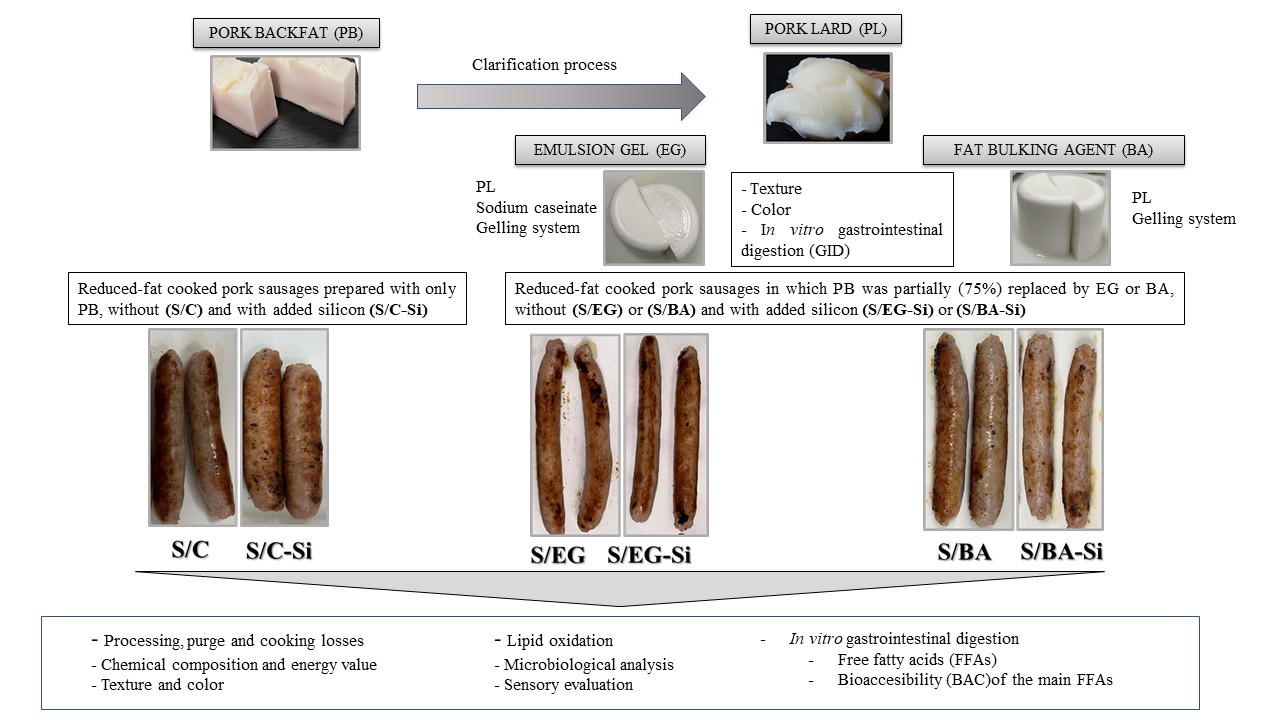
1. Introduction
Fresh pork sausages are a highly popular processed meat product, widely consumed for their affordability, ease of preparation, and notable nutritional benefits. With approximately 60% meat content, they provide proteins of biological value, essential B vitamins, and highly bioavailable minerals, among others. However, these products raise health concerns due to their elevated animal fat content (over 27%), particularly saturated fats. Excessive consumption of cholesterol and saturated fats has been associated with negative health effects (e.g., cardiovascular disease). Nevertheless, saturated fats play a crucial role in the technological properties and sensory characteristics of this type of products. To address these concerns, the meat industry has been searching for fat analogues that not only improve fat content, but also simulate the characteristics of saturated fats. Recent research has focused on structuring healthy oils of vegetable or marine origin to create oleogels (OGs), emulsions gels (EGs) and oil bulking agents (BAs) [
1] to be used as animal fat replacers. Generally, EGs are defined as complex colloidal materials where emulsions and gels (hydrogels) coexist. In contrast, BAs are based on the dispersion of a large amount of oil droplets in a continuous aqueous matrix-forming gel. In this regard, alginate gels present interesting opportunities as fat-bulking agents for its ability to form gels in the presence of calcium salt [
2]. Alginate gels involve polymeric molecules that are cross-linked to form a rigid three-dimensional macromolecular network containing a great proportion of water within its structure, presenting solid structures with characteristics close to those of the animal fat they are intended to replace. While EGs consist mostly of proteins (emulsifying agent) and hydrocolloids (gelling agent), BAs comprise polysaccharides as gelling agents. The incorporation of these lipid materials has improved the lipid content in numerous reformulated meat products, but it has also led to processing issues, such as fatting out and oil exudation. These defects often compromise the desired quality parameters of the reformulated products [
3,
4]. To overcome these issues, there is a new strategy based on modifying animal fat itself by developing emulsions with pork lard (PL), which has a balanced lipid profile between saturated and unsaturated fats. Furthermore, Siri-Tarino et al. [
5] concluded that there is insufficient evidence to definitively link saturated fat intake with cardiovascular disease. In this context, the development of gelled PL lipid materials, such as EGs and fat-based BAs, has emerged as an ideal approach to replace animal fats. These gelled structures, not only have a lower fat content than PL but also exhibit significant cross-linking, which hinders hydrolytic enzymes from accessing fat droplets, therefore reducing lipid digestibility [
6,
7]. While EGs and OGs have been previously used as fat analogues to reduce lipid content and absorption, BAs have only been used to improve the lipid content in meat products, rather than to limit fat digestibility.
Another approach to reformulating healthier meat derivatives involves the incorporation of bioactive compounds. Silicon (Si) is an essential micronutrient, known for its important health effects, including its role as a bone mineralization inducer and neuroprotector. Also, and as evidenced in animal experimental studies, Si exhibits other lesser-known characteristics, including antioxidant activity, as well as hypoglycemic and hypolipemic properties [
8]. In this context, Si has been incorporated into emulsions stabilized with a protein and cellulose ether mixture, showing an important reduction or delay in lipid digestibility at the end of GID compared to emulsions without Si [
9]. The Si-containing emulsions were then used as a complete replacement of pork backfat (PB) in the reformulation of reduced-fat pâtés, resulting in lower fatty acid (FA) contents in the bioaccessible phase compared to their counterparts without Si [
10].
To date, there are no studies comparing two types of fat analogues elaborated with PL (EG vs. BA) for their subsequent incorporation into widely consumed processed meat products. Therefore, the objective of this study was to formulate two different lipid materials using PL and the alginate-based gelling system, to be employed as PB substitutes in reduced-fat fresh pork sausages, without and with added Si as a bioactive compound. For this purpose, the study evaluated the technological, nutritional, microbial, sensory characteristics, as well as lipid digestibility after in vitro GID, of the fresh pork sausages.
2. Results and Discussion
2.1. Visual Appearance, Physicochemical Characteristics and Lipolysis During In Vitro GID of Pork Backfat, Pork Lard and Fat Analogues
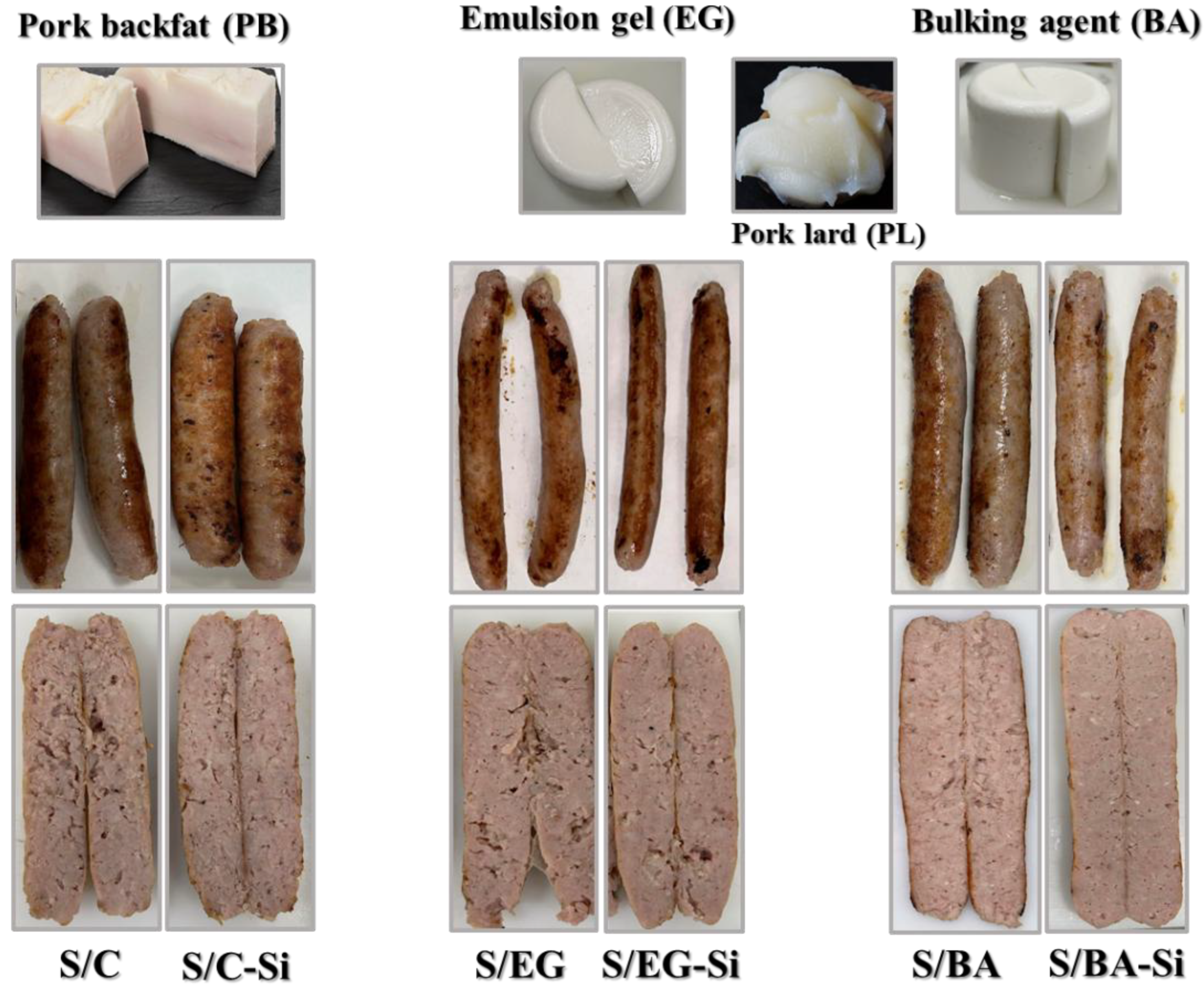
Figure 1 shows the visual appearance of pork backfat (PB), pork lard (PL) and the two fat analogues: the emulsion gel (EG) and the fat bulking agent (BA). Both fat analogues exhibited a similar whitish appearance at room temperature, visually resembling PL but slightly different from PB. In addition, the incorporation of the gelling system - formed by the sodium alginate, CaSO
4 and pyrophosphate - promoted the gelation process in EG and BA, conferring a homogeneous appearance and solid-like characteristics more similar to those of PB than those of PL (
Figure 1).
In terms of texture, both fat analogues exhibited a maximum breaking force (
Figure S1) typical of gel structures. However, the textural properties of the EG sample were significantly lower than those of BA (
Table 1). In contrast, neither PB nor PL presented a maximum force peak, as their structures did not break during the test, and PB had much higher penetration parameter values at the end of the test compared to PL (
Figure S1).
Due to these differences, the textural parameters of PB and PL were not statistically compared to those of EG and BA (
Table 1). The textural differences observed between EG and BA may be attributed to variations in oil droplet size resulting from their respective preparation processes. In this sense, the creation of a bulking system, such as BA, implies the dispersion and physical entrapment of oil droplets within a hydrogel matrix, forming a structured system without emulsifiers. As a result, BA features larger oil droplet sizes compared to conventional gelled emulsions, where the emulsification process with an emulsifying protein, in our case SC, leads to smaller droplets. These texture results are consistent with those reported for oil-in-water (O/W) emulsions formulated with proteins [
11], and for O/W emulsions stabilized with SC, without and with microbial transglutaminase (MTG) [
12]. Other authors have examined the stability of O/W emulsions containing caseinate-coated droplets by adding sodium alginate [
13]. These authors observed that at pH 6 and 7, alginate was not adsorbed to the droplet surfaces due to electrostatic repulsion between anionic groups, either on the alginate or on the adsorbed caseinate (far from the isoelectric point of the protein). Similar electrostatic repulsions between negatively charged droplets and alginate molecules could explain the lower textural gel properties of EG compared to BA (
Table 1).
Regarding the objective color parameters (
Table 1), L* and b* values were significantly higher in EG and BA than in PB and PL, with EG displaying the highest L* and b* values. In contrast, PB exhibited the highest a* value, lower brightness, and a greater tendency to red. This is likely due to the presence of traces of meat and other biological components in this raw material (
Figure 1;
Table 1), while the higher L* and b* values of EG compared to BA could be attributed to the presence of SC as the emulsifying protein.
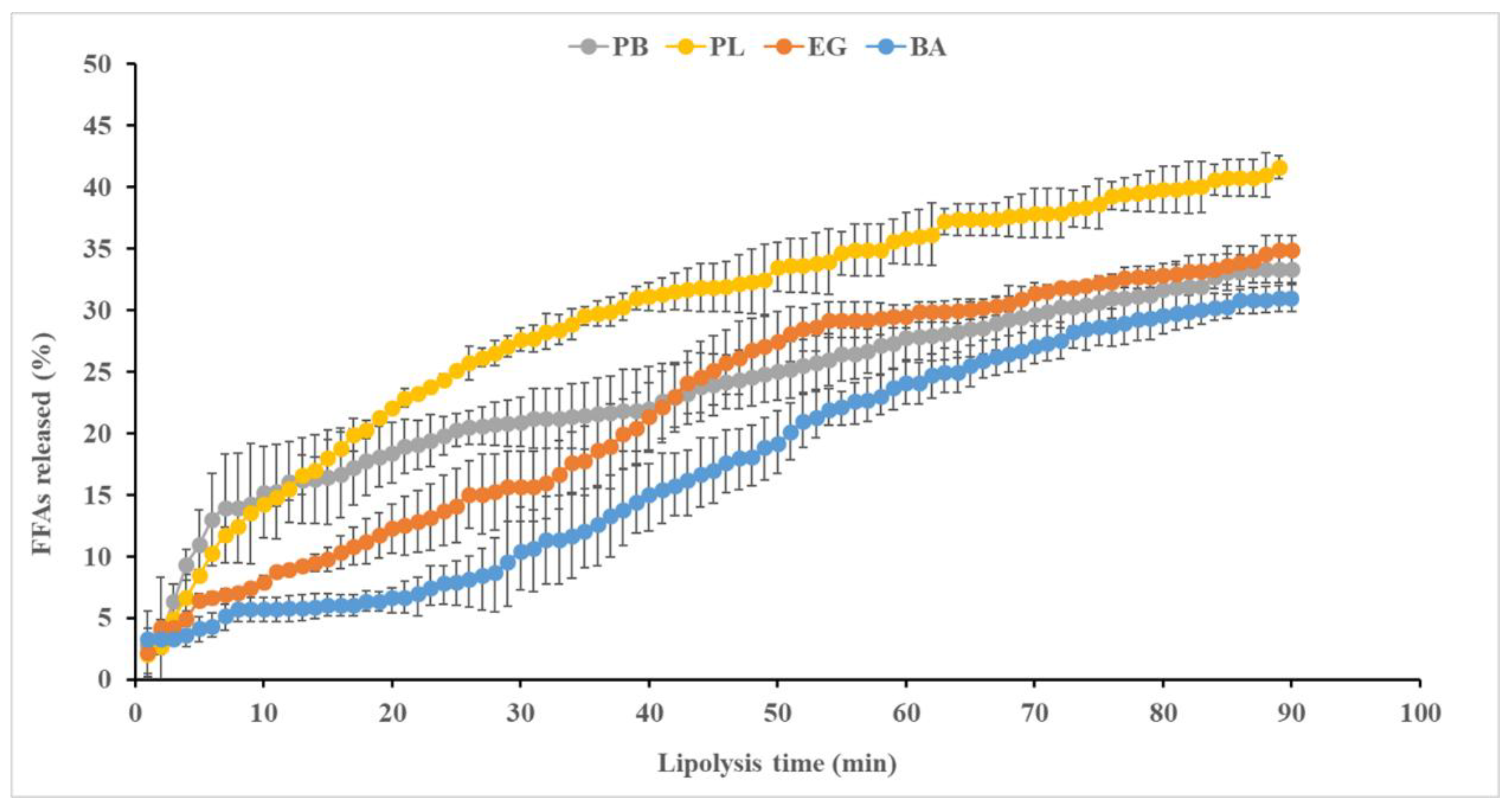
Figure 2 illustrates the rate of FFA release corresponding to PB, PL and EG and BA during lipolysis. Both PB and PL exhibited a similar trend in FFA release during the first 13 min of intestinal digestion. However, after this period, the FFA release from PB was slower than that from PL, reaching lipolysis levels of ~ 33% and ~ 41.61%, respectively, after 90 min. These differences could be attributed to variations in the physical properties in PB and PL fatty materials, as a result of the clarification process to obtain PL from PB. During clarification, changes in the positional distribution of triacylglycerols (TAGs) result in PL having a semi-solid consistency, a lower melting point and a lower solid fat content compared to PB at the GID temperature. Fats with lower melting points have higher
in vitro digestibility and are more easily absorbed over prolonged digestion. In this context, the highly digestible PL exhibited a less compact microstructure and smaller fat crystals than PB. Thus, PB was digested less efficiently, as fats containing stearoyl-richer TAGs require higher temperatures to melt [
14].
Regarding the lipolysis of the fat analogues (
Figure 2), both showed a lower release of FFAs during the first 40 min of digestion compared to PB and PL, with BA exhibiting a lower FFA release than EG. This was followed by a gradual and similar increase in the release profile over time, although some differences between the two persisted; while EG showed a rapid increase in FFA release, BA exhibited a significant delay in lipolysis until min 70, whereas up to min 90 of digestion differences in FFA release decreased. Notably, at the end of intestinal digestion, the FFAs released from both fat analogues and PB were very similar (ranging from 31-35%) and significantly lower than those from PL. These lipolysis percentages were lower than those previously reported for emulsions stabilized with soy protein concentrate but without gelation [
9], as well as for other gelled emulsions formulated with MTG and ĸ-carrageenan [
6]. On the other hand, the initial delay in lipolysis observed in BA could be attributed to its higher consistency (
Table 1); softer gels like EG could degrade faster during
in vitro GID than harder gels like BA [
15]. These authors reported that harder gels have a denser, more compact particulate gel structure with increased cross-linking, making it more difficult for hydrolytic enzymes to access fat droplets. In addition, the higher rate and extent of lipolysis observed in EG could be associated with the fact that emulsifying proteins such as casein, which are commonly used in food-grade emulsions, form an interfacial protein film that can be easily displaced by bile salts, thereby facilitating lipid digestion by lipases [
16].
2.2. Characterization of Reformulated Fresh/Cooked Sausages
2.2.1. Proximate Composition and Energy Content
Table 2 presents the proximate composition of the cooked sausages formulated with PB alone (S/C and S/C-Si), with the emulsion gel (S/EG and S/EG-Si) and with the fat bulking agent (S/BA and S/BA-Si), both of which were used as partial PB replacers at a high substitution percentage (75%). All fresh sausages were formulated with a theoretical reduced-fat content (~ 13.43%) and a protein content ranging from 12.48 to 13.94%. The partial substitution of PB with EG or BA, along with the incorporation of Si, resulted in significant modifications in the proximate composition of the cooked sausages (
Table 2). The sausages formulated without Si (S/C, S/EG and S/BA) exhibited higher moisture content than their counterparts containing Si (S/C-Si, S/EG-Si and S/BA-Si), which was expected, given that Si was added by replacing water (
Table 8). Also, and as anticipated, the cooking process induced an increase in protein content across all samples, particularly in both control sausages (
Table 2). Conversely, the fat content of the control sausages was significantly lower than that of sausages containing EG and BA as fat replacers, with no significant differences observed between the latter. In addition, samples containing Si exhibited a significantly higher ash content.
Given that fresh commercial pork sausages typically contain ~ 30% fat, all the reformulated samples qualify as reduced-fat sausages (fat content ranging from 13.11 to 15.87%). Consequently, their energy content (from 192.79 to 209.47 kcal/100 g product) (
Table 2), was lower than that of commercial and other formulated pork sausages (~ 325 kcal/100 g product) [
17,
18].
2.2.2. Physicochemical Properties
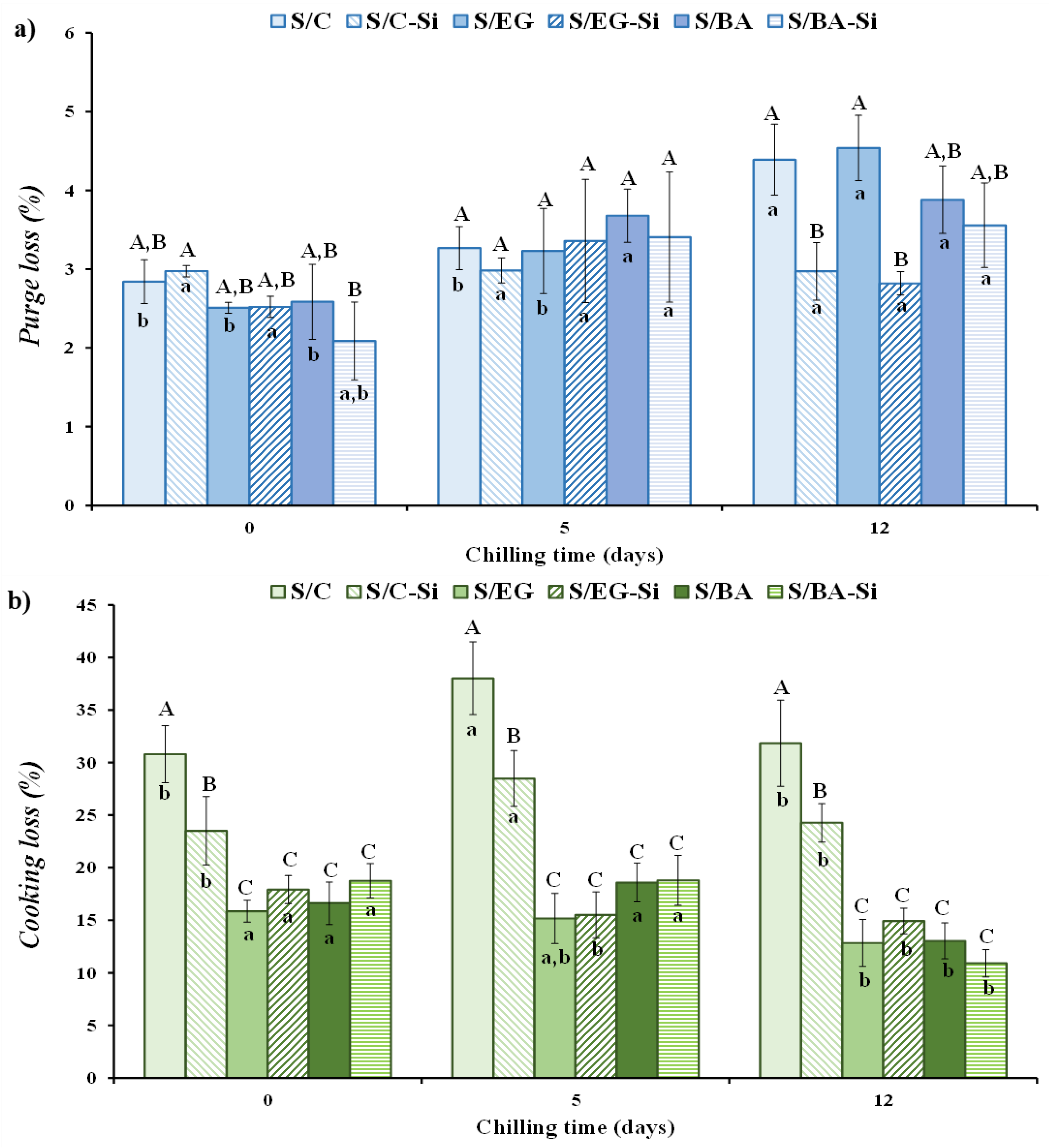
The processing losses in the fresh sausages were minimal, ranging from 1.45 to 2.52% with no significant differences among formulations. The purge and cooking losses during chilled storage are shown in
Figure 3a and
Figure 3b, respectively. The purge loss varied between 2.09% and 4.54% and, in general, up to 5 days of storage, neither the partial substitution of PB with EG or BA nor the incorporation of Si had a significant effect on this parameter. All samples exhibited similar exudate values (
Figure 3a). However, after 12 days of chilled storage, the purge loss increased in all samples without added Si (S/C, S/EG and S/BA) compared to values recorded at days 0 and 5. In contrast, the purge loss values in sausages with added Si (S/C-Si, S/EG-Si and S/BA-Si), remained stable throughout the storage period, indicating that Si reduced exudate losses at the end of the storage period.
Regarding cooking loss, it is well known that the cooking process in meat products results in significant mass loss, primarily influenced (
p < 0.05) by formulation and, to a lesser extent, by storage time (
Figure 3b). Although reported weight losses during sausage cooking vary considerably in the literature, the values recorded in this experiment (ranging from 11% to 38%), fall within the normal range (15% to 40%) for comparable ground meat products [
19]. The highest cooking losses were observed in the control samples (S/C and S/C-Si) throughout the entire storage period (0, 5 and 12 days) (
Figure 3b). In this case, the presence of Si reduced cooking losses only in the control sample (S/C-Si vs. S/C). The partial substitution of PB with either EG or BA resulted in a significant reduction in the cooking loss, with no differences between the values obtained for sausages containing these two fat analogues. This suggests that a relatively high proportion of the fat and water present in both fat analogues was effectively retained after thermal treatment. In contrast, the presence of Si resulted in a loss-reducing effect only in formulations in which losses were substantial (control samples). These differences could be attributed to the fact that, although all cooked sausages had very similar moisture, protein and fat contents (
Table 2), formulations containing EG and BA retained water more effectively within their gel structure, whereas the free water added during the formulation of S/C and S/C-Si was less well retained. The processing, purge and cooking losses values observed in this study are consistent with those reported by other authors in pork sausages [
17,
20]. The latter authors found that replacing PB with chia and oat emulsion gels in reduced-fat sausages significantly reduced the cooking losses.
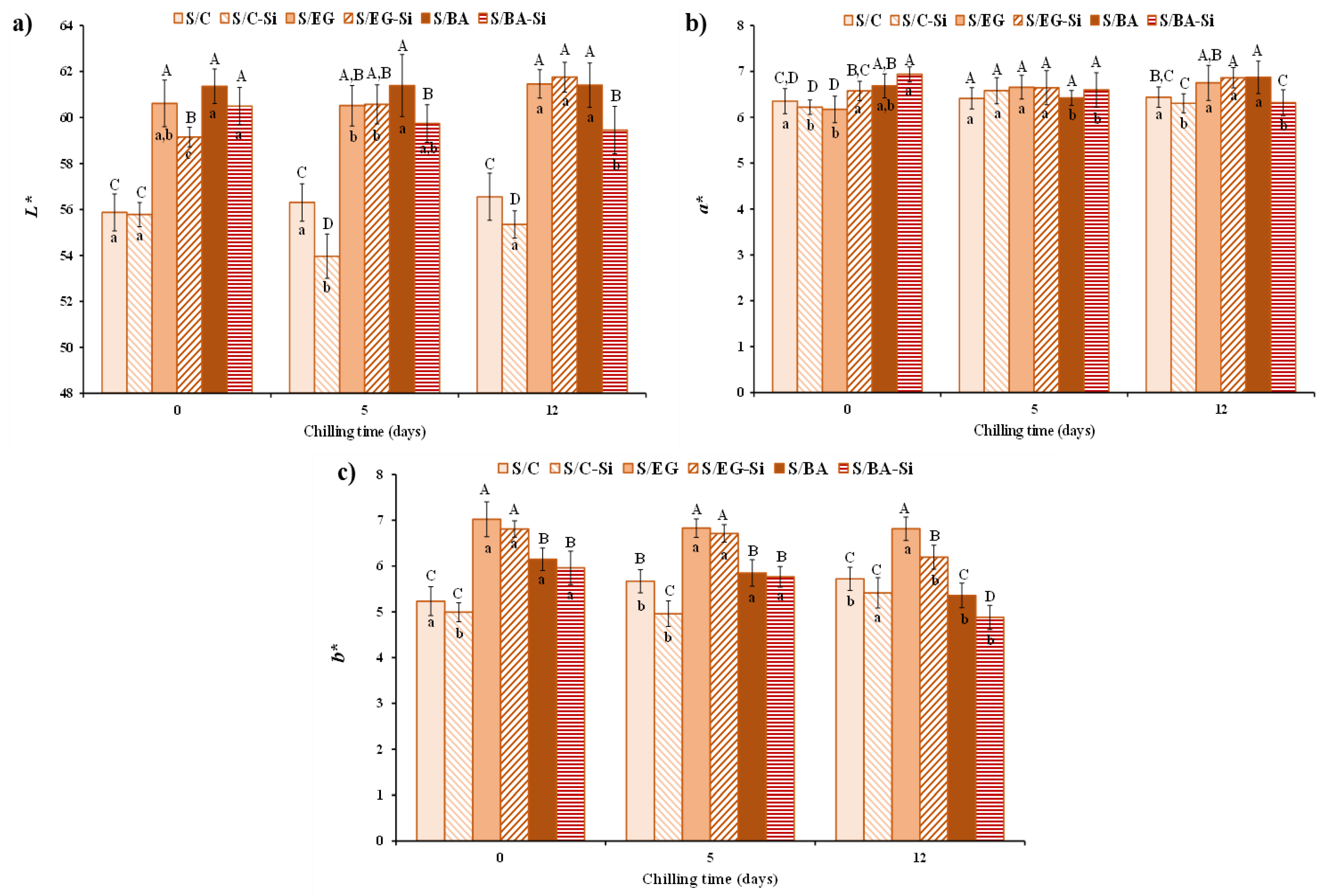
The color parameters of the cooked sausages are shown in
Figure 4. The partial replacement of PB with EG or BA increased lightness (L*) and yellowness (b*) values, which remained relatively stable throughout the storage period (
Figure 4a and
Figure 4c).
The cooked sausages containing BA (S/BA) exhibited significantly (
p < 0.05) lower b* values compared to those containing EG (S/EG) (
Figure 4c). This is consistent with the b* values observed in both BA and EG (
Table 1). The effect of adding Si on color parameters depended on the type of fat analogue used but, in general, Si tended to reduce L* values. However, only minor differences were found in a* as consequence of either formulation or chilled storage, with values ranging from 6.17 to 6.94. Differences in L* and b* can be attributed to variations in color and composition of PB and PL compared to EG and BA. Specifically, PB contained approx. 86% fat, and may have also contained trace amounts of meat and very little water content (~ 5%), whereas the fat analogues contained clarified PL, which is devoid of meat residues and has a high-water content (60%). Despite the significant differences found in L* and b* parameters in the cooked sausages, their technological relevance is negligible, as evidenced by the visual appearance (
Figure 1) and the sensory evaluation described below. Similar changes in pork sausages resulting from PB replacement with different fat analogues (emulsions, oil bulking agents, etc.) have been previously reported [
17,
20].
The textural properties of the cooked sausages were significantly (
p < 0.05) affected by formulation and storage time (
Figure 5). However, variations in springiness and cohesiveness were minimal, with values ranging from 0.831 to 0.898 and from 0.703 to 0.790, respectively (
Figure 5b and
Figure 5c). Hardness and chewiness, which were positive and significantly correlated, were significantly lower in the control sausages formulated with PB, compared to those prepared with EG or BA, throughout all the storage period (
Figure 5a and
Figure 5d). The increased hardness observed in the sausages containing EG or BA can be attributed to the fact that the components included in these fat analogues (mainly water and fat) were better retained within the meat protein gel during cooking compared to the control sausages with PB. This contributed to the higher hardness and chewiness of these products [
21]. The reduction in cooking loss caused by Si in the control samples (S/C-Si vs S/C;
Figure 3b) caused a significant increase in the textural properties at 0, 5 and 12 days of chilled storage. In general terms, the presence of Si tended to increase the hardness of the sausages compared to their counterparts without added Si. Furthermore, hardness and chewiness also increased with increasing storage time, although the magnitude of change (typically minor) and the specific time points at which it occurred (5 or 12 days) varied depending on the fat analogue used, probably due to a slight water loss (
Figure 5a, and
Figure 5d). A similar textural behavior has been observed in different meat products, including cooked pork sausages, in which different fat analogues such as gelled emulsions or oil bulking agents were used as animal fat replacers [
22,
23].
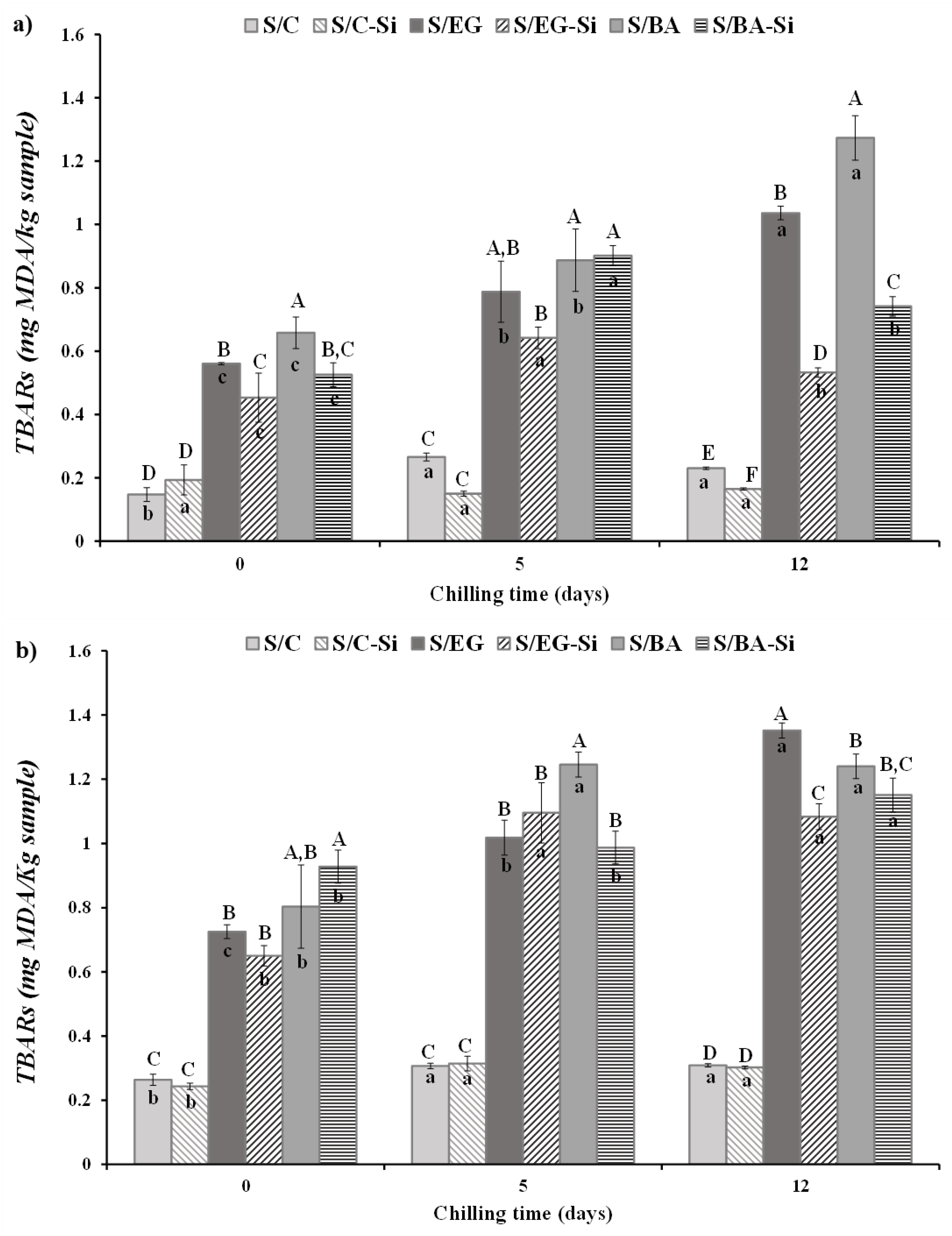
2.2.3. Lipid Oxidation
The results of lipid oxidation, assessed by malondialdehyde levels (TBARs), in fresh and cooked sausages during chilled storage are shown in
Figure 6. Lipid oxidation was significantly lower in fresh sausages (
Figure 6a) compared to cooked sausages (
Figure 6b), which aligns with the well-known fact that thermal processing increases lipid oxidation [
24]. The replacement of PB with either EG or BA caused a significant increase in TBARs values in both fresh and cooked samples (
p < 0.05), with no significant differences between EG and BA. These results were expected, as the control sausages contained only PB, which did not undergo any treatment, thus exhibiting low susceptibility to oxidation. Conversely, the sausages formulated with EG or BA contained PL, obtained from the above-mentioned PB clarification process (heating at ~ 100 °C for at least 30 min). This heat treatment likely explains the higher TBARs values found in the sausages containing fat analogues. Initially, the addition of Si had no effect on the oxidation levels in the cooked samples, while in the fresh samples Si was associated with reduced TBARs levels in the reformulated sausages compared to their counterparts without Si.
The changes in TBARs during storage were formulation dependent, and although they were significant, they were not particularly relevant in the case of S/C and S/C-Si. However, different behavior in terms of lipid oxidation during storage was observed in reformulated products containing EG or BA. In these products the highest TBARs values were detected after 12 days of storage, particularly in S/EG. Notably, in this sample, Si had a significant antioxidative effect, reducing oxidation levels (
Figure 6b). These results are consistent with those observed by other authors in meat products formulated with pork fat, and remain below the reported threshold for sensory detection of undesirable flavors in processed meat products [
25,
26].
2.2.4. Sensory Analysis
Overall, the sensory evaluation of pork sausages was unaffected by formulation (
Table 3). Panelists were unable to distinguish (
p > 0.05) among samples in terms of appearance, color, odor or texture acceptability. The only significant difference was observed in flavor acceptability and overall acceptability, where S/BA received lower scores than S/C-Si. However, there were very little differences in the overall acceptability of the different sausages, regardless the presence of Si or the substitution of PB with a fat analogue (
Table 3), either in the form of a gelled emulsion or a bulking agent (except for S/BA, which was rated lower in acceptability compared to S/C-Si). These results are consistent with those obtained in a pâté formulated with biopolymeric emulsions containing PL, which exhibited sensory attributes comparable to the control pâtés [
10]. Similar results have also been reported in burger patties reformulated with an EG containing polyunsaturated oil as a PB replacer, where no significant differences in sensory attributes were observed [
27].
2.2.5. Microbiological Analysis
Microbiological counts of the fresh sausages are shown in
Table 4. Initially, the control samples (S/C and S/C-Si) exhibited the lowest levels of total viable counts (TVC), lactic acid bacteria (LAB) and
Enterobacteriaceae, with values ranging from 5.95-6.31, 3.95-3.86, and 2.75-2.63 Log cfu/g, respectively. These values align with those reported by others authors for similar products [
17,
28,
29], and, as counts were below 6 Log cfu/g, are acceptable according to the total microbial quality standard for pork sausages and ground beef [
30]. This is noteworthy, given the fact that sausage preparation involved a high level of handling of all raw materials, increasing susceptibility to contamination [
28]. However, the partial replacement of PB with BA, and particularly with EG in the reformulated sausages (S/EG, S/EG-Si, S/BA and S/BA-Si), caused a significant increase of TVC, LAB and
Enterobacteriaceae counts. This increase could be attributed to the manual processing of PL during clarification, in contrast to the control products, which were prepared using PB without additional handling.
During chilled storage (
Table 4) and under vacuum packaging, microbiological counts remained relatively stable across all groups of refrigerated fresh sausages. In this regard, a slight yet significant increase in TVC and LAB levels in the control sausages was observed after 5 days of storage; however, the values remained constant until the end of the storage period. This increase is associated with protein and fat degradation occurring during storage mainly due to endogenous meat enzymes. However, the observed microbial growth was generally lower than that observed in similar fresh products [
29,
30], where after 3–5 days of storage, levels of 8 Log cfu/g were reported. Nevertheless, the reformulated sausages with EG or BA, without and with added Si, hardly changed their TVC, LBA and
Enterobacteriaceae counts throughout the storage period (
Table 4). The low microbial growth rate observed across all samples during storage may be mainly attributed to the preservative effects of vacuum packaging in combination with low storage temperatures.
2.3. Total FFAs Released from Digested Cooked Sausages
The total FFAs (mg/g fat) released after
in vitro GID ranged from 454.8 to 554.0 mg FFAs/g fat, with the highest values observed in the S/C sample and the lowest in the S/EG-Si product (
Table 5).
These findings are consistent with those reported by Asensio-Grau et al. [
31] for different meat matrices (hamburger, sausage, etc.) after
in vitro GID under similar duodenal conditions. The replacement of PB added as bulk fat in the control samples with either of the fat analogues (EG or BA) resulted in a similar reduction of the FFAs released. As all samples are classified as coarsely processed meat products, the differences found between them can be attributed to variations in the structural organization of the lipid material added during processing, leading to distinct fat distribution patterns in the final products. Previous studies have suggested that lipid bioavailability depends on physicochemical properties as well as on the structural organization of lipids in the matrix [
32]. In this context, the lower amount of FFAs released after the
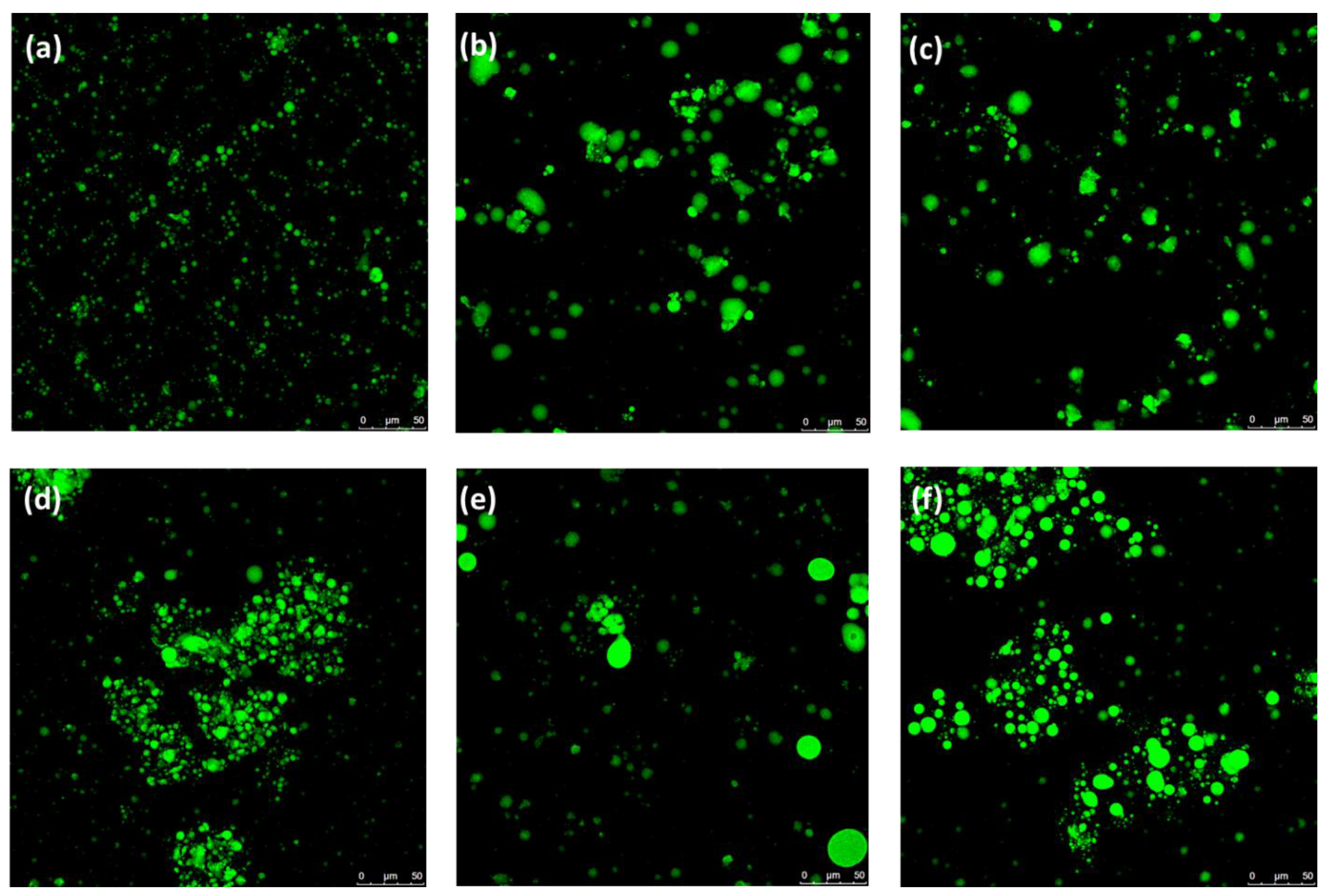
in vitro GID in sausages where PB was replaced with EG or BA is presumably due to the embedding of fat within a gel matrix in both fat analogues. As a result, although partially digested in the gastric and intestinal phase, the oil droplets would remain trapped within the gel network and dispersed in the meat matrix. This structural constraint limits exposure to bile and lipase activity, thus delaying digestion. This result highlights the role of lipid structural organization in limiting the degree of digestion. To confirm this hypothesis, microscopic evaluation of the samples was performed following intestinal digestion (
Figure 7). The digests of the samples containing EG or BA (
Figure 7b and
Figure 7c) exhibited larger oil droplets, probably due to the reduced lipid digestibility caused by the restricted enzymatic access to the surface of the oil droplets embedded in the gel matrices. In contrast, the digest of S/C (
Figure 7a) displayed smaller oil droplets, as the droplets from bulk fat are more accessible to lipase. This was consistent with results found by Diao et al. [
33]. However, the different structural characteristics of EG and BA (
Figure 7) had a minimal impact on lipid digestion (
Table 5).
In addition, the presence of Si in the sausages delayed lipid digestion, with samples containing Si presenting lower FFA release values than their counterparts without Si (
Table 5). The most significant effect was observed in S/EG-Si. This could be due to the partial digestion of the emulsifying protein (SC) in EG during the gastric and intestinal phases, leading to the formation of smaller oil droplets compared to those confined in the bulk fat of BA. Consequently, Si may have further delayed lipid digestion. Previous studies have reported the ability of Si to reduce lipolysis in both biopolymeric emulsions and pâtés elaborated with these systems [
10]. These results are supported by microscopic images, where larger oil droplets were observed in S/EG-Si (
Figure 7e) and S/BA-Si (
Figure 7f) compared to S/C-Si (
Figure 7d).
2.4. FA Profile of Undigested and Digested Cooked Sausages
Table 5 also presents the main FA content of the reduced-fat cooked pork sausages before and after
in vitro GID. As all sausages were produced with the same fat type and content, and considering that Si addition does not affect the lipid profile, the reported data for the FA profiles of the cooked, undigested samples represent the mean values of all six sausage types. Oleic acid (monounsaturated) was the most abundant FA in all samples, followed by palmitic and stearic acids (saturated), linoleic acid (polyunsaturated) and vaccenic acid (monounsaturated), in that order, comprising about 90% of total FAs in TAG form. These results align with FA profiles found in meat and fat handled in other pork meat products [
34,
35].
Regarding the main FA content released after
in vitro GID of all cooked sausages (
Table 5), oleic acid was released in the highest quantity, followed by stearic, palmitic, linoleic, and vaccenic acids. This shows a strong and positive correlation with the lipid composition of the undigested samples. In general, after 90 min of intestinal digestion, the total release of these five main FAs was higher in the control sausage (S/C) than in the samples containing EG or BA, being this consistent with the total FFA results. It should be noted that Si significantly reduced oleic acid solubilization in the micellar phase, with the greatest reduction being observed in S/C-Si (13.94% lower than in S/C), followed by S/EG-Si and S/BA-Si (10.84 and 4.29% lower, respectively), as compared to their counterparts S/EG and S/BA. This may once again be attributed to the more homogeneous fat distribution in the control product, whereas fat gelation favors the delaying effect of Si on lipid digestibility.
Additionally, the bioaccessibility (BAC) of the five main FAs at the end of
in vitro GID of the different pork sausages was calculated (
Table 6). BAC values in the pork sausages ranged from 30.0% to 87.6%, with a mean of 57.8%. These values align with previous digestion studies with fat, where BAC ranges of 50–90% [
36] and 34–71% [
37] were reported for intestinal FA hydrolysis. Regarding the BAC of the five specific FAs studied (
Table 6), it is noteworthy that among the main SFAs, stearic acid had greater BAC values than palmitic acid for all six sausage types. Notably, both of these SFAs had significantly higher BAC in control sausages S/C and S/C-Si than in the reformulated samples with EG or BA as PB partial substitutes. The lower BAC of palmitic acid may offer beneficial health effects, as its adverse impact on cardiovascular health is well-documented, while stearic acid is considered neutral in this regard. The two main MUFAs, vaccenic and oleic acids, displayed very similar BAC values across all samples.
Despite vaccenic acid being a minor FA compared to oleic acid either before or after
in vitro digestion (
Table 5), its BAC values were significantly higher in S/C than in the rest of the sausages (
Table 6). The effect of Si was particularly notable, since its presence in the formulations significantly reduced the BAC of most FAs, confirming the hypotriglyceridemic effect of Si observed in previous works [
6,
9].
3. Conclusions
Healthy fat-reduced fresh pork sausages were developed using two fat analogues (EG or BA) prepared with pork lard (PL) and a cold gelling system, and without and with added Si. The physicochemical, lipid oxidation and sensory characteristics of the reformulated products closely resembled those of the control samples entirely formulated with pork backfat (PB). In vitro digestion assays, performed on both fat analogues as well as on the pork sausages prepared with EG or BA, revealed reduced lipid digestion compared to the control product (S/C). In addition, the presence of Si further reduced or delayed lipid digestibility at the end of GID, with a more pronounced effect in products containing EG. This finding could have significant implications for designing functional meat products based on the reduction of fat content and lipid absorption, providing groundwork for precision nutrition strategies to improve individual health.
4. Materials and Methods
4.1. Materials and Chemicals
For the development of fat analogues (an emulsion gel, EG and a fat bulking agent, BA), pork lard (PL), with 99.9% fat content, was obtained after a clarification process of Iberian pork backfat (PB), which was purchased at a local supermarket (Madrid, Spain). Sodium caseinate (SC), 81% protein content, was provided by Sosa Ingredients S.L. (Barcelona, Spain). The cold gelling system to obtain the gel structures was formed by the hydrocolloid sodium alginate (SA), with 70% carbohydrates, was from TRADES (Barcelona, Spain), and tetra-sodium pyrophosphate anhydrous and calcium sulfate from Panreac Química, S.A. (Madrid Spain). A texturizing mixture (Fibrext 01), containing a blend of fibre, starch and pea protein, was provided by Sancan (Barcelona, Spain). In addition, the ingredients used to prepare the fresh sausages included pork meat and PB, which were both purchased at a local supermarket (Madrid, Spain). A food grade preparation of diatomaceous earth powder (DP) “Tierra de Diatomeas®” with a SiO2 content of 85%, thus equivalent to 40% Si, was kindly offered by Vitality Gesf S.L. (Valencia, Spain). A commercial seasoning mix (Salch color 1041), containing an appropriate combination of authorized preservatives substances and antioxidants, was provided by Sancan (Barcelona, Spain). Natural lamb casings (Type C-22/24) were supplied by Collelldevall S.L.U. (Girona, Spain). Pepsin (≥2500 U/mg, P7012), pancreatin (8xUSP, P7545), and bile extract (B8631), all of porcine origin, as well as dehydrated calcium chloride, potassium chloride, sodium bicarbonate, and sodium hydrogen carbonate, were all supplied by Sigma Aldrich Chemie GmbH (Steinheim, Germany). Methanol/chloroform were obtained from Fisher Scientific S.L. (Madrid, Spain). Trichloroacetic acid (TCA) was supplied from Panreac Química, SA (Barcelona, Spain), while 2-thiobarbituric acid reagent was acquired from (Merck KGaA, Madrid, Spain). Finally, 1,1,3,3-tetraethoxypropane (TEP) was supplied by Sigma Chemical Co. (St. Louis, MO, USA).
4.2. Preparation of Fat Analogues (EG and BA)
Table 7 shows the formulation used to prepare both fat analogues (EG and BA). Briefly, for EG preparation, different solutions were first prepared (12% SC, 5% SA, and another solution containing 0.15 and 0.10% of CaSO
4 and tetra-sodium pyrophosphate anhydrous, respectively). Then, the SC solution was added to a homogenizer (Thermomix TM 31, Vorwerk España M.S.L., S.C, Madrid, Spain) along with the corresponding amount of Milli-Q water (
Table 1) and mixed at ~ 300 rpm for 30 s. Next, the texturizing mixture was added and mixed at ~ 1100 rpm for 1 min. To form the primary emulsion, the melted PL was gradually added while increasing the mixing speed from 1100 and 2000 rpm over 3.5 min. The cold gelling system was then added, and the final mixture was further homogenized for 3 min, gradually increasing the speed from 3100 to 4400 rpm. For BA elaboration, the procedure was similar to that of EG, but without the emulsifying protein (SC) solution.
Finally, each type of fat analogue was placed in a metal container under pressure to compact it and prevent bubble formation. The samples were then stored in a chilled room at 4 ± 2 °C until their incorporation into the meat products (no more than 4 days). For physicochemical analyses, samples were placed in cylindrical containers (3.5 cm height × 2.5 cm diameter) with lids and stored at 4 ± 2 °C to form the final EG and BA until analysis after 3 days of storage.
4.3. Physicochemical Characteristics of PB, PL and Fat Analogues (EG and BA)
The physicochemical characteristics of the fat analogues, PB and PL were analyzed after 72 h of chilled storage at 4 °C. Penetration tests were performed using a Texture Analyzer (TA.HDPlus, Stable Micro Systems, Ltd., Godalming, UK), equipped with a 5 kg load cell and the Texture Exponent Software (version 6.1.23.0). Samples were penetrated (20±1 °C) as previously described by Cofrades et al. [
10]. From the force-distance curves, the following parameters were derived: force at 10 mm (N), total work at 10 mm (mJ), breaking force (N) and breaking work (mJ) (when detected). Color measurements of the lipid materials were conducted on a glass plate using a Konica Minolta CM-3500 D spectrophotometer (Konica Minolta Business Technologies, Tokyo, Japan), set to D65 illuminant/10° observer. Lightness (L*), redness (+a*) and yellowness (+b*) were recorded to determine color coordinates. All tests were carried out in quintuplicate.
4.4. Preparation of Fresh Sausage
Fresh post-rigor meat (30 kg of a mixture of biceps femoris, semimembranosus, semitendinosus, gracilis and adductor muscles) and PB (6 kg), each from different animals, were obtained from a local market on different days. Both visible fat and connective tissue were removed. Batches of approximately 700 g were vacuum packed, frozen and stored at −20 °C until use. Six different batches of fresh sausages were prepared (
Table 8) and each formulation was replicated three times: Two control sausages, elaborated exclusively with PB, and without and with added DP as a source of Si (S/C and S/C-Si, respectively).
Table 8.
Ingredients (g/100 g) used in the preparation of fresh pork sausages.
Table 8.
Ingredients (g/100 g) used in the preparation of fresh pork sausages.
| Fresh sausages |
Meat |
Pork backfat (PB) |
EG |
BA |
DP |
Water |
Seasoning |
| S/C |
60.00 |
13.00 |
0 |
0 |
0 |
23.00 |
4.00 |
| S/C-Si |
60.00 |
13.00 |
0 |
0 |
1.50 |
21.50 |
4.00 |
| S/EG |
60.00 |
3.25 |
21.45 |
0 |
0 |
11.30 |
4.00 |
| S/EG-Si |
60.00 |
3.25 |
21.45 |
0 |
1.50 |
9.80 |
4.00 |
| S/BA |
60.00 |
3.25 |
0 |
21.45 |
0 |
11.30 |
4.00 |
| S/BA-Si |
60.00 |
3.25 |
0 |
21.45 |
1.50 |
9.80 |
4.00 |
Two batches in which 75% of PB was replaced with EG, without and with added Si (S/EG and S/EG-Si, respectively). Two more batches in which 75% of PB was replaced with BA, again without and with Si (S/BA and S/BA-Si, respectively). For the formulation of these sausages, the meat and PB were thawed for 24 h at 4 ± 2 °C. The chilled meat, PB, and EG and BA were minced using a meat mincer equipped with a 6 mm plate (Vam.Dall. Srl., FTSIII, Treviglio, Italy). For all formulations, the required ingredients (
Table 2) and 4% of a commercial seasoning preparation were added to a mixer (MAINCA, Granollers, Barcelona, Spain) and homogenized for a total of 4 min. The mixture was kept at 4 °C for 1.5 h, manually stuffed into 22 mm-diameter natural lamb casings, and hand-linked into sausages of 10 ± 2 cm in length. The resulting strings of sausages were weighed, hung and stored in a chest at 4 ± 2 °C overnight. After overnight storage, each string of sausages was weighed again to calculate processing losses. Individual sausages were then weighed, and vacuum-packaged (Cryovac® OSB3050, Sealead Air, Elmwood Park, NJ, USA) in bags containing 5 sausages each. The packages were stored at 4 ± 2 °C for 12 days. To evaluate the effect of formulation and storage time on quality characteristics, five packages from each treatment were randomly selected for analysis after 0, 5 and 12 days of chilled storage. As this type of sausages are typically consumed cooked, some parameters were analyzed in cooked samples to assess how PB substitution with EG or BA affected quality and lipid
in vitro GID. For this purpose, and before analysis, fresh sausages were cooked at 210 °C for 2 min per side using a contact electric grill (Jata classic multigrill model JT950, Spain).
4.5. Proximate Analysis and Energy Content of Cooked Sausages
Moisture and ash contents of cooked samples were determined according to AOAC [
38]. Protein was measured with a Nitrogen Determinator LECO FP-2000 (Leco Corporation, St Joseph, MI. USA), while fat content was evaluated following the method of Bligh and Dyer [
39]. All tests were performed in triplicate. Energy content was calculated using conversion factors of 4 kcal/g for protein and 9 kcal/g for fat [
40].
4.6. Physicochemical Parameters of Fresh/Cooked Pork Sausages
4.6.1. Processing, Purge and Cooking Losses
Processing loss was evaluated by weighing at least five strings of fresh sausages per formulation before and after overnight refrigeration. Results were expressed as a percentage of the initial weight. Purge loss was determined as follows: three bags with 5 sausages (previously weighed) per formulation were tempered for 10 min. The sausages were then removed from the bag, their surfaces were blotted with a paper towel to eliminate surface exudate and they were weighed. The purge loss was expressed as a percentage of the initial weight. Cooking loss was measured after grilling the sausages to a temperature of 72 °C. At least seven sausages per formulation were weighed before and after cooking, to determine the cooking loss by weight difference. Purge and cooking losses were measured after 0, 5 and 12 days of chilled storage.
4.6.2. Texture Profile Analysis (TPA) and Color Measurements of Cooked Pork Sausages
TPA was conducted on the same Texture Analyzer mentioned above, equipped with a 35-mm diameter cylindrical aluminium probe (P/35). Cylindrical sausage samples with ~ 20-mm diameter and 20-mm height were compressed twice to 25% of their original height to prevent breakage, with a rest period of 2 s between cycles. The TPA test was performed at room temperature with a trigger force of 0.020 N (2 g) and a test speed of 2 mm/s. The textural properties derived from force-time curves included hardness (N), springiness (dimensionless), cohesiveness (dimensionless) and chewiness (N). Definitions of TPA parameters can be found in Wee et al. [
41]. The color measurements of the cooked sausages were performed on their cross-sectional surfaces at each storage time using the previously mentioned spectrophotometer. Five pieces of sausage per batch were evaluated at 0, 5 and 12 days of chilled storage.
4.7. Lipid Oxidation
The thiobarbituric acid-reactive substances (TBARs) method was used to determine (in triplicate) the secondary lipid oxidation [
10], in both fresh and cooked sausages after 0, 5 and 12 days of chilled storage. Results were expressed as mg MDA/kg sample.
4.8. Microbiological Analyses
Microbiological analyses were conducted to determine total viable counts (TVC), for lactic acid bacteria (LAB) and for
Enterobacteriaceae in fresh sausages during chilled storage, following the methodology described by Pintado et al. [
17]. All microbial counts were expressed as logarithms of colony-forming units per gram (Log cfu/g).
4.9. Sensory Analysis
Sensory analysis was carried out on day 0. Each grilled sausage was cut into ~ 4 cm pieces and served immediately after cooking. Sensory evaluation was performed by 33 semi-trained panelists, recruited from the ICTAN staff. These panelists had prior experience assessing various meat products (hamburgers, frankfurters, pâtés, sausages, etc.) and were widely familiar with both the sausages and the attributes tested. A hedonic scale rating test was used to evaluate appearance, acceptability of color, odor, flavor, texture, and overall acceptability for each of the 6 types of sausages. A non-structured scale with fixed extremes (0 = dislike extremely, 9 = like extremely) was used, with each point assigned a corresponding numerical value. All panelists signed a written consent before participation. The study was approved by the CSIC Ethics Committee (063/2019).
4.10. In Vitro GID of PB, PL, EG, BA and Cooked Pork Sausages
EG, BA and the cooked sausages (S/C, S/C-Si, S/EG, S/EG-Si, S/BA and S/BA-Si) were subjected to an
in vitro INFOGEST 2.0 digestion procedure based on the protocol described by Brodkorb et al. [
42] with slight modifications. Each formulation was analyzed within two days of preparation. The complete simulated
in vitro GID procedure, including the oral, gastric, and intestinal phases was performed three times per sample as described in Cofrades et al. [
6,
10]. Digest solutions were kept frozen at -20 °C until analysis.
4.11. Rate and Extent of Lipolysis of PB, PL, EG and BA During In Vitro GID
The extent and rate of lipolysis were determined by measuring the content of free fatty acids (FFAs) released during the
in vitro intestinal digestion. In brief, 0.1 M NaOH was used to neutralize the FFAs (pH = 7.0) and the FFAs released were monitored by a pH-stat automatic potentiometric titrator (T7, Mettler Toledo, Zurich, Switzerland). Finally, the FFA release rate was calculated using the following equation in accordance with Sarkar et al. [
43]:
where
VNaOH is the volume of NaOH solution required,
mNaOH is the molarity (0.1 M),
Mlipid is the average molecular weight of lipid,
Wlipid is the weight of lipid in the mixtures.
4.12. Fatty Acid (FA) Profile of the Undigested and Digested Cooked Sausages and Bioaccessibility (BAC) of the Main FAs After In Vitro GID
The fatty acid (FA) profile of the undigested cooked sausages was determined according to the method of Lee et al. [
44] as described in Cofrades et al. [
10]. FA composition was also evaluated from the micellar fraction obtained after
in vitro GID of the different cooked sausages. The final digested solutions of the six types of sausages were centrifuged (Sorvall Lynx 4000 centrifuge, Thermo Scientific, Waltham, MA, USA) at 12,000 rpm for 30 min at 20 °C. The resulting micellar fraction (the bioaccessible fraction) was collected and used to determine the degree of lipolysis (expressed as the percentage of FFAs released), their composition and the bioaccessibility (BAC) of the main FFAs, following the methodology outlined in Cofrades et al. [
9,
10].
4.13. Microstructure Measurements
The microstructure of the digested solutions after 90 min of
in vitro GID was determined by confocal laser scanning microscopy (CLSM) using a confocal microscope (Leica TCS SP5 AOBS, Mannheim, Germany) with 20 × optics. Fluorophores (Fast Green and Red Nile) were added to a drop (≈10 μL) of sample on a microscope slide, as previously described in Cofrades et al. [
6].
4.14. Statistical Analysis
A one-way analysis of variance (ANOVA) was performed to evaluate the statistical significance (p < 0.05) of the effect of sample formulation. As well, a two-way ANOVA was conducted to evaluate the interaction between formulation and storage time, both using the SPSS program (v.22, IBM SPSS Inc.; Chicago, IL, USA). Formulation and storage time were considered fixed effects and replication a random effect. The entire experimental design was conducted in triplicate, and differences between replicates were not significant (p < 0.05). Results were expressed as mean and standard deviation. Tukey’s HSD test was used to identify significant differences (p < 0.05) between formulations and across storage times.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org, Figure S1: Force-distance curves of fat analogues (EG and BA) as compared to pork backfat (PB) and pork lard (PL).
Author Contributions
Conceptualization, M.D.Á. and S.C.; methodology, M.D.Á., A.S. and S.C.; investigation, M.D.Á., A.S. and S.C.; validation, M.D.Á., A.S. and S.C.; formal analysis, M.D.Á., A.S. and S.C.; data curation, M.D.Á., A.S. and S.C.; writing – original draft preparation, writing – review & editing, M.D.Á. and S.C.; project administration, S.C.; funding acquisition, S.C.
Funding
This work was supported by the Project with reference PID2019-103872RB-I00/AEI/10.13039/501100011033 from the Ministerio de Ciencia e Innovación, and the Project TEC-2024/BIO-307 from Comunidad de Madrid.
Institutional Review Board Statement
The study was approved by the CSIC Ethics Committee (063/2019).
Informed Consent Statement
Written consent was signed by all subjects involved in the sensory analysis.
Data Availability Statement
The original contributions presented in this study are included in the article/
supplementary material. Further inquiries can be directed to the corresponding author.
Acknowledgments
We are grateful to the Analysis Service Unit facilities of ICTAN-CSIC for the determination of silicon and fatty acid profile. Thanks to Rita Shimano (native English speaker) by providing language help and writing assistance.
References
- Jimenez-Colmenero, F.; Salcedo-Sandoval, L.; Bou, R.; Cofrades, S.; Herrero, A.M.; Ruiz-Capillas, C. Novel Applications of Oil Structuring Methods as a Strategy to Improve the Fat Content of Meat Products. Trends Food Sci. Technol. 2015, 44, 177–188. [Google Scholar] [CrossRef]
- Herrero, A.M.; Carmona, P.; Jiménez-Colmenero, F.; Ruíz-Capillas, C. Polysaccharide Gels as Oil Bulking Agents: Technological and Structural Properties. Food Hydrocoll. 2014, 36, 374–381. [Google Scholar] [CrossRef]
- Manzoor, S.; Masoodi, F.A.; Rashid, R.; Naqash, F.; Ahmad, M. Oleogels for the Development of Healthy Meat Products: A Review. Applied Food Res. 2022, 2, 100212. [Google Scholar] [CrossRef]
- Ren, Y.; Huang, L.; Zhang, Y.; Li, H.; Zhao, D.D.; Cao, J.; Xinqui, L. Application of Emulsion Gels as Fat Substitutes in Meat Products. Foods 2022, 11, 1950. [Google Scholar] [CrossRef] [PubMed]
- Siri-Tarino, P.W.; Sun, Q.; Hu, F.B.; Krauss, R.M. Saturated Fatty Acids and Risk of Coronary Heart Disease: Modulation by Replacement Nutrients. Curr. Atheroscler. Rep. 2010, 12, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Cofrades, S.; Hernández-Martín, M.; Garcimartín, A.; Saiz, A.; López-Oliva, M.E.; Benedí, J.; et al. Impact of Silicon Addition on the Development of Gelled Pork Lard Emulsions with Controlled Lipid Digestibility for Application as Fat Replacers. Gels 2023, 9, 728. [Google Scholar] [CrossRef] [PubMed]
- Poyato, C.; Ansorena, D.; Berasategi, I.; Navarro-Blasco, I.; Astiasarán, I. Optimization of a Gelled Emulsion Intended To Supply ω-3 Fatty Acids into Meat Products by Means of Response Surface Methodology. Meat Sci. 2014, 98, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Santos-López, J.A.; Garcimartín, A.; Merino, P.; López-Oliva, M.E.; Bastida, S.; Benedí, J.; Sánchez-Muniz, F.J.; Portero-Otin, M. Effects of Silicon vs. Hydroxytyrosol-Enriched Restructured Pork on Liver Oxidation Status of Aged Rats Fed High-Saturated/High-Cholesterol Diets. PLoS ONE 2016, 11, e0147469. [Google Scholar] [CrossRef] [PubMed]
- Cofrades, S.; Garcimartín, A.; Pérez-Mateos, M.; Saiz, A.; Redondo-Castillejo, R.; Bocanegra, A.; et al. Stabilized Soy Protein Emulsion Enriched with Silicon and Containing or Not Methylcellulose as Novel Technological Alternatives to Reduce Animal Fat Digestion. Food Res. Int. 2023, 170, 112833. [Google Scholar] [CrossRef] [PubMed]
- Cofrades, S.; Saiz, A.; Álvarez, M.D. Biopolymeric Emulsions with Added Silicon as Pork Fat Substitutes in the Reformulation of Healthy Pâtés: Effect on Technological, Nutritional and Sensory Properties and on Lipid Digestibility after In Vitro Gastrointestinal Digestion. LWT - Food Sci. Technol. 2024, 210, 116865. [Google Scholar] [CrossRef]
- Patel, A.R.; Dewettinck, K. Edible Oil Structuring: An Overview and Recent Updates. Food Funct. 2016, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Herrero, A.M.; Carmona, P.; Pintado, T.; Jiménez-Colmenero, F.; Ruíz-Capillas, C. Olive Oil-in-Water Emulsions Stabilized with Caseinate: Elucidation of Protein-Lipid Interactions by Infrared Spectroscopy. Food Hydrocoll. 2011, 25, 12–18. [Google Scholar] [CrossRef]
- Pallandre, S.; Decker, E.A.; McClements, D.J. Improvement of Stability of Oil-in-Water Emulsions Containing Caseinate-Coated Droplets by Addition of Sodium Alginate. J. Food Sci. 2007, 72, E518–E524. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.-S.; Lee, J.-H. Melting, Crystallization, and In Vitro Digestion Properties of Fats Containing Stearoyl-Rich Triacylglycerols. Molecules 2022, 27, 191. [Google Scholar] [CrossRef] [PubMed]
- Luo, N.; Ye, A.; Wolber, F.M.; Singh, H. Effect of Gel Structure on the In Vitro Gastrointestinal Digestion Behaviour of Whey Protein Emulsion Gels and the Bioaccessibility of Capsaicinoids. Molecules 2021, 26, 1379. [Google Scholar] [CrossRef] [PubMed]
- Bellesi, F.A.; Pizones Ruiz-Henestrosa, V.M.; Pilosof, A.M.R. Lipolysis of Soy Protein and HPMC Mixed Emulsion as Modulated by Interfacial Competence of Emulsifiers. Food Hydrocoll. 2020, 99, 105328. [Google Scholar] [CrossRef]
- Pintado, T.; Herrero, A.M.; Jiménez-Colmenero, F.; Pasqualin Cavalheiro, C.; Ruiz-Capillas, C. Chia and Oat Emulsion Gels as New Animal Fat Replacers and Healthy Bioactive Sources in Fresh Sausage Formulation. Meat Sci. 2018, 135, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guo, Y.; Liu, H.; Liang, B.; He, H.; Fu, X.; Sun, C.; Li, X.; Ji, C. Preparation, Characterization of Curdlan-Based Emulsion Micro-Gel Particles and its Application in Low-Fat Pork Sausages. LWT - Food Sci. Technol. 2023, 185, 115160. [Google Scholar] [CrossRef]
- Boles, J.A.; Shand, P.J. Effects of Raw Binder System, Meat Cut and Prior Freezing on Restructured Beef. Meat Sci. 1999, 53, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Park, G.; Jin, S.; Choi, J. Effects of Physicochemical Characteristics and Storage Stability of Porcine Albumin Protein Hydrolysates in Pork Sausage. Current Res. Nutr. Food Sci. 2022, 10, 1007–1019. [Google Scholar] [CrossRef]
- Paglarini, C.S.; Furtado, G.F.; Honório, A.R.; Mokarzel, L.; Vidal, V.A.S.; Ribeiro, A.P.B.; Cunha, R.L.; Pollonio, M.A.R. Functional Emulsion Gels as Pork Back Fat Replacers in Bologna Sausage. Food Struct. 2019, 20, 100105. [Google Scholar] [CrossRef]
- Jimenez-Colmenero, F.; Herrero, A.; Pintado, T.; Solas, M.T.; Ruiz-Capillas, C. Influence of Emulsified Olive Oil Stabilizing System Used for Pork Backfat Replacement in Frankfurters. Food Res. Int. 2010, 43, 2068–2076. [Google Scholar] [CrossRef]
- Pintado, T.; Cofrades, S. Quality Characteristics of Healthy Dry Fermented Sausages Formulated with a Mixture of Olive and Chia Oil Structured in Oleogel or Emulsion Gel as Animal Fat Replacer. Foods 2020, 9, 830. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.E.; Stepanyan, V.; Allen, P.; O’Grady, M.N.; Kerry, J.P. Evaluation of the Effects of Selected Plant-Derived Nutraceuticals on the Quality and Shelf-Life Stability of Raw and Cooked Pork Sausages. LWT - Food Sci. Technol. 2011, 44, 164–172. [Google Scholar] [CrossRef]
- Alirezalu, K.; Hesari, J.; Yaghoubi, M.; Khaneghah, A.M.; Alirezalu, A.; Pateiro, M.; Lorenzo, J.M. Combined Effects of ε-Polylysine and ε-Polylysine Nanoparticles with Plant Extracts on the Shelf Life and Quality Characteristics of Nitrite-Free Frankfurter-Type Sausages. Meat Sci. 2021, 172, 108318. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Holman, B.W.; Ponnampalam, E.N.; Kerr, M.G.; Bailes, K.L.; Kilgannon, A.K.; Collins, D.; Hopkins, D.L. Understanding Beef Flavour and Overall Liking Traits Using Two Different Methods for Determination of Thiobarbituric Acid Reactive Substance (TBARS). Meat Sci. 2019, 149, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Poyato, C.; Astiasaran, I.; Barriuso, B.; Ansorena, D. A New Polyunsaturated Gelled Emulsion as Replacer of Pork Back-Fat in Burger Patties: Effect on Lipid Composition, Oxidative Stability and Sensory Acceptability. LWT- Food Sci. Technol. 2015, 62, 1069–1075. [Google Scholar] [CrossRef]
- Mizzi, L.; Cofrades, S.; Bou, R.; Pintado, T.; López-Caballero, M.E.; Zaide, F.; Jiménez-Colmenero, F. Antimicrobial and Antioxidant Effects of Combined High Pressure Processing and Sage in Beef Burgers during Prolonged Chilled Storage. Innov. Food Sci. Emerg. Technol. 2019, 51, 32–40. [Google Scholar] [CrossRef]
- Mathenjwa, S.A.; Hugo, C.J.; Bothma, C.; Hugo, A. Effect of Alternative Preservatives on the Microbial Quality, Lipid Stability and Sensory Evaluation of Boerewors. Meat Sci. 2012, 91, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Capillas, C.; Cofrades, S.; Serrano, A.; Jiménez-Colmenero, F. Biogenic amines in restructured beef steaks as affected by added walnuts and cold storage. J. Food Protect. 2004, 67, 607–609. [Google Scholar] [CrossRef] [PubMed]
- Asensio-Grao, A.; Calvo-Lerma, J.; Heredia, A.; Andrés, A. Fat Digestibility in Meat Products: Influence of Food Structure and Gastrointestinal Conditions. Int. J. Food Sci. Nutr. 2018, 70, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; McClements, D.J. Modulating Lipid Droplet Intestinal Lipolysis by Electrostatic Complexation with Anionic Polysaccharides: Influence of Cosurfactants. Food Hydrocoll. 2014, 35, 367–374. [Google Scholar] [CrossRef]
- Diao, X.; Ke, W.; Li, S.; Mao, X.; Shan, K.; Zhang, M.; Zhao, D.; Li, C. Effect of Wheat Aleurone on Lard Emulsions during In Vitro Digestion. Food Chem. 2024, 435, 137530. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Pando, G.; Cofrades, S.; Rodríguez-Salas, L.; Jiménez-Colmenero, F. A Healthier Oil Combination and Konjac Gel as Functional Ingredients in Low-Fat Pork Liver Pâté. Meat Sci. 2011, 88, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Lucas-González, R.; Pérez-Álvarez, J.A.; Viuda-Martos, M.; Fernández-López, J. Pork Liver Pâté Enriched with Persimmon Coproducts: Effect of In Vitro Gastrointestinal Digestion on its Fatty Acid and Polyphenol Profile Stability. Nutrients 2021, 13, 1332. [Google Scholar] [CrossRef] [PubMed]
- Lairon, D. Designing Functional Foods. In Woodhead publishing series in food science, technology and nutrition; McClements, D.J., Decker, E.A., Eds.; Woodhead Publ. Ltd.: Cambridge, U.K., 2009; 177, pp. 68–93. [Google Scholar]
- Solomando, J.C.; Antequera, T.; Perez-Palacios, T. Lipid Digestion Products in Meat Derivatives Enriched with Fish Oil Microcapsules. J. Funct. Foods 2020, 68, 103916. [Google Scholar] [CrossRef]
- AOAC. In Official methods of analysis of AOAC International; AOAC international, 2005.
- Bligh, E.G.; Dyer, W.J. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- European Union (EU). Regulation (EU) No. 1169/2011 of the European Parliament and of the Council on the provision of food information to consumers. Official Journal of the European Union, European Commission 2011, 304, 18–6. [Google Scholar]
- Wee, M.S.M.; Goh, A.T.; Stieger, M.; Forde, C.G. Correlation of Instrumental Texture Properties from Textural Profile Analysis (TPA) with Eating Behaviours and Macronutrient Composition for a Wide Range of Solid Foods. Food Funct. 2018, 9, 5301–5312. [Google Scholar] [CrossRef] [PubMed]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; et al. INFOGEST Static In Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Ye, A.; Singh, H. On the Role of Bile Salts in the Digestion of Emulsified Lipids. Food Hydrocoll. 2016, 60, 77–84. [Google Scholar] [CrossRef]
- Lee, M.R.F.; Tweed, J.K.S.; Kim, E.J.; Scollan, N.D. Beef, Chicken and Lamb Fatty Acid Analysis-a Simplified Direct Bimethylation Procedure Using Freeze-Dried Material. Meat Sci. 2012, 92, 863–866. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).