Submitted:
16 July 2025
Posted:
18 July 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Convolution Estimator
2.1. Toy Example
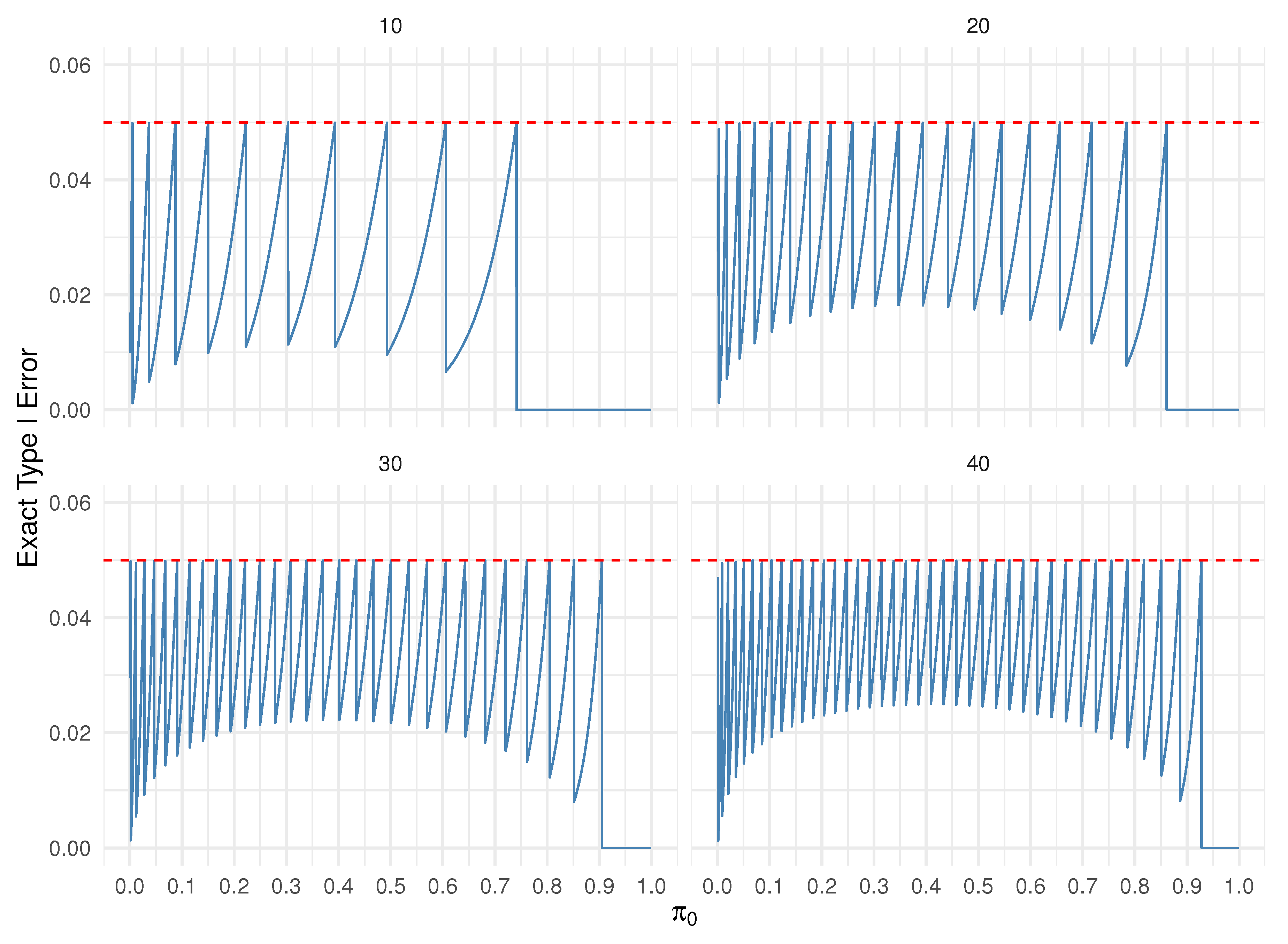
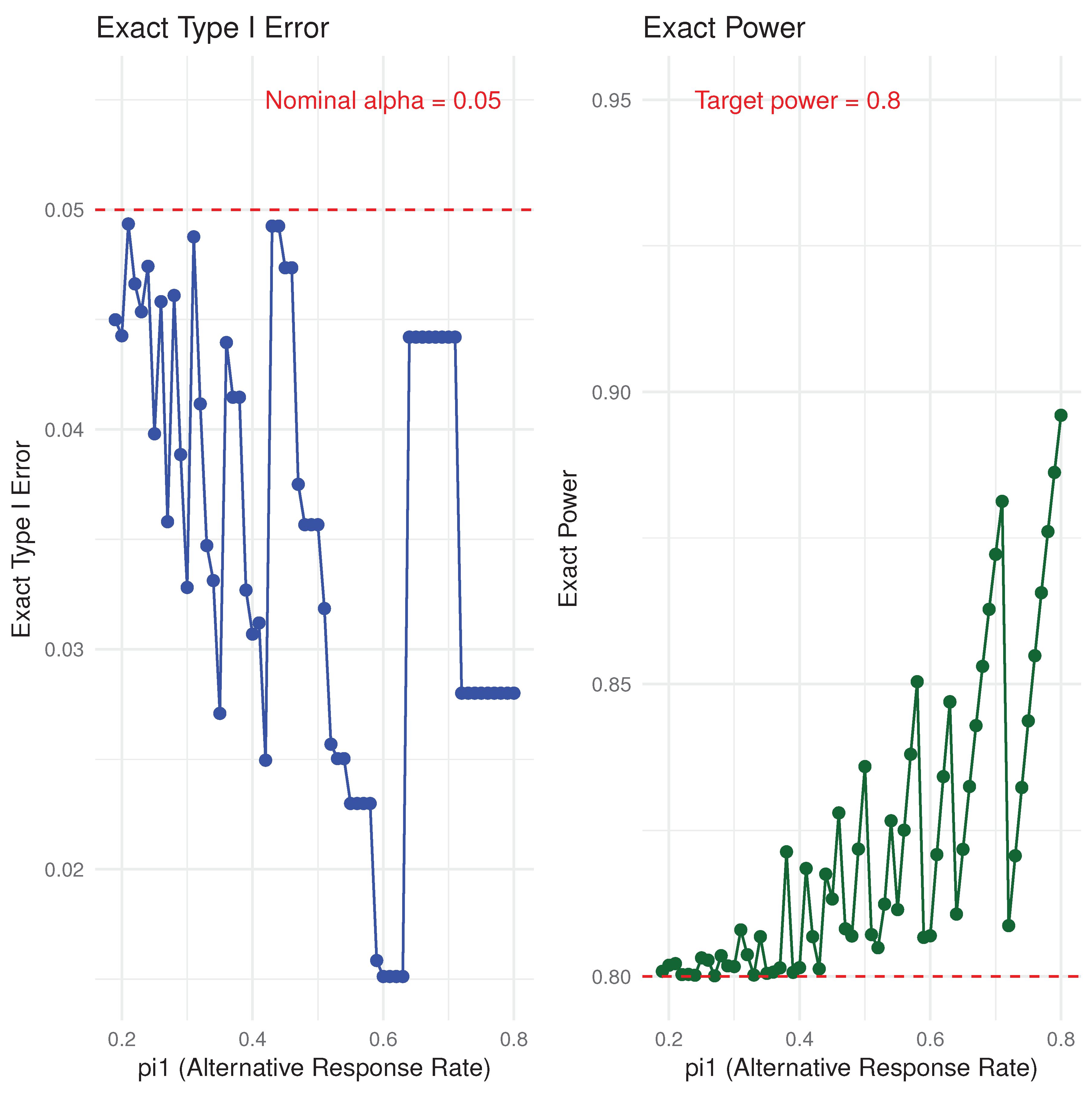
2.2. Convolution Approach and Exact Binomial Test Comparison
3. Two Stage Design
3.1. Convolution Approach and Simon Two-Stage Design Comparison
4. Real World Examples
4.1. One-Stage Designs
4.1.1. Example 1
4.1.2. Example 2
4.2. Two-Stage Designs
4.2.1. Example 1
- For , : Assuming 7 out of 27 responses, the futility p-value was 0.011, below the stopping threshold . The final-stage p-value, assuming 8 out of 35 total responses, was 0.0158, significant at the adjusted level .
- For , : Assuming 6 out of 24 responses, the futility p-value was 0.002, below . The final-stage p-value based on 8 of 36 responses was 0.0162, also significant at .
- For , : Assuming 5 out of 18 responses, the futility p-value was 0.015, which is less than . The final p-value with 8 of 37 responses was 0.020, significant at .
4.2.2. Example 2
5. Conclusions
Acknowledgments
References
- Prepared by Battelle Technology Partnership Practice. Biopharmaceutical Industry-Sponsored Clinical Trials: Impact on State Economies. Prepared for Pharmaceutical Research and Manufacturers of America (PhRMA). 2015.
- Leighl, N.B., Nirmalakumar, S., Ezeife, D. A. and Gyawali, B. (2021) An Arm and a Leg: The Rising Cost of Cancer Drugs and Impact on Access. American Society of Clinical Oncology educational book. American Society of Clinical Oncology. Annual Meeting 41 1-12. [CrossRef]
- Kapinos, K. A., Hu, E., Trivedi, J., Geethakumari, P. R. and Kansagra, A. (2023) Cost-Effectiveness Analysis of CAR T-Cell Therapies vs Antibody Drug Conjugates for Patients with Advanced Multiple Myeloma. Cancer Control 30 1-8. [CrossRef]
- Hoover, A. , Reimche, P., Watson, D., Tanner, L., Gilchrist, L., Finch, M., Messinger, Y. H. and Turcotte, L. M. (2024) Healthcare cost and utilization for chimeric antigen receptor (CAR) T-cell therapy in the treatment of pediatric acute lymphoblastic leukemia: A commercial insurance claims database analysis. Cancer Reports. Epub ahead of print. [CrossRef]
- Simon, R. (1989) Optimal two-stage designs for phase II clinical trials. Controlled Clinical Trials 10 1-10. [CrossRef]
- Hamaker, H. C. Attributes. Journal of the American Statistical Association 50 830–849.
- Duncan, A. J. (1986) Quality Control and Industrial Statistics. Irwin, Homewood, IL.
- Chernick, M. R. and Christine Y. Liu, C. Y. (2002) The Saw-Toothed Behavior of Power Versus Sample Size and Software Solutions: Single Binomial Proportion Using Exact Methods. American Statistician 56 149–155.
- Hutson, A. D. (2006) Modifying the Exact Test for a Binomial Proportion and Comparisons with Other Approaches. Journal of Applied Statistics 33 679-690. [CrossRef]
- Whitlock, M. C. (2005) Combining probability from independent tests: the weighted Z-method is superior to Fisher’s approach. Journal of Evolutionary Biology 18 1368-73.
- Asahina, H. , Oizumi, S., Takamura, K., Harada, T., Harada, M., Yokouchi, H., Kanazawa, K., Fujita, Y., Kojima, T., Sugaya, F., Tanaka, H., Honda, R., Kikuchi, E., Ikari, T., Ogi, T., Shimizu, K., Suzuki, M., Konno, S., Dosaka-Akita, H., Isobe, H., Nishimura, M.; Hokkaido Lung Cancer Clinical Study Group. (2019) A prospective phase II study of carboplatin and nab-paclitaxel in patients with advanced non-small cell lung cancer and concomitant interstitial lung disease (HOT1302). Lung Cancer 138 65-71. [CrossRef]
- Boudadi, K. , Suzman, D. L., Anagnostou, V., Fu, W., Luber, B., Wang, H., Niknafs, N., White, J. R., Silberstein, J. L., Sullivan, R., Dowling, D., Harb, R., Nirschl, T. R., Veeneman, B. A., Tomlins, S. A., Wang, Y., Jendrisak, A., Graf, R. P., Dittamore, R., Carducci, M. A., Eisenberger, M. A., Haffner, M. C., Meeker, A. K., Eshleman, J. R., Luo, J., Velculescu, V. E., Drake, C. G. and Antonarakis, E. S. (2018) Ipilimumab plus nivolumab and DNA-repair defects in AR-V7-expressing metastatic prostate cancer. Oncotarget 9 28561-28571. [CrossRef]
- Gridelli, C. , Perrone, F., Gallo, C., De Marinis, F., Ianniello, G., Cigolari, S., Cariello, S., Di Costanzo, F., D’Aprile, M., Rossi, A., Migliorino, R., Bartolucci, R., Bianco, A. R., Pergola, M. and Monfardini, S. (1997) Vinorelbine is well tolerated and active in the treatment of elderly patients with advanced non-small cell lung cancer. A two-stage phase II study. European Journal of Cancer bf 33 392-397.
- Sundquist, F. , Georgantzi, K., Jarvis, K. B., Brok, J., Koskenvuo, M., Rascon, J., van Noesel, M., Grybäck,.P, Nilsson, J., Braat, A., Sundin, M., Wessman, S., Herold, N., Hjorth, L., Kogner, P., Granberg, D., Gaze, M. and Stenman, J. (2022) A Phase II Trial of a Personalized, Dose-Intense Administration Schedule of 177Lutetium-DOTATATE in Children With Primary Refractory or Relapsed High-Risk Neuroblastoma-LuDO-N. Frontiers in Pediatrics 10 836230.
| Run | y | x | Convoution p-value | Exact binomial p-value | ||
|---|---|---|---|---|---|---|
| 1 | 4 | 0.007968 | 4.007968 | 0.5832 | 0.4168 | 0.5886 |
| 2 | 11 | 0.014024 | 11.01402 | 0.9999 | 0.0001 | 0.0006 |
| 3 | 3 | 0.008362 | 3.008362 | 0.3701 | 0.6299 | 0.7939 |
| Critical c | Mixture Power | Rejection k | Binomial Power | ||
|---|---|---|---|---|---|
| 0.1 | 0.1 | 2.9962 | 0.050000 | 4 | 0.012795 |
| 0.1 | 0.2 | 2.9962 | 0.251377 | 4 | 0.120874 |
| 0.1 | 0.3 | 2.9962 | 0.523352 | 4 | 0.350389 |
| 0.1 | 0.4 | 2.9962 | 0.757080 | 4 | 0.617719 |
| 0.1 | 0.5 | 2.9962 | 0.904088 | 4 | 0.828125 |
| 0.2 | 0.2 | 4.0086 | 0.050000 | 5 | 0.032793 |
| 0.2 | 0.3 | 4.0086 | 0.189362 | 5 | 0.150268 |
| 0.2 | 0.4 | 4.0086 | 0.415895 | 5 | 0.366897 |
| 0.2 | 0.5 | 4.0086 | 0.663109 | 5 | 0.623047 |
| 0.2 | 0.6 | 4.0086 | 0.855538 | 5 | 0.833761 |
| 0.3 | 0.3 | 5.0195 | 0.050000 | 6 | 0.047349 |
| 0.3 | 0.4 | 5.0195 | 0.171407 | 6 | 0.166239 |
| 0.3 | 0.5 | 5.0195 | 0.383292 | 6 | 0.376953 |
| 0.3 | 0.6 | 5.0195 | 0.638272 | 6 | 0.633103 |
| 0.3 | 0.7 | 5.0195 | 0.852383 | 6 | 0.849732 |
| 0.4 | 0.4 | 6.9878 | 0.050000 | 8 | 0.012295 |
| 0.4 | 0.5 | 6.9878 | 0.158735 | 8 | 0.054688 |
| 0.4 | 0.6 | 6.9878 | 0.358174 | 8 | 0.167290 |
| 0.4 | 0.7 | 6.9878 | 0.619691 | 8 | 0.382783 |
| 0.4 | 0.8 | 6.9878 | 0.856551 | 8 | 0.677800 |
| 0.5 | 0.5 | 7.9876 | 0.050000 | 9 | 0.010742 |
| 0.5 | 0.6 | 7.9876 | 0.154390 | 9 | 0.046357 |
| 0.5 | 0.7 | 7.9876 | 0.357879 | 9 | 0.149308 |
| 0.5 | 0.8 | 7.9876 | 0.645587 | 9 | 0.375810 |
| 0.5 | 0.9 | 7.9876 | 0.909147 | 9 | 0.736099 |
| Critical c | Mixture Power | Rejection k | Binomial Power | ||
|---|---|---|---|---|---|
| 0.1 | 0.1 | 4.0143 | 0.050000 | 5 | 0.043174 |
| 0.1 | 0.2 | 4.0143 | 0.386941 | 5 | 0.370352 |
| 0.1 | 0.3 | 4.0143 | 0.772408 | 5 | 0.762492 |
| 0.1 | 0.4 | 4.0143 | 0.951708 | 5 | 0.949048 |
| 0.1 | 0.5 | 4.0143 | 0.994442 | 5 | 0.994091 |
| 0.2 | 0.2 | 7.0045 | 0.050000 | 8 | 0.032143 |
| 0.2 | 0.3 | 7.0045 | 0.281501 | 8 | 0.227728 |
| 0.2 | 0.4 | 7.0045 | 0.638410 | 8 | 0.584107 |
| 0.2 | 0.5 | 7.0045 | 0.892613 | 8 | 0.868412 |
| 0.2 | 0.6 | 7.0045 | 0.983738 | 8 | 0.978971 |
| 0.3 | 0.3 | 9.0186 | 0.050000 | 10 | 0.047962 |
| 0.3 | 0.4 | 9.0186 | 0.249643 | 10 | 0.244663 |
| 0.3 | 0.5 | 9.0186 | 0.593093 | 10 | 0.588099 |
| 0.3 | 0.6 | 9.0186 | 0.874692 | 10 | 0.872479 |
| 0.3 | 0.7 | 9.0186 | 0.983230 | 10 | 0.982855 |
| 0.4 | 0.4 | 11.9910 | 0.050000 | 13 | 0.021029 |
| 0.4 | 0.5 | 11.9910 | 0.229635 | 13 | 0.131588 |
| 0.4 | 0.6 | 11.9910 | 0.562559 | 13 | 0.415893 |
| 0.4 | 0.7 | 11.9910 | 0.865636 | 13 | 0.772272 |
| 0.4 | 0.8 | 11.9910 | 0.985944 | 13 | 0.967857 |
| 0.5 | 0.5 | 13.9918 | 0.050000 | 15 | 0.020695 |
| 0.5 | 0.6 | 13.9918 | 0.224232 | 15 | 0.125599 |
| 0.5 | 0.7 | 13.9918 | 0.568302 | 15 | 0.416371 |
| 0.5 | 0.8 | 13.9918 | 0.890702 | 15 | 0.804208 |
| 0.5 | 0.9 | 13.9918 | 0.995777 | 15 | 0.988747 |
| n | Type I Error | Power | EN0 | P(early stop) | Method | ||||
|---|---|---|---|---|---|---|---|---|---|
| 0.3 | 25 | 15 | 1 | 5 | 0.033 | 0.802 | 19.5 | 0.549 | Minimax |
| 0.3 | 26 | 12 | 1 | 5 | 0.036 | 0.805 | 16.8 | 0.659 | |
| 0.3 | 27 | 11 | 1 | 5 | 0.040 | 0.806 | 15.8 | 0.697 | |
| 0.3 | 29 | 10 | 1 | 5 | 0.047 | 0.805 | 15.0 | 0.736 | Optimal |
| 0.4 | 13 | 8 | 1 | 3 | 0.031 | 0.802 | 8.9 | 0.813 | Minimax |
| 0.4 | 15 | 4 | 0 | 3 | 0.043 | 0.818 | 7.8 | 0.656 | Optimal |
| 0.5 | 8 | 4 | 0 | 2 | 0.036 | 0.836 | 5.4 | 0.656 | Minimax |
| 0.5 | 9 | 3 | 0 | 2 | 0.041 | 0.828 | 4.6 | 0.729 | Optimal |
| 0.6 | 6 | 3 | 0 | 2 | 0.015 | 0.807 | 3.8 | 0.729 | Minimax |
| 0.6 | 8 | 2 | 0 | 2 | 0.025 | 0.819 | 3.1 | 0.810 | Optimal |
| n | Power | ESN | P(early stop) | |||||
|---|---|---|---|---|---|---|---|---|
| 0.3 | 23 | 16 | 7 | 0.34 | 0.810 | 18.4 | 0.055 | 0.66 |
| 0.3 | 23 | 17 | 6 | 0.32 | 0.807 | 18.9 | 0.056 | 0.68 |
| 0.3 | 23 | 17 | 6 | 0.30 | 0.806 | 18.8 | 0.057 | 0.70 |
| 0.3 | 23 | 15 | 8 | 0.31 | 0.806 | 17.5 | 0.056 | 0.69 |
| 0.3 | 23 | 14 | 9 | 0.37 | 0.806 | 17.3 | 0.054 | 0.63 |
| 0.3 | 23 | 14 | 9 | 0.45 | 0.806 | 18.0 | 0.052 | 0.55 |
| 0.3 | 23 | 14 | 9 | 0.46 | 0.806 | 18.1 | 0.052 | 0.54 |
| 0.3 | 23 | 16 | 7 | 0.69 | 0.806 | 20.8 | 0.050 | 0.31 |
| 0.3 | 23 | 16 | 7 | 0.36 | 0.805 | 18.5 | 0.054 | 0.64 |
| 0.3 | 23 | 15 | 8 | 0.37 | 0.805 | 18.0 | 0.054 | 0.63 |
| 0.3 | 23 | 13 | 10 | 0.50 | 0.805 | 18.0 | 0.051 | 0.50 |
| 0.3 | 23 | 13 | 10 | 0.53 | 0.805 | 18.3 | 0.051 | 0.47 |
| 0.3 | 23 | 12 | 11 | 0.70 | 0.805 | 19.7 | 0.050 | 0.30 |
| 0.4 | 11 | 8 | 3 | 0.20 | 0.801 | 8.6 | 0.066 | 0.80 |
| 0.4 | 11 | 9 | 2 | 0.21 | 0.801 | 9.4 | 0.064 | 0.79 |
| 0.5 | 7 | 5 | 2 | 0.20 | 0.809 | 5.4 | 0.066 | 0.80 |
| 0.5 | 7 | 5 | 2 | 0.25 | 0.805 | 5.5 | 0.060 | 0.75 |
| 0.5 | 7 | 5 | 2 | 0.30 | 0.801 | 5.6 | 0.057 | 0.70 |
| 0.6 | 5 | 3 | 2 | 0.29 | 0.810 | 3.6 | 0.057 | 0.71 |
| 0.6 | 5 | 3 | 2 | 0.38 | 0.804 | 3.8 | 0.053 | 0.62 |
| 0.6 | 5 | 3 | 2 | 0.40 | 0.801 | 3.8 | 0.053 | 0.60 |
| 0.6 | 5 | 3 | 2 | 0.52 | 0.801 | 4.0 | 0.051 | 0.48 |
| n | Power | ESN | ||||
|---|---|---|---|---|---|---|
| 35 | 27 | 8 | 0.23 | 0.803 | 28.8 | 0.062 |
| 36 | 24 | 12 | 0.24 | 0.802 | 26.9 | 0.061 |
| 37 | 18 | 19 | 0.56 | 0.801 | 28.6 | 0.051 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





