1. Introduction
Isolated extra-cranial internal carotid artery occlusions (i-ICAO) account for 7-8% of total subjects presenting with an acute onset ischemic stroke (AIS)[
1]. Clinical presentation and the volume of the infarcted brain areas varies substantially in this group of subjects: they range from mild symptomatic cases to large and fatal hemispheric strokes. This variability in symptoms severity depends on several factors affecting intracranial hemodynamics after an i-ICAO, such as collateral flows through the patent circle of Willis, the speed of progression to complete vessel occlusion, the presence of a pre-occlusion stenosis and the status of the contralateral carotid artery[
2].
Endovascular therapy (EVT) and intravenous thrombolysis (IVT) are standard treatments for patients with AIS associated with intracranial large vessel occlusion (LVO)[
3].
No clear recommendations, however, have been established on how to manage these subjects with AIS and i-ICAO. On one hand, this subgroup of patients have not been extensively evaluated in previous clinical trials of EVT for LVO-related AIS[
4]. On the other hand, in patients presenting with i-ICAO and strokes of minor severity (as defined by a National Institutes of Health Stroke Scale score NIHSS <6) the efficacy of IVT as opposite to best medical treatment (BMT, no acute reperfusion treatment) remains undetermined[
5].
Perfusion CT (PCT) is a technique that provides important information on the hemodynamic changes of the brain after an AIS[
6]. PCT allows visualization of cerebral infarction indirectly based on changes in perfusion. It enables the differentiation between brain tissue that is likely irreversibly infarcted (core) and tissues that experiences significant hypoperfusion but can be saved in case of treatment (penumbra)[
7]. According to recent guidelines, the application of these techniques is particularly useful in the extended time window for both EVT and IVT[
8].
In this study we hypothesized that evaluating cerebral hemodynamic changes in subjects with AIS due to i-CAO helps in characterizing the extent of the suffering brain parenchyma. From this perspective, a patient-specific approach considering both the clinical examination, and the data derived from PCT provides useful information to guide the therapeutic management. Taking into account possible and serious consequences related to EVT[
9,
10], in fact, it is essential to correctly select patients in which could be beneficial EVT with re-opening of the occluded ICA.
We aimed to conduct an exploratory retrospective study to evaluate differences in PCT findings and clinical outcomes between subjects affected AIS due to i-ICAO and treated with EVT and those treated with Best Medical Treatment (BMT) only.
2. Methods
2.1. Population
This is a single center retrospective study. The study cohort included all consecutive patients admitted for an AIS to the Neuroradiology and Neurology Units based in “Vito Fazzi” Hospital (Lecce, Italy) between January 2020 and February 2025.
Inclusion criteria were: subjects presented with an AIS; subjects suffering from i-ICAO with patency of intracranial arteries confirmed by a Computed Tomography Angiography (CTA); Alberta Stroke Program Early CT scores (ASPECTS) ≥ 6.
Demographics, baseline clinical and procedural characteristics were assessed, including: NIHSS score at hospital admission ad at discharge; concomitant diseases; home therapies; acute reperfusion therapies (BMT, IVT or EVT), the modified Rankin Scale (mRS) at hospital discharge.
We compared patients receiving EVT with those who were treated with BMT only. Clinical evaluations (NIHSS and mRS scores) as well as PCT-derived data were analyzed. Finally, we analyzed the usefulness of clinical and PCT-derived data in discriminating patients undergoing EVT from those undergoing BMT only.
Informed consent from patients and approval from the ethics committee were not required for retrospective analyses of routinely collected medical records and the collection and analysis of fully anonymized data. This report complies with the guidelines outlined in the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement[
11]
2.2. Image Analysis
Imaging was performed on a GE HealthCare CT optima 64 scanner. Carotid occlusion location was assessed by experienced neuroradiologists on CTA images. i-ICAO was defined as occlusion of the ICA with patency of the carotid terminus, the anterior and middle cerebral arteries, assessed as contrast filing on CTA images.
PCT were analyzed using the Brainomix Stroke Solution (Brainomix Ltd., Oxford, UK). It was accessed via a webserver into which anonymized images were uploaded.
The ischemic core was defined by a cerebral blood flow (CBF) less than 30% of the contralateral side. The penumbra was the region with a Tmax greater than 6 s not belonging to the ischemic core region. The mismatch ratio was defined as the perfusion deficit (Tmax >6 s) volume divided by the core volume (rCBV <30%)[
7].
2.3. Outcomes
The following main outcomes were evaluated: in-hospital mortality; NIHSS and mRS scores at hospital discharge; successful reperfusion evaluated by means of modified thrombolysis in cerebral infarction score (TICI) and defined as a TICI ≥2b.
2.4. Statistical Analysis
Continuous variables are expressed as median with standard deviation. Categorical variables are summarized as percentages. Differences between the two groups was assessed by ANOVA test for continuous variables whereas comparison of frequencies was performed with the χ2-test. Results with p values < 0.05 were considered statistically significant.
The performance of clinical and neuroradiological variables (NIHSS at disease onset and ischemic penumbra volume, respectively) in discriminating patients undergoing EVT from those undergoing BMT only, was assessed through the Receiver Operating Characteristic (ROC) analysis and Area Under the Curve (AUC) with 95% Confidence Interval (95% CI) was calculated.
All analyses were carried out using IBM SPSS Statistics software 29.0.
3. Results
A total of 698 patients were admitted with an AIS. Among them, 25 patients met the inclusion criteria. Baseline patients’ characteristics are detailed in
Table 1. 17/25 patients underwent EVT, and successful recanalization was achieved in all subjects; in this group, IVT was administered in 6 subjects. The remaining 8/25 patients were treated with BMT only (3 subjects were treated with IVT; in the remaining 5 subjects, antiplatelet agents were administered). No rescue thrombectomy was performed.
Patients undergoing EVT compared to those treated only with BMT showed higher mean NIHSS score at hospital admission (14.1 ± 5.6 Vs 6.2 ± 4.8 respectively, p<0.05), wider mean penumbra volume (193.8 ± 147.1 ml Vs 30.0 ± 23.0 ml respectively, p<0.05) and a larger core volume (7.9 ± 8.6 ml Vs 0.9 ± 1.6 ml respectively, p<0.05).
Although there was a difference between the two groups in NIHSS at hospital discharge, this finding did not reach statistical significance: NIHSS score at discharge was 8.6 ± 7.8 for the EVT group and 2.9 ± 2.2 for the BMT group (p=0.0547); one patient died in the endovascular group.
No significant difference in functional independence at discharge emerged: mean mRS was 2.4 ± 2.1 in the EVT group and 1.4 ± 0.9 in the BMT group (p=0.081 n.s.).
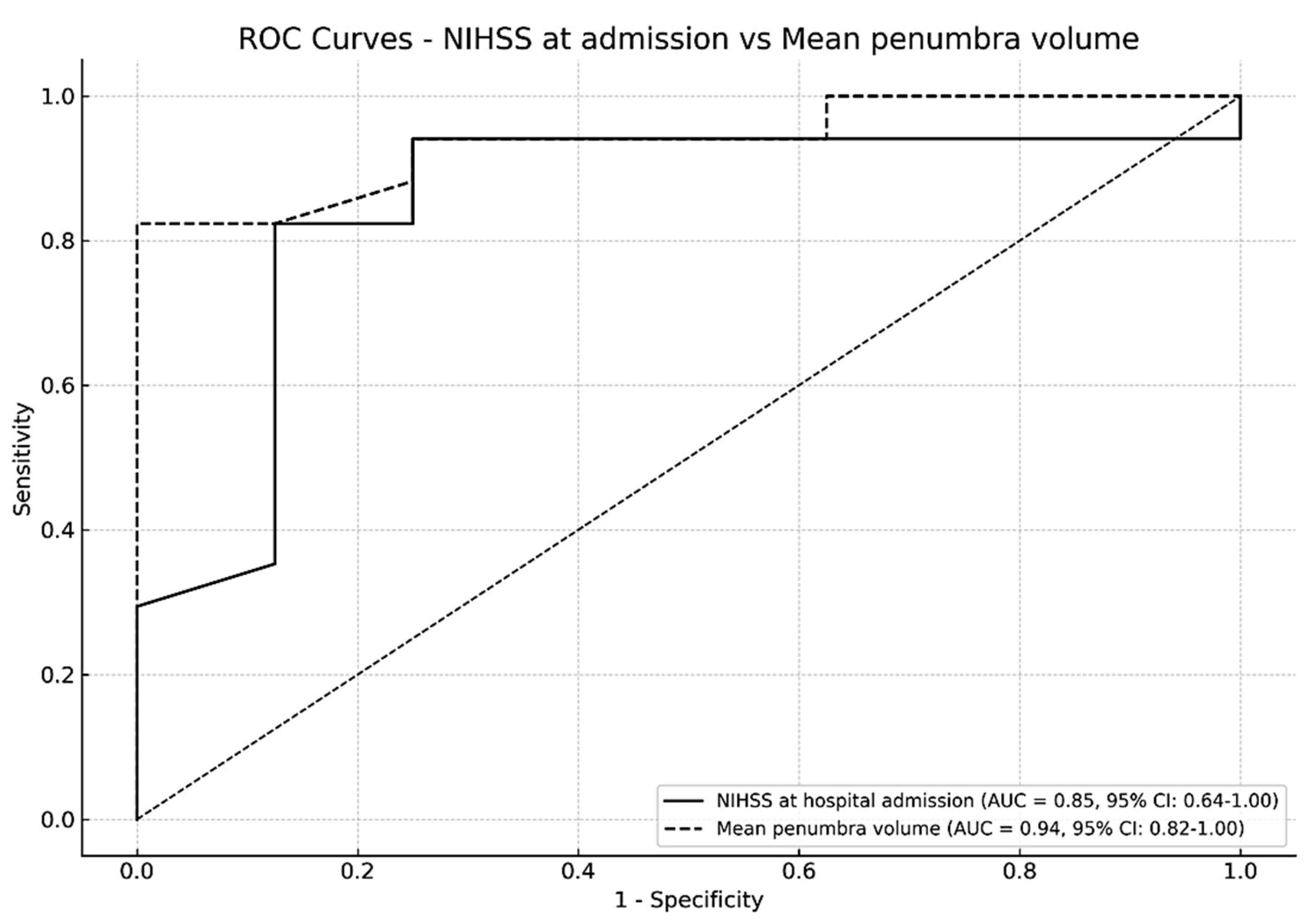
ROC analysis showed the diagnostic accuracy in differentiating patients undergoing EVT to those treated only with BMT: the AUC for the volume of ischemic penumbra (0.94, CI 95% 0.82-1.00) was higher than the AUC for NIHSS at disease onset (AUC 0.85, CI 95% 0.64 - 1.00) (
Figure 1).
4. Discussion
In our retrospective study of ischemic stroke subjects due to i-ICAO, EVT was performed in more severe cases characterized by a higher NIHSS score and a wider penumbra volume at hospital admission. Our findings align with other published research: data from a previous study supported the use of EVT in subjects with AIS due to symptomatic i-ICAO[
12]. A recent meta-analysis found a higher probability of favorable outcome for subjects undergoing EVT[
13] and an analysis of the MR CLEAN registry further confirmed these findings[
9].
On the one hand, a recent retrospective study pointed out that in subject with AIS and symptomatic acute occlusions of the ICA below the circle of Willis, EVT was not superior over BMT, whereas EVT had a risk for periprocedural distal embolization with poor outcomes[
10]. Patients included in this study, however, were not stratified either by neuroimaging data or by severity of clinical presentation, considering that the median NIHSS before treatment initiation was not different in the EVT and BMT groups[
10].
On the other hand, a recently published European retrospective multicenter cohort study involving 998 patients with an AIS due to isolated cervical internal carotid artery occlusion, showed that subjects receiving EVT had a higher admission NIHSS score compared to those treated with BMT only[
14]; conversely, there was no difference between EVT and BMT groups in 3-month mRS shift and favorable outcome[
14]. In this study, however, stroke severity was quantified only by admission NIHSS score. Authors did not take into account data derived from neuroimaging; in a more patient-centered approach, in fact, these findings might help in the evaluation of collateral flow and in the prediction of possible clinical deterioration[
14].
In the treatment of subjects with i-ICAO, therefore, it is crucial the proper selection of subjects with AIS who may benefit from EVT; in particular, they can be patients with more severe presentations or those with fluctuating symptoms that may worsen. When treating subjects with mild or moderate stroke symptoms, in fact, it should be important to consider possible complications of EVT, namely intracerebral bleeding or to distal thrombus migration that can worsen the clinical picture. Some studies reported this complication to affect approximately 20% of EVT procedures in this setting[
9].
This complication acquires great relevance particularly in subjects presenting with a minor stroke. A French study evaluated the usefulness of BMT as opposite to EVT in patients with i-ICAO and minor stroke (as defined by median NIHSS score of 3): authors suggested that in this group of patients, prognosis was favorable also with a medical strategy[
15].
According to current guidelines the decision whether to perform EVT is primarily based on the severity of clinical impairment as assessed by the NIHSS score[
16]; none of the previously mentioned studies had so far considered perfusion imaging data to stratify subjects.
In this perspective, we hypothesized that a patient-specific approach aimed to evaluate the stroke severity through both the clinical examination and the extend of the potential at risk tissue through the penumbra volume, might provide useful information to guide the therapeutic management. Advanced neuroimaging allows for more accurate capture of changes in cerebral hemodynamics after an i-ICAO: whereas the core represents brain tissue that is likely irreversibly infarcted, the penumbra is part of brain tissue that is at risk of progressing to infarction but still salvageable if reperfused[
7].
In the present study, therefore, we focused on PCT data, and we hypothesized that more severe cases were characterized by both a higher NIHSS and a wider penumbra volume. Our findings showed that EVT was performed in subjects with higher NIHSS score and wider penumbra volume with involvement of the vascular territory of the internal carotid.
A higher penumbra volume in i-ICAO can be hypothesized to result from inefficient collateral support via both the Circle of Willis and leptomeningeal pathways. This flow, however, may reduce over time as hemodynamic compensation decreases; therefore, a more aggressive therapy is warranted in these cases. We hypothesize that PCT data and particularly the penumbra volume (representing at-risk tissue) may serve as adjunctive imaging markers to consider in order to predict infarct growth and the necessity of endovascular reperfusion techniques[
10]. In our analysis, ROC curves support our hypothesis as they showed that the volume of the penumbra might be a better predictor of EVT than clinical assessment alone.
Although PCT is nowadays confided to the treatment of AIS in the extended time window, we suggest that incorporating these parameters in the routine setting of AIS patients with i-ICAO might provide important information to estimate the overall size of both the affected (core) and at-risk (penumbra) brain parenchyma. If the findings of the present study will be further confirmed in larger cohorts, PCT data might be used in the future as an adjunctive parameter helping to guide the therapeutic management in subjects suffering from AIS due to i-ICAO.
This study is limited by its retrospective design, lack of standardization and small sample size.
5. Conclusion
Our findings suggested that in cases of AIS due to i-ICAO, a subject-specific characterization of the changes in intracranial hemodynamics with the use of PCT-derived data might provide useful information on the severity of the stroke and the risk of progression; together with the clinical evaluation, we suggest that it could be one of the parameters to take into consideration in order to orient towards the best therapeutic option.
To achieve a more comprehensive understanding of the evolving patterns of cerebral hemodynamics, larger and longitudinal studies are essential, covering patients with a broad spectrum of disease and integrating PCT scans with other neuroimaging parameters.
Funding
No specific founds were received for this article.
Ethics Approval
Ethical review and approval were waived for this study, due to this report complies with the guidelines outlined in the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.
Consent to Participate
Not applicable.
Availability of Data and Material
The Corresponding Author has full access to all the data (data transparency).
Informed Consent
Ethical review and approval were waived for this study, due to this report complies with the guidelines outlined in the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Sallustio, F.; Saia, V.; Marrama, F.; Pracucci, G.; Gandini, R.; Koch, G.; Mascolo, A.P.; D’Agostino, F.; Rocco, A.; Argiro’, R.; et al. Mechanical Thrombectomy for Acute Intracranial Carotid Occlusion with Patent Intracranial Arteries : The Italian Registry of Endovascular Treatment in Acute Stroke. Clin Neuroradiol 2021, 31, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Hendrikse, J.; Hartkamp, M.J.; Hillen, B.; Mali, W.P.; van der Grond, J. Collateral Ability of the Circle of Willis in Patients with Unilateral Internal Carotid Artery Occlusion: Border Zone Infarcts and Clinical Symptoms. Stroke 2001, 32, 2768–2773. [Google Scholar] [CrossRef] [PubMed]
- Majoie, C.B.; Cavalcante, F.; Gralla, J.; Yang, P.; Kaesmacher, J.; Treurniet, K.M.; Kappelhof, M.; Yan, B.; Suzuki, K.; Zhang, Y.; et al. Value of Intravenous Thrombolysis in Endovascular Treatment for Large-Vessel Anterior Circulation Stroke: Individual Participant Data Meta-Analysis of Six Randomised Trials. Lancet 2023, 402, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Raha, O.; Hall, C.; Malik, A.; D’Anna, L.; Lobotesis, K.; Kwan, J.; Banerjee, S. Advances in Mechanical Thrombectomy for Acute Ischaemic Stroke. BMJ Medicine 2023, 2. [Google Scholar] [CrossRef] [PubMed]
- Boulenoir, N.; Turc, G.; Ter Schiphorst, A.; Heldner, M.R.; Strambo, D.; Laksiri, N.; Girard Buttaz, I.; Papassin, J.; Sibon, I.; Chausson, N.; et al. Should Patients With Acute Minor Ischemic Stroke With Isolated Internal Carotid Artery Occlusion Be Thrombolysed? Stroke 2022, 53, 3304–3312. [Google Scholar] [CrossRef] [PubMed]
- Vagal, A.; Wintermark, M.; Nael, K.; Bivard, A.; Parsons, M.; Grossman, A.W.; Khatri, P. Automated CT Perfusion Imaging for Acute Ischemic Stroke: Pearls and Pitfalls for Real-World Use. Neurology 2019, 93, 888–898. [Google Scholar] [CrossRef] [PubMed]
- Mallon, D.; Fallon, M.; Blana, E.; McNamara, C.; Menon, A.; Ip, C.L.; Garnham, J.; Yousry, T.; Cowley, P.; Simister, R.; et al. Real-World Evaluation of Brainomix e-Stroke Software. Stroke Vasc Neurol 2023, svn. [Google Scholar] [CrossRef] [PubMed]
- Albers, G.W.; Marks, M.P.; Kemp, S.; Christensen, S.; Tsai, J.P.; Ortega-Gutierrez, S.; McTaggart, R.A.; Torbey, M.T.; Kim-Tenser, M.; Leslie-Mazwi, T.; et al. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. N Engl J Med 2018, 378, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Hoving, J.W.; Kappelhof, M.; Schembri, M.; Emmer, B.J.; Berkhemer, O.A.; Groot, A.E.D.; Dippel, D.W.J.; van Zwam, W.H.; Coutinho, J.M.; Marquering, H.A.; et al. Thrombectomy for Acute Ischemic Stroke Patients with Isolated Distal Internal Carotid Artery Occlusion: A Retrospective Observational Study. Neuroradiology 2021, 63, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Meyer, L.; Broocks, G.; Alexandrou, M.; Lüttich, Á.; Larrea, J.Á.; Schwindt, W.; Krähling, H.; Naziri, W.; Behme, D.; Thormann, M.; et al. Endovascular versus Best Medical Treatment for Acute Carotid Occlusion BelOw Circle of Willis (ACOBOW): The ACOBOW Study. Radiology 2025, 314, e240293. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. ; STROBE Initiative The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, D.P.O.; Reiff, T.; Mansmann, U.; Schoene, D.; Strambo, D.; Michel, P.; Abdalkader, M.; Nguyen, T.N.; Gawlitza, M.; Möhlenbruch, M.A.; et al. Endovascular Treatment for Acute Isolated Internal Carotid Artery Occlusion. Clin Neuroradiol 2024, 34, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Romoli, M.; Mosconi, M.G.; Pierini, P.; Alberti, A.; Venti, M.; Caso, V.; Vidale, S.; Lotti, E.M.; Longoni, M.; Calabresi, P.; et al. Reperfusion Strategies in Stroke Due to Isolated Cervical Internal Carotid Artery Occlusion: Systematic Review and Treatment Comparison. Neurol Sci 2021, 42, 2301–2308. [Google Scholar] [CrossRef] [PubMed]
- Marto, J.P.; Riegler, C.; Gebert, P.; Reiff, T.; Sykora, M.; Wiącek, M.; Pakizer, D.; Araújo, A.; Ter Schiphorst, A.; Sousa, J.A.; et al. Endovascular Treatment for Isolated Cervical Internal Carotid Artery Occlusion: ETIICA Study. Eur Stroke J 2025, 23969873251323488. [Google Scholar] [CrossRef] [PubMed]
- Ter Schiphorst, A.; Gaillard, N.; Dargazanli, C.; Mourand, I.; Corti, L.; Charif, M.; Ayrignac, X.; Lippi, A.; Bouly, S.; Thibault, L.; et al. Symptomatic Isolated Internal Carotid Artery Occlusion with Initial Medical Management: A Monocentric Cohort. J Neurol 2021, 268, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Turc, G.; Bhogal, P.; Fischer, U.; Khatri, P.; Lobotesis, K.; Mazighi, M.; Schellinger, P.D.; Toni, D.; de Vries, J.; White, P.; et al. European Stroke Organisation (ESO) – European Society for Minimally Invasive Neurological Therapy (ESMINT) Guidelines on Mechanical Thrombectomy in Acute Ischaemic StrokeEndorsed by Stroke Alliance for Europe (SAFE). European Stroke Journal 2019, 4, 6–12. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).