Submitted:
22 July 2025
Posted:
23 July 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
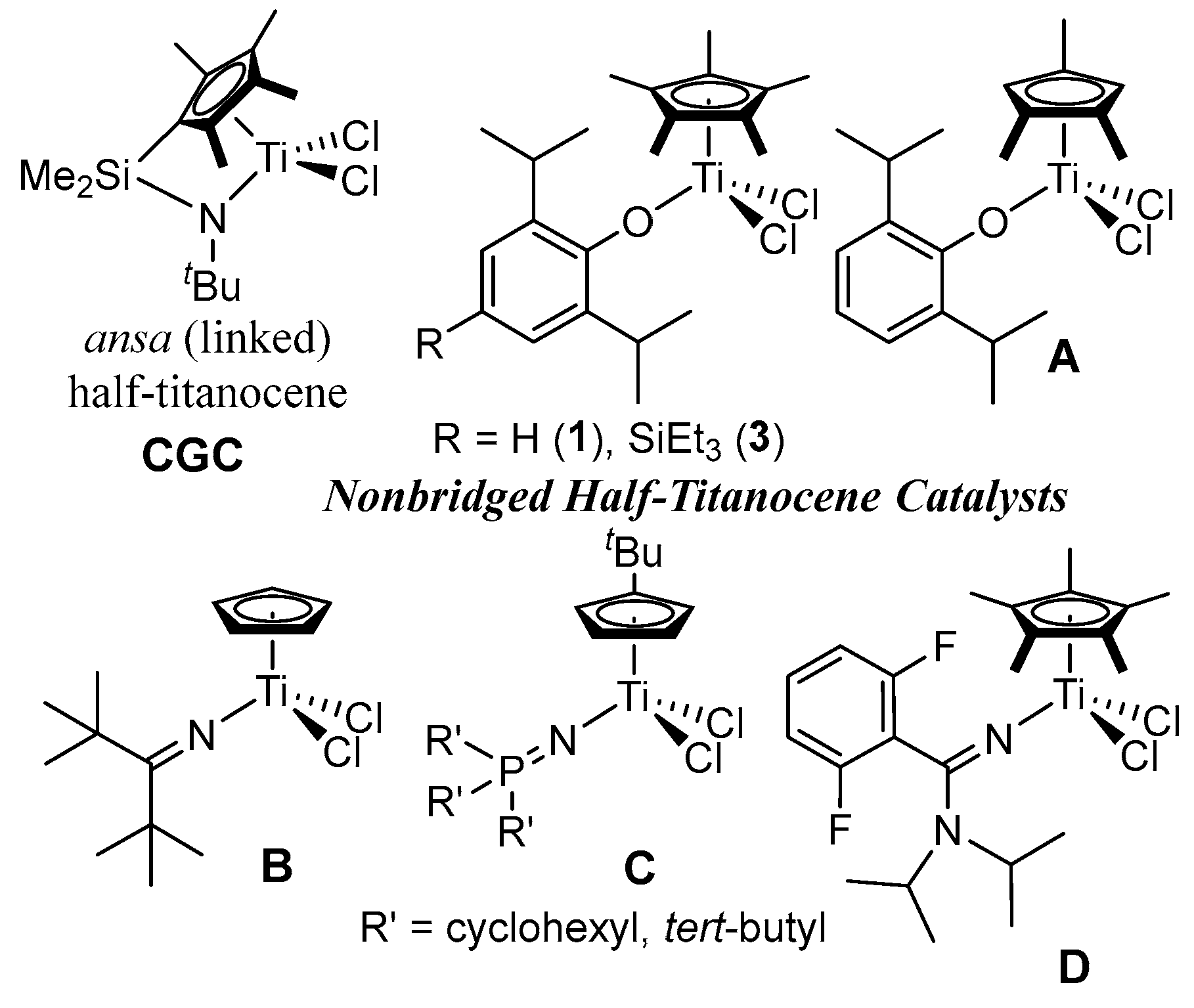
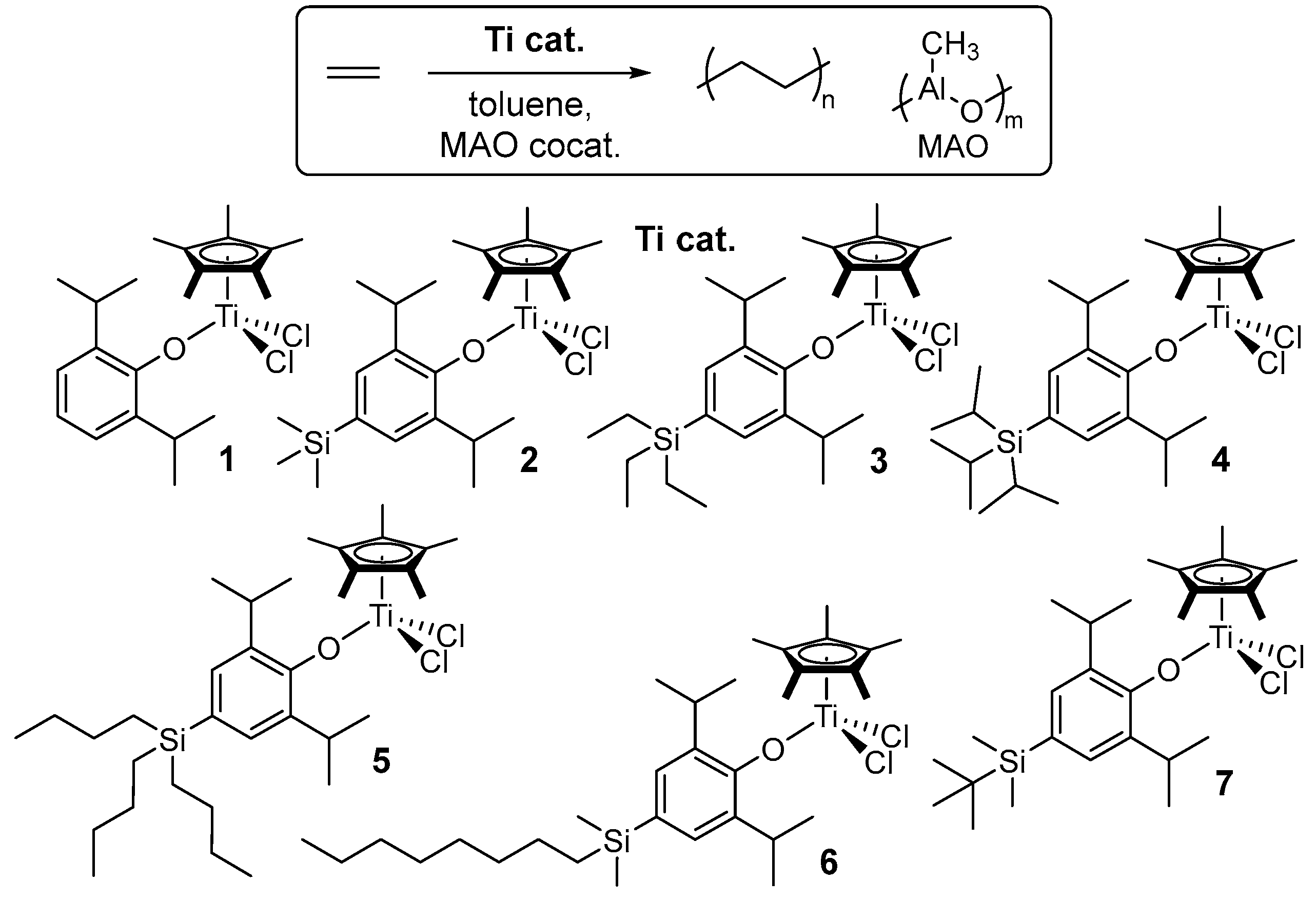
2.1. Synthesis of Half-Titanocenes Containing Different Trialkylsilyl Para-Phenoxy Substituents, Cp*TiCl2(O-2,6-iPr2-4-R-C6H2) (5-7)
2.2. Ethylene Polymerization by Cp*TiCl2(O-2,6-iPr2-4-R-C6H2) (1-7)
2.3. Ethylene Copolymerization with 1-Dodecene by Cp*TiCl2(O-2,6-iPr2-4-R-C6H2) (4-7)
3. Conclusions
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Selected pioneering reviews, see refs 1-6: Brintzinger, H. H.; Fischer, D.; Mülhaupt, R.; Rieger, B.; Waymouth, R. M. A Tailor-made metallocene for the copolymerization of ethene with bulky cycloalkenes. Angew. Chem. Int. Ed. Engl. 1995, 34, 1143–1170. [CrossRef]
- Kaminsky, W. New polymers by metallocene catalysis Macromol. Chem. Phys. 1996, 197, 3907–3945. [Google Scholar] [CrossRef]
- Kaminsky, W.; Arndt, M. Metallocenes for polymer catalysis. Adv. Polym. Sci. 1997, 127, 143–187. [Google Scholar] [CrossRef]
- Suhm, J.; Heinemann, J.; Wörner, C.; Müller, P.; Stricker, F.; Kressler, J.; Okuda, J.; Mülhaupt, R. Novel polyolefin materials via catalysis and reactive processing. Macromol. Symp. 1998, 129, 1–28. [Google Scholar] [CrossRef]
- McKnight, A. L.; Waymouth, R. M. Group 4 ansa-cyclopentadienyl-amido catalysts for olefin polymerization Chem. Rev. 1998, 98, 2587–2598. [Google Scholar] [CrossRef]
- Gibson, V. C.; Spitzmesser, S. K. Advances in non-metallocene olefin polymerization catalysis. Chem. Rev. 2003, 103, 283–316. [Google Scholar] [CrossRef] [PubMed]
- Nomura, K.; Liu, J.; Padmanabhan, S.; Kitiyanan, B. Nonbridged half-metallocenes containing anionic ancillary donor ligands: New promising candidates as catalysts for precise olefin polymerization. J. Mol. Catal. A: Chem. 2007, 267, 1–29. [Google Scholar] [CrossRef]
- Nomura, K. Half-titanocenes containing anionic ancillary donor ligands as promising new catalysts for precise olefin polymerization. Dalton Trans. 2009, 38, 8811–8823. [Google Scholar] [CrossRef] [PubMed]
- Nomura, K.; Liu, J. Half-titanocenes for precise olefin polymerisation: Effects of ligand substituents and some mechanistic aspects. Dalton Trans. 2011, 40, 7666–7682. [Google Scholar] [CrossRef] [PubMed]
- Redshaw, C.; Tang, Y. Tridentate ligands and beyond in group IV metal α-olefin homo-/co-polymerization catalysis. Chem. Soc. Rev. 2012, 41, 4484–4510. [Google Scholar] [CrossRef] [PubMed]
- Baier, M. C.; Zuideveld, M. A.; Mecking, S. Post-metallocenes in the industrial production of polyolefins. Angew. Chem. Int. Ed. 2014, 53, 9722–9744. [Google Scholar] [CrossRef] [PubMed]
- van Doremaele, G.; van Duin, M.; Valla, M.; Berthoud, A. On the development of titanium κ1-amidinate complexes, commercialized as Keltan ACETM technology, enabling the production of an unprecedented large variety of EPDM polymer structures. J. Polym. Sci. PartA: Polym. Chem. 2017, 55, 2877–2891. [Google Scholar] [CrossRef]
- Yuan, S.-F.; Yan, Y.; Solan, G. A.; Ma, Y.; Sun, W.-H. Recent advancements in N-ligated group 4 molecular catalysts for the (co)polymerization of ethylene. Coord. Chem. Rev. 2020, 411, 213254. [Google Scholar] [CrossRef]
- Organometallic Reactions and Polymerization; Osakada, K., Ed.; The Lecture Notes in Chemistry 85; Springer-Verlag: Berlin, 2014. [Google Scholar]
- Handbook of Transition Metal Polymerization Catalysts (2nd Ed.); Hoff, R., Ed.; Hoff R. Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018. [Google Scholar]
- Nomura, K.; Kitphaitun, S. Catalysis for a Sustainable Environment: Reactions, Processes and Applied Technologies; Pombeiro, A.J.L., Sutradhar, M., Alegria, E.C.B.A, Eds.; John Wiley & Sons, Ltd.: Chichester, West Sussex, UK, 2024; pp. 323–338. [Google Scholar]
- For recent examples, references 17-19: Kitphaitun, S.; Yan, Q.; Nomura, K. Effect of SiMe3, SiEt3 para-substituents for exhibiting high activity, introduction of hydroxy group in ethylene copolymerization catalyzed by phenoxide-modified half-titanocenes. Angew. Chem. Int. Ed. 2020, 59, 23072–23076. [CrossRef] [PubMed]
- Kawamura, K.; Nomura, K. Ethylene copolymerization with limonene, β-pinene: New bio-based polyolefins prepared by coordination polymerization. Macromolecules 2021, 54, 4693–4703. [Google Scholar] [CrossRef]
- Kitphaitun, S.; Chaimongkolkunasin, S.; Manit, J.; Makino, R.; Kadota, J.; Hirano, H.; Nomura, K. Ethylene/myrcene copolymer as new bio-based elastomers prepared by coordination polymerization using titanium catalysts. Macromolecules 2021, 54, 10049–10058. [Google Scholar] [CrossRef]
- For recent examples, references 20-22: Kawatsu, M.; Fujioka, T.; Losio, S.; Tritto, I.; Nomura, K. (Trialkylsilyl-cyclo-pentadienyl)titanium(IV) dichloride complexes containing ketimide ligands, Cp′TiCl2(N=CtBu2) (Cp′ = Me3SiC5H4, Et3SiC5H4), as efficient catalysts for ethylene copolymerisation with norbornene and tetracyclododecene. Catal. Sci. Technolog. 2025, 15, 2757–2765. [CrossRef]
- Wang, Q.; Chen, M.; Zou, C.; Chen, C. L. Direct synthesis of polar-functionalized polyolefin elastomers. Angew. Chem. Int. Ed. 2025, 64, e202423814. [Google Scholar] [CrossRef] [PubMed]
- Losio, S.; Boggioni, L.; Vignali, A.; Bertini, F.; Nishiyama, A.; Nomura, K.; Tritto, I. Poly(propene-co-norbornene)s with high molar masses, tunable norbornene contents and properties, in high yield by ketimide-modified half-titanocene catalysts. Polym. Chem. 2025, 16, accepted. [Google Scholar] [CrossRef]
- Selected examples, references 23-25: Stephan, D. W.; Stewart, J. C.; Guérin, F.; Spence, R. E. v. H.; Xu, W.; Harrison, D. G. Organometallics 1999, 18, 1116–1118. [CrossRef]
- Stephan, D. W.; Stewart, J. C.; Guérin, F.; Courtenay, S.; Kickham, J.; Hollink, E.; Beddie, C.; Hoskin, A.; Graham, T.; Wei, P.; Spence, R. E. v. H.; Xu, W.; Koch, L.; Gao, X.; Harrison, D. G. An Approach to Catalyst Design: Cyclopentadienyl-titanium phosphinimide complexes in ethylene polymerization. Organometallics 2003, 22, 1937–1947. [Google Scholar] [CrossRef]
- Stephan, D. W. The road to early-transition-metal phosphinimide olefin polymerization catalysts. Organometallics 2005, 24, 2548–2560. [Google Scholar] [CrossRef]
- Selected known the other reports, references 26-28: Kretschmer, W. P.; Dijkhuis, C.; Meetsma, A.; Hessen, B.; Teuben, J. H. A highly efficient titanium-based olefin polymerisation catalyst with a monoanionic iminoimidazolidide π-donor ancillary ligand. Chem. Commun. 2002, 608–609. [CrossRef]
- Tamm, M.; Randoll, S.; Herdtweck, E.; Kleigrewe, N.; Kehr, G.; Erker, G.; Rieger, B. Imidazolin-2-iminato titanium complexes: synthesis, structure and use in ethylenepolymerization catalysis. Dalton Trans 2006, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Ijpeij, E. G.; Coussens, B.; Zuideveld, M. A.; van Doremaele, G. H. J.; Mountford, P.; Lutz, M.; Spek, A. L. Synthesis, solid state and DFT structure and olefin polymerization capability of a unique base-free dimeric methyl titanium dication. Chem. Commun. 2010, 46, 3339–3341. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Shimoyama, D.; Gao, J.; Nomura, K. Synthesis of ethylene copolymers with 2-allylphenol by half-titanocene catalysts containing SiEt3-, SiiPr3-substituted phenoxide ligands, Cp*TiCl2(O-2,6-iPr2-4-SiR3-C6H2) (R = Et, iPr). Catal. Sci. Technolog. 2023, 14, 3800–3806. [Google Scholar] [CrossRef]
- Dodecene content (mol%) in poly(ethylene-co-1-hexene)s and triad sequence distributions were estimated from 13C NMR spectra. These calculations were made by the following paper, Randall, J. C. A review of high resolution liquid 13carbon nuclear magnetic resonance characterizations of ethylene based polymers. J. Macromol. Sci., Rev. Macromol. Chem. Phys. 1989, C29, 201–317. [CrossRef]
- The calculations of rE and rH values are based on dyads and the initial monomer concentrations. Ethylene concentrations under the reaction conditions were estimated by the equation quoted by Kissin (Kissin, Y.V. Isospecific Polymerization of Olefin with Heterogeneous Ziegler-Natta Catalysts, Springer-Verlag, NewYork, 1985), and the ethylene solubilities in the reaction mixture (1 atm) were used as those in toluene reported in the following article, Sahgal, A.; La, H. M.; Hayduk, W. Solubility of ethylene in several polar and non-polar solvents. Can. J. Chem. Eng. 1978, 56, 354–357. [CrossRef]
- Heiland, K.; Kaminsky, W. Comparison of zirconocene and hafnocene catalysts for the polymerization of ethylene and 1-butene. Makrmol. Chem. 1992, 193, 601. [Google Scholar] [CrossRef]
- Suhm, J.; Schneider, M. J.; Mülhaupt, R. Temperature dependence of copolymerization parameters in ethene/1-octene copolymerization using homogeneous rac-Me2Si(2-MeBenz[e]Ind)2ZrCl2/MAO catalyst. J. Polym. Sci., Part A: Polym. Chem. 1997, 35, 735. [Google Scholar] [CrossRef]
- Kakinuki, K.; Fujiki, M.; Nomura, K. Copolymerization of ethylene with α-olefins containing various substituents catalyzed by half-titanocenes: Factors affecting the monomer reactivities. Macromolecules 2009, 42, 4585–4595. [Google Scholar] [CrossRef]
- Kitphaitun, S.; Yan, Q.; Nomura, K. Effect of para-substituents in ethylene copolymerizations with 1-decene, 1-dodecene, and with 2-methyl-1-pentene using phenoxide modified half-titanocenes-MAO catalyst systems. ChemistryOpen 2021, 10, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Nomura, K.; Naga, N.; Miki, M.; Yanagi, K. Olefin polymerization by (cyclopentadienyl)(aryloxy)titanium(IV) complexes-cocatalyst systems. Macromolecules 1998, 31, 7588–7597. [Google Scholar] [CrossRef]
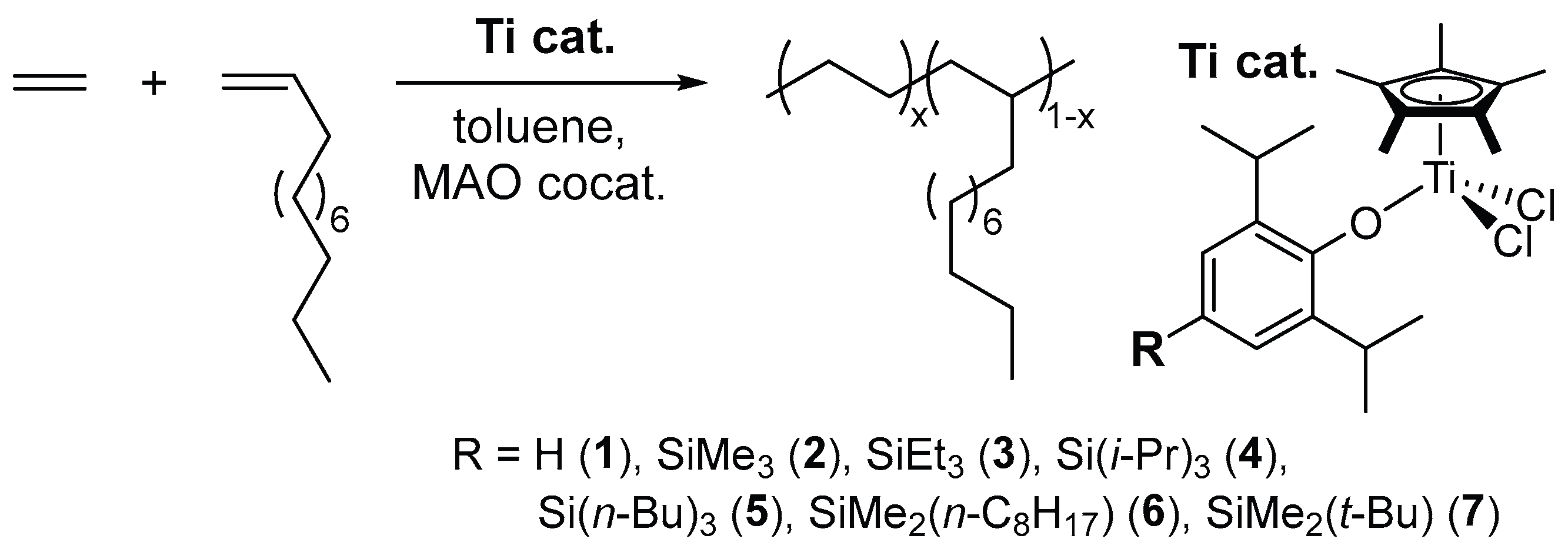
| run | cat. |
temp / °C |
yield / mg |
activity / kg-PE/mol-Ti·h |
Mn2 ×10-6 |
Mw/Mn2 |
|---|---|---|---|---|---|---|
| 1 | 1 | 25 | 124 | 49600 | 1.06 | 3.18 |
| 2 | 1 | 50 | 120 | 48000 | 0.67 | 2.41 |
| 3 | 2 | 25 | 136 | 54400 | ||
| 4 | 2 | 50 | 147 | 58800 | ||
| 5 | 3 | 25 | 139 | 55600 | 1.64 | 3.84 |
| 6 | 3 | 50 | 161 | 64400 | 0.55 | 2.96 |
| 7 | 4 | 25 | 141 | 56400 | 1.17 | 2.67 |
| 8 | 4 | 50 | 127 | 50800 | 0.43 | 2.31 |
| 9 | 5 | 25 | 164 | 65600 | 0.88 | 2.84 |
| 10 | 5 | 25 | 170 | 68000 | 1.21 | 2.78 |
| 11 | 5 | 50 | 157 | 62800 | 0.53 | 2.44 |
| 12 | 6 | 25 | 154 | 61600 | 0.78 | 2.89 |
| 13 | 6 | 25 | 157 | 62800 | 0.67 | 2.49 |
| 14 | 6 | 50 | 179 | 71600 | ||
| 15 | 7 | 25 | 147 | 58800 | 1.06 | 2.43 |
| 16 | 7 | 50 | 157 | 62800 | 0.44 | 2.72 |
| run | cat. / μmol |
temp / oC |
MAO / mmol |
yield / mg |
activity / kg-PE/mol-Ti·h |
Mn2 ×10-6 |
Mw/ Mn2 |
|---|---|---|---|---|---|---|---|
| 17 | 4 (0.005) | 25 | 2.0 | 40 | 48000 | 1.23 | 2.98 |
| 18 | 4 (0.005) | 25 | 3.0 | 60 | 72000 | ||
| 19 | 4 (0.005) | 25 | 4.0 | 73 | 87600 | 0.65 | 2.18 |
| 20 | 5 (0.005) | 25 | 2.0 | 80 | 96000 | 0.65 | 2.81 |
| 21 | 5 (0.005) | 25 | 3.0 | 108 | 129600 | ||
| 22 | 5 (0.005) | 25 | 4.0 | 120 | 144000 | 0.55 | 2.20 |
| 23 | 5 (0.015) | 50 | 3.0 | 157 | 628000 | 0.53 | 2.44 |
| 24 | 5 (0.015) | 50 | 4.0 | 161 | 644000 | 0.37 | 1.95 |
| 25 | 5 (0.015) | 50 | 5.0 | 175 | 700000 | 0.29 | 1.75 |
| 26 | 5 (0.015) | 50 | 6.0 | 161 | 644000 | 0.24 | 2.28 |
| 27 | 5 (0.015) | 80 | 2.0 | 107 | 428000 | 0.28 | 2.85 |
| 28 | 5 (0.015) | 80 | 3.0 | 124 | 496000 | 0.27 | 1.87 |
| 29 | 5 (0.015) | 80 | 4.0 | 132 | 528000 | 0.23 | 2.14 |
| 30 | 5 (0.015) | 80 | 5.0 | 151 | 604000 | 0.17 | 2.05 |
| 31 | 5 (0.015) | 80 | 6.0 | 144 | 576000 | 0.15 | 2.22 |
| run | cat. | temp / °C |
MAO / mmol |
yield / mg |
activity2 / kg-polymer/mol-Ti·h |
Mn3 ×10-5 |
Mw/ Mn3 |
cont.4 / mol% |
|---|---|---|---|---|---|---|---|---|
| 32 | 1 | 25 | 2.0 | 16 | 160000 | 2.54 | 1.90 | |
| 33 | 1 | 50 | 2.0 | 89 | 890000 | |||
| 34 | 2 | 25 | 2.0 | 85 | 850000 | 1.50 | 1.54 | |
| 35 | 2 | 50 | 2.0 | 105 | 1050000 | |||
| 36 | 3 | 25 | 2.0 | 109 | 1090000 | 1.67 | 1.62 | |
| 37 | 3 | 50 | 2.0 | 155 | 1550000 | |||
| 38 | 4 | 25 | 2.0 | 207 | 2070000 | 1.95 | 1.86 | 19.8 |
| 39 | 4 | 50 | 2.0 | 60 | 600000 | |||
| 40 | 4 | 50 | 3.5 | 72 | 720000 | 1.59 | 2.03 | 25.2 |
| 41 | 5 | 25 | 2.0 | 340 | 3400000 | 2.29 | 1.72 | 18.9 |
| 425 | 5 | 25 | 2.0 | 108 | 4320000 | |||
| 43 | 5 | 50 | 2.0 | 298 | 2980000 | |||
| 44 | 5 | 50 | 3.5 | 350 | 3500000 | 1.46 | 2.07 | 25.9 |
| 45 | 5 | 50 | 3.5 | 355 | 3550000 | |||
| 46 | 6 | 25 | 2.0 | 310 | 3100000 | 1.99 | 1.84 | 19.8 |
| 47 | 6 | 50 | 2.0 | 40 | 400000 | |||
| 48 | 6 | 50 | 2.5 | 75 | 750000 | |||
| 49 | 6 | 50 | 3.0 | 309 | 3090000 | |||
| 50 | 6 | 50 | 3.5 | 328 | 3280000 | 1.31 | 2.01 | 21.8 |
| 51 | 6 | 50 | 4.0 | 225 | 2250000 | |||
| 52 | 7 | 25 | 2.0 | 156 | 1560000 | 1.96 | 1.84 | 18.3 |
| 53 | 7 | 50 | 2.0 | 90 | 900000 | |||
| 54 | 7 | 50 | 3.5 | 120 | 1200000 | 1.68 | 2.07 | 23.2 |
| run | cat. | temp. | DD2 | triad sequence distribution3/ % | dyads4/ % | rE5 | rD5 | rE·rD6 | rE·rD7 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| / °C | / mol% | EEE | EED+DEE | DED | EDE | DDE+EDD | DDD | EE | ED+DE | DD | ||||||
| 38 | 4 | 25 | 19.8 | 46.4 | 27.5 | 6.2 | 15.3 | 3.6 | 0.9 | 60.1 | 37.1 | 2.7 | 3.34 | 0.14 | 0.48 | 0.48 |
| 40 | 4 | 50 | 25.2 | 48.1 | 20.6 | 6.0 | 19.6 | 5.6 | trace | 58.5 | 38.7 | 2.8 | 4.05 | 0.11 | 0.44 | 0.44 |
| 41 | 5 | 25 | 18.9 | 52.5 | 25.3 | 3.3 | 15.4 | 2.8 | 0.7 | 65.1 | 32.8 | 2.1 | 4.09 | 0.12 | 0.50 | 0.50 |
| 44 | 5 | 50 | 25.9 | 42.9 | 26.7 | 4.4 | 20.4 | 3.6 | 1.9 | 56.3 | 40.0 | 3.7 | 3.77 | 0.14 | 0.51 | 0.51 |
| 46 | 6 | 25 | 19.7 | 47.3 | 28.7 | 4.3 | 16.3 | 1.1 | 2.3 | 61.6 | 35.5 | 2.9 | 3.57 | 0.16 | 0.56 | 0.57 |
| 50 | 6 | 50 | 21.8 | 46.7 | 27.7 | 3.7 | 17.3 | 4.0 | 0.5 | 60.6 | 36.9 | 2.5 | 4.40 | 0.10 | 0.44 | 0.45 |
| 52 | 7 | 25 | 18.3 | 54.5 | 23.9 | 3.2 | 15.4 | 2.4 | 0.5 | 66.5 | 31.7 | 1.7 | 4.32 | 0.11 | 0.46 | 0.46 |
| 54 | 7 | 50 | 23.2 | 48.2 | 22.9 | 5.6 | 17.3 | 5.4 | 0.5 | 59.7 | 37.1 | 3.2 | 4.31 | 0.13 | 0.55 | 0.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).