Submitted:
01 August 2025
Posted:
05 August 2025
You are already at the latest version
Abstract
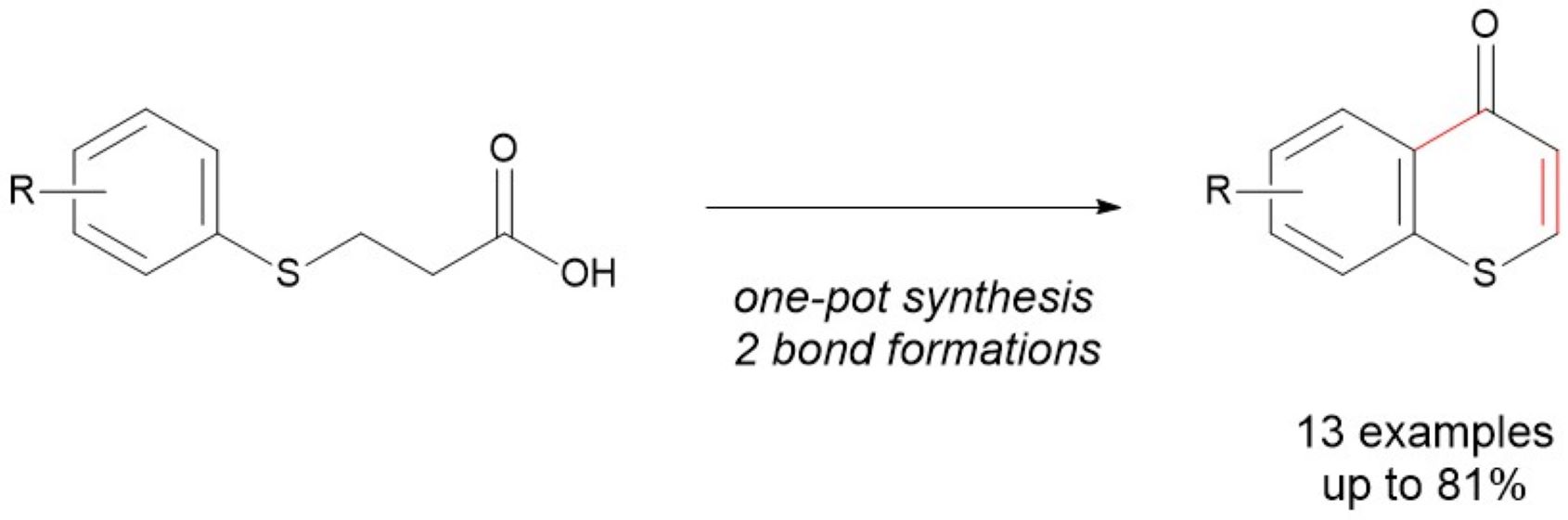
Keywords:
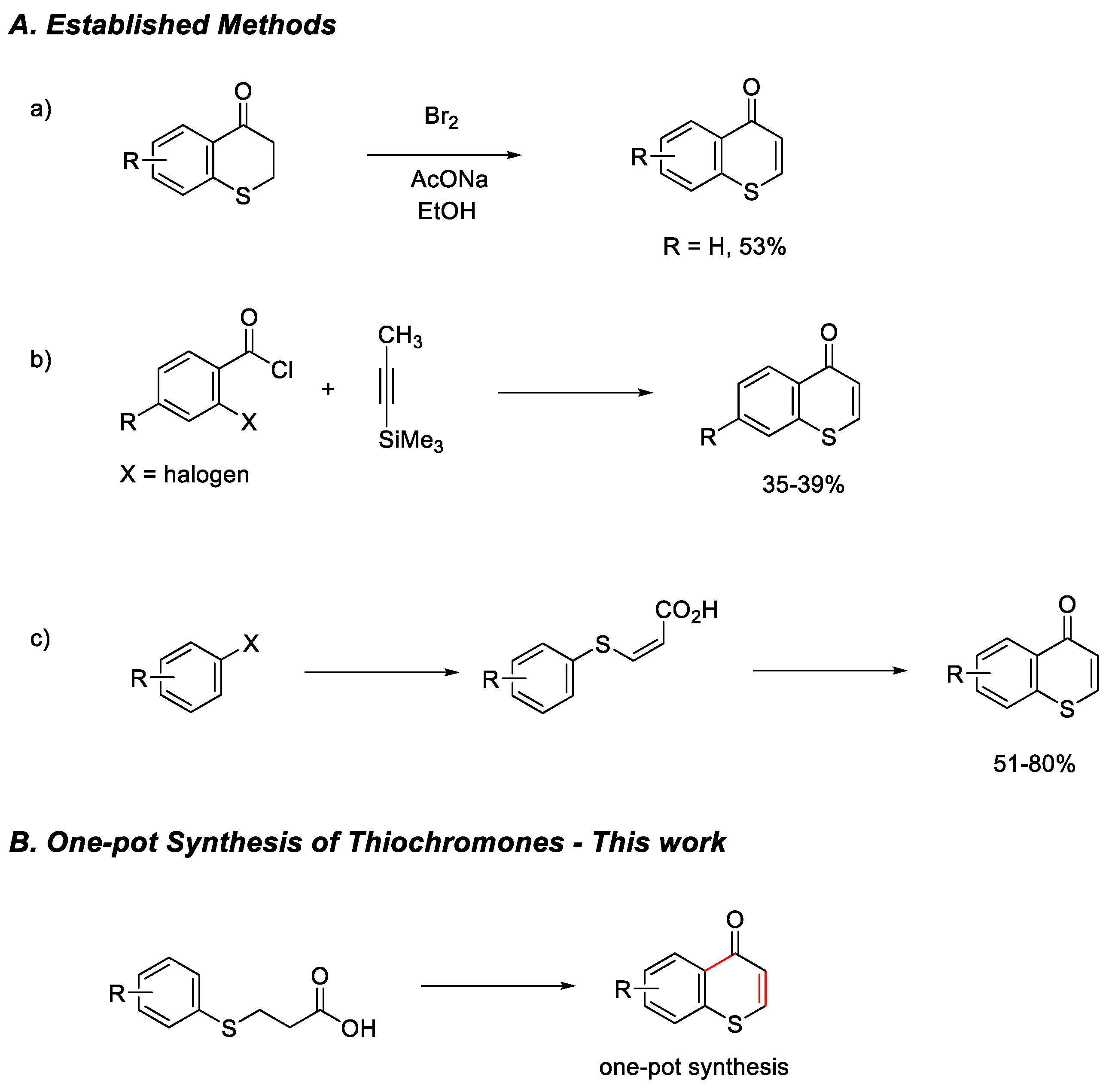
1. Introduction
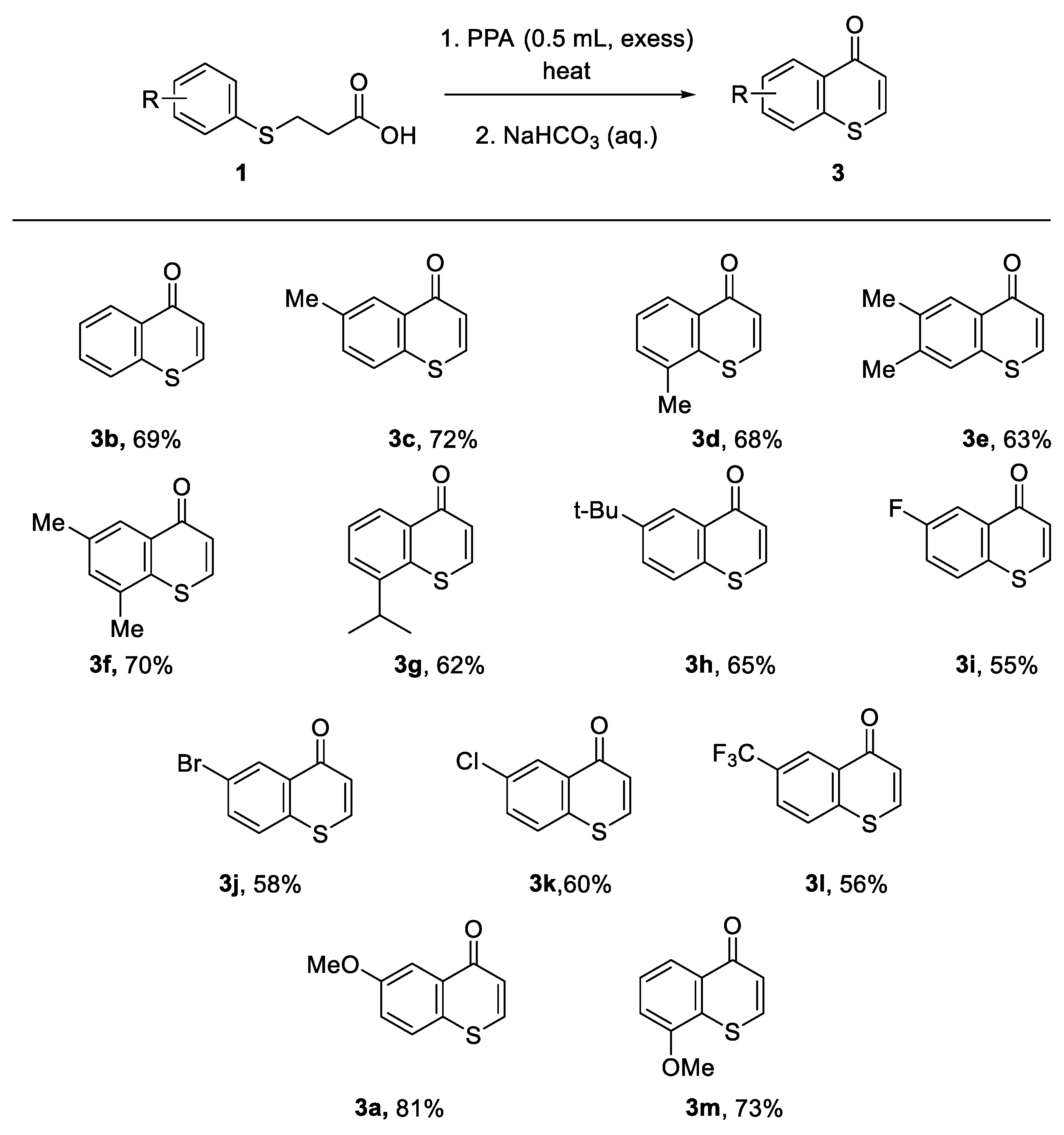
2. Results and Discussions
3. Materials and Methods
3.1. General Methods
3.2. Materials
3.3. General Procedures
3.3.1. General Procedures A
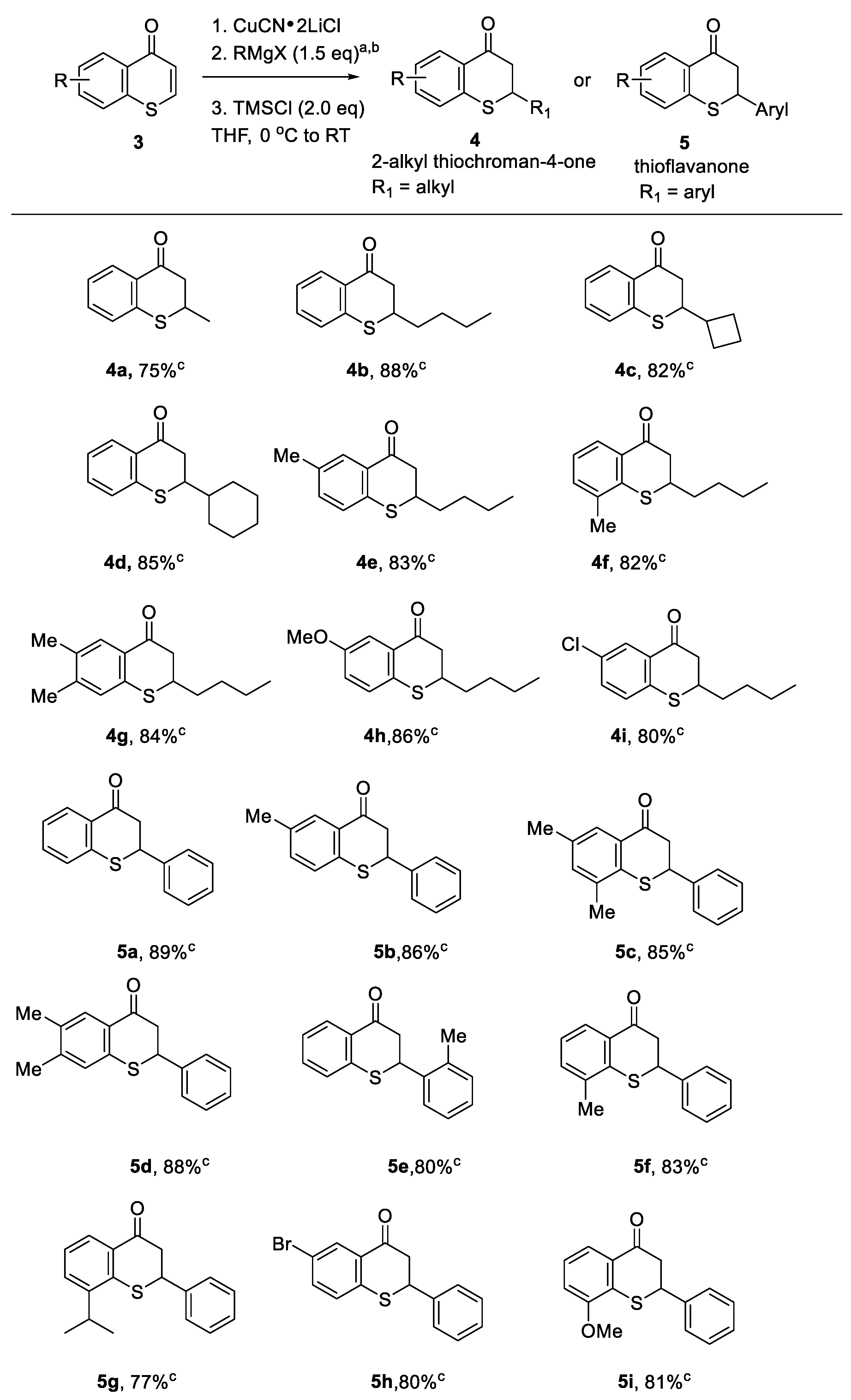
3.3.2. General Procedures B: Synthetic Applications of Thiochromones - Conjugate Addition Reactions of Grignard Reagents (RMgX or ArMgX; X = Cl or Br) to Thiochromones Catalyzed by CuCN∙2LiCl (0.2 eq)
3.4. Synthesis
3.4.1. Synthesis of 6-Methoxylthiochromone (3a)
3.4.2. Synthesis of 8-Methylthiochromone (3d)
3.4.3. Synthesis of 6, 8-Dimethylthiochromone (3f)
3.4.4. Synthesis of 8-Isopropylthiochromone (3g)
3.4.5. Synthesis of 8-Methoxylthiochromone (3m)
3.4.6. Synthesis of 2-Cyclopropylthiochroman-4-one (4c)
3.4.7. Synthesis of 8-Methyl-2-n-butylthiochroman-4-one (4f) [16]
3.4.8. Synthesis of 6-Isopropyl-2-phenylthiochroman-4-one (5g) [14]
3.4.9. Synthesis of 8-Methoxy-2-phenylthiochroman-4-one (5i) [14]
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Costa, M.; Dias, T.A.; Brito, A.; Proença, F. Biological importance of structurally diversified chromenes. Eur. J. Med. Chem. 2016, 123, 487–507.
- Keri, R.S.; Budagumpi, S.; Pai, R.K.; Balakrishna, R.G. Chromones as a privileged scaffold in drug discovery: A review. Eur. J. Med. Chem. 2014, 78, 340–374.
- Welsch, M.E.; Snyder, S.A.; Stockwell, B.R. Privileged scaffolds for library design and drug discovery. Curr. Opin. Chem. Biol. 2010, 14, 347–361.
- Csepanyi, E.; Szabados-Furjesi, P.; Kiss-Szikszai, A.; Frensemeier, L.M.; Karst, U.; Lekli, I.; Haines, D.D.; Tosaki, A.; Bak, I. Antioxidant properties and oxidative transformation of different chromone derivatives. Molecules 2017, 22, 588.
- Presley, C.C.; Valenciano, A.L.; Fernández-Murga, M.L.; Du, Y.; Shanaiah, N.; Cassera, M.B.; Goetz, M.; Clement, J.A.; Kingston, D.G.I. Antiplasmodial chromanes and chromenes from the monotypic plant species Koeberlinia spinosa. J. Nat. Prod. 2018, 81, 475-483.
- Payen, L.; Honorat, M.; Guitton, J.; Gauthier, C.; Bouard, C.; Lecerf-Schmidt, F.; Peres, B.; Terreux, R.; Gervot, H.; Rioufol, C.; et al. MBL-II-141, a chromone derivative, enhances irinotecan (CPT-11) anticancer efficiency in ABCG2-positive xenografts. Oncotarget 2014, 5, 11957–11970.
- China Raju, B.; Nageswara Rao, R.; Suman, P.; Yogeeswari, P.; Sriram, D.; Shaik, T.B.; Kalivendi, S.V. Synthesis, structure-activity relationship of novel substituted 4H-chromen-1,2,3,4-tetrahydropyrimidine-5-carboxylates as potential anti-mycobacterial and anticancer agents. Bioorg. Med. Chem. Lett. 2011, 21, 2855–2859.
- Shaw, A.Y.; Chang, C.Y.; Liau, H.H.; Lu, P.J.; Chen, H.L.; Yang, C.N.; Li, H.Y. Synthesis of 2-styrylchromones as a novel class of antiproliferative agents targeting carcinoma cells. Eur. J. Med. Chem. 2009, 44, 2552–2562.
- Li, N.-G.; Shi, Z.-H.; Tang, Y.-P.; Ma, H.-Y.; Yang, J.-P.; Li, B.-Q.; Wang, Z.-J.; Song, S.-L.; Duan, J.-A. Synthetic strategies in the construction of chromones. J. Heterocycl. Chem. 2010, 47, 785-799.
- Sosnovskikh, V. Y. Synthesis and chemical properties of thiochromone and its 3-subsituted derivatives. Chemistry of Heterocyclic compounds 2016, 52, 427-440.
- Wang, H.-K.; Bastow, K. F.; Cosentino, L. M.; Lee, K.-H. J. Med. Chem. 1996, 39, 1975.
- Schneller, S. W. Adv. Heterocycl. Chem. 1975, 18, 79.
- Guo, F.; Young, J.A.; Perez, M.S.; Hankerson, H.A.; Chavez, A.M. Progress on the Cu-Catalyzed 1,4-Conjugate Addition to Thiochromones. Catalysts 2023, 13, 713.
- Bellinger, T.J.; Harvin, T.; Pickens-Flynn, T.; Austin, N.; Whitaker, S.H.; Tang Yuk Tutein, M.L.C.; Hukins, D.T.; Deese, N.; Guo, F. Conjugate Addition of Grignard Reagents to Thiochromones Catalyzed by Copper Salts: A Unified Approach to Both 2-Alkylthiochroman-4-One and Thioflavanone. Molecules 2020, 25, 2128.
- In Lee, J. Synthetic Approaches to 2-Alkylthiochroman-4-ones and Thioflavanones. Bull. Korean Chem. Soc. 2021, 42, 852–862.
- Bass, S.A.; Parker, D.M.; Bellinger, T.J.; Eaton, A.S.; Dibble, A.S.; Koroma, K.L.; Sekyi, S.A.; Pollard, D.A.; Guo, F. Development of Conjugate Addition of Lithium Dialkylcuprates to Thiochromones: Synthesis of 2-Alkylthiochroman-4-ones and Additional Synthetic Applications. Molecules 2018, 23, 1728.
- Guo, F.; Jeffries, M.C.; Graves, B.N.; Graham, S.A.; Pollard, D.A.; Pang, G.; Chen, H.Y. A rapid entry into thioflavanones via conjugate additions of diarylcuprates to thiochromones. Tetrahedron 2017, 73, 5745–5750.
- Nakazumi, H.; Endo, T.; Nakaue, T.; Kitao, T. J. Heterocycl. Chem. 1985, 22, 89.
- Willy, B.; Müller, T. J. J. A novel consecutive three-component Coupling-Addition-SNAr (CASNAR) synthesis of 4H-thiochromen-4-ones, Synlett. 2009, 1255-1260.
- Willy, B.; Frank, W.; Müller, T. J. J. Microwave-assisted three-component coupling-addition-SNAr (CASNAR) sequences to annelated 4H-thiopyran-4-ones. Org. Biomol. Chem. 2010, 8, 90.
- Palani, T.; Park, K.; Song, K. H.; Lee, S. Palladium-catalyzed synthesis of (Z)-3-arylthioacrylic acids and thiochromenones, Adv. Synth. Catal. 2013, 355, 1160.
- Hayashi, Y. Pot economy and one-pot synthesis, Chem. Sci., 2016, 7, 866–880.
- Giles, P. R.; Marson, C. M. Aust. J. Chem. 1992, 45, 439-443.
- Jia, W.; Y.-J.; Li, W.; Liu, Y.; Zhang, D.-J.; Zhang, P.; Gong, P. Bioorg. Med. Chem. 2009, 17, 4569-4574.
- Choi, E.J., Lee, J.I., Kim, G.H. Int. J. Mol. Med., 2012, 29, 252-256.
- Song, Y.-L., Wu, F., Zhang, C.-C., Liang, G.-C., Zhou, G., Yu, J.-J. Bioorg Med Chem Lett, 2015, 25, 259-261.
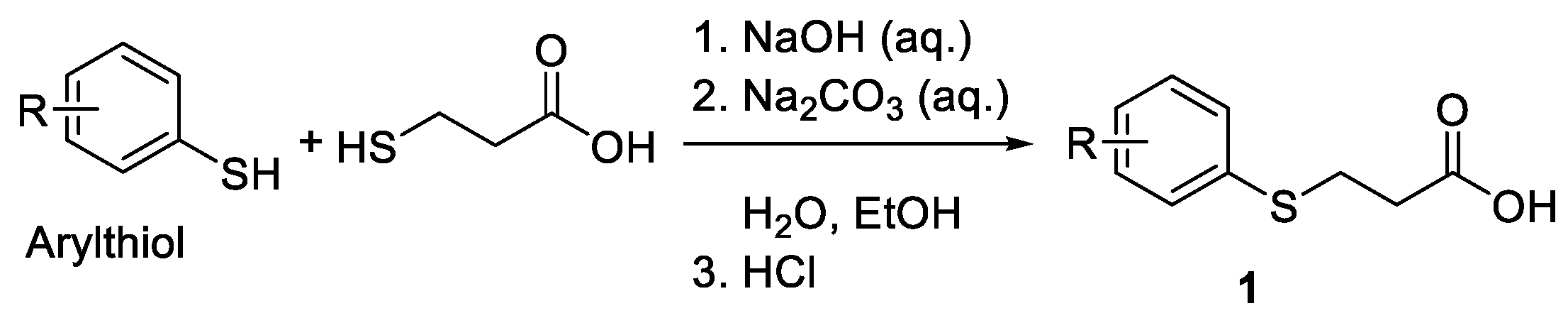
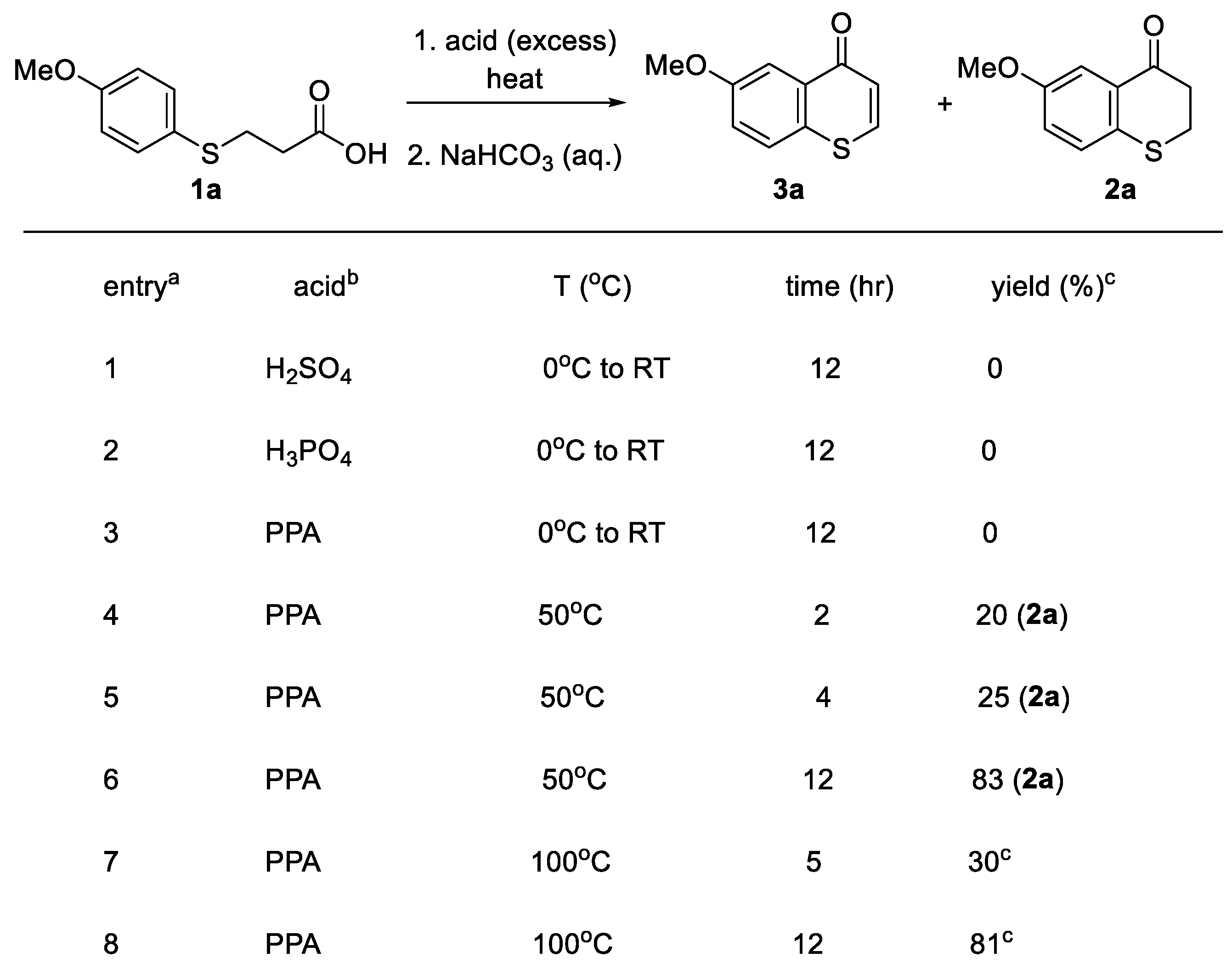 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





