Submitted:
09 July 2025
Posted:
10 July 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
2.1. Physicochemical Properties
2.1.1. Moisture Content
2.1.2. Polarity
2.1.3. Conductivity
2.1.4. pH
2.1.5. Viscosity
2.2. Antioxidant Property
2.2.1. DPPH Radical Scavenging Activity
2.2.2. ABTS Radical Scavenging Activity
2.2.3. Fe2+ Chelating Activity
2.3. Antibacterial Property
2.3.1. Antibacterial Zone
2.3.1. The MIC of CADESs
2.4. The CADES on the Germination Toxicity of Mung Beans
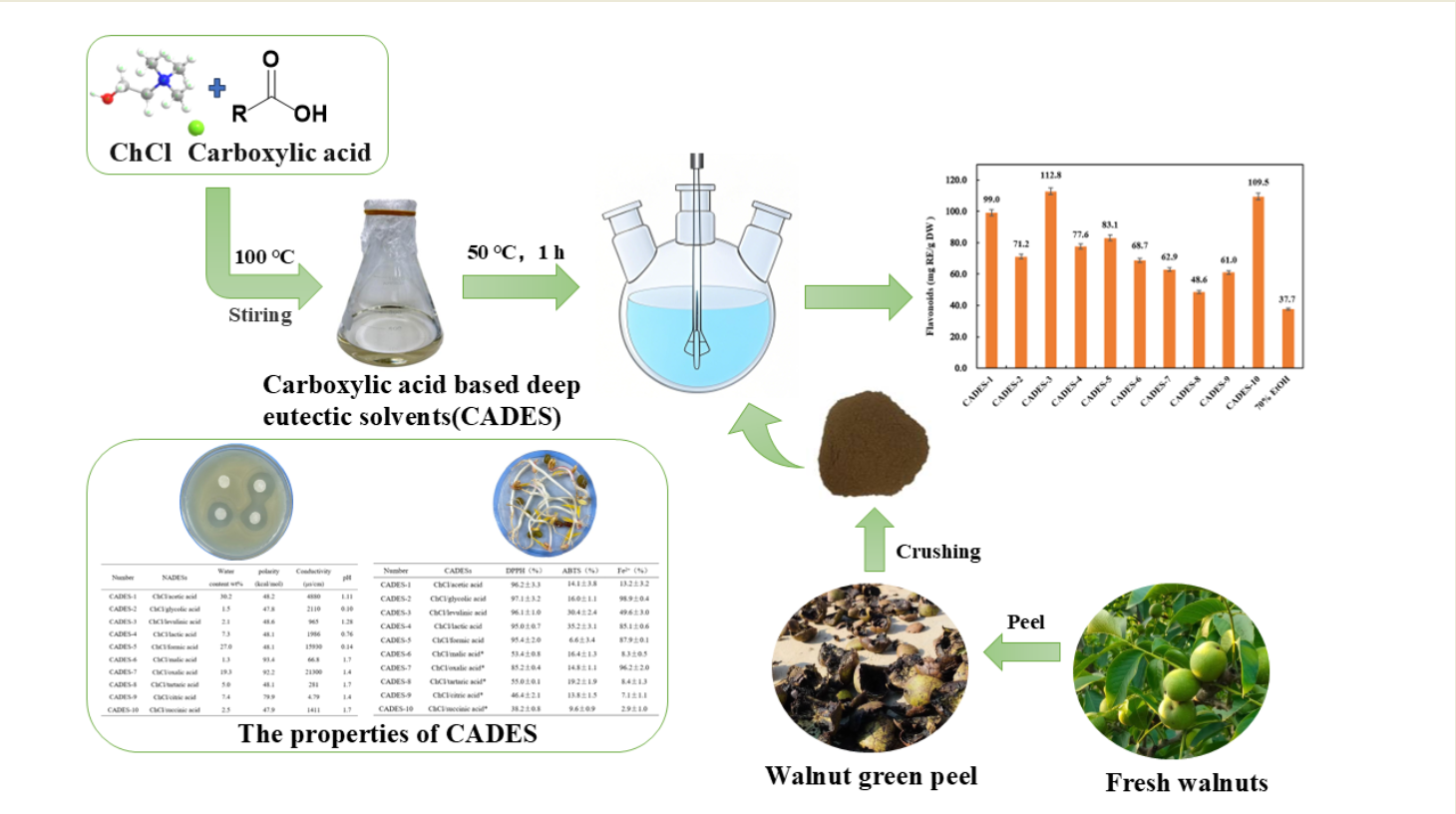
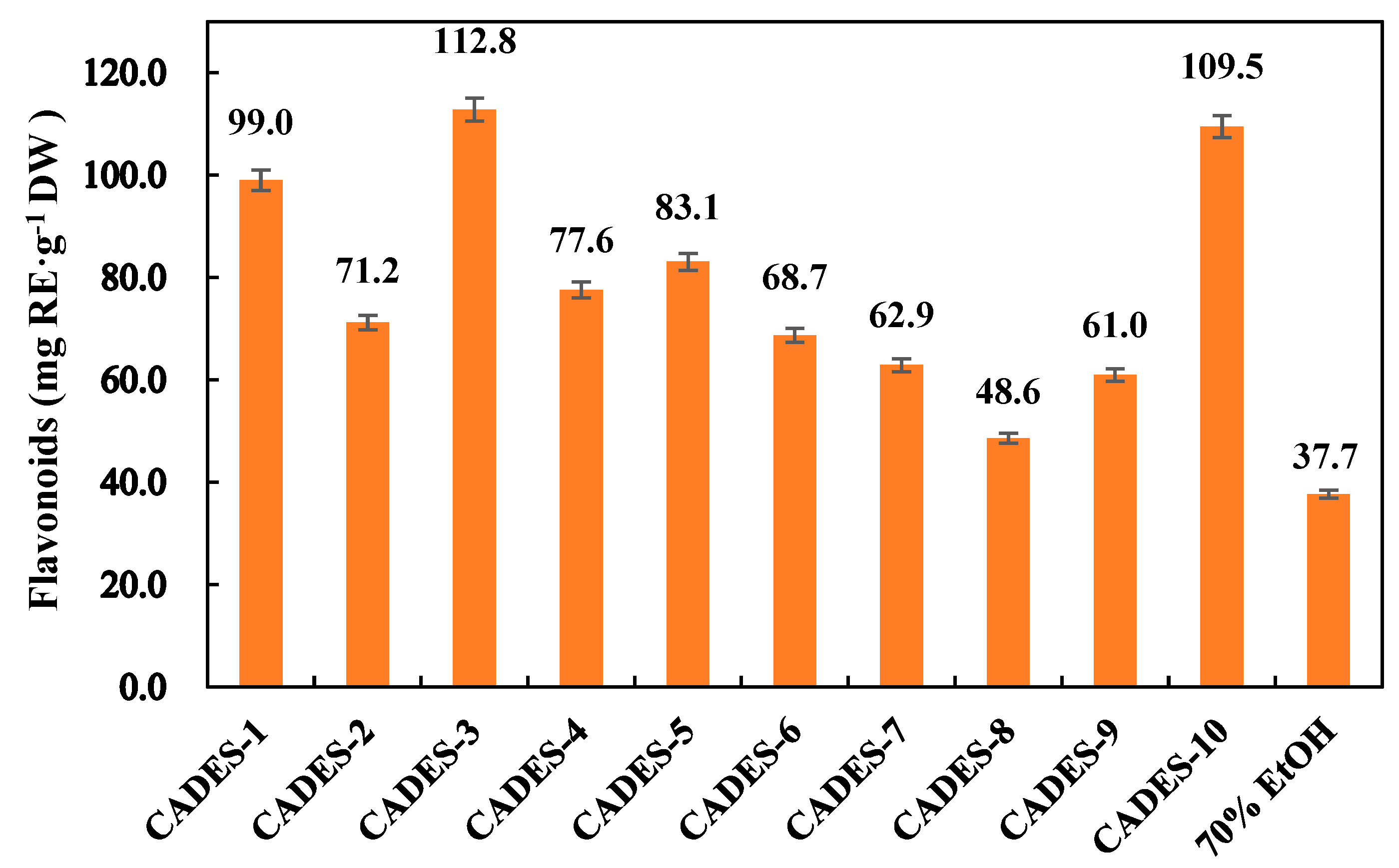
2.5. Extract Flavonoids from Walnut Green Peel Using CADESs
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of CADESs
3.3. Physicochemical Properties
3.3.1. pH Measurement
3.3.2. Electrical Conductivity Determination
3.3.3. Viscosity Determination
3.3.4. Moisture Content Determination
3.3.5. Polarity Determination
3.4. Antioxidant Activity
3.4.1. DPPH Radical Scavenging Activity
3.4.2. ABTS Radical Scavenging Activity
3.4.3. Fe2+ Chelating Activity
3.5. Antibacterial Property
3.5.1. Antibacterial Zone
3.5.2. Determination of the MIC of CADES
3.6. The Impact of CADES on the Germination Toxicity of Mung Beans
3.7. Extraction and Determination of Flavonoids from Walnut Green Peel
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DESs | Deep eutectic solvents |
| CADESs | Carboxylic acid-based deep eutectic solvents |
| NADESs | Natural deep eutectic solvents |
| HBAs | Hydrogen bond acceptors |
| HBDs | Hydrogen bond donors |
| ILs | Ionic liquids |
| ChCl | Choline chloride |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| ABTS | 3-ethylbenzothiazoline-6-sulfonic acid |
| LB | Luria-Bertani |
| TFC | Total flavonoid content |
| MIC | Minimum inhibitory concentration |
| RGP | Relative germination rate |
| AGR | Average germination rate |
| HAT | Hydrogen atom transfer |
| mg RE·g-1 DW | mg Rutin Equivalent·g-1 Dry Weight |
References
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 2014, 114, 11060-11082. [CrossRef]
- Dong, Q.H.; Cao, J.; Chen, L.Y.; Cao, J.R.; Wang, H.M.; Cao, F.L.; Su, E.Z. Solubilization of phytocomplex using natural deep eutectic solvents: A case study of Ginkgo biloba leaves extract. Ind. Crops. Prod. 2022, 177, 114455. [CrossRef]
- Fan, Y.C.; Luo, H.; Zhu, C.Y.; Li, W.J.; Wu, D.; Wu, H.W. Hydrophobic natural alcohols based deep eutectic solvents: Effective solvents for the extraction of quinine. Sep. Purif. Technol. 2021, 275, 119112. [CrossRef]
- Vanda, H.; Dai, Y.T.; Wilson, E.G.; Verpoorte, R.; Choi, Y.H. Green solvents from ionic liquids and deep eutectic solvents to natural deep eutectic solvents. CR. Chim. 2018, 21, 628-638. [CrossRef]
- Pan, Z.C.; Bo, Y.Y.; Liang, Y.H.; Lu, B.B.; Zhan, J.B.; Zhang, J.C.; Zhang, J.H. Intermolecular interactions in natural deep eutectic solvents and their effects on the ultrasound-assisted extraction of artemisinin from Artemisia annua. J. Mol. Liq. 2021, 326, 115283. [CrossRef]
- Chen, X.Q.; Li, Z.H.; Liu, L.L.; Wang, H.; Yang, S.H.; Zhang, J.S.; Zhang, Y. Green extraction using deep eutectic solvents and antioxidant activities of flavonoids from two fruits of Rubia species. LWT-food. Sci. Technol. 2021, 148, 111708. [CrossRef]
- Zhang, X.J.; Liu, Z.T.; Chen, X.Q.; Zhang, T.T.; Zhang, Y. Deep eutectic solvent combined with ultrasound technology: A promising integrated extraction strategy for anthocyanins and polyphenols from blueberry pomace. Food. Chem. 2023, 422, 136224. [CrossRef]
- Hou, Y.J.; Wang, P.W.; Zhang, H.; Fan, Y.Y.; Cao, X.; Luo, Y.Q.; Li, Q.; Njolibimi, M.; Li, W.J.; Hong, B. A high-permeability method for extracting purple yam saponins based on ultrasonic-assisted natural deep eutectic solvent. Food. Chem. 2024, 457, 140046. [CrossRef]
- Oliveira, I.; Sousa, A.; Ferreira, I.C.F.R.; Bento, A.; Estevinho, L.; Pereira, J.A. Total phenols, antioxidant potential and antimicrobial activity of walnut (Juglans regia L.) green husks. Food. Chem. Toxicol. 2008, 46, 2326-2331. [CrossRef]
- ZamaniBahramabadi, E.; Afshar, H.M.; Rezanejad, F. Chemical constituents of green peel of Persian walnut (Juglans regia L.) fruit. Biomass. Convers. Bior. 2024, 14, 27519-27524. [CrossRef]
- Karadaş F., Gültepe N. Effects of walnut (Juglans regia) green peel extract on growth performance and challenge to enteric redmouth disease (Yersinia ruckeri) in rainbow trout (Oncorhynchus mykiss). Isr. J. Aquacult-Bamid. 2025, 77, 90-96. [CrossRef]
- Moreira, C.D.; Santos, T.B.; Freitas, R.H.C.N.; Pacheco, P.A.F.; da Rocha, D.R. Juglone: A versatile natural platform for obtaining new bioactive compounds. Curr. Top. Med. Chem. 2021, 21, 2018-2045. [CrossRef]
- Liga, S.; Paul, C.; Peter, F. Flavonoids: Overview of biosynthesis, biological activity, and current extraction techniques. Plants-Basel. 2023, 12, 2732. [CrossRef]
- Okoye, C.O.; Jiang, H.F.; Wu, Y.F.; Li, X.; Gao, L.; Wang, Y.L.; Jiang, J.X. Bacterial biosynthesis of flavonoids: Overview, current biotechnology applications, challenges, and prospects. J. Cell. Physiol. 2024, 239, e31006. [CrossRef]
- Dai, Y.T.; van Spronsen, J.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta. 2013, 766, 61-68. [CrossRef]
- Wei, J.H.; Zhao, G.J.; Wu, G.D.; Bo, Y.K.; Yang, D.; Guo, J.J.; Ma, Y.; An, M. An ultrasound-assisted extraction using an alcohol-based hydrophilic natural deep eutectic solvent for the determination of five flavonoids from Platycladi Cacumen. Microchem. J. 2024, 198, 110076. [CrossRef]
- Rashid, R.; Wani, S.M.; Manzoor, S.; Masoodi, F.A.; Dar, M.M. Green extraction of bioactive compounds from apple pomace by ultrasound assisted natural deep eutectic solvent extraction: Optimisation, comparison and bioactivity. Food. Chem. 2023, 398, 133871. [CrossRef]
- Meenu, M.; Bansal, V.; Rana, S.; Sharma, N.; Kumar, V.; Arora, V.; Garg, M. Deep eutectic solvents (DESs) and natural deep eutectic solvents (NADESs): Designer solvents for green extraction of anthocyanin. Sustain. Chem. Pharm. 2023, 34, 101168. [CrossRef]
- Roda, A.; Santos, F.; Chua, Y.Z.; Kumar, A.; Do, H.T.; Paiva, A.; Duarte, A.R.C.; Held, C. Unravelling the nature of citric acid: l-arginine: water mixtures: the bifunctional role of water. Phys. Chem. Chem. Phys. 2021, 23, 1706-1717. [CrossRef]
- Wang, Y.B.; Li, Q.; Hong, H.; Yang, S.; Zhang, R.; Wang, X.Q.; Jin, X.; Xiong, B.; Bai, S.C.; Zhi, C.Y. Lean-water hydrogel electrolyte for zinc ion batteries. Nat. Commun. 2023, 14, 3890. [CrossRef]
- Omar, K.A.; Sadeghi, R. Physicochemical properties of deep eutectic solvents: A review. J. Mol. Liq. 2022, 360, 119524. [CrossRef]
- Manasi, I.; Bryant, S.J.; Hammond, O.S.; Edler, K.J. Interactions of water and amphiphiles with deep eutectic solvent nanostructures. Eutectic Solvents and Stress in Plants. 2021, 97, 41-68. [CrossRef]
- Shelembe, J.S.; Cromarty, D.; Bester, M.; Minnaar, A.; Duodu, K.G. Effect of acidic condition on phenolic composition and antioxidant potential of aqueous extracts from sorghum (Sorghum bicolor) bran. J. Food. Biochem. 2014, 38, 110-118. [CrossRef]
- Bashir, I.; Dar, A.H.; Dash, K.K.; Pandey, V.K.; Fayaz, U.; Shams, R.; Srivastava, S.; Singh, R. Deep eutectic solvents for extraction of functional components from plant-based products: A promising approach. Sustain. Chem. Pharm. 2023, 33, 101102. [CrossRef]
- Pinho, M.R.; Lima, A.S.; Oliveira, G.D.R.; Liao, L.M.; Franceschi, E.; da Silva, R.; Cardozo, L. Choline chloride- and organic acids-based deep eutectic solvents: Exploring chemical and thermophysical properties. J. Chem. Eng. Data. 2024, 69, 3403-3414. [CrossRef]
- Yusof, R.; Abdulmalek, E.; Sirat, K.; Rahman, M.B.A. Tetrabutylammonium bromide (TBABr)-based deep eutectic solvents (DESs) and their physical properties. Molecules. 2014, 19, 8011-8026. [CrossRef]
- Maciel, E.N.; Almeida, S.K.C.; da Silva, S.C.; de Souza, G.L.C. Examining the reaction between antioxidant compounds and 2,2-diphenyl-1-picrylhydrazyl (DPPH) through a computational investigation. J. Mol. Model. 2018, 24, 218. [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical methods used in determining antioxidant activity: A review. Int. J. Mol. Sci. 2021, 22, 3380. [CrossRef]
- Dai, J.; Liu, Y.M.; Li, J.; Lu, Z.Y.; Yang, W.S. Phenanthroline method for quantitative determination of surface carboxyl groups on carboxylated polystyrene particles with high sensitivity. Surf. Interface. Anal. 2009, 41, 577-580. [CrossRef]
- Bedair, H.M.; Samir, T.M.; Mansour, F.R. Antibacterial and antifungal activities of natural deep eutectic solvents. Appl. Microbiol. Biot. 2024, 108, 198. [CrossRef]
- Wen, Q.; Chen, J.X.; Tang, Y.L.; Wang, J.; Yang, Z. Assessing the toxicity and biodegradability of deep eutectic solvents. Chemosphere. 2015, 132, 63-69. [CrossRef]
- Taysun, M.B.; Sert, E.; Atalay, F.S. Effect of hydrogen bond donor on the physical properties of benzyltriethylammonium chloride based deep eutectic solvents and their usage in 2-ethyl-hexyl acetate synthesis as a catalyst. J. Chem. Eng. Data. 2017, 62, 1173-1181. [CrossRef]
- Silva, J.M.; Silva, E.; Reis, R.L.; Duarte, A.R.C. A closer look in the antimicrobial properties of deep eutectic solvents based on fatty acids. Sustain. Chem. Pharm. 2019, 14, 100192. [CrossRef]
- Martínez, G.M.; Townley, G.G.; Martínez-Espinosa, R.M. Controversy on the toxic nature of deep eutectic solvents and their potential contribution to environmental pollution. Heliyon. 2022, 8, e12567. [CrossRef]
- Thinh, B.B.; Thin, D.B.; Ogunwande, I.A. Chemical composition, antimicrobial and antioxidant properties of essential oil from the leaves of Vernonia volkameriifolia DC. Nat. Prod. Commun. 2024, 19, 1934578X241239477. [CrossRef]
- Oke, E.A.; Ijardar, S.P. Advances in the application of deep eutectic solvents based aqueous biphasic systems: An up-to-date review. Biochem. Eng. J. 2021, 176, 108211. [CrossRef]
- Li, P.F.; Liu, C.L.; Luo, Y.; Shi, H.N.; Li, Q.; PinChu, C.E.; Li, X.J.; Yang, J.L.; Fan, W. Oxalate in plants: Metabolism, function, regulation, and application. J. Agric. Food. Chem. 2022, 70, 16037-16049. [CrossRef]
- Atilhan, M.; Costa, L.T.; Aparicio, S. On the behaviour of aqueous solutions of deep eutectic solvents at lipid biomembranes. J. Mol. Liq. 2017, 247, 116-125. [CrossRef]
- Stanisz, M.; Stanisz, B.J.; Cielecka-Piontek, J. A comprehensive review on deep eutectic solvents: Their current status and potential for extracting active compounds from adaptogenic plants. Molecules. 2024, 29, 4767. [CrossRef]
- Wu, C.Y.; Wang, B.R.; Han, S.Y.; Zhang, Y.H.; Mu, Z.S. Extraction of hydroxy safflower yellow A and anhydroxysafflor yellow B from safflower florets using natural deep eutectic solvents: Optimization, biological activity and molecular docking. Food. Biosci. 2025, 68, 106418. [CrossRef]
- Hao, Y.; Pei, F.X.; Huang, J.J.; Li, G.Z.; Zhong, C.L. Application of deep eutectic solvents on extraction of flavonoids. J. Sep. Sci. 2024, 47, 2300925. [CrossRef]
- Chen, J.N.; Li, Y.; Wang, X.P.; Liu, W. Application of deep eutectic solvents in food analysis: A review. Molecules. 2019, 24, 4594. [CrossRef]
- Airouyuwa, J.O.; Mostafa, H.; Ranasinghe, M.; Maqsood, S. Influence of physicochemical properties of carboxylic acid-based natural deep eutectic solvents (CA-NADES) on extraction and stability of bioactive compounds from date (Phoenix dactylifera L.) seeds: An innovative and sustainable extraction technique. J. Mol. Liq. 2023, 388, 122767. [CrossRef]
- Ogihara, W.; Aoyama, T.; Ohno, H. Polarity measurement for ionic liquids containing dissociable protons. Chem. Lett. 2004, 33, 1414-1415. [CrossRef]
- Sarikurkcu, C.; Kirkan, B.; Ozer, M.S.; Ceylan, O.; Atilgan, N.; Cengiz, M.; Tepe, B. Chemical characterization and biological activity of onosma gigantea extracts. Ind. Crop. Prod. 2018, 115, 323-329. [CrossRef]
- Jia, M.Z.; Fu, X.Q.; Deng, L.; Li, Z.L.; Dang, Y.Y. Phenolic extraction from grape (Vitis vinifera) seed via enzyme and microwave co-assisted salting-out extraction. Food Biosci. 2021, 40, 100919. [CrossRef]
- Papuc, C.; Crivineanu, M.; Goran, G.; Nicorescu, V.; Durdun, N. Free radicals scavenging and antioxidant activity of European Mistletoe (Viscum album) and European Birthwort (Aristolochia clematitis). Rev. Chim-Bucharest. 2010, 61, 619-622. .
- Dlugosz, O.; Chmielowiec-Korzeniowska, A.; Drabik, A.; Tymczyna, L.; Banach, M. Bioactive selenium nanoparticles synthesized from propolis extract and quercetin based on natural deep eutectic solvents (NDES). J. Clust. Sci. 2023, 34, 1401-1412. [CrossRef]
- Dai, Y.T.; Varypataki, E.M.; Golovina, E.A.; Jiskoot, W.; Witkamp, G.J.; Choi, Y.H.; Verpoorte, R. Natural deep eutectic solvents in plants and plant cells: In vitro evidence for their possible functions. Eutectic Solvents and Stress in Plants. 2021, 97, 159-184. [CrossRef]
- Wang, Z.W.; Wang, D.D.; Fang, J.X.; Song, Z.X.; Geng, J.M.; Zhao, J.F.; Fang, Y.F.; Wang, C.T.; Li, M. Green and efficient extraction of flavonoids from perilla frutescens (l.) britt. Leaves based on natural deep eutectic solvents: Process optimization, component identification, and biological activity. Food. Chem. 2024, 452, 139508. [CrossRef]
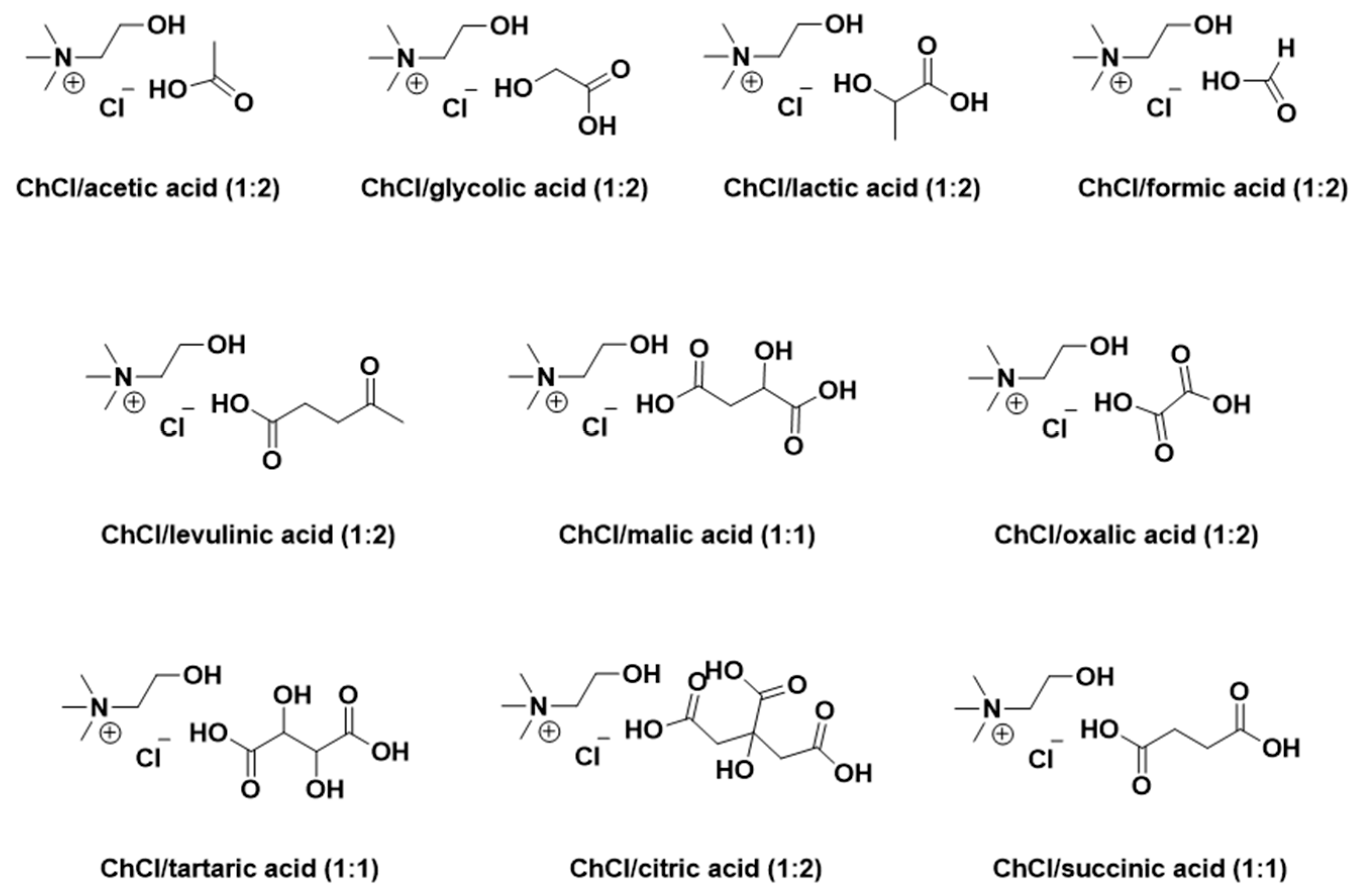
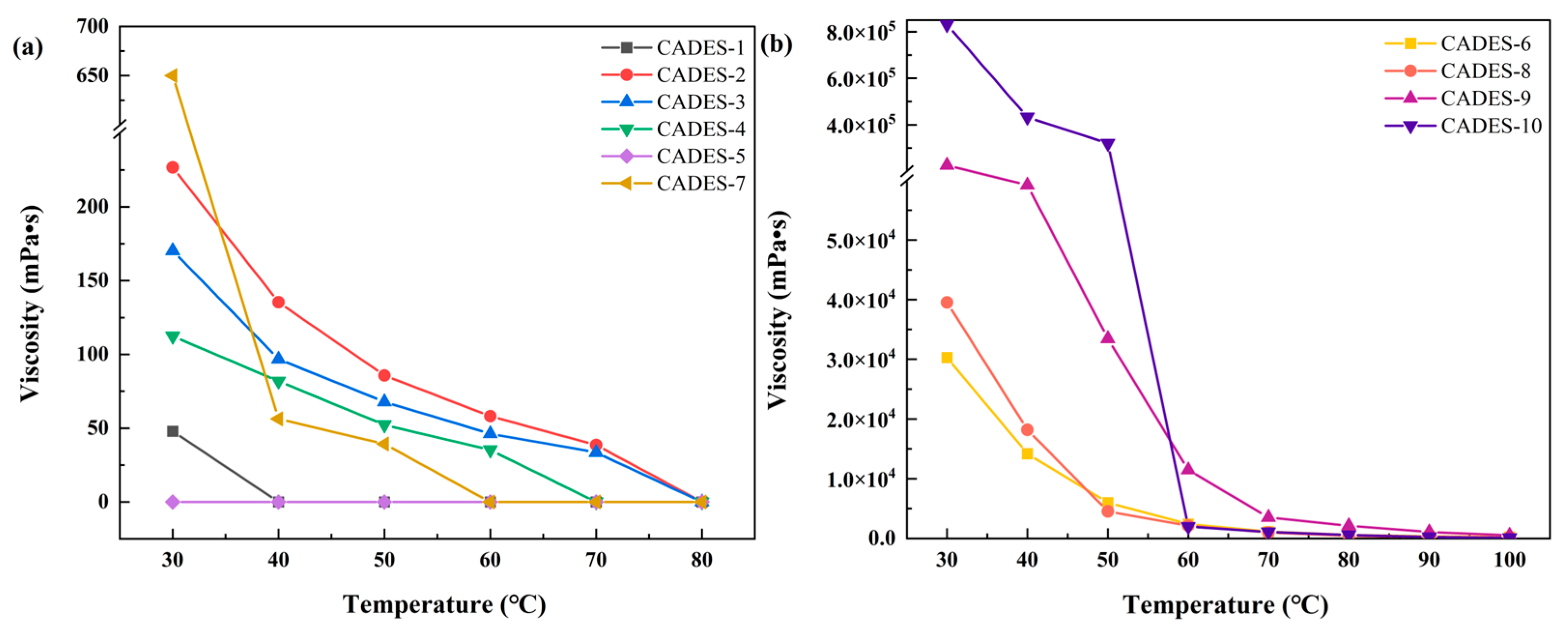
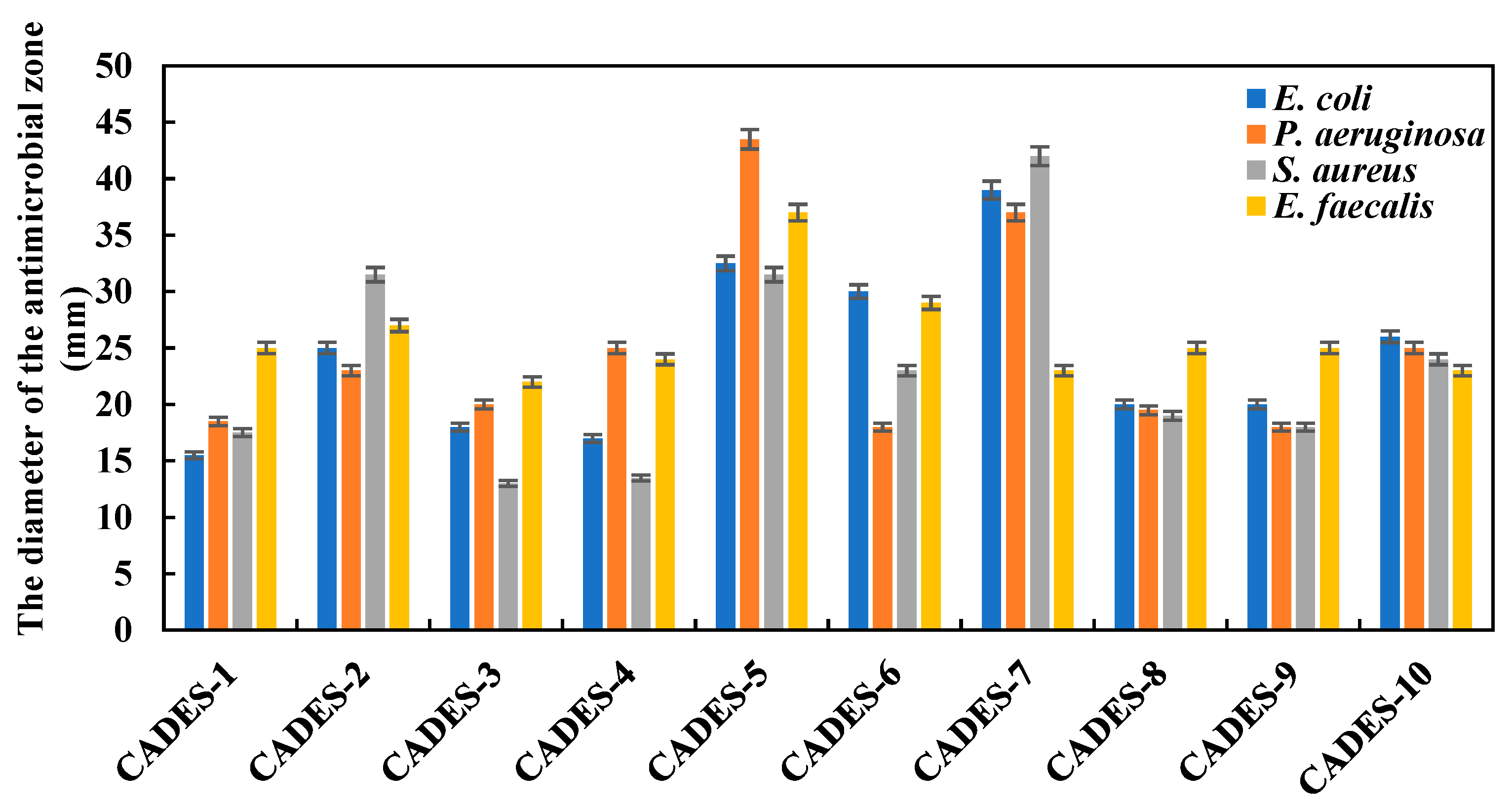

| Number | CADESs | Moisture content (wt%) | Polarity (kcal·mol-1) |
Conductivity (μs·cm-1) |
pH |
|---|---|---|---|---|---|
| CADES-1 | ChCl/acetic acid | 30.2 | 48.2 | 4880 | 1.11 |
| CADES-2 | ChCl/glycolic acid | 1.5 | 47.8 | 2110 | 0.10 |
| CADES-3 | ChCl/levulinic acid | 2.1 | 48.6 | 965 | 1.28 |
| CADES-4 | ChCl/lactic acid | 7.3 | 48.1 | 1986 | 0.76 |
| CADES-5 | ChCl/formic acid | 27.0 | 48.1 | 15930 | 0.14 |
| CADES-6 | ChCl/malic acid | 1.3 | 93.4 | 66.8 | 1.7 |
| CADES-7 | ChCl/oxalic acid | 19.3 | 92.2 | 21300 | 1.4 |
| CADES-8 | ChCl/tartaric acid | 5.0 | 48.1 | 281 | 1.7 |
| CADES-9 | ChCl/citric acid | 7.4 | 79.9 | 4.79 | 1.4 |
| CADES-10 | ChCl/succinic acid | 2.5 | 47.9 | 1411 | 1.7 |
| Number | CADESs | DPPH(%) | ABTS(%) | Fe2+(%) |
|---|---|---|---|---|
| CADES-1 | ChCl/acetic acid | 96.2±3.3 | 14.1±3.8 | 13.2±3.2 |
| CADES-2 | ChCl/glycolic acid | 97.1±3.2 | 16.0±1.1 | 98.9±0.4 |
| CADES-3 | ChCl/levulinic acid | 96.1±1.0 | 30.4±2.4 | 49.6±3.0 |
| CADES-4 | ChCl/lactic acid | 95.0±0.7 | 35.2±3.1 | 85.1±0.6 |
| CADES-5 | ChCl/formic acid | 95.4±2.0 | 6.6±3.4 | 87.9±0.1 |
| CADES-6* | ChCl/malic acid | 53.4±0.8 | 16.4±1.3 | 8.3±0.5 |
| CADES-7* | ChCl/oxalic acid | 85.2±0.4 | 14.8±1.1 | 96.2±2.0 |
| CADES-8* | ChCl/tartaric acid | 55.0±0.1 | 19.2±1.9 | 8.4±1.3 |
| CADES-9* | ChCl/citric acid | 46.4±2.1 | 13.8±1.5 | 7.1±1.1 |
| CADES-10* | ChCl/succinic acid | 38.2±0.8 | 9.6±0.9 | 2.9±1.0 |
| CADESs | E. coli | P. aeruginosa | S. aureus | E. faecalis |
|---|---|---|---|---|
| ChCl/acetic acid | 7.5 | 3.75 | 7.5 | 7.5 |
| ChCl/glycolic acid | 7.5 | 7.5 | 15 | 7.5 |
| ChCl/levulinic acid | 7.5 | 7.5 | 15 | 15 |
| ChCl/lactic acid | 30 | 30 | 15 | 30 |
| ChCl/formic acid | 3.75 | 3.75 | 3.75 | 3.75 |
| ChCl/malic acid | 15 | 7.5 | 30 | 30 |
| ChCl/oxalic acid | 7.5 | 7.5 | 7.5 | 7.5 |
| ChCl/tartaric acid | 30 | 15 | 15 | 15 |
| ChCl/citric acid | 15 | 7.5 | 7.5 | 15 |
| ChCl/succinic acid | 15 | 15 | 15 | 15 |
| Solvents | Dilution factor | Number of days (bud length: mm) | RGP % | |||
|---|---|---|---|---|---|---|
| 1 day | 2 days | 3 days | 4 days | |||
| Deionized water | / | 6.5 | 21.8 | 27.1 | 41.5 | 100 |
| ChCl/acetic acid | 400 | 1.4 | 9.4 | 13.1 | 14.6 | 60 |
| 1200 | 5 | 25.5 | 33.5 | 35.2 | 90 | |
| 2000 | 2.7 | 15.8 | 30.6 | 29.7 | 100 | |
| ChCl/glycolic acid | 400 | 3.6 | 13.5 | 20.9 | 22.7 | 100 |
| 1200 | 7.4 | 23.5 | 35.7 | 37.6 | 90 | |
| 2000 | 2.3 | 17.7 | 31.5 | 34.2 | 100 | |
| ChCl/levulinic acid | 400 | 1.9 | 3.2 | 3.8 | 3.4 | 100 |
| 1200 | 4.9 | 11.5 | 15.5 | 23.7 | 100 | |
| 2000 | 7.1 | 22.1 | 35.8 | 60 | 100 | |
| ChCl/lactic acid | 400 | 6.2 | 13.6 | 19.8 | 31.1 | 100 |
| 1200 | 7.7 | 23.4 | 40.3 | 52 | 100 | |
| 2000 | 9.4 | 28.1 | 45.8 | 65.6 | 100 | |
| ChCl/formic acid | 400 | 2.6 | 3.4 | 7.2 | 7.8 | 100 |
| 1200 | 8.9 | 20.3 | 35.7 | 54.8 | 100 | |
| 2000 | 7.5 | 23.8 | 62.4 | 74.3 | 100 | |
| ChCl/malic acid | 400 | 7.8 | 19.1 | 25.4 | 25.6 | 100 |
| 1200 | 8.2 | 24.3 | 31.5 | 32.3 | 100 | |
| 2000 | 10.6 | 26.2 | 36.1 | 40.2 | 100 | |
| ChCl/oxalic acid | 400 | 0 | 0.3 | 0.3 | 0.3 | 20 |
| 1200 | 2.7 | 7.7 | 12.3 | 12.8 | 100 | |
| 2000 | 7.8 | 28.3 | 40.3 | 41.5 | 100 | |
| ChCl/tartaric acid | 400 | 1.8 | 2.8 | 3.4 | 3.4 | 100 |
| 1200 | 4 | 12.3 | 23.3 | 33.3 | 100 | |
| 2000 | 5.9 | 15.4 | 33.8 | 57.7 | 100 | |
| ChCl/citric acid | 400 | 4.5 | 12.4 | 17.3 | 19.9 | 100 |
| 1200 | 4.7 | 16.4 | 25.9 | 42.1 | 100 | |
| 2000 | 7.1 | 26.3 | 52.2 | 69 | 100 | |
| ChCl/succinic acid | 400 | 3.3 | 6 | 9.6 | 12.8 | 100 |
| 1200 | 9.1 | 22.4 | 51.9 | 71.1 | 100 | |
| 2000 | 8.3 | 27.7 | 49.5 | 76.2 | 100 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




