1. Introduction
From the onset of puberty through menopause, progesterone and estradiol interact in complex ways — sometimes independently, at times synergistically, but most often antagonistically — to regulate physiological processes and promote health across the reproductive lifespan. Acting in synergy, progesterone enhances estradiol’s cardioprotective role in reducing exercise-induced myocardial ischemia [
1], illustrating how the coordinated signaling between these two reproductive hormones from the steroid hormone biosynthesis pathway supports physiological homeostasis. Even when acting in opposition, their dynamic interplay can be essential for the coordination of normal physiological function. Indeed, one of the most well-characterized examples of functional antagonism is progesterone’s regulatory role in the endometrium: following estradiol-induced proliferation in the follicular phase, progesterone acts to stabilize the endometrial lining in the luteal phase by suppressing further proliferation while in parallel promoting the secretion of proteins, lipids, and growth factors necessary for implantation and tissue homeostasis [
2].
Disruption of this tightly regulated antagonism — whether due to abnormal systemic levels of estradiol and progesterone or cellular level dysfunction — can have widespread consequences for reproductive and systemic health. According to the widely accepted unopposed estrogen theory, first described by Key et al. (1988) [
3], estrogen that is not opposed by an adequate progesterone concentration can exert unregulated mitogenic effects, leading to excessive endometrial proliferation and, ultimately, the development of endometrial hyperplasia and adenocarcinoma. This concept has informed therapeutic strategies that leverage progesterone’s antiproliferative effects on the endometrium. Although progesterone treatment is not indicated for all forms of endometrial cancer, it is incorporated into the management of
complex atypical hyperplasia and
clinical stage 1A low-grade endometrial tumors in patients who are not surgical candidates. In such cases, reversal of endometrial hyperplasia can be observed in as little as 10 weeks following the initiation of treatment [
4].
Epidemiological studies investigating the association between circulating levels of estradiol, progesterone, and endometrial cancer have been difficult to execute, largely due to historical reliance on immunoassay technologies that lack the specificity and sensitivity to accurately differentiate steroid hormones. The more recent adoption of mass spectrometry has overcome these limitations by offering highly specific, sensitive, and reproducible hormone quantification, making it the preferred method in both research and clinical settings. Using this approach, and in line with the strong biological premise discussed above, recent findings indicate that pre-diagnostic levels of progesterone relative to estradiol (P4:E2 ratio) in postmenopausal women are inversely associated with endometrial cancer risk [
5].
Given the growing recognition of the P4:E2 ratio as a biologically meaningful marker of endometrial cancer risk, this study aimed to identify its predictors among postmenopausal women in the United States. Leveraging data from the National Health and Nutrition Examination Survey (NHANES), the approach implemented here uniquely combined two major methodological strengths. First, it relied upon the NHANES-implemented gold-standard mass spectrometry—specifically, isotope dilution liquid chromatography-tandem mass spectrometry (ID LC-MS/MS)—for the measurement of circulating hormone concentrations with high specificity and sensitivity, thus overcoming the limitations of traditional immunoassay-based approaches. Second, it employed a supervised machine learning framework to model the relationship between the P4:E2 ratio and a broad array of features spanning hormonal, demographic, dietary, and inflammatory domains. This approach enabled the identification of complex, potentially nonlinear relationships, while ensuring rigorous model validation through cross-validation and performance benchmarking.
In addition to modeling the P4:E2 ratio, estradiol and progesterone were analyzed as individual outcomes to disentangle the distinct pathways governing each hormone. While the ratio offers a useful integrative marker, its components may be influenced by partially independent biological processes where estradiol and progesterone exhibit different temporal dynamics and sources of production. Modeling estradiol and progesterone separately allowed for the detection of unique predictors and clarified whether shared or divergent mechanisms underlie their ratio.
Taken together, the use of high-resolution hormone quantification and interpretable machine learning models positioned this study to advance our understanding of hormonal regulation in postmenopausal women and generate hypotheses for future clinical and epidemiological investigations.
2. Materials and Methods
Study Design
The present study applied a cross-sectional design using publicly available NHANES hormone data. The NHANES is a research program that gathers information about health and nutritional profile of the population in the United States. It involves in-person interviews, questionnaire administration, physical examinations, and laboratory tests. NHANES data is publicly available on the Center for Disease Control website. The present study utilized the NHANES databases that contain Sex Steroid Hormone Panel – Serum and both estradiol and progesterone: 2021-2023, 2017-2020, and 2017-2018 (Surplus). The NHANES project is approved by the National Center for Health Statistics (NCHS) Ethics Review Board. All participants signed the informed consent.
Study Population
Women reporting absence of regular menstrual cycles in the past 12 months due to menopause were included in the study. The exclusion criteria was use of any hormones, specified as hormone/hormone modifiers in the Prescription Medications - Drug Information (RXQ_DRUG) NHANES codebook.
Target Variable Derivation and Measurement
The target variable for the study supervised machine learning model was the natural log-transformed ratio of progesterone to estradiol concentrations (P4:E2), calculated as log (progesterone / estradiol). As a supplemental analysis, estradiol and progesterone were modeled as individual target variables to further explore their unique patterns of regulation and predictors. Progesterone and estradiol concentrations were measured using isotope dilution liquid chromatography-tandem mass spectrometry (ID LC-MS/MS). This method involves dissociating hormones from serum binding proteins, followed by sequential liquid-liquid extraction and quantification using mass spectrometry with isotopically labeled internal standards. The ID LC-MS/MS approach is considered the gold standard for steroid hormone measurement due to the precision of the method and minimal cross-reactivity. Progesterone and estradiol values above the limit of detection (LOD), 0.86 ng/dL and 1.72 pg/mL, respectively, were included in the analysis.
Feature Selection
Anthropomorphic. The study anthropomorphic measure was waist circumference (WC). It was measured to the nearest 0.1 cm just above the iliac crest by trained NHANES technicians using a standardized protocol.
Metabolic. Total cholesterol in NHANES was measured enzymatically in serum samples using the Roche Modular P chemistry analyzer, with results reported in mg/dL. The lower limit of detection (LLOD) for total cholesterol in this method was 4 mg/dL, and the values in the present studies were filtered as such.
Demographics. Demographic features included age, and age at menarche. Age was recorded at the time of screening, and age at menarche was self-reported during the reproductive health interview.
Dietary. Dietary intake features (total kilocalories, protein, carbohydrate, fat, sugar, and fiber) were derived from the NHANES 24-hour dietary recall interviews, which were conducted on two non-consecutive days using the USDA’s validated Automated Multiple-Pass Method. Day 1 dietary data were collected in-person at the Mobile Examination Center (MEC), while Day 2 data were collected by telephone 3 to 10 days later. Data were extracted from NHANES Day 1 and Day 2 total nutrient files and mean daily intake for each nutrient was calculated across the two recall days to improve dietary exposure estimation and minimize intra-individual variability. All dietary variables were included as continuous features in the model.
Hormonal. Hormone features included estrone, estrone sulfate, follicle-stimulating hormone (FSH), and luteinizing hormone (LH). The ratio of estrone sulfate to estrone (sulfation ratio) was calculated to reflect estrogen storage capacity, as estrone sulfate serves as a circulating reservoir that can be converted to bioactive estrone and subsequently estradiol. Hormone values were filtered to retain values above LOD: estrone (0.13 ng/dL), estrone sulfate (2.04 pg/mL), FSH (0.30 mIU/mL) and LH (0.10 mIU/mL).
Inflammatory. High-sensitivity C-reactive protein (hs-CRP) was included as an indicator of systemic low-grade inflammation. In NHANES, CRP was measured in serum using a high-sensitivity assay, following standardized laboratory protocols. Values within the analytic range (0.15-20 mg/L) were included as continuous variables to capture the full distribution of inflammatory status.
Statistical Analysis
Statistical Analysis Framework and Data Preparation. The primary modeling objective was a prediction of the P4:E2 ratio as a continuous outcome based on demographic, anthropometric, hormonal, dietary, and inflammatory predictors using regression. The dataset was randomly partitioned into a training set, comprising 70% of the observations, and a test set, comprising the remaining 30%. Stratified sampling was used to ensure balanced representation of the target variable across both sets. In addition to modeling the P4:E2 ratio, two secondary models were constructed to predict serum estradiol and serum progesterone concentrations independently, using the same set of predictors. These secondary analyses were conducted to decompose the P4:E2 ratio signal and help identify whether the model’s predictive power was primarily driven by estradiol or progesterone. Each hormone was log-transformed prior to modeling to correct for skewness and stabilize variance.
Missing Data. Missing values in the dataset were handled using median imputation to minimize the influence of outliers and to maintain the central tendency of each variable. Using the pre-processing capabilities of the caret package [
6], the imputation model was developed on the training data and subsequently applied to both the training and test datasets to prevent information leakage.
Model Development and Hyperparameter Tuning. All predictive models were developed using the native xgboost package [
7]. To reduce the risk of overfitting, the following conservative model complexity and regularization settings were implemented: tree depth was capped at 4 (max_depth = 4), minimum child weight was set to 10 (min_child_weight = 10), and both L1 (alpha = 2) and L2 (lambda = 2) regularization penalties were applied. A small learning rate (eta = 0.03) and dropout-like subsampling of observations and features (subsample = 0.6, colsample_bytree = 0.6) were used to further enhance generalizability. Model hyperparameters were optimized using Bayesian optimization via the ParBayesianOptimization package, which efficiently searches the hyperparameter space by leveraging a Gaussian process surrogate model. Five-fold cross-validation with early stopping (patience = 50 rounds) was used during tuning to identify the optimal number of boosting rounds.
Final Model Training and Evaluation. Final XGBoost models for the P4:E2 ratio, estradiol, and progesterone were each trained on their respective training sets using the optimal hyperparameters identified during cross-validation and Bayesian optimization. Predictions were then generated on the held-out test sets to assess generalizability. Model performance was evaluated using root mean squared error (RMSE), mean absolute error (MAE), and the coefficient of determination (R2). Residual diagnostics were conducted for each model to assess assumptions and detect any prediction error patterns.
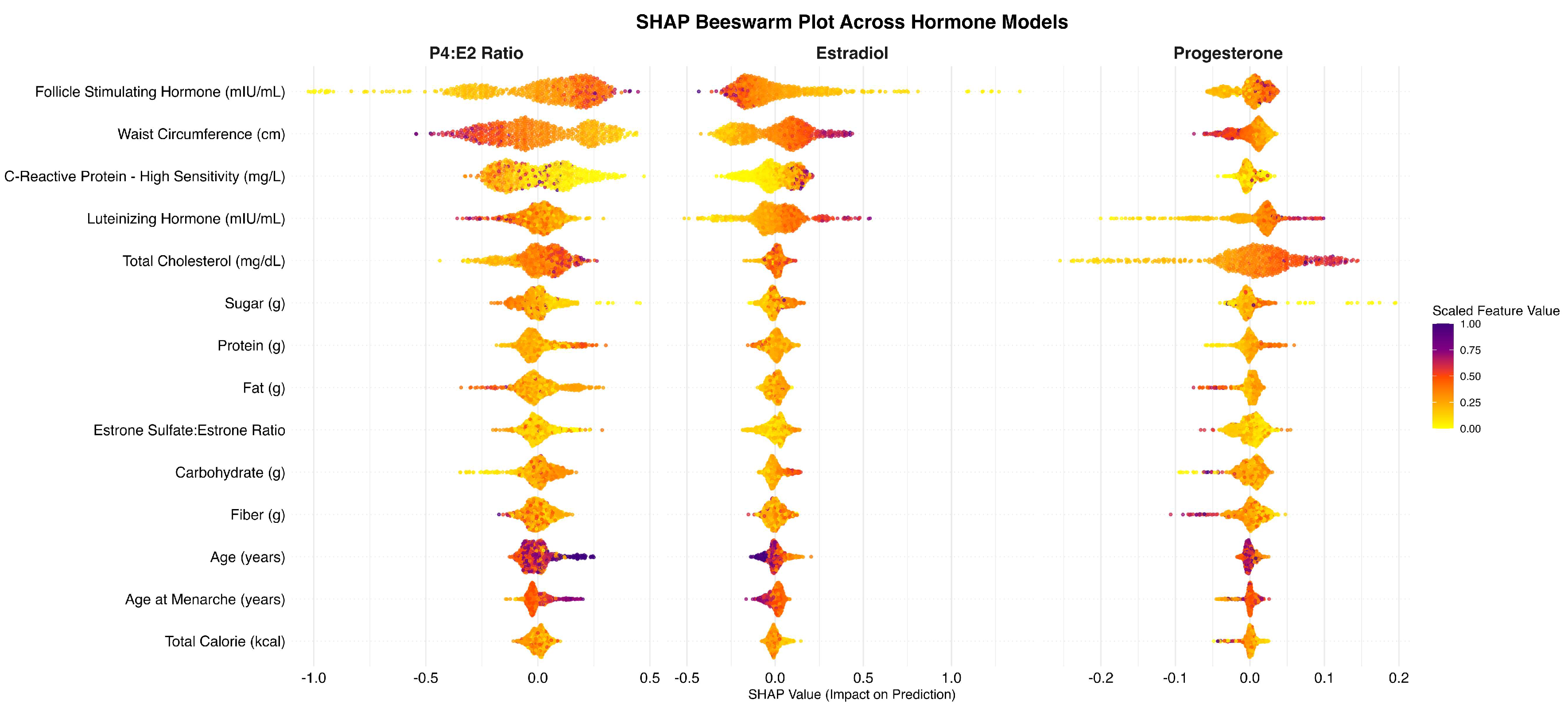
Model Interpretation Using SHapley Additive exPlanations (SHAP) Values. To further interpret the final XGBoost models, SHAP values were computed to quantify the contribution of each predictor to individual predictions. The fastshap package was used to calculate SHAP values for the test dataset. SHAP values were generated using Monte Carlo sampling with 200 simulations to ensure stable and reliable estimates of feature contributions. Separate SHAP analyses were performed for each of the three models (P4:E2 ratio, estradiol, and progesterone), and the resulting feature importance rankings were compared to assess the differential influence of predictors across hormone outcomes.
4. Discussion
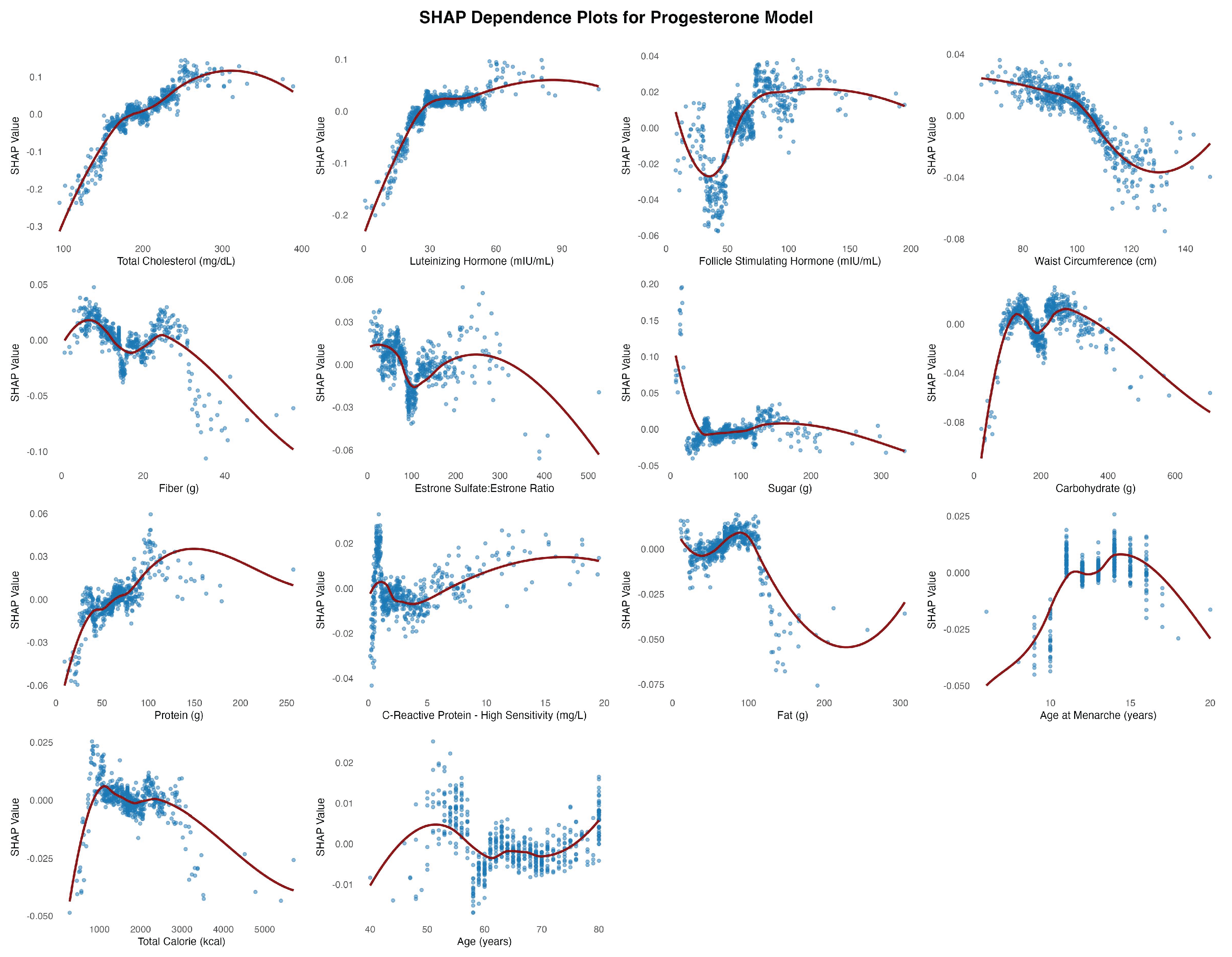
In this study of 1,902 postmenopausal women from the NHANES dataset, we developed a machine learning model to identify key predictors of the P4:E2 ratio—an emerging risk factor for endometrial cancer. The model achieved an R2 of 0.298 on the test set, indicating that approximately 30% of the variance in the log-transformed P4:E2 ratio could be explained by the selected predictors. We found that FSH, waist circumference, and CRP were the most influential predictors, followed by total cholesterol, LH, and intake of dietary fat, protein, and sugar. Additional models revealed that FSH and waist circumference primarily predicted estradiol levels, while progesterone was more strongly influenced by cholesterol and LH. These findings offer new insights into the hormonal, metabolic, and lifestyle correlates of the P4:E2 ratio and provide a foundation for future work aimed at understanding its role in postmenopausal health and disease risk.
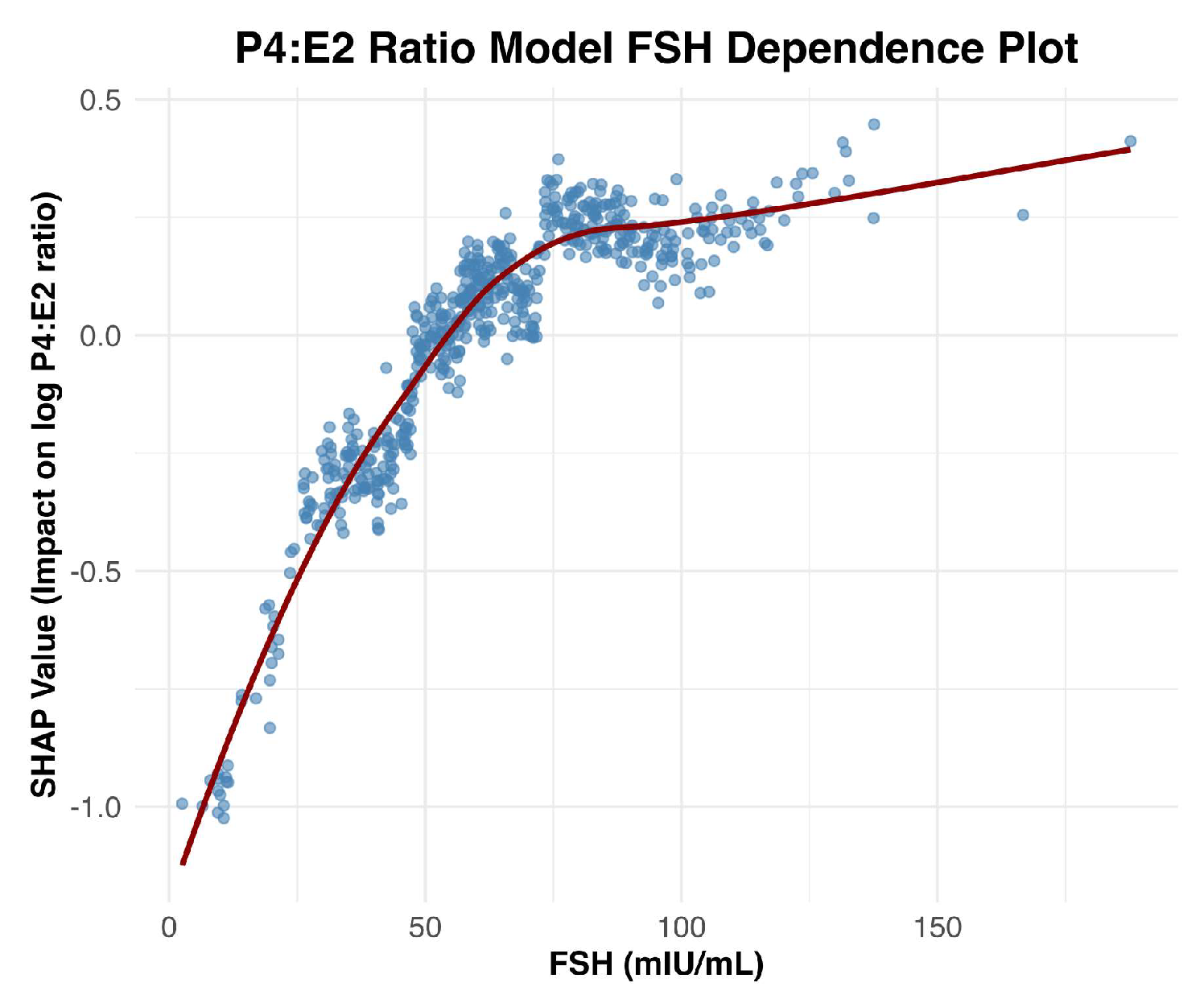
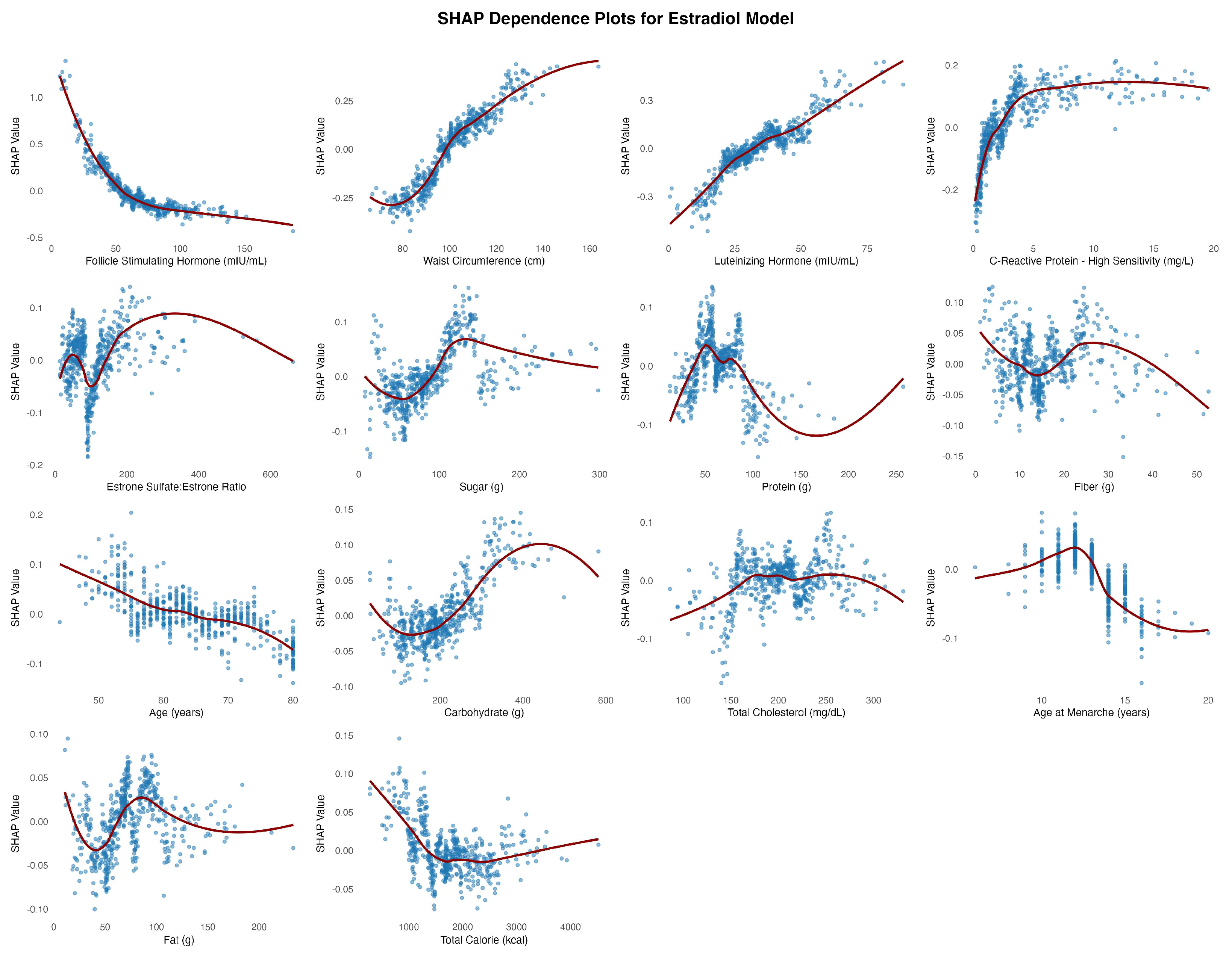
The observed inverse association between FSH and the P4:E2 ratio is biologically consistent with known endocrine adaptations to menopause [
8]. As ovarian function declines, circulating levels of both progesterone and estradiol decrease, resulting in diminished negative feedback on the hypothalamic–pituitary–gonadal axis. Notably, progesterone production stabilizes at low levels by the onset of menopause, whereas estradiol continues to be produced in more variable quantities, with progressively lower and more stable levels as menopause progresses. As a result, with the progression of menopause, the P4:E2 ratio increases, leading to compensatory increases in FSH. This relationship is visualized in the SHAP dependence plot (
Figure 2), which reveals a strong, nonlinear positive association between FSH and the predicted log-transformed P4:E2 ratio. SHAP values increase sharply with FSH concentrations up to approximately 75 mIU/mL, after which the curve plateaus, indicating a deflection point beyond which additional increases in FSH contribute minimally to the model’s output. This plateau may reflect a biological “ceiling effect,” wherein estradiol levels have already reached minimal postmenopausal values, thereby limiting the predictive utility of further increases in FSH.
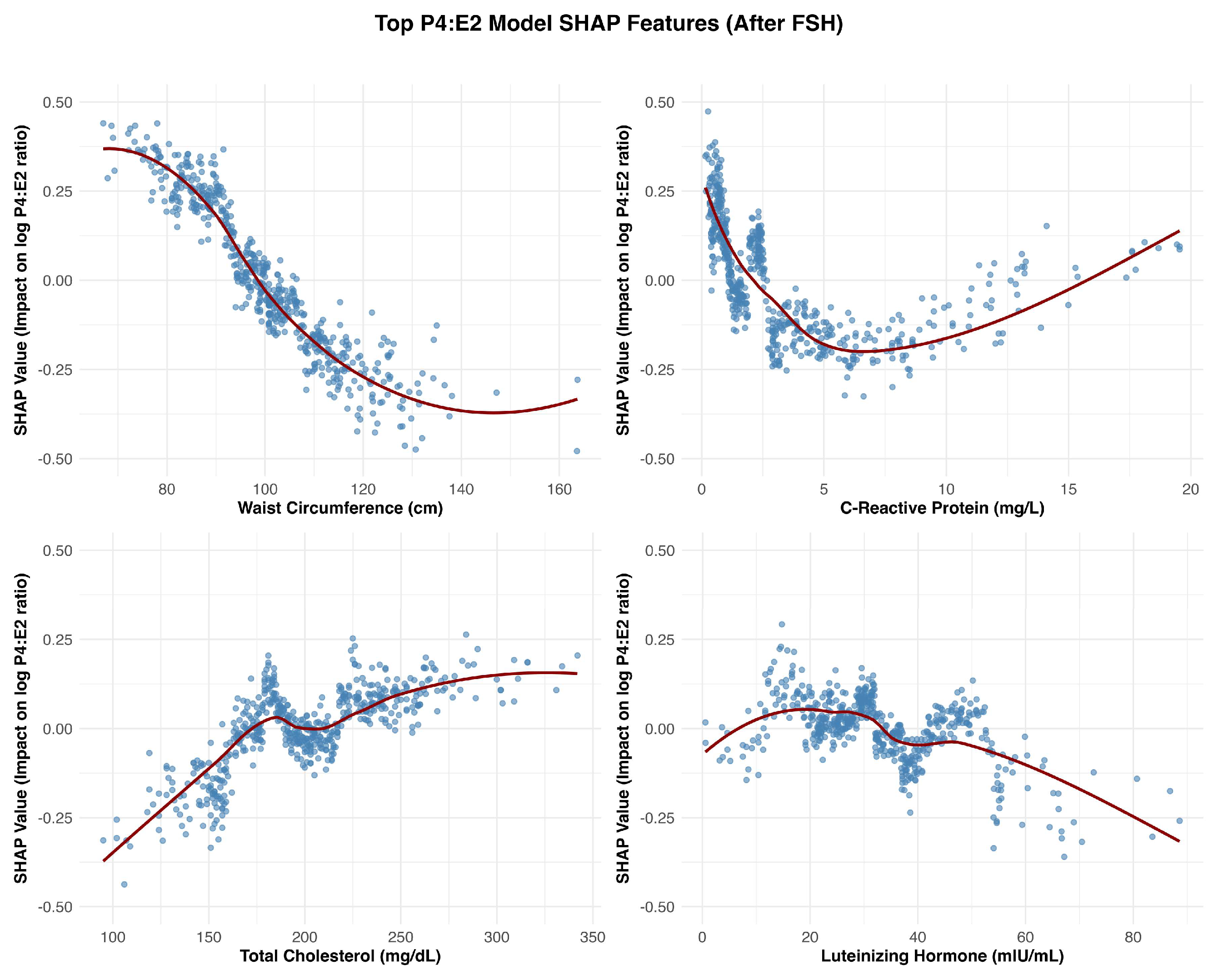
Waist circumference emerged as a key feature influencing the P4:E2 ratio, with SHAP dependence plots suggesting that this effect was primarily driven by estradiol elevations associated with adiposity, while progesterone contributed a waist circumference–restricted effect that emerged only beyond higher thresholds of central adiposity. In the estradiol model, SHAP values increased steadily with waist circumference, reflecting the well-established role of adipose tissue as a site of peripheral estrogen biosynthesis via aromatization [
9]. This adiposity-related rise in estradiol exerts downward pressure on the P4:E2 ratio by disproportionately elevating estradiol relative to progesterone. Notably, starting at waist circumferences of approximately 100 cm, a modest progesterone decline also emerged, suggesting that at this level of adiposity the influence of central adiposity may extend to both hormones, with progesterone dynamics contributing a secondary, waist circumference–dependent effect that reinforces the downward slope of the ratio.
C-reactive protein (CRP) emerged as one of the strongest non-hormonal predictors of the P4:E2 ratio, with SHAP dependence plots revealing a sharp decline in the ratio at lower CRP concentrations, followed by a plateau beyond approximately 5 mg/L. In the estradiol model, SHAP values increased steeply below this threshold and then stabilized, indicating a nonlinear relationship. The effects of estradiol on inflammation are context-dependent and can be pro- or anti-inflammatory depending on the cytokine profile, immune cell type, and estrogen receptor expression patterns [
10]. Pro-inflammatory actions of estradiol, as suggested in the present study assessing the hormone’s association with CRP, are mediated through estrogen receptor signaling pathways that activate transcription factors, particularly in immune and endothelial cells [
11]. The pattern observed in the present study underscores the importance of accounting for the concentration-sensitive interactions between estrogenic activity and inflammatory signaling in postmenopausal physiology, especially considering the altered distribution and function of estrogen receptor subtypes that occur with aging. In fact, prior studies examining the CRP–estradiol associations in postmenopausal women yielded mixed results—some reporting a positive association, others finding no significant relationship (reviewed in [
12]). The use of machine learning in the present analysis enabled detection of threshold-dependent, positive nonlinear associations that helps to reconcile these discrepancies and offer a more nuanced understanding of inflammation–estradiol dynamics.
Total cholesterol exhibited a nonlinear, predominantly positive association in both the P4:E2 and progesterone models. The relationship between estradiol and total cholesterol was relatively weak and more linear, suggesting a limited role for cholesterol in estradiol regulation. The divergence in the association between the two reproductive hormones (i.e., estradiol and progesterone) and total cholesterol likely reflects differences in their positions within the steroid biosynthesis pathway, with progesterone situated upstream and closer to cholesterol than estradiol. In the P4:E2 ratio model, SHAP values increased notably between approximately 140 and 200 mg/dL, with a plateau observed at higher concentrations, indicating a threshold-dependent effect on the ratio. The progesterone model revealed a strong and pronounced positive association, with SHAP values rising steeply between 120 and 220 mg/dL before stabilizing, highlighting cholesterol as a key metabolic predictor of progesterone levels (and the higher P4:E2 ratio) in postmenopausal women.
Although ovulatory cycles cease after menopause, the pulsatile release of LH often mirrors that of FSH, albeit its secretory amplitude is smaller [
13]. The non-linear relationship between LH and the P4:E2 ratio appears to reflect distinct—and at times opposing—contributions from estradiol and progesterone. In the present study, the stimulatory effect of LH on progesterone output plateaued at approximately 40 mIU/mL, contributing to an increase in the ratio within this range. Conversely, estradiol demonstrated a continuous positive association with LH across the entire range of values, exerting a countervailing influence that tempered the rise in the ratio up to 40 mIU/mL. Beyond this threshold, the persistent rise in estradiol—combined with the plateauing of progesterone—drove the ratio downward.
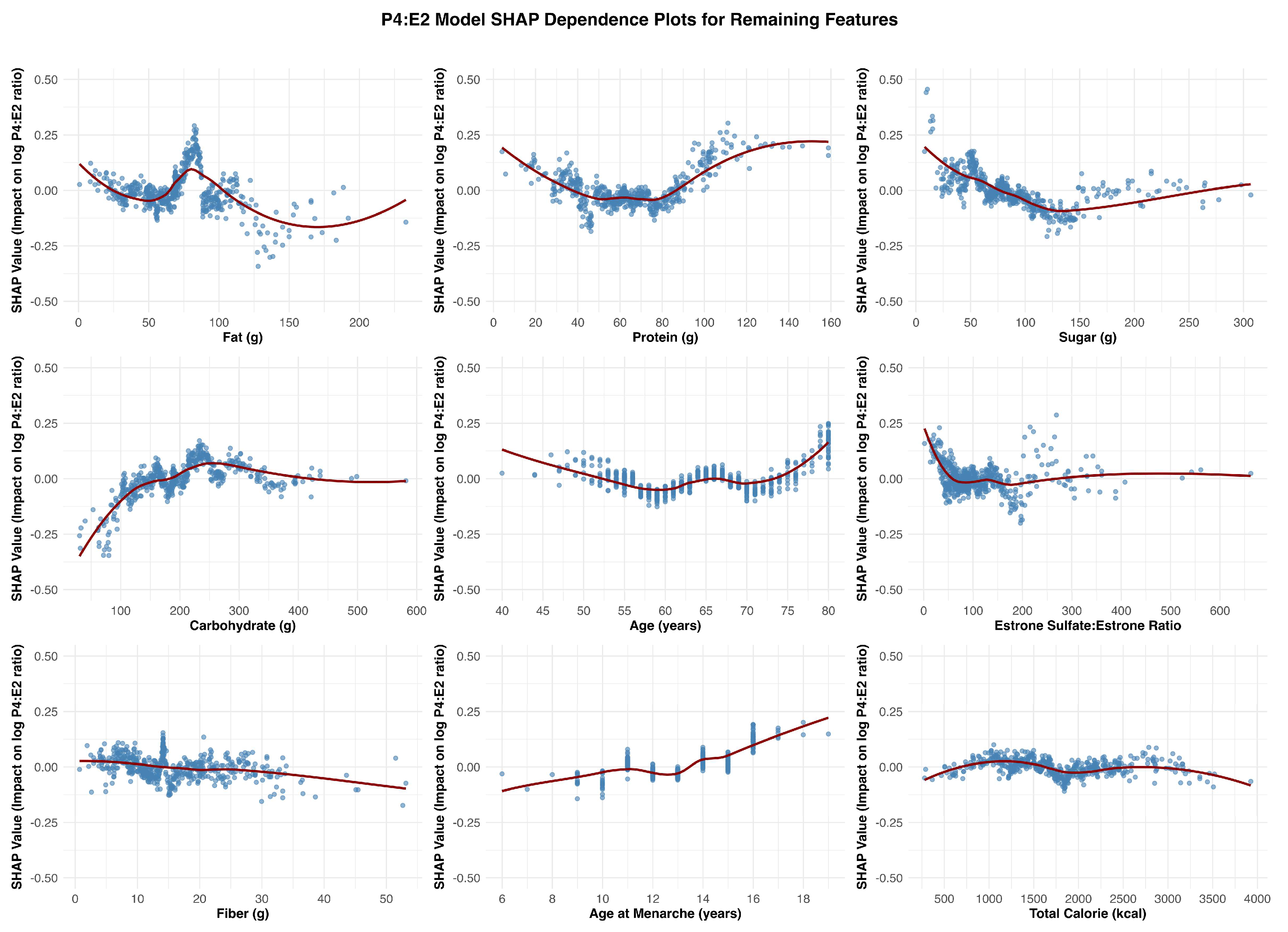
Carbohydrate intake emerged as the most consistent and meaningful dietary contributor across the three models. It showed a positive association with estradiol, particularly within the ~100 to ~250 g/day range, beyond which the effect plateaued. A similar but more attenuated pattern was observed in the P4:E2 ratio model, with SHAP values increasing gradually and leveling off beyond ~200 g/day. In the progesterone model, the association with carbohydrate intake was relatively flat, with only minor positive effects observed at lower intake levels, indicating a less consistent relationship. The remaining dietary measures exhibited weaker, inconsistent, or minimal effects on hormonal outcomes. The remaining dietary features tended to show nonlinear but shallow SHAP profiles, often centering near zero or demonstrating fluctuating associations that lacked clear thresholds or sustained impact across models.
The SHAP dependence plot for age at menarche in the P4:E2 ratio model revealed a U-shaped pattern, with a modest decline in the ratio observed between approximately ages 10 and 13, followed by a steady increase beyond this range. This shape appears to reflect contrasting associations in the component hormone models: in the estradiol model, earlier age at menarche is associated with higher estradiol levels, while in the progesterone model, a positive association emerges at later menarche ages. These opposing trends result in a biphasic effect on the ratio, where the influence of estradiol predominates at younger menarcheal ages, lowering the ratio, and progesterone’s influence becomes more apparent at later ages, pushing the ratio upward. This composite pattern underscores how developmental timing may impart lasting effects on postmenopausal hormonal balance through divergent trajectories of individual steroid hormones. The SHAP dependence plots for age across the three models—estradiol, progesterone, and the P4:E2 ratio—show largely modest and inconsistent effects, suggesting limited explanatory value of chronological age alone in postmenopausal hormone variability.
Estrone sulfate serves as a circulating estrogen reservoir that can be converted to bioactive estradiol via the intermediate estrone conversion step. This process appears to be limited in postmenopausal women as the SHAP dependence plots reveal subtle associations across the estradiol and P4:E2 models. In the estradiol model, there is a mild nonlinear relationship, with SHAP values increasing slightly at low ratio values, followed by a plateau, suggesting a modest positive influence of a higher sulfate-to-parent hormone balance on estradiol levels. Similarly, the P4:E2 ratio model exhibits minimal SHAP variation across the estrone sulfate:estrone ratio range, indicating limited influence on the ratio itself. These findings suggest that while estrone sulfate may contribute to estradiol availability, its impact is not strong enough to meaningfully affect the balance between progesterone and estradiol in a postmenopausal context.
Having examined the individual SHAP dependence patterns above, a more integrated understanding takes shape regarding the interplay between global feature importance and context-specific hormonal dynamics. Estradiol consistently emerged as the dominant hormonal driver across the examined features, demonstrating the highest SHAP magnitudes and most pronounced associations in both the individual estradiol model and the P4:E2 ratio model (Table 2). However, although the overall SHAP magnitude for the progesterone model was modest, the hormone nonetheless exerted a meaningful influence on the P4:E2 ratio in specific contexts. This was particularly evident for features such as total cholesterol LH, and waist circumference where SHAP dependence plots showed that progesterone altered the shape and direction of the ratio’s response. These findings underscore the importance of considering biological relevance alongside global model performance metrics. Progesterone’s sensitivity to upstream metabolic and gonadotropic signals—even if less predictive in isolation—can meaningfully modulate hormonal balance, particularly in systems modeled as ratios. Thus, while estradiol was the dominant driver of SHAP variance in most cases, progesterone’s context-specific contributions add interpretive depth to mechanistic inferences.
The mechanistic insights presented here align with epidemiological evidence linking distinct patterns of progesterone and estradiol concentrations to hormone-sensitive cancer risk in postmenopausal women. Notably, endogenous progesterone appears to play divergent roles in relation to estradiol —
reducing risk in the endometrium but potentially increasing it in the breast. In a case-cohort study nested within the Breast and Bone Follow-up to the Fracture Intervention Trial examining endometrial cancer incidence in relation to progesterone to estradiol ratio in postmenopausal women during a 12-year follow-up, Trabert et al. (2021) [
5] reported that postmenopausal women with high estradiol and low progesterone had the highest risk of developing endometrial cancer, while those with higher progesterone levels exhibited reduced risk. In contrast, in the same cohort,
analysis of 405 incident breast cancer cases revealed that elevated progesterone concentrations were associated with an
increased risk of invasive breast cancer—particularly when estradiol levels were also high [
14]. Together, these data underscore the complex and tissue-specific roles of progesterone in hormone-sensitive cancers—
exerting protective effects in the endometrium while potentially promoting tumorigenesis in the breast. Indeed, although both clinical and epidemiological studies support a synergistic role of estradiol and progesterone in elevating breast cancer risk,
disentangling their individual contributions remains challenging due to the partial dependence of progesterone receptor transcription on estrogen receptor α–mediated signaling [
15].
Thus, evaluating their combined hormonal interaction may be more informative than attempting to isolate independent effects [
15].
The divergent role of progesterone in endometrial versus breast cancer risk underscores the importance of contextualizing hormonal balance within specific biological outcomes and disease pathways, reinforcing the need for mechanistically informed, tissue-targeted research in postmenopausal women. In this regard, the present study’s feature-level SHAP modeling offers a framework for disentangling the nuanced, context-dependent effects of individual hormones. As an example, the analysis of waist circumference revealed how central adiposity may elevate estradiol and reduce progesterone in a threshold-dependent manner (i.e., waist circumference >100 cm) to promote endometrial proliferation while potentially mitigating breast carcinogenesis.
Despite offering valuable insights into the determinants of the P4:E2 ratio, several limitations should be noted. The cross-sectional nature of the NHANES dataset limits causal inference, as the temporal ordering between predictors and hormone levels cannot be established. Although the P4:E2 model explained a moderate proportion of variance (R2 = 0.298), the progesterone model demonstrated low predictive performance (R2 = 0.022), suggesting that relevant biological or behavioral variables may be unmeasured or inadequately captured. Finally, residual confounding remains a concern, as unmeasured factors such as stress and circadian timing could influence hormonal dynamics.