1. Introduction
1.1. Dysphagia
Dysphagia, or swallowing disorder, is the difficult passage of a food bolus through all the swallowing phases [
1], and can involve physiological complications including difficulty swallowing, dehydration and nutrient deficiency, choking or pneumonia due to regurgitated food entering the respiratory tract [
1]. This swallowing dysfunction can be oropharyngeal or esophageal; oropharyngeal dysphagia being caused by the delay of the liquid or solid bolus to enter the oropharyngeal phase of swallowing and difficulty in bringing the bolus into the neck of the esophagus [
2], with 80% of cases of oropharyngeal dysphagia being mainly caused by a neuromuscular problem [
3].
Approximately 14% to 18% of hospitalized patients suffer dysphagia, while between 30% and 60% of patients in nursing homes report symptomatic dysphagia [
2]. Oropharyngeal dysphagia is quite common and occurs in up to 50% of the elderly and up to 50% of individuals with neurological involvement, while most of these go undiagnosed and receive no treatment [
4]. The main cause of dysphagia in neurodegenerative diseases is Parkinson's Disease, in which 50% of the patients show oropharyngeal symptoms [
4]. Dysphagia affects more than 50% of stroke survivors, with approximately 12% of patients suffering this disorder 6 months after the stroke [
5]. A systematic review of the costs of dysphagic patients in the USA reported that the annual cost of caring for this type of patient is
$12,715, somewhat higher than other groups (40.36% per annum) [
6]. A significant increase in costs was also reported for patients with oropharyngeal dysphagia [
6].
1.2. Actual Diagnosis and Treatment of Dysphagia
Diagnosing dysphagia is challenging because of the complexity of swallowing and its multifactorial causes, including neurological, structural, and functional disorders, often requiring specialized multidisciplinary evaluation. The gold standard techniques include videofluroscopy (VFS) and fibroscopic swallowing evaluation (FEES), while the usual method is volume-viscosity clinical examination (V-VST) [
7,
8]. VFS obtains detailed images of swallowing dynamics using X-rays and contrast, although its drawbacks include radiation exposure, high costs, the need for specialized equipment and subjective expert evaluation [
7,
8]. FEES involves direct visualization of the pharyngeal structures by a flexible endoscope and is useful for detecting silent aspiration and anatomical abnormalities. However, it cannot visualize the oral swallowing phase and patient discomfort [
7,
8]. V-VST is the most widely used method in clinical practice for assessing oropharyngeal dysphagia. The procedure requires the patient to ingest boluses ranging from 5 to 20 mL with viscosity adjustments based on the patient's swallowing difficulty, including the consistencies of water, nectar, and puddings [
7], but relies heavily on the evaluator’s expertise and does not provide visual anatomical information, making this test highly subjective and somewhat deficient in diagnosing dysphagia both quantitatively and objectively.
Current treatments, including neurorehabilitation, diet modification, percutaneous endoscopic gastrostomy and surgical interventions, focus on minimizing the risk of aspiration pneumonia and ensuring the patient’s safety [
9]. No standardized tool is currently available for evaluating the progression of dysphagia or the effectiveness of therapeutic interventions, which hinders the patient's recovery process and delays clinical decisions while ensuring safety. Ultimately, a standardized approach is essential for the comprehensive management of the disorder.
1.3. Entropy in EEG Signals
In the literature, swallowing event-related desynchronization (ERD) in beta band power has been found in the motor cortex, which may be associated with the activation of neural circuits involved in motor control [
10] which reveals that the brain is actively preparing or coordinating the motor activity needed for swallowing. While ERD has proven to be a valuable source of information, the development of a comprehensive diagnostic tool remains elusive due to the variability in response observed between subjects and epochs, so that the scientific community is actively seeking novel quantitative biomarkers that could offer a more reliable dysphagia diagnosis.
Many studies have shown that swallowing is primarily regulated by the cerebral cortex, requiring the coordination of both cortical and subcortical neural activity [
11,
12,
13,
14,
15], which may be reflected in the degree of organization of EEG signals. Swallowing impairment may result from disrupted coordination of cortical and subcortical neural activity, leading to increased EEG signal complexity. In this regard, Sample Entropy (SampEn) measures signal irregularity by assessing how often patterns of a given length (
m) remain similar when extended to
m+1 [
16]. Higher entropy values indicate greater complexity, while values near zero reflect highly predictable patterns. Entropy metrics have proven valuable in understanding various neurological disorders and aid early diagnosis, disease monitoring, and treatment evaluations. They have been applied in studies on healthy aging, Parkinson’s and Alzheimer’s disease. SampEn may also provide critical insights into the neural dysfunction associated with swallowing impairments, supporting early detection and clinical assessment.
The primary objective of this study was thus to identify entropy-based EEG biomarkers for the quantitative assessment of post-stroke dysphagia to obtain novel tools for monitoring swallowing dysfunction and tracking the progress of the disease throughout neurorehabilitation.
2. Materials and Methods
2.1. Database
A 32-channel EEG was recorded from 36 post-stroke patients with dysphagia (dysphagic group), 22 post-stroke patients without dysphagia (control group), and 21 healthy elderly individuals (healthy group) at the Paré Jofré and La Pedrera Hospitals in the Valencian Community, Spain. The criteria for diagnosing a stroke were magnetic resonance imaging and subsequently the criterion used to diagnose patients with dysphagia (DG) was the volume-viscosity clinical examination (V-VST). In compliance with the Declaration of Helsinki, the study followed a protocol approved by the ethics committees of both hospitals and the Universitat Politècnica de València.The participants’ ages were 59.9 ± 6.3 years for the healthy group, 69.0 ± 12.9 years for the dysphagic group, and 68.5 ± 8.8 years for the control group, with statistically significant differences observed between groups. The proportion of female participants was 41.94% in the dysphagic group, 52.38% in the control group, and 40% in the healthy group. EEG signal acquisition was conducted on all patients on hospital admission.
2.2. Recording Protocol
The patients' facial skin and mastoid areas were first cleansed with an exfoliating gel, followed by alcohol for thorough preparation. A BrainWave cap, featuring 32 electrodes arranged according to the standard 10-20 system, was then carefully positioned on the subject. The signals were captured with the TMSi SAGA 32/64+ Amplifier at a sampling frequency of 2 kHz. The TMSi user interface was implemented to check acceptable impedances up to 10 kΩ. Four disposable Ag/AgCl bipolar electrodes (MN00005 Duotrodes, Bisico) were placed on the submental area with an inter-electrode distance of 21 mm for additional recording of electromyographic (EMG) activity from infrahyoid and suprahyoid swallowing muscles.
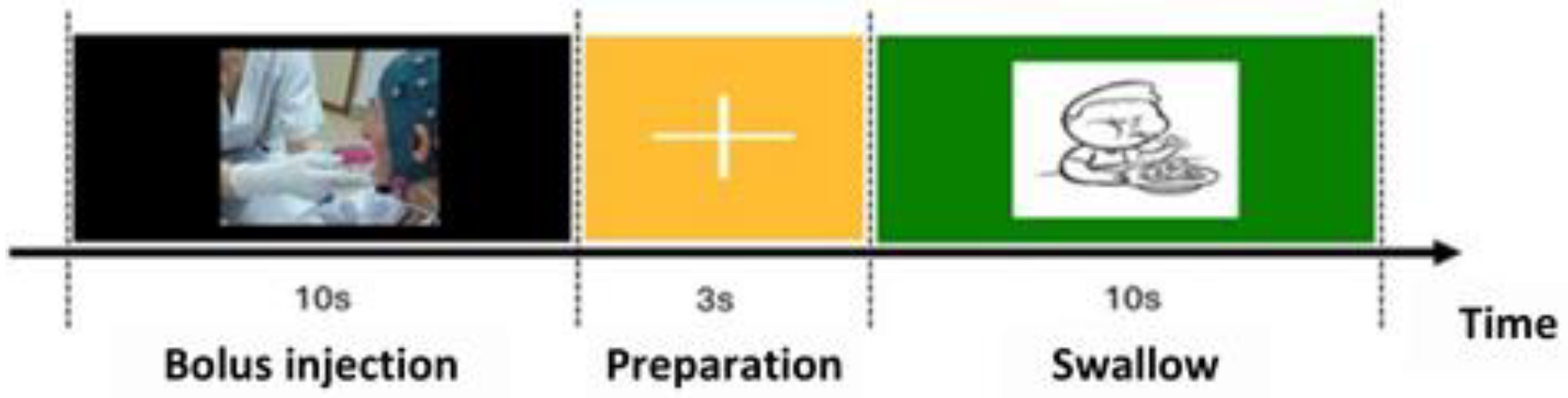
The swallowing protocol consisted of 3 stages: in the first a liquid bolus was placed in the patient’s mouth while a black screen was displayed on the computer; in the second there was a fixation cross instructing the patient to remain still. In the final stage, an image of a child appeared as a cue for the patient to initiate swallowing (swallowing marker). After verifying the patient's safety by simple questions and assessing the appropriate responses, the recording protocol was manually restarted and continued until reaching a total of 40 repetitions. A summary of the test sequence and the described procedures is provided in
Figure 1.
2.3. Preprocessing
EEG data were preprocessed with a 0.5 Hz high-pass filter and an anti-aliasing low-pass filter with a cutoff at 128 Hz and downsampled at 256Hz. The next step involved the MATLAB FASTER tool ("Fully Automated Statistical Thresholding for EEG Artifact Rejection") [
17], to automatically eliminate defective channels. Epochs were then extracted, each comprising 3 seconds of the EEG signal preceding the swallowing marker (order to swallow) and 8 seconds afterwards. Artifact-contaminated epochs were identified by the FASTER algorithm and excluded from further analysis. To mitigate biological interference, including ocular, muscular, and cardiac artifacts, the EEG signals were band-pass filtered between 1 and 30 Hz, re-referenced to the average of all EEG channels and processed by Independent Component Analysis (ICA). Subsequently, non-neural components were removed using the EEGLAB toolbox in MATLAB [
18] through a meticulous visual inspection of the independent components to eliminate any residual muscle activity.
2.4. Obtaining Biomarkers Based on Sample Entropy (SampEn)
Figure 2 contains a flowchart outlining the process followed in this study.
Fragmented swallow frequently occurred in dysphagia patients, which is characterized by multiple separate muscle activations during a single functional swallow [
19,
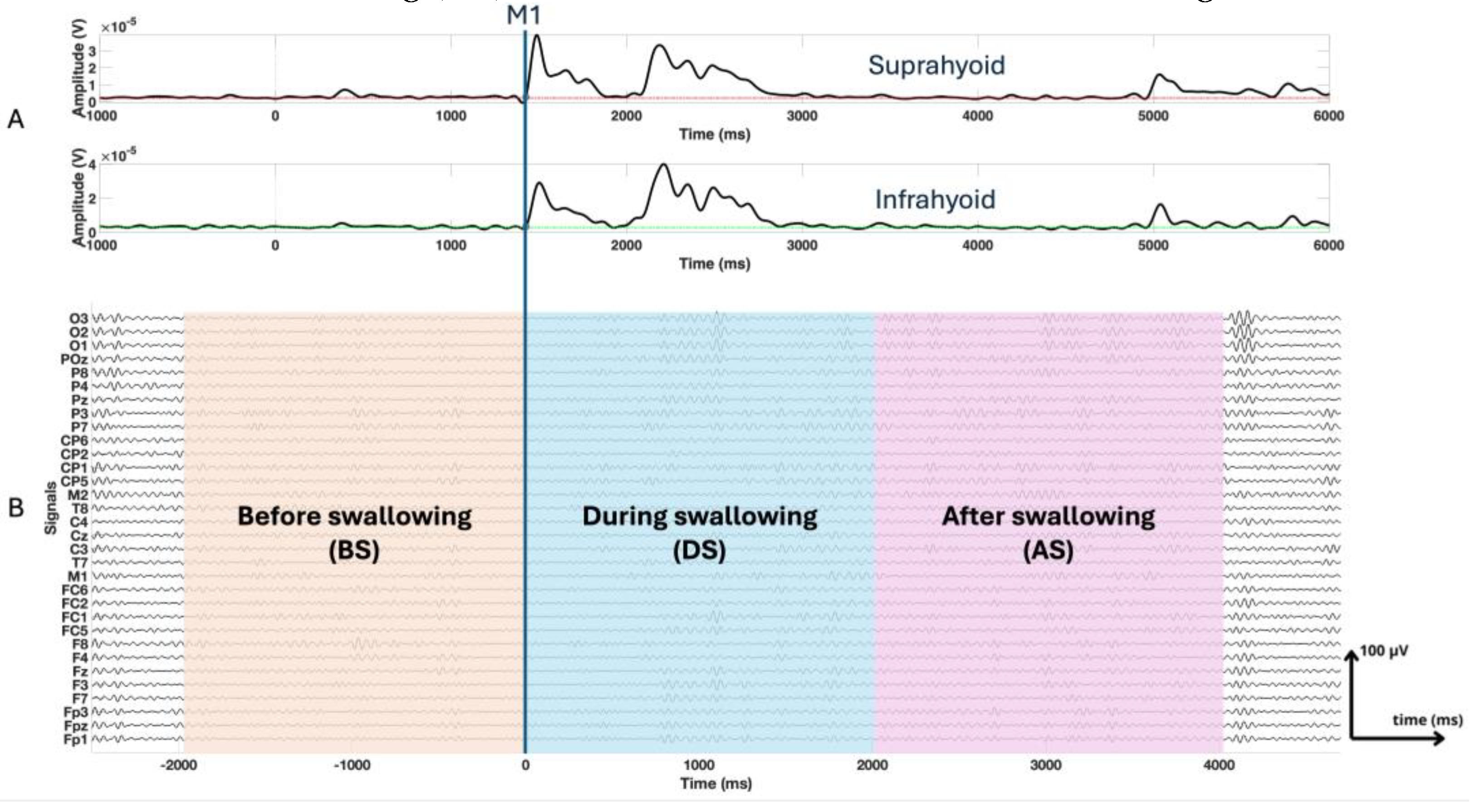
20], making precise onset and offset detection difficult. Consequently, fixed-length epochs aligned to the M1 event [
21], the onset of muscle activation in the suprahyoid muscles during the swallowing process determined by EMG signals, were used to ensure consistency across all subjects. Since healthy subjects typically exhibit swallowing durations of 1 to 2 seconds [
22], we extracted epochs extending from -2000 ms to 4000 ms, M1 being the temporal reference (0 ms) [
23,
24]. We further divided each epoch into 3 segments: before swallowing (BS), from -2000 ms to 0 ms prior to M1; during swallowing (DS), from 0 ms to 2000 ms; after the swallowing (AS), from 2000 ms to 4000 ms, as shown in
Figure 3.
Sample entropy was used to characterize the EEG signals. This entropy measure is not only simple and easy to interpret but also well-established and widely validated in the literature. For a time series x[n], n=1…Nr, SampEn was worked out using the following Eq. (1) [
25]:
Where Vi(r) the number of pattern pairs of length m+1 similar to a pattern in the time series x, starting at position i, according to threshold r, which is multiplied by the standard deviation of the signal, and Ui(r) the number of pattern pairs of length m similar to a pattern in the time series x, starting at position i, according to threshold r. These parameters count patterns m+1 and m long, respectively, that meet the similarity criterion, i.e. how many of these fragments resemble each other according to a given threshold (r). Considering other previous studies in literature for the EEG complexity assessment [
26,
27,
28,
29,
30,
31], we performed a parameter sweep over a range of SampEn hyperparameters, specifically testing embedding dimensions m = 2 and 3, and tolerance values r = 0.1, 0.15, 0.2, 0.25, and 0.3. Specifically, m = 3 and r = 0.2 was ultimately selected based on its performance stability across subjects and its physiological consistency.
Event-related desynchronization (ERD) in alpha and beta band are well known process during swallowing tasks [
10,
32]. Theta band activity is expected to be related with sensorimotor integration, attention, and cognitive control, all of which are crucial for coordinating voluntary and reflexive aspects of swallowing [
33]. In this work, SampEn metrics were calculated for the frequency bands to characterize swallowing-related cortical dynamics: theta (4 to 8 Hz), alpha (8 to 13 Hz) and beta (13 to 30 Hz).
All the above processes were carried out following the following formula:
Where j is the swallowing state (BS, DS and AS), k corresponds to the frequency band (theta, alpha and beta), l the patient, N is the total number of epochs per patient, p the epoch and m each electrode in the cortical surface.
2.5. Statistical analysis
Nonparametric procedures were used to assess differences between groups because of the lack of normality in the data distribution, confirmed by the Kolmogorov-Smirnov test. The corresponding statistical test identified a significant difference in participant age between groups. Therefore, a generalized additive model (GAM) was applied to cancel out the influence of this confounding variable on the SampEn of each channel. GAM allowed us to flexibly model the potential nonlinear influence of age on signal complexity [
34]. The GAM model used was as follows:
Where SampEnij represents the sample entropy value for subject i at electrode j and s1(Age) is a smooth function (spline) that models the nonlinear effect of age. The adjusted residual, which corresponds to age-adjusted SampEn, represents the variations that cannot be explained by age.
Subsequently, a nonparametric cluster-based permutation test, as described by[
35], was applied to assess statistical differences between groups. This approach effectively controls for multiple comparisons by leveraging the spatial dependence between electrodes, making it particularly well-suited for EEG data analysis. Pairwise comparisons were made between groups (DG vs. HG, DG vs. CG and CG vs. HG).
Once significant clusters were identified (p<0.05), the age-adjusted SampEn values from all electrodes within each cluster were extracted, and a mean value per subject was calculated for each cluster. These cluster-specific means were then visualized using boxplots to convey both the central tendency and variability of the data across groups. Aditionally, Cliff's Delta [
36] was computed as a nonparametric effect-size metric, yielding a scale-independent estimate of the magnitude of the observed group differences.
3. Results
Figure 4,
Figure 5 and
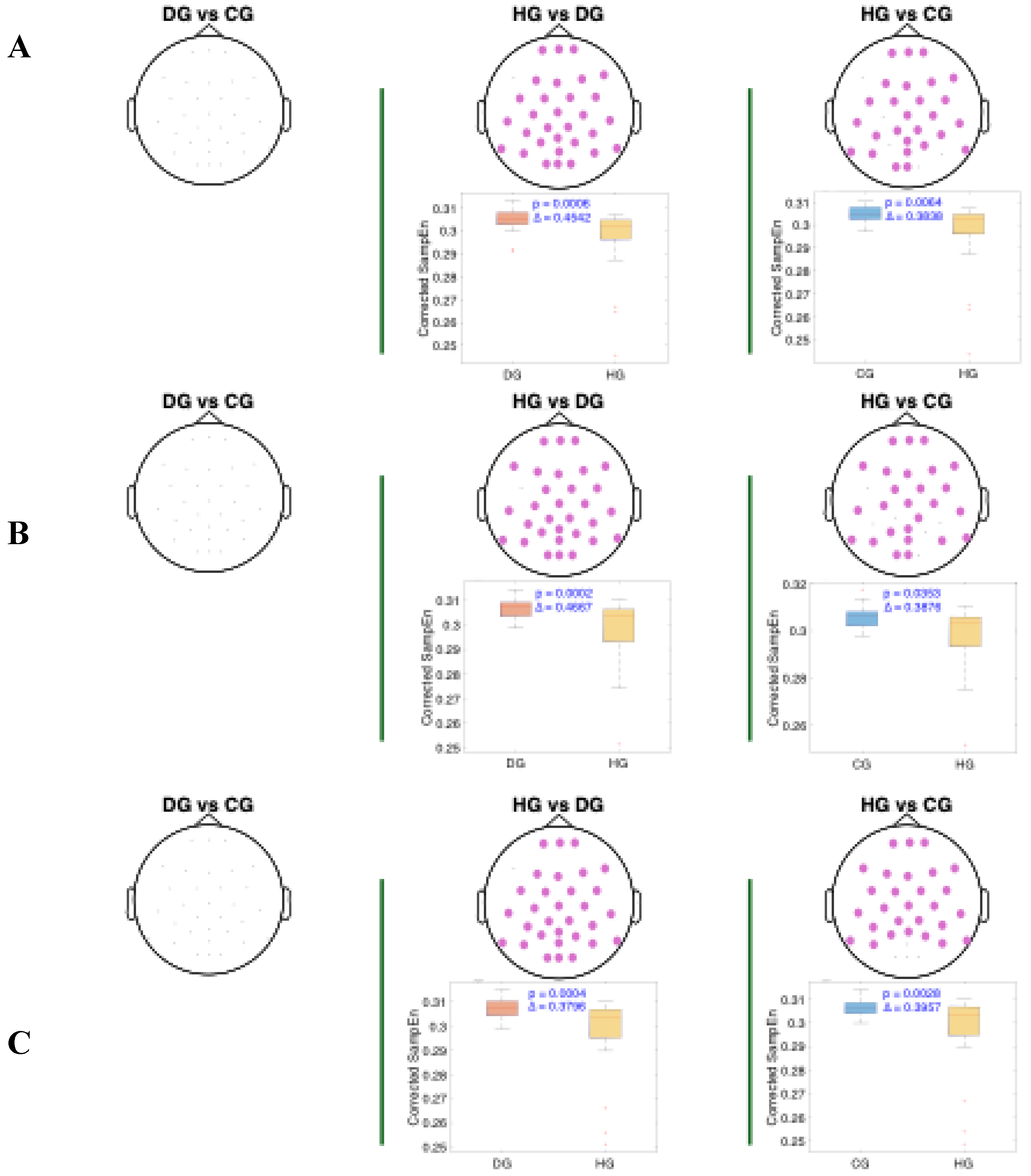
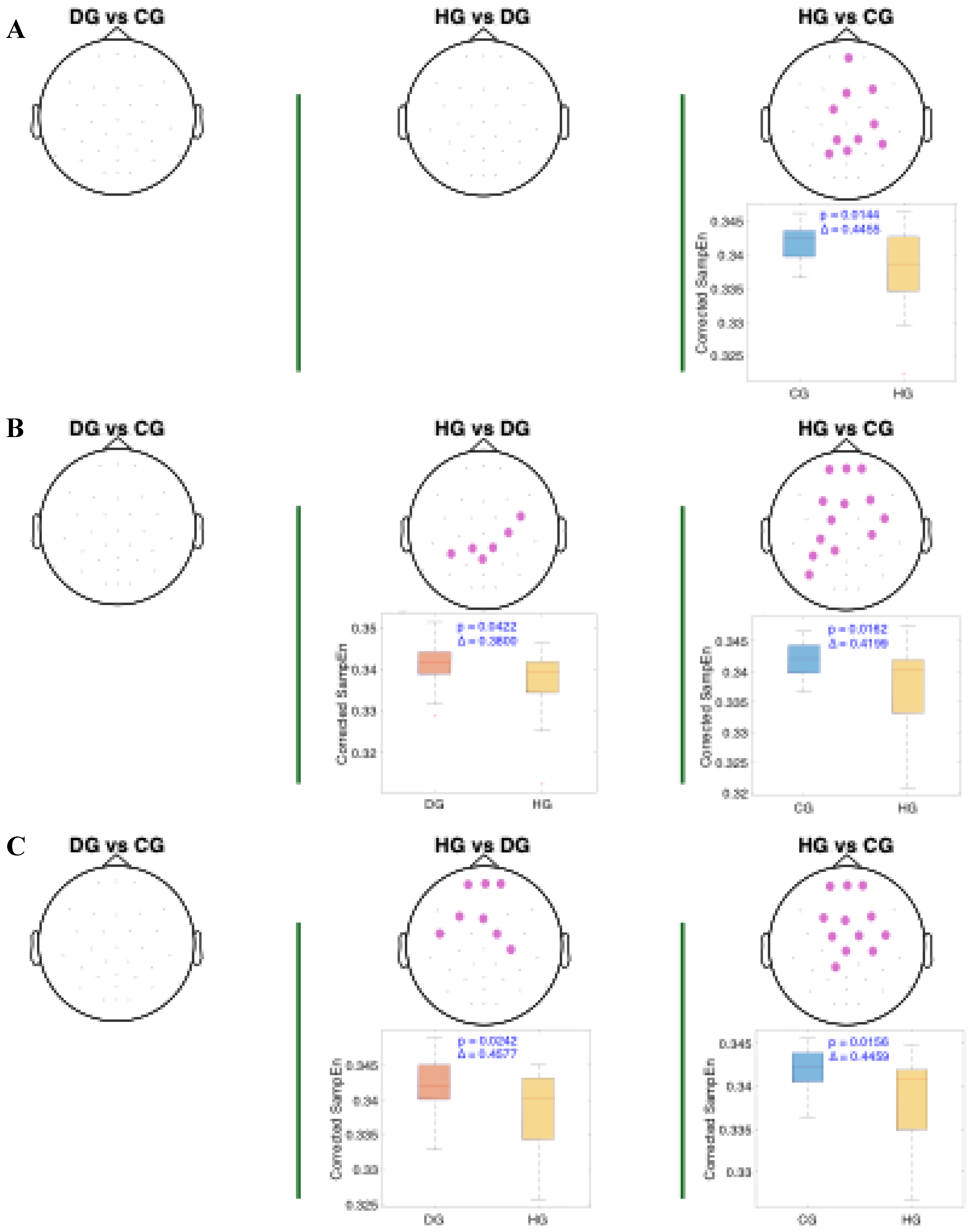
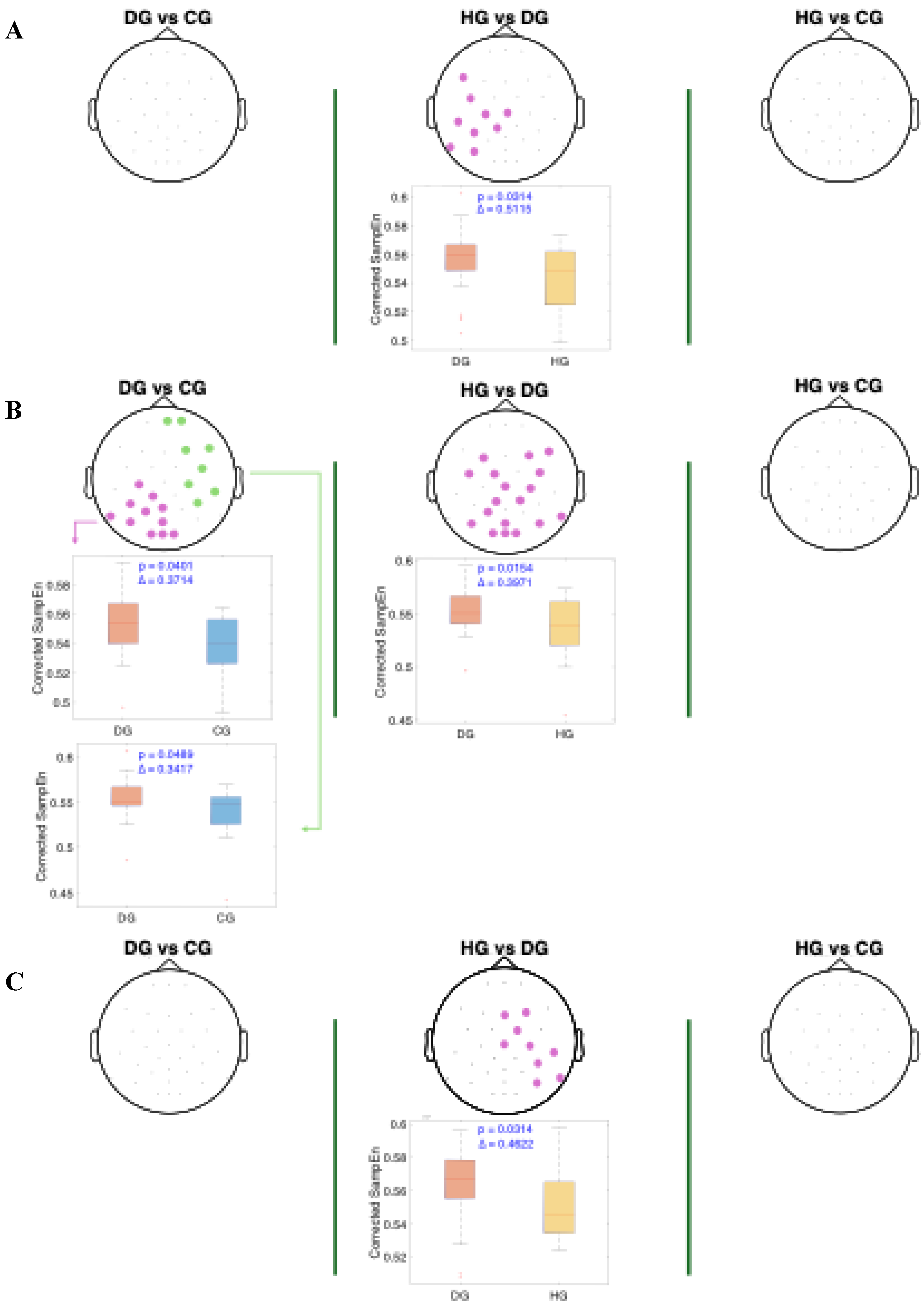
Figure 6 present the results of the statistical analysis of age-adjusted Sample Entropy (SampEn) values measured before, during, and after swallowing, across the theta, alpha, and beta frequency bands, respectively. This analysis identified spatial electrode clusters in which statistically significant differences were observed between groups in the theta, alpha, and beta frequency bands.
For both the alpha and theta bands (
Figure 4 and
Figure 5), notable similarities are observed, significant clusters were observed in nearly all phases when comparing the healthy group (HG) with the other groups. The HG group consistently shows lower age-adjusted SampEn values. In the theta band, the significant clusters tend to be spatially broader, involving a larger number of electrodes and covering nearly the entire cerebral cortex, while the alpha band covers mainly the central and frontocentral areas. Regarding effect sizes, higher Cliff’s delta values are observed in the theta band for the HG vs. DG comparison, and in the alpha band for the HG vs. CG comparison.
For the beta band (
Figure 6), distinct patterns can be observed across all swallowing phases. Significant clusters are present in the comparison between HG and DG, with high Cliff's delta values. A notable finding in the DS phase is the presence of two distinct clusters in the DG vs. CG comparison: one with a higher Cliff's delta located in the left parietal and occipital regions, and another with a lower effect size involving the right frontotemporal area. Additionally, across all comparisons, the distributions of age-adjusted SampEn consistently show higher values in the DG group compared to the other groups.
4. Discussion
In this study we evaluated using sample entropy (SampEn) metrics from EEG signals as biomarkers for the quantification and assessment of neurogenic dysphagia.
Our findings show that theta and alpha frequency bands are particularly sensitive markers for distinguishing stroke (CG and DG) patients from HG group across all phases of the protocol (BS, DS and AS). This pattern aligns with existing literature identifying alterations in these bands as robust indicators of stroke-related cortical dysfunction [
37]. Specifically, alpha band abnormalities were predominantly observed in frontal and central regions, consistent with previous studies that associate alpha power reductions or disruptions in these areas with impaired cortical regulation and motor deficits following stroke [
38]. The frontal and central alpha oscillations have been linked to attentional processes and motor preparation, both critical in swallowing control, which explains their particular relevance in our swallowing paradigm. In contrast, differences in the theta band extended across nearly the entire scalp, reflecting widespread cortical alterations. Elevated theta activity is a well-established marker of post-stroke cortical dysfunction, often associated with cortical slowing and reduced neural efficiency [
39,
40]. Importantly, during the swallowing task, stroke patients must exert greater attentional, sensorimotor, and cognitive control to compensate for impaired automatic motor function. This increased demand likely contributes to the observed global theta enhancement, reflecting both neural dysfunction and heightened task-related engagement.
It is crucial to highlight the ongoing debate concerning the role of the cerebral cortex in regulating swallowing. We found that in dysphagia patients, brain activity differed significantly from healthy controls in beta band—beginning in the left hemisphere before swallowing, shifting to the central region during swallowing, and moving to the right hemisphere post-swallow. Our results support previous findings that the degree of cortical involvement differs depending on the swallowing phase: the oral phase is predominantly governed by the left hemisphere, while dominance shifts during the pharyngeal phase, with the right hemisphere taking control in the esophageal phase [
41,
42].
The parietal neocortex plays a central role in swallowing by integrating sensory input from the oropharynx with motor functions necessary for deglutition, effectively channeling sensory signals to motor centers and coordinating activity with other motor regions [
43,
44]. Functional imaging studies in healthy individuals have shown that motor planning and execution of voluntary swallowing primarily involve the lateral pericentral cortex, frontoparietal operculum, and specifically the left postcentral gyrus when tongue movement is excluded [
45]. Furthermore, neuroimaging and electrophysiological studies report significant beta-band event-related desynchronization (ERD) in the inferior precentral gyrus and left parietal cortex during swallowing, indicating cortical activation [
10,
32]. Notably, our preliminary data also confirmed ERD in this area, highlighting its relevance. Our findings showed that dysphagic patients exhibit reduced EEG signal organization (i.e., higher Sample Entropy values) in the left parietal region—an area crucial for monitoring and executing swallowing—suggesting disruption in the neural mechanisms governing these processes. The absence or reduction of ERD and increased entropy may reflect disorganized neural activity and impaired motor coordination during swallowing [
46]. This is consistent with prior studies identifying the left postcentral gyrus and Sylvian fissure as key regions in swallowing control [
41,
42]. These findings support the potential of beta-band Sample Entropy, particularly in the left parietal and motor cortex, as a biomarker for detecting dysphagia, in agreement with prior evidence [
10,
32].
Interestingly, our results revealed significant differences in SampEn values in the right frontotemporal region between DG and the other groups in the beta band. This finding suggests a possible role for this cortical area in the altered neuronal processing associated with swallowing dysfunction. Furthermore, functional imaging studies have shown that these regions become more active in post-stroke patients with dysphagia, possibly reflecting a reorganization of the cortical swallowing network to recruit additional resources for motor planning and execution [
47].
Swallowing has also been associated with increased gamma activity in the Sylvian fissure, as demonstrated by intracranial EEG studies [
32]. In this work, we did not assess the alteration in gamma band in dysphagia patients. This is mainly motivated by the fact that due to the relatively low signal-to-noise ratio, the gamma activity remains challenging in scalp electrodes.
ERD-based metrics limit the ability to capture more complex aspects of brain dynamics, such as temporal variability or the complexity of the organization of neural activity, whereas entropy metrics offer a complementary perspective by quantifying the degree of disorder, unpredictability, or complexity of the EEG signal. The integrated use of power and entropy metrics not only enhances the interpretation of neuronal activity changes but also provides a more comprehensive understanding of the mechanisms underlying cognitive and pathological processes, which in turn increase the physiological significance of the findings derived from EEG signals.
Our findings reinforce the hypothesis that dysfunction in cortical modulation in dysphagic subjects could be related to altered neuronal connectivity, affecting both the planning and execution of the swallowing motor act. In addition, the beta band, known for its role in regulating motor and cognitive tasks [
48,
49,
50,
51], could be a sensitive marker of functional changes in dysphagia-related cortical activity. This study on entropy proposes an effective biomarker for distinguishing dysphagic patients, facilitating a more precise characterization of the disorder and informing of the development of targeted therapeutic approaches. SampEn assessment during swallowing has the potential to be established as a crucial tool for identifying early abnormalities and formulating specialized rehabilitation strategies for individuals with dysphagia.
This study has several limitations. First, our relatively small sample may underestimate the discriminatory power of SampEn, and a larger cohort would not only strengthen statistical robustness but also allow exploration of more subtle group differences. Future work should recruit diverse, larger patient populations to validate and refine SampEn for clinical use. Second, although we incorporated Cliff's Delta to gauge effect size independently of sample size and distribution assumptions, we did not correlate EEG biomarkers with dysphagia severity. Longitudinal studies that track patients before and after specific therapeutic interventions—using both SampEn and complementary complexity measures—could elucidate how these metrics evolve with disease progression and recovery, thereby establishing their prognostic value. Third, the high inter-subject variability inherent to our cross-sectional design highlights the need for repeated measures within individuals over time; Such designs would clarify within-subject changes and help disentangle trait versus state effects in neurogenic dysphagia. Finally, integrating SampEn with other EEG features (e.g., spectral power, connectivity), peripheral physiological metrics, and clinical scales in multimodal predictive models represents a promising avenue for enhancing diagnostic precision and tailoring personalized rehabilitation strategies. By addressing these points, future research can move beyond proof-of-concept toward robust, clinically actionable biomarkers for dysphagia.
Despite these limitations, the present work paves the way towards an objective and quantitative assessment of neurogenic dysphagia using the non-invasive and cost-effective EEG technique. It could have an impact on social and health care that would decongest health care centers and an improvement in dysphagia patients’ quality of life, as neurorehabilitative and pharmacological treatments could be personalized. On the other hand, a range of research is now open for quantifying dysphagia using other entropy metrics or frequency-based entropies to obtain complementary biomarkers to those provided by SampEn.
5. Conclusions
Post-stroke patients—regardless of dysphagia status—exhibited elevated age-adjusted SampEn across multiple cortical regions, with particularly widespread pronounced increases in the theta band and the frontal–central alpha band, both before, during, and after swallowing, relative to healthy controls. These findings point to a generalized disruption of neural dynamics following stroke, likely stemming from impaired sensorimotor integration and compensatory cognitive effort during swallowing. Crucially, further analysis revealed that beta band dysphagia patients exhibited significantly higher in age-adjusted SampEn in the right frontotemporal and left parietal cortices than non-dysphagic stroke patients. The lack of reduced entropy during swallowing, particularly in the left parietal cortex, may reflect cortical discoordination and impaired sensorimotor processing associated with swallowing dysfunction, serving as a potential neurophysiological marker of dysphagia.
Quantifying these alterations provides potential objective and quantitative biomarkers for diagnosing and assessing neurogenic dysphagia and using EEG as a non-invasive and cost-effective technique.
Author Contributions
Adrian Velasco Hernandez: Conceptualization, Methodology, Software, Investigation, Validation, Formal analysis, Writing - Original Draft, Writing - Review & Editing. Javier Imaz Higuera: Resources, Data Curation, Methodology, Software, Writing - Review & Editing. Jose L. Martinez-de-Juan: Conceptualization, Investigation, Validation, Methodology, Writing - Review & Editing, Supervision. Yiyao Ye-Lin: Conceptualization, Methodology, Investigation, Validation, Writing - Review & Editing, Supervision. Javier Garcia Casado: Conceptualization, Investigation, Validation, Methodology, , Writing - Review & Editing, Supervision, Funding acquisition, Project administration. Marta Gutierrez Delgado: Resources, Data Curation, Writing - Review & Editing. Jenny Prieto House: Resources, Data Curation, Writing - Review & Editing. Gema Mas Sese: Resources, Data Curation, Writing - Review & Editing. Araceli Belda Calabuig: Resources, Data Curation, Writing - Review & Editing. Gema Prats-Boluda: Conceptualization, Methodology, Investigation, Validation, Writing - Review & Editing, Supervision, Funding acquisition, Project administration.
Funding
Supported by grants from the Generalitat Valenciana, the Universitat Politècnica de València and the promotors POLISABIO (Polisabio2021-A02, Polisabio2022_PI03) and INVESTIGO (Invest/2022/67). Thanks to the clinical staff whose involvement permitted to develop this project
Institutional Review Board Statement
The study was approved by the ethical committee of the Universitat Politècnica de València and the La Pedrera and Pare Jofre Hospitals. Informed signed consent was obtained from the subjects.
Data Availability Statement
The data supporting the findings of this study are not publicly available due to privacy and ethical restrictions, as they contain personal and sensitive information. Data can be made available upon reasonable request to the corresponding author, subject to approval by the relevant ethics committee and compliance with data protection regulations.
Acknowledgments
We would like to thank the Generalitat Valenciana, the Universitat Politécnica de València and the promoters POLISABIO (Polisabio2021-A02, Polisabio2022_PI03) and INVESTIGO (Invest/2022/67) for their support. Thanks to the clinical staff whose involvement has allowed us to develop the project.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Panara, K.; Ahangar, E.R.; Padalia, D. Physiology, Swallowing. StatPearls 2023. [Google Scholar]
- Ujiki, M.B.; Hedberg, H.M. Dysphagia. Dysphagia: A Clinical Guide, 2024; 1–217. [Google Scholar] [CrossRef]
- Wang, E.Q. Dysphagia. Encyclopedia of Movement Disorders, Three-Volume Set, 2010; V1-365–V1-367. [Google Scholar] [CrossRef]
- Clavé, P.; Shaker, R. Dysphagia: Current Reality and Scope of the Problem. Nat Rev Gastroenterol Hepatol 2015, 12, 259–270. [Google Scholar] [CrossRef] [PubMed]
- González-Fernández, M.; Ottenstein, L.; Atanelov, L.; Christian, A.B. Dysphagia after Stroke: An Overview. Curr Phys Med Rehabil Rep 2013, 1, 187. [Google Scholar] [CrossRef]
- Attrill, S.; White, S.; Murray, J.; Hammond, S.; Doeltgen, S. Impact of Oropharyngeal Dysphagia on Healthcare Cost and Length of Stay in Hospital: A Systematic Review. BMC Health Serv Res 2018, 18, 1–18. [Google Scholar] [CrossRef]
- Clavé, P.; Arreola, V.; Velasco, M.; Quer, M.; Castellví, J.M.; Almirall, J.; Peris, P.G.; Carrau, R. Diagnóstico y Tratamiento de La Disfagia Orofaríngea Funcional. Aspectos de Interés Para El Cirujano Digestivo. Cir Esp 2007, 82, 62–76. [Google Scholar] [CrossRef]
- Ertekin, C.; Aydogdu, I.; Yüceyar, N.; Tarlaci, S.; Kiylioglu, N.; Pehlivan, M.; Çelebi, G. Electrodiagnostic Methods for Neurogenic Dysphagia. Electroencephalography and Clinical Neurophysiology/Electromyography and Motor Control 1998, 109, 331–340. [Google Scholar] [CrossRef]
- Simons, A.; Hamdy, S. The Use of Brain Stimulation in Dysphagia Management. Dysphagia 2017, 32, 209–215. [Google Scholar] [CrossRef]
- Furlong, P.L.; Hobson, A.R.; Aziz, Q.; Barnes, G.R.; Singh, K.D.; Hillebrand, A.; Thompson, D.G.; Hamdy, S. Dissociating the Spatio-Temporal Characteristics of Cortical Neuronal Activity Associated with Human Volitional Swallowing in the Healthy Adult Brain. Neuroimage 2004, 22, 1447–1455. [Google Scholar] [CrossRef]
- Zald, D.H.; Pardo, J. V. Cortical Activation Induced by Intraoral Stimulation with Water in Humans. Chem Senses 2000, 25, 267–275. [Google Scholar] [CrossRef]
- Vasant, D.H.; Hamdy, S. Cerebral Cortical Control of Deglutition. Principles of Deglutition: A Multidisciplinary Text for Swallowing and its Disorders, 2013; 55–65. [Google Scholar] [CrossRef]
- Hamdy, S.; Aziz, Q.; Rothwell, J.C.; Singh, K.D.; Barlow, J.; Hughes, D.G.; Tallis, R.C.; Thompson, D.G. The Cortical Topography of Human Swallowing Musculature in Health and Disease. Nature Medicine 1996 2:11 1996, 2, 1217–1224. [Google Scholar] [CrossRef]
- Rangarathnam, B.; Kamarunas, E.; McCullough, G.H. Role of Cerebellum in Deglutition and Deglutition Disorders. Cerebellum 2014, 13, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, S.; Aziz, Q.; Rothwell, J.C.; Power, M.; Singh, K.D.; Nicholson, D.A.; Tallis, R.C.; Thompson, D.G. Recovery of Swallowing after Dysphagic Stroke Relates to Functional Reorganization in the Intact Motor Cortex. Gastroenterology 1998, 115, 1104–1112. [Google Scholar] [CrossRef]
- Bein, B. Entropy. Best Pract Res Clin Anaesthesiol 2006, 20, 101–109. [Google Scholar] [CrossRef]
- Nolan, H.; Whelan, R.; Reilly, R.B. FASTER: Fully Automated Statistical Thresholding for EEG Artifact Rejection. J Neurosci Methods 2010, 192, 152–162. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. EEGLAB: An Open Source Toolbox for Analysis of Single-Trial EEG Dynamics Including Independent Component Analysis. J Neurosci Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Monaco, A.; Cattaneo, R.; Spadaro, A.; Giannoni, M. Surface Electromyography Pattern of Human Swallowing. BMC Oral Health 2008, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Roubeau, B.; Morinière, S.; Périé, S.; Martineau, A.; Falières, J.; St Guily, J.L. Use of Reaction Time in the Temporal Analysis of Normal Swallowing. Dysphagia 2008, 23, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Suntrup, S.; Teismann, I.; Wollbrink, A.; Warnecke, T.; Winkels, M.; Pantev, C.; Dziewas, R. Altered Cortical Swallowing Processing in Patients with Functional Dysphagia: A Preliminary Study. PLoS One 2014, 9, e89665–e89665. [Google Scholar] [CrossRef]
- Omari, T.I.; Ferris, L.; Schar, M.; Cock, C.; Doeltgen, S. Multiple Swallow Behaviour during High Resolution Pharyngeal Manometry: Prevalence and Sub-Typing in Healthy Adults. Speech, Language and Hearing 2022, 25, 1–7. [Google Scholar] [CrossRef]
- Imaz-Higuera, J.; Prats-Boluda, G.; Ye-Lin, Y.; Martinez-de-Juan, J.L.; Gutierrez-Delgado, M.; Prieto-House, J.; Más-Sesé, G.; Belda-Calabuig, A.; Garcia-Casado, J. Post-Stroke Dysphagia: Evaluating Myoelectric Alterations in Swallowing Muscles. Measurement 2025, 249, 117010. [Google Scholar] [CrossRef]
- Imaz-Higuera, J.; Beltran-Sanchez, J.; Garcia-Casado, J.; Ye-Lin, Y.; Martinez-de-Juan, J.L.; Gutierrez-Delgado, M.; Prieto-House, J.; Más-Sesé, G.; Belda-Calabuig, A.; Prats-Boluda, G. Comparison of Supra and Infrahyoid Muscle Activity in Healthy and Dysphagic Elderly Populations. IFMBE Proc 2024, 110, 221–229. [Google Scholar] [CrossRef]
- Molina Picó, A. Caracterizacion de Medidas de Regulariadad En Señales Biomedicas; 2014; ISBN 9788490482582.
- Song, Y.; Crowcroft, J.; Zhang, J. Automatic Epileptic Seizure Detection in EEGs Based on Optimized Sample Entropy and Extreme Learning Machine. J Neurosci Methods 2012, 210, 132–146. [Google Scholar] [CrossRef]
- Thomas, K.P.; Vinod, A.P. Biometric Identification of Persons Using Sample Entropy Features of EEG during Rest State. 2016 IEEE International Conference on Systems, Man, and Cybernetics, SMC 2016 - Conference Proceedings, 2017; 3487–3492. [Google Scholar] [CrossRef]
- Liu, Q.; Ma, L.; Fan, S.Z.; Abbod, M.F.; Shieh, J.S. Sample Entropy Analysis for the Estimating Depth of Anaesthesia through Human EEG Signal at Different Levels of Unconsciousness during Surgeries. PeerJ 2018, 6. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, Y.F.; Fan, S.Z.; Abbod, M.F.; Shieh, J.S. EEG Signals Analysis Using Multiscale Entropy for Depth of Anesthesia Monitoring during Surgery through Artificial Neural Networks. Comput Math Methods Med 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Karmakar, C.; Yearwood, J.; Venkatesh, S.; Palaniswami, M.; Liu, C. Detection of Epileptic Seizure Based on Entropy Analysis of Short-Term EEG. PLoS One 2018, 13. [Google Scholar] [CrossRef] [PubMed]
- Dastgoshadeh, M.; Rabiei, Z. Detection of Epileptic Seizures through EEG Signals Using Entropy Features and Ensemble Learning. Front Hum Neurosci 2022, 16, 1084061. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, H.; Takahashi, K.; Kameda, S.; Yoshida, F.; Maezawa, H.; Oshino, S.; Tani, N.; Khoo, H.M.; Yanagisawa, T.; Yoshimine, T.; et al. Swallowing-related Neural Oscillation: An Intracranial EEG Study. Ann Clin Transl Neurol 2021, 8, 1224. [Google Scholar] [CrossRef]
- Cavanagh, J.F.; Frank, M.J. Frontal Theta as a Mechanism for Cognitive Control. Trends Cogn Sci 2014, 18, 414–421. [Google Scholar] [CrossRef]
- Hastie, T.; Tibshirani, R. Generalized Additive Models. Statistical Science 1986, 1, 297–318. [Google Scholar]
- Maris, E.; Oostenveld, R. Nonparametric Statistical Testing of EEG- and MEG-Data. J Neurosci Methods 2007, 164, 177–190. [Google Scholar] [CrossRef]
- Kitchenham, B.; Madeyski, L. Recommendations for Analysing and Meta-Analysing Small Sample Size Software Engineering Experiments. Empir Softw Eng 2024, 29, 1–46. [Google Scholar] [CrossRef]
- Sato, Y.; Schmitt, O.; Ip, Z.; Rabiller, G.; Omodaka, S.; Tominaga, T.; Yazdan-Shahmorad, A.; Liu, J. Pathological Changes of Brain Oscillations Following Ischemic Stroke. Journal of Cerebral Blood Flow & Metabolism 2022, 42, 1753. [Google Scholar] [CrossRef]
- Schleiger, E.; Sheikh, N.; Rowland, T.; Wong, A.; Read, S.; Finnigan, S. Frontal EEG Delta/Alpha Ratio and Screening for Post-Stroke Cognitive Deficits: The Power of Four Electrodes. International Journal of Psychophysiology 2014, 94, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Luu, P.; Tucker, D.M.; Englander, R.; Lockfeld, A.; Lutsep, H.; Oken, B. Localizing Acute Stroke-Related EEG Changes: Assessing the Effects of Spatial Undersampling. J Clin Neurophysiol 2001, 18, 302–317. [Google Scholar] [CrossRef]
- Sato, Y.; Schmitt, O.; Ip, Z.; Rabiller, G.; Omodaka, S.; Tominaga, T.; Yazdan-Shahmorad, A.; Liu, J. Pathological Changes of Brain Oscillations Following Ischemic Stroke. Journal of Cerebral Blood Flow and Metabolism 2022, 42, 1753–1776. [Google Scholar] [CrossRef]
- Teismann, I.K.; Steinstraeter, O.; Stoeckigt, K.; Suntrup, S.; Wollbrink, A.; Pantev, C.; Dziewas, R. Functional Oropharyngeal Sensory Disruption Interferes with the Cortical Control of Swallowing. BMC Neurosci 2007, 8. [Google Scholar] [CrossRef]
- Teismann, I.K.; Steinsträter, O.; Warnecke, T.; Suntrup, S.; Ringelstein, E.B.; Pantev, C.; Dziewas, R. Tactile Thermal Oral Stimulation Increases the Cortical Representation of Swallowing. BMC Neurosci 2009, 10. [Google Scholar] [CrossRef]
- Yamamura, K.; Kurose, M.; Okamoto, K. Guide to Enhancing Swallowing Initiation: Insights from Findings in Healthy Subjects and Dysphagic Patients. Curr Phys Med Rehabil Rep 2018, 6, 178–185. [Google Scholar] [CrossRef]
- Frank, U.; Radtke, J.; Nienstedt, J.C.; Pötter-Nerger, M.; Schönwald, B.; Buhmann, C.; Gerloff, C.; Niessen, A.; Flügel, T.; Koseki, J.C.; et al. Dysphagia Screening in Parkinson’s Disease. A Diagnostic Accuracy Cross-Sectional Study Investigating the Applicability of the Gugging Swallowing Screen (GUSS). Neurogastroenterology and motility 2021, 33. [Google Scholar] [CrossRef]
- Martin, R.E.; MacIntosh, B.J.; Smith, R.C.; Barr, A.M.; Stevens, T.K.; Gati, J.S.; Menon, R.S. Cerebral Areas Processing Swallowing and Tongue Movement Are Overlapping but Distinct: A Functional Magnetic Resonance Imaging Study. J Neurophysiol 2004, 92, 2428–2443. [Google Scholar] [CrossRef]
- Hager, B.M.; Yang, A.C.; Gutsell, J.N. Measuring Brain Complexity During Neural Motor Resonance. Front Neurosci 2018, 12, 758–758. [Google Scholar] [CrossRef]
- Dziewas, R.; Sörös, P.; Ishii, R.; Chau, W.; Henningsen, H.; Ringelstein, E.B.; Knecht, S.; Pantev, C. Neuroimaging Evidence for Cortical Involvement in the Preparation and in the Act of Swallowing. Neuroimage 2003, 20, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.W.; Ofori, E.; Misra, G.; Hess, C.W.; Vaillancourt, D.E. Beta-Band Activity and Connectivity in Sensorimotor and Parietal Cortex Are Important for Accurate Motor Performance. Neuroimage 2017, 144, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Tzagarakis, C.; Ince, N.F.; Leuthold, A.C.; Pellizzer, G. Beta-Band Activity during Motor Planning Reflects Response Uncertainty. The Journal of Neuroscience 2010, 30, 11270. [Google Scholar] [CrossRef] [PubMed]
- Coleman, S.C.; Seedat, Z.A.; Whittaker, A.C.; Lenartowicz, A.; Mullinger, K.J. Beyond the Beta Rebound: Post-Task Responses in Oscillatory Activity Follow Cessation of Working Memory Processes. Neuroimage 2023, 265, 119801. [Google Scholar] [CrossRef]
- Schmidt, R.; Ruiz, M.H.; Kilavik, B.E.; Lundqvist, M.; Starr, P.A.; Aron, A.R. Beta Oscillations in Working Memory, Executive Control of Movement and Thought, and Sensorimotor Function. The Journal of Neuroscience 2019, 39, 8231. [Google Scholar] [CrossRef]
Figure 1.
Diagram of visual instructions during the swallowing protocol.
Figure 1.
Diagram of visual instructions during the swallowing protocol.
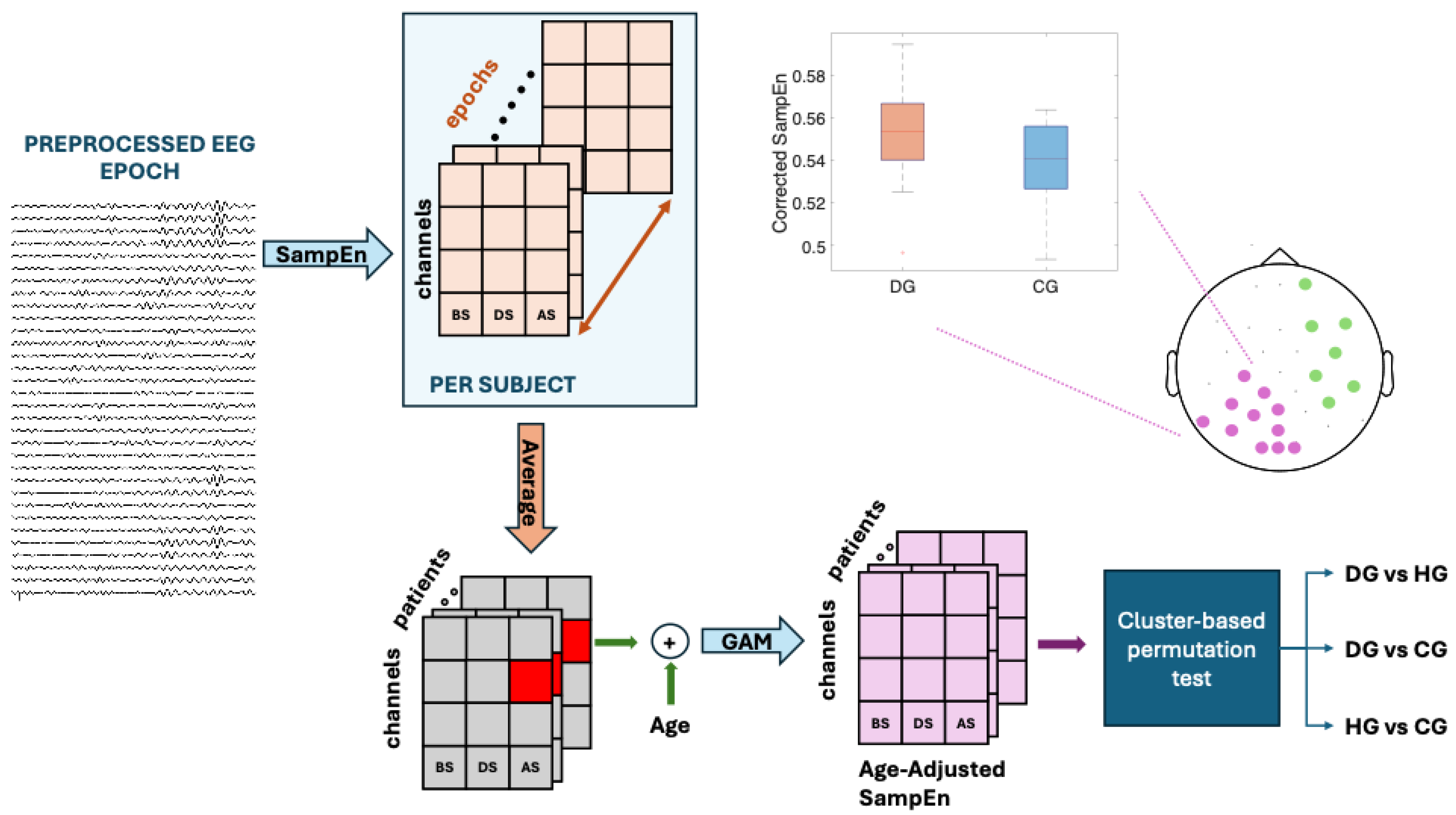
Figure 2.
Data analysis flowchart for theta, alpha and beta band. BS, DS and AS are the swallowing states: before swallowing (BS), during swallowing (DS) and after swallowing (AS). The study populations are dysphagics (DG), controls (CG) and healthy subjects (HG). Sample Entropy (SampEn) was used to assess signal predictability, Generalized Additive Model (GAM) was used to obtaining Age-Adjusted SampEn (Corrected SampEn) by removing the influence of age.
Figure 2.
Data analysis flowchart for theta, alpha and beta band. BS, DS and AS are the swallowing states: before swallowing (BS), during swallowing (DS) and after swallowing (AS). The study populations are dysphagics (DG), controls (CG) and healthy subjects (HG). Sample Entropy (SampEn) was used to assess signal predictability, Generalized Additive Model (GAM) was used to obtaining Age-Adjusted SampEn (Corrected SampEn) by removing the influence of age.
Figure 3.
Panel A presents the electromyographic signals of the suprahyoid and infrahyoid muscles, with a temporal reference based on muscle activation during swallowing (M1). Panel B displays the windowed electroencephalography signals (BS, DS, and AS).
Figure 3.
Panel A presents the electromyographic signals of the suprahyoid and infrahyoid muscles, with a temporal reference based on muscle activation during swallowing (M1). Panel B displays the windowed electroencephalography signals (BS, DS, and AS).
Figure 4.
Spatial distribution of the significant cluster in the theta band, identified using a nonparametric cluster-based permutation test (parameters: m = 3, r = 0.2), across three swallowing phases: A) before swallowing (BS), B) during swallowing (DS), and C) after swallowing (AS). Electrodes forming the significant cluster are highlighted for each group comparison: DG (dysphagic group), CG (control group), and HG (healthy group). Cluster is displayed in purple. Corresponding box plots show the distribution of mean SampEn values within the identified cluster for each subject. The p-value from the cluster-based permutation test and the Cliff’s delta (effect size) are reported for each comparison.
Figure 4.
Spatial distribution of the significant cluster in the theta band, identified using a nonparametric cluster-based permutation test (parameters: m = 3, r = 0.2), across three swallowing phases: A) before swallowing (BS), B) during swallowing (DS), and C) after swallowing (AS). Electrodes forming the significant cluster are highlighted for each group comparison: DG (dysphagic group), CG (control group), and HG (healthy group). Cluster is displayed in purple. Corresponding box plots show the distribution of mean SampEn values within the identified cluster for each subject. The p-value from the cluster-based permutation test and the Cliff’s delta (effect size) are reported for each comparison.
Figure 5.
Spatial distribution of the significant cluster in the alpha band, identified using a nonparametric cluster-based permutation test (parameters: m = 3, r = 0.2), across three swallowing phases: A) before swallowing (BS), B) during swallowing (DS), and C) after swallowing (AS). Electrodes forming the significant cluster are highlighted for each group comparison: DG (dysphagic group), CG (control group), and HG (healthy group). Cluster is displayed in purple. Corresponding box plots show the distribution of mean SampEn values within the identified cluster for each subject. The p-value from the cluster-based permutation test and the Cliff’s delta (effect size) are reported for each comparison.
Figure 5.
Spatial distribution of the significant cluster in the alpha band, identified using a nonparametric cluster-based permutation test (parameters: m = 3, r = 0.2), across three swallowing phases: A) before swallowing (BS), B) during swallowing (DS), and C) after swallowing (AS). Electrodes forming the significant cluster are highlighted for each group comparison: DG (dysphagic group), CG (control group), and HG (healthy group). Cluster is displayed in purple. Corresponding box plots show the distribution of mean SampEn values within the identified cluster for each subject. The p-value from the cluster-based permutation test and the Cliff’s delta (effect size) are reported for each comparison.
Figure 6.
Spatial distribution of the significant clusters in the beta band, identified using a nonparametric cluster-based permutation test (parameters: m = 3, r = 0.2), across three swallowing phases: A) before swallowing (BS), B) during swallowing (DS), and C) after swallowing (AS). Electrodes forming the significant cluster are highlighted for each group comparison: DG (dysphagic group), CG (control group), and HG (healthy group). When multiple significant clusters are detected, the first is shown in purple and the second in green. Corresponding box plots show the distribution of mean SampEn values within the identified cluster for each subject. The p-value from the cluster-based permutation test and the Cliff’s delta (effect size) are reported for each comparison.
Figure 6.
Spatial distribution of the significant clusters in the beta band, identified using a nonparametric cluster-based permutation test (parameters: m = 3, r = 0.2), across three swallowing phases: A) before swallowing (BS), B) during swallowing (DS), and C) after swallowing (AS). Electrodes forming the significant cluster are highlighted for each group comparison: DG (dysphagic group), CG (control group), and HG (healthy group). When multiple significant clusters are detected, the first is shown in purple and the second in green. Corresponding box plots show the distribution of mean SampEn values within the identified cluster for each subject. The p-value from the cluster-based permutation test and the Cliff’s delta (effect size) are reported for each comparison.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).