1. Introduction
Cardiovascular disease (CVD) is one of the leading causes of death worldwide and manifests itself in various forms, including hypertension, atherosclerosis, and myocardial infarction [
1]. These diseases are mainly caused by multiple pathological mechanisms, including oxidative stress, inflammatory response, and dyslipidemia, and there is an ongoing need to develop effective therapeutics to modulate these mechanisms [
2,
3]. Recently, natural substances from the flavonoid family have gained attention in the prevention and management of CVDs, and taxifolin has emerged as a promising candidate for its efficacy and safety [
4]. Taxifolin is a flavonoid primarily derived from plants that exhibits potent antioxidant and anti-inflammatory activities, inhibiting the production of reactive oxygen species and inflammatory cytokines, which are major contributors to CVD [
5,
6]. In addition, taxifolin plays an important role in maintaining vascular health by protecting vascular endothelial cell function and regulating cholesterol metabolism [
7]. These properties suggest that taxifolin may have preventive or therapeutic potential in a variety of CVDs, including hypertension, atherosclerosis, and hyperlipidemia.
Focusing on the protective effects of taxifolin on the cardiovascular system, this review synthesizes recent studies to explore its mechanism of action and clinical potential. In doing so, this review aims to further analyze the potential role of taxifolin in the prevention and treatment of CVDs and provide directions for future research and clinical applications.
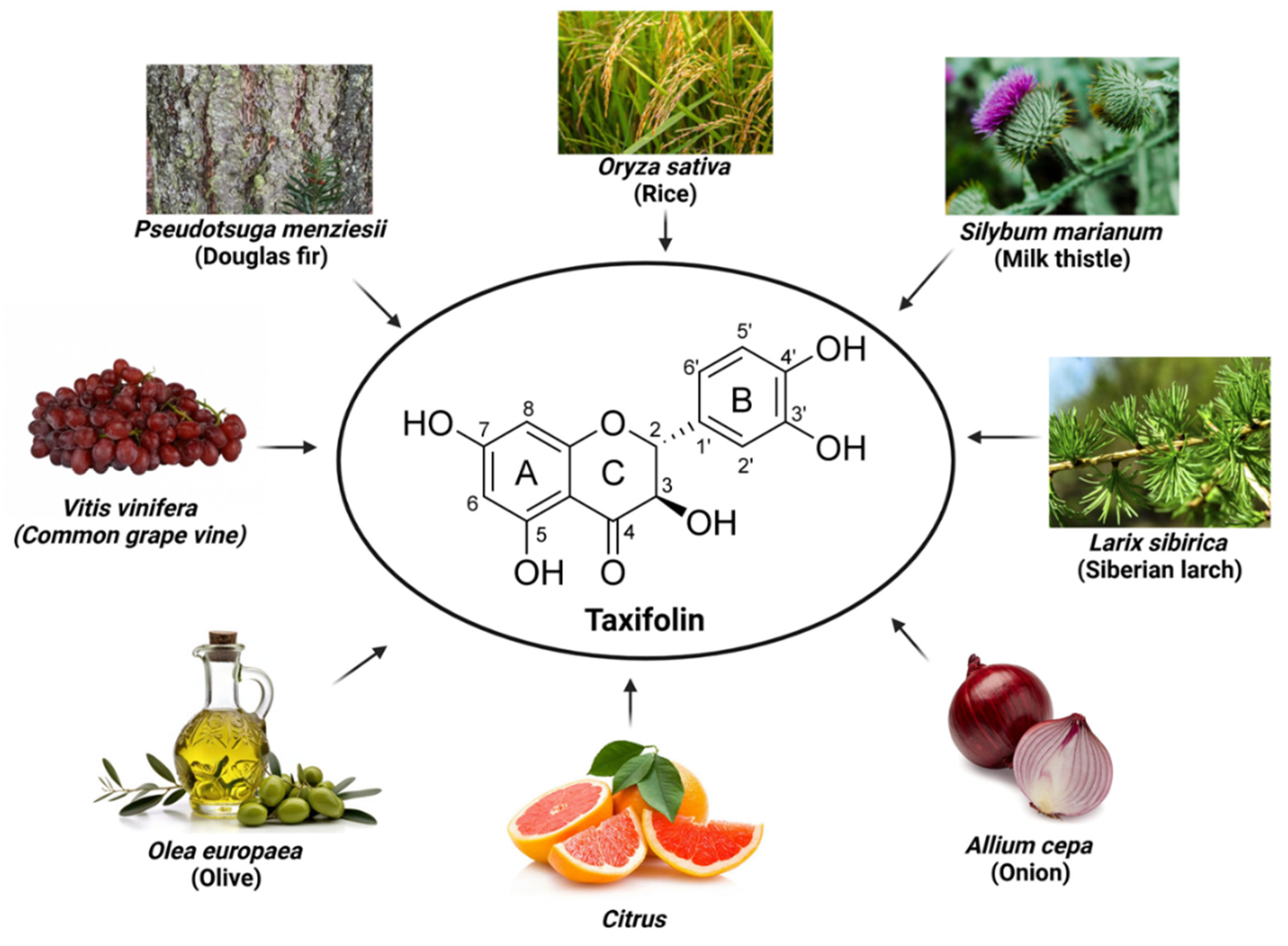
2. Taxifolin
Originally isolated from the bark of Douglas pine wood (
Pseudotsuga menziesii, Pinaceae) [
8], taxifolin, also known as dihydroquercetin or 3,5,7,3′,4′-pentahydroxyflavanone, has since been found in a variety of plants. It is widely distributed in nature, including Siberian larch (
Larix sibirica, Pinaceae), milk thistle (
Silybum marianum, Asteraceae), and onions (
Allium cepa, Amaryllidaceae), and is recognized as an important bioactive substance [
9,
10] (
Figure 1). Milk thistle and onion, in particular, have been recognized for their beneficial effects on cardiovascular health, further highlighting the therapeutic potential of taxifolin [
11,
12,
13]. In addition, taxifolin exhibits a variety of pharmacological activities, including antioxidant, anti-inflammatory, anticancer, and neuroprotective properties. Due to these pharmacological activities, it is widely studied in various therapeutic applications such as cancer, CVD, liver disease, and as a food additive. It is also used as an ingredient in dietary supplements for its health-promoting properties [
14].
Taxifolin (C
15H
12O
7, molar mass 304.25 g/mol) belongs to flavanonol (2,3-dihydroflavonol), a subclass of flavonoids [
15]. The structure of taxifolin consisting of two phenyl groups (A and B) bonded to O-heterocycle C, which is a key factor in determining the chemical and biological properties of taxifolin [
16]. Specifically, the A ring of taxifolin contains hydroxyl groups bonded at positions C-5 and C-7, while hydroxyl groups at the C-3' and C-4' positions of the B ring contribute to mitigating oxidative stress by neutralizing free radicals and stabilizing electron distribution [
17,
18]. The C ring has a hydroxyl group at position C-3 and a carbonyl group at position C-4, which plays an important role in chelating metal ions [
19]. The stereoisomeric structure of taxifolin is characterized by the two chiral centers (C-2 and C-3) in the C ring. It was known that the dihydroflavonols with
trans-diaxial H-2/H-3 position are more stable than the
cis-compounds (Trouillas et al., 2004), and the absolute structure of a major isomeric compound is (2
R,3
R)-(+)-taxifolin [
20,
21] (
Figure 1). The multiple hydroxyl groups in taxifolin exert potent antioxidant activity by increasing its high solubility in water and ability to form hydrogen bonds. In addition, the carbonyl group in the C ring and the dihydroxyl group in the B ring are involved in metal ion chelation, which inhibits reactive oxygen species (ROS) generation and reduces metal-catalyzed oxidation reactions, thus preventing oxidative damage [
19]. The unique arrangement of hydroxyl and carbonyl groups shows that it exhibits antioxidant and anti-inflammatory effects, making it a promising compound for therapeutic use [
22]. Research into the structure-activity relationship of taxifolin continues to reveal its potential to combat oxidative stress and inflammation, highlighting its importance in the realm of natural antioxidants.
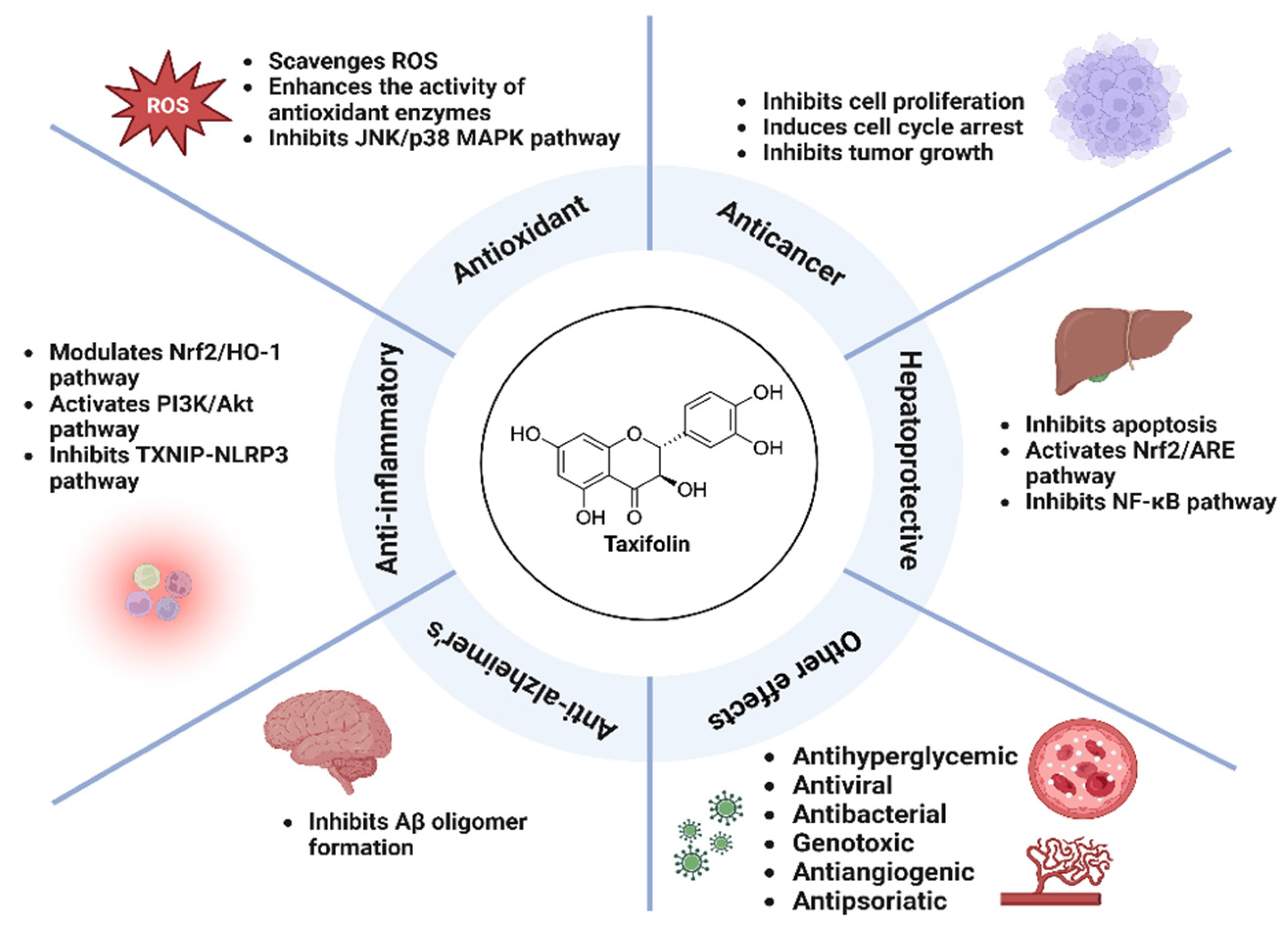
3. Pharmacological Activity of Taxifolin
Taxifolin exhibits a variety of pharmacological effects, including antioxidant, anti-inflammatory, hepatoprotective, anticancer, and neuroprotective. It effectively scavenges ROS, enhances antioxidant enzymes, and inhibits inflammatory responses through the NF-κB and PI3K/Akt pathways. Taxifolin protects the liver by reducing oxidative stress and apoptosis, while also demonstrating anticancer effects through cell cycle arrest and tumor suppression [
23]. In Alzheimer's disease models, it attenuates amyloid beta accumulation and neuroinflammation. Taxifolin also exhibits antibacterial, antiviral, and antiangiogenic effects, showing broad therapeutic potential.
3.1. Antioxidant Activity
Flavonoids exhibit potent antioxidant activity in the body as free radical scavengers and complexing agents for metal ions [
24,
25]. Among flavonoids, taxifolin is known to have particularly potent antioxidant activity [
26]. This compound effectively scavenges ROS to reduce oxidative stress, which prevents cell damage and plays an important role in the prevention of a variety of chronic diseases. Studies have shown that taxifolin has greater antioxidant capacity than common flavonoids and is more effective than quercetin, the leading antioxidant. At a dose of 100 mg/kg, it was 3.4 times more effective than quercetin and 4.9 times more effective than quercetin at 300 mg/kg [
27]. Studies have shown that taxifolin has a potent antioxidant effect in a CCl
4-induced hepatitis model [
28]. The study showed that taxifolin enhances the activity of antioxidant enzymes such as superoxide dismutase (SOD) and catalase, which are important for neutralizing ROS. Another study also demonstrated the antioxidant efficacy of taxifolin to scavenge ROS and prevent cell death in bmMSCs (bone marrow-derived mesenchymal stem cells) damaged by hydroxyl radicals by inhibiting the JNK/p38 MAPK signaling pathway [
29]. The ability to chelate iron ions has also been identified as one of the antioxidant effects of taxifolin [
19]. It was found that taxifolin inhibits the production of ROS by binding to iron ions and exerts its antioxidant activity through its ability to chelate iron ions.
3.2. Anti-Inflammatory Activity
Protection against liver injury is one of taxifolin’s notable benefits, achieved primarily through the inhibition of oxidative stress and inflammatory responses. Studies have shown that taxifolin mitigates cisplatin-induced nephrotoxicity by modulating the Nrf2/HO-1 pathway, an important regulator of oxidative stress and inflammation [
30]. Nrf2 is a key defense mechanism against oxidative stress by promoting the expression of antioxidant enzymes within cells. Taxifolin activates Nrf2 to induce the expression of HO-1, which plays a role in suppressing oxidative stress and inflammation. This regulation leads to a reduction in pro-inflammatory cytokines such as TNF-α and IL-6 and decreases markers of oxidative stress. By inhibiting these inflammatory mediators, taxifolin protects kidney tissue. Taxifolin has also been shown to modulate inflammatory responses through the PI3K/Akt signaling pathway [
31]. In a rat model with metabolic syndrome, taxifolin ameliorated impairments in glucose metabolism and water-salt metabolism by activating the PI3K/Akt pathway. Notably, modulating this pathway reduced the production of pro-inflammatory cytokines, effectively suppressing inflammatory responses in kidney tissue. In another study, taxifolin was shown to play an important role in suppressing inflammatory responses in high glucose-stimulated rat microglia [
32]. The study demonstrated that taxifolin attenuates inflammatory responses by modulating the TXNIP-NLRP3 axis. In a high-glucose environment, TXNIP expression increased, which activated NLRP3 inflammasomes, leading to the release of inflammatory cytokines. However, taxifolin effectively suppressed the excessive inflammatory response in microglia by inhibiting TXNIP expression and reducing the activation of NLRP3 inflammasomes. This reduced the production of inflammatory mediators and prevented neuronal damage. Taxifolin prevented various tissue damage by inhibiting the production of inflammatory cytokines and alleviating oxidative stress and inflammatory responses.
3.3. Hepatoprotective Activity
Taxifolin protects against liver injury through its primary mechanism of inhibiting oxidative stress and inflammatory responses. Studies have shown that taxifolin attenuates liver injury in a mouse model of CCl
4-induced acute liver injury by reducing oxidative stress through activation of antioxidant enzymes and inhibiting inflammatory responses in hepatocytes [
33]. Specifically, taxifolin maintained glutathione levels, reduced the accumulation of malondialdehyde, and prevented damage to cell membranes. In addition, a study was published showing that taxifolin enhances the antioxidant defense system of hepatocytes and inhibits hepatocyte apoptosis by activating the Nrf2/ARE pathway in an acetaminophen-induced acute liver injury model [
34]. In this study, taxifolin reduced ROS in liver cells and inhibited apoptosis by regulating the Bax/Bcl-2 ratio. Taxifolin was found to suppress inflammatory responses and promote cell survival in acute alcohol-induced liver injury by regulating the NF-κB pathway and PI3K/Akt pathway. Taxifolin attenuated liver inflammation by decreasing the expression of pro-inflammatory cytokines and increasing the expression of anti-inflammatory cytokines. The protective effects of taxifolin were evaluated in mice with induced hepatic encephalopathy and found that it contributed to alleviating the symptoms of hepatic encephalopathy by suppressing inflammation and oxidative stress in the liver and brain [
35]. The study confirmed that taxifolin alleviated hepatic encephalopathy by maintaining the integrity of the blood-brain barrier and reducing neuroinflammation. These studies demonstrate the potential of taxifolin to protect the liver through its antioxidant, anti-inflammatory, and cytoprotective effects in various liver injury models.
3.4. Anticancer Activity
As a flavonoid, taxifolin has gained significant attention in cancer research for its potent anticancer properties, primarily through interactions with key signaling pathways and regulation of the cell cycle. Studies have shown that treatment of various cancer cell lines, including HeLa (human cervical cancer), HepG2 (human liver cancer), and MDA-MB-231 (human breast cancer), with taxifolin at concentrations ranging from 10 μM to 100 μM, exhibits anti-cancer effects [
36]. The study demonstrated that taxifolin inhibits cancer cell proliferation in a dose-dependent manner by inducing cell cycle arrest, mainly in the G
2/M phase. Cell cycle arrest is mediated by the regulation of important cell cycle regulators such as cyclins and cyclin-dependent kinase; specifically, taxifolin inhibits these regulators to prevent the transition from G
2 phase to M phase, effectively halting cell cycle progression and thus inhibiting cancer cell proliferation. The study also examined the effects of taxifolin on the Wnt/β-catenin signaling pathway, a pathway that is often dysregulated in cancer. Taxifolin was shown to inhibit tumor growth by enhancing the nuclear translocation of β-catenin, while inducing the activation of Wnt target genes that promote cell death pathways. This cell cycle inhibition and activation of cell death signaling contributed to a significant reduction in tumor growth in the models used in the study. A study in SKH-1 hairless mice demonstrated that topical application of taxifolin significantly reduced the development and proliferation of UVB-induced skin tumors [
37]. By inhibiting the phosphorylation of EGFR, taxifolin effectively downregulated the subsequent activation of the PI3K/Akt signaling pathway, which is known to promote cell survival, proliferation, and tumor growth.
3.5. Anti-Alzheimer’s Activity
Alzheimer’s disease (AD) is a neurodegenerative disease characterized by the gradual death of brain nerve cells due to the buildup of abnormal proteins, such as amyloid-β (Aβ) protein, in the brain. Taxifolin has attracted attention for its neuroprotective effects related to AD, and several studies have demonstrated its promising anti-AD effects. Studies have shown that taxifolin is effective in inhibiting Aβ oligomer formation, restoring vascular structural integrity, and improving memory function in an Aβ-injected rat model [
38]. This study highlighted the mechanisms by which taxifolin improves vascular permeability and restores cognitive function in cerebral amyloid angiopathy. In addition, the effectiveness of taxifolin in preventing synapse formation impairment and memory deficits in the H9c2 cardiomyocyte model induced by β-amyloid was also demonstrated [
39]. Taxifolin reduced inflammatory responses by inhibiting the cPLA₂/PGE₂ pathway, resulting in neuroprotection and improved cognitive function. Studies have also shown that taxifolin exhibited multifaceted neuroprotective effects in an Aβ-induced mouse model, enhancing neuronal survival and reducing damage through the PI3K/Akt pathway [
40]. In this study, taxifolin significantly reduced Aβ protein accumulation, alleviated vascular damage through improved vascular permeability and normalization of vascular structure, and restored the function of vascular endothelial cells. In addition, taxifolin reduced neuronal death by activating the PI3K/Akt pathway and regulated neuroinflammatory responses by inhibiting the NF-κB pathway. It was also demonstrated that the combined treatment of taxifolin and cilostazol reduced Aβ accumulation and neurotoxicity by inhibiting the p-JAK2/p-STAT3/NF-κB/BACE1 signaling pathway [
41].
3.6. Other Pharmacological Activities
In addition to its antihyperglycemic effects, which improve insulin sensitivity and lower blood glucose levels [
42,
43,
44], taxifolin has demonstrated a variety of pharmacologic actions. These include antiviral [
45], antibacterial [
46,
47], and genotoxic activities [
48], as well as antiangiogenic activity that inhibits abnormal blood vessel formation [
49]. Anti-psoriatic effects have also been shown, providing potential benefits in psoriasis management by modulating additional inflammatory pathways [
50].
Figure 2.
Schematic representation of the pharmacological effects and mechanism of action of taxifolin.
Figure 2.
Schematic representation of the pharmacological effects and mechanism of action of taxifolin.
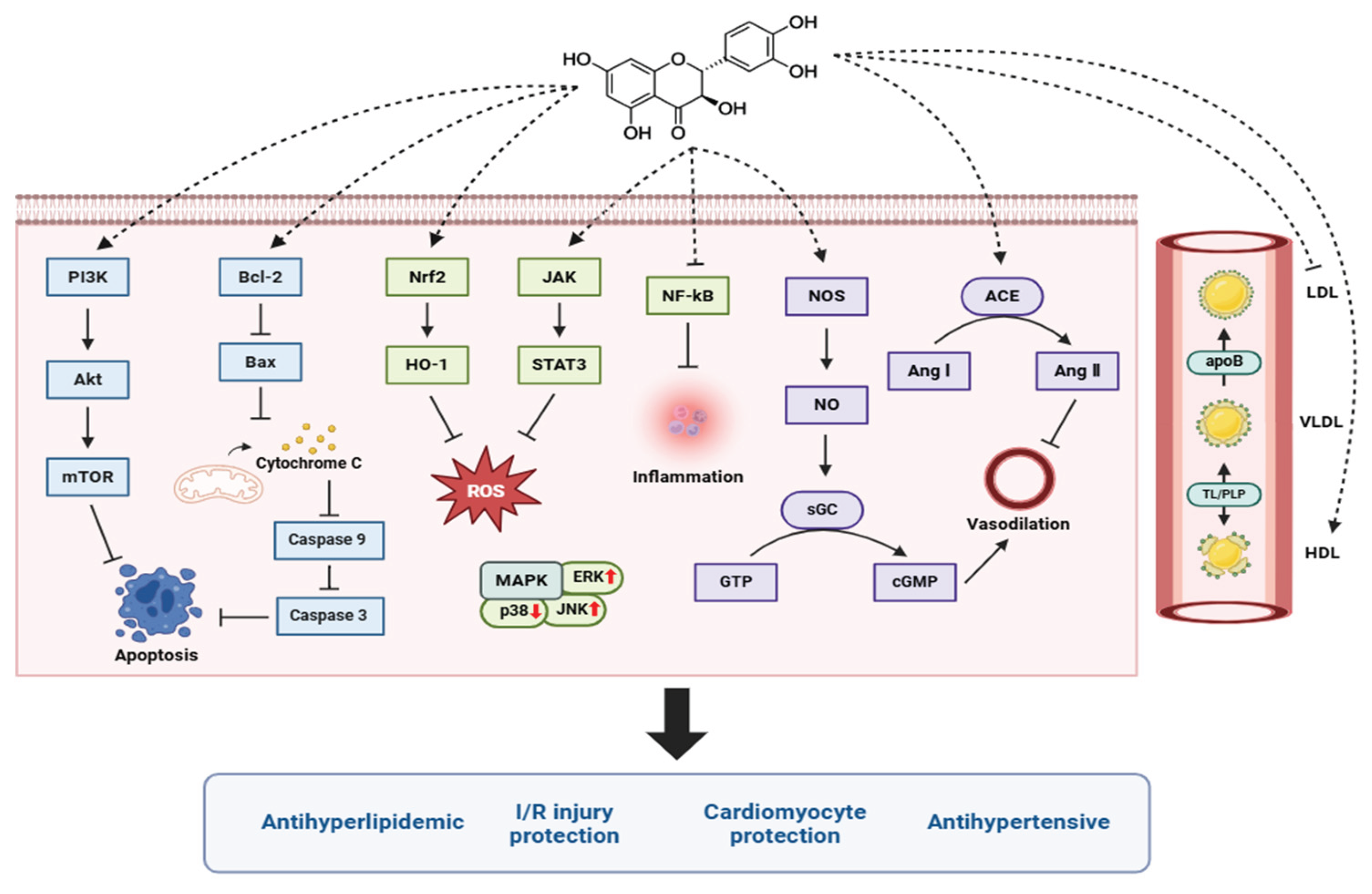
4. Taxifolin and the Cardiovascular System
Research on taxifolin has explored its potential benefits in cardiovascular health, liver protection, cancer prevention, and other areas. Various experimental and epidemiologic studies have shown that consumption of flavonoid-rich foods is associated with a reduced risk of CVD [
51]. Taxifolin has been recognized for its antioxidant, anti-inflammatory, and antihyperlipidemic properties, which may play an important role in the prevention and treatment of CVD, including antihypertensive effects and cardiomyocyte protection [
52,
53]. Recently, various mechanisms of action have been investigated, showing promising results in blood pressure regulation, cholesterol metabolism, and protection against myocardial ischemia/reperfusion injury (
Table 1 and
Figure 3). Therefore, taxifolin is expected to contribute to the alleviation of CVDs such as atherosclerosis, hypertension, and myocardial infarction.
4.1. Antihypertensive
Taxifolin has shown antihypertensive activity in experimental models of hypertension. Studies have shown that taxifolin lowers blood pressure by enhancing vasorelaxant function and inhibiting the activity of inflammatory cytokines in the blood vessels [
59]. Endothelial function was significantly improved in the taxifolin-treated group, with an increased vasorelaxant response to acetylcholine and a decreased phenylephrine-induced vasoconstrictor response. Taxifolin also inhibited vessel wall thickening, reduced damage to endothelial cells, and induced structural improvements in blood vessels. Studies have also demonstrated the antihypertensive effects of taxifolin in hypertension models [
62]. In SHR and IHR models, taxifolin effectively lowered blood pressure, and its mechanism of action was shown to be through the improvement of vasorelaxant responses and the alleviation of oxidative stress and inflammatory responses. Another study used a radiation-induced hypertension model and found that taxifolin effectively suppressed hypertension by inhibiting angiotensin-converting enzyme (ACE) activity and preventing excessive production of angiotensin II, which causes vasoconstriction [
61]. By inhibiting ACE activity and preventing the overproduction of angiotensin II, which causes vasoconstriction and elevated blood pressure, taxifolin shows promise as a treatment for hypertension. In a rat model of aging and increased oxidative stress and inflammatory response, taxifolin also inhibited ACE activity [
58]. Taxifolin demonstrated antihypertensive effects by lowering blood pressure in elderly hypertensive rat [
60]. Taxifolin treatment significantly lowered systolic blood pressure through mechanisms related to modulation of endothelial function and reduction of oxidative stress. Specifically, taxifolin increases the bioavailability of nitric oxide (NO), which promotes vasodilation and reduces vascular resistance. This demonstrates the therapeutic potential of taxifolin in the management of hypertension in the elderly population, who are vulnerable to oxidative stress and vascular dysfunction.
4.2. Cardiomyocyte Protection
In various cardiac injury models, protective effects on cardiomyocytes have been demonstrated by taxifolin. Studies have demonstrated that taxifolin attenuates oxidative stress and cardiac structural damage in an acrylamide-induced cardiac injury model [
70]. It has also demonstrated protective effects against isoproterenol-induced cardiac injury [
6]. Taxifolin exerted its cardioprotective effects by regulating oxidative stress and inflammatory responses by activating the Nrf2/HO-1 pathway. Additionally, taxifolin has been found to prevent cardiac hypertrophy and fibrosis while improving cardiac function under pressure overload via the ERK1/2, JNK1/2, and Smad signaling pathways [
52]. Furthermore, taxifolin was effective in preventing and alleviating cardiac damage in diabetic cardiomyopathy by activating the JAK2/STAT3 pathway, suppressing oxidative stress, and inhibiting apoptosis through decreased caspase-3 expression and increased Bcl-2 expression [
72]. Diabetic cardiomyopathy, a complication of diabetes that causes structural and functional damage to the heart, was significantly alleviated by taxifolin treatment.
4.3. Myocardial Ischemia/Reperfusion (I/R) Injury Protection
Several studies have demonstrated the cardioprotective effects of taxifolin against ischemia/reperfusion injury via modulation of oxidative stress and apoptotic pathways. Taxifolin exerted myocardial protective effects by inhibiting oxidative stress and endoplasmic reticulum stress-induced apoptosis through activation of the PI3K/Akt signaling pathway, resulting in a significant reduction in cardiac tissue damage [
73]. In addition, taxifolin reduced apoptosis by preventing the loss of mitochondrial membrane potential, inhibiting the release of cytochrome C, and inhibiting the activation of caspase-9 and caspase-3 [
5]. Taxifolin alleviated oxidative stress and apoptosis by reducing ROS production, increasing the expression of Bcl-2, and inhibiting the expression of the pro-apoptotic protein Bax. In addition, blood levels of LDH and CK-MB were significantly reduced in the taxifolin-treated group, confirming that myocardial injury was inhibited. Morphological analysis of myocardial tissue showed that taxifolin preserved myocardial structure by reducing myofibrillar destruction, edema, and inflammatory cell infiltration caused by I/R injury.
4.4. Antihyperlipidemic
Hyperlipidemia is an abnormally high level of triglycerides and low-density lipoprotein (LDL) cholesterol in the blood, which puts a strain on the cardiovascular system and increases the risk of conditions like atherosclerosis and heart attack. Taxifolin has been shown to be effective in lowering serum cholesterol and LDL cholesterol levels in several studies. Taxifolin has been shown to be effective in lowering serum cholesterol and LDL cholesterol levels in several studies. Studies have shown that taxifolin lowers serum cholesterol levels by lowering LDL cholesterol and improving high-density lipoprotein (HDL) cholesterol balance [
55]. In addition, the antioxidant properties of taxifolin reduce oxidative stress in lipids, which not only prevents oxidation of LDL but also benefits overall cardiovascular health. In addition to this, taxifolin reduces oxidative stress and prevents oxidative damage associated with fat accumulation in the liver. Administration of taxifolin-rich tea to rats increased the activity of the antioxidant enzymes SOD and catalase, which contributed to the reduction of cellular damage caused by oxidative stress [
54]. In another study, taxifolin was shown to reduce the production and secretion of LDL and very low-density lipoprotein (VLDL) in the liver [
57]. This was achieved by inhibiting apoptosis and the activity of lipid metabolism regulatory enzymes, suggesting that taxifolin may be effective in the management of hyperlipidemia.
4.5. Clinical Studies
Various preclinical studies have recognized the cardioprotective effects of taxifolin, including improving cardiovascular health through antioxidant and anti-inflammatory actions, preventing cardiac injury, and enhancing vascular endothelial function. According to the recent systematic review for natural and semi-synthetic flavonoid drugs, total of 19 flavonoid-based drugs have been approved for medical prescription, and 30% of them are used for the treatment of cardiovascular diseases [
74]. However, despite these positive results, to date, few clinical studies have been conducted on the effects of taxifolin on CVD. There is also a lack of data on the safety of taxifolin, appropriate dosage, and possible side effects of long-term use. Therefore, clinical studies in patients with CVD will be an important step in developing taxifolin into a real treatment. Future studies should systematically validate the cardioprotective effects of taxifolin in different patient populations, evaluate its combination with other cardiovascular drugs, and assess its long-term efficacy. This will play an important role in broadening the clinical applicability of taxifolin and establishing its safety.
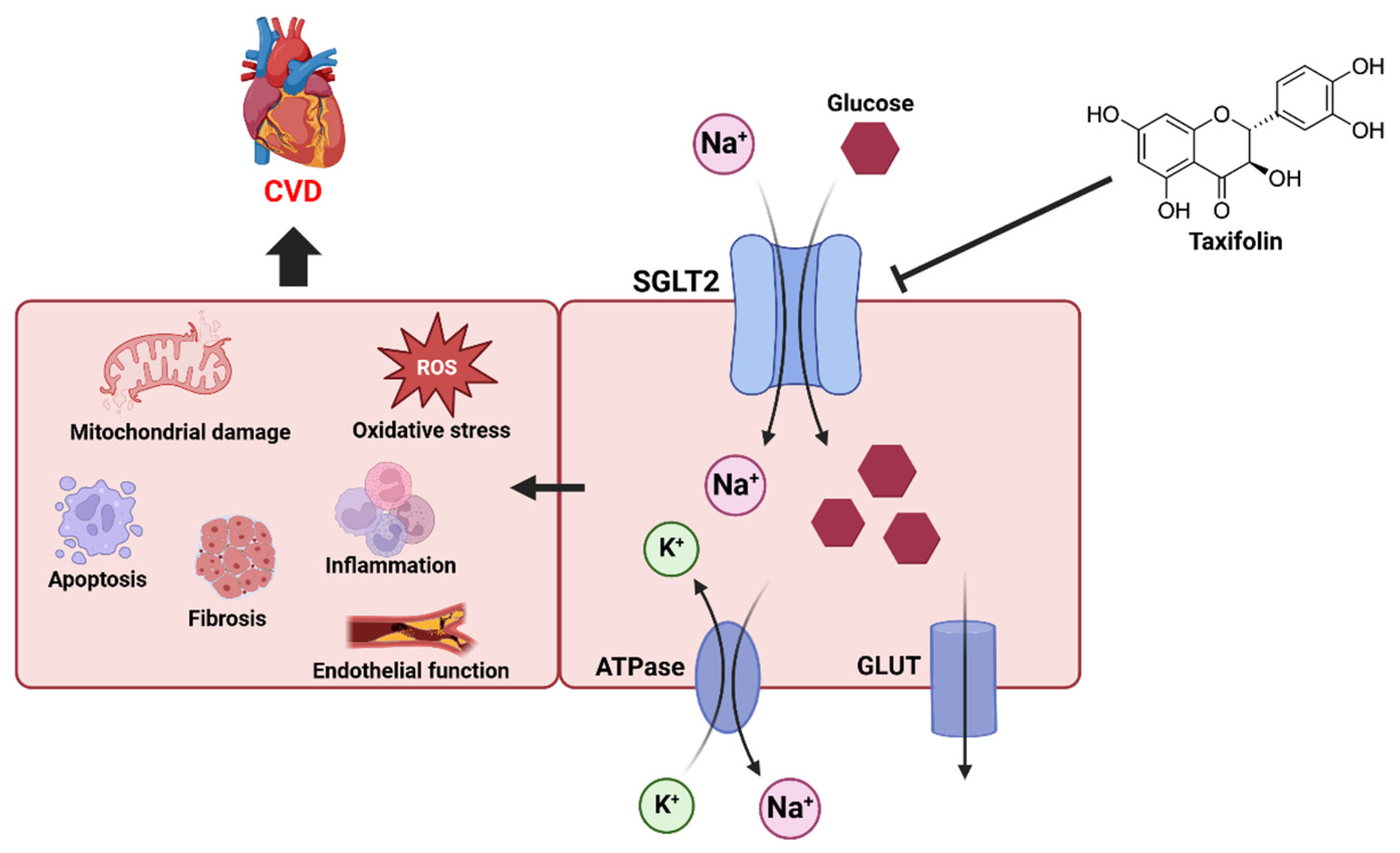
5. Future Research Directions
Sodium-glucose transporter (SGLT) and cardiovascular health have been the focus of recent research. The SGLT is an important protein that regulates the intracellular transport of glucose and has two main forms, SGLT1 and SGLT2. SGLT1 is primarily responsible for the uptake of glucose and galactose in the small intestine, while SGLT2 is responsible for the reabsorption of glucose in the kidneys [
75]. These transporters utilize sodium concentration gradients to efficiently move glucose into cells, which is essential for the regulation of glucose metabolism in the body [
76] (
Figure 4). SGLT2 inhibitors work primarily to lower blood sugar by blocking the reabsorption of glucose in the kidneys. By inhibiting SGLT2's ability to move sodium ions and glucose together into cells, glucose is excreted in the urine [
77]. This reduces the concentration of glucose in the body and improves insulin secretion, which is effective in diabetes management [
78]. However, SGLT2 inhibitors do more than just control blood sugar; they have also been shown to have a positive impact on cardiovascular health [
79]. In addition to glycemic control, recent studies have shown that SGLT2 inhibitors provide significant cardiovascular benefits, including reduced risk of heart failure and improved endothelial function [
80,
81]. Given the known cardioprotective properties of taxifolin, studies elucidating its effects on these mechanisms may contribute to the development of new therapeutic strategies. In future studies, it will be important to further explore the mechanisms of its potential interaction with SGLT2 and its inhibitors.
5.1. CVD and Sodium-Glucose Transporter
SGLT2 inhibitors exert their cardioprotective effects by reducing inflammatory responses, decreasing oxidative stress, improving endothelial dysfunction, and regulating sodium excretion and fluid [
82,
83,
84] (
Figure 4). SGLT2 inhibitors inhibit atherosclerosis by normalizing the expression of inflammation-related genes such as TNF-α, IL-6, ICAM-1, MMP2, and MMP9 [
85]. These drugs reduce oxidative stress by reducing ROS production and improve cardiac function by preventing cellular damage [
86]. They have also demonstrated therapeutic effects on endothelial dysfunction by reducing plasma levels of CAM, a marker of endothelial dysfunction [
87]. Additionally, SGLT2 inhibitors promote the excretion of sodium from the kidneys, which reduces water in the body, which in turn lowers the pressure on the heart, helping to alleviate symptoms in patients with heart failure [
88]. Through these mechanisms, SGLT2 inhibitors play an important role in promoting cardiovascular health.
Several clinical studies have confirmed the cardioprotective effects of SGLT2 inhibitors. In the EMPA-REG OUTCOME study, empagliflozin was shown to reduce the risk of cardiovascular death by 38% and hospitalization for heart failure by 35% in patients with type 2 diabetes [
89]. The CANVAS program also found that canagliflozin reduced the risk of cardiovascular death by 20% and reduced the incidence of cardiovascular events by 14% [
90]. In the DECLARE-TIMI 58 study, dapagliflozin was found to be effective in reducing heart failure hospitalization rates by 27% [
91]. Similar results were confirmed by ertugliflozin in the VERTIS CV study, which found that while the drug did not significantly reduce the direct occurrence of cardiovascular events, it played a role in reducing heart failure-related hospitalizations [
92]. These studies suggest that SGLT2 inhibitors may be important therapeutic agents in the prevention and management of CVD, not just in the management of diabetes. These mechanisms have important implications for the prevention of cardiovascular and renal disease, especially in diabetic patients. However, research involving SGLTs is still in its infancy, and future studies are needed. It is important to elucidate how SGLT inhibitors interact with different metabolic pathways and the mechanisms by which they contribute to the prevention of CVD. In addition, research is needed on the effects of natural substances such as taxifolin on the function of SGLT and their interactions with SGLT inhibitors. This may contribute to the development of new therapeutic strategies. These research directions will contribute to a deeper understanding of the physiological functions of SGLTs and clarify their role in the prevention and treatment of CVDs. By exploring the various functions of SGLTs and their association with the cardiovascular system, new insights into the prevention and treatment of related diseases may be gained.
5.2. SGLT and Taxifolin
Research has shown that taxifolin plays an important role in ameliorating glucose and water-salt metabolic disturbances in metabolic syndrome rats [
31]. The study found that taxifolin exerts these effects through the PI3K/Akt signaling pathway, suggesting that taxifolin influences metabolic regulation. Specifically, taxifolin reduces the expression of SGLT2 and GLUT2, which prevents excessive reabsorption of glucose, promotes the excretion of glucose, and contributes to glycemic control. This suggests that taxifolin may act in a similar manner to SGLT inhibitors to help regulate blood sugar. In addition, by lowering SGLT2 levels and reducing proximal glucose reabsorption, normal tubuloglomerular feedback mechanisms are restored, which may contribute to reduced glomerular filtration. Another study examined the combined effect of taxifolin and dapagliflozin and found that it lowered intrarenal blood pressure and improved blood flow [
93]. Dapagliflozin is an SGLT2 inhibitor that blocks the reabsorption of glucose and sodium in the kidneys, reducing the body's inflammatory response and promoting sodium and water excretion. In this study, the combination of taxifolin and dapagliflozin was more effective than monotherapy in improving colistin-induced nephrotoxicity. The combination of taxifolin and dapagliflozin effectively alleviated colistin-induced nephrotoxicity by inhibiting inflammatory cytokine expression in kidney tissue, increasing antioxidant enzyme activity, significantly reducing kidney damage, and lowering blood creatinine and urea nitrogen levels, which are markers of kidney function. These mechanisms suggest that the synergistic effects of the two compounds may play an important role in kidney protection. Further studies are needed to better understand the interaction of SGLT2 inhibitors and taxifolin, especially their effects on CVD. Such studies may clarify the effects of taxifolin on SGLTs and provide new approaches to the treatment of CVD.
6. Conclusions
Taxifolin is a natural flavonoid with great potential for improving cardiovascular health through its antioxidant, anti-inflammatory, and vasculoprotective effects, and various studies have demonstrated its potential in the prevention and treatment of cardiovascular disease. In particular, taxifolin plays an important role in reducing blood pressure and mitigating cardiac injury and vascular structural damage through a variety of pharmacological effects. The preclinical studies presented in this review have demonstrated that taxifolin has cardioprotective effects, including blood pressure reduction, myocardial protection, and prevention of ischemic injury, and that these effects are exerted primarily through ROS reduction, anti-inflammatory mechanisms, and modulation of various cellular pathways.
Nevertheless, clinical studies of taxifolin in the prevention and treatment of cardiovascular disease are currently limited, and there is a lack of data on its long-term safety and efficacy in various patient populations. Therefore, it will be important for future studies to clinically validate the effectiveness of taxifolin, evaluate its potential for combination with other cardiovascular therapies, and the optimal dose and method of administration. These studies are expected to establish taxifolin as a promising therapeutic agent for cardiovascular healthcare.
Author Contributions
Conceptualization, H.-H.S., J.-Y.K. and M.-H.O.; investigation, D.-S.G. and H.-K.L.; resources, D.-W.K. and J.J.K.; writing—original draft preparation, H.-H.S. and J.-Y.K.; writing—review and editing, H.-H.S., J.-Y.K., D.-W.K., H.J.K. and M.-H.O.; visualization, H.-H.S., D.-S.G. and J.J.K.; supervision, H.J.K. and M.-H.O.; project administration, H.J.K. and M.-H.O.; funding acquisition, H.-K.L. and M.-H.O. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Glocal University Project of Mokpo National University in 2025 and was supported by Korea Institute of Marine Science & Technology Promotion (KIMST) funded by the Ministry of Oceans and Fisheries (RS-2022-KS221671).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data available on request from the authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Thiriet, M. Cardiovascular disease: an introduction. Vasculopathies: Behavioral, Chemical, Environmental, and Genetic Factors 2018, 1-90.
- Rader, D.J.; Daugherty, A. Translating molecular discoveries into new therapies for atherosclerosis. Nature 2008, 451, 904–913. [Google Scholar] [CrossRef]
- Asgary, S.; Rastqar, A.; Keshvari, M. Functional food and cardiovascular disease prevention and treatment: a review. Journal of the American College of Nutrition 2018, 37, 429–455. [Google Scholar] [CrossRef]
- Hooper, L.; Kroon, P.A.; Rimm, E.B.; Cohn, J.S.; Harvey, I.; Le Cornu, K.A.; Ryder, J.J.; Hall, W.L.; Cassidy, A. Flavonoids, flavonoid-rich foods, and cardiovascular risk: a meta-analysis of randomized controlled trials. The American journal of clinical nutrition 2008, 88, 38–50. [Google Scholar] [CrossRef]
- Tang, Z.; Yang, C.; Zuo, B.; Zhang, Y.; Wu, G.; Wang, Y.; Wang, Z. Taxifolin protects rat against myocardial ischemia/reperfusion injury by modulating the mitochondrial apoptosis pathway. PeerJ 2019, 7, e6383. [Google Scholar] [CrossRef]
- Obeidat, H.M.; Althunibat, O.Y.; Alfwuaires, M.A.; Aladaileh, S.H.; Algefare, A.I.; Almuqati, A.F.; Alasmari, F.; Aldal’in, H.K.; Alanezi, A.A.; Alsuwayt, B. Cardioprotective effect of taxifolin against isoproterenol-induced cardiac injury through decreasing oxidative stress, inflammation, and cell death, and activating Nrf2/HO-1 in mice. Biomolecules 2022, 12, 1546. [Google Scholar] [CrossRef]
- Das, A.; Baidya, R.; Chakraborty, T.; Samanta, A.K.; Roy, S. Pharmacological basis and new insights of taxifolin: A comprehensive review. Biomedicine & Pharmacotherapy 2021, 142, 112004. [Google Scholar]
- Pew, J.C. A Flavonone from Douglas-Fir Heartwood2. Journal of the American Chemical Society 1948, 70, 3031–3034. [Google Scholar] [CrossRef]
- Sunil, C.; Xu, B. An insight into the health-promoting effects of taxifolin (dihydroquercetin). Phytochemistry 2019, 166, 112066. [Google Scholar] [CrossRef]
- Liu, X.; Ma, Y.; Luo, L.; Zong, D.; Li, H.; Zeng, Z.; Cui, Y.; Meng, W.; Chen, Y. Dihydroquercetin suppresses cigarette smoke induced ferroptosis in the pathogenesis of chronic obstructive pulmonary disease by activating Nrf2-mediated pathway. Phytomedicine 2022, 96, 153894. [Google Scholar] [CrossRef]
- Vilahur, G.; Casaní, L.; Peña, E.; Crespo, J.; Juan-Babot, O.; Ben-Aicha, S.; Mendieta, G.; Béjar, M.T.; Borrell, M.; Badimon, L. Silybum marianum provides cardioprotection and limits adverse remodeling post-myocardial infarction by mitigating oxidative stress and reactive fibrosis. International journal of cardiology 2018, 270, 28–35. [Google Scholar] [CrossRef]
- Kendler, B.S. Garlic (Allium sativum) and onion (Allium cepa): a review of their relationship to cardiovascular disease. Preventive medicine 1987, 16, 670–685. [Google Scholar] [CrossRef]
- Wang, C.K. Health benefits of onion bioactives on hypercholesterolemia, cardiovascular diseases, and bone mineral density. Food Frontiers 2020, 1, 107–108. [Google Scholar] [CrossRef]
- Ding, T.-m.; Tian, S.-j.; Zhang, Z.-x.; Gu, D.-z.; Chen, Y.-f.; Shi, Y.-h.; Sun, Z.-p. Determination of active component in silymarin by RP-LC and LC/MS. Journal of pharmaceutical and biomedical analysis 2001, 26, 155–161. [Google Scholar] [CrossRef]
- Reyes, V.; Martínez, O.; Hernández, G. National center for biotechnology information. Plant Breeding. Universidad Autónoma Agraria Antonio Narro, Calzada Antonio Narro 1923.
- Beecher, G.R. Overview of dietary flavonoids: nomenclature, occurrence and intake. The Journal of nutrition 2003, 133, 3248S–3254S. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free radical biology and medicine 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Trouillas, P.; Fagnère, C.; Lazzaroni, R.; Calliste, C.; Marfak, A.; Duroux, J.-L. A theoretical study of the conformational behavior and electronic structure of taxifolin correlated with the free radical-scavenging activity. Food chemistry 2004, 88, 571–582. [Google Scholar] [CrossRef]
- Topal, F.; Nar, M.; Gocer, H.; Kalin, P.; Kocyigit, U.M.; Gülçin, İ.; Alwasel, S.H. Antioxidant activity of taxifolin: an activity–structure relationship. Journal of enzyme inhibition and medicinal chemistry 2016, 31, 674–683. [Google Scholar] [CrossRef]
- Nifant’ev, E.; Koroteev, M.; Kaziev, G.; Uminskii, A.; Grachev, A.; Men’shov, V.; Tsvetkov, Y.; Nifant’ev, N.; Bel’skii, V.; Stash, A. On the problem of identification of the dihydroquercetin flavonoid. Russian journal of general chemistry 2006, 76. [Google Scholar] [CrossRef]
- Terekhov, R.P.; Melnikov, E.S.; Nikitin, I.D.; Tokareva, M.A.; Rodina, T.A.; Savina, A.D.; Pankov, D.I.; Zhevlakova, A.K.; Beloborodov, V.L.; Selivanova, I.A. Diastereomers of spheroidal form and commercially available taxifolin samples. Scientia Pharmaceutica 2024, 92, 5. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, X.; Tian, Y.; Zhai, S.; Liu, Y.; Xiong, Z.; Chu, S. An insight into novel therapeutic potentials of taxifolin. Frontiers in Pharmacology 2023, 14, 1173855. [Google Scholar] [CrossRef]
- Butt, S.S.; Khan, K.; Badshah, Y.; Rafiq, M.; Shabbir, M. Evaluation of pro-apoptotic potential of taxifolin against liver cancer. PeerJ 2021, 9, e11276. [Google Scholar] [CrossRef]
- Mondal, S.; Rahaman, S. Flavonoids: A vital resource in healthcare and medicine. Pharm. Pharmacol. Int. J 2020, 8, 91–104. [Google Scholar]
- Hao, B.; Yang, Z.; Liu, H.; Liu, Y.; Wang, S. Advances in Flavonoid Research: Sources, Biological Activities, and Developmental Prospectives. Current Issues in Molecular Biology 2024, 46, 2884–2925. [Google Scholar] [CrossRef]
- Zu, Y.; Wu, W.; Zhao, X.; Li, Y.; Wang, W.; Zhong, C.; Zhang, Y.; Zhao, X. Enhancement of solubility, antioxidant ability and bioavailability of taxifolin nanoparticles by liquid antisolvent precipitation technique. International journal of pharmaceutics 2014, 471, 366–376. [Google Scholar] [CrossRef]
- Kolhir, V.; Bykov, V.; Baginskaja, A.; Sokolov, S.; Glazova, N.; Leskova, T.; Sakovich, G.; Tjukavkina, N.; Kolesnik, Y.A.; Rulenko, I. Antioxidant activity of a dihydroquercetin isolated from Larix gmelinii (Rupr.) Rupr. wood. Phytotherapy research 1996, 10, 478–482. [Google Scholar] [CrossRef]
- Teselkin, Y.O.; Babenkova, I.; Kolhir, V.; Baginskaya, A.; Tjukavkina, N.; Kolesnik, Y.A.; Selivanova, I.; Eichholz, A. Dihydroquercetin as a means of antioxidative defence in rats with tetrachloromethane hepatitis. Phytotherapy Research 2000, 14, 160–162. [Google Scholar] [CrossRef]
- Li, X.; Xie, H.; Jiang, Q.; Wei, G.; Lin, L.; Li, C.; Ou, X.; Yang, L.; Xie, Y.; Fu, Z. The mechanism of (+) taxifolin’s protective antioxidant effect for• OH-treated bone marrow-derived mesenchymal stem cells. Cellular & Molecular Biology Letters 2017, 22, 1–11. [Google Scholar]
- Alanezi, A.A.; Almuqati, A.F.; Alfwuaires, M.A.; Alasmari, F.; Namazi, N.I.; Althunibat, O.Y.; Mahmoud, A.M. Taxifolin prevents cisplatin nephrotoxicity by modulating Nrf2/HO-1 pathway and mitigating oxidative stress and inflammation in mice. Pharmaceuticals 2022, 15, 1310. [Google Scholar] [CrossRef]
- Gao, L.; Yuan, P.; Zhang, Q.; Fu, Y.; Hou, Y.; Wei, Y.; Zheng, X.; Feng, W. Taxifolin improves disorders of glucose metabolism and water-salt metabolism in kidney via PI3K/AKT signaling pathway in metabolic syndrome rats. Life Sciences 2020, 263, 118713. [Google Scholar] [CrossRef]
- Iwasa, M.; Kato, H.; Iwashita, K.; Yamakage, H.; Kato, S.; Saito, S.; Ihara, M.; Nishimura, H.; Kawamoto, A.; Suganami, T. Taxifolin Suppresses Inflammatory Responses of High-Glucose-Stimulated Mouse Microglia by Attenuating the TXNIP–NLRP3 Axis. Nutrients 2023, 15, 2738. [Google Scholar] [CrossRef]
- Yang, C.-L.; Lin, Y.-S.; Liu, K.-F.; Peng, W.-H.; Hsu, C.-M. Hepatoprotective mechanisms of taxifolin on carbon tetrachloride-induced acute liver injury in mice. Nutrients 2019, 11, 2655. [Google Scholar] [CrossRef]
- Chen, X.; Huang, J.; Hu, Z.; Zhang, Q.; Li, X.; Huang, D. Protective effects of dihydroquercetin on an APAP-induced acute liver injury mouse model. International Journal of Clinical and Experimental Pathology 2017, 10, 10223. [Google Scholar]
- Okkay, U.; Ferah Okkay, I.; Cicek, B.; Aydin, I.C.; Ozkaraca, M. Hepatoprotective and neuroprotective effect of taxifolin on hepatic encephalopathy in rats. Metabolic Brain Disease 2022, 37, 1541–1556. [Google Scholar] [CrossRef]
- Razak, S.; Afsar, T.; Ullah, A.; Almajwal, A.; Alkholief, M.; Alshamsan, A.; Jahan, S. Taxifolin, a natural flavonoid interacts with cell cycle regulators causes cell cycle arrest and causes tumor regression by activating Wnt/β-catenin signaling pathway. BMC cancer 2018, 18, 1–18. [Google Scholar] [CrossRef]
- Oi, N.; Chen, H.; Ok Kim, M.; Lubet, R.A.; Bode, A.M.; Dong, Z. Taxifolin suppresses UV-induced skin carcinogenesis by targeting EGFR and PI3K. Cancer prevention research 2012, 5, 1103–1114. [Google Scholar] [CrossRef]
- Saito, S.; Yamamoto, Y.; Maki, T.; Hattori, Y.; Ito, H.; Mizuno, K.; Harada-Shiba, M.; Kalaria, R.N.; Fukushima, M.; Takahashi, R. Taxifolin inhibits amyloid-β oligomer formation and fully restores vascular integrity and memory in cerebral amyloid angiopathy. Acta neuropathologica communications 2017, 5, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Q.; Bao, X.; Ding, Y.; Shentu, J.; Cui, W.; Chen, X.; Wei, X.; Xu, S. Taxifolin prevents β-amyloid-induced impairments of synaptic formation and deficits of memory via the inhibition of cytosolic phospholipase A 2/prostaglandin E 2 content. Metabolic Brain Disease 2018, 33, 1069–1079. [Google Scholar] [CrossRef]
- Inoue, T.; Saito, S.; Tanaka, M.; Yamakage, H.; Kusakabe, T.; Shimatsu, A.; Ihara, M.; Satoh-Asahara, N. Pleiotropic neuroprotective effects of taxifolin in cerebral amyloid angiopathy. Proceedings of the National Academy of Sciences 2019, 116, 10031–10038. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, H.Y.; Park, H.J.; Shin, H.K.; Hong, K.W.; Kim, C.D. Concurrent treatment with taxifolin and cilostazol on the lowering of β-amyloid accumulation and neurotoxicity via the suppression of P-JAK2/P-STAT3/NF-κB/BACE1 signaling pathways. PLoS One 2016, 11, e0168286. [Google Scholar] [CrossRef]
- Taldaev, A.; Savina, A.D.; Olicheva, V.V.; Ivanov, S.V.; Terekhov, R.P.; Ilyasov, I.R.; Zhevlakova, A.K.; Selivanova, I.A. Protective Properties of Spheroidal Taxifolin Form in Streptozotocin-Induced Diabetic Rats. International Journal of Molecular Sciences 2023, 24, 11962. [Google Scholar] [CrossRef]
- Rehman, K.; Chohan, T.A.; Waheed, I.; Gilani, Z.; Akash, M.S.H. Taxifolin prevents postprandial hyperglycemia by regulating the activity of α-amylase: Evidence from an in vivo and in silico studies. Journal of cellular biochemistry 2019, 120, 425–438. [Google Scholar] [CrossRef]
- Gurumayum, S.; Bharadwaj, S.; Sheikh, Y.; Barge, S.R.; Saikia, K.; Swargiary, D.; Ahmed, S.A.; Thakur, D.; Borah, J.C. Taxifolin-3-O-glucoside from Osbeckia nepalensis Hook. mediates antihyperglycemic activity in CC1 hepatocytes and in diabetic Wistar rats via regulating AMPK/G6Pase/PEPCK signaling axis. Journal of Ethnopharmacology 2023, 303, 115936. [Google Scholar] [CrossRef]
- Gogoi, N.; Chowdhury, P.; Goswami, A.K.; Das, A.; Chetia, D.; Gogoi, B. Computational guided identification of a citrus flavonoid as potential inhibitor of SARS-CoV-2 main protease. Molecular diversity 2021, 25, 1745–1759. [Google Scholar] [CrossRef]
- Artem’Eva, O.; Pereselkova, D.; Fomichev, Y.P. Dihydroquercetin, the bioactive substance, to be used against pathogenic microorganisms as an alternative to antibiotics. Сельскoхoзяйственная биoлoгия 2015, 513-519.
- Cushnie, T.T.; Lamb, A.J. Antimicrobial activity of flavonoids. International journal of antimicrobial agents 2005, 26, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Zhanataev, A.; Kulakova, A.; Nasonova, V.; Durnev, A. In vivo study of dihydroquercetin genotoxicity. 2008.
- Haque, W.; Pattanayak, S.P.; Sinha, B.N. Evaluation of taxifolin and phloretin as antiangiogenic flavonoids: An in vivo, in vitro experimental analysis. Int. J. Pharm. Sci 2015, 7, 5–12. [Google Scholar]
- Yuan, X.; Li, N.; Zhang, M.; Lu, C.; Du, Z.; Zhu, W.; Wu, D. Taxifolin attenuates IMQ-induced murine psoriasis-like dermatitis by regulating T helper cell responses via Notch1 and JAK2/STAT3 signal pathways. Biomedicine & Pharmacotherapy 2020, 123, 109747. [Google Scholar]
- Feliciano, R.P.; Pritzel, S.; Heiss, C.; Rodriguez-Mateos, A. Flavonoid intake and cardiovascular disease risk. Current Opinion in Food Science 2015, 2, 92–99. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, X.; Cui, Y.; Zhou, H.; Xu, D.; Shan, T.; Zhang, F.; Guo, Y.; Chen, Y.; Wu, D. Taxifolin protects against cardiac hypertrophy and fibrosis during biomechanical stress of pressure overload. Toxicology and Applied Pharmacology 2015, 287, 168–177. [Google Scholar] [CrossRef]
- Bernatova, I.; Liskova, S. Mechanisms modified by (−)-epicatechin and taxifolin relevant for the treatment of hypertension and viral infection: Knowledge from preclinical studies. Antioxidants 2021, 10, 467. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, K.; Uchida, Y.; Murakami, N.; Mizutani, K.; Masuda, H. Effect of astilbin in tea processed from leaves of engelhardtia chrysolepis., on the serum and liver lipid concentrations and on the erythrocyte and liver antioxidative enzyme activities of rats. Bioscience, biotechnology, and biochemistry 1996, 60, 513–515. [Google Scholar] [CrossRef]
- Itaya, S.; Igarashi, K. Effects of taxifolin on the serum cholesterol level in rats. Bioscience, biotechnology, and biochemistry 1992, 56, 1492–1494. [Google Scholar] [CrossRef]
- Kostyuk, V.A.; Kraemer, T.; Sies, H.; Schewe, T. Myeloperoxidase/nitrite-mediated lipid peroxidation of low-density lipoprotein as modulated by flavonoids. FEBS letters 2003, 537, 146–150. [Google Scholar] [CrossRef]
- Theriault, A.; Wang, Q.; Van Iderstine, S.C.; Chen, B.; Franke, A.A.; Adeli, K. Modulation of hepatic lipoprotein synthesis and secretion by taxifolin, a plant flavonoid1. Journal of Lipid Research 2000, 41, 1969–1979. [Google Scholar] [CrossRef]
- Arutyunyan, T.V.; Korystova, A.F.; Kublik, L.N.; Levitman, M.K.; Shaposhnikova, V.V.; Korystov, Y.N. Effects of taxifolin on the activity of angiotensin-converting enzyme and reactive oxygen and nitrogen species in the aorta of aging rats and rats treated with the nitric oxide synthase inhibitor and dexamethasone. Age 2013, 35, 2089–2097. [Google Scholar] [CrossRef]
- Liskova, S.; Cacanyiova, S.; Cebova, M.; Berenyiova, A.; Kluknavsky, M.; Micurova, A.; Valachova, K.; Soltes, L.; Bernatova, I. Taxifolin reduces blood pressure via improvement of vascular function and mitigating the vascular inflammatory response in spontaneously hypertensive rats. International Journal of Molecular Sciences 2023, 24, 12616. [Google Scholar] [CrossRef]
- Tukhovskaya, E.; Slashcheva, G.; Shaykhutdinova, E.; Ismailova, A.; Palikova, Y.A.; Palikov, V.; Rasskazova, E.; Semushina, S.; Perepechenova, N.; Sadovnikova, E. Taxifolin reduces blood pressure in elderly hypertensive male Wistar rats. Bulletin of Experimental Biology and Medicine 2022, 174, 29–32. [Google Scholar] [CrossRef]
- Korystova, A.; Kublik, L.; Kim, Y.A.; Levitman, M.K.; Shaposhnikova, V.; Korystov, Y.N. Dihydroquercetin and fucoidin inhibit the increase of angiotensin-converting enzyme activity in the rat aorta after irradiation. Bulletin of Experimental Biology and Medicine 2018, 165, 360–363. [Google Scholar] [CrossRef]
- Plotnikov, M.; Aliev, O.; Sidekhmenova, A.; Shamanaev, A.Y.; Anishchenko, A.; Nosarev, A.; Pushkina, E. Modes of hypotensive action of dihydroquercetin in arterial hypertension. Bulletin of experimental biology and medicine 2017, 162, 353–356. [Google Scholar] [CrossRef]
- Seong, E.-H.; Gong, D.-S.; Shiwakoti, S.; Adhikari, D.; Kim, H.J.; Oak, M.-H. Taxifolin as a major bioactive compound in the vasorelaxant effect of different pigmented rice bran extracts. Frontiers in Pharmacology 2022, 13, 799064. [Google Scholar] [CrossRef]
- Cao, X.; Bi, R.; Hao, J.; Wang, S.; Huo, Y.; Demoz, R.M.; Banda, R.; Tian, S.; Xin, C.; Fu, M. A study on the protective effects of taxifolin on human umbilical vein endothelial cells and THP-1 cells damaged by hexavalent chromium: a probable mechanism for preventing cardiovascular disease induced by heavy metals. Food & function 2020, 11, 3851–3859. [Google Scholar]
- Lin, Z.; Wang, J. Taxifolin protects against doxorubicin-induced cardiotoxicity and ferroptosis by adjusting microRNA-200a-mediated Nrf2 signaling pathway. Heliyon 2023, 9. [Google Scholar] [CrossRef]
- ZENG, Z.; WANG, X.; YE, Y.; WANG, T.; SU, Q.; ZHAN, J.; SHEN, J.; ZENG, M.; ZHAO, M. Oxidative Stress Protection Mechanism of Taxifolin in H9C2 Cells. Chinese General Practice 2019, 22, 1794. [Google Scholar]
- Zhang, Y.; Shi, G.; Cai, J.; Yang, J.; Zheng, Y.; Yu, D.; Liu, Q.; Gong, Y.; Zhang, Z. Taxifolin alleviates apoptotic injury induced by DEHP exposure through cytochrome P450 homeostasis in chicken cardiomyocytes. Ecotoxicology and Environmental Safety 2019, 183, 109582. [Google Scholar] [CrossRef]
- Zheng, Y.; Shi, G.; Cai, J.; Yang, J.; Zhang, Y.; Gong, Y.; Liu, Q.; Yu, D.; Zhang, Z. Di-(2-ethyl hexyl) phthalate induces necroptosis in chicken cardiomyocytes by triggering calcium overload. Journal of hazardous materials 2020, 387, 121696. [Google Scholar] [CrossRef]
- Cai, J.; Shi, G.; Zhang, Y.; Zheng, Y.; Yang, J.; Liu, Q.; Gong, Y.; Yu, D.; Zhang, Z. Taxifolin ameliorates DEHP-induced cardiomyocyte hypertrophy via attenuating mitochondrial dysfunction and glycometabolism disorder in chicken. Environmental Pollution 2019, 255, 113155. [Google Scholar] [CrossRef]
- Coşgun, M.S.; Çoşkun, R.; Celik, A.I. The preventive effect of taxifolin on acrylamide-induced heart damage in rats. Revista de Nutrição 2022, 35, e210079. [Google Scholar] [CrossRef]
- Feng, E.; Wang, J.; Wang, X.; Wang, Z.; Chen, X.; Zhu, X.; Hou, W. Inhibition of HMGB1 might enhance the protective effect of taxifolin in cardiomyocytes via PI3K/AKT signaling pathway. Iranian Journal of Pharmaceutical Research: IJPR 2021, 20, 316. [Google Scholar]
- Sun, X.; Chen, R.-c.; Yang, Z.-h.; Sun, G.-b.; Wang, M.; Ma, X.-j.; Yang, L.-j.; Sun, X.-b. Taxifolin prevents diabetic cardiomyopathy in vivo and in vitro by inhibition of oxidative stress and cell apoptosis. Food and Chemical Toxicology 2014, 63, 221–232. [Google Scholar] [CrossRef]
- Shu, Z.; Yang, Y.; Yang, L.; Jiang, H.; Yu, X.; Wang, Y. Cardioprotective effects of dihydroquercetin against ischemia reperfusion injury by inhibiting oxidative stress and endoplasmic reticulum stress-induced apoptosis via the PI3K/Akt pathway. Food & function 2019, 10, 203–215. [Google Scholar]
- Xu, K.; Ren, X.; Wang, J.; Zhang, Q.; Fu, X.; Zhang, P.-C. Clinical development and informatics analysis of natural and semi-synthetic flavonoid drugs: A critical review. Journal of Advanced Research 2024, 63, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Augustin, R.; Mayoux, E. Mammalian sugar transporters. In Glucose homeostasis; IntechOpen: 2014.
- Wright, E.M.; Ghezzi, C.; Loo, D.D. Novel and unexpected functions of SGLTs. Physiology 2017, 32, 435–443. [Google Scholar] [CrossRef]
- Gajewska, A.; Wasiak, J.; Sapeda, N.; Młynarska, E.; Rysz, J.; Franczyk, B. SGLT2 Inhibitors in Kidney Diseases—A Narrative Review. International Journal of Molecular Sciences 2024, 25, 4959. [Google Scholar] [CrossRef]
- Xie, L.; Xiao, Y.; Tai, S.; Yang, H.; Zhou, S.; Zhou, Z. Emerging roles of sodium glucose cotransporter 2 (SGLT-2) inhibitors in diabetic cardiovascular diseases: focusing on immunity, inflammation and metabolism. Frontiers in Pharmacology 2022, 13, 836849. [Google Scholar] [CrossRef] [PubMed]
- Moparthi, K.P.; Al Rushaidi, M.T.; Muddam, M.R.; Obajeun, O.A.; Abaza, A.; Jaramillo, A.P.; Idris, F.S.; Shaikh, H.A.; Vahora, I.; Nath, T.S. Efficacy and Safety of Sodium-Glucose Cotransporter 2 Inhibitors to Decrease the Risk of Cardiovascular Diseases: A Systematic Review. Cureus 2023, 15. [Google Scholar] [CrossRef]
- Margonato, D.; Galati, G.; Mazzetti, S.; Cannistraci, R.; Perseghin, G.; Margonato, A.; Mortara, A. Renal protection: a leading mechanism for cardiovascular benefit in patients treated with SGLT2 inhibitors. Heart failure reviews 2021, 26, 337–345. [Google Scholar] [CrossRef]
- Lee, D.M.; Battson, M.L.; Jarrell, D.K.; Hou, S.; Ecton, K.E.; Weir, T.L.; Gentile, C.L. SGLT2 inhibition via dapagliflozin improves generalized vascular dysfunction and alters the gut microbiota in type 2 diabetic mice. Cardiovascular Diabetology 2018, 17, 1–14. [Google Scholar] [CrossRef]
- Salim, H.M.; Fukuda, D.; Yagi, S.; Soeki, T.; Shimabukuro, M.; Sata, M. Glycemic control with ipragliflozin, a novel selective SGLT2 inhibitor, ameliorated endothelial dysfunction in streptozotocin-induced diabetic mouse. Frontiers in cardiovascular medicine 2016, 3, 43. [Google Scholar] [CrossRef]
- Hallow, K.M.; Helmlinger, G.; Greasley, P.J.; McMurray, J.J.; Boulton, D.W. Why do SGLT2 inhibitors reduce heart failure hospitalization? A differential volume regulation hypothesis. Diabetes, Obesity and Metabolism 2018, 20, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, F.; Scheen, A. Effects of SGLT2 inhibitors on systemic and tissue low-grade inflammation: the potential contribution to diabetes complications and cardiovascular disease. Diabetes & metabolism 2018, 44, 457–464. [Google Scholar]
- Nakatsu, Y.; Kokubo, H.; Bumdelger, B.; Yoshizumi, M.; Yamamotoya, T.; Matsunaga, Y.; Ueda, K.; Inoue, Y.; Inoue, M.-K.; Fujishiro, M. The SGLT2 inhibitor luseogliflozin rapidly normalizes aortic mRNA levels of inflammation-related but not lipid-metabolism-related genes and suppresses atherosclerosis in diabetic ApoE KO mice. International journal of molecular sciences 2017, 18, 1704. [Google Scholar] [CrossRef]
- Li, X.; Flynn, E.R.; do Carmo, J.M.; Wang, Z.; da Silva, A.A.; Mouton, A.J.; Omoto, A.C.; Hall, M.E.; Hall, J.E. Direct cardiac actions of sodium-glucose cotransporter 2 inhibition improve mitochondrial function and attenuate oxidative stress in pressure overload-induced heart failure. Frontiers in Cardiovascular Medicine 2022, 9, 859253. [Google Scholar] [CrossRef]
- Tahara, A.; Takasu, T.; Yokono, M.; Imamura, M.; Kurosaki, E. Characterization and comparison of SGLT2 inhibitors: Part 3. Effects on diabetic complications in type 2 diabetic mice. European journal of pharmacology 2017, 809, 163–171. [Google Scholar] [CrossRef]
- Vallon, V.; Verma, S. Effects of SGLT2 inhibitors on kidney and cardiovascular function. Annual review of physiology 2021, 83, 503–528. [Google Scholar] [CrossRef]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. New england journal of medicine 2015, 373, 2117–2128. [Google Scholar] [CrossRef]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; De Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R. Canagliflozin and cardiovascular and renal events in type 2 diabetes. New England Journal of Medicine 2017, 377, 644–657. [Google Scholar] [CrossRef]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. New England Journal of Medicine 2019, 380, 347–357. [Google Scholar] [CrossRef]
- Cosentino, F.; Cannon, C.P.; Cherney, D.Z.; Masiukiewicz, U.; Pratley, R.; Dagogo-Jack, S.; Frederich, R.; Charbonnel, B.; Mancuso, J.; Shih, W.J. Efficacy of ertugliflozin on heart failure–related events in patients with type 2 diabetes mellitus and established atherosclerotic cardiovascular disease: results of the VERTIS CV Trial. Circulation 2020, 142, 2205–2215. [Google Scholar] [CrossRef]
- Kabel, A.; Salama, S.A. Effect of taxifolin/dapagliflozin combination on colistin-induced nephrotoxicity in rats. Human & Experimental Toxicology 2021, 40, 1767–1780. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).