investigated by questionnaire at the first visit. Subjects answered the questionnaire consisting of questions about the efficacy of the test product and product satisfaction.
Adverse reactions during the study period are assessed through interview and observation by investigator under the supervision of a dermatologist. They were subdivided in objective reactions (erythema, edema, scaling, other) and subjective reactions (itching, poking, burning, stinging, stiffness, other) and classified as follows: 0: none, 1: mild, 2 severe.
Among the subjects who completed the study, only subjects who satisfied the compliance criteria of 80% were included in the data analysis.
2.6. Statistical Analysis
The Shapiro-Wilk test assessed normality. Parametric tests were used if p > 0.05, otherwise, non-parametric tests were applied. Triplicate measurements of pigmentation, brightness, elasticity, TEWL, and hydration were averaged for analysis. Randomization validity was tested by comparing baseline age and measurements using t-tests (parametric) or Mann-Whitney U (non-parametric) when p > 0.1. Post-treatment changes were analyzed using paired t-tests or Wilcoxon signed-rank tests, depending on normality. Inter-group comparisons used independent t-tests or Mann-Whitney U, with significance at p < 0.05. Analyses were performed using SPSS 29.0 (IBM SPSS Statistics, USA).
4. Discussion
There is no simple treatment routine for managing the skin changes induced by solar radiation. Beyond topical treatments, the concomitant use of systemic active agents for the management of pigmentation disorders is increasingly gaining recognition and scientific support. [
2,
9]
The rationale for combining both topical and oral photoprotection lies in their complementary modes of action. Topical sunscreens offer immediate surface-level defense, while oral supplements provide internal support, enhancing the skin’s overall resilience to UV damage. This combined strategy not only overcomes the limitations of relying solely on sunscreen but also delivers broader protection against the full spectrum of solar radiation. [
29]
Among all the depigmenting active ingredients, not all are suitable for the formulation of oral photoprotection. Given that one of the most commonly known oral agents, such as tranexamic acid, raise concerns due to their potential prothrombotic effects, [
9,
30,
31] the introduction of alternative products that combine effective actives targeting the tyrosinase pathway or having direct or indirect anti-inflammatory effect, with a favorable safety profile, may represent a valuable approach for managing pigmentation and other skin changes induced by solar exposure. [
9] The combination of the active ingredients of the study product Heliocare
® Luminance (PLE, Glutathione, Niacinamide, rosemary extract and magnesium stearate) has demonstrated, in the present study, improvements not only in pigmentation-related parameters but also in other aspects associated with skin aging and skin health, such as firmness, elasticity, wrinkles and the reduction of transepidermal water loss.
PLE (Fernblock
®), a tropical fern extract, has demonstrated significant antioxidant and photoprotective properties, making it a promising adjunct treatment for pigmentary disorders such as vitiligo, melasma, and post-inflammatory hyperpigmentation (PIH). PLE contains multiple bioactive compounds that neutralize reactive oxygen species, reduce UV-induced damage, and modulate inflammatory pathways. [
11] Clinical studies, including randomized, double-blind, placebo-controlled trials, have shown that oral administration of PLE significantly improves melasma severity, particularly when combined with sunscreen and standard topical therapies. [
12] PLE has exhibited an exceptional safety profile, with no significant adverse effects reported in clinical trials. Given its multiple protective mechanisms, PLE appears to be a valuable systemic option for managing pigmentary disorders. [
11] PLE has shown to regulate opsin-3 and prevent photooxidation of melanin precursors on skin cells exposed to blue light emitted by digital devices. [
13] In addition, in a randomized, double-blind, placebo-controlled trial, 40 Asian patients received either oral PLE or a placebo for 12 weeks while continuing treatment with 4% hydroquinone and SPF 50+ sunscreen. Results showed a statistically significant improvement in mMASI scores in the PLE group compared to the placebo group, with differences observed as early as week 8 (p≤0.05) and further enhanced at week 12. PLE also improved MelasQoL scores, suggesting a beneficial effect on patients’ quality of life. No significant side effects were reported, reinforcing the safety of oral PLE as a treatment. [
14] In summary, PLE has shown to protect against melanogenesis and aging caused by exposure to UVB-UVA-VL (including blue light)-IR.
Glutathione has gained popularity as a systemic skin-lightening agent, particularly in populations with darker skin tones, despite limited clinical evidence supporting its efficacy. Glutathione is one of the most powerful antioxidants produced by cells in the human body and it is involved in many important biological processes (DNA & protein synthesis, cell protection through the metabolism of xenobiotics and carcinogens, protection against intracellular free radicals & ROS). [
15] The anti-melanogenic effects of glutathione are attributed to its ability to inhibit tyrosinase activity, shift melanin production from eumelanin to pheomelanin, and neutralize oxidative stress. It also interferes with the cellular transfer of tyrosinase to premelanosomes. However, studies assessing its effectiveness and safety remain scarce. Clinical trials evaluating oral and topical glutathione formulations have demonstrated mild but reversible depigmenting effects with a good safety profile, whereas intravenous glutathione has been associated with serious adverse effects, including liver dysfunction, renal impairment, and potential neurotoxicity. [
16,
17] In a study of 47 people diagnosed with melasma, plasma glutathione level was shown to have a strong negative correlation with MASI score. [
18,
19] Other study controlled with placebo in 60 healthy medical students, taking 500mg of glutathione/ day vs placebo for 4 weeks showed a significant decrease in melanin index at all sites vs placebo. [
20] Another study performed in 30 Filipino female subjects who took 500mg of glutathione daily for 8 weeks showed a significant decrease in melanin index from T0 to T8, with evident improvements visible at just 2 weeks. [
21] Oral glutathione is generally well-tolerated, with very few side effects. [
22]
Nicotinamide (niacinamide), a derivative of vitamin B3, has emerged as a multifunctional cosmeceutical agent with demonstrated efficacy in skin aging and pigmentation control. It plays a crucial role in NAD+ metabolism, supporting cellular energy homeostasis, DNA repair, and antioxidative defense mechanisms. Nicotinamide has been shown to enhance the skin barrier function, improve hydration, and reduce oxidative stress-induced damage. Additionally, it exhibits anti-inflammatory properties by inhibiting pro-inflammatory cytokines, thereby reducing erythema, hyperpigmentation, and photoaging-related skin deterioration. Its depigmenting effects are attributed to the inhibition of melanosome transfer from melanocytes to keratinocytes, making it a promising treatment for melasma and post-inflammatory hyperpigmentation. There is growing interest in its oral administration. Preliminary findings suggest that systemic niacinamide may reduce melanosome transfer and mitigate the inflammatory processes that contribute to uneven skin pigmentation. [
23]
The extract of Rosmarinus officinalis (rosemary extract) is a natural reservoir of bioactive molecules with notable antioxidant activity, rich in polyphenols, especially rosmarinic acid, and diterpenes. Among these compounds, rosmarinic acid is highly regarded for its strong antioxidant, anti-inflammatory, antimicrobial, and neuroprotective effects, establishing it as a key element in both traditional remedies and contemporary medical research. [
24] Hoskin et al. published ex-vivo data demonstrating the ability of rosemary extract prevent an inflammatory phenomenon in cutaneous tissue limited the loss of filaggrin, suggesting their role to prevent pollution-induced skin aging/damage. [
25] Park et al. showed the protection against UV-induced photoaging, through the downregulation of MMP-1, 3, 9) and pollution-induced skin aging. [
26]
Other authors claimed also about the antiaging properties of rosmery extract due to anti-glycation activity leading to anti-elastase, anti-tyrosinase, anti-collagenase properties. [
27,
28]
We hypothesize that the combination of the different mechanisms of action discussed in the introduction attributed to each of these active compounds, may contribute in a synergistic manner to the positive outcomes observed in this study, according to published data regarding the benefits of active combination. [
3,
7,
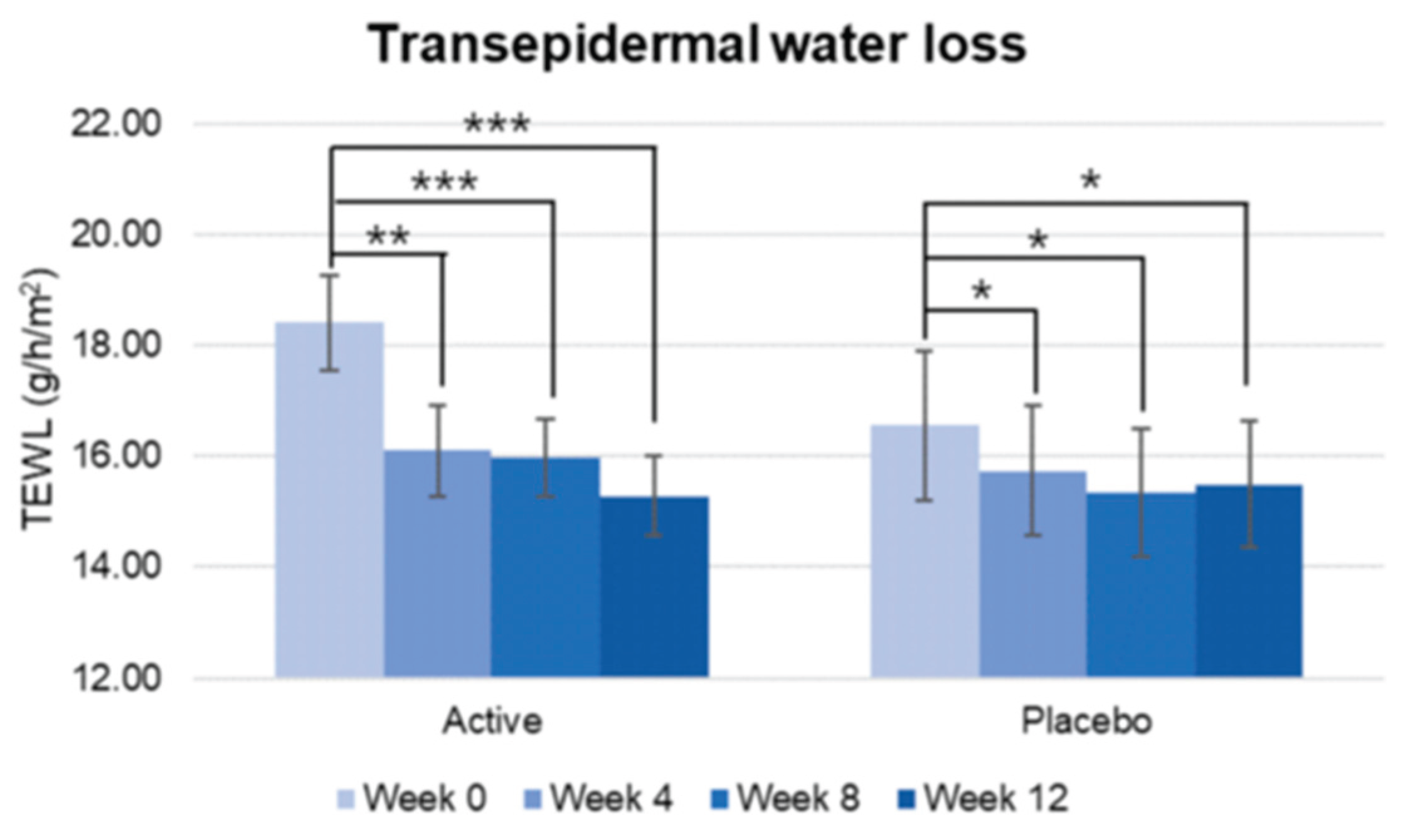
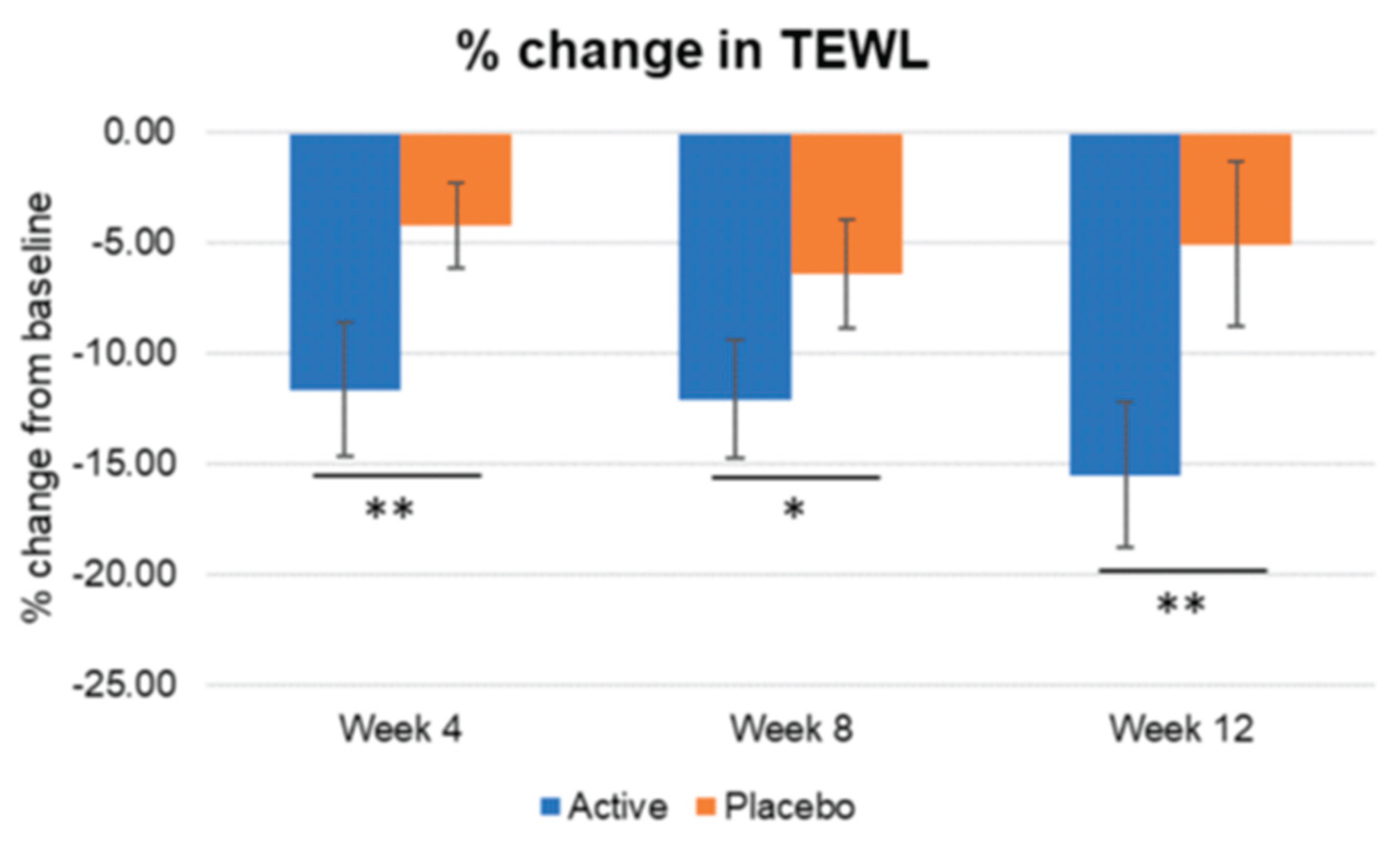
8] In our study, this combination of actives provided a significantly greater pigmentation decreased and higher skin brightness increase since week 4, compared with placebo group. Skin elasticity and firmness also improved along the study with significant improvement being observed since week 4. The firmness statistically increased reaching statistical differences vs placebo since week 8. As for elasticity (R2) the significant difference vs placebo was found as fast as week 4. Total wrinkle area, total length, and mean wrinkle depth also showed a significant improvement since week 4 and was maintained until the end of the study and with significant differences vs placebo for wrinkle area and mean depth as fast as week 4 and in wrinkle length since week 8. TEWL decrease significantly more in the active treated group since week 4 reflecting that Heliocare
® Luminance significantly improves the skin barrier function.
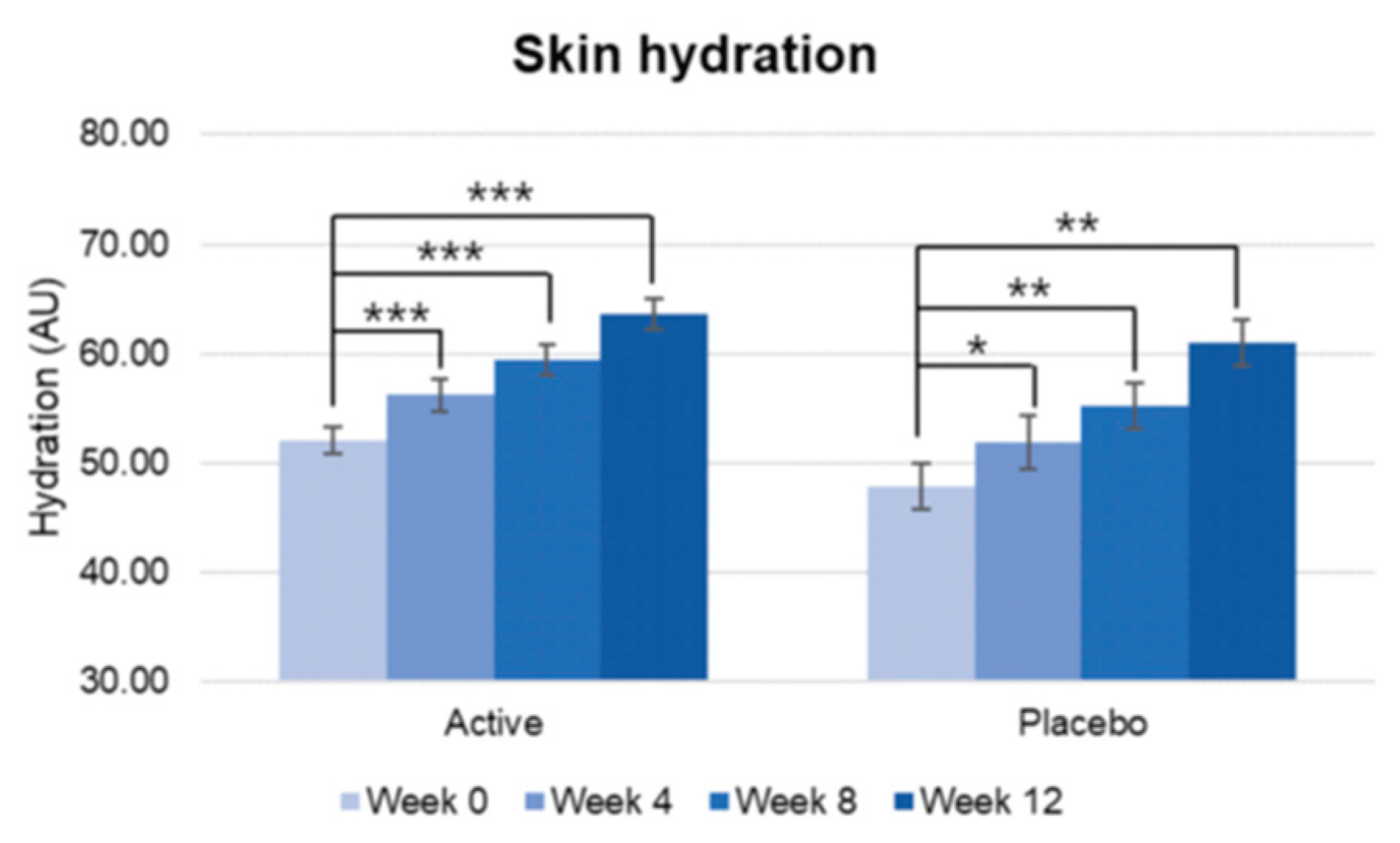
Skin hydration increased over the study period in both Active and Placebo group, and no statistically significant difference was observed in the change rate from baseline between groups. Therefore, it was difficult to determine that the changes in skin hydration were solely due to the test product. However, the percentage of subjects who perceived that their skin condition improved was significantly higher in the active group over study period.
During the study period, no adverse reactions were observed or reported confirming the safety of the product.
Limitations of the study were small sample size. Although no adverse reactions were reported during the study, long-term safety data are lacking.
Further clinical studies are needed to establish standardized protocols and long-term efficacy and safety for systemic depigmenting therapies.
Conclusions: This clinical study reflects the efficacy and safety of the combination of specific oral actives (Heliocare® Luminance) to improve skin hyperpigmentation and brightness. In addition, an improvement in skin barrier function was also shown. Moreover, it also improved skin aging by enhancing wrinkles, skin elasticity and firmness. These changes justify the better subjective outcome evaluated by patients.
Figure 1.
Pre-post comparison of skin Hydration, showing significant improvement since week 4, p<0.001.
Figure 1.
Pre-post comparison of skin Hydration, showing significant improvement since week 4, p<0.001.
Figure 2.
Pre-post comparison of TEWL. Week 0 vs. Week 4, Week 8, Week 12, Significant decrease was found since week 4 in active group. Although the decrease reached lower percentages in the placebo group, it also had significant improvement since week 4. Wilcoxon signed-rank test, Significance: *p<0.05, **p<0.01, ***p<0.001, Bar: S.E.
Figure 2.
Pre-post comparison of TEWL. Week 0 vs. Week 4, Week 8, Week 12, Significant decrease was found since week 4 in active group. Although the decrease reached lower percentages in the placebo group, it also had significant improvement since week 4. Wilcoxon signed-rank test, Significance: *p<0.05, **p<0.01, ***p<0.001, Bar: S.E.
Figure 3.
Inter-comparison of TEWL. Active group vs. Placebo group, Mann-Whitney U test, Significance: *p<0.05, **p<0.01, Bar: S.E. A significantly greater decrease in TEWL was found in the active treated group vs placebo since week4.
Figure 3.
Inter-comparison of TEWL. Active group vs. Placebo group, Mann-Whitney U test, Significance: *p<0.05, **p<0.01, Bar: S.E. A significantly greater decrease in TEWL was found in the active treated group vs placebo since week4.
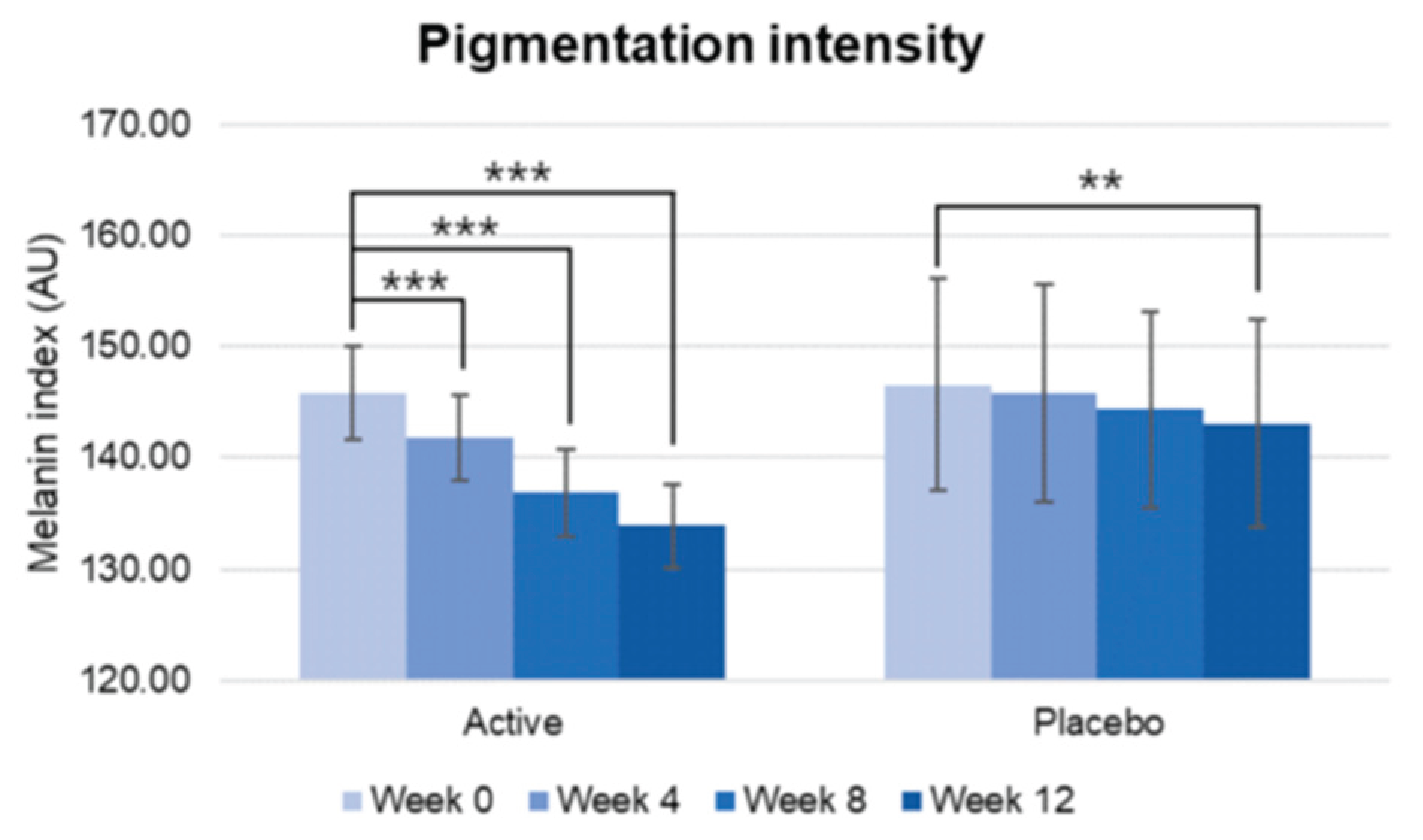
Figure 4.
Pre-post comparison of pigmentation intensity. Week 0 vs. Week 4, Week 8, Week 12, Wilcoxon signed-rank test, Significance: **p<0.01, ***p<0.001, Bar: S.E.
Figure 4.
Pre-post comparison of pigmentation intensity. Week 0 vs. Week 4, Week 8, Week 12, Wilcoxon signed-rank test, Significance: **p<0.01, ***p<0.001, Bar: S.E.
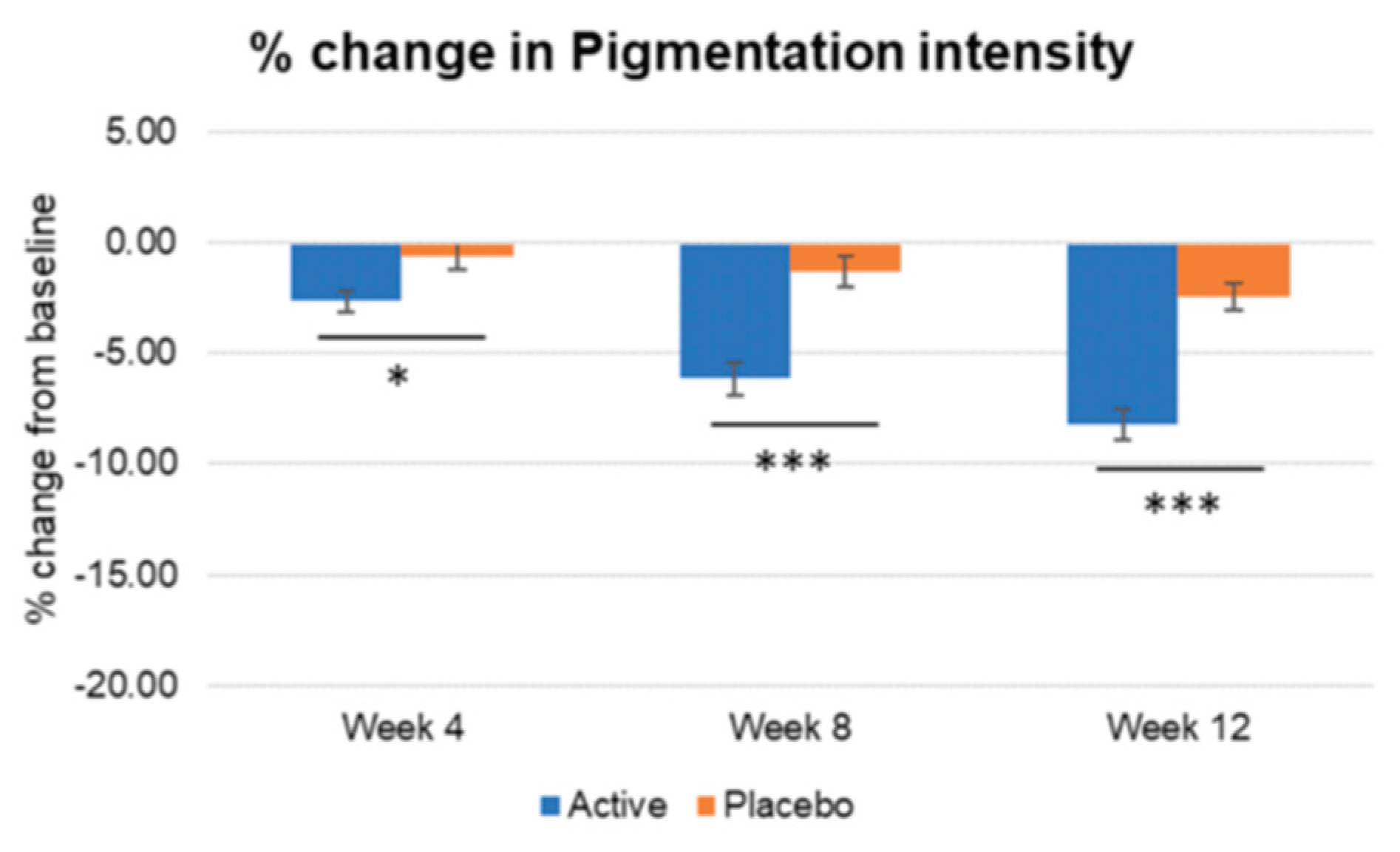
Figure 5.
Inter-group comparison of pigmentation intensity. Active group vs. Placebo group, Mann-Whitney U test, Significance: *p<0.05, ***p<0.001, Bar: S.E.
Figure 5.
Inter-group comparison of pigmentation intensity. Active group vs. Placebo group, Mann-Whitney U test, Significance: *p<0.05, ***p<0.001, Bar: S.E.
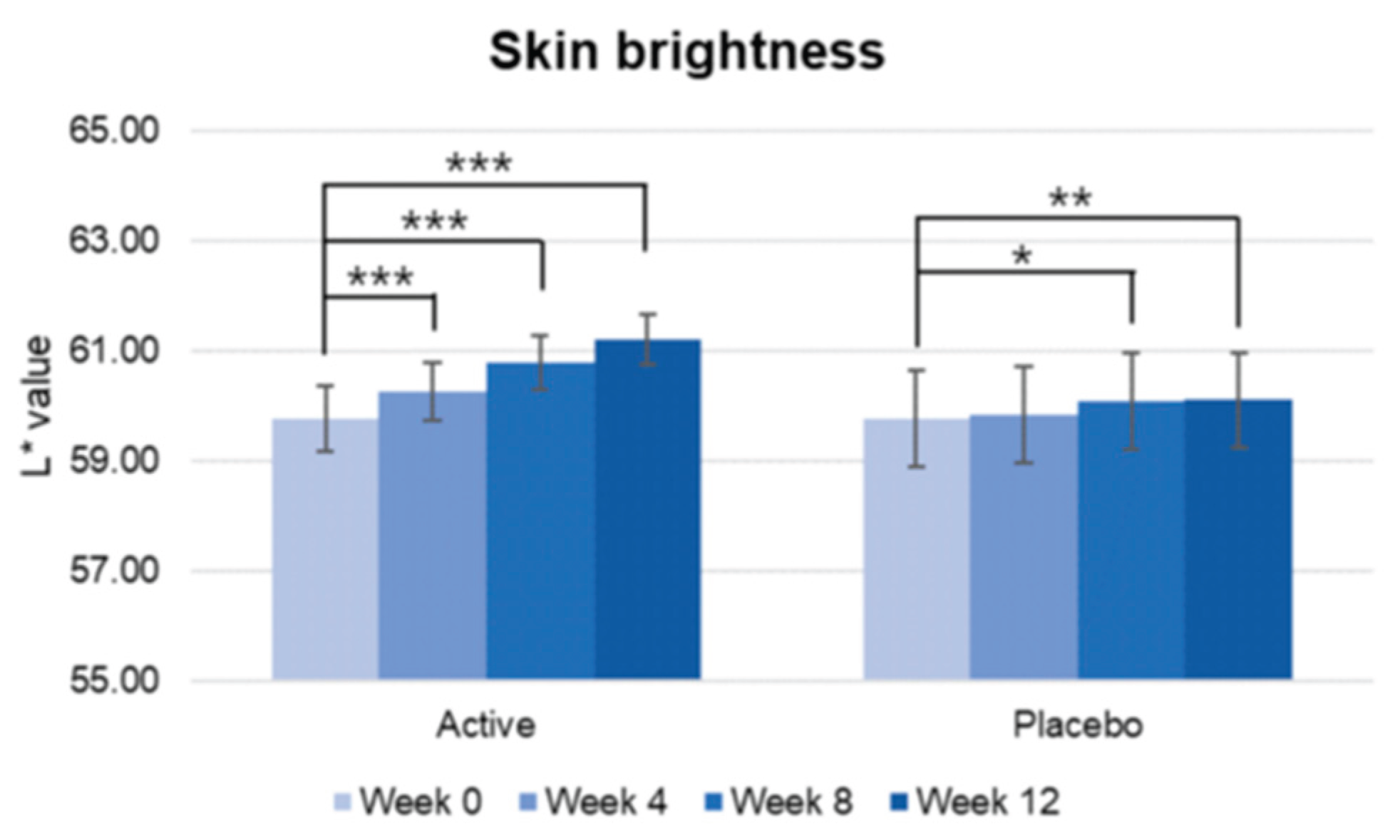
Figure 6.
Pre-post comparison of skin brightness. Week 0 vs. Week 4, Week 8, Week 12, Wilcoxon signed-rank test, Significance: *p<0.05, **p<0.01, ***p<0.001, Bar: S.E.
Figure 6.
Pre-post comparison of skin brightness. Week 0 vs. Week 4, Week 8, Week 12, Wilcoxon signed-rank test, Significance: *p<0.05, **p<0.01, ***p<0.001, Bar: S.E.
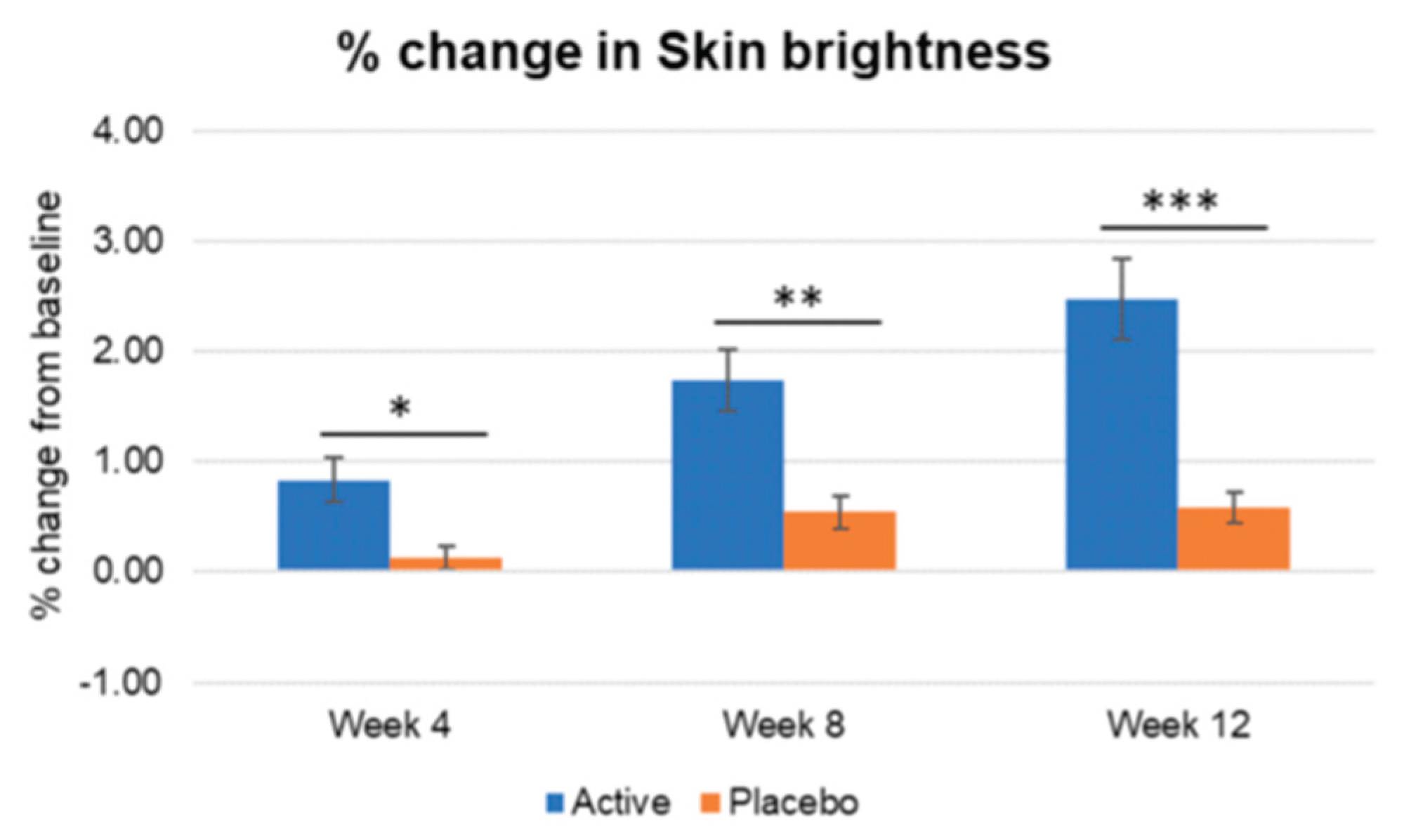
Figure 7.
Inter-group comparison of skin brightness. Active group vs. Placebo group, Mann-Whitney U test, Significance: *p<0.05, **p<0.01, ***p<0.001, Bar: S.E. A significantly higher increase in skin brightness is shown in the active treated group.
Figure 7.
Inter-group comparison of skin brightness. Active group vs. Placebo group, Mann-Whitney U test, Significance: *p<0.05, **p<0.01, ***p<0.001, Bar: S.E. A significantly higher increase in skin brightness is shown in the active treated group.
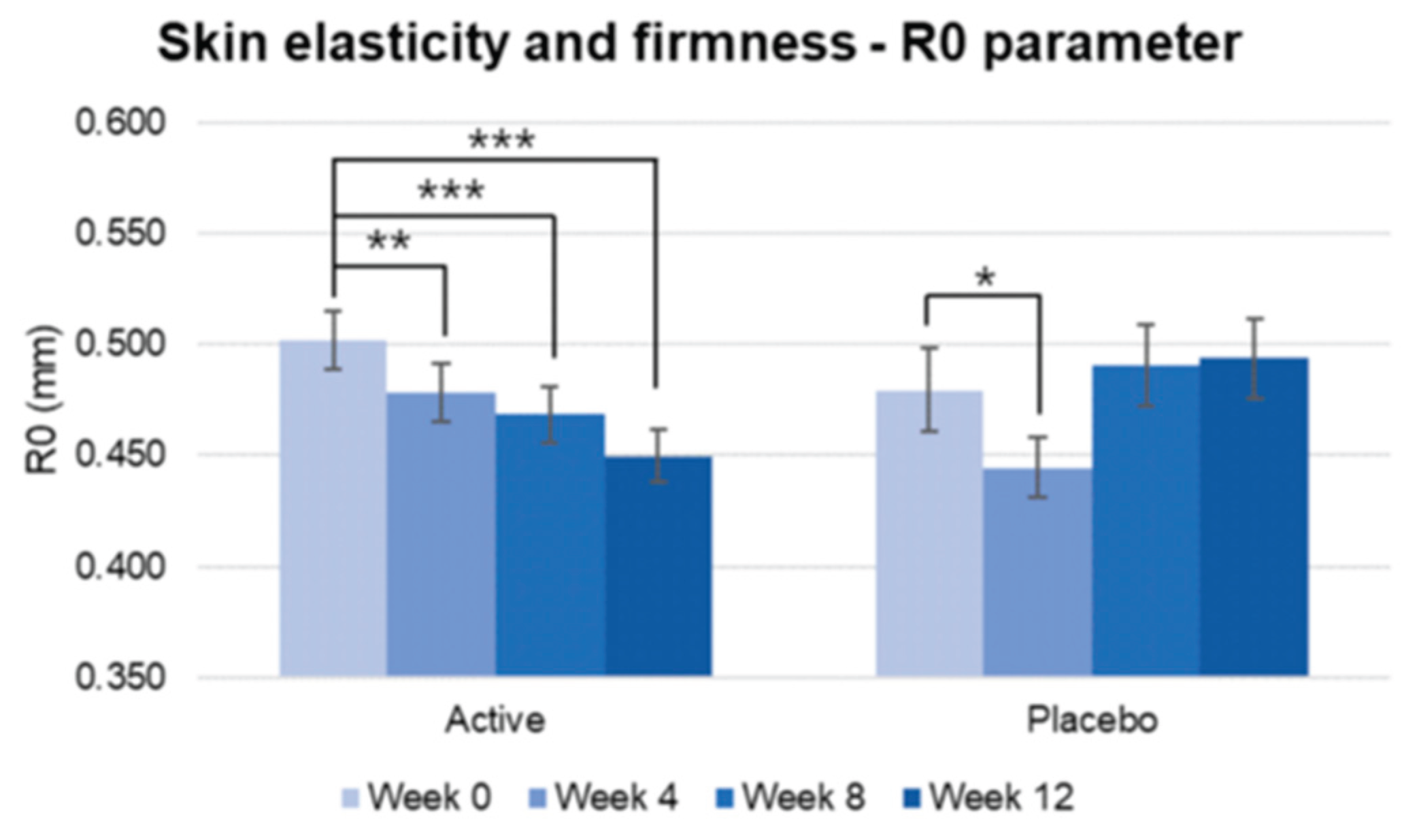
Figure 8.
Pre-post comparison of R0 parameter regarding firmness. A lower Ro score correlates with greater skin firmness. Week 0 vs. Week 4, Week 8, Week 12, paired t-test, Significance: *p<0.05, **p<0.01, ***p<0.001, Bar: S.E. A significantly higher increase in skin firmness is shown in the active treated group.
Figure 8.
Pre-post comparison of R0 parameter regarding firmness. A lower Ro score correlates with greater skin firmness. Week 0 vs. Week 4, Week 8, Week 12, paired t-test, Significance: *p<0.05, **p<0.01, ***p<0.001, Bar: S.E. A significantly higher increase in skin firmness is shown in the active treated group.
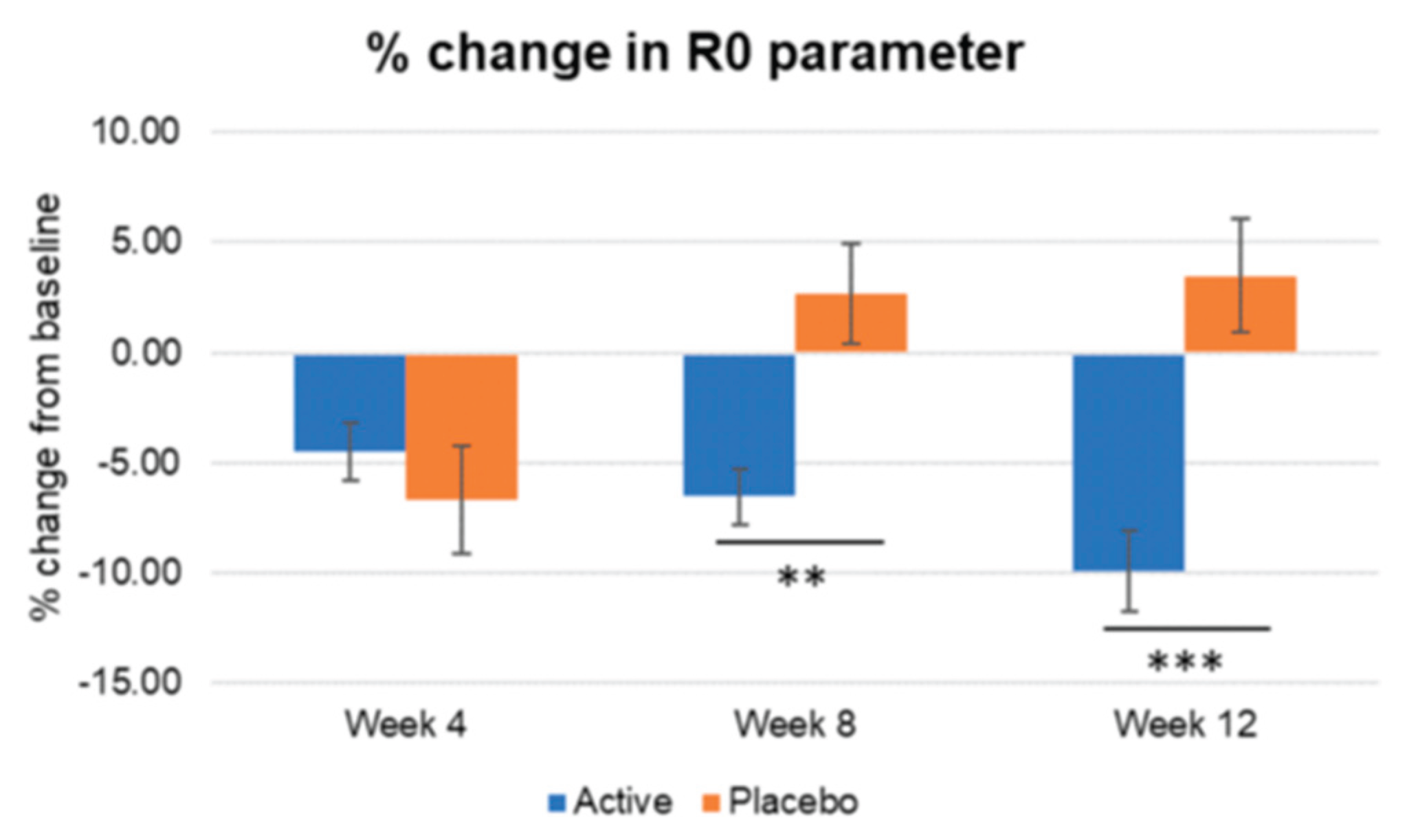
Figure 9.
Inter-group comparison of R0 parameter regarding firmness. Active group vs. Placebo group, Mann-Whitney U test, Significance: **p<0.01, ***p<0.001, Bar: S.E. A significantly lower punctuation in R0 parameter reflecting a higher increase in skin firmness is shown in the active treated group.
Figure 9.
Inter-group comparison of R0 parameter regarding firmness. Active group vs. Placebo group, Mann-Whitney U test, Significance: **p<0.01, ***p<0.001, Bar: S.E. A significantly lower punctuation in R0 parameter reflecting a higher increase in skin firmness is shown in the active treated group.
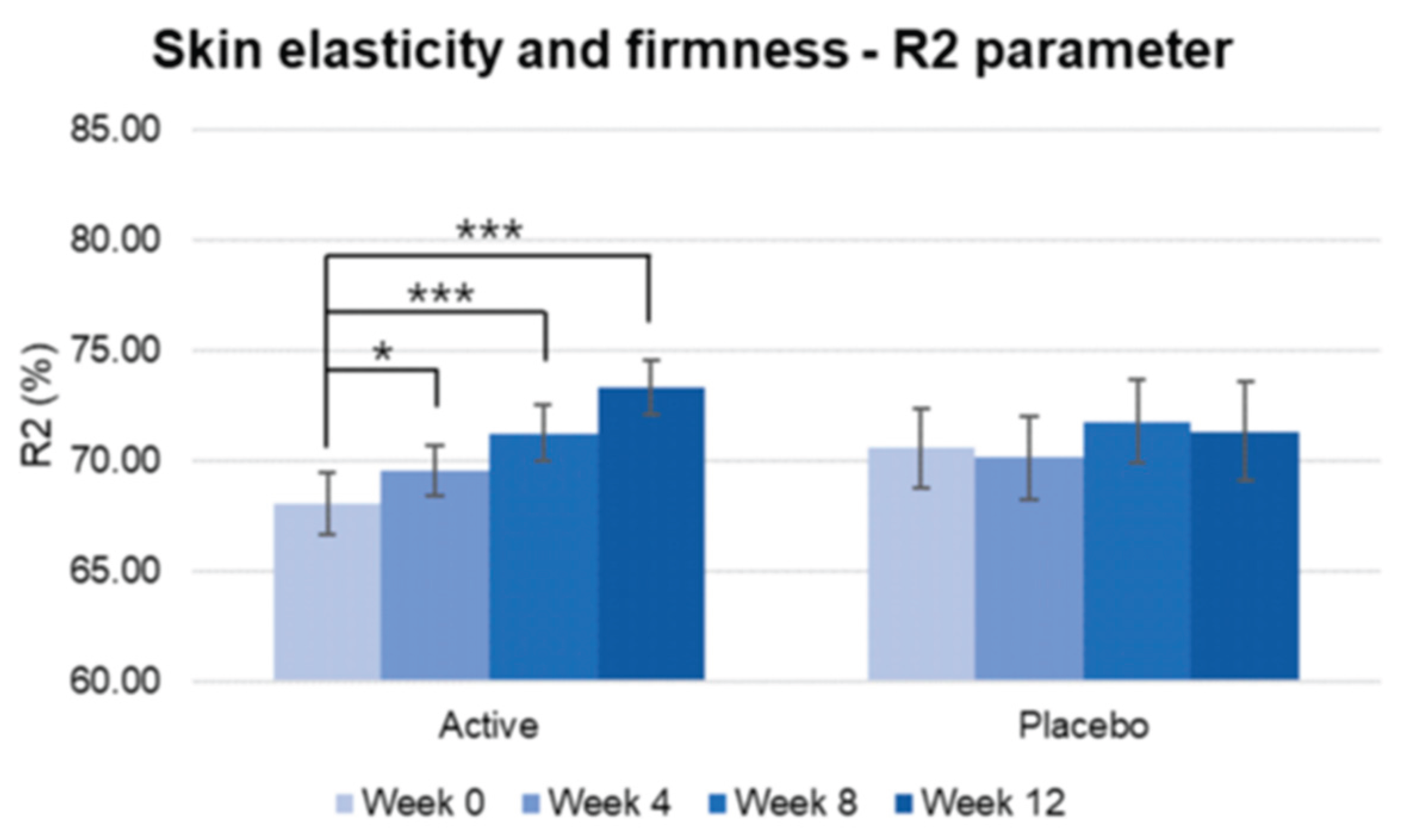
Figure 10.
Pre-post comparison of R2 parameter. Week 0 vs. Week 4, Week 8, Week 12, Wilcoxon signed-rank test, Significance: *p<0.05, ***p<0.001, Bar: S.E. A significantly higher increase in skin elasticity is shown in the active treated group.
Figure 10.
Pre-post comparison of R2 parameter. Week 0 vs. Week 4, Week 8, Week 12, Wilcoxon signed-rank test, Significance: *p<0.05, ***p<0.001, Bar: S.E. A significantly higher increase in skin elasticity is shown in the active treated group.
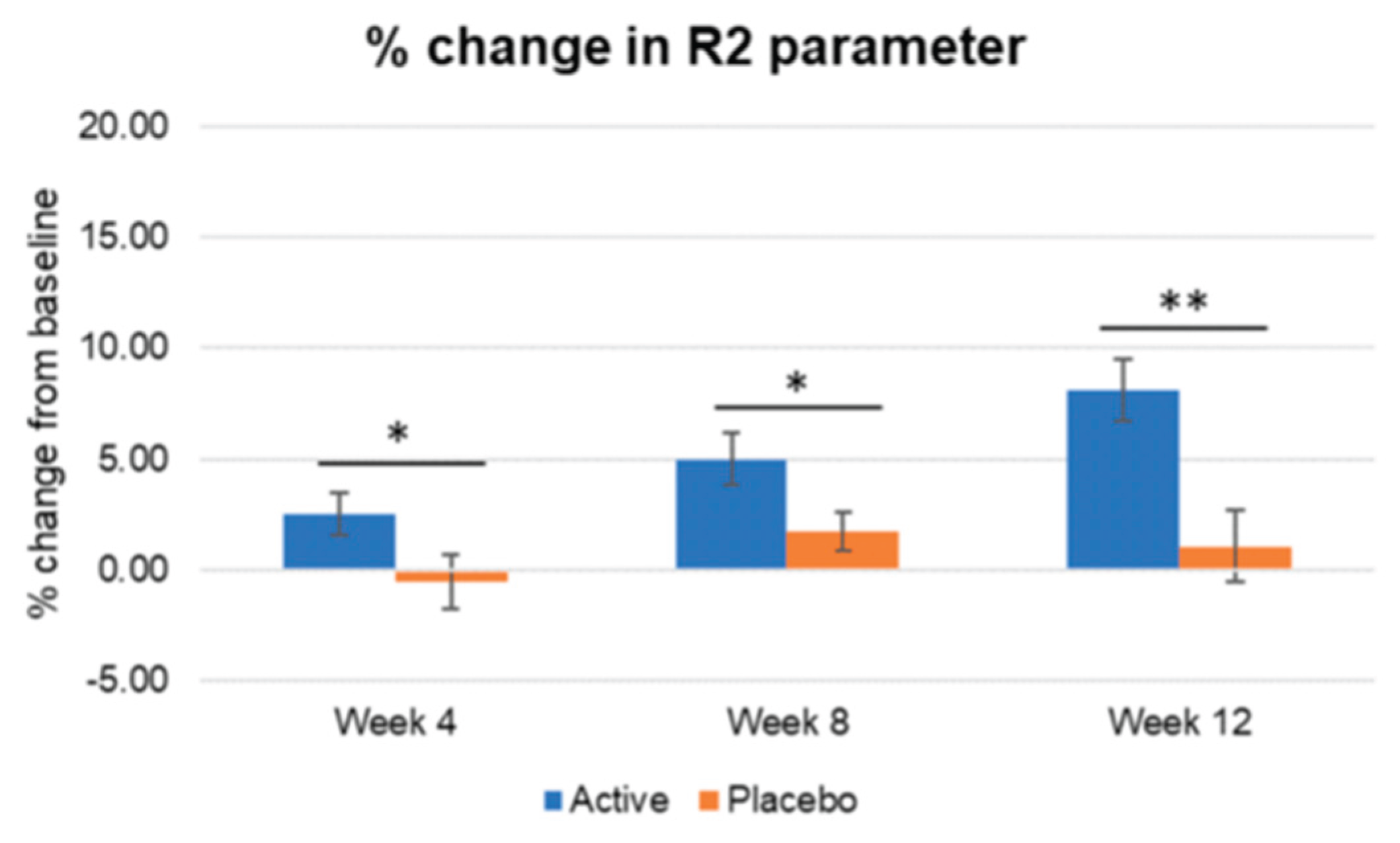
Figure 11.
Inter-group comparison of R2 parameter. Active group vs. Placebo group, Mann-Whitney U test, Significance: *p<0.05, **p<0.01, Bar: S.E. A significantly higher increase in skin elasticity is shown in the active treated group compared to placebo since week 4.
Figure 11.
Inter-group comparison of R2 parameter. Active group vs. Placebo group, Mann-Whitney U test, Significance: *p<0.05, **p<0.01, Bar: S.E. A significantly higher increase in skin elasticity is shown in the active treated group compared to placebo since week 4.
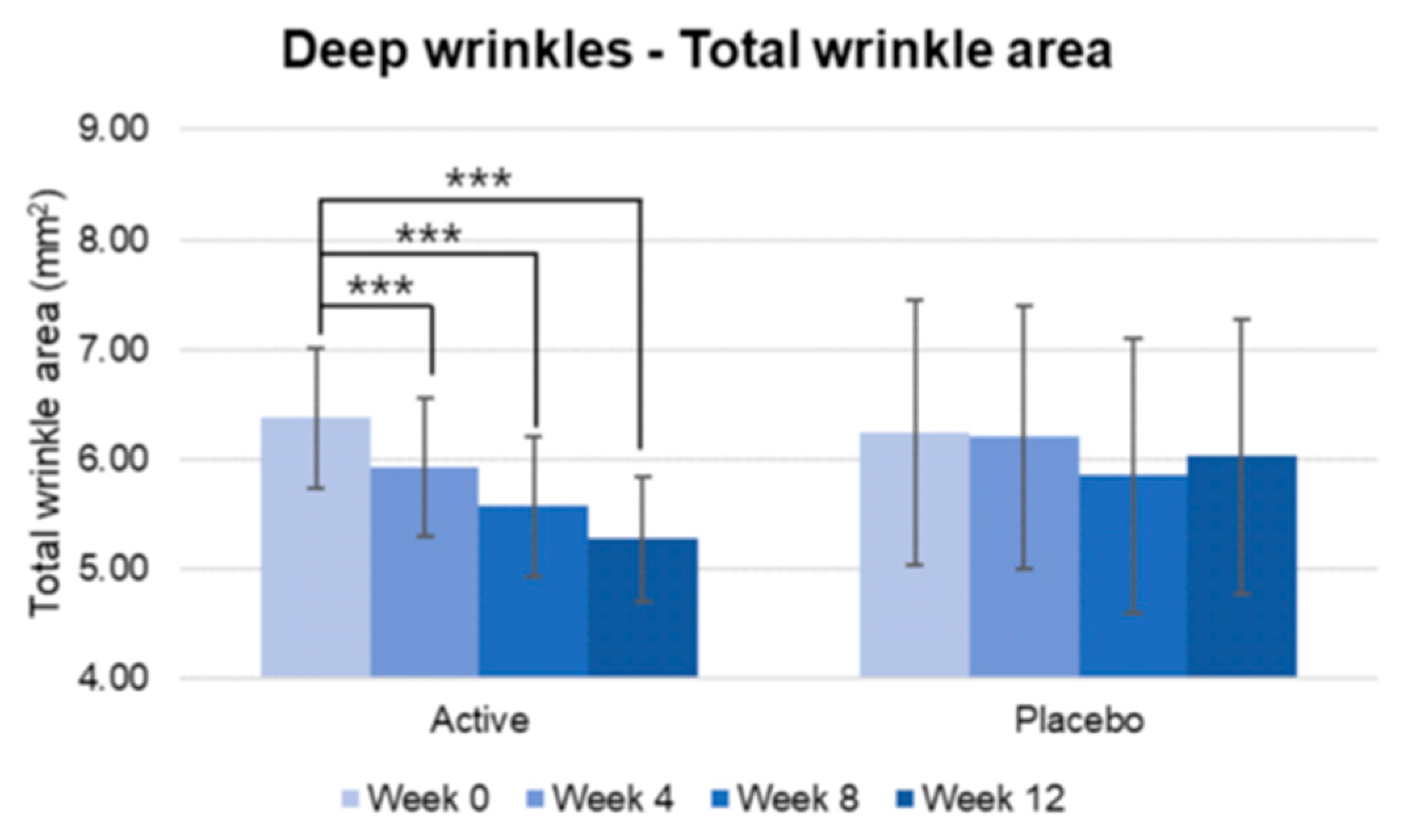
Figure 12.
Pre-post comparison of total wrinkle area. Week 0 vs. Week 4, Week 8, Week 12, Wilcoxon signed-rank test, Significance: ***p<0.001, Bar: S.E. A significantly higher decrease in wrinkle area was shown in the active group.
Figure 12.
Pre-post comparison of total wrinkle area. Week 0 vs. Week 4, Week 8, Week 12, Wilcoxon signed-rank test, Significance: ***p<0.001, Bar: S.E. A significantly higher decrease in wrinkle area was shown in the active group.
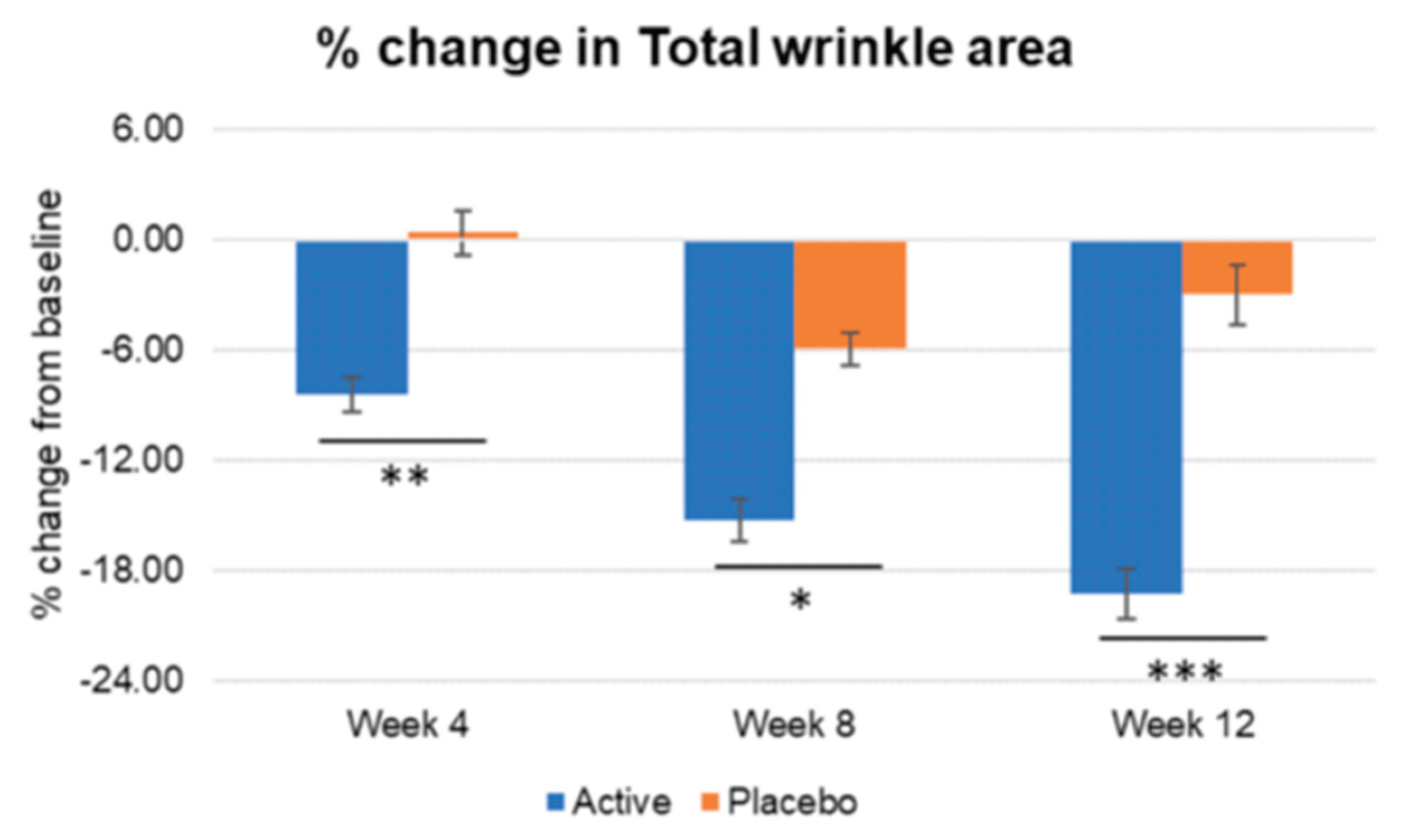
Figure 13.
Inter-group comparison of total wrinkle area. Active group vs. Placebo group, Mann-Whitney U test, Significance: *p<0.05, **p<0.01, ***p<0.001, Bar: S.E. Significantly higher percentage of wrinkle area reduction was detected in the active group vs placebo, since week 4.
Figure 13.
Inter-group comparison of total wrinkle area. Active group vs. Placebo group, Mann-Whitney U test, Significance: *p<0.05, **p<0.01, ***p<0.001, Bar: S.E. Significantly higher percentage of wrinkle area reduction was detected in the active group vs placebo, since week 4.
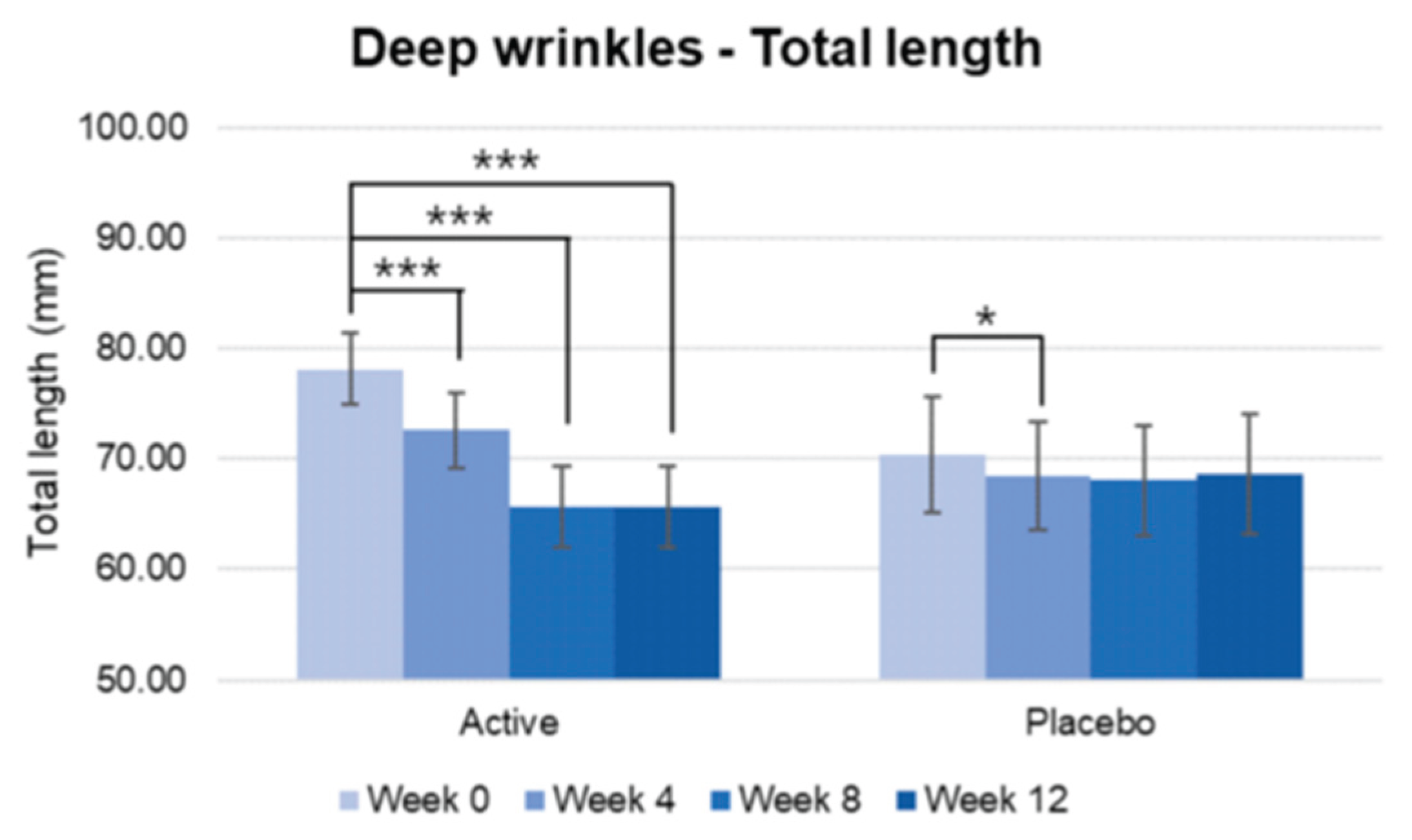
Figure 14.
Pre-post comparison of total length. Week 0 vs. Week 4, Week 8, Week 12, paired t-test, Significance: *p<0.05, ***p<0.001, Bar: S.E. A significantly higher decrease in wrinkle length was shown in the active group.
Figure 14.
Pre-post comparison of total length. Week 0 vs. Week 4, Week 8, Week 12, paired t-test, Significance: *p<0.05, ***p<0.001, Bar: S.E. A significantly higher decrease in wrinkle length was shown in the active group.
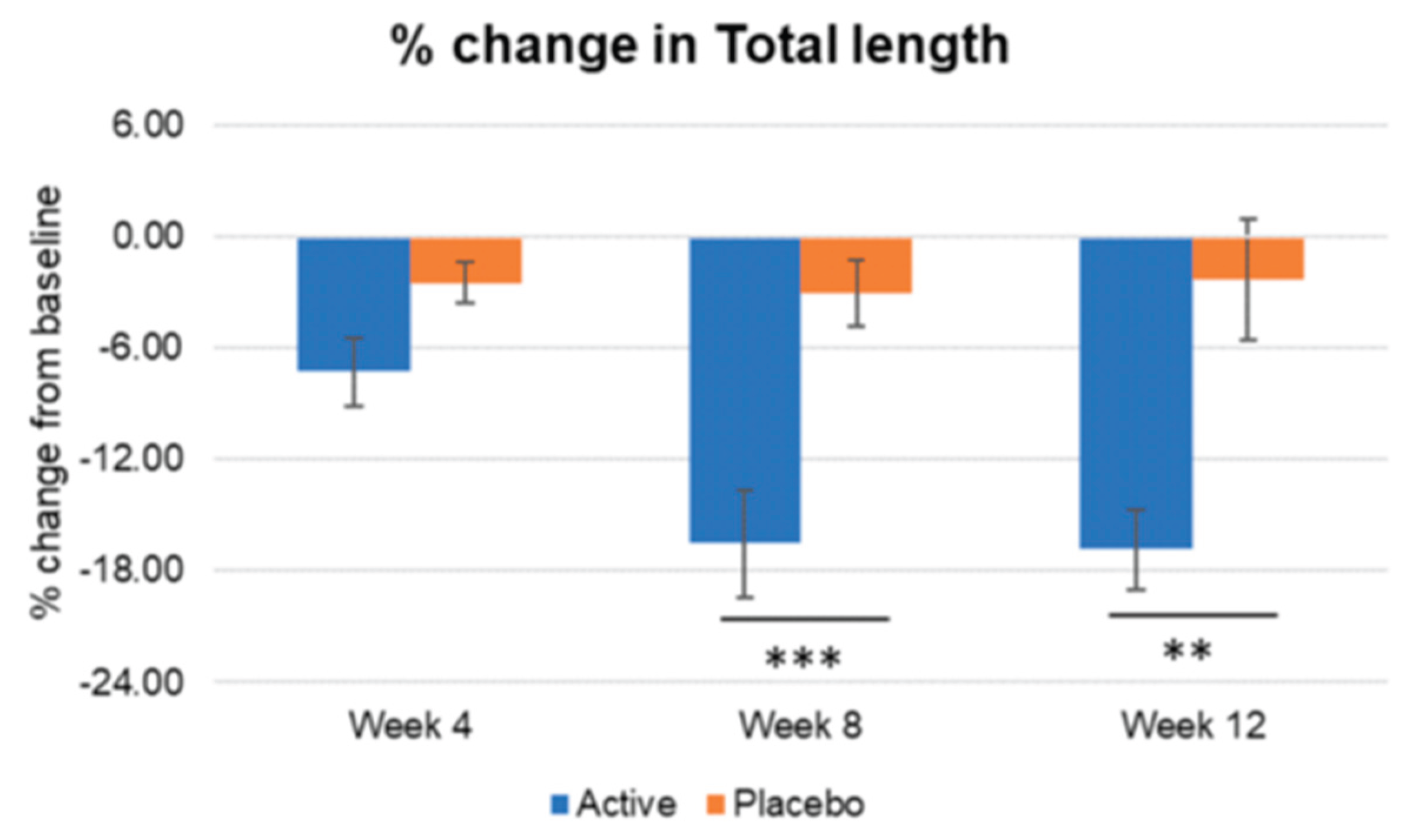
Figure 15.
Inter-group comparison of total length. Active group vs. Placebo group, Mann-Whitney U test, Significance: **p<0.01, ***p<0.001, Bar: S.E. Significantly higher percentage of wrinkle length reduction was detected in the active group vs placebo, since week 8.
Figure 15.
Inter-group comparison of total length. Active group vs. Placebo group, Mann-Whitney U test, Significance: **p<0.01, ***p<0.001, Bar: S.E. Significantly higher percentage of wrinkle length reduction was detected in the active group vs placebo, since week 8.
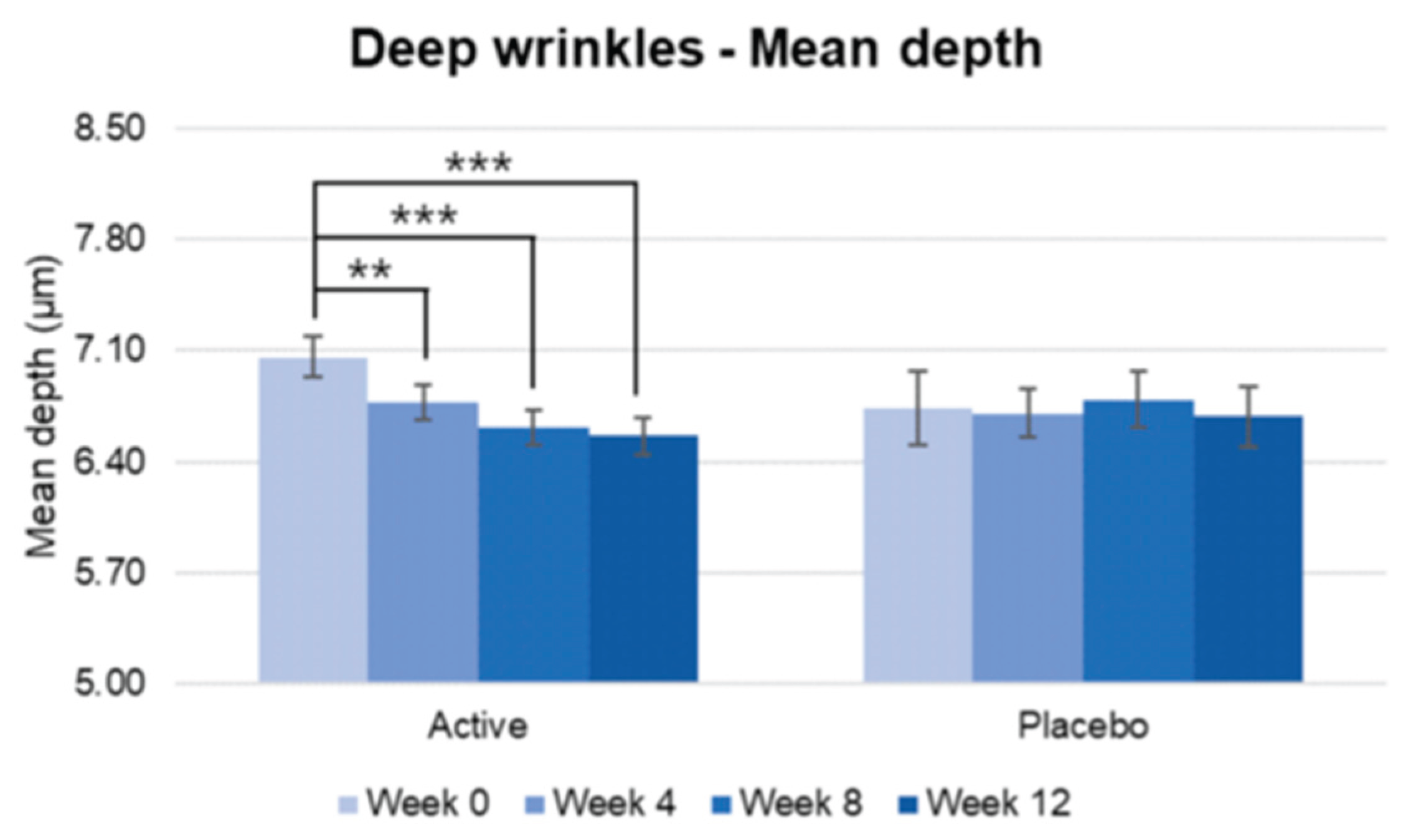
Figure 16.
Pre-post comparison of mean depth. Week 0 vs. Week 4, Week 8, Week 12, Wilcoxon signed-rank test, Significance: **p<0.01, ***p<0.001, Bar: S.E. A significantly reduction in the mean depth wrinkles were detected for active group treatment from week 4 while no significant reduction was detected in the placebo group.
Figure 16.
Pre-post comparison of mean depth. Week 0 vs. Week 4, Week 8, Week 12, Wilcoxon signed-rank test, Significance: **p<0.01, ***p<0.001, Bar: S.E. A significantly reduction in the mean depth wrinkles were detected for active group treatment from week 4 while no significant reduction was detected in the placebo group.
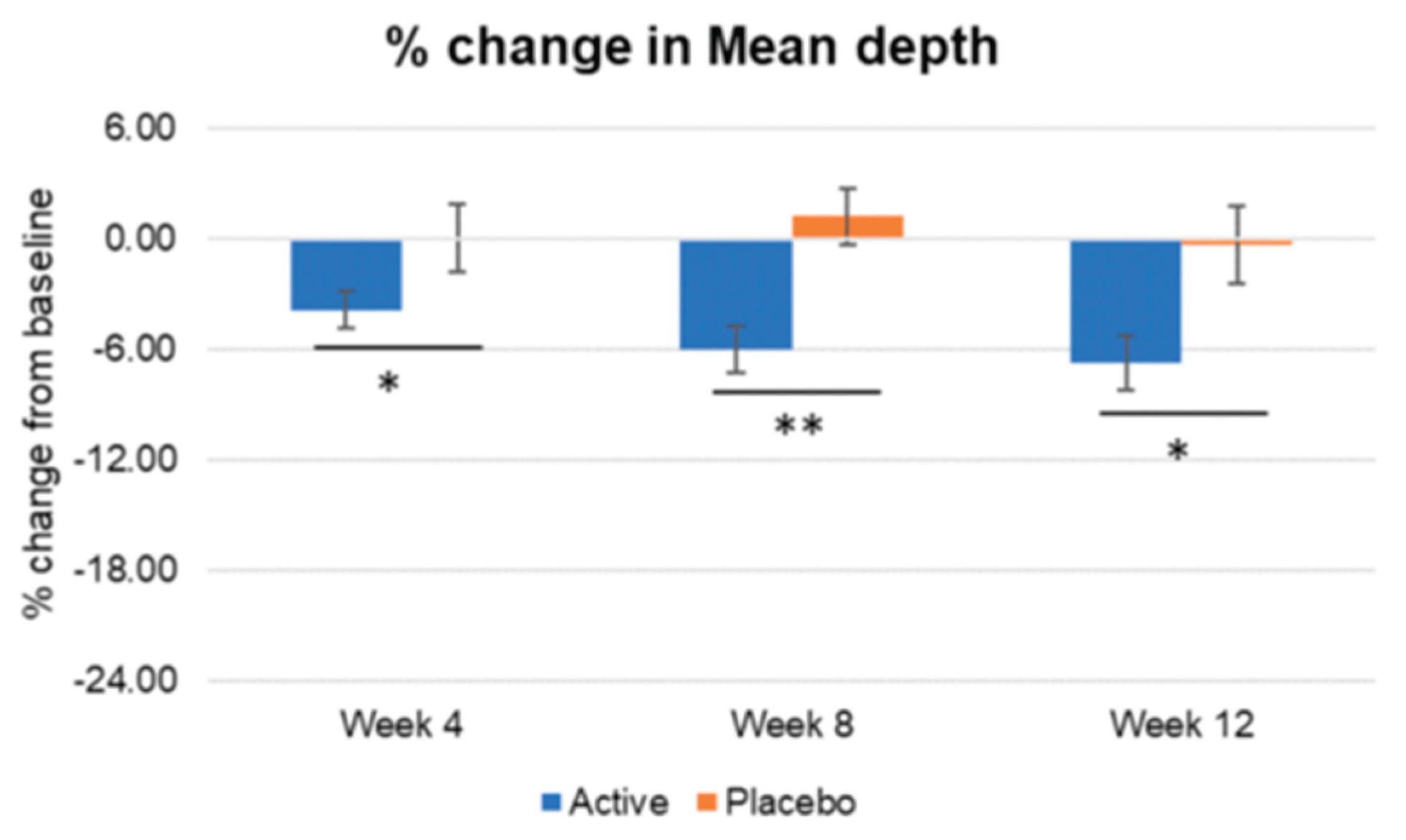
Figure 17.
Inter-group comparison of mean depth. Active group vs. Placebo group, Mann-Whitney U test, Significance: *p<0.05, **p<0.01, Bar: S.E. A significantly higher percentages of wrinkle mean depth reduction were detected in the active group compared to placebo group, since week 4.
Figure 17.
Inter-group comparison of mean depth. Active group vs. Placebo group, Mann-Whitney U test, Significance: *p<0.05, **p<0.01, Bar: S.E. A significantly higher percentages of wrinkle mean depth reduction were detected in the active group compared to placebo group, since week 4.
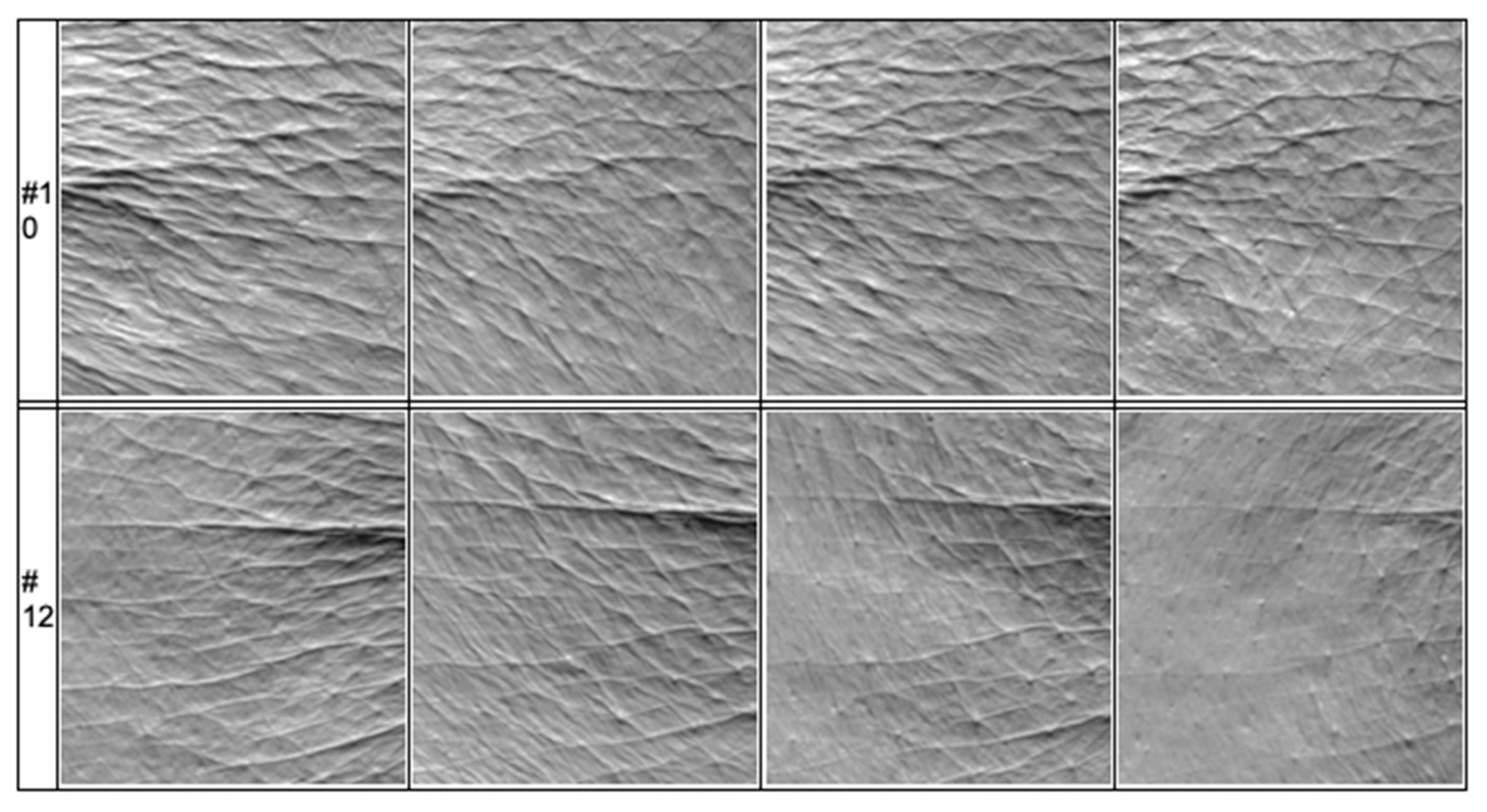
Figure 18.
The image shows mean wrinkle depth improvement in two different patients.
Figure 18.
The image shows mean wrinkle depth improvement in two different patients.
Table 1.
Show the different evaluations performed at each visit of the study.
Table 1.
Show the different evaluations performed at each visit of the study.
| Study period |
Visit 1 |
Visit 2 |
Visit 3 |
Visit 4 |
| Week 0 |
Week 4 |
Week 8 |
Week 12 |
| Test product intake period |
|---|
| Evaluation items |
Pigmentation |
+ |
+ |
+ |
+ |
| Skin brightness |
+ |
+ |
+ |
+ |
| Skin elasticity and firmness |
+ |
+ |
+ |
+ |
| Deep wrinkles |
+ |
+ |
+ |
+ |
| TEWL |
+ |
+ |
+ |
+ |
| Skin hydration |
+ |
+ |
+ |
+ |
| Subject questionnaire |
|
+ |
+ |
+ |
| Skin safety assessment |
|
+ |
+ |
+ |
Table 2.
Description of the different instrumental evaluations that were performed.
Table 2.
Description of the different instrumental evaluations that were performed.
| Evaluation item |
Instrument |
| Pigmentation intensity |
Mexameter® [Mexameter® MX18] (Courage + Khazaka Electronic GmbH, Germany) |
| Skin brightness |
Spectrophotometer [CM-2600d] (Konika Minolta, Inc., Japan) |
| Skin elasticity and firmness |
Cutometer® [Cutometer® Dual MPA 580] (Courage + Khazaka Electronic GmbH, Germany) |
| Deep wrinkles |
Visioline® [Visioline® VL650]
(Courage + Khazaka Electronic GmbH, Germany) |
| Transepidermal water loss |
Tewameter® [Tewameter® TM Hex] (Courage + Khazaka Electronic GmbH, Germany) |
| Skin hydration |
Skin-O-Mat® [Skin-O-Mat®] (COSMOMED, Germany) |
| Subject questionnaire |
- |
| Skin safety assessment |
- |
Table 3.
Description of the instrumental wrinkle evaluation.
Table 3.
Description of the instrumental wrinkle evaluation.
| Parameters |
Description |
| Total wrinkle area (mm2) |
Total wrinkle area of the skin which produce measurable shadows |
| Total length (mm) |
Total length of the wrinkle which produce measurable shadows |
| Mean depth (μm) |
Mean depth of the wrinkle which produce measurable shadows |