1. Introduction
Obstructive sleep apnea (OSA) is a sleep-related breathing disorder characterized by recurrent partial or complete upper airway obstructions despite ongoing respiratory efforts [
1]. These episodes lead to disrupted breathing, impaired ventilation, and fragmented sleep patterns [
1,
2]. The OSA spectrum ranges from simple snoring to more severe manifestations, such as upper airway resistance syndrome and true obstructive sleep apnea syndrome [
3].
The pathogenesis of OSA is multifactorial and not yet fully understood, involving both anatomical and functional factors [
3]. In children, upper airway obstruction is often associated with anatomical issues, primarily adenoid and tonsillar hypertrophy [
4]. Additionally, skeletal constraints, such as a retrognathic mandible, a reduced bony pharynx, and a narrow maxilla, can further compromise the airway. The association between certain craniofacial anomalies and the severity of OSA underscores the pivotal role of anatomical factors in its pathogenesis [
5]. Moreover, functional impairments, including deficits in neuromuscular control or underlying neurological conditions, may further compromise airway patency. These factors directly affect the tone and function of the pharyngeal dilator muscles, contributing to the complex pathophysiology of the disorder [
6,
7,
8,
9,
10]. During wakefulness, individuals with OSA already rely on increased activity of the airway dilator muscles as a compensatory mechanism to maintain airway patency due to their anatomically narrow and more collapsible pharyngeal airway. However, with sleep onset, this augmented dilator muscle activity diminishes or is lost, leading to pharyngeal collapse. Therefore, the reduction in muscle tone during sleep onset is considered a crucial factor in the pathogenesis of OSA [
11]. These anatomical and functional factors are modulated by additional variables, including body mass index (BMI), gender, medical conditions, and age [
12].
OSA has wide-ranging implications for the pediatric population, significantly affecting cognitive and behavioral development leading to long-term neurodevelopmental consequences. It notably impairs attention, memory, behavior, and academic performance. Its prevalence is particularly concerning among children with learning and behavioral disabilities, where it is estimated to be about six to nine times higher than in the general pediatric population. Because of its association with multiple health complications, OSA represents a serious public health concern [13].
Therefore, OSA in children should never be overlooked, and treatment should be initiated promptly after diagnosis. Comprehensive management requires close collaboration among pediatricians, otolaryngologists, sleep medicine experts, speech therapists, and orthodontists to ensure coordinated, multidisciplinary care.
Orthodontists play a crucial role in the early identification and intervention of pediatric OSA. Through growth modification, orthodontists may optimize compromised airway patterns and support long-term treatment outcomes. This collaboration is vital in ensuring effective and individualized care tailored to the patient’s needs [16].
While early orthodontic intervention is promising, the timing and extent of treatment remain the subject of debate. Some experts advocate for early interceptive management as early as 2-3 years of age, while others call for caution against overtreatment due to the absence of robust, long-term data.
Thus, this narrative review aims to provide a balanced perspective on the role of orthodontists in the interdisciplinary management of pediatric OSA, focusing on early diagnosis, craniofacial growth modification, and personalized treatment planning that aligns with current recommendations [15]. Moreover, it outlines current treatment strategies, addresses clinical challenges, and proposes future research directions. A further key objective is to promote effective communication and collaboration within the interdisciplinary team while identifying future research priorities to improve outcomes in this patient population.
2. Epidemiology and Risk Factors
It is estimated that 3-26% of children are habitual snorers [16-18], with 1.2-5.7% of the general pediatric population exhibiting OSA [
19,
20]. The peak incidence of OSA occurs between the ages of 2 and 8 years. In pre-pubertal children, OSA affects both genders equally [
21]. However, after puberty, its prevalence increases in males [
22]. Among adolescents who seek orthodontic treatment, up to 13.3% exhibit a risk for sleep-disordered breathing [
23,
24]. OSA is significantly more prevalent among specific pediatric populations with underlying risk profiles, like anatomical, neuromuscular, genetic, and environmental contributors.
OSA affects up to 36% of children with obesity [
25], and the prevalence may exceed 60% in those who habitually snore [
26]. Pediatric obesity is strongly associated with increased subcutaneous fat deposition in the neck surrounding the airway and fatty infiltration in the tongue. This reduces airway patency and contributes to OSA in children. Additionally, fat deposition around the thoracic, abdominal, and viscera regions decreases lung volume and oxygen reserve [
27]. Notably, a 10% increase in body weight correlates with a 32% increase in the Apnea-Hypopnea Index (AHI) [
28].
Among children with Down syndrome [
29], prevalence ranges from 30% to 63%, linked to midface hypoplasia, macroglossia, and generalized muscle hypotonia.
In craniofacial syndromes [
30,
31], such as craniofacial dysostosis (e.g., Treacher Collins syndrome, Goldenhar syndrome, craniofacial microsomia) [
32] and syndromic craniosynostosis (e.g., Crouzon and Apert syndromes) [
33,
34], OSA prevalence ranges between 40–60% [
35]. Similarly, children with Robin sequence [
36] and cleft lip and palate [
37] exhibit increased susceptibility. In all conditions associated with micrognathia, including secondary micrognathia related to temporomandibular joint ankylosis, trauma, or juvenile idiopathic arthritis, the prevalence of OSA increases with the severity of mandibular deformity [
38,
39,
40]. Other syndromes associated with an elevated OSA risk include, among others, Prader-Willi syndrome [
41], achondroplasia, and pycnodysostosis [
42].
Neuromuscular conditions, including muscle tone disorders or dysfunction in central breathing control, further contribute to OSA. Additionally, children with a history of prematurity or seizure disorders are more prone to OSA. Chronic upper airway inflammation, including asthma, allergies, recurrent respiratory infections, or exposure to environmental factors (e.g., parental smoking, air pollution), also significantly increases the risk [
43]. Among children with ADHD, the risk of OSA may reach 62.5%, with sleep bruxism observed in up to 40% [
44].
Ethnic variation in craniofacial morphology and airway anatomy influences the risk of OSA. For example, African populations with macroglossia and low tongue posture are at higher risk. Asian and Hispanic populations with maxillary or bimaxillary retrognathism demonstrate increased susceptibility [
45,
46]. Since craniofacial morphology and airway structure differ between ethnic groups, these factors must be considered when assessing OSA risk and planning treatment [
47].
3. Craniofacial Growth and Functional Impact of Mouth Breathing
Craniofacial and dentofacial development is influenced by genetics [
48] and environmental factors, including functional influences, such as breathing mode, tongue posture, and orofacial muscle tone [
49,
50]. Today, it is accepted that cartilage is the primary determinant of craniofacial growth at the cranial base synchondroses. According to the functional matrix theory (Moss et al.) [
51], craniofacial and dentofacial growth occur in response to functional needs, such as mastication, swallowing, and breathing, and likely in response to the growth of the nasal cartilage [
51]. Favorable dental arch growth depends on nasal breathing with a closed mouth posture and the tongue in contact with the palate, acting as a mold for development [
52,
53].
Conversely, increased airway resistance, often caused by adenoidal hypertrophy, has been associated with craniofacial disharmony and malocclusion, as demonstrated by Linder-Aronson [
49]. Mouth breathing has a multifactorial etiology, often stemming from anatomical obstructions such as adenotonsillar hypertrophy, midfacial hypoplasia, enlarged turbinates, or a deviated nasal septum. It can also result from neuromuscular hypotonia or may persist as a habit after treatment of anatomical obstructions. (
Figure 1)
The soft tissue stretch theory by Solow and Kreiborg [
54] postulates that mouth breathing alters head posture and muscle recruitment, negatively affecting craniofacial growth. Mouth-breathing children often exhibit features collectively referred to as “adenoid facies” or long-face syndrome, along with reduced orofacial muscle tone [
49,
55,
56,
57]. The low and anterior position of the tongue, related to mouth breathing (
Figure 2), results in a lack of internal tongue pressure on the palate and a predominant cheek pressure, leading to a reduction in transverse growth of the maxilla and the development of a high-arched palate and lateral crossbites [
58].
Importantly, some craniofacial changes related to mouth breathing may be partially reversible in young children [
59]. These findings have been demonstrated in animal studies using Rhesus monkeys with experimentally induced nasal obstruction [
60,
61,
62].
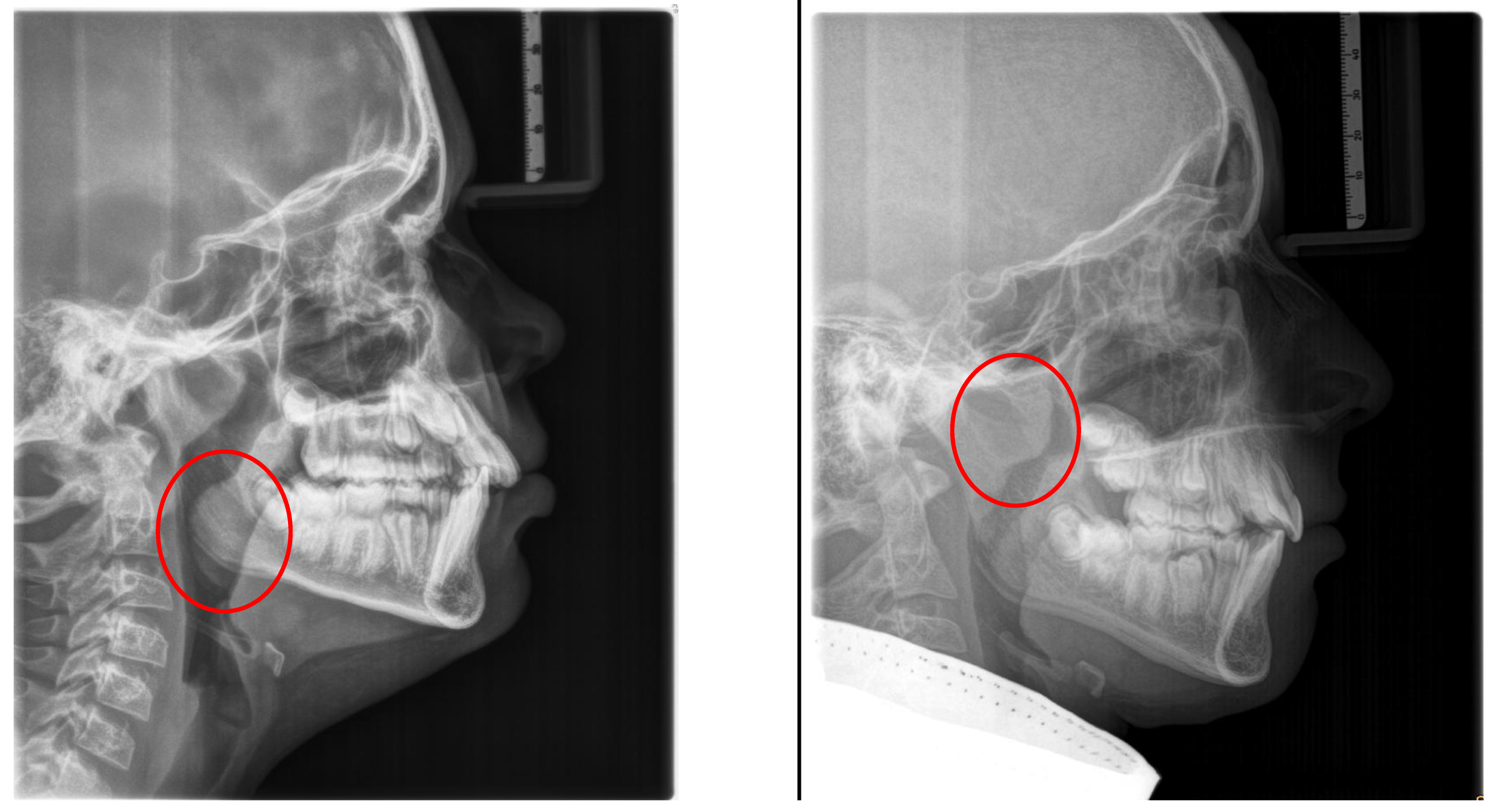
4. Craniofacial Anatomy in OSA Patients
Craniofacial anatomy is considered an underlying risk factor contributing to the development of OSA. Initially, cephalometric abnormalities in patients with OSA were described by Riley et al. [
63] and Guilleminault et al. [
64]. Notably, craniofacial morphology in adults with OSA has been observed to resemble that of children with OSA or chronic mouth breathing [
65]. However, altered craniofacial anatomy might represent a physiologic compensation to the underlying clinical condition rather than a primary causative factor [
66].
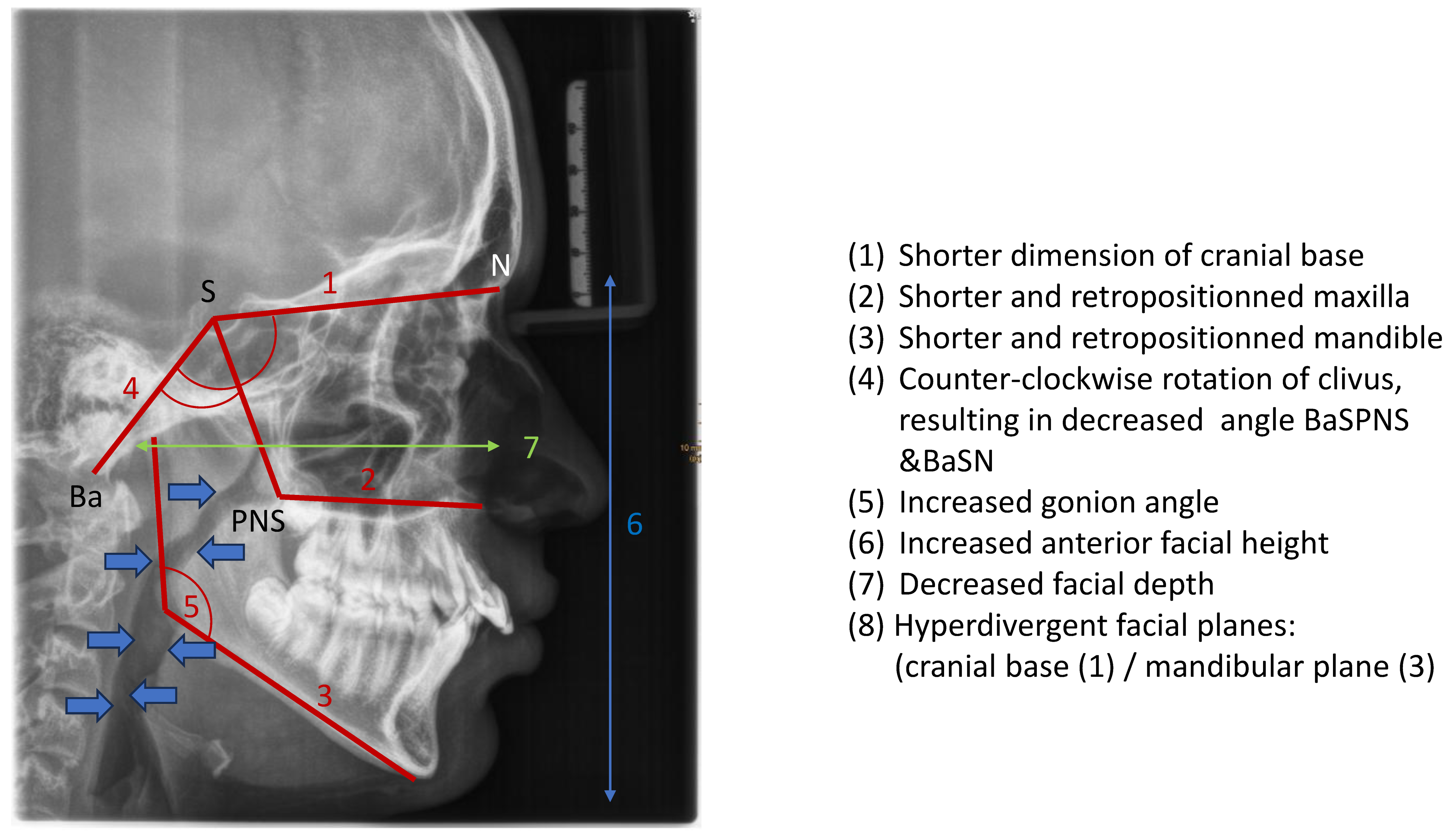
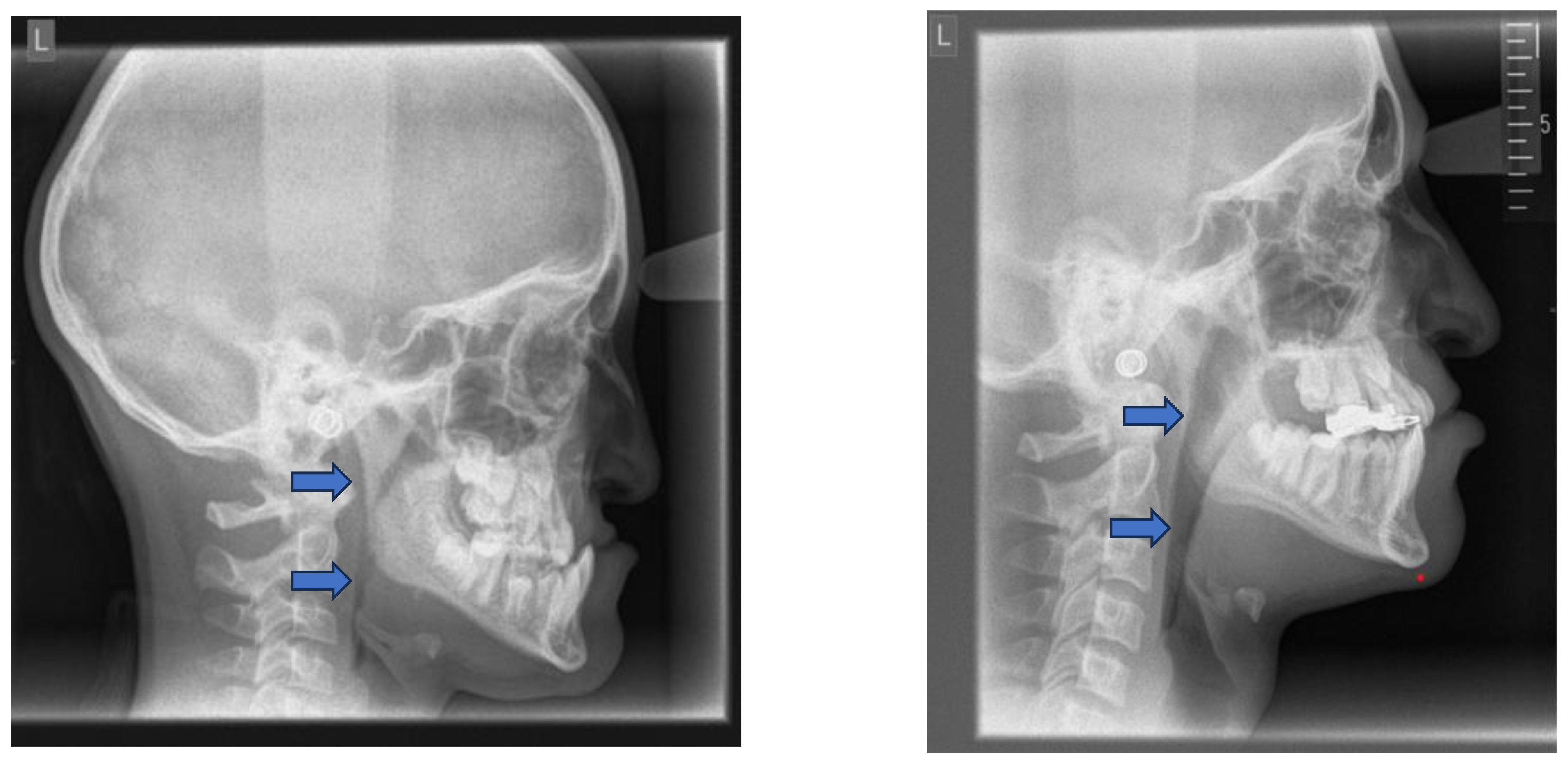
Cephalometric evaluations of patients with OSA have revealed several skeletal (a) and soft tissue (b) morphological differences compared to the normative values (
Table 1) [
67,
68].
The consequences of these aberrations result in reduced size of the bony pharynx and a hyperdivergent facial pattern characterized by a larger anterior facial height. A bimaxillary retrognathic pattern, which is more pronounced in the mandible, further decreased facial depth and contributed to a narrowed posterior airway space [
69]. Together, these structural changes significantly reduce upper airway volume, thereby predisposing affected individuals to OSA. (
Figure 3)
5. Clinical Characteristics in Children with OSA
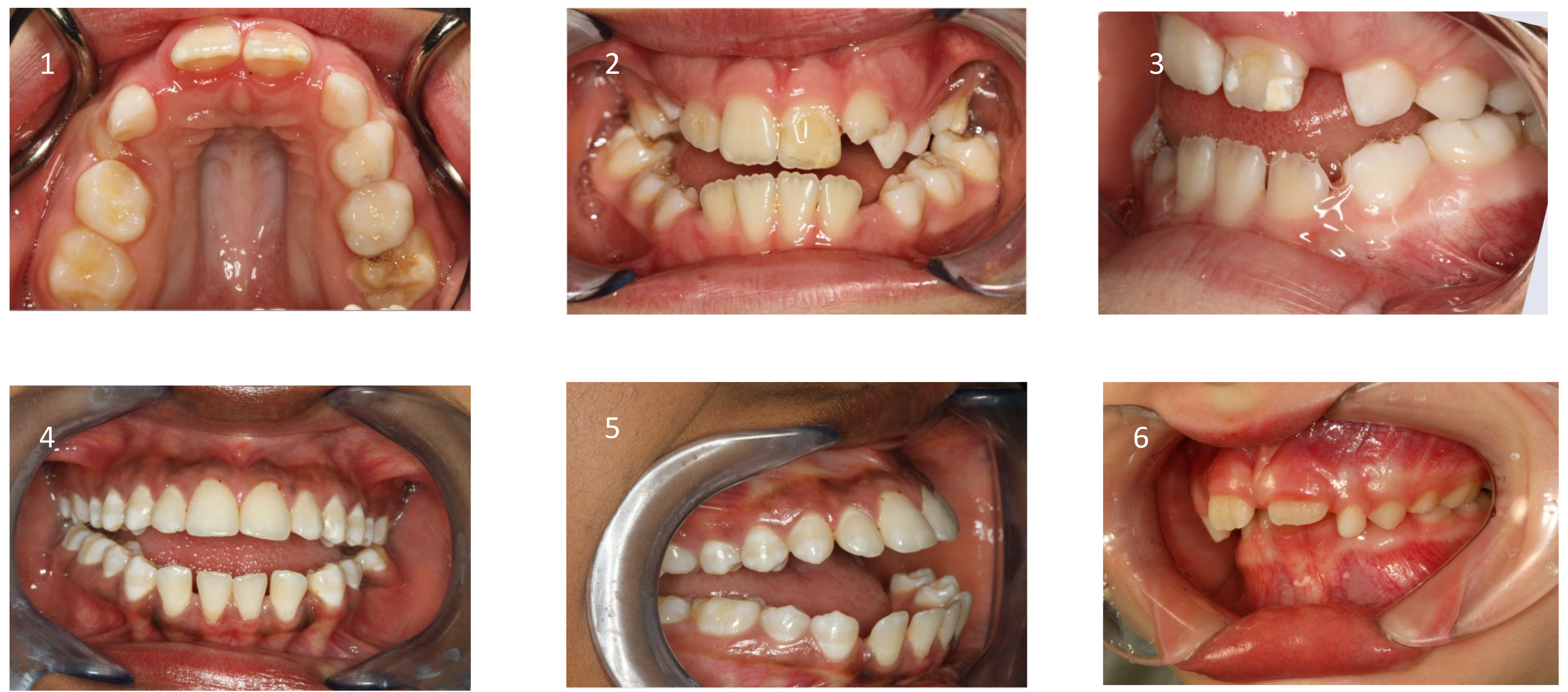
5.1. Extraoral Findings
These patients often exhibit “adenoid facies” characteristics and present with dark circles around the eyes, flattened cheekbones, dry lips, an open-mouth posture, a lowered mandibular posture, a low tongue position, labial incompetence, underdeveloped nasal bones, pronounced nasolabial furrows, which collectively complete the typical appearance [
49,
55,
56,
57]. They often present a convex profile due to a retrognathic mandible (
Figure 4) and an increased mandibular angle. The lower facial third is frequently longer than the average (long-face, dolichofacial morphology) [
55]. (
Table 2). Additionally, they exhibit an altered head position resulting from hyperextension of the cervical spine and an overall reduction in orofacial muscle tonicity.
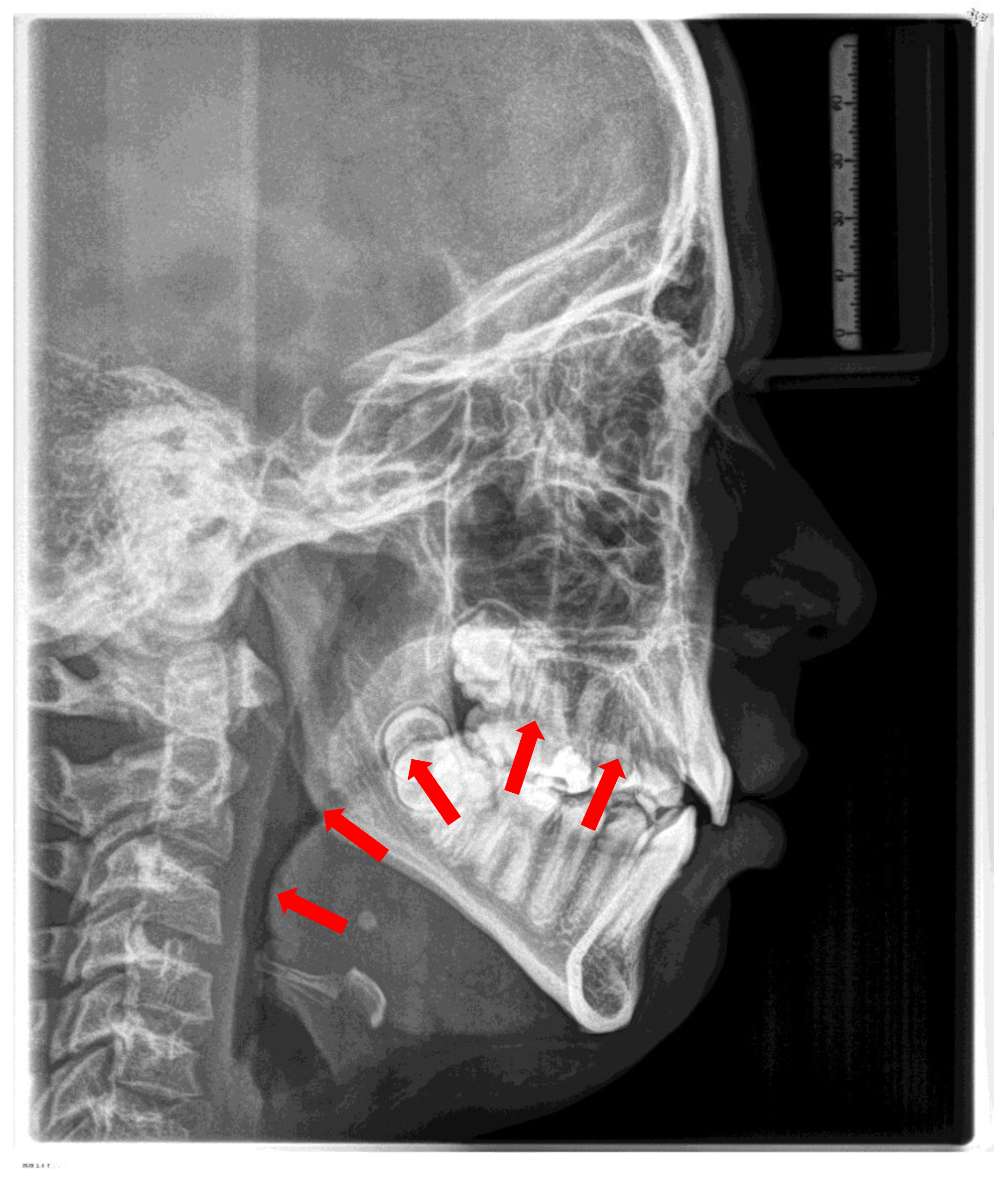
5.2. Intraoral Findings
Malocclusion is highly prevalent in children with OSA. They often present posterior crossbites in addition to lateral functional shifts due to a narrow maxilla [
70,
71,
72]. Regarding the palatal vault, it is higher and narrower than in not affected children [
73]. This is related to the altered equilibrium between the tongue and cheeks [
58]. In addition, an anterior open bite (reduced overbite) and sometimes a deep bite (increased overbite), an increased overjet due to a retrognathic mandible, protruded upper incisors, and crowding in the maxilla and the mandible are constant findings [
55,
74,
75]. (
Figure 5 and
Table 2)
5.3. The Typical “At-Risk Patient”
The typical “at-risk patient” presents as follows (
Table 3) [
76]: All these characteristics should alert health professionals, and further diagnostics should be initiated.
6. The Role of Orthodontics in OSA Diagnosis
6.1. Role of the Orthodontist
The orthodontist plays a critical role in collaboration with multidisciplinary professionals (e.g., ENT, pediatrics, sleep medicine) to assess craniofacial structures that may contribute to OSA.
In fulfilling this role, the orthodontist’s contributions include the following:
- (1)
Collaborative assessment: The orthodontist works with other healthcare providers to assess craniofacial structures that may contribute to pediatric OSA.
- (2)
Diagnostic referral: Upon detecting signs of sleep-disordered breathing, the orthodontist refers the patient to a physician for a definitive diagnosis.
- (3)
Airway-focused treatment: The orthodontist may initiate treatment to address skeletal discrepancies that contribute to airway narrowing.
- (4)
Caregiver education: Orthodontists inform caregivers about the potential risks of untreated OSA and the role of orthodontic therapy in improving airway function.
- (5)
Ongoing monitoring: The orthodontist continues to monitor patients’ craniofacial development and collaborates with the multidisciplinary team to ensure comprehensive care.
6.2. Screening for OSA
Screening requires a comprehensive clinical evaluation that focuses on medical history and behavioral indicators of sleep related breathing disorders. Key aspects to consider are: previous diagnosis of OSA, snoring, mouth breathing during sleep, witnessed pauses in breathing during sleep, sleep bruxism [
77], sleep position (hyperextension of the neck), nasal obstruction, age, height and weight, neurodevelopmental signs (developmental delays, poor school performance, attention problems, hyperactivity disorder), behavioral concerns (aggressive behavior, inappropriate bed wetting), medication, sleep-related issues (challenging to weak-up in the morning, morning headaches, daytime sleepiness or fall asleep quickly). Additionally, the Pediatric Sleep Questionnaire (PSG), which has a high diagnostic value, can be used as a screening tool for sleep problems in children. The questionnaire has 22 items comprising three symptom complexes: snoring, excessive daytime sleepiness, and inattentive or hyperactive behavior [
78,
79].
During screening, a thorough and comprehensive orthodontic examination should be conducted. In addition to the above-mentioned extra- and intraoral features, oral functions, such as breathing and swallowing patterns, tongue size, function and rest position, speech, temporomandibular joint disorders, signs of bruxism [
80], and size of tonsils should be investigated. Compared to a control group, mouth-breathing children with obstructive sleep apnea exhibited differences in oral microbiota, higher acidity, and poorer dental status [
81].
Recently, a comprehensive “Pediatric Obstructive Sleep Apnea Diagnostic Examination Form” was developed by a German working group. This form is based on clinical experience and current literature addressing craniofacial and functional items linked to OSA [
82]. This form comprises two pages and captures craniofacial and functional characteristics associated with OSA in a well-structured and comprehensive manner. It is self-explanatory and can be used by all health professionals involved in OSA treatment.
6.3. Aims of Orthodontic Treatment
Orthodontic treatment plans for patients with OSA should follow established principles used for treating dental and skeletal deformities, just as those not affected by OSA. Treatment planning must consider both anatomic and functional risk factors for OSA that compromise airway volume. The treatment goal is to ensure a sufficiently large intraoral space and maintain proper tongue posture against the hard palate. Orthodontic devices should be used only in patients with specific structural issues, such as a narrow maxilla, and combined with myofunctional therapy if necessary.
Orthodontic treatment can mitigate or even treat OSA by correcting the underlying skeletal disharmony and enlarging the upper airway space. Treatment should align with growth phases and guide development toward a favorable facial and functional growth pattern.
Once growth is complete, underlying skeletal disharmony can only be corrected through orthognathic surgery and surgically assisted rapid maxillary expansion [
83].
Thus, orthodontic treatment may be curative or at least reduce the symptoms of OSA and may even prevent the onset of OSA in later life.
7. Imaging and Radiologic Assessment
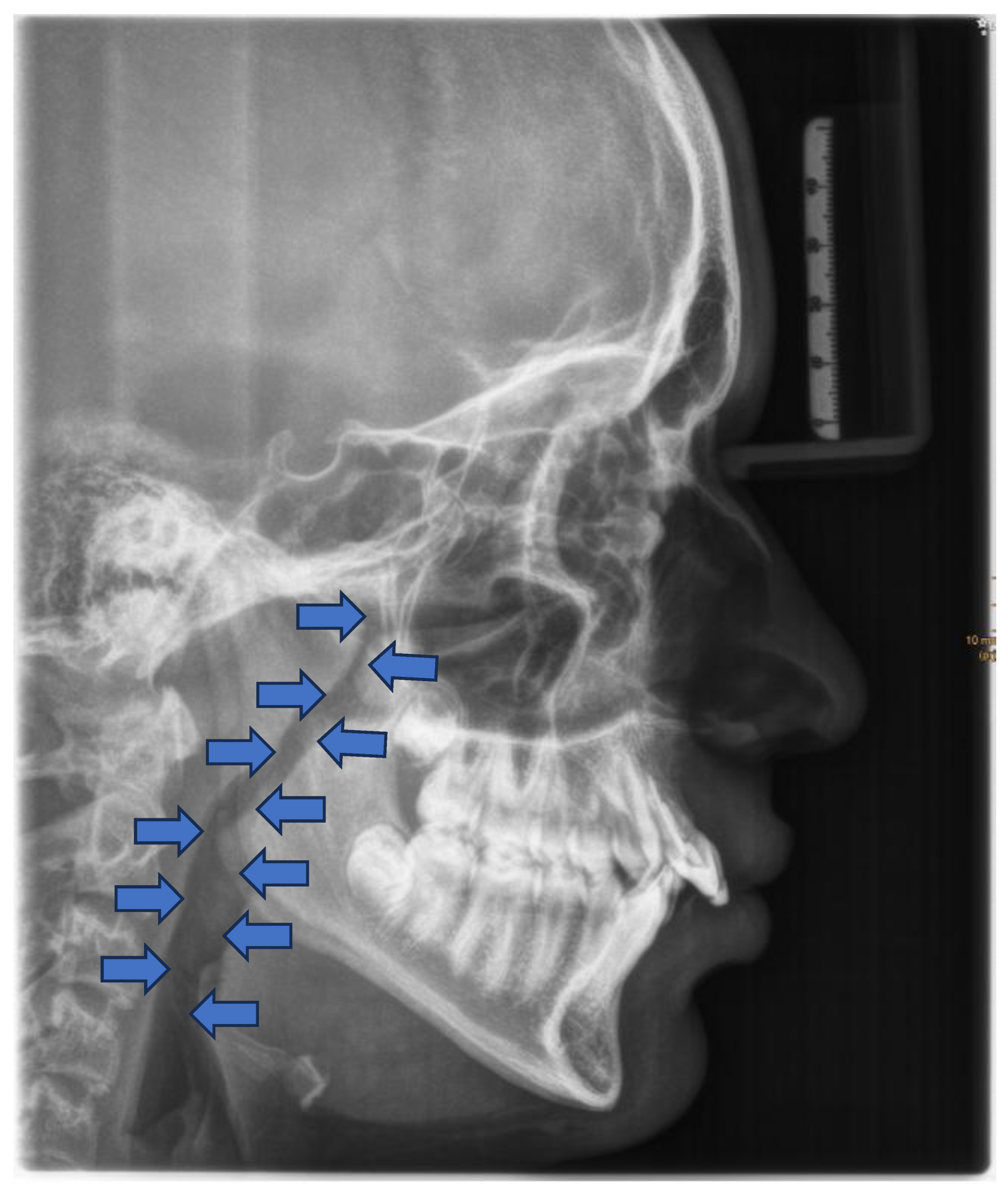
Lateral cephalometry has several advantages in assessing craniofacial morphology, including both hard and soft tissues, and identifying the sagittal dimension of the posterior airway space [
84,
85]. This diagnostic tool is reproducible, affordable, easily accessible in an orthodontic office, involves minimal radiation exposure, and is non-invasive [
2]. (
Figure 6) The cephalometric radiographs are taken in an upright and natural head position, where eyes focus ahead with a horizontal visual axis parallel to the floor (Frankfort horizontal plane). The occlusion should be the habitual bite (not forced into maximum intercuspation) and the lips in gentle contact (not forcefully closed). Lateral cephalometry can be used as a screening tool for assessing craniofacial morphology of hard and soft tissues and airway and identifying the sagittal dimension of the smallest airway dimension [
84,101].
Nevertheless, it is essential to note that soft tissues in the upper airway behave differently when a person is asleep, in a supine position, compared to an upright position [86-89]. Several studies attempted to establish a relation between oropharyngeal dimensions and craniofacial structures in subjects with sleep disorders and with OSA through cephalometric assessment [90-92].
However, a comprehensive assessment of the airway is better achieved with cone-beam computed tomography (CBCT). This three-dimensional evaluation offers a detailed visualization of the airway and the surrounding structures [
93]. Though no universally accepted airway volume threshold exists to predict OSA risk, patients with OSA generally have smaller airway volumes compared to their unaffected counterparts [
94]. No radiographic methods have been reported to have high specificity and sensitivity, serving as actual risk-assessing tools for OSA [
83]. Moreover, the challenges of studying a functional airway using static images are inherently limited [
95].
In contrast, drug-induced sleep endoscopy is a technique used to evaluate the airway under sleep-like sedation. It is considered the most reliable functional airway assessment method and helps guide treatment decisions, typically reserved for special diagnostic questions [
96].
8. Craniofacial and Orthodontic Treatment Strategies for Pediatric OSA
This section presents orthodontic treatment options for pediatric OSA across the full developmental spectrum from birth to adolescence. Once growth is complete, only surgical options, such as orthognathic surgery and surgically assisted maxillary expansion, are effective in correcting the underlying skeletal deformity of OSA patients in a curative way. When identified early, many craniofacial and functional discrepancies can be addressed conservatively through growth-guided functional orthodontic treatment. This section provides also an overview of the various functional orthodontic treatment modalities that can be employed to manage pediatric OSA during growth.
8.1. Prevention
Abnormal oral habits play a significant role in the development of malocclusions in children. Habits such as thumb and finger sucking, prolonged pacifier use, mouth breathing, a persistent infantile swallowing pattern, and low tongue posture exert abnormal forces on developing dentition and facial structures. Management of these habits involves behavioral modification, child education, and parental counseling [
114].
While sucking habits are considered acceptable during infancy, their persistence beyond 2-4 years of age, particularly into the mixed dentition phase, raises concern due to their potential impact on craniofacial and dental structures. Insofar as sucking habits, mouth breathing, malocclusions, low tongue position and dysfunction are interdependent factors [
76] and may interfere with craniofacial and dental development, they should be addressed early. Otherwise, these factors may also contribute to the development of OSA.
From a preventive perspective, promoting nasal breathing and eliminating these habits early are essential. Myofunctional therapy should be initiated to restore proper orofacial function, including optimal tongue posture and breathing patterns. Early interception of oral habits, combined with restoration of nasal breathing and myofunctional therapy, constitutes a proactive strategy for preventing craniofacial growth disturbances and potentially reducing the risk of OSA.
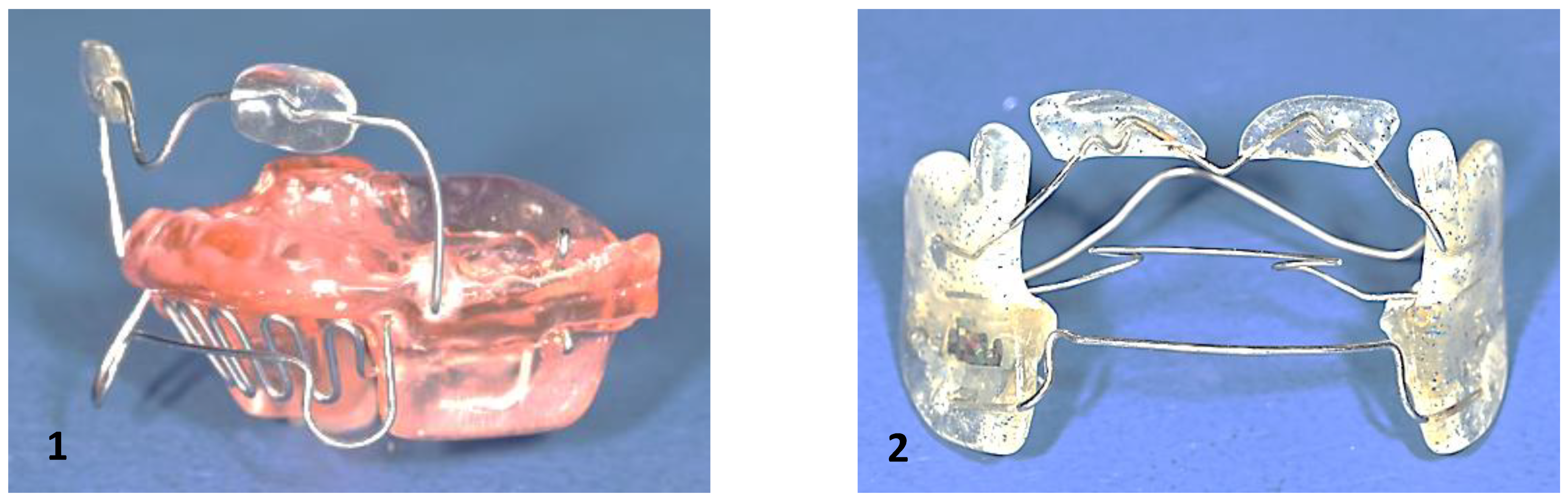
8.2. Neonatal Intervention: Robin Sequence
Robin sequence is a malformative triad characterized by mandibular micro- and retrognathia, glossoptosis, and upper airway obstruction. A U-shaped cleft palate may be present but is not obligatory [
98]. A birth prevalence of 12.4 per 100,000 live births has been reported, classifying Robin sequence as a rare disease [
99]. Robin sequence may occur as an isolated entity, as part of a syndrome, or in association with other malformations. Several early treatment options exist to address upper airway obstruction in affected neonates. These range from less invasive measures, such as prone positioning, nasopharyngeal airway placement, non-invasive CPAP ventilation, and the use of Tübingen palatal plate [
100,
101], to more invasive surgical treatments, including mandibular distraction osteogenesis [
102,
103] or tracheostomy. Tübingen palatal plate has demonstrated its effectiveness in neonates with isolated and syndromic Robin sequence [
100,
101].
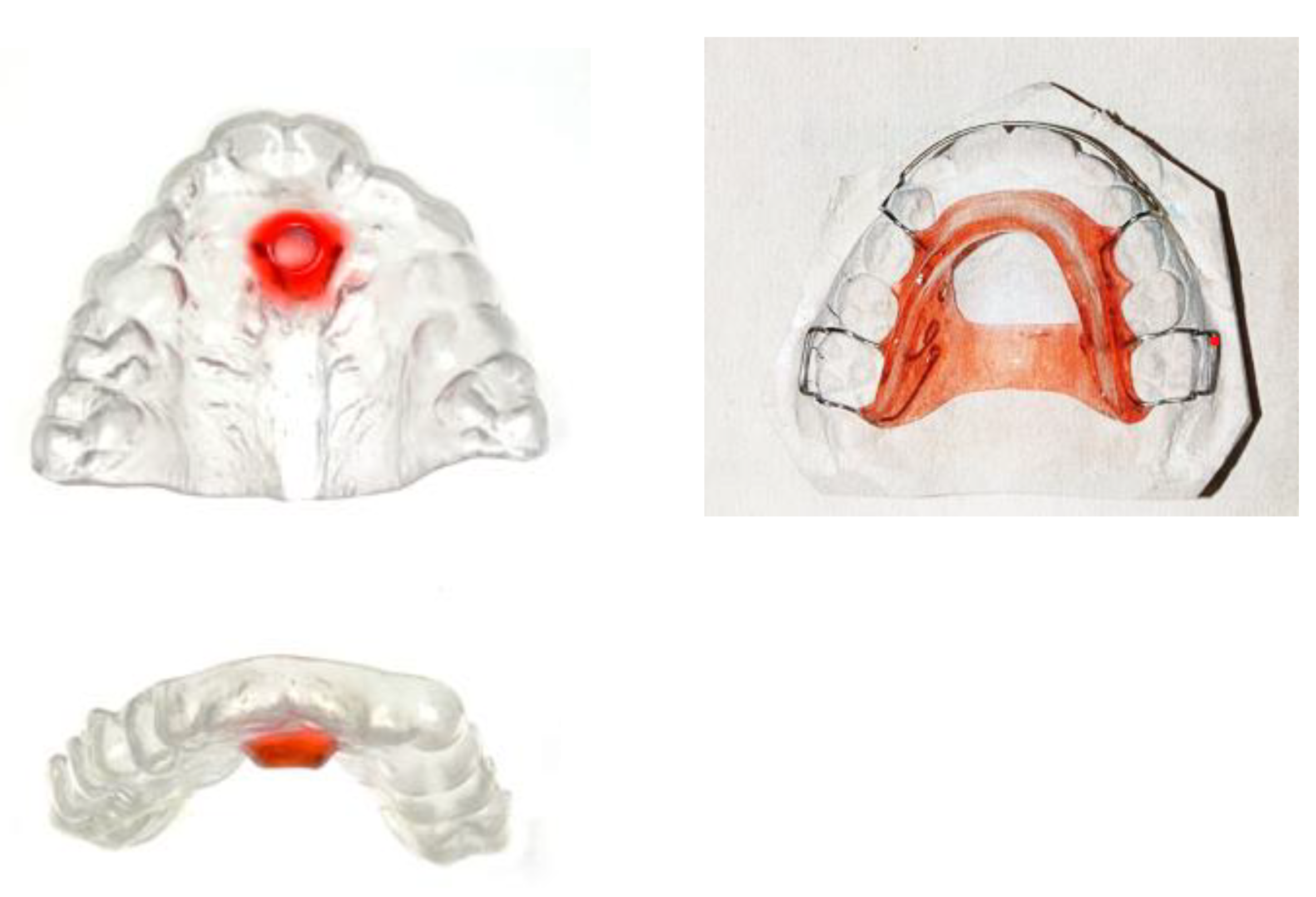
The Tübingen palatal plate consists of a palatal part and an attached spur (
Figure 7). The spur repositions the tongue anteriorly and horizontally, thereby enlarging the pharyngeal airway (immediate effect). Functioning as a functional orthodontic appliance, it also promotes condylar growth, leading to catch-up growth of the micrognathic mandible over time (long-term effect) [
104]. The Tübingen palatal plate treatment is accompanied by myofunctional therapy and feeding training.
8.3. Myofunctional Therapy
Myofunctional therapy aims to reduce the frequency and severity of pediatric OSA and snoring [
105,
106] by improving labial seal and lip tone and nasal breathing while promoting favorable tongue positioning within the oral cavity. By re-establishing tongue-to-palate contact, a stable posterior oral seal [
107] is created, preventing posterior displacement of the tongue during sleep. Additionally, respiratory muscle therapy enhances coordination between the upper and lower airway muscles, thereby improving airway patency. Patients are instructed to perform daily orofacial exercises to strengthen the tongue and orofacial muscles and promote correct tongue posture (active myofunctional treatment). A recent systematic review and meta-analysis support the role of oropharyngeal muscle therapy as adjunct management of pediatric OSA, showing improvement in key respiratory parameters such as the Apnea-Hypopnea Index (AHI) and Apnea Index (AI) and patient-reported outcomes, including the Epworth Sleepiness Scale, the Pittsburgh Sleep Quality Index, and snoring frequency) [
108]. Although these exercises are well-tolerated and straightforward, patient cooperation and adherence are essential for any potential benefits related to this type of treatment [
109]. Several studies have also proposed passive myofunctional treatment using intraoral appliances as an alternative or adjunct to active exercises [
110,
111].
8.4. Interceptive Functional Appliances and Screening Devices
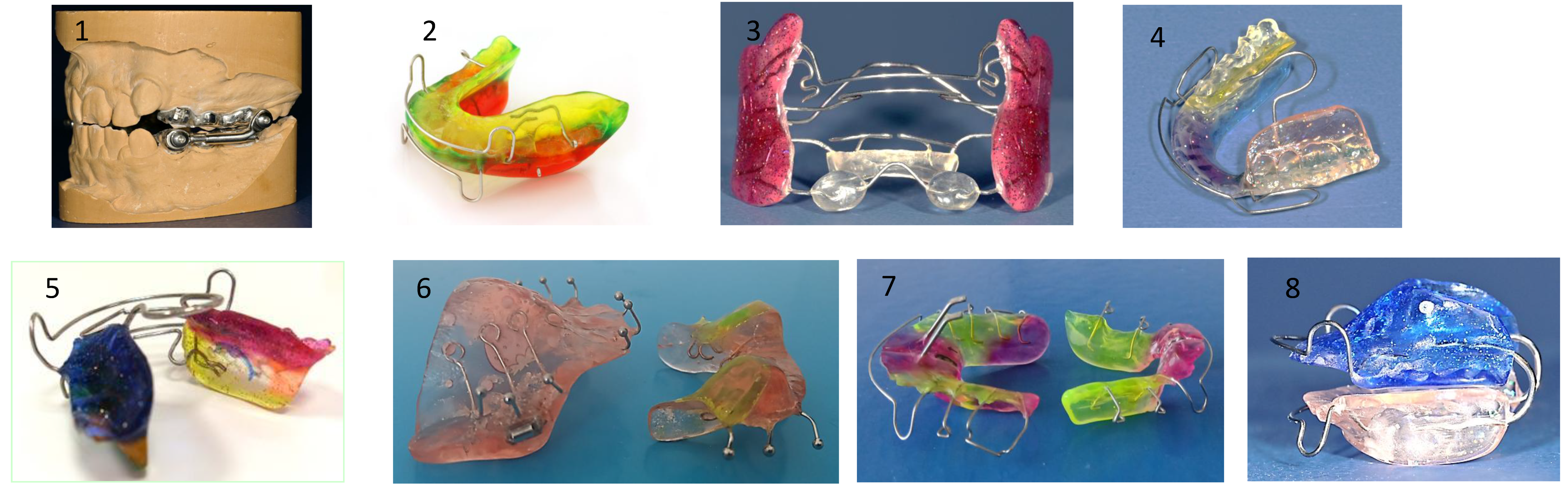
8.4.1. Interceptive Functional Appliances
Interceptive functional appliances aim to promote proper tongue posture by stimulating the tongue to rest directly behind the upper incisors, thereby improving habitual tongue positioning (
Figure 8).
In a study, an oral device incorporating a tongue stimulation element improved after 12 months of treatment, nasal breathing during sleep, mandibular linear growth, airway morphology, and patient-reported quality of life [
112].
Recently, new prefabricated myofunctional devices have been introduced to support oral habit correction and functional training. The “Froggy Mouth®” device has demonstrated efficacy in correcting atypical swallowing patterns and eliminating dysfunctional oral habits [
113].
Another prefabricated myofunctional appliance, the “Myobrace®” device, is designed for cessation of habits, occlusal guidance, and orofacial muscle training. This one appears particularly beneficial in managing Class II.1 malocclusion [
114].
8.4.2. Screening Devices
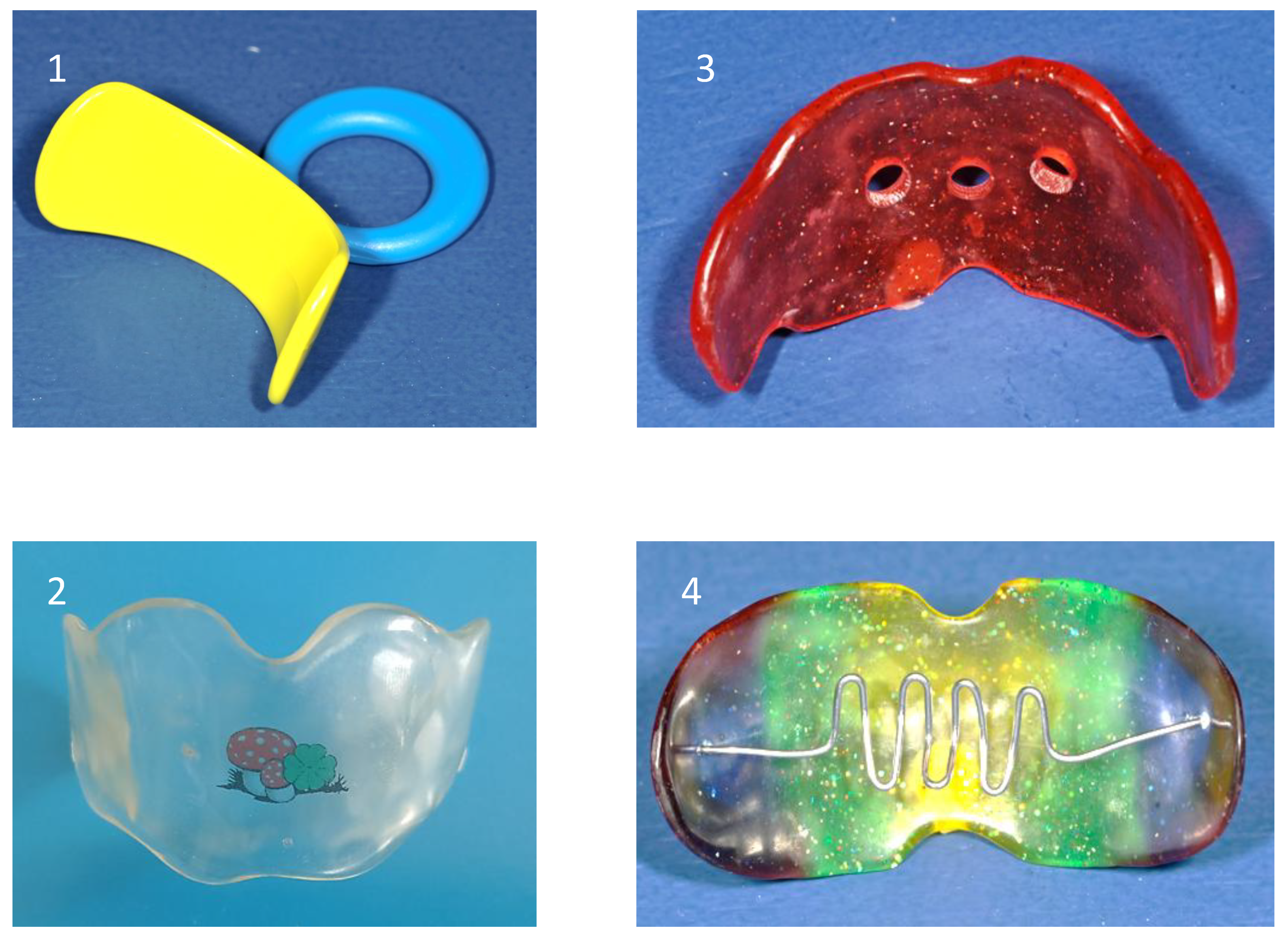
The vestibular plate is a functional appliance designed to eliminate abnormal perioral forces and support the undisturbed development of the orofacial system, particularly during the deciduous and early mixed dentition stages. It primarily influences the function of the lip, cheek, and tongue muscles to counteract the deformative effects of soft tissue dysfunctions, such as tongue thrust, low tongue posture, and habitual mouth breathing. The appliance may help eliminate sucking habits.
In deciduous dentition, the vestibular plate helps correct acquired malocclusions resulting from abnormal habits and mouth breathing, such as anterior open bites associated with persistent finger sucking and retained infantile swallowing patterns. In mixed dentition, it is frequently employed as an adjunct before comprehensive orthodontic treatment to reduce abnormal perioral muscle influences.
This treatment is most effective when initiated during periods of active growth potential. Additional myofunctional exercises are often helpful during screening therapy.
The vestibular plate may also promote anterior mandibular positioning. In cases of tongue dysfunction, additional elements, such as a tongue grid or shield, may be incorporated. At the same time, breathing holes can be progressively reduced to facilitate the transition from habitual mouth breathing to nasal breathing. This is particularly relevant, as some children continue mouth breathing even after adenotonsillectomy, increasing the risk of adenoidal regrowth. Thus, the vestibular screen is typically custom-fabricated but is also available in prefabricated designs. (
Figure 9)
8.5. Maxillary Expansion
Transverse maxillary deficiency requires skeletal maxillary expansion, which is achieved by separating and stimulating growth at the midpalatal suture. As individuals age, the midpalatal maxillary suture becomes increasingly interdigitated, increasing its resistance to separation.
In adolescence, expansion may involve fracturing the bony interdigitations, which can reduce both the extent and stability of expansion. Therefore, maxillary expansion is ideally performed at an early age when skeletal responsiveness is optimal [
115].
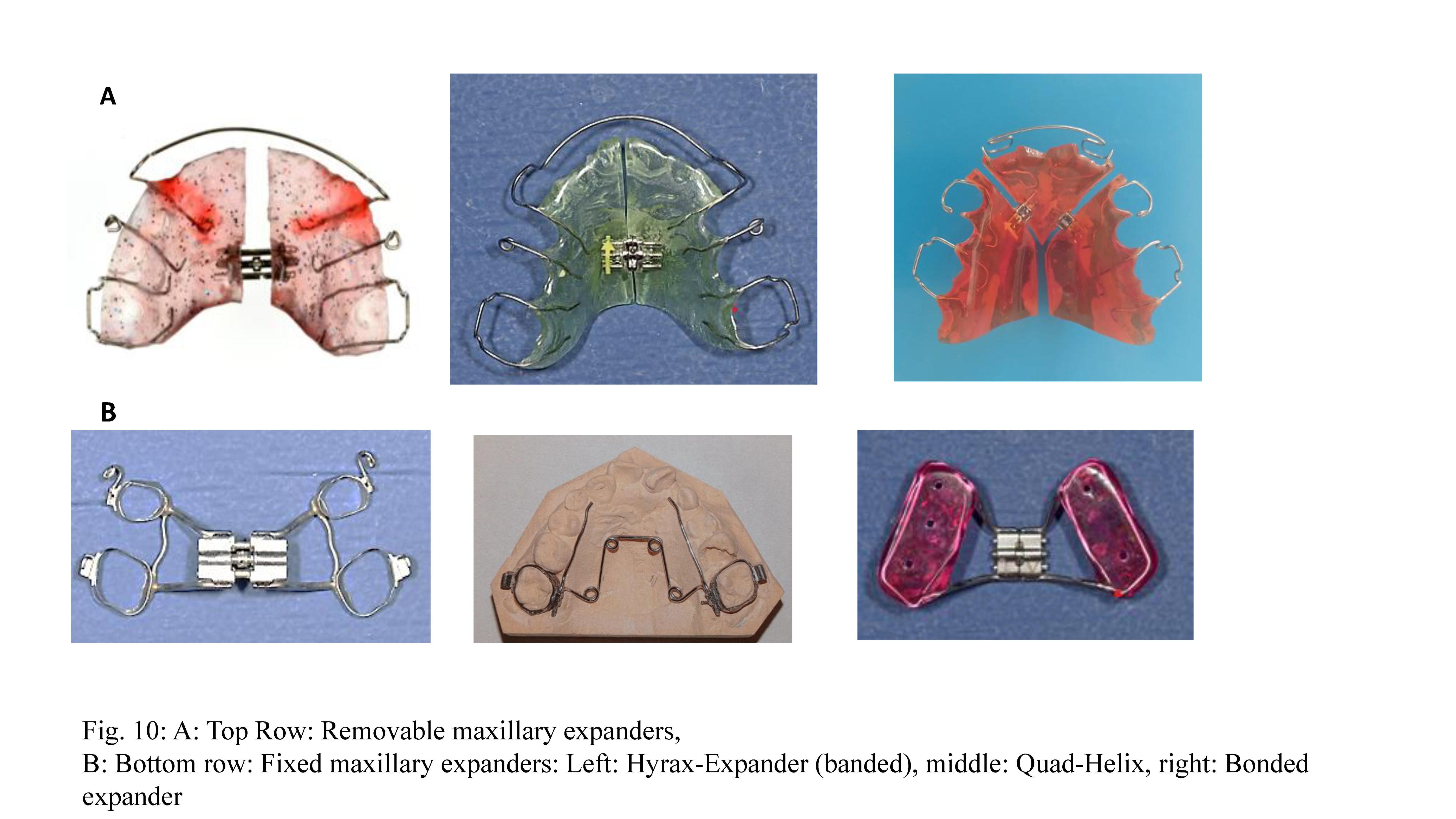
8.5.1. Removable Expanders
These appliances are designed for slow maxillary expansion. They are mainly indicated in cases of unilateral, bilateral, or localized dental arch expansion. In young children, expansion remains predominantly skeletal, whereas, in late adolescence, the effects are mainly dental. Palatal expansion protocols necessitate meticulous control to prevent dislodging of the appliance. The activation of the screw should not exceed one turn every 5 to 7 days. In the case of a high palate, up to 1 turn (90°) every 5 days is possible. (
Figure 10). Patient compliance is paramount, as the appliance requires 15-hour daily wear to achieve the desired expansion of about 0.25mm per week.
8.5.2. Fixed Maxillary Expanders
Fixed maxillary expanders are designed to produce rapid skeletal maxillary expansion and are indicated for unilateral or bilateral transverse deficiencies. Fixed expanders may be anchored with bands (banded expanders) or acrylic blocks (bonded expanders). A bonded expander can be used in any dentition stage, provided adequate root support remains. Otherwise, a banded expander anchored to the first molars or first molars and first premolars is recommended in the late mixed phase. In late adolescence, skeletal anchorage is a good treatment option to avoid dental tipping. Adults typically require surgically assisted maxillary expansion. For patients with sagittal deficiencies, rapid maxillary expansion may be combined with orthopedic sagittal traction using a Delaire’s mask.
Fixed maxillary expanders have two types of force delivery mechanisms: screw expansion or spring expansion. The Hyrax-Expander is the most commonly used screw expander, and Quad-Helix is the most common spring expander. The Hyrax-Expander is indicated for severe skeletal deficiencies, with activation occurring 1-2 times daily. The range of expansion is 0.25 mm to 0.5 mm per activation until an overcorrection of approximately 25% is achieved. The Quad-Helix is less bulky, requires less frequent reactivation, and does not rely on patient cooperation for adjustments. Its indication is a mild skeletal deficiency. Both expander devices require a minimum retention period of at least 3 to 6 months. (
Figure 10)
Rationale
Maxillary constriction increases nasal airway resistance, often leading to mouth breathing and altered tongue posture, which can contribute to retroglossal airway narrowing. (
Figure 2) Rapid maxillary expansion (RME) increases maxillary width, enlarges the nasal floor and cavity, reduces nasal resistance, and favors nasal breathing by expanding the nasomaxillary complex. Additionally, it improves tongue posture and enlarges the pharyngeal airway [
116]. Several studies report significant reductions in AHI after RME [
117,
118]. In the short term, RME may aid in improving the quality of life for children with a narrow maxilla [
119]. Additionally, improvements in behavioral disturbances, cognitive abilities, and nasal function were reported after rapid maxillary expansion in children affected by snoring or OSA [
120,
121]. A systematic review and meta-analysis concluded that RME might help eliminate predisposing factors to OSA [
122].
Nevertheless, current evidence on RME for treating pediatric OSA is inconclusive [
123]. While some studies suggest RME may improve snoring and quality of life in children with persistent symptoms after adenotonsillectomy, overall findings are limited by methodological weaknesses and lack of control groups [
120]. Interceptive orthodontic treatments may offer benefits but cannot yet be recommended as standalone therapies for OSA [
124].
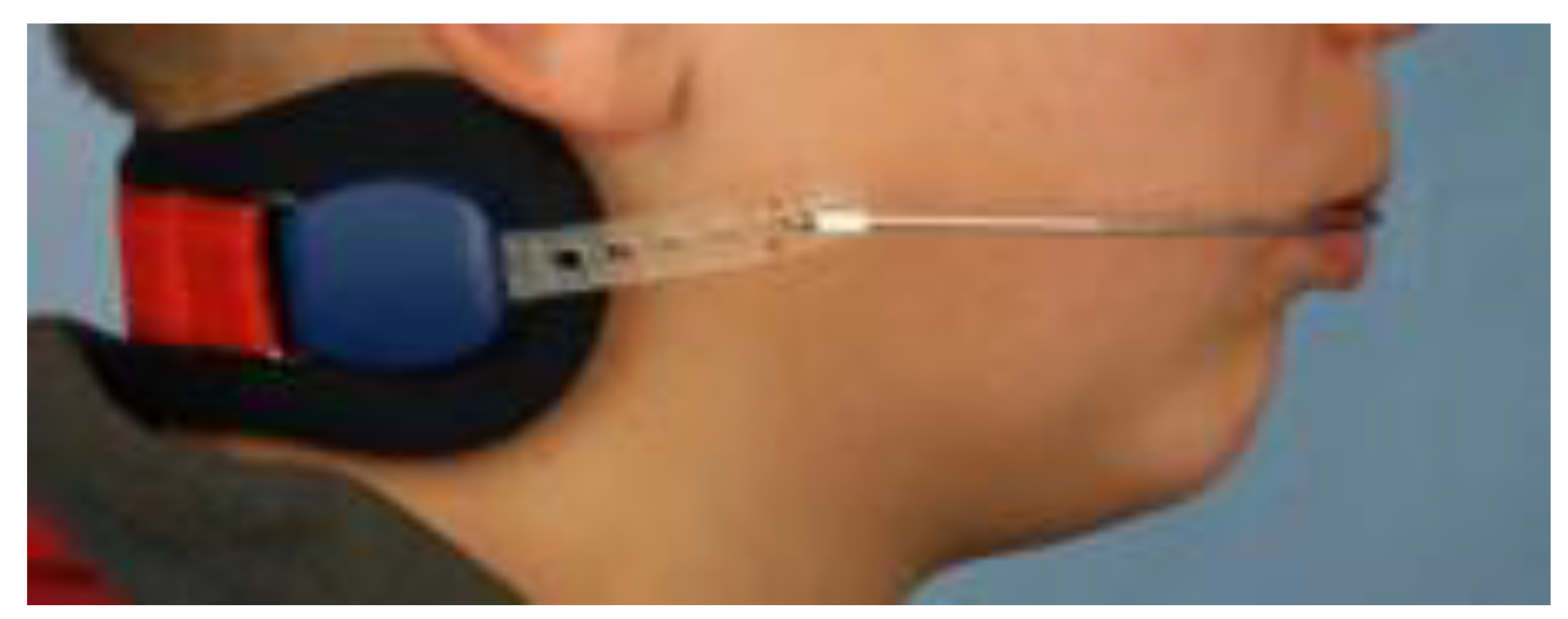
8.6. Maxillary Protraction Treatment (face mask)
Retrusive maxilla and Class III malocclusion can result in a decreased retropalatal airway space [
125]. Sagittal correction of the maxilla can be achieved through protraction treatment utilizing a Delaire’s mask (reverse pull headgear). The optimal time to initiate protraction of the retrognathic maxilla and to achieve the best results is between 6 and 9 years of age, before the fusion of the circummaxillary sutures. Early intervention allows for the mobilization of sutures and the maxillary complex, as well as protraction osteogenesis [
126]. If the patient is an adolescent, maxillary protraction can still be achieved through a miniplate-assisted facemask or maxillomandibular bone-to-bone traction techniques. On average, the maxilla can be protracted by approximately 5.5 mm with protraction treatment. (
Figure 11) The most severely affected patients are often those with syndromic craniosynostosis, resulting in severe midfacial hypoplasia, where more aggressive approaches may be required.
8.7. Functional Appliances for Class III Treatment
Functional orthodontic appliances can be used for minor forms of maxillary retrognathia. Several functional appliances, such as Fraenkel III or Class III activator, can achieve growth-promoting treatment. (
Figure 13) Pads act as a shield and keep the pressure of the upper lip and the cheeks away, thereby initiating bone formation and anterior growth of the maxilla. These appliances are mainly used for retention after protraction treatment.
8.8. Appliance for Redirecting Vertical Growth Pattern
A bite plate in the lower jaw can be used to attenuate the vertical growth pattern. Through this, condylar growth is directed in a more cranio-ventral direction, leading to a counterclockwise rotation of the mandible. However, the effect is only discrete. (
Figure 14)
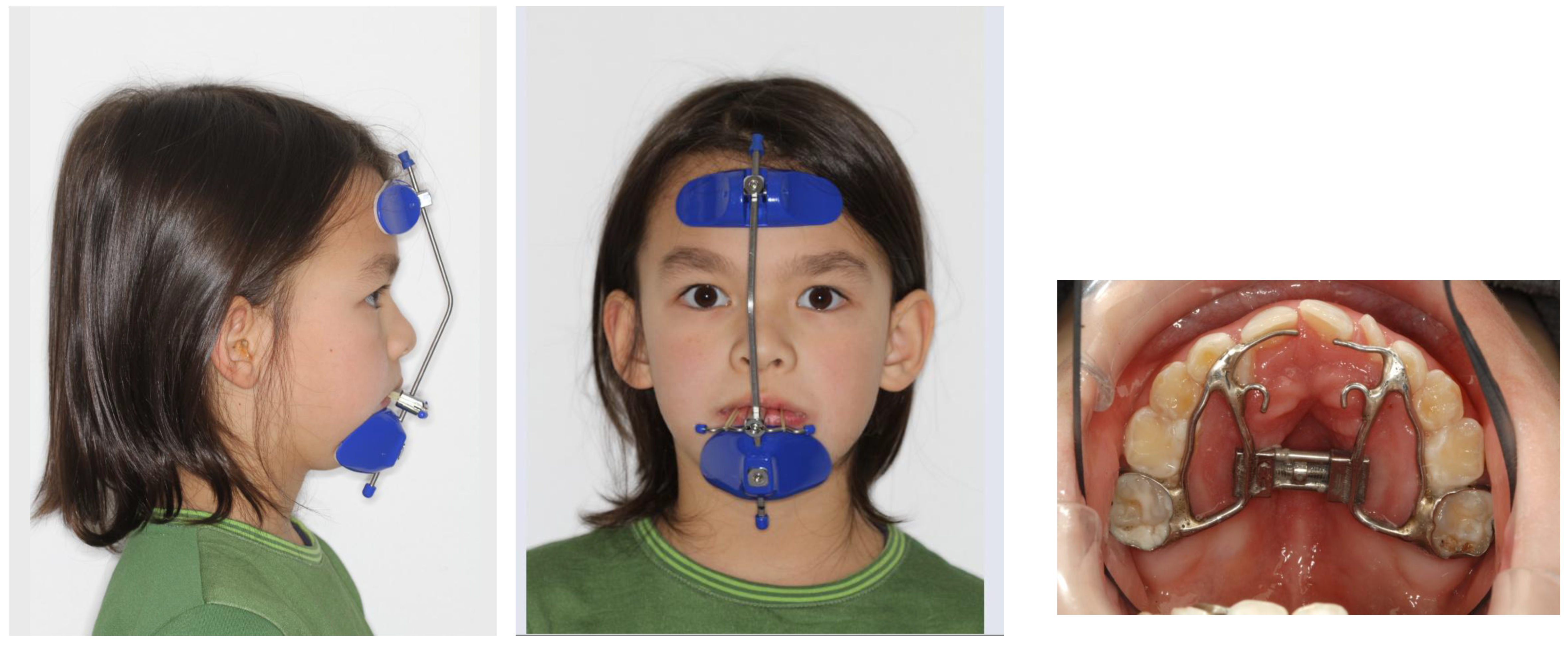
8.9. Mandibular Advancement
A retrognathic mandible induces retrodisplacement of the tongue, thereby reducing the upper airway volume. Orthopedic mandibular advancement was first introduced by Dr. Kingley with the “bite-jumping” appliance in 1879 [
129]. This type of appliance aims to correct skeletal mandibular retrognathia. It redirects mandibular growth into a more forward and downward position, either passively or actively, while being worn either fixed or removably (for approximately 15 hours). There are many functional Class II appliances, such as the classical activator and its modifications, the Fraenkel II appliance, the Herbst appliance, the Bionator, the Twin Block, and the bite-jumping appliance (VDP-Sander II appliance). In addition, some can be combined with maxillary expansion (Herbst-RME) appliances. All devices have in common that they bring the lower jaw into the desired therapeutic position. They are worn until the orthopedic effect is achieved. (
Figure 15)
Dental and bony changes associated with the use of functional appliances in growing patients are well-documented [
130,
131]. When devices are worn during growth, mandibular length increases (long-term effect). Expected advancement will be from half cusp to full cusp. The advancement period during treatment typically lasts 6 to 9 months.
9. Limitations and Contraindications in Orthodontic Treatment for Pediatric OSA
9.1. Extractions
Orthodontic interventions, such as tooth extractions and distalization (moving teeth backward) that alter the anterior-posterior dimensions of tongue space, have been blamed for potentially exacerbating the risk of OSA. Thus, premolar extractions and bimaxillary retraction decrease the oral cavity size, restrict tongue space, and lead to posterior tongue displacement, resulting in a constriction of the upper airway, especially in the retroglossal space.
A study compared changes in posterior airway space (PAS) after treatment of Class II anomalies, both with and without premolar extractions, in normodivergent and hyperdivergent facial patterns. The most pronounced airway reduction was observed in the hyperdivergent extraction group, although it was not statistically significant [
137].
In a systematic review, patients with bimaxillary protrusion who underwent extraction of four first premolars and significant retraction of the anterior teeth showed reduced airway dimensions thereafter. Controversially, in patients with four premolar extractions related to severe crowding, followed by forward (mesial) movement of the molars, increased upper airway dimensions were observed after relieving crowding [
138].
In further systematic reviews [
135,
139] and meta-analysis [
140], no substantial evidence has been reported linking dental extractions to airway space restriction. Nevertheless, it is essential to note that the patients included had a mean age of 19.1 years and had long passed the peak of craniofacial growth. In growing individuals, there are indications that tooth extractions result in reduced growth of the jaws and decreased airway space [
141]. It is well established that each tooth bud promotes the growth of the jaws. Thus, for airway considerations, extractions should be avoided before the peak of growth to achieve a maximum intraoral volume to accommodate the tongue intraorally. Serial extractions should also be viewed critically in this context.
9.2. Headgear and Maxillary Growth Restriction
Headgear treatment is used for skeletal Class II correction by affecting the anterior-posterior position of the maxilla. Cervical headgear, used for at least 12 hours daily with an average force of 450g per side for one year, can effectively restrict anterior maxillary growth [
142,
143,
144,
145,
146]. This restriction may have an adverse effect on nasopharyngeal airway dimensions and the intraoral tongue space. Children receiving combined activator-headgear treatment for skeletal Class II correction presented a significant negative impact on sagittal development of the maxilla (reduction of SNA angle, compared to controls without headgear treatment) [
147]. Thus, treatment with headgear might be a risk factor for dimensions of not only nasopharyngeal airway [
148]. A further study concluded that headgear treatment may contribute to OSA during nighttime wear. Therefore, headgear treatment in children with retrognathic mandibles should be carried out with special care. If a patient already presents OSA, headgear treatment may aggravate OSA [
149]. (
Figure 16)
9.3. Craniofacial Adverse Effect of Continuous Positive Airway Pressure (CPAP) Therapy
Even if there are clear benefits to CPAP therapy, when it is implemented at a very young age, flattening of the midface or maxillary retrusion from the mask’s prolonged pressure may occur and must be carefully monitored [
109,
150]. This may reduce nasopharyngeal airway and intraoral volume, leading to deep tongue rest position and its consequences. In addition, unwanted tooth movement is a further side effect of CPAP therapy, which may affect quality of life [
151].
These dental and skeletal side effects may impact both function and aesthetics, highlighting the need for orthodontic monitoring in children undergoing prolonged CPAP therapy.
10. Discussion
10.1. Clinical Implications and Future Implementation Strategies
This review highlights the multifactorial pathophysiology of OSA, emphasizing the complex interplay of craniofacial anatomy, neuromuscular dysfunction, and upper airway patency that contribute to disease onset and progression [
3,
8]. The craniofacial features of OSA patients include mandibular retrognathia, maxillary constriction, a high-arched palate, vertical growth patterns, and reduced airway volume [20,67-73]. Myofunctional disorders are associated with mouth breathing, tongue dysfunction, and open-mouth posture [
49,
56,
57], which can further exacerbate upper airway obstruction and contribute to the heterogeneity of clinical presentations.
Despite the relatively high prevalence of OSA in specific high-risk groups, particularly those with craniofacial syndromes [
30,
31], the condition remains underdiagnosed in pediatric populations due to the lack of standardized diagnostic protocols, small observational groups and heterogeneous diagnostic criteria.
Orthodontists are strategically well positioned to identify craniofacial risk factors and implement interceptive orthopedic measures, contributing to the early detection, risk stratification, and intervention in the interdisciplinary management of pediatric OSA [
14,
83,
153]. Early orthodontic screening enables the timely identification of anatomical and functional risk factors [
82], allowing for targeted interventions that can alter growth trajectories, mitigate airway obstruction, and potentially reduce the need for future orthognathic surgery.
Orthodontic therapies, including rapid maxillary expansion, mandibular advancement, and maxillary orthopedic protraction, combined with myofunctional therapy, offer preventive and therapeutic benefits by addressing underlying skeletal discrepancy and optimizing skeletal development [
122,
124]. These treatment interventions further contribute to enlarging the upper airway space. Therefore, these approaches should be carefully planned in close collaboration with pediatricians, sleep specialists, otolaryngologists, and speech-language pathologists, forming an integrated multidisciplinary approach. Treatment planning should consider the child’s age, developmental status, and ability to comply. Importantly, parental and caregiver education should accompany clinical interventions to enhance adherence and outcomes.
Myofunctional therapy [
105,
106], both active (e.g., orofacial exercises) and passive (e.g., intraoral appliances) [
110,
111], has emerged as a valuable non-invasive adjunctive strategy for improving airway patency and respiratory parameters, as well as enhancing sleep quality and daytime function [
108]. However, success depends heavily on patient cooperation and individualized treatment planning [
109]. Orthodontists are well-equipped to deliver and monitor these therapies as part of a comprehensive airway-centered treatment strategy.
According to the American Association of Orthodontists 2019 White Paper, the primary role of orthodontists in managing OSA is to screen for signs, recognize potential cases, and refer patients to appropriate physician specialists for diagnosis and treatment [
83]. Orthodontic treatment may support OSA management as part of a multidisciplinary team but should only be initiated after a thorough medical evaluation. Diagnosis is outside the orthodontist’s scope, and treatments such as palatal expansion or mandibular advancement devices should be carefully considered in light of their indications, effectiveness, and potential side effects [
14]. It is crucial to screen children for OSA as part of their orthodontic assessment because a high risk of OSA was identified in almost 30% of children undergoing orthodontic treatment [
152].
Importantly, even after adenotonsillectomy, the current first-line surgical approach, sleep-disordered breathing frequently persists, especially in patients with underlying craniofacial risk factors. This observation further highlights the importance of orthodontic assessment and intervention in comprehensive care pathways for pediatric OSA.
Young patients with craniofacial risk factors may experience a more substantial impact [67-72]. Rapid maxillary expansion (RME) is a key intervention that widens the nasal cavity, reduces nasal resistance, and improves tongue posture and breathing [116-122].
While interventions like rapid maxillary expansion or mandibular advancement have been proposed, robust long-term evidence is lacking. Nonetheless, integrating dentists and orthodontists into the multidisciplinary teams is essential to improving the recognition and care of pediatric OSA [
153] and reducing the burden of undiagnosed pediatric OSA. Furthermore, early orthodontic assessment and sleep diagnostics may enable earlier detection across populations, underscoring the need for more preventive care strategies.
In addition, optimizing care pathways, standardizing diagnostic protocols [
82] —including routine airway assessments during orthodontic examinations—and providing clear referral guidelines are essential, as they can improve early detection and treatment outcomes. Orthodontists should be integrated into pediatric OSA care networks for treatment, prevention, and long-term follow-up.
This review also addresses current limitations in the literature, such as the lack of long-term data on orthodontic interventions in pediatric OSA and the variability in diagnostic criteria and treatment protocols.
10.2. Research Implications and Future Implementation Strategies
Despite the growing clinical interest and promising short-term outcomes, the long-term efficacy and stability of orthodontic interventions for OSA remain insufficiently characterized. Existing studies are often limited, with varying diagnostic criteria, treatment protocols, heterogeneous study populations, inconsistent follow-up periods, and a lack of long-term data. This variability compromises the ability to draw definitive conclusions and translate findings into standardized care models.
Future research should focus on prospective, multicenter trials that evaluate the efficacy of combined therapeutic approaches across diverse populations, particularly in growth outcomes and quality-of-life measures. Inconsistent diagnostic and treatment protocols underline the need for standardized longitudinal research.
Collaborative research is crucial for developing standardized, personalized treatment protocols and enhancing long-term outcomes [
154,
155]. High-quality prospective trials should evaluate the long-term impact of combined therapeutic approaches on airway function, craniofacial growth, and overall quality of life across diverse pediatric populations [
156].
This narrative review provides a foundation for future research, highlights the need for evidence-based clinical practice, and identifies knowledge gaps regarding the prevention, early detection, and the evolving needs of pediatric patients with OSA.
11. Conclusion
Orthodontists play an integral role in the interdisciplinary management of pediatric OSA through the early identification of craniofacial and functional risk factors and the implementation of growth-modifying interventions, thereby supporting airway expansion. Frequently co-existing myofunctional disorders should also be addressed to ensure treatment success. These interventions, when carefully selected and executed within a multidisciplinary framework, may not only support favorable craniofacial development but also serve as a preventive strategy against adult-onset OSA.
Pediatric OSA, if left untreated, carries significant consequences ranging from neurocognitive impairments to behavioral, cardiovascular, and metabolic dysfunction.
Orthodontic treatment offers the potential to modify craniofacial growth, optimize tongue posture, and improve airway patency through orthopedic interventions such as rapid maxillary expansion, mandibular advancement, and myofunctional therapy.
Special attention must be given to patients with mouth breathing, snoring, open-mouth posture, low tongue position, lateral crossbite, long face, and mandibular retrognathism. Additionally, children with craniofacial syndromes carry an increased risk for OSA and require early recognition and closer monitoring. After growth completion, correcting the skeletal discrepancies in patients with OSA can only be targeted by orthognathic surgery.
However, orthodontic therapies must be guided by robust clinical evidence, clear indications, and collaborative care to avoid treatment where long-term benefits remain unproven. The implementation of early screening and intervention must carefully balance the potential benefits with the current limitations of available evidence to prevent overexertion of orthodontic practices in the absence of long-term data.
Continued interdisciplinary research, collaborative care models, and high-quality clinical trials are paramount to refining treatment protocols, optimizing patients’ outcomes, and advancing precision medicine approaches for pediatric OSA.
Author Contributions
SMH and TB contributed equally to the conceptualization and drafting of the manuscript. SMH conducted the initial literature search, prepared the first draft and the photographic material. VA revised the manuscript for important intellectual content. TB performed the updated literature search, critically revised the manuscript for intellectual content. All authors have read and approved the final version of the manuscript. Correspondence concerning this manuscript may be addressed to SMH and TB.
Funding
This research received no external funding
Institutional Review Board Statement
Not applicable
Informed Consent Statement
Written informed consent was obtained from participating patients (and their caregivers) to use their photographs
Data Availability Statement
Not applicable
Conflicts of Interest
The authors declare no conflict of interest
Abbreviations
| AHI |
Apnea-Hypopnea index |
| AI |
Apnea index |
| ANS |
Anterior nasal spine |
| Ba |
Basion |
| CPAP |
Continuous positive airway pressure |
| MCA |
Minimal cross-sectional area |
| N |
Nasion |
| OSA |
Obstructive sleep apnea |
| PAS |
Posterior airway space |
| PNS |
Posterior nasal spine |
| RME |
Rapid maxillary expansion |
| S |
Sella |
| SN-GoGn |
Angle formed by sella-nasion to gonion-gnathion |
| TPP |
Tübingen palatal plate |
References
- Moore, K.E.; Esther, M.S. Current medical management of sleep related breathing disorders. Oral Maxillofac Surg Clin North Am. 2002, 14, 297–304. [Google Scholar] [CrossRef]
- Fleisher, K.E.; Krieger, A.C. Current trends in the treatment of obstructive sleep apnea. J Oral Maxillofac Surg. 2007, 65, 2056–2068. [Google Scholar] [CrossRef]
- Giuca, M.R.; Carli, E.; Lardani, L.; Pasini, M.; Miceli, M.; Fambrini, E. Pediatric Obstructive Sleep Apnea Syndrome: Emerging Evidence and Treatment approach. The Scientific World journal. 2021. [Google Scholar] [CrossRef]
- Guilleminault, C.; Stroohs, R. Obstructive sleep apnea syndrome in children. Paediatrician. 1990, 17, 46–51. [Google Scholar]
- Katz, E.S.; D’Ambrosio, C.M. Pathophysiology of Pediatric Obstructive Sleep Apnea. Proc Am Thorac Soc. 2008, 5, 253–262. [Google Scholar] [CrossRef]
- Goldberg, A.N.; Schwab, R.J. Identifying the patient with sleep apnea. Upper airway assessment and physical examination. Otolaryngol Clin North Am. 1998, 31, 919–930. [Google Scholar] [CrossRef]
- Badr, M.S. Pathophysiology of obstructive sleep apnea. Oral Maxillofac Surg Clin North Am. 2002, 14, 285–292. [Google Scholar] [CrossRef]
- Arens, R.; Marcus, C.L. Pathophysiology of upper airway obstruction: a developmental perspective. Sleep. 2004, 27, 997–1019. [Google Scholar] [CrossRef] [PubMed]
- Marcus, C.L.; Katz, E.S.; Lutz, J.; Black, C.A.; Galster, P.; Carson, K.A. Upper airway dynamic responses in children with obstructive sleep apnea syndrome. Pediatr Res. 2005, 57, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Tapia, I.E.; Marcus, C.L.; McDonough, J.M.; Kim, J.Y.; Cornaglia, M.A.; Xiao, R.; Allen, J.L. Airway resistance in children with obstructive sleep apnea syndrome. Sleep. 2016, 39, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Fogel, R.B.; Trinder, J.; White, D.P.; Malhotra, A.; Raneri, J.; Schory, K.; Kleverlaan, D.; Pierce, R.J. The effect of sleep onset on upper airway muscle activity in patients with sleep apnoea versus controls. J Physiol. 2005, 564, 549–562. [Google Scholar] [CrossRef]
- McNicholas, W.T. Obstructive Sleep Apnoea: Focus on Pathophysiology. Adv Exp Med Biol. 2022, 1384, 31–42. [Google Scholar] [CrossRef]
- Gozal, D. Sleep disordered breathing and school performance in children. Pediatrics. 1998, 102, 616–620. [Google Scholar] [CrossRef]
- Kazmiersky, R.H. Obstructive sleep apnea: What is an orthodontist’s role? Prog Orthod. 2024, 25, 21. [Google Scholar] [CrossRef]
- Ahn, Y.M. Treatment of obstructive sleep apnea in children. Korean J Pediatr. 2010, 53, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.J.; Pitson, D.J.; Stradling, J.R. Snoring, sleep disturbance, and behaviour in 4-5 year olds. Arch Dis child. 1993, 68, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Gislason, T.; Benediktsdottir, B. Snoring, apneic episodes, and nocturnal hypoxemia among children 6 months to 6 years old. An epidemiologic study of lower limit of prevalence. Chest. 1995, 107, 963–966. [Google Scholar] [CrossRef]
- Hultcrantz, E.; LÖfstrand-TidestrÖm, B.; Ahlquist-Rastad, J. The epidemiology of sleep related sleep breathing disorders in children. Int J Pediatr Otorhinolaryngol. 1995, 32, S63–S66. [Google Scholar] [CrossRef]
- Bixler, E.O.; Vgontzas, A.N.; Lin, H.-M.; Liao, D.; Calhoun, S.; Vela-Bueno, A.; Fedoc, F.; Vlasic, V.; Graff, G. Sleep disordered breathing in children in a general population sample. Sleep. 2009, 32, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Cornelis, M.A.; Stoustrup, P.B. Cattaneo. Sleep-Disordered Breathing in Early Adolescent Orthodontic Patients: Prevalence and Correlation with Dentofacial Features. J Oral Rehabil. 2025, 0, 1–10. [Google Scholar]
- Carroll, J. Sleep-related upper airway obstruction in children and adolescents. Child Adolec Psychiatric Clin N Am. 1996, 5, 617–647. [Google Scholar] [CrossRef]
- Kim, C.H.; Reinertsen, E.; Dang, C.; Nkutshweu, D.; Sathekge, R.; Choi, Y.J.; Cha, J.-Y.; Alturki, G.; Jamel, A.; Suzuki, A.; Arai, K.; Amm, E.; Motro, M.; Parsi, G. Association between craniofacial morphology, ethnicity, and risk of pediatric sleep-related breathing disorders: A multicenter study. Am J Orthod Dentofacial Orthop. 2024, 165, 414–422. [Google Scholar] [CrossRef]
- Rohra, A.K.; Demko, C.A.; Hans, M.G.; Rosen, C.; Palomo, J.M. Sleep disorderd breathing in children seeking orthodontic care. Am J Orthod Dentofacial Orthop. 2018, 154, 65–71. [Google Scholar] [CrossRef]
- Abtahi, S.; Witmans, M.; Alsufyani, N.A.; Major, M.P.; Major, P.W. Pediatric sleep-disordered breathing in the orthodontic population: Prevalence of positive risk and associations. Am J Orthod Dentofacial Orthop. 2020, 157, 466–473. [Google Scholar] [CrossRef]
- Marcus, C.L.; Curtis, S.; Koerner, C.B.; Joffe ASerwint, J.R.; Loughlin, G.M. Evaluation of pulmonary function and polysomnography in obese children and adolescents. Pediatr Pulmonol. 1996, 21, 176–183. [Google Scholar] [CrossRef]
- Silvestri, J.M.; Weese-Meyer, D.E.; Bass, M.T.; Kenny, A.S.; Hauptmann, S.A.; Pearsall, S.M. Polysomnography in obese children with a history of sleep-associated breathing disorders. Pediatr Pulmonol. 1993, 16, 124–129. [Google Scholar] [CrossRef]
- Blechner, M.; Williamson, A.A. Consequences of Obstructive Sleep Apnea in Children. Curr Probl Pediatr Adolesc Health Care. 2016, 46, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Peppard, P.E.; Young, T.; Palta, M.; Dempsey, J.; Skatrud, J. Longitudinal study of moderate weight change and sleep disordered breathing. JAMA. 2000, 284, 3015–3021. [Google Scholar] [CrossRef] [PubMed]
- Horne, R.; Wijayaratne, P.; Nixon, G.M.; Walter, L.M. Sleep and sleep disordered breathing in children with Down syndrome. Sleep Med Rev. 2019, 44, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cielo, C.M.; Marcus, C.L. Obstructive sleep apnea in children with craniofacial syndromes. Paediatr Respir Rev. 2015, 16, 189–196. [Google Scholar] [PubMed]
- Müller-Hagedorn, S.; Giarrana-Kaufmann, M.L. Kraniofaziale Anomalien und assoziierte Atmungsstörungen. Atemwegs- Lungenkr. 2019, 45, 22–38. [Google Scholar] [CrossRef]
- Caron, C.J.J.M.; Pluijmers, B.I.; Joosten, K.F.M.; Mathijssen, I.M.J.; van der Schroeff, M.P.; Dunaway, D.J.; Wolvius, E.B. , Koudstaal Obstructive sleep apnea in craniofacial microsomia. Int J Oral Maxillofac Surg. 2015, 44, 592–598. [Google Scholar] [CrossRef]
- Müller-Hagedorn, S.; Wiechers, C.; Arand, J.; Buchenau, W.; Bacher, M.; Krimmel, M.; Reinert, S.; Poets, C. Less Invasive Treatment of Sleep-Disordered Breathing in Children with Syndromic Craniosynostosis. Orphanet J Rare Dis. 2018, 13, 63 doi 10. [Google Scholar] [CrossRef]
- Yu, W.; Wang, M.; Yao, K.; Cai, M.; Sun, H.; Lu, L.; Zhu, M.; Lu, X. Individualized Therapy for treating obstructive sleep apnea in pediatric Crouzon syndrome patients. Sleep Breath. 2016, 20, 1119–1129. [Google Scholar] [CrossRef]
- Cielo, C.M.; Konstantinopoulou, S.; Hoque, R. OSAS in Specific Pediatric Populations. Curr Probl Pediatr Adolesc Health Care. 2016, ;46, 11–18. [Google Scholar] [CrossRef]
- Staudt, C.B.; Gnoinski, W.M.; Peltomäki, T. italic>Upper airway changes in Pierre Robin sequence from childhood to adulthood Orthod Craniofac Res. 2013, 16, 202–213.
- Iwasaki, T.; Suga, H.; Minami-Yanagisawa, A.; Hashiguchi-Sato, M.; Sato, H.; Yamamoto, Y.; Shirazawa, Y.; Tsujii, T.; Kanomi, R.; Yamasaki, Y. Upper airway in children with unilateral cleft lip and palate evaluated with computational fluid dynamics. Am J Orthod Dentofacial Orthop. 2019, 156, 257–265. [Google Scholar] [CrossRef]
- Shang, H.; Xue, Y.; Liu, Y.; Zhao, J.; He, L. Modified internal mandibular distraction osteogenesis in the treatment of micrognathia secondary to temporomandibular joint ankylosis: 4-Year follow-up of a case. J Craniomaxillofac Surg. 2012, 40, 373–378. [Google Scholar] [CrossRef]
- Ma, K.S.-K.; Illescas Ralda, M.M.; Veeravalli, J.J.; Wang, L.-T.; Thota, E.; Huang, J.-J.; Kao, C.-T.; Wie, J.C.-C.; Resnick, C.M. Patients with juvenile arthritis are at increased risk for obstructive sleep apnoea: a population-based cohort study. Eur J Orthod. 2022, 226–231. [Google Scholar] [CrossRef]
- Gibson, M.; Cron, R.Q.; Stoll Ml Kinard, B.E.; Patterson, T.; Kau, C.H. A 3D CBCT Analysis of Airway and Cephalometric Values in Patients Diagnosed with Juvenile Idiopathic Arthritis Compared to a Control Group. Appl. Sci. 2022, 12, 4286. [Google Scholar] [CrossRef]
- Richards, A.; Quaghebeur, G.; Clift, S.; Holland, A.; Dahlitz, M.; Parkes, D. Sleep and breathing in Prader-Willi syndrome. Clin Otolaryngol Allied Sci. 1994, 34, 193–197. [Google Scholar] [CrossRef]
- Ferlias, N.; Gjorup, H.; Doherty, M.A.; Haagerup, A.; Petersen, T.K. Obstructive sleep apnoea in pycnodysostosis: A three-dimensional upper airway analysis. Orthod Craniofac Res. 2022, 25, 494–501. [Google Scholar] [CrossRef]
- Seailles, T.; Couloigner, V.; Cohen-Levy, J. Savoir dépister le syndrome d’ apnées obstructives de sommeil (SAOS) de l’enfant. Rev. Orthop Dento Faciale. 2009, 43, 261–277. [Google Scholar] [CrossRef]
- Alessandri-Bonetti, A.; Guglielmi, F.; Deledda, G.; Sangalli, L.; Brogna, C.; Gallenzi, P. Malocclusions, Sleep Bruxism, and Obstructive Sleep Apnea Risk in Pediatric ADHD Patients: A Prospective Study. J Atten Disord. 2024, 28, 1017–23. [Google Scholar] [CrossRef]
- Will, M.J.; Ester, M.A.; Ramirez, S.G.; Tiner, B.D.; McAnear, J.T.; Epstein, L. Comparison of Cephalometric Analysis With Ethnicity in Obstructive Sleep Apnea Syndrome. Sleep 1995, 18, 873–875. [Google Scholar] [CrossRef]
- Galievsky, M.; Lambert, A. Sleep respiratory problems in children: Diagnosis and contribution of the orthodontist. Int Orthod. 2017, 15, 405–423. [Google Scholar] [CrossRef]
- Liu, Y.; Lowe, A.A.; Zeng, X.; Fu, M.; Fleetham, J.A. Cephalometric comparisons between Chinese and Caucasian patients with obstructive sleep apnea. Am J Orthod Dentofacial Orthop. 2000, 117, 479–485. [Google Scholar] [CrossRef]
- Billing, H.; Leighton, B.C.; Linder-Aronson, S.; Lundström, A.; McWilliam, J. The development of the pharyngeal space and lymphoid tissue on the posterior nasopharyngeal wall – an assessment with regard to heritability. Eur J Orthod. 1988, 10, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Linder-Aronson, S. Adenoids. Their effect on mode of breathing and their relationship to characteristics of the facial skeleton and the dentition. A biometric, rhino-manometric and cephalometro-radiographic study on children with and without adenoids. Acta Otolaryngol Suppl. 1970, 265, 1–132. [Google Scholar] [PubMed]
- Vig, K.W. Nasal obstruction and facial growth: the strength of evidence for clinical assumptions. Am J Orthod Dentofacial Orthop. 1998, 113, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Moss, M.L.; Salentijn, L. The primary of functional matrices in facial growth. Am J Orthod 1969, 55, 566–577. [Google Scholar] [CrossRef] [PubMed]
- Cooper, B.C. Nasorespiratory function and orofacial development. Otolaryngol Clin North Am. 1989, 22, 413–441. [Google Scholar] [CrossRef]
- Yamada, T.; Tanne, K.; Miyamoto, K.; Yamauchi, K. Influences of nasal respiratory obstruction on craniofacial growth in young Macaca fuscata monkeys. Am J Orthod Dentofacial Orthop 1997, 11, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Solow, B.; Kreiborg, S. Soft tissue stretching: a possible control factor in craniofacial morphogenesis. Scand. J. Dent. Res. 1977, 85, 506–507. [Google Scholar] [CrossRef]
- Huynh, N.T.; Morton, P.D.; Rompré, P.H.; Papadakis, A.; Remise, C. Associations between sleep-disordered breathing symptoms and facial and dental morphometry, assessed with screening examinations. Am J Orthod Dentofacial Orthop. 2011, 140, 762–770. [Google Scholar] [CrossRef]
- Principato, J.J. Upper airway obstruction and craniofacial morphology. Otolaryngol Head Neck Surg. 1991, 104, 881–890. [Google Scholar] [CrossRef]
- McNamara, J.A. Influence of respiratory pattern on craniofacial growth. Angle Orthod 1981, 51, 269–300. [Google Scholar] [CrossRef]
- Ovsenik, M. Incorrect orofacial functions until 5 years of age and their association with posterior crossbite. Am J Orthod Dentofacial Orthop. 2009, 136, 375–381. [Google Scholar] [CrossRef]
- Hultcrantz, E.; Hellequist, M.L.; Ahlquist-Rastad, J.; Svanholm, H.; Jakobson, O.P. The influence of tonsillar obstruction and tonsillectomy on facial growth and dental arch morphology. Int J Pediatr Otorhinolaryngol. 1991, 22, 125–134. [Google Scholar] [CrossRef]
- Yamada, T.; Tanne, K.; Miyamoto, K.; Yamauchi, K. Influences of nasal respiratory obstruction on craniofacial growth in young Macaca fuscata monkeys. Am J Orthod Dentofacial Orthop. 1997, 11, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Vargervik, K.; Harvold, E.P. Experiments on the Interaction Between Orofacial Function and Morphology. ENT journal. 1987, 66, 26–39. [Google Scholar]
- Harvold, E.P.; Chierici, G.; Vargervik, K. Experiments on the development of dental malocclusions. Am J Orthod. 1972, 61, 38–44. [Google Scholar] [CrossRef]
- Riley, R.W.; Guilleminault, C.; Herran, J.; Powell, N.B. Cephalometric analysis and flow volume loops in obstructive sleep apneic patients. Sleep. 1983, 6, 304–317. [Google Scholar] [CrossRef] [PubMed]
- Guilleminault, C.; Riley, R.; Powell, N.B. Obstructive sleep apnea and cephalometric roentgenograms. Am Rev Respir Dis. 1984, 130, 145–146. [Google Scholar]
- Djupesland, G.; Lyberg, T.; Krogstadt, O. Craniofacial abnormalities and surgical treatment of patients with Obstructive Sleep Apnea Syndrome. Acta otolaryngol. 1987, 103, 551–557. [Google Scholar] [PubMed]
- Pracharktam, N.; Nelson, S.; Hans, M.G.; Broadbent, B.H.; Redline SRosenberg, C.; Strohl, K.P. Cephalometric assessment in obstructive sleep apnea. Am J Orthod Dentofacial Orthop. 1996, 109, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Tangugsorn, V.; Skatvedt, O.; Krogstad, O.; Lyberg, T. Obstructive sleep apnoea: a cephalometric study. Part 1. Cervico-craniofacial skeletal morphology. Eur J Orthod. 1995, 17, 45–56. [Google Scholar] [CrossRef]
- Tangugsorn, V.; Skatvedt, O.; Krogstad, O.; Lyberg, T. Obstructive sleep apnoea: a cephalometric study. Part 2. Uvulo-glossopharyngeal morphology. Eur J Orthod. 1995, 17, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Flores-Mir, C.; Korayem, M.; Heo, G.; Witmans, M.; Major, M.P.; Major, P.W. Craniofacial morphological characteristics in children with obstructive sleep apnea syndrome A systematic review and meta-analysis. J Am Dent Assoc. 2012, 144, 269–277. [Google Scholar] [CrossRef]
- Hultcrantz, E.; Lofstrand Tidestrom, B. The development of sleep disordered breathing from 4 to 12 years and dental arch morphology. Int J Pediatr Otorhinolaryngol. 2009, 73, 1234–1241. [Google Scholar] [CrossRef]
- Seto, B.H.; Gotsopoulos, H.; Sims, M.R.; Cistulli, P.A. Maxillary morphology in obstructive sleep apnea syndrome. Eur J Orthod. 2001, 23, 703–714. [Google Scholar] [CrossRef]
- Löfstrand-Tideström, B.; Thilander, B.; Ahlqvist-Radstad, J.; Jakobsson, O.; Hultcrantz, E. Breathing obstruction in relation to craniofacial and dental arch morphology in 4-yeard old children. Eur J Orthod. 1999, 21, 323–332. [Google Scholar] [CrossRef]
- Yu, C.; Ahn, H.-W.; Kim, S.-H. Three-dimensional morphological evaluation of the hard palate in Korean adults with mild-to-moderate obstructive sleep apnea. Korean journal of orthodontics. 2018. [CrossRef]
- Galeotti, A.; Festa, P.; D’Anto, V.; Sitzia, E.; Piga, S.; Pavone, M. Prevalence of malocclusion in children with obstructive sleep apnoea. Orthod Craniofac Res. 2018, 21, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Pirilä-Parkkinen, K.; Pirttiniemi, P.; Nieminen, P.; Tolonen, U.; Pelttari Löppönen, H. Dental arch morphology in children with sleep-disordered breathing. Eur J Orthod. 2009, 31, 160–167. [Google Scholar] [CrossRef]
- Müller-Hagedorn, S.; Koos, B. Das pädiatrische obstruktive Schlafapnoesyndrom. Somnologie. 2016, 20, 297–308. [Google Scholar] [CrossRef]
- Sambale, J.; Koehler, U.; Conradt, R.; Kesper, K.; Cassel, W.; Degerli, M.; Viniol, C.; Korbmacher-Steiner, H.M. Is sleep bruxism in obstructive sleep apnea only an oral health related problem? BMC Oral Health. 2024, 24, 565. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.R.; Tu, Y.K.; Chuang, L.P.; Gordon, C.; Chen, N.H.; Chen, P.Y.; Chiu, H.Y. Diagnostic meta-analysis of the Pediatric Sleep Questionnaire, OSA-18, and pulse oximetry in detecting pediatric obstructive sleep apnea syndrome. Sleep Med Rev. 2020, 101355. [Google Scholar] [CrossRef]
- Ferry, A.M.; Wright, A.E.; Ohlstein, J.F.; Khoo, K.; Pine, H.S. Efficacy of a Pediatric Sleep Questionnaire for the diagnosis of Obstructive Sleep Apnea in Children. Cureus. 2020, 12, e12244. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.S.; Zaghi, S.; Ghodousi, N.; Peterson, C.; Silva, D.; Lavigne, G.J.; Yoon, A.J. Determinants of probable sleep bruxism in a pediatric mixed dentition population: a multivariate analysis of mouth vs. nasal breathing, tongue mobility, and tonsil size. Sleep Med.
- Davidovich, E.; Hevroni, A.; Gadassi, L.T.; Spierer-Weil, A.; Yitschaky, O.; Polak, D. Dental, oral pH, orthodontic and salivary values in children with obstructive sleep apnea. Clin Oral Invest. 2022, 26, 2503–11. [Google Scholar] [CrossRef]
- Sambale, J.; Birk, R.; Koehler, U.; Hildebrandt, W.; Korbmacher-Steiner, H.M. An Interdisciplinary Approach: Presentation of the Pediatric Obstructive Sleep Apnea Diagnostic Examination Form (POSADEF). Diagnostics. 2024, 14, 1593. [Google Scholar] [CrossRef]
- Behrents, R.G.; Shelgikar, A.V.; Conley, R.S.; Flores-Mir, C.; Hans, M.; Levine, M.; McNamara, J.A.; Palomo, J.M.; Pliska, B.; Stockstill, J.W.; Wise, J.; Murphy, S.; Nagel, N.J.; Hittner, J. Obstructive sleep apnea and orthodontics: An American Association of Orthodontists White Paper. Am J Orthod Dentofacial Orthop. 2019, 156, 13–28. [Google Scholar] [CrossRef]
- Alwadei, A.H.; Galang-Boquiren, T.S.; Kusnoto, B.; Costa Viana, M.G.; Lin, E.Y.; Evans, C.A.; Masoud, A.I. Computerized measurement of the location and valus of the minimum sagittal linear dimension of the upper airway on reconstructed lateral cephalograms compared with 3-dimensional values. Am J Orthod Dentofacial Orthop. 2018, 154, 780–787. [Google Scholar] [CrossRef]
- Feng, X.; Li, G.; Liu, L.; Näsström, K.; Shi, X.-Q. Comparative analysis of upper airway volume with lateral cephalograms and cone-beam computed tomography. Am J Orthod Dentofacial Orthop. 2014, 147, 197–204. [Google Scholar] [CrossRef]
- Alsufyani NA, Flores-Mir C, Major PW: Three-dimensional segmentation of the upper airway using cone beam CT: a systematic review. Dentomaxillofac Radiol. 2012, 41, 276–284. [CrossRef] [PubMed]
- Lenza, M.G.; Lenza, M.M.; Dalstra, M.; Melsen, B.; Cattaneo, P.M. An analysis of different approaches to the assessment of upper airway morphology: a CBCT study. Orthod Craniofac Res. 2010, 13, 96–105. [Google Scholar] [CrossRef]
- Hsu, W.E.; Wu, T.Y. Comparison of upper airway measurement by lateral cephalogram in upright position and CBCT in supine position. J Dent Sci. 2019, 14, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Ingman, T.; Nieminen, T.; Hurmerinta, K. Cephalometric comparison of pharyngeal changes in subjects with upper airway resistance syndrome or obstructive sleep apnoea in upright and supine positions. Eur J Orthod. 2004, 26, 321–326. [Google Scholar] [CrossRef]
- Battagel JM, L’Estrange PR, The cephalometric morphology of patients with obstructive sleep apnea. Eur J Orthod. 1996, 18, 557–569. [CrossRef]
- Tangugsorn, V.; Krogstad, O.; Espeland, L.; Lyberg, T. Obstructive sleep apnea (OSA): a cephalometric analysis of severe and non-severe OSA patients. Part I multiple comparisons of cephalometric variables. Int J Adult Orthod Orthognath Surg. 2000, 15, 139–152. [Google Scholar]
- Cillo Jr, J.E.; Thayer, S.; Dasheiff, R.M.; Finn, R. Relations between obstructive sleep apnea syndrome and specific cephalometric measurements, body mass index, and apnea-hypopnea index. Int J Oral Maxillofac Surg. 2012, 70, 278–283. [Google Scholar] [CrossRef]
- Kapila, S.D.; Nervina, J.M. CBCT in orthodontics: assessment of treatment outcomes and indications for its use. Dentomaxillofac. Radiol. 2015, 44, 20140282. [Google Scholar] [CrossRef]
- Mouhanna-Fattal, C.; Papadopoulos, M.; Bouserhal, J.; Tauk, A.; Bassil-Nassif, N.; Athanasiou, A. Evaluation of upper airway volume and craniofacial volume and craniofacial structures in obstructive sleep apnoea adults: A descriptive CBCT study. Int Orthod. 2019, 17, 678–686. [Google Scholar] [CrossRef]
- Antosz, M. CBCT volumetric analyses have no value in assessing functional airway. Am J Orthod Dentofacial Orthop. 2015, 147, 10–11. [Google Scholar] [CrossRef]
- Damian, A.; Gozal, D. Innovations in the Treatment of Pediatric Obstructive Sleep Apnea. Adv Exp Med Biol. 2022, 1384, 339–50. [Google Scholar] [PubMed]
- Koaban, A.; Al-Harbi, S.K.; Al-Sheheri, A.Z.; Al Shamri, B.S.; Aburazizah, M.F.; Al Qatani, G.H.; Al-Wusaybie, L.H.; Alkhalifa, L.B.; Al-Saad, M.M.; Al-Nehab, A.A.; Al-Halimi, F.M. Current trends in Pediatric Orthodontics: A comprehensive Review. Cureus 2024, 16, e68537. [Google Scholar] [CrossRef] [PubMed]
- Breugem, C.C.; Evans, K.N.; Poets, C.F.; Suri, S.; Picard, A.; Filip, C.; et al. Best practices for the diagnosis and evaluation of infants with Robin sequence: a critical consensus report. JAMA pediatr. 2016, 170, 894–902. [Google Scholar] [CrossRef]
- Vatlach, S.; Maas, C.; Poets, C.F. Birth prevalence and initial treatment of Robin sequence in Germany: a prospective epidemiologic study. Orphanet J Rare Dis. 2014, 9, 9–13. [Google Scholar] [CrossRef]
- Poets, C.F.; Maas, C.; Buchenau, W.; Arand, J.; Vierzig, A.; Braumann, B.; Müller-Hagedorn, S. Multicenter study on the effectiveness of the pre-epiglottic baton plate for airway obstruction and feeding problems in Robin sequence. Orphanet J Rare Dis. 2017, 12, 46. [Google Scholar] [CrossRef]
- Müller-Hagedorn, S.; Buchenau, W.; Arand, J.; Bacher, M.; Poets, C.F. Treatment of infants with Syndromic Robin sequence with modified palatal plates: a minimally invasive treatment option. Head Face Med. 2017, 13. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, J.G.; Schreiber, J.; Karp, N.; Thorne, C.H.; Grayson, B.H. Lengthening the human mandible by gradual distraction. Plast Reconstr Surg. 1992, 89, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Jie, C.; Chen JZou, J.; Ji, Y. Mandibular distraction osteogenesis to relieve Pierre Robin severe airway obstruction in neonates: indication and operation. J Craniofac Surg. 2009, 20, 1812–1816. [Google Scholar] [CrossRef]
- Wiechers C, Sowula J, Kreutzer K, Schwarz CE, Weismann C, Krimmel M, Poets CF, Koos B: Prospective cohort study on facial profile changes in infants with Robin sequence and healthy controls. World J Pediatr. 2024. [CrossRef]
- Camacho, M.; Certal, V.; Abdullatif, J.; Zaghi, S.; Ruoff, C.M.; Capasso, R.; Kushida, C.A. Myofunctional Therapy to treat Obstructive Sleep Apnea: A Systematic Review and Meta-analysis. Sleep. 2015, 38, 669–675. [Google Scholar] [CrossRef]
- Camacho M, Guilleminault C, Wei JM, Song SA, Noller MW, Reckley LR, Fernandez-Salvador C, Zaghi. Oropharyngeal and tongue exercises (myofunctional therapy) for snoring: a systematic review and meta-analysis. Eur Arch Otorhinolaryngol. 2018, 275, 849–855. [CrossRef]
- Fränkel, R. Die Dynamik des interokklusalen Unterdrucks. Deutsche Zahnärztliche Zeitschrift. 1967, 22, 1282. [Google Scholar]
- Hsu, B.; Emperumal, C.P.; Grbach, V.X.; Padilla, M.; Enciso, R. Effects of respiratory muscle therapy on obstructive sleep apnea: a systematic review and meta-analysis. J Clin Sleep med. 2020, 16, 785–801. [Google Scholar] [CrossRef] [PubMed]
- Gozal, D.; Tan, H.-L. Kheirandish-Gozal LTreatment of Obstructive Sleep Apnea in Children Handling the Unknown with Precision. J. Clin. Med. 2020, 9, 888. [Google Scholar] [CrossRef]
- Huang, Y.-S.; Hsu, S.C.; Guilleminault, C.; Chuang, L.C. Myofunctional Therapy: Role in Pediatric OSA. Sleep Med Clin. 2018, 14, 135–142. [Google Scholar] [CrossRef]
- Ribeiro Nunes jr, W.; Gozal, D.; Di Francesco, R.C. Cephalometric and Pharyngometric Evaluation in Snoring Children with Sleep disordered Breathing and Adenotonsillar Hypertrophy Under Orthodontic or Orthopedic Treatment. J. Child Sci. 2019, 9, e68–e74. [Google Scholar] [CrossRef]
- Chuang, L.-C.; Hwang, Y.-J.; Lian, Y.C.; Hervy-Auboiron, M.; Pirelli, P.; Huang, Y.-S.; Guilleminault, C. Changes in craniofacial and airway morphology as well as quality of life in children with obstructive sleep apnea: a comparative cohort study. Sleep Breath. 2019, 23, 1359–1369. [Google Scholar] [CrossRef] [PubMed]
- Di Vecchio, S.; Manzini, P.; Candida, E.; Gargari, M. Froggy Mouth: a new myofunctional approach to atypical swallowing. Eur J Paediatr Dent. 2019, 20, 35–37. [Google Scholar] [CrossRef]
- Mohammed, H.; Čigic, E.; Rizk, M.Z.; Vandevska-Radunovic, V. Effectiveness of prefabricated myofunctional appliances in the treatment of Class II Division I malocclusion: a systematic review. Eur J Orthod. 2020, 42, 125–134. [Google Scholar] [CrossRef]
- Darendeliler MAEarly Maxillary Expansion in: Rakosi, T.; Graber, T.M. Orthodontic and Dentofacial Orthopedic Treatment. Thieme, Stuttgart, New York 2010 pp 155-176.
- Iwasaki, T.; Saitoh, I.; Takemoto, Y.; Inanda, E.; Kakuno, E.; Kanomi, R.; Hayasaki, H.; Yamasaki, Y. Tongue posture improvement and pharyngeal airway enlargement as secondary effects of rapid maxillary expansion: A cone-beam computed tomography study. Am J Orthod Dentofacial Orthop. 2013, 143, 235–245. [Google Scholar] [CrossRef]
- Cistulli, P.A.; Palmisano, R.G.; Poole, M.D. Treatment of Obstructive Sleep Apnea Syndrome by Rapid Maxillary Expansion. Sleep. 1998, 21, 831–835. [Google Scholar] [CrossRef]
- Cerritelli, L.; Hatzopoulos, S.; Catalano, A.; Bianchini, C.; Cammaroto, G.; Meccariello, G.; Iannella, G.; Vicini, C.; Pelucchi, S.; Skarzynski, P.H.; Ciorba, A. Rapid Maxillary Expansion (RME): An Otolaryngologic Perspective J. Clin. Med. 2022, 11, 5243. [Google Scholar] [CrossRef] [PubMed]
- Katyal, V.; Pamula, Y.; Daynes, C.N.; Martin, J.; Dreyer, C.W.; Kennedy, D.; Sampson, W.J. Craniofacial and upper airway morphology in pediatric sleep-disordered breathing and changes in quality of life with rapid maxillary expansion. Am J Orthod Dentofacial Orthop. 2013, 144, 860–871. [Google Scholar] [CrossRef] [PubMed]
- Bariani, B.R.C.; Bigliazzi, R.; Gobbo Messa, M.; Roim Micieli, A.P.; Tufik, S.; Fujita, R.R.; Berlim de Mello, C.; Moreira, G.A. Changes in behavioral and cognitive abilities after rapid maxillary expansion in children affected by persistent snoring after long-term adenotonsillectomy: A noncontrolled study. Am J Orthod Dentofacial Orthop. 2024, 165, 344–356. [Google Scholar] [CrossRef]
- Ottaviano, G.; Maculan, P.; Borghetto, G.; Favero, V.; Galletti, B.; Savietto, E.; Scarpa, B.; Martini, A.; Stellini, E.; De Filippis, C.; Favero, L. Nasal function before and after rapid maxillary expansion in children: A randomized, prospective, controlled study. Int J Pediatr Otorhinolaryngol. 2018, 115, 133–138. [Google Scholar] [CrossRef]
- Huynh, N.T.; Desplats, E.; Almeida, F.R. Orthodontic treatments for managing obstructive sleep apnea syndrome in children: A systematic review and meta-analysis. Sleep Med Rev. 2016, 25, 84–94. [Google Scholar] [CrossRef]
- Barbosa, D.F.; Bana, L.F.; Michel, M.C.B.; Meira, E.C.M.; Zancanella, E.; Machado Júnior, A.J. Rapid maxillary expansion in pediatric patients with obstructive sleep apnea: an umbrella review. Braz J Otorhinolaryngol. 2023, 89, 494–502. [Google Scholar] [CrossRef]
- Bucci, R.; Rongo, R.; Zunino, B.; Michelotti, A.; Bucci, P.; Alessandri-Bonetti, G.; et al. Effect of orthopedic and functional orthodontic treatment in children with obstructive sleep apnea: A systematic review and meta-analysis. Sleep Med Rev. 2023, 67, 101730. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, R.L.; Schendel, S.A. Treating obstructive sleep apnea: The case for surgery. Am J Orthod Dentofac Orthop. 2012, 142, 435–442. [Google Scholar] [CrossRef]
- Wells, A.P.; Sarver, D.M.; Proffit, W.R. Long-term efficacy of reverse pull headgear therapy. Angle Orthod. 2006, 76, 915–922. [Google Scholar] [CrossRef]
- Ming, Y.; Hu, Y.; Li, Y.; Yu, J.; He, H.; Zheng, L. Effects of maxillary protraction appliances on airway dimensions in growing class III maxillary retrognathic patients: a systematic review and meta-analysis. Int J Pediatr Otorhinolaryngol. 2018, 105, 138–145. [Google Scholar] [CrossRef]
- Smyth, R.S.; Ryan, F.S. Early treatment of class III malocclusion with facemask. Evid Based Dent. 2017, 18107–118. [Google Scholar] [CrossRef]
- Wahl N: Orthodontics in 3 millenia. Chapter 9, Functional appliances to mid-century: Am J Orthod Dentofac Orthop. 2006, 129, 829–833.
- Ruf S, Pancherz H, Long-term TMJ effects of Herbst treatment: a clinical and MRI study. Am J Orthod Dentofacial Orthop. 1998, 114, 475–483. [CrossRef] [PubMed]
- Ruf, S.; Pancherz, H. Temporomandibular joint remodelling in adolescents and young adults during Herbst treatment: a prospective longitudinal magnetic resonance imaging and cephalometric radiographic investigation. Am J Orthod Dentofacial Orthop. 1999, 115, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Isono, S.; Tanaka, A.; Tagaito, Y.; Sho, Y.; Nishino, T. Pharyngeal patency in response to advancement of the mandible in obese anesthesized persons. Anesthesiology 1997, 87, 1055–1062. [Google Scholar] [CrossRef]
- Li, L.; Liu, H.; Cheng Het, a.l. CBCT evaluation of the upper airway morphological changes in growing patients of Class II division I malocclusion using twin block appliance: a comparative research. PLoS One 2014, 9, e94578. [Google Scholar] [CrossRef] [PubMed]
- Xiang, M.L.; Hu, B.; Liu, Y.; Sun, J.; Song, J. Changes in airway dimensions following functional appliances in growing patients with skeletal Class II malocclusion: A systematic review and meta-analysis. Int J Pediatr Otorhinolaryngol. 2017, 97, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, Y.; Sonnesen, L. Association between orthodontic treatment and upper airway changes in children assessed with cone-beam computed tomography (CBCT): A systematic review. J Oral Rehabil. 2024, 51, 2195–2208. [Google Scholar] [CrossRef] [PubMed]
- Bariani, R.C.B.; Bigliazzi, R.; Cappellette Jr, M.; Moreira, G.; Fujita, R.R. Effectiveness of functional orthodontic appliances in obstructive sleep apnea treatment in children: literature review. Braz J Otorhinolaryngol. 2022, 88, 263–78. [Google Scholar] [CrossRef]
- Vejwarakul, W.; Ko, E.W.-C.; Lin, C.-H. Evaluation of pharyngeal airway space after orthodontic extraction treatment in Class II malocclusion integration with the subjective sleep quality assessment. Scientific Reports. 2023, 13, 9210. [Google Scholar] [CrossRef]
- Hu, Z.; Yin, X.; Liao, J.; Zhou, C.; Yang, Z.; Zou, S. The effect of teeth extraction for orthodontic treatment on the upper airway: a systematic review. Sleep Breath. 2015, 19, 441–451. [Google Scholar] [CrossRef]
- Papageorgiou, S.N.; Zyli, M.; Papadopoulou, A.K. Extraction of premolars in orthodontic treatment does not negatively affect upper airway volume and minimum cross-sectional area: a systematic review with meta-analysis. Eur J Orthod 2025, 47, cjaf012. [Google Scholar] [CrossRef]
- Orabi, N.; Flores-Mir Elshebiny, T.; Elkordy, S.; Palomo, J.M. Pharyngeal airway dimensional changes after orthodontic treatment with premolar extractions: A systematic review with meta-analysis. Am J Orthod Dentofacial Orthop 2021, 160, 503–515. [Google Scholar] [CrossRef]
- Kikuchi, M. Orthodontic treatment in children to prevent sleep disordered breathing in adulthood. Sleep Breath. 2006, 9, 146–158. [Google Scholar] [CrossRef]
- Cangialosi, T.J.; Meistrell MEJr Leung, M.A.; Ko, J.Y. A cephalometric appraisal of edgewise Class II non-extraction treatment with extraoral force. Am Orthod Dentofacial Orthop. 1988, 93, 315–324. [Google Scholar] [CrossRef]
- Keeling, S.D.; Wheeler, T.T.; King, G.J.; Garvan, C.W.; Cohen, D.A.; Cabassa Set, a.l. Anteriorposterior skeletal and dental changes after early Class II treatments with bionators and headgear. Am J Orthod Dentofacial Orthop. 1998, 113, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Gautam, P.; Valiathan, A.; Adhikari, R. Craniofacial displacement in response to varying headgear forces evaluated biomechanically with finite element analysis. Am J Orthod Dentofacial Orthop. 2009, 135, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Freitas, M.R.; Lima, D.V.; Freitas, K.M.; Janson, G.; Henriques, J.F. Cephalometric evaluation of Class II malocclusion treatment with cervical headgear and mandibular fixed appliances. Eur J Orthod 2008, 30, 477–482. [Google Scholar] [CrossRef]
- Baumrind, S.; Korn, E.L.; Molthen, R.; West, E.E. Changes in facial dimensions associated with the use of forces to retract the maxilla. Am J Orthod. 1981, 80, 17–30. [Google Scholar] [CrossRef]
- Hänggi, M.P.; Teuscher, U.M.; Roos, M.; Peltomäki, T.A. Long-term changes in pharyngeal airway dimensions following activator-headgear and fixed appliance treatment. Eur J Orthod. 2008, 30, 598–605. [Google Scholar] [CrossRef]
- Godt, A.; Koos, B.; Hagen, H.; Göz, G. Changes in upper airway width associated with Class II treatments (Headgear vs Activator) and different growth patterns. Angle Orthod. 2011, 81, 440–446. [Google Scholar] [CrossRef]
- Pirilä-Parkkinen, K.; Pirttiniemi, P.; Nieminen, P.; Löppönen, H.; Tolonen, U.; Uotila, R.; Huggare, J. Cervical headgear therapy as a factor in obstructive sleep apnea syndrome. Pediatr Dent. 1999, 21, 39–45. [Google Scholar]
- Fauroux, B.; Lavis, J.F.; Nicot, F.; Picard, A.; Boelle, P.Y.; Clément, A.; Vazquez, M.P. Facial side effects during noninvasive positive pressure ventilation in children. Intensive Care Med. 2005, 31, 965–969. [Google Scholar] [CrossRef]
- Pliska, B.T.; Almeida, F.R. Tooth Movement Associated With CPAP Therapy. J Clin Sleep Med. 2018, 14, 701–702. [Google Scholar] [CrossRef] [PubMed]
- Choong, W.Y.; Teh, K.W.; Lau, M.N.; Wey, M.C.; Abd Rahman, A.N.A.; Ashari, A. A multicenter study on the prevalence of adults and children seeking orthodontic treatment at high risk of obstructive sleep apnea. Cranio. 2023, 41, 340–7. [Google Scholar] [CrossRef]
- Fagundes, N.C.F.; Flores-Mir, C. Pediatric obstructive sleep apnea-Dental professionals can play a crucial role. Pediatr Pulmonol. 2022, 57, 1860–8. [Google Scholar] [CrossRef]
- Cohen-Levy, J.; Quintal, M.C.; Rompré, P.; Almeida, F.; Huynh, N. Prevalence of malocclusions and oral dysfunctions in children with persistent sleep-disordered breathing after adenotonsillectomy in the long term. J Clin Sleep Med. 2020, 16, 1357–68. [Google Scholar] [CrossRef] [PubMed]
- Faber, J.; Mota, A.; Ho, L.I.; Darendeliler, M.A. The role of orthodontists in the multidisciplinary management of obstructive sleep apnea. Prog Orthod. 2024, 25, 40. [Google Scholar] [CrossRef] [PubMed]
- Jost, P.; Conte, A.L.; Lira, A.O.; Pugliese, F.; Palomo, J.M.; Quevedo, B.; et al. Risk of sleep-disordered breathing in orthodontic patients: comparison between children and adolescents. Eur J Orthod. 2024, 46. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Radiographic cephalograms: left side: enlarged tonsils; right side: enlarged adenoids.
Figure 1.
Radiographic cephalograms: left side: enlarged tonsils; right side: enlarged adenoids.
Figure 2.
Radiographic cephalogram: Low tongue position, the tongue protrudes into the oropharynx (red arrows indicate tongue shadow).
Figure 2.
Radiographic cephalogram: Low tongue position, the tongue protrudes into the oropharynx (red arrows indicate tongue shadow).
Figure 3.
Radiographic cephalogram: Typical craniofacial morphology in OSA patients (blue arrows indicate PAS).
Figure 3.
Radiographic cephalogram: Typical craniofacial morphology in OSA patients (blue arrows indicate PAS).
Figure 4.
Left side: Extraoral photograph of a patient with OSA, convex profile, with retrognathic mandible; right side: intraoral photograph of the same patient. Deep bite and increased overjet. (Photographs shown with parental permission).
Figure 4.
Left side: Extraoral photograph of a patient with OSA, convex profile, with retrognathic mandible; right side: intraoral photograph of the same patient. Deep bite and increased overjet. (Photographs shown with parental permission).
Figure 5.
Intraoral findings: (1) narrow maxilla; (2) bilateral posterior crossbites, (3) low tongue position, (4) anterior open bite; (5) protruded incisors; (6) deep bite and increased overjet.
Figure 5.
Intraoral findings: (1) narrow maxilla; (2) bilateral posterior crossbites, (3) low tongue position, (4) anterior open bite; (5) protruded incisors; (6) deep bite and increased overjet.
Figure 6.
Radiographic cephalogram: Posterior airway space.
Figure 6.
Radiographic cephalogram: Posterior airway space.
Figure 7.
Tübingen Palatal Plate for neonates with Robin sequence.
Figure 7.
Tübingen Palatal Plate for neonates with Robin sequence.
Figure 8.
Custom-made myofunctional appliances.
Figure 8.
Custom-made myofunctional appliances.
Figure 9.
Vestibular screens: (1) prefabricated screen, (2) Individual screen, (3) screen with breathing holes, (4) screen with tongue grid.
Figure 9.
Vestibular screens: (1) prefabricated screen, (2) Individual screen, (3) screen with breathing holes, (4) screen with tongue grid.
Figure 10.
Top Row: Removable maxillary expanders, bottom row: Fixed maxillary expanders: Left: Hyrax-Expander, middle: Quad-Helix, right: Bonded expander.
Figure 10.
Top Row: Removable maxillary expanders, bottom row: Fixed maxillary expanders: Left: Hyrax-Expander, middle: Quad-Helix, right: Bonded expander.
Figure 11.
Protraction treatment: Extraoral and intraoral appliance. Photographs shown with parental and patient’s permission.
Figure 11.
Protraction treatment: Extraoral and intraoral appliance. Photographs shown with parental and patient’s permission.
Figure 12.
PAS before (left) and after (right) protraction treatment.
Figure 12.
PAS before (left) and after (right) protraction treatment.
Figure 13.
Functional Class III appliances: Left: Class III activator, right: Fraenkel III.
Figure 13.
Functional Class III appliances: Left: Class III activator, right: Fraenkel III.
Figure 14.
Bite plate for lower jaw.
Figure 14.
Bite plate for lower jaw.
Figure 15.
Most common appliances for mandibular advancement: (1) Herbst, (2) Activator, (3) Fraenkel II, (4) Bionator, (5) elastic activator, (6) Twin Block, (7) Double plate, (8) activator modification.
Figure 15.
Most common appliances for mandibular advancement: (1) Herbst, (2) Activator, (3) Fraenkel II, (4) Bionator, (5) elastic activator, (6) Twin Block, (7) Double plate, (8) activator modification.
Table 1.
Cervico-craniofacial skeletal (a) and soft tissue morphology (b) in patients with OSA.
Table 1.
Cervico-craniofacial skeletal (a) and soft tissue morphology (b) in patients with OSA.
-
a.
Skeletal variables |
Shorter dimension of the cranial base (distance of S-N decreased) Shorter maxillary and mandibular lengths Slight counter-clockwise rotation of clivus (angle S-N-Ba decreased) Pronounced maxillary and mandibular retrognathia Increased anterior facial height and mandibular plane angle (angle SN-GoGn) Increased gonion angle Deviated head posture with larger cranio-cervical angle (hyperextension of cervical spine) |
-
b.
Soft tissue variables |
Increased length and thickness of soft palate Increased sagittal area of the tongue with more oral area occupation Caudally extended tongue mass∙ Decreased sagittal dimensions:
-
o
nasopharynx (due to adenoids) -
o
velopharynx (due to soft palate) -
o
oropharynx with reduced distance between the base of the tongue and the posterior pharyngeal wall (due to tongue)
|
Table 2.
Extraoral and intraoral findings in children with Obstructive Sleep Apnea (OSA).
Table 2.
Extraoral and intraoral findings in children with Obstructive Sleep Apnea (OSA).
| Extraoral findings |
Intraoral findings |
-
Adenoid facies:
-
o
Dark circles around the eyes -
o
Flattened cheekbones -
o
Dry lips -
o
Labial incompetence -
o
Underdeveloped nasal bones -
o
Pronounced nasolabial furrows -
o
Open mouth posture -
o
Reduced orofacial muscle tonicity
|
|
|
|
|
|
|
|
Table 3.
Intraoral and extraoral typical features of “at-risk” children.
Table 3.
Intraoral and extraoral typical features of “at-risk” children.
| Typical features of “at-risk patient” |
|
|
|
|
|
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).