Submitted:
13 July 2025
Posted:
15 July 2025
You are already at the latest version
Abstract

Keywords:
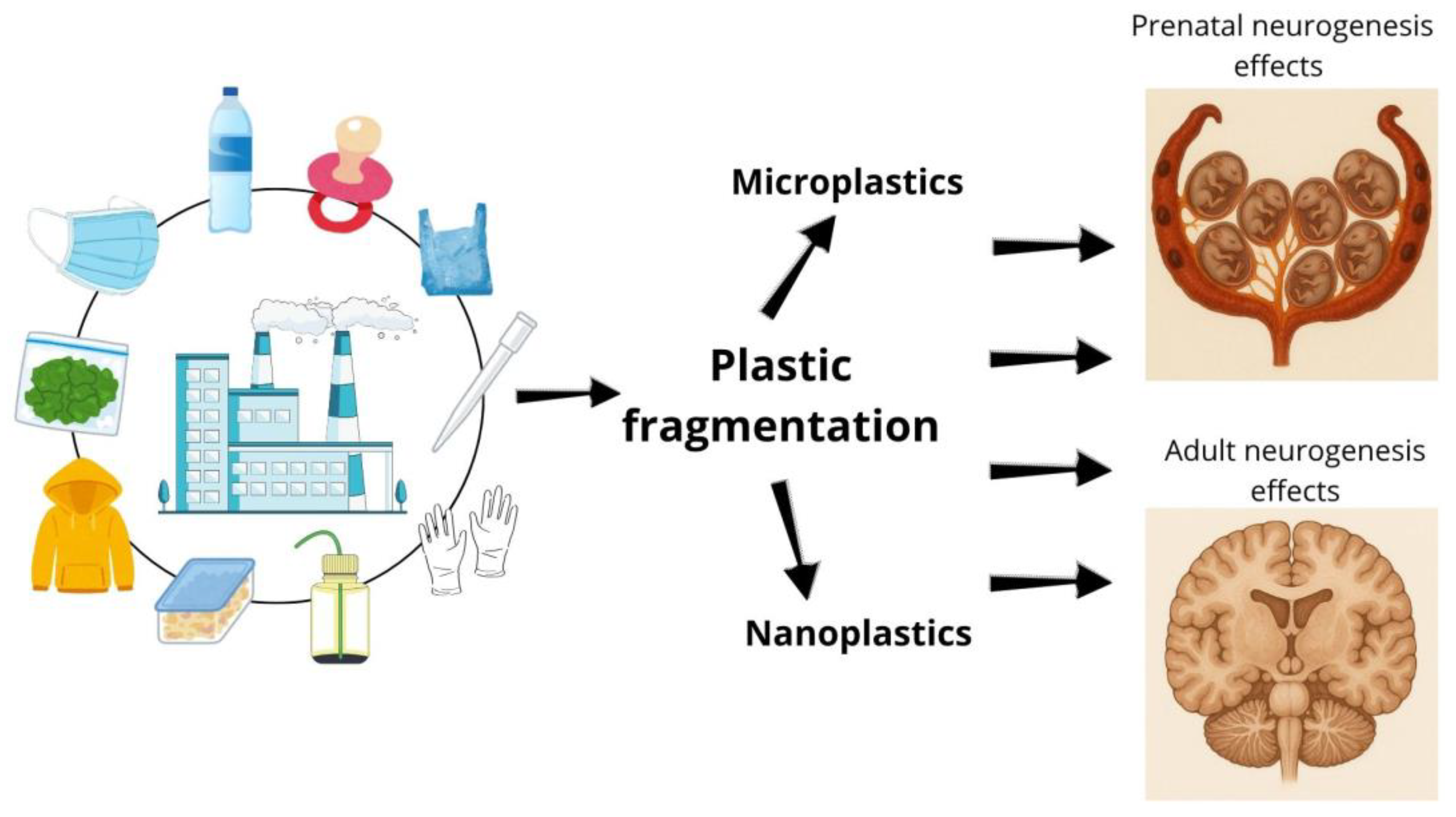
1. Introduction
2. Harmful Effects of Plastic Particle Exposure on the Central Nervous System
3. Detrimental Consequences of Plastic Particle Exposure on the Production of Neurons Both in the Prenatal and Adult Life
4. Plastic Particles Exposure and Neurogenesis in the Context of the Brdu-Labeling
4.1. Plastic Particles and 5-BROMO-2´-Deoxyuridine Induce Apoptosis
4.2. Plastic Particles and 5-BROMO-2´-Deoxyuridine Induce Senescence
4.3. Plastic Particles and 5-BROMO-2´-Deoxyuridine Alter DNA Methylation
5. Conclusions and Future Perspectives
Author contribution
Acknowledgments
Conflicts of Interest
Declaration of Generative AI and AI-assisted technologies in the writing process
Abbreviations
| BrdU | 5-bromo-2’-deoxyuridine |
| CNS | Central Nervous System |
| BBB | Blood-brain barrier |
References
- Baby, M. G.; Gerritse, J.; Beltran-Sanahuja, A.; Wolter, H.; Sébastien, Rohais; Maria-Fernanda, Romero-Sarmiento. Aging of Plastics and Microplastics in the Environment: A Review on Influencing Factors, Quantification Methods, Challenges, and Future Perspectives. In Environmental Science and Pollution Research; 2024. [Google Scholar] [CrossRef]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J. H.; Abu-Omar, M.; Scott, S. L.; Suh, S. Degradation Rates of Plastics in the Environment. ACS Sustainable Chemistry & Engineering 2020, 8, 3494–3511. [Google Scholar] [CrossRef]
- Usman, S.; Abdull Razis, A. F.; Shaari, K.; Azmai, M. N. A.; Saad, M. Z.; Mat Isa, N.; Nazarudin, M. F. The Burden of Microplastics Pollution and Contending Policies and Regulations. International Journal of Environmental Research and Public Health 2022, 19, 6773. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J. R.; Law, K. L. Production, Use, and Fate of All Plastics Ever Made. Science Advances 2017, 3. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.; Martins, R.; Marques, C. Critical Review on Microplastics Characterisation in Aquatic Environments: Recent Trends in the Last 10 Years. Analytical Methods 2024. [Google Scholar] [CrossRef] [PubMed]
- Andrady, A.L. Microplastics in the marine environment. Mar Pollut Bull 2011. Aug;62(8), 1596-605. [CrossRef]
- Wang, F.; Zhang, M.; Sha, W.; Wang, Y.; Hao, H.; Dou, Y.; Li, Y. Sorption Behavior and Mechanisms of Organic Contaminants to Nano and Microplastics. Molecules 2020, 25, 1827. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; He, Y.; Jiang, L.; Shi, Y.; Hao, L.; Huang, L.; Lyu, M.; Wang, S. Plastic Additives as a New Threat to the Global Environment: Research Status, Remediation Strategies and Perspectives. Environmental Research 2024, 263, 120007. [Google Scholar] [CrossRef]
- Gigault, J.; Halle, A. ter; Baudrimont, M.; Pascal, P.-Y.; Gauffre, F.; Phi, T.-L.; El Hadri, H.; Grassl, B.; Reynaud, S. Current Opinion: What Is a Nanoplastic? Environmental Pollution 2018, 235, 1030–1034. [Google Scholar] [CrossRef]
- Shan, S.; Zhang, Y.; Zhao, H.; Zeng, T.; Zhao, X. Polystyrene Nanoplastics Penetrate across the Blood-Brain Barrier and Induce Activation of Microglia in the Brain of Mice. Chemosphere 2022, 298, 134261. [Google Scholar] [CrossRef]
- Kopatz, V.; Wen, K.; Tibor, Kovács; Keimowitz, A. S.; Pichler, V.; Widder, J.; A. Dick, Vethaak; Oldamur, Hollóczki; Kenner, L. Micro- and Nanoplastics Breach the Blood–Brain Barrier (BBB): Biomolecular Corona’s Role Revealed. Nanomaterials 2023, 13, 1404–1404. [Google Scholar] [CrossRef]
- Li, J.; Zhang, K.; Zhang, H. Adsorption of Antibiotics on Microplastics. Environmental Pollution 2018, 237, 460–467. [Google Scholar] [CrossRef]
- Gerdes, Z.; Ogonowski, M.; Nybom, I.; Ek, C.; Adolfsson-Erici, M.; Barth, A.; Gorokhova, E. Microplastic-Mediated Transport of PCBs? A Depuration Study with Daphnia Magna. PLOS ONE 2019, 14. [Google Scholar] [CrossRef] [PubMed]
- Toussaint, B.; Raffael, B.; Angers-Loustau, A.; Gilliland, D.; Kestens, V.; Petrillo, M.; Rio-Echevarria, I. M.; Van den Eede, G. Review of Micro- and Nanoplastic Contamination in the Food Chain. Food Additives & Contaminants: Part A 2019, 36, 639–673. [Google Scholar] [CrossRef] [PubMed]
- Barceló, D.; Picó, Y.; Alfarhan, A. H. Microplastics: Detection in Human Samples, Cell Line Studies, and Health Impacts. Environmental Toxicology and Pharmacology 2023, 101, 104204. [Google Scholar] [CrossRef] [PubMed]
- Nihart, A. J.; Garcia, M. A.; El Hayek, E.; Liu, R.; Olewine, M.; Kingston, J. D.; Castillo, E. F.; Gullapalli, R. R.; Howard, T.; Bleske, B.; Scott, J.; Gonzalez-Estrella, J.; Gross, J. M.; Spilde, M.; Adolphi, N. L.; Gallego, D. F.; Jarrell, H. S.; Dvorscak, G.; Zuluaga-Ruiz, M. E.; West, A. B. Bioaccumulation of Microplastics in Decedent Human Brains. Nature Medicine 2025, 31. [Google Scholar] [CrossRef]
- Ghosh, A.; Bapi Gorain. Mechanistic Insight of Neurodegeneration due to Micro/Nano-Plastic-Induced Gut Dysbiosis. Archives of Toxicology 2024. [CrossRef]
- Duque, A.; Rakic, P. Different Effects of Bromodeoxyuridine and [3H]Thymidine Incorporation into DNA on Cell Proliferation, Position, and Fate. Journal of Neuroscience 2011, 31, 15205–15217. [Google Scholar] [CrossRef]
- Joaquín, Martí; Wills, K. V.; Bernardino, Ghetti; Bayer, S. A. A Combined Immunohistochemical and Autoradiographic Method to Detect Midbrain Dopaminergic Neurons and Determine Their Time of Origin. Brain research protocols 2002, 9, 197–205. [Google Scholar] [CrossRef]
- Martí, J.; Santa-Cruz, M. C.; Bayer, S. A.; Ghetti, B.; Hervás, J. P. Purkinje Cell Age-Distribution in Fissures and in Foliar Crowns: A Comparative Study in the Weaver Cerebellum. Brain Structure and Function 2007, 212, 347–357. [Google Scholar] [CrossRef]
- Solius, G. M.; Maltsev, D. I.; Belousov, V. V.; Podgorny, O. V. Recent Advances in Nucleotide Analogue-Based Techniques for Tracking Dividing Stem Cells: An Overview. Journal of Biological Chemistry 2021, 297, 101345. [Google Scholar] [CrossRef]
- Joaquín Martí-Clúa. 5-Bromo-2’-Deoxyuridine Labeling: Historical Perspectives, Factors Influencing the Detection, Toxicity, and Its Implications in the Neurogenesis. Neural Regeneration Research 2024, 19, 302–308. [Google Scholar] [CrossRef]
- Lehner, B.; Sandner, B.; Marschallinger, J.; Lehner, C.; Furtner, T.; Couillard-Despres, S.; Rivera, F. J.; Brockhoff, G.; Bauer, H.-C.; Weidner, N.; Aigner, L. The Dark Side of BrdU in Neural Stem Cell Biology: Detrimental Effects on Cell Cycle, Differentiation and Survival. Cell and Tissue Research 2011, 345, 313–328. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, S.; Liu, Q.; Wei, J.; Jin, Y.; Wang, X.; Zhang, L. Polystyrene Microplastics Cause Cardiac Fibrosis by Activating Wnt/β-Catenin Signaling Pathway and Promoting Cardiomyocyte Apoptosis in Rats. Environmental Pollution 2020, 265, 115025. [Google Scholar] [CrossRef]
- Dong, C.-D.; Chen, C.-W.; Chen, Y.-C.; Chen, H.-H.; Lee, J.-S.; Lin, C.-H. Polystyrene Microplastic Particles: In Vitro Pulmonary Toxicity Assessment. Journal of Hazardous Materials 2020, 385, 121575. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, L.; Chen, J.; Liu, X.; Li, K.; Liu, H.; Lai, W.; Shi, Y.; Lin, B.; Xi, Z. Selective Bioaccumulation of Polystyrene Nanoplastics in Fetal Rat Brain and Damage to Myelin Development. Ecotoxicology and environmental safety 2024, 278, 116393–116393. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Han, Q.; Wei, Z.; Wang, Y.; Xie, J.; Chen, M. Polystyrene Microplastics Affect Learning and Memory in Mice by Inducing Oxidative Stress and Decreasing the Level of Acetylcholine. Food and Chemical Toxicology 2022, 162, 112904. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Lee, S.; Lee, Y.; Cho, J.-H.; Sou Hyun, Kim; Ha, E.-S.; Jung, Y.-S.; Hae Young, Chung; Kim, M.-S.; Hyung Sik, Kim; Chang, S.-C.; Min, K.-J.; Lee, J. Cationic Nanoplastic Causes Mitochondrial Dysfunction in Neural Progenitor Cells and Impairs Hippocampal Neurogenesis. Free radical biology & medicine 2023, 208, 194–210. [Google Scholar] [CrossRef]
- Nishimura, Y.; Kanda, Y.; Sone, H.; Aoyama, H. Oxidative Stress as a Common Key Event in Developmental Neurotoxicity. Oxidative Medicine and Cellular Longevity 2021, 2021, 1–10. [Google Scholar] [CrossRef]
- Usman, S.; Abdull Razis, A. F.; Shaari, K.; Amal, M. N. A.; Saad, M. Z.; Mat Isa, N.; Nazarudin, M. F. Polystyrene Microplastics Exposure: An Insight into Multiple Organ Histological Alterations, Oxidative Stress and Neurotoxicity in Javanese Medaka Fish (Oryzias Javanicus Bleeker, 1854). International Journal of Environmental Research and Public Health 2021, 18, 9449. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, X.; Leng, Y.; Wang, J. Defense Responses in Earthworms (Eisenia Fetida) Exposed to Low-Density Polyethylene Microplastics in Soils. Ecotoxicology and Environmental Safety 2020, 187, 109788. [Google Scholar] [CrossRef]
- Rodríguez-Ithurralde, D.; Maruri, A.; Xiliana, Rodríguez. Motor Neurone Acetylcholinesterase Release Precedes Neurotoxicity Caused by Systemic Administration of Excitatory Amino Acids and Strychnine. Journal of the Neurological Sciences 1998, 160, S80–S86. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, S.; Razanajatovo, R. M.; Zou, H.; Zhu, W. Accumulation, Tissue Distribution, and Biochemical Effects of Polystyrene Microplastics in the Freshwater Fish Red Tilapia (Oreochromis Niloticus). Environmental Pollution 2018, 238, 1–9. [Google Scholar] [CrossRef]
- Torres-Ruiz, M.; de Alba González, M.; Morales, M.; Martin-Folgar, R.; González, Ma. C.; Cañas-Portilla, A. I.; De la Vieja, A.; González, Ma. C.; Cañas-Portilla, A. I.; De la Vieja, A. Neurotoxicity and Endocrine Disruption Caused by Polystyrene Nanoparticles in Zebrafish Embryo. Science of The Total Environment 2023, 874, 162406. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, Y.; Dou, J.; Hou, Q.; Cheng, J.; Jiang, X. Bioeffects of Inhaled Nanoplastics on Neurons and Alteration of Animal Behaviors through Deposition in the Brain. Nano Letters 2022, 22, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Jeong, C.-B.; Won, E.-J.; Kang, H.-M.; Lee, M.-C.; Hwang, D.-S.; Hwang, U.-K.; Zhou, B.; Souissi, S.; Lee, S.-J.; Lee, J.-S. Microplastic Size-Dependent Toxicity, Oxidative Stress Induction, and P-JNK and P-P38 Activation in the Monogonont Rotifer (Brachionus Koreanus). Environmental Science & Technology 2016, 50, 8849–8857. [Google Scholar] [CrossRef]
- Trevisan, R.; Voy, C.; Chen, S.; Di, R. T. Nanoplastics Decrease the Toxicity of a Complex PAH Mixture but Impair Mitochondrial Energy Production in Developing Zebrafish. 2019, 53 (14), 8405–8415. [CrossRef]
- Huang, Y.; Liang, B.; Li, Z.; Zhong, Y.; Wang, B.; Zhang, B.; Du, J.; Ye, R.; Xian, H.; Min, W.; Yan, X.; Deng, Y.; Feng, Y.; Bai, R.; Fan, B.; Yang, X.; Huang, Z. Polystyrene Nanoplastic Exposure Induces Excessive Mitophagy by Activating AMPK/ULK1 Pathway in Differentiated SH-SY5Y Cells and Dopaminergic Neurons in Vivo. Particle and Fibre Toxicology 2023, 20. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Huang, Y.; Zhong, Y.; Li, Z.; Ye, R.; Wang, B.; Zhang, B.; Meng, H.; Lin, X.; Du, J.; Hu, M.; Wu, Q.; Sui, H.; Yang, X.; Huang, Z. Brain Single-Nucleus Transcriptomics Highlights That Polystyrene Nanoplastics Potentially Induce Parkinson’s Disease-like Neurodegeneration by Causing Energy Metabolism Disorders in Mice. Journal of Hazardous Materials 2022, 430, 128459. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Yu, H.; Ye, L.; Tian, L.; Zhang, X.; Wang, C.; Li, P.; Ji, H.; Gao, Q.; Dong, S. Nanoplastics Promote Arsenic-Induced ROS Accumulation, Mitochondrial Damage and Disturbances in Neurotransmitter Metabolism of Zebrafish (Danio Rerio). Science of The Total Environment 2023, 863, 161005–161005. [Google Scholar] [CrossRef]
- Kwon, W.; Kim, D.; Kim, H.-Y.; Jeong, S. W.; Lee, S.-G.; Kim, H.-C.; Lee, Y.-J.; Kwon, M. K.; Hwang, J.-S.; Han, J. E.; Park, J.-K.; Lee, S.-J.; Choi, S.-K. Microglial Phagocytosis of Polystyrene Microplastics Results in Immune Alteration and Apoptosis in Vitro and in Vivo. Science of The Total Environment 2022, 807, 150817. [Google Scholar] [CrossRef]
- Wang, G.; Lin, Y.; Shen, H. Exposure to Polystyrene Microplastics Promotes the Progression of Cognitive Impairment in Alzheimer’s Disease: Association with Induction of Microglial Pyroptosis. Molecular neurobiology 2023, 61, 900–907. [Google Scholar] [CrossRef]
- Wang, C.; Lin, K.; Zhang, Z.; Pan, Y.; Miao, Q.; Han, X.; Zhang, Z.; Zhu, P.; Yang, J.; Peng, Y.; Yung, K. K.-L.; Shi, L.; Zhang, S. Adolescent Exposure to Micro/Nanoplastics Induces Cognitive Impairments in Mice with Neuronal Morphological Damage and Multi-Omic Alterations. Environment International 2025, 197, 109323–109323. [Google Scholar] [CrossRef]
- Zhang, R.; Quan, H.; Wang, Y.; Luo, F. Neurogenesis in Primates versus Rodents and the Value of Non-Human Primate Models. National Science Review 2023, 10. [Google Scholar] [CrossRef]
- Obernier, K.; Alvarez-Buylla, A. Neural Stem Cells: Origin, Heterogeneity and Regulation in the Adult Mammalian Brain. Development 2019, 146, dev156059. [Google Scholar] [CrossRef]
- Bonfanti, L.; Chiara La Rosa; Ghibaudi, M.; Sherwood, C. C. Adult Neurogenesis and “Immature” Neurons in Mammals: An Evolutionary Trade-off in Plasticity?. Brain Structure & Function 2023. [CrossRef]
- Arantxa Cebrian-Silla; Nascimento, M. A.; Mancia, W.; Gonzalez-Granero, S.; Romero-Rodriguez, R.; Obernier, K.; Steffen, D. M.; Lim, D. A.; Garcia-Verdugo, J. M.; Alvarez-Buylla, A. Neural Stem Cell Relay from B1 to B2 Cells in the Adult Mouse Ventricular-Subventricular Zone. Cell Reports 2025, 44, 115264–115264. [Google Scholar] [CrossRef]
- Rendell-Bhatti, F.; Paganos, P.; Pouch, A.; Mitchell, C.; D’Aniello, S; Godley, B.J.; Pazdro, K.; Arnone, M.I.; Jimenez-Guri, E. Developmental Toxicity of Plastic Leachates on the Sea Urchin Paracentrotus Lividus. Environmental Pollution 2020, 115744. [CrossRef]
- Santos, D.; Luzio, A.; Félix, L.; Cabecinha, E.; Bellas, J.; Monteiro, S. M. Microplastics and Copper Induce Apoptosis, Alter Neurocircuits, and Cause Behavioral Changes in Zebrafish (Danio Rerio) Brain. Ecotoxicology and Environmental Safety 2022, 242, 113926. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-Y.; Kim, M. S.; Oh, N. Cytotoxicity of Amine-Modified Polystyrene MPs and NPs on Neural Stem Cells Cultured from Mouse Subventricular Zone. Heliyon 2024, 10, e30518–e30518. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Lee, S.; Lee, Y.; Cho, J.-H.; Sou Hyun, Kim; Ha, E.-S.; Jung, Y.-S.; Hae Young, Chung; Kim, M.-S.; Hyung Sik, Kim; Chang, S.-C.; Min, K.-J.; Lee, J. Cationic Nanoplastic Causes Mitochondrial Dysfunction in Neural Progenitor Cells and Impairs Hippocampal Neurogenesis. Free radical biology & medicine 2023, 208, 194–210. [Google Scholar] [CrossRef]
- Prosperi, G.; Marchetti, N.; D’Elia, A.; Massari, R.; Giusto, M.; Pietrodangelo, A.; Rossi, T.; Nucara, A.; Scavizzi, F.; Strimpakos, G.; Marinelli, S.; Mandillo, S.; D’Amato, F. R.; Farioli-Vecchioli, S. Inhalation of Nanoplastics in the Mouse Model: Tissue Bio-Distribution and Effects on the Olfactory System. The Science of the total environment 2025, 968, 178853. [Google Scholar] [CrossRef]
- Prüst, M.; Meijer, J.; Westerink, R. H. S. The Plastic Brain: Neurotoxicity of Micro- and Nanoplastics. Particle and Fibre Toxicology 2020, 17. [Google Scholar] [CrossRef]
- Winiarska, E.; Jutel, M.; Zemelka-Wiacek, M. The Potential Impact of Nano- and Microplastics on Human Health: Understanding Human Health Risks. Environmental Research 2024, 251, 118535–118535. [Google Scholar] [CrossRef]
- Santos, D.; Luzio, A.; Bellas, J.; Monteiro, S. M. Microplastics- and Copper-Induced Changes in Neurogenesis and DNA Methyltransferases in the Early Life Stages of Zebrafish. Chemico-Biological Interactions 2022, 363, 110021. [Google Scholar] [CrossRef]
- Jung, B.-K.; Han, S.-W.; Park, S.-H.; Bae, J.-S.; Choi, J.; Ryu, K.-Y. Neurotoxic Potential of Polystyrene Nanoplastics in Primary Cells Originating from Mouse Brain. NeuroToxicology 2020, 81, 189–196. [Google Scholar] [CrossRef]
- So, Y. H.; Shin, H. S.; Lee, S. H.; Moon, H. J.; Jang, H. J.; Lee, E.-H.; Jung, E.-M. Maternal Exposure to Polystyrene Microplastics Impairs Social Behavior in Mouse Offspring with a Potential Neurotoxicity. NeuroToxicology 2023, 99, 206–216. [Google Scholar] [CrossRef]
- Yang, D.; Zhu, J.; Zhou, X.; Pan, D.; Nan, S.; Yin, R.; Lei, Q.; Ma, N.; Zhu, H.; Chen, J.; Han, L.; Ding, M.; Ding, Y. Polystyrene Micro- and Nano-Particle Coexposure Injures Fetal Thalamus by Inducing ROS-Mediated Cell Apoptosis. Environment International 2022, 166, 107362. [Google Scholar] [CrossRef]
- Ryoo, H. D.; Bergmann, A. The Role of Apoptosis-Induced Proliferation for Regeneration and Cancer. Cold Spring Harbor Perspectives in Biology 2012, 4, a008797–a008797. [Google Scholar] [CrossRef]
- Petrenko, V.; Mihhailova, J.; Salmon, P.; Kiss, J. Z. Apoptotic Neurons Induce Proliferative Responses of Progenitor Cells in the Postnatal Neocortex. Experimental Neurology 2015, 273, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Gratzner, H. Monoclonal Antibody to 5-Bromo- and 5-Iododeoxyuridine: A New Reagent for Detection of DNA Replication. Science 1982, 218, 474–475. [Google Scholar] [CrossRef] [PubMed]
- Nowakowski, R. S.; Lewin, S. B.; Miller, M. W. Bromodeoxyuridine Immunohistochemical Determination of the Lengths of the Cell Cycle and the DNA-Synthetic Phase for an Anatomically Defined Population. Journal of Neurocytology 1989, 18, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Altman, J.; Bayer, S.A. Development of the cerebellar system in relation to its evolution. structure, and functions. CRC Press, Boca Raton, 1997.
- Joaquín Martí-Clúa. Developmental Timetables and Gradients of Neurogenesis in Cerebellar Purkinje Cells and Deep Glutamatergic Neurons: A Comparative Study between the Mouse and the Rat. The Anatomical Record 2021, 304, 2856–2864. [Google Scholar] [CrossRef]
- J., Martí-Clua. Times of Neuron Origin and Neurogenetic Gradients in Mice Purkinje Cells and Deep Cerebellar Nuclei Neurons during the Development of the Cerebellum. A Review. Tissue and Cell 2022, 78, 101897–101897. [Google Scholar] [CrossRef]
- Dori, I.; Bekiari, C.; Grivas, I.; Tsingotjidou, A.; Papadopoulos, G. C. Birth and Death of Neurons in the Developing and Mature Mammalian Brain. The International Journal of Developmental Biology 2022, 66, 9–22. [Google Scholar] [CrossRef]
- Svandova, E.; Lesot, H.; Sharpe, P.; Matalova, E. Making the Head: Caspases in Life and Death. Frontiers in Cell and Developmental Biology 2023, 10. [Google Scholar] [CrossRef]
- Ryu, J. R.; Hong, C. J.; Kim, J. Y.; Kim, E.-K.; Sun, W.; Yu, S.-W. Control of Adult Neurogenesis by Programmed Cell Death in the Mammalian Brain. Molecular Brain 2016, 9. [Google Scholar] [CrossRef]
- Cameron, H. A.; Mckay, R. D. G. Adult Neurogenesis Produces a Large Pool of New Granule Cells in the Dentate Gyrus. The Journal of Comparative Neurology 2001, 435, 406–417. [Google Scholar] [CrossRef]
- Nandakumar, S.; Rozich, E.; Buttitta, L. Cell Cycle Re-Entry in the Nervous System: From Polyploidy to Neurodegeneration. Frontiers in Cell and Developmental Biology 2021, 9. [Google Scholar] [CrossRef]
- Pavulraj, S.; Stout, R. W.; Paulsen, D. B.; Chowdhury, S. I. Live Triple Gene-Deleted Pseudorabies Virus-Vectored Subunit PCV2b and CSFV Vaccine Undergoes an Abortive Replication Cycle in the TG Neurons Following Latency Reactivation. Viruses 2023, 15, 473. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Blain, S. W. S Phase Entry Causes Homocysteine-Induced Death While Ataxia Telangiectasia and Rad3 Related Protein Functions Anti-Apoptotically to Protect Neurons. Brain 2010, 133, 2295–2312. [Google Scholar] [CrossRef] [PubMed]
- Kuan, C.-Y. Hypoxia-Ischemia Induces DNA Synthesis without Cell Proliferation in Dying Neurons in Adult Rodent Brain. Journal of Neuroscience 2004, 24, 10763–10772. [Google Scholar] [CrossRef]
- Taupin, P. BrdU Immunohistochemistry for Studying Adult Neurogenesis: Paradigms, Pitfalls, Limitations, and Validation. Brain Research Reviews 2007, 53, 198–214. [Google Scholar] [CrossRef]
- Lucía Rodríguez-Vázquez; Joaquín Martí. Administration of 5-Bromo-2′-Deoxyuridine Interferes with Neuroblast Proliferation and Promotes Apoptotic Cell Death in the Rat Cerebellar Neuroepithelium. The Journal of Comparative Neurology 2020, 529, 1081–1096. [Google Scholar] [CrossRef]
- Calcinotto, A.; Kohli, J.; Zagato, E.; Pellegrini, L.; Demaria, M.; Alimonti, A. Cellular Senescence: Aging, Cancer, and Injury. Physiological Reviews 2019, 99, 1047–1078. [Google Scholar] [CrossRef]
- Rhinn, M.; Ritschka, B.; Keyes, W. M. Cellular Senescence in Development, Regeneration and Disease. Development 2019, 146, dev151837. [Google Scholar] [CrossRef]
- Wang, Y.; Kuca, K.; You, L.; Nepovimova, E.; Heger, Z.; Valko, M.; Adam, V.; Wu, Q.; Klaudia Jomova. The Role of Cellular Senescence in Neurodegenerative Diseases. Archives of Toxicology 2024. [Google Scholar] [CrossRef] [PubMed]
- Saugat Shiwakoti; Ko, J. -Y.; Gong, D.-S.; Bikalpa Dhakal; Jeong Min Lee; Adhikari, R.; Yeonhyang Gwak; Park, S.-H.; Ik Jun Choi; Schini-Kerth, V. B.; Kang, K.-W.; Oak, M.-H. Effects of Polystyrene Nanoplastics on Endothelium Senescence and Its Underlying Mechanism. 2022, 164, 107248–107248. [CrossRef]
- Jin, W.; Zhang, W.; Tang, H.; Wang, P.; Yan, Z.; Liu, S.; Qiu, J.; Chen, H.; Wang, L.; Wang, R.; Sun, Y.; Liu, P.; Tang, H.; Zhu, Y. Microplastics Exposure Causes the Senescence of Human Lung Epithelial Cells and Mouse Lungs by Inducing ROS Signaling. Environment international 2024, 108489–108489. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Xiao, T.; Sun, X.; Song, Y.; Shi, W.; Lu, K.; Chen, D.; Sun, C.; Bian, Q. The Regulation of CircRNA_kif26b on Alveolar Epithelial Cell Senescence via MiR-346-3p Is Involved in Microplastics-Induced Lung Injuries. Science of The Total Environment 2023, 882, 163512–163512. [Google Scholar] [CrossRef]
- Pan, C.; Wang, X.; Fan, Z.; Mao, W.; Shi, Y.; Wu, Y.; Liu, T.; Xu, Z.; Wang, H.; Chen, H. Polystyrene Microplastics Facilitate Renal Fibrosis through Accelerating Tubular Epithelial Cell Senescence. Food and Chemical Toxicology 2024, 191, 114888. [Google Scholar] [CrossRef]
- Michishita Eriko; Nakabayashi, K. ; Suzuki, T.; Kaul, S. C.; Ogino, H.; Fujii, M.; Mitsui, Y.; Dai Ayusawa. 5-Bromodeoxyuridine Induces Senescence-like Phenomena in Mammalian Cells regardless of Cell Type or Species. 1999, 126, 1052–1059. [CrossRef]
- Ross, H. H.; Levkoff, L. H.; Marshall, G. P.; Caldeira, M.; Steindler, D. A.; Reynolds, B. A.; Laywell, E. D. Bromodeoxyuridine Induces Senescence in Neural Stem and Progenitor Cells. STEM CELLS 2008, 26, 3218–3227. [Google Scholar] [CrossRef]
- Wataru, Satou; Suzuki, T.; Noguchi, T.; Ogino, H.; Fujii, M.; Dai, Ayusawa. AT-Hook Proteins Stimulate Induction of Senescence Markers Triggered by 5-Bromodeoxyuridine in Mammalian Cells. Experimental Gerontology 2003, 39, 173–179. [Google Scholar] [CrossRef]
- Guerrero, A. Nucleosome Disruption by 5-Bromodeoxyuridine Leads to Senescence. The FEBS Journal 2022, 290, 684–687. [Google Scholar] [CrossRef]
- En, A.; Watanabe, K.; Dai, Ayusawa; Fujii, M. The Key Role of a Basic Domain of Histone H2B N-Terminal Tail in the Action of 5-Bromodeoxyuridine to Induce Cellular Senescence. The FEBS Journal 2022, 290, 692–711. [Google Scholar] [CrossRef]
- Mattei, A. L.; Bailly, N.; Meissner, A. DNA Methylation: A Historical Perspective. Trends in Genetics 2022, 38, 676–707. [Google Scholar] [CrossRef] [PubMed]
- Chera, A.; Mircea, Stancu-Cretu; Nicolae Radu, Zabet; Bucur, O. Shedding Light on DNA Methylation and Its Clinical Implications: The Impact of Long-Read-Based Nanopore Technology. Epigenetics & Chromatin 2024, 17. [Google Scholar] [CrossRef]
- Masiá, P.; Ardura, A.; García-Vázquez, E. Virgin Polystyrene Microparticles Exposure Leads to Changes in Gills DNA and Physical Condition in the Mediterranean Mussel Mytilus Galloprovincialis. Animals 2021, 11, 2317. [Google Scholar] [CrossRef]
- Menéndez, D.; Blanco-Fernandez, C.; Machado-Schiaffino, G.; Ardura, A.; Garcia-Vazquez, E. High Microplastics Concentration in Liver Is Negatively Associated with Condition Factor in the Benguela Hake Merluccius Polli. Ecotoxicology and Environmental Safety 2023, 262, 115135. [Google Scholar] [CrossRef]
- Wright, S. L.; Thompson, R. C.; Galloway, T. S. The Physical Impacts of Microplastics on Marine Organisms: A Review. Environmental Pollution 2013, 178, 483–492. [Google Scholar] [CrossRef]
- Im, J.; Eom, H.-J.; Choi, J. Effect of Early-Life Exposure of Polystyrene Microplastics on Behavior and DNA Methylation in Later Life Stage of Zebrafish. Archives of Environmental Contamination and Toxicology 2022. [Google Scholar] [CrossRef]
- Ortiz-Moriano, M. P.; Masiá, P.; Acle, S.; Ardura, A.; Garcia-Vazquez, E.; Machado-Schiaffino, G. Changes in Global Methylation Patterns of Mytilus Galloprovincialis Exposed to Microplastics. Aquatic Toxicology 2024, 276, 107115–107115. [Google Scholar] [CrossRef]
- Farag, A. A.; Youssef, H. S.; Sliem, R. E.; El, B.; Nashwa, Nabil; Mouktar, M. M.; Marei, Y. M.; Ismail, N. S.; Radwaan, S. E.; Badr, A. M.; Sayed, H. Hematological Consequences of Polyethylene Microplastics Toxicity in Male Rats: Oxidative Stress, Genetic, and Epigenetic Links. Toxicology 2023, 492, 153545–153545. [Google Scholar] [CrossRef]
- Jiang, Z.; Lai, Y.; Beaver, J. M.; Tsegay, P. S.; Zhao, M.-L.; Horton, J. K.; Zamora, M.; Rein, H. L.; Miralles, F.; Shaver, M.; Hutcheson, J. D.; Agoulnik, I.; Wilson, S. H.; Liu, Y. Oxidative DNA Damage Modulates DNA Methylation Pattern in Human Breast Cancer 1 (BRCA1) Gene via the Crosstalk between DNA Polymerase β and a de Novo DNA Methyltransferase. Cells 2020, 9, 225–225. [Google Scholar] [CrossRef]
- Schneider, L.; d’Adda di Fagagna, F. Neural Stem Cells Exposed to BrdU Lose Their Global DNA Methylation and Undergo Astrocytic Differentiation. Nucleic Acids Research 2012, 40, 5332–5342. [Google Scholar] [CrossRef]
- Morris, S. M.; Domon, O. E.; McGarrity, L. J.; Kodelo, R. L.; Casciano, D. A. Effect of Bromodeoxyuridine on the Proliferation and Growth of Ethyl Methanesulfonate-Exposed P3 Cells: Relationship to the Induction of Sister-Chromatid Exchanges. Cell Biology and Toxicology 1992, 8, 75–87. [Google Scholar] [CrossRef] [PubMed]
- En, A.; Watanabe, K.; Dai, Ayusawa; Fujii, M. The Key Role of a Basic Domain of Histone H2B N-Terminal Tail in the Action of 5-Bromodeoxyuridine to Induce Cellular Senescence. The FEBS Journal 2022, 290, 692–711. [Google Scholar] [CrossRef]
- Zhao, X.; van Praag, H. Steps towards Standardized Quantification of Adult Neurogenesis. Nature Communications 2020, 11. [Google Scholar] [CrossRef]
- Sorrells, S. F.; Paredes, M. F.; Zhang, Z.; Kang, G.; Pastor-Alonso, O.; Biagiotti, S.; Page, C. E.; Sandoval, K.; Knox, A.; Connolly, A.; Huang, E. J.; Garcia-Verdugo, J. M.; Oldham, M. C.; Yang, Z.; Alvarez-Buylla, A. Positive Controls in Adults and Children Support That Very Few, If Any, New Neurons Are Born in the Adult Human Hippocampus. Journal of Neuroscience 2021, 41, 2554–2565. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




