1. Introduction
The textile and dyeing industries consume vast amounts of water and release considerable quantities of untreated dyes into aquatic ecosystems. It is estimated that nearly 20% of global dye production is discharged into the environment without adequate treatment (Osterloh et al., 2018; Walter et al., 2018; Kudo et al., 2011). The persistence and chemical stability of many modern dyes—especially those used in growing textile applications make them resistant to conventional degradation methods. Consequently, textile wastewater poses significant environmental and health hazards, necessitating advanced and targeted treatment technologies (Maeda et al., 2013; Bagtache et al., 2014; Bulte et al., 2004).
Among synthetic dyes, azo compounds represent over 60% of those used in industrial applications (Bagtache et al., 2019). These molecules exhibit high stability and low biodegradability due to their conjugated double bonds and mesomeric structures, and many are classified as toxic or carcinogenic (Helaili et al., 2010; Sabri et al., 2020). To address the ecological risks associated with dye-contaminated effluents, several treatment approaches have been proposed, including biological processes (Djaballah et al., 2022), adsorption (Bagtache et al., 2021), sonolysis (Sahmi et al., 2024), and photocatalysis.
Photocatalysis has emerged as a particularly effective method for the degradation of a broad range of organic pollutants, including dyes (Bagtache et al., 2020), pesticides (Bagtache et al., 2022), pharmaceutical residues (Banat et al., 1996), and antibiotics (Kaci et al., 2021). Various semiconductor materials have been employed for this purpose, with metal oxides such as bismuth-based compounds gaining attention due to their cost-effectiveness, ease of synthesis, chemical stability, and environmental compatibility (Ben Aziza et al., 2021; Baaloudj et al., 2021; Vijay Kumar et al., 2018).
In this context, the present work focuses on the synthesis and detailed physical, chemical, and photoelectrochemical characterization of the selenite phase Bi₁₂NiO₁₉. The material was prepared via a sol–gel route and applied for the first time to the visible-light-induced degradation of Methyl Violet (MV), a model pollutant representative of synthetic dyes.
2. Experimental
2.1. Material and Methods
2.1.1. Synthesis of Bi12NiO19
One widely used synthesis approach for complex oxides involves the decomposition of metal nitrates. In this study, Bi₁₂NiO₁₉ was synthesized via a citrate-assisted sol–gel route. High-purity bismuth nitrate [Bi (NO₃) ₃·9H₂O] and nickel nitrate [Ni (NO₃) ₂·6H₂O] (≥99%) were dissolved in distilled water. To the resulting clear solution, 5% excess citric acid was added under continuous stirring, followed by gentle heating until the formation of a viscous gel. This gel was subsequently heated at 350 °C for 2 hours on a hot plate to promote the removal of nitrogen oxides (NOₓ) fumes.
The obtained precursor was further calcined in air at 400 °C for 3 hours, then ground into fine powder. The powder underwent a first calcination step at 600 °C for 3 hours, followed by a second calcination at 800 °C for 3 hours. The final product was air-quenched to facilitate the incorporation of bismuth into the lattice and to obtain the crystalline Bi₁₂NiO₁₉ phase.
2.2. Characterisation
The crystal structure of the synthesized Bi₁₂NiO₁₉ powder was elucidated using X-ray diffraction (XRD) performed on an X'Pert Pro diffractometer with Cu Kα radiation (λ = 1.54 Å). The morphology of the powder was examined by scanning electron microscopy (SEM) using a Schottky field emission microscope (FESEM; JSM-7610F Plus, JEOL). Optical properties were investigated through diffuse reflectance measurements recorded on a Jasco 650 UV/Vis spectrophotometer within the 190–900 nm wavelength range. Photoelectrochemical characterization was carried out in a conventional three-electrode cell filled with 0.1 M Na₂SO₄ electrolyte solution. A saturated calomel electrode (SCE, Hg/Hg₂Cl₂/Cl⁻) served as the reference electrode, while a platinum gauze functioned as the counter electrode. The working electrode consisted of an annealed Bi₁₂NiO₁₉ pellet (1.32 cm²) electrically connected via a copper wire to its back face and sealed inside a Teflon tube with hardening araldite to ensure proper insulation. Current–potential (J–E) characteristics were recorded at a scan rate of 10 mV·s⁻¹. The flat band potential (E_fb), critical for understanding photocatalytic activity, was determined from Mott–Schottky plots of the inverse square capacitance (C⁻²) versus applied potential at a frequency of 10 kHz to suppress the influence of the electrical double layer. Additionally, electrochemical impedance spectroscopy (EIS) measurements were performed over a frequency range from 10⁻² to 10⁵ Hz at the stabilized open circuit potential to further probe the interfacial charge transfer processes.
2.2.1. Point of Zero Charge (pHPZC)
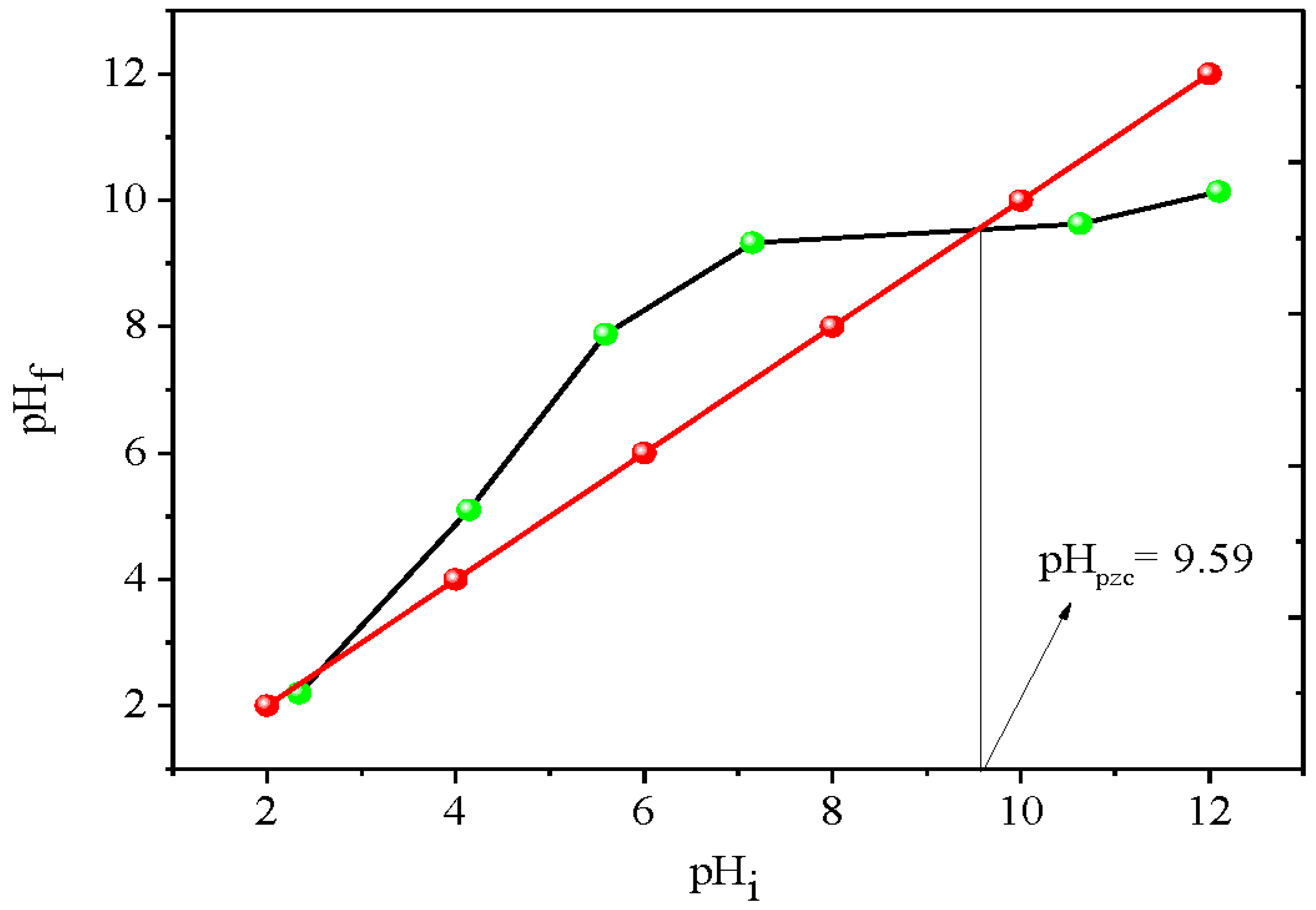
The zero charge point (pHpzc) of Bi12NiO19 was: 25 mg of Bi12NiO19 was mixed with 25 mL of NaNO3 solutions (0.1 M), the pH was adjusted in the range (2 – 12) by the addition of HCl or NaOH (0.1 N). The solutions were kept stirring (48 h) and the final pH was measured; the curves were plotted and pHpzc corresponds to the intersection point of the curves (pHfinal = pHinitial).
2.2.2. Photo Degradation
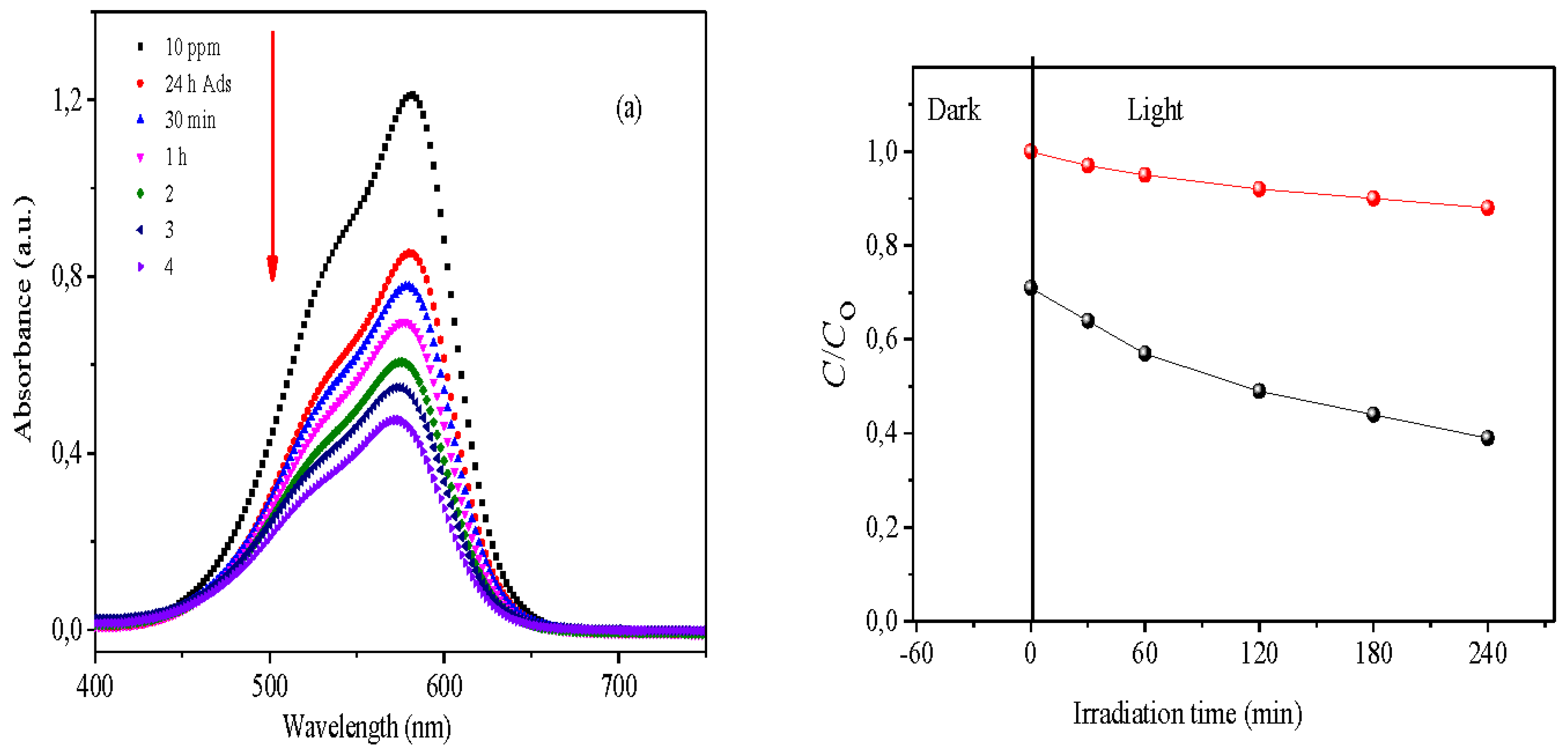
The photocatalytic performance of Bi₁₂NiO₁₉ was assessed through the visible-light-driven oxidation of Methyl Violet (MV). Experiments were conducted in a double-walled Pyrex photoreactor designed to maintain the reaction temperature at 25 ± 1 °C, minimizing thermal effects. A suspension of 10 mg of Bi₁₂NiO₁₉ was magnetically stirred at 400 rpm in 10 mL of MV solution (10 ppm) in the dark for 24 hours to achieve adsorption–desorption equilibrium. Subsequently, the solution was irradiated with a 14 W LED lamp emitting visible light at an intensity of 23 mW·cm⁻², positioned 5 cm from the reaction mixture. The pH of the solution was kept at its natural value (~7) throughout the experiment. The concentration of MV was periodically monitored using UV-Vis spectrophotometry (Jasco 650) by measuring absorbance at 582 nm (λ_max). Concentration values were accurately determined via linear interpolation from a pre-established calibration curve. For comparison and control, photolysis tests without the catalyst were also performed under identical conditions.
3. Results and Discussion
3.1.1. Characterization of Bi12NiO19
3.1.1. Phase Identification
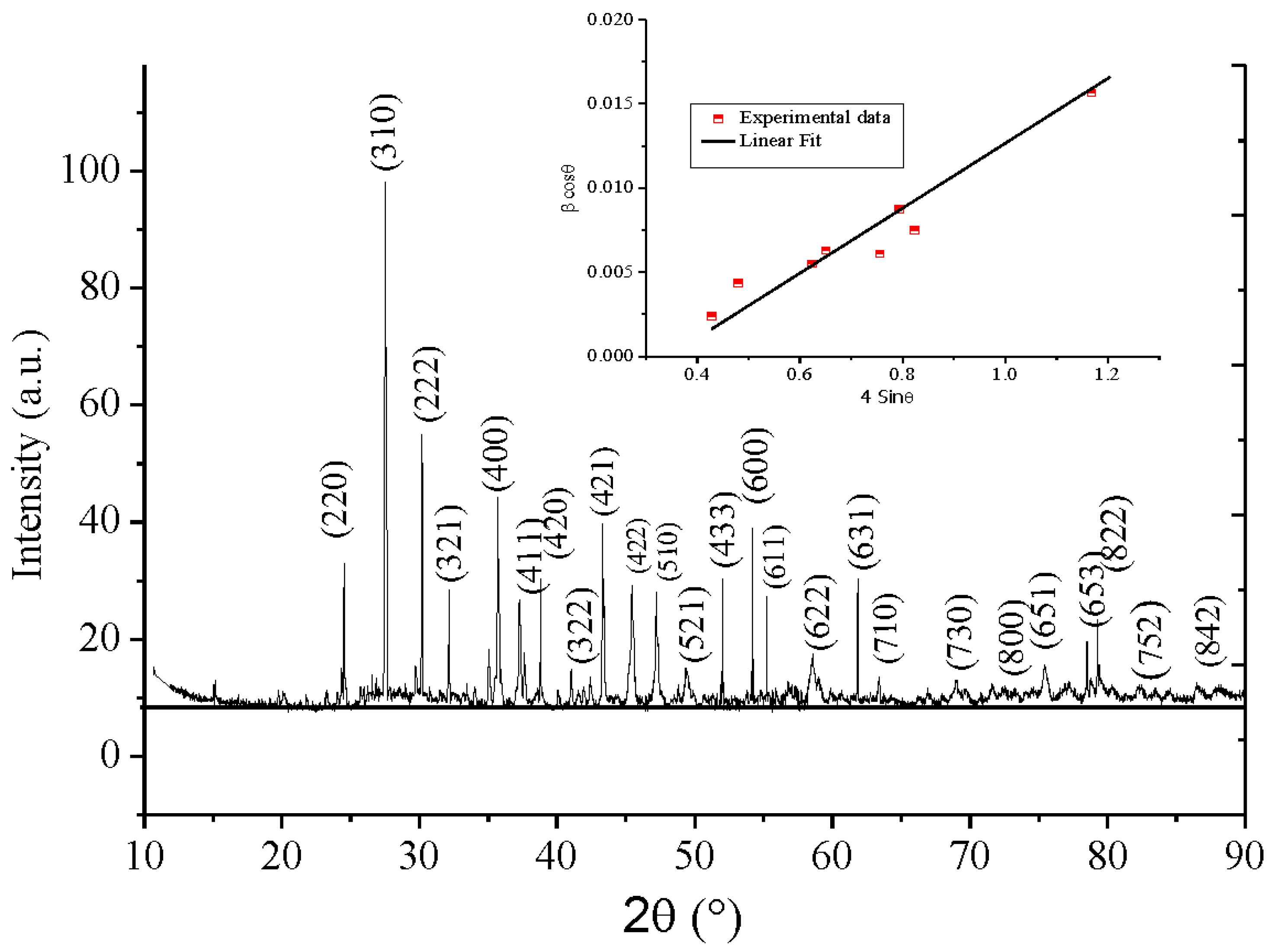
To identify the selenite phase Bi
12NiO
19, XRD analysis was used (
Figure 1). All peaks belong to single phase Bi
12NiO
19 according to the JCPDS Card N
o: 43-0448 (SG: I 23) (Brahimi et al. 2021). The least square method was used to refine the lattice parameters (a= 10.2406 Å). The good crystallization is evidenced from the narrow peak. The crystallite size calculated from.
The average dimension of the crystallites was calculated from the Williamson Hall (W-H) relation (Equ. 1), which shows the contribution of the size broadening (kλ/d) and strain broadening (η sinθ) (Nithya et al. 2020; Hosseinzadeh et al. 2022):
The β broadening is a function of instrumental parameters, induced defects and strain, in the crystal structure (Inset
Figure 1). A size of 32.38 nm was deduced from the extrapolation of the line “β cosθ“ with the y-axis
3.1.2. Optical Study
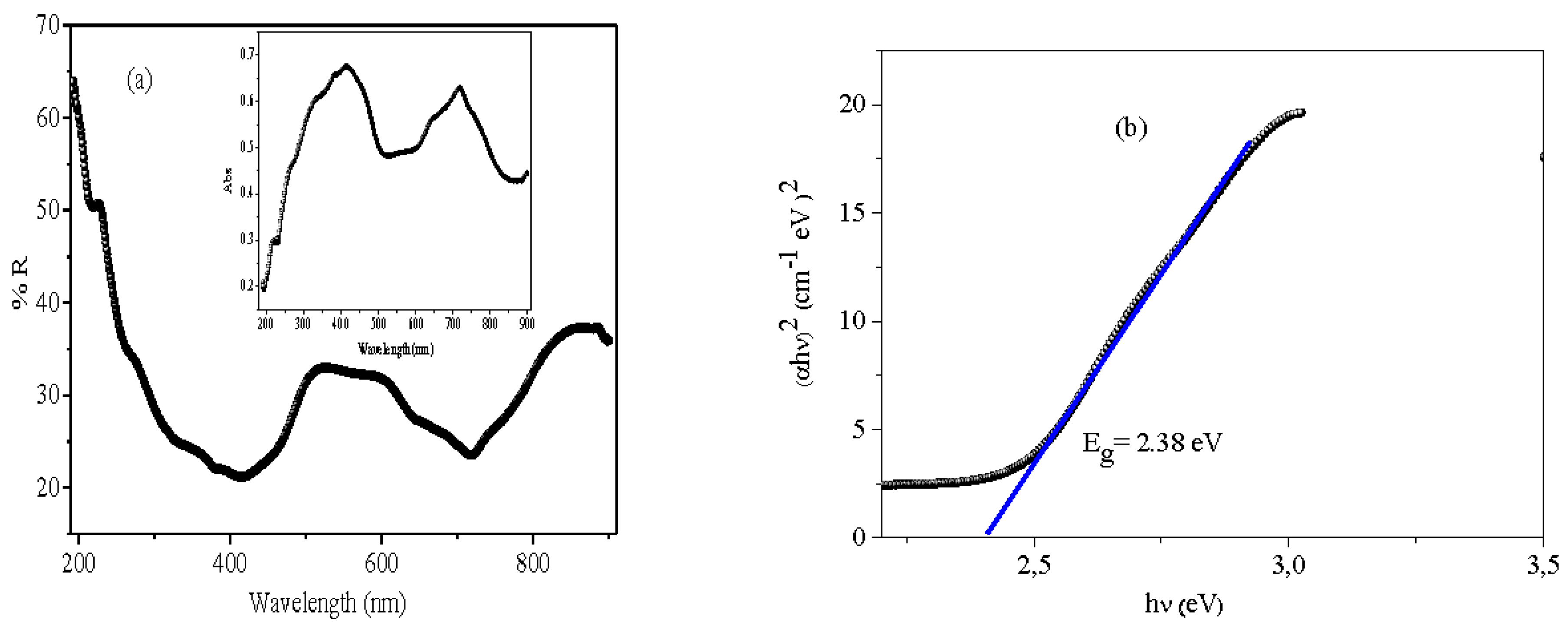
Photoactivity is primarily conditioned by the band gap (E
g) value (
Figure 2). The optical characteristics of Bi
12NiO
19 were determined by UV-Vis diffuse reflectance by studying the dependence of the absorption coefficient (α
λ) on the photon energy (hν) using the relation:
(Thi Mai Tho et al. 2020 ; Hsieh et al. 2013):
This technique is based on the value of the absorption coefficient (α
λ, cm
-1) which is a function of λ, R∞ is obtained from the diffuse refectance data (R%), taken from the diffuse reflectance spectrum. The optical gap (E
g) is deduced by extrapolation of the line with the abscissa axis in accordance with the relation (Meng et al. 2015):
where K is an intrinsic factor while the transition nature is indicated by the
n value, equal to 0.5 and 2 respectively for direct and indirect optical transition. The line (
Figure 2 a, Inset) shows that the selenite Bi
12NiO
19 with a yellow colour has a high absorption in the UV-Visible range, with two bands at 400 and 715 nm where the fraction of the light irradiance is converted into electrical and/or chemical energy (Zhoua et al. 2021). An additional direct transition at 2.38 eV (
n = 2) is also observed (
Figure 2b). The narrow gap makes the selenite an attractive photocatalyst for water treatment or photovoltaic conversion (Pei et al. 2016, Rajamoorthy et al. 2021).
3.1.3. Morphology
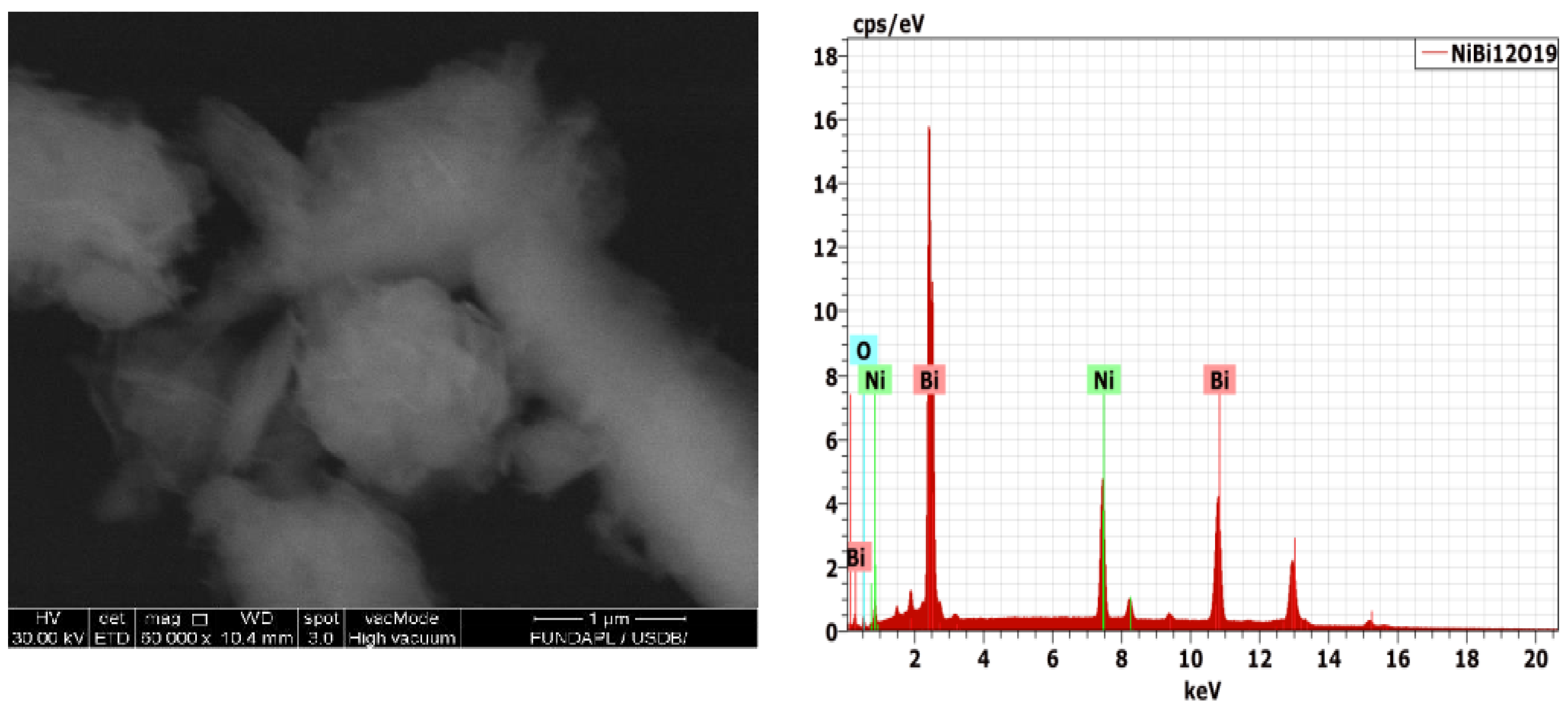
Scanning Electron Microscopy (SEM) was used to capture the morphology of the Bi
12NiO
19 powder. The SEM images show small agglomeration can be seen due to the ultrafine nature of the sample, prepared hydrothermally (
Figure 3a) with a non-uniform distribution of crystals of different shapes. The existence of the constituting elements (Ni, Bi, and O) of the selenite was confirmed by the EDX spectrum (
Figure 3b), with a composition close to the nominal one.
3.1.4. Electrochemical Properties
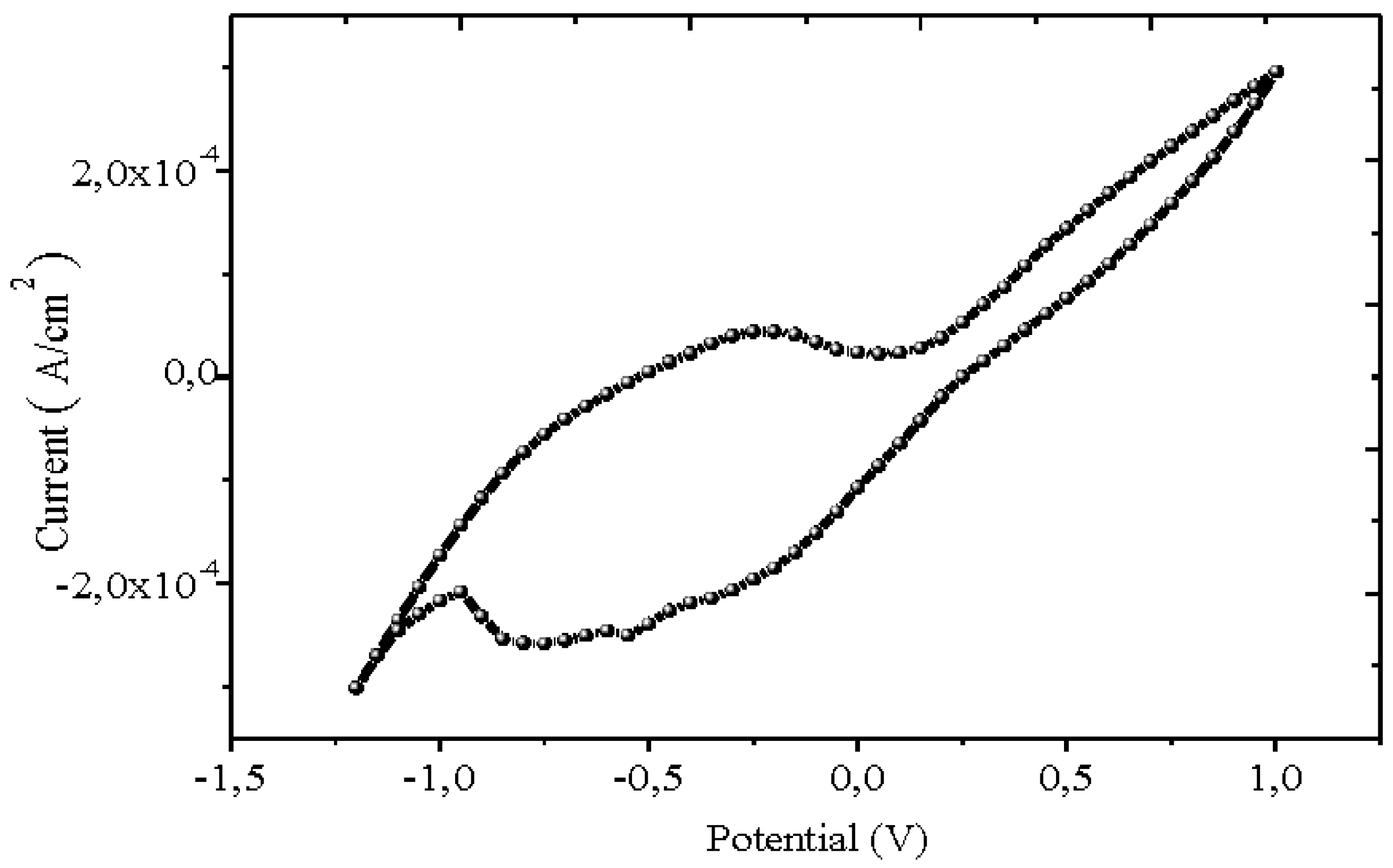
Cyclic voltammetry of Bi
12NiO
19 is plotted to examine its behaviour and stability in the working solution (Na
2SO
4).
Figure 4 presents the J(E) characteristics in the dark. The current averages at ~ 0.2 mA cm
2 and decreases below -0.9 V without a diffusion plateau, due to the evolution of H
2; the band at ~ -0.5 V corresponds to the reduction of oxygen
Selenite is used in photocatalysis and electrochemistry is required to delimit the electroactivity domain and localize the electronic bands in the potential scale. Cyclic voltammetry of Bi
12NiO
19 is undertaken to elucidate the behaviour and its stability in the working solution (Na
2SO
4). J(E) characteristics plotted in the dark and under visible light are shown in
Figure 4. The current density is around 0.2 mA cm
2 and decreases below -0.9 V without reaching a diffusion plateau, due to the evolution of H
2. The band at about -0.5 V corresponds to the reduction of oxygen (2 H
2O + O
2 + 2 e
- → H
2O
2 + 2 OH
-) (Ma et al. 2019). To corroborate the
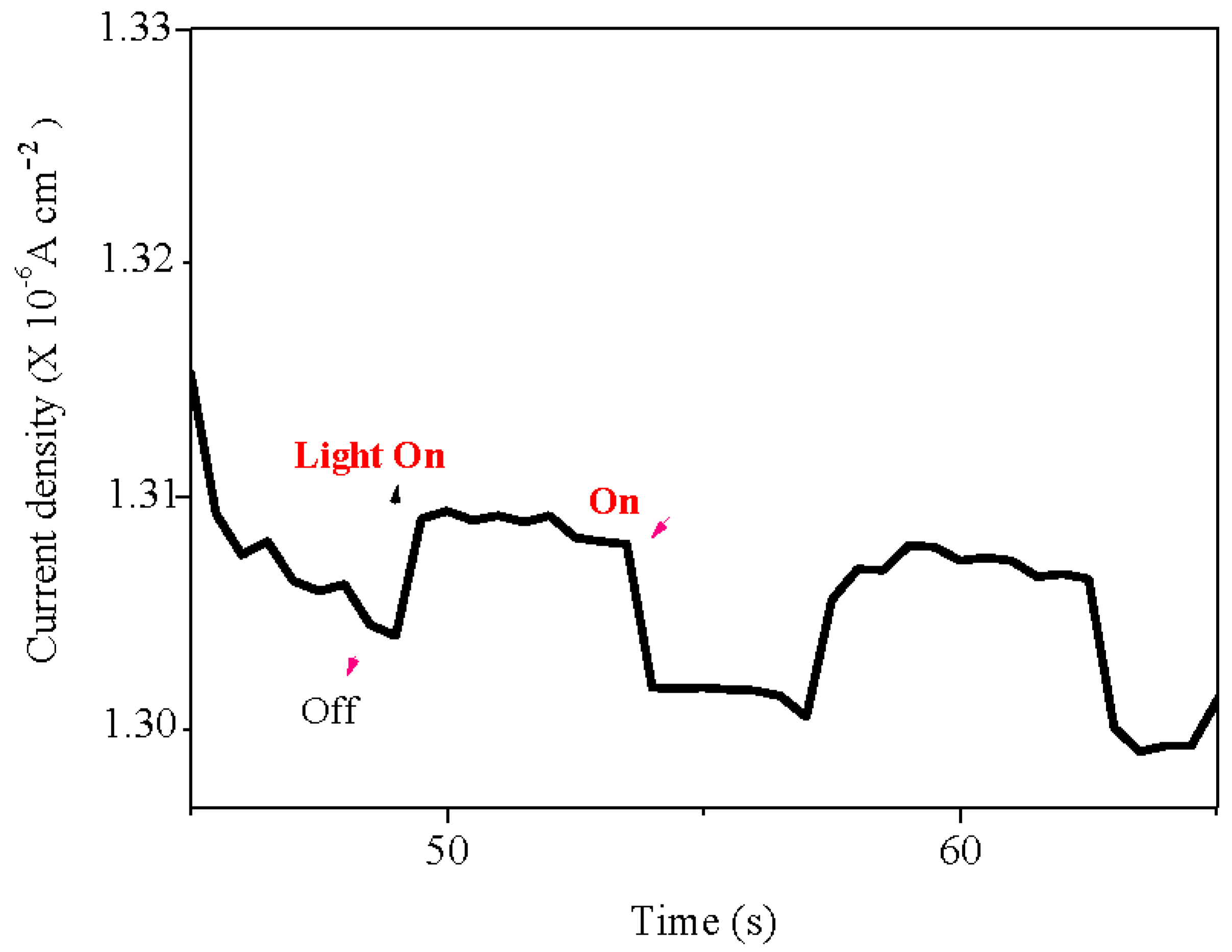
p type nature of the Selenite, we have undertaken a chrono-amperometry under chopped visible light (
Figure 5); the electrode was polarized at -0.25 V, a potential more cathodic than the potential E
fb. The increased cathodic current brings an unambiguous confirmation of
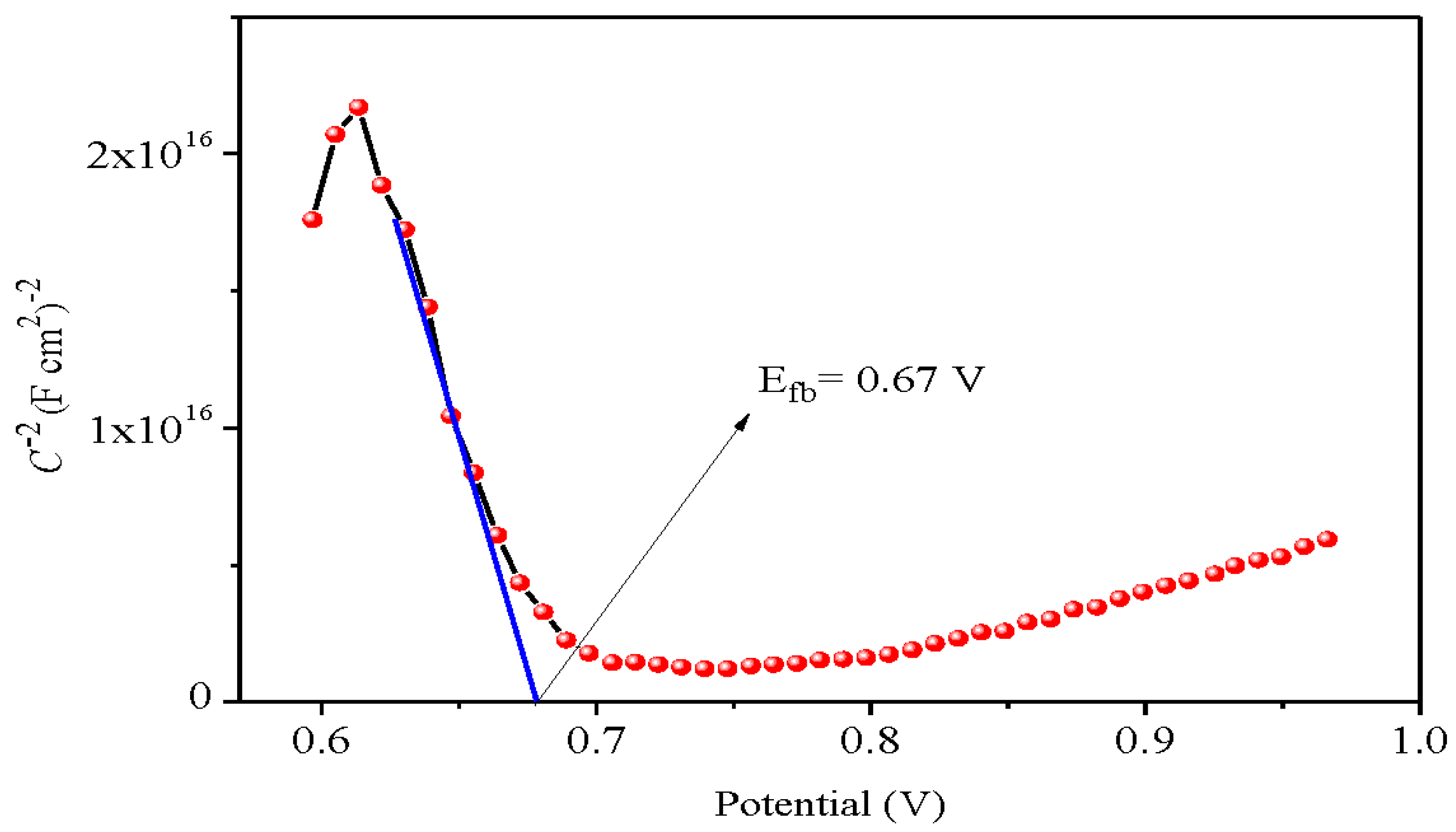
p-type behaviour. The flat band potential (E
fb) is essential for predicting the photocatalytic reactions (Pant et al. 2013) and is reliably determined from the capacitance measurement et high frequency (10 kHz) using the relation (Pant et al. 2019):
The
p-type conduction is corroborated by the negative slope with holes as dominant charge carriers. A potential E
fb of 0.67 V was determined by extrapolating the line to infinite capacity (C
-2 = 0) (
Figure 6). The energy/potential of the valence band (E
VB: 5.62 eV/0.87 V
SCE) indicates that VB is formed by the orbital O
2-:
2p while the conduction band (E
CB 3.24 eV/- 1.51 V
SCE) is made up of Bi
3+:
6s orbital. The potential of the bands CB and VB are given by (Li et al. 2017):
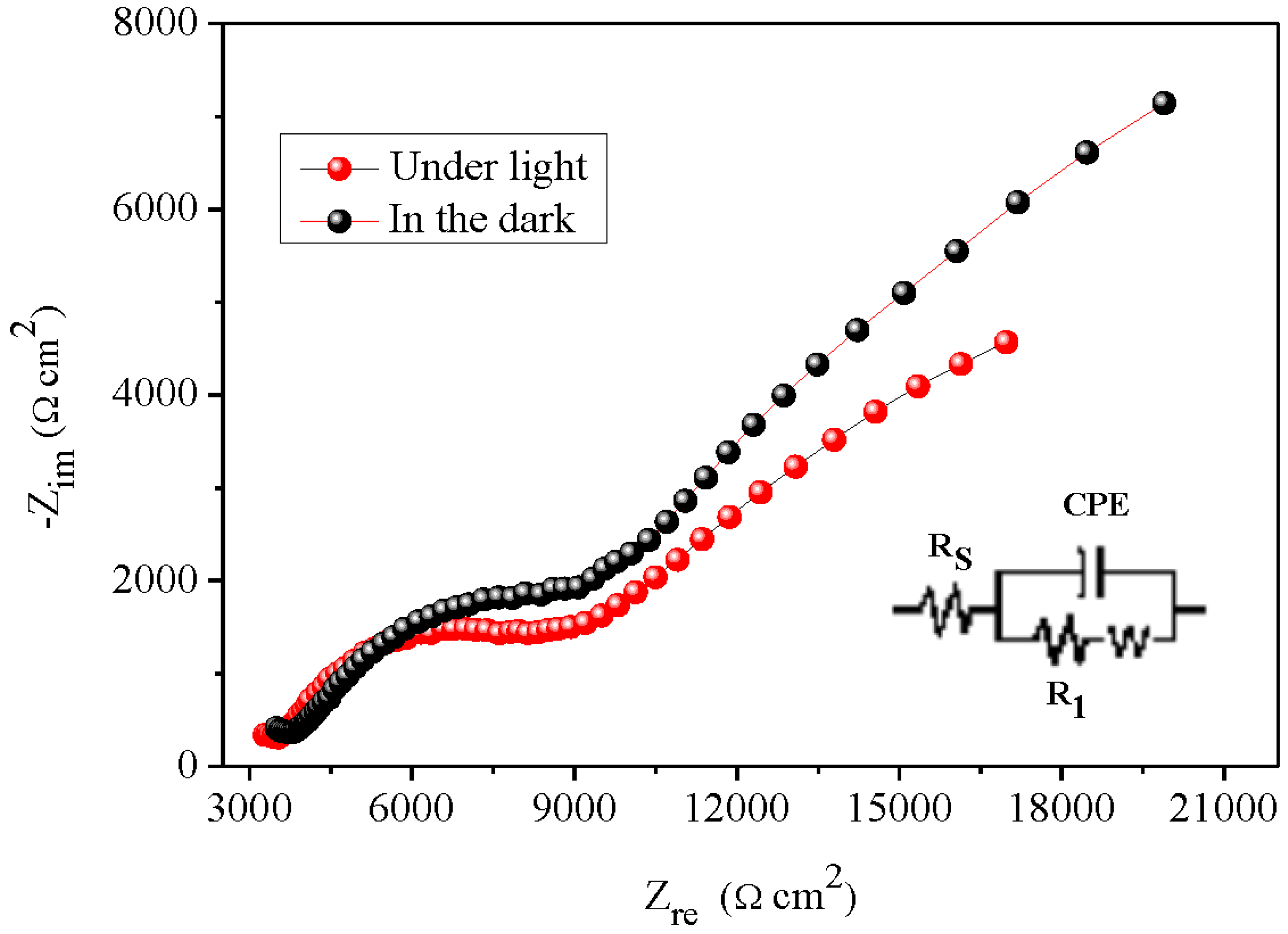
The EIS analysis was used to elucidate the electron transfer across the Bi
12NiO
19/liquid interface over a large frequency range in Na
2SO
4 solution at stabilized free potential (OCP = 0.11 V). The complex diagram i.e. the imaginary part (-Z
imag) against the real part (Z
real) both in the obscurity and under visible light (
Figure 7). The centres of the semicircles are not positioned on the real axis and this implies a deviation from a purely capacitive behaviour with existence of a constant phase element (CPE) (Djellal et al. 2009):
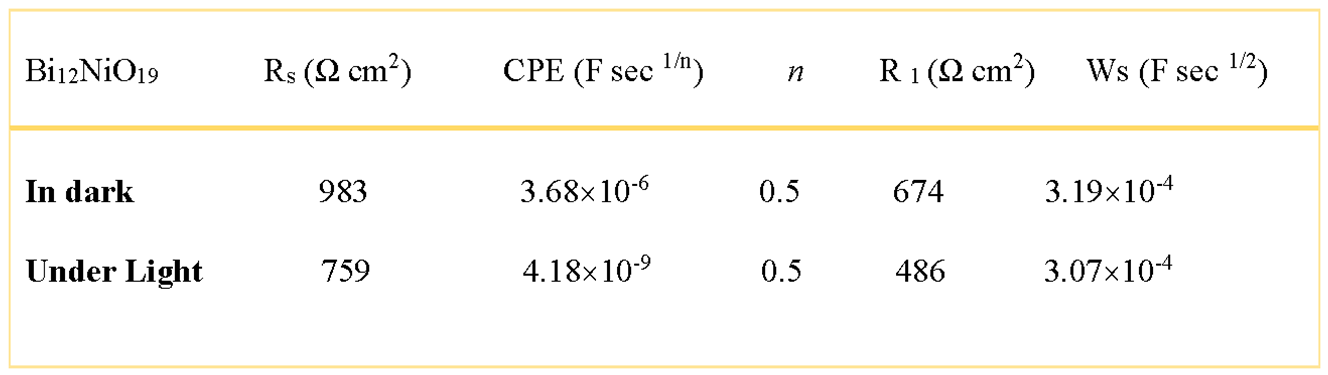
the homogeneity factor m, between 0 and 1, is related to the phase angle φ {= (mπ/2)} and Q a constant independent of the frequency. EIS correlates the morphology to the interfacial mechanism and quantifies the contributions of the bulk, grain boundaries and ion diffusion as well as the microstructure of the compound to the transport properties (Su et al. 2018).
The semi-circle is associated to the charge transfer resistance (R
1 = 674 Ω cm
2) whose diameter falls down to 485 Ω cm
2 under visible irradiation, corroborating the semi conductivity of Bi
12NiO
19, The offset near the origin is attributed to the electrolytic solution R
s = 759 Ω cm
2 (
Table 1). The data are fitted to an equivalent electrical circuit by the Zview software (
Figure 7, Inset). The formation of the double layer due to the adsorption of ions generates a potential gradient, coming mainly from H
+ and OH
- ions; the quantity ΔpH = 0.059 (pH − pzc) at 25 °C can be neglected. The point of zero charge (pHpzc = 9.59) is determined from the drift method (
Figure 8).
3.2. Photoactivity
Currently, the removal of pollutants from wastewater is a hot topic and photocatalysis is one of our research themes. Bi
12NiO
19 was chosen because of its band gap, allowing it to generate O
2•- radicals (Zhang et al. 2017). However,
•OH species cannot be formed because the BV potential (0.87 V) is not anodic enough to oxidize water to
•OH capable of oxidizing the MV dye to mineral end products. An initial concentration of MV (10 mg/L), commonly encountered in effluents, was used and the reaction mechanism on the surface of Bi
12NiO
19 was proposed (Zhang et al. 2016):
Adsorption, the first step in photocatalysis, depends on the surface charge of Bi
12NiO
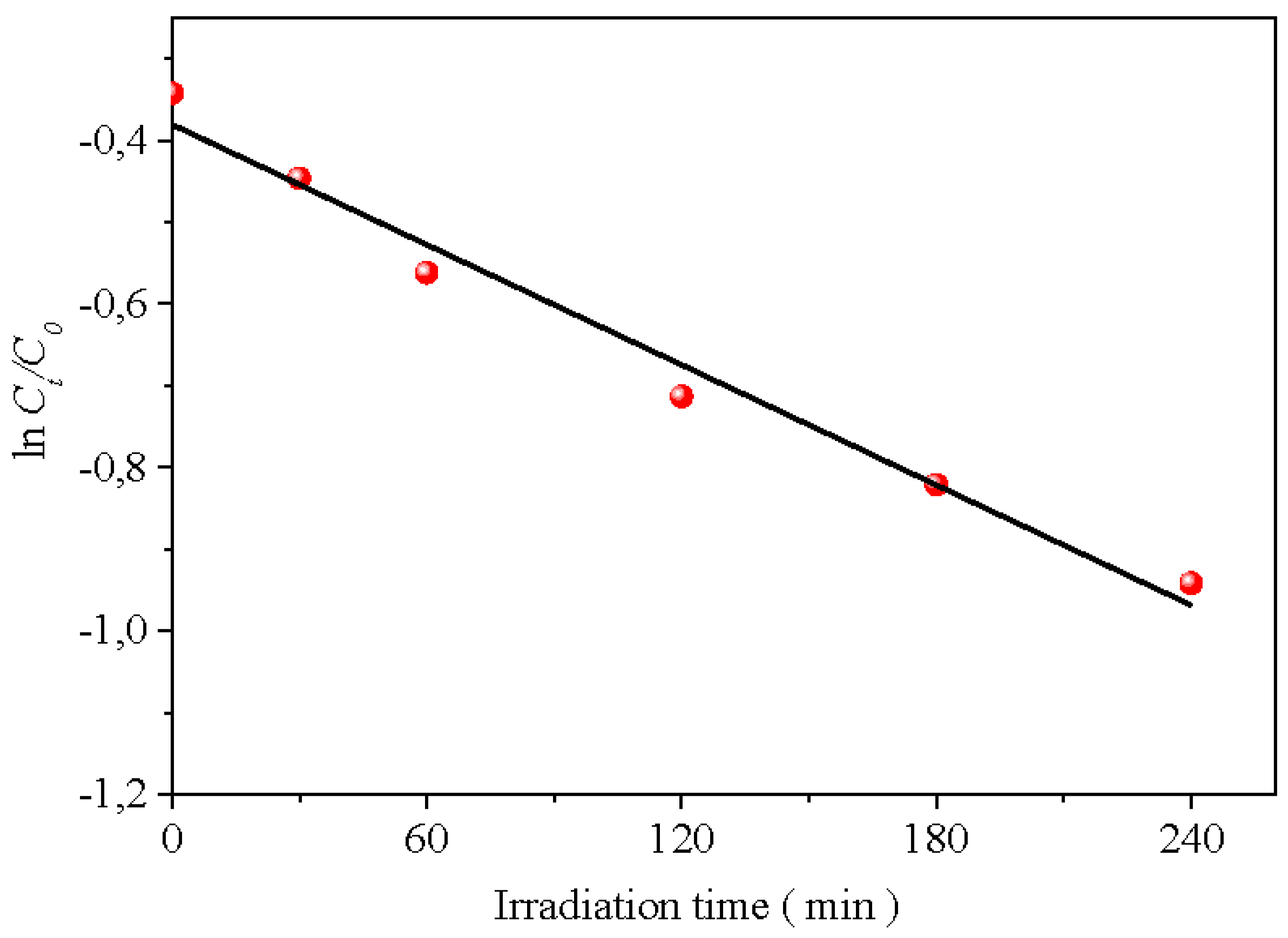
19, governed by pHpzc. A pseudo-second-order kinetic has been successfully tested for photo-oxidation (Chang et al. 2018):
where
t is the reaction time (min) and
k the degradation rate constant (min
-1). It is worthwhile to mention that 13 % of MV are removed by photolysis (without catalyst) (
Figure 9b) while an elimination percentage of 60% was reached after 240 min under visible irradiation (
Figure 9a). The rate constant (
k) is calculated from the slope of the line 1/C
t vs. t (
Figure 10); giving a photocatalytic half-life of 30 min. The H
2 production onto the selenite under visible light irradiation will be the objective of our next paper.
where
t is the reaction time (min) and
k the rate constant (min
-1). It is worth noting that 13% of MV was eliminated by photolysis (
Figure 9b). In contrast, 60% reduction was reached after 4 h under visible light illumination (
Figure 9a). The constant
k is deduced from the slope of the line 1/C
t vs.
t (
Figure 10); a photocatalytic half-life of 30 min was deduced. More interestingly, we noticed that the H
2 evolution occurs simultaneously, due the conduction band (-1.5 V) cathodic than the H
2 level (~ -0.8 V) on the selenite. Therefore, the H
2 production on selenite under visible light irradiation will be the focus of our next article.
4. Conclusion
In this study, Bi₁₂NiO₁₉ was successfully synthesized via a citrate-assisted sol–gel route, producing a well-crystallized cubic-phase p-type semiconductor with an average crystallite size of approximately 32 nm. Structural characterization confirmed the high purity and crystallinity of the material, while electron microscopy revealed particle agglomeration into heterogeneous grains. Optical investigations identified a direct band gap transition at around 2.38–2.87 eV, suitable for visible-light absorption. Electrochemical analyses established the semiconductor’s p-type nature, with holes as majority carriers, and determined key parameters including a flat band potential of 0.67 V vs. SCE, alongside valence and conduction band edges formed mainly by O²⁻ (2p) and Bi³⁺ (6p) orbitals, respectively. The material exhibited excellent electrochemical stability in Na₂SO₄ solution, as evidenced by capacitance and chronoamperometric measurements.
Importantly, Bi₁₂NiO₁₉ demonstrated significant photocatalytic activity under visible light for the degradation of the synthetic dye Methyl Violet. A 60% degradation efficiency was achieved within 4 hours, driven primarily by reactive superoxide radicals (O₂•⁻). The photocatalytic reaction kinetics followed a pseudo-second-order model, with the high-energy Bi 6p orbitals playing a crucial role in facilitating effective charge transfer and dye oxidation. These findings not only highlight the potential of Bi₁₂NiO₁₉ as a cost-effective and environmentally friendly photocatalyst but also contribute valuable insights into the design of advanced p-type semiconductors for wastewater treatment applications.
Future work could explore doping strategies and composite formation to further enhance the visible-light absorption and charge separation efficiency of Bi₁₂NiO₁₉, aiming to optimize its photocatalytic performance for a broader range of organic pollutants. This study paves the way for the rational development of bismuth-based selenite oxides as promising candidates in sustainable environmental remediation technologies.
Data Availability Statement
The date presented in this manuscript is available with the corresponding author and can be provided on reasonable request.
Acknowledgments
This study was funded by the Faculty of Chemistry Grant N° B00L01UN160420190020 (USTHB; Algiers)
Conflicts of Interest
The authors declare that there is no known competing interests or personal relationships that could have appeared to influence the research work reported in this manuscript.
References
- Al-Gheethi, A.A.; S. H. Z. Removal of Methylene Blue Dye from Aqueous Solution Using an Eco-Friendly Bio-adsorbent: A Review. Journal of Water Process Engineering. 2018, 28, 143–153. [Google Scholar]
- Bagtache, R.; Abdmeziem, K.; Rekhila, G.; Trari, M. Synthesis, physical and electrochemical characterizations of organically templated cobalt-aluminophosphate. Application to oxygen evolution. Journal of Materials Science: Materials in Electronics 2019, 30, 14928–14934. [Google Scholar] [CrossRef]
- Bagtache, R.; Boudjedien, K.; Djaballah, A.M.; Trari, M. Photoelectrochemical characterization of Ag/CuCo2O4 prepared at low temperature: application to solar light oxidation of methyl orange. International Journal of Environmental Science and Technology 2022, 19, 1–10. [Google Scholar] [CrossRef]
- Bagtache, R.; Zahra, S.; Abdi, A.; Trari, M. Characterization of CuCo2O4 prepared by nitrate route: application to Ni2+ reduction under visible light. Journal of Photo Chemistry and Photobiology A: Chemistry 2020, 400, 112728. [Google Scholar] [CrossRef]
- Banat, I.M.; Nigam, P.; Singh, D.; Marchant, R. Microbial decolorization of textile-dye containing effluents: a review. Bioresource Technology 1996, 58, 217–227. [Google Scholar] [CrossRef]
- Barakat, M.; Badawi, M.; Trari, M. Electrochemical characterization of CuCr2O4 photoanodes and their application in photoelectrochemical water splitting. Journal of Energy Storage 2020, 30, 101459. [Google Scholar]
- Ben Aziza, Meriem, et al. The Influence of Different Stabilizers on Properties of Sol–Gel Spin-Coated Zinc Oxide Films. Brazilian Journal of Physics 2021, 51, 722–730. [Google Scholar] [CrossRef]
- Boulhired, A.; Halima, S.; Trari, M. Green synthesis and characterization of ZnO/Ag3PO4 nanocomposite with enhanced photocatalytic activity. Journal of Environmental Chemical Engineering 2023, 11, 108174. [Google Scholar]
- Bousse, M.A.; Mahmoud, A.K.; Trari, M. Effect of doping on the photocatalytic activity of TiO2: A review. Materials Science for Energy Technologies 2022, 8, 242–261. [Google Scholar]
- Brahimi, B.; Kenfoud, H.; Benrighi, Y.; Baaloudj, O. Structural and optical properties of Bi12NiO19 sillenite crystals: Application for the removal of Basic Blue 41 from wastewater. Photochem 2021, 1, 319–329. [Google Scholar] [CrossRef]
- Bulte, J.W.; Kraitchman, D.L. Iron oxide MR contrast agents for molecular and cellular imaging. NMR in Biomedicine: An International Journal Devoted to the Development and Application of Magnetic Resonance In Vivo 2004, 17, 484–499. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.J.; Wang, C.W.; Wei, Y.H.; Chen, C.Y. Enhanced photocatalytic H2 production activity of Ag-doped Bi2WO6-graphene based photocatalysts. Int. J. Hydrogen Energy 2018, 43, 11345–11354. [Google Scholar] [CrossRef]
- Djaballah, A.M.; R. Bagtache, M. Trari. Physical and electrochemical properties of the spinel ZnBi2O4 prepared by citrate route: application towards photo degradation of methyl violet. Journal of Materials Science: Materials in Electronics 2022, 33, 18410–18419. [Google Scholar]
- Djemel, B.; M. Trari. Photocatalytic properties of CuBi2O4 under visible light: A review. Journal of Environmental Chemical Engineering 2023, 11, 107634. [Google Scholar]
- Hosseinzadeh, G.; Zinatloo-Ajabshir, S.; Yousefi, A. Innovative synthesis of a novel ZnO/ZnBi2O4/graphene ternary heterojunction nanocomposite photocatalyst in the presence of tragacanth mucilage as natural surfactant. Ceramics International 2022, 48, 6078–6086. [Google Scholar] [CrossRef]
- Hsieh, S.H.; Lee, G.J.; Davies, S.H.; Masten, S.J.; Wu, J.J. Synthesis of Cr2O3 and Pt doped RuO2/Bi2O3 Photocatalysts for Hydrogen Production from Water Splitting. Am. J. Environ. Eng. 2013, 3, 115–120. [Google Scholar]
- Kaci, M.M.; et al. Enhanced photocatalytic performance of CuAl2O4 nanoparticles spinel for dye degradation under visible light. Research on Chemical Intermediates 2021, 47, 3785–3806. [Google Scholar] [CrossRef]
- Kudo, A. Z-scheme photocatalyst systems for water splitting under visible light irradiation. MRS Bulletin 2011, 36, 32–38. [Google Scholar] [CrossRef]
- Ma, Y.; Qiu, F.L.; Wei, T.; Lin, F.F.; Yan, L.; Wu, H.; Zhang, Y.; Pei, L.Z.; Fan, C.G. Facile Synthesis of Polyaniline/Bismuth Nickelate Nanorod Composites for Sensitive Tartaric Acid Detection. Surf. Eng. Appl. Electrochem. 2019, 55, 335–341. [Google Scholar] [CrossRef]
- Maeda, K. Z-scheme water splitting using two different semiconductor photocatalysts. ACS Catalysis 2013, 3, 1486–1503. [Google Scholar] [CrossRef]
- Meng, Yuying, Deyang Chen, Yitao Sun, Dongling Jiao, Dechang Zeng, Zhongwu Liu. Applied Surface Science, 2015, 324, 745–750.
- N. ; Helaili, Y.; Bessekhouad, A.; Bouguelia, M. Trari. p-Cu2O/n-ZnO heterojunction applied to visible light orange II degradation. Solar Energy 2010, 84, 1187–1192. [Google Scholar] [CrossRef]
- Nithya, R.; Ayyappan, S. Novel exfoliated graphitic-C3N4 hybridised ZnBi2O4 (g-C3N4/ZnBi2O4) nanorods for catalytic reduction of 4-Nitrophenol and its antibacterial activity. J. Photochem. Photobiol. A Chem. 2020, 398. [Google Scholar] [CrossRef]
- N. Thi Mai Tho, et al. Mechanism of Visible-Light Photocatalytic Mineralization of Indigo Carmine Using ZnBi2O4-Bi2S3 Composites. Chemistry Select 2018, 3, 9986–9994. [Google Scholar]
- Pant, B.; Pant, H.R.; Barakat, N.A.M.; Park, M.; Jeon, K.; Choi, Y.; Kim, H.Y. Carbon nanofibers decorated with binary semiconductor (TiO2/ZnO) nanocomposites for the effective removal of organic pollutants and the enhancement of antibacterial activities. Ceram. Int. 2013, 39, 7029–7035. [Google Scholar] [CrossRef]
- Pant, B.; Ojha, G.P.; Kim, H.Y.; Park, M.; Park, S.J. Fly-ash-incorporated electrospun zinc oxide nanofibers: Potential material for environmental remediation. Environ. Pollut. 2019, 245, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Pei, L.Z.; Wei, T.; Lin, F.F.; Chen, X.J.; Qiu, F.L.; Ma, Y.; Zhang, Y.; Zhao, L.; Fan, C.G. A Novel Polyaniline/Bismuth Nickelate Nanocomposite for Effective Photocatalytic Degradation of Dyes. J. Chem. Eng. 2020, 399. [Google Scholar]
- Pham, T.K.; Ha, H.K.; Kim, H.Y.; Son, D.T.; Kim, S.K. Synthesis of CeO2 nanoparticles using different methods: The structural and optical properties. Powder Technology 2020, 368, 611–619. [Google Scholar]
- Saad, A.; Bouguelia, A.; Trari, M. Copper oxide nanoparticles: synthesis, characterization, and photocatalytic properties under visible light. Environmental Science and Pollution Research 2023. [Google Scholar]
- Taha, A.; Bouslama, R.; Trari, M. Preparation and characterization of silver and copper nanoparticles for visible light photocatalysis. Arabian Journal of Chemistry 2021, 14, 103279. [Google Scholar]
- Tazout, M.; Nasser, A.; Merbouh, N.; Trari, M. Recent advances in visible light photocatalytic activity of ZnO and its composite materials. Journal of Environmental Chemical Engineering 2022, 10, 107049. [Google Scholar]
- Thi Mai Tho, N. ; Nguyen Nha, Khanh, D.; Quoc Thang, N.; Yong-Ill Lee, Thi Kim Phuong Nguyen. Novel reduced graphene oxide/ZnBi2O4 hybrid photocatalyst for visible light degradation of 2,4-dichlorophenoxyacetic acid. Environmental Science and Pollution Research 2020. [Google Scholar]
- Trari, M.; Bagtache, R.; Djaballah, A.M. Recent advances in photocatalysis for water treatment. Materials Today: Proceedings 2020, 27, 879–884. [Google Scholar]
- Trari, M.; Djemel, B.; Zahra, S. Characterization and photocatalytic activity of synthesized ZnBi2O4 for dye degradation. Journal of Environmental Chemical Engineering 2022, 10, 106998. [Google Scholar]
- Trari, M.; Saad, A. Photocatalytic performance of Cu2O nanoparticles for the degradation of organic pollutants. Materials Today: Proceedings 2019, 16, 1673–1679. [Google Scholar]
- Wang, S.; Wang, P.; Zhang, L. Nanostructured Photocatalysts for Environmental Remediation: Recent Advances and Future Challenges. Journal of Hazardous Materials 2020, 382, 121195. [Google Scholar]
- Zhang, Y.; Wang, C.; Wang, Y.; Wu, Z. Fabrication of Bi2WO6/Ag3PO4 heterojunctions with enhanced photocatalytic activity. RSC Advances 2016, 6, 11091–11097. [Google Scholar]
- Zhang, Z.; Zhao, Y.; Zhang, Q.; Wang, Y. Synthesis of Bi2S3/Bi2WO6 heterojunctions with enhanced photocatalytic activity under visible light. Journal of Hazardous Materials 2017, 331, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Yang, H.; Zhai, Y. Effects of temperature and humidity on the performance of TiO2 photocatalytic materials. Journal of Photochemistry and Photobiology A: Chemistry 2015, 318, 64–70. [Google Scholar]
- Zhoua, P.; Xiaoa, F.; Jia, L. Bi 12 NiO 19 micro-sheets grown on graphene oxide:Temperature-dependent facile synthesis and excellent electrochemical behavior forsupercapacitor electrode. J. Electroanal. Chem., 2021, 884, 115075. [Google Scholar] [CrossRef]
- Zou, G.; Chen, W.; Yang, Y.; Wang, J. Novel preparation of nitrogen-doped TiO2 photocatalysts and their photocatalytic performance. Journal of Materials Science 2019, 54, 14133–14142. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).