1. Introduction
The increasing global demand for renewable and sustainable energy sources has intensified interest in the valorization of lignocellulosic biomass as a feedstock for solid biofuels. Among various herbaceous species, Sorghum halepense (L.) Pers., Poaceae (Johnsongrass), has gained attention not only for its agronomic performance and influence, but also for its content of bioactive compounds, traditionally utilized in some pharmaceutical applications [
1]. Sorghum halepense, native to a broad region stretching from Macaronesia through Central Asia to Indochina [
2], is recognized as a major noxious weed worldwide, affecting more than 30 crops in at least 53 countries [
3]. It was introduced as a forage crop in regions such as the United States in the early 1800s. Its aggressive nature led to it escaping cultivation, a common pathway for invasive species [
4].
Its invasiveness is attributed to a dual reproductive strategy: extensive creeping rhizomes that ensure local persistence and prolific seed production that enables long-distance dispersal. Rhizome fragments, easily spread through tillage, readily regenerate, with a single plant capable of developing a substantial underground network—rhizomes often account for the majority of the plant’s biomass [
5,
6]. Simultaneously, S. halepense can produce up to 80,000 seeds per growing season, many of which remain viable for several years [
6]. These seeds disperse via wind, water, animals, and contaminated crop seed, complicating control efforts. The species also forms dense monospecific stands that outcompete surrounding vegetation, possibly supported by allelopathic interactions [
3,
6]. Being a well-known weed, Johnsongrass has been the subject of extensive and often futile eradication campaigns that have placed a heavy financial burden both on farmers and governments. The scientific community has predominantly focused on its harmful effects on crops, soils and the environment, neglecting its potential benefits. This omission underlines the urgent need to research and promote the positive aspects of Johnsongrass, fostering a more balanced understanding in agriculture and scientific sectors, as the possibilities of its use instead of extinction are far from being exausted [
6].
This study addresses the existing gap in the energetic valorization of Johgsongrass biomass in form of residues derived from pharmaceutical processing. While the raw Johnsongrass biomass has been previously studied for fuel applications, no systematic evaluation has been conducted on the energy characteristics of the biomass following bioactive compound extraction. Pharmaceutical plant waste, encompassing residues from production processes and unused or expired medicines, remains largely underutilized for energy recovery [
7]. Despite its substantial organic content, this biomass is often treated as low-value residue, with limited application in energy generation. This underutilization persists due to factors such as the ecotoxicity of active pharmaceutical ingredients (APIs) and the complexity of pharmaceutical waste streams, which complicate their valorization. Consequently, pharmaceutical waste is frequently disposed of through conventional methods like incineration or landfilling, rather than being harnessed for energy recovery. Addressing these challenges requires the development of specialized technologies and strategies to mitigate environmental risks and enhance the economic viability of utilizing pharmaceutical plant waste as a renewable energy source [
8].
This study challenges that notion by demonstrating that bioactive-extracted biomass can be significantly upgraded through hydrothermal carbonization (HTC), yielding solid fuels with enhanced combustion performance. Hydrothermal carbonization (HTC) represents an increasingly recognized thermochemical route for the valorization of wet lignocellulosic biomass, offering a direct conversion process that operates in aqueous environments at moderate temperatures (typically 180–260 °C) and under autogenous pressure [
9]. This process simulates accelerated coalification by facilitating a series of dehydration, decarboxylation, and condensation reactions, ultimately yielding a carbon-rich solid product known as hydrochar [
10,
11]. Compared to conventional dry thermochemical processes such as pyrolysis or torrefaction, HTC is particularly well-suited for high-moisture biomass, as it eliminates the need for prior drying, thereby reducing overall energy input and improving process economics [
12].
The hydrochars produced via HTC typically exhibit enhanced energy density, reduced oxygen and hydrogen contents (as reflected in lower O/C and H/C atomic ratios), and improved physical properties such as hydrophobicity and grindability. These characteristics contribute to their suitability as solid biofuels and facilitate their handling, storage, and combustion performance [
13,
14]. Furthermore, HTC offers additional environmental advantages, including the stabilization of organic matter, partial retention of nutrients in the solid phase, and a significant reduction in biomass volume, making it attractive for integrated waste-to-energy strategies. In this context, HTC has shown considerable promise for the conversion of unconventional and underutilized biomass streams, including agro-industrial and biorefinery residues [
15]. One such category, often overlooked in energy recovery frameworks, includes biomass residues from pharmaceutical plant processing. Although these residues frequently possess high organic content and latent calorific value, they are commonly treated as low-value waste due to their heterogeneous composition and the presence of trace bioactive compounds [
16]. The application of HTC to these residues not only offers a pathway to recover energy in the form of solid biofuels but also aligns with broader objectives of circular bioeconomy by promoting the sustainable utilization of sector-specific waste streams. Thus, HTC emerges as a versatile and scalable platform for converting complex biowastes-such as post-extraction pharmaceutical biomass-into functional energy carriers.
By applying HTC to both raw and extracted Johnsongrass, this study aims to (i) assess the influence of pharmaceutical extraction on the physicochemical and fuel properties of the biomass and (ii) evaluate how HTC treatment further enhances its bioenergy potential. Detailed analyses-including elemental composition, and Van Krevelen atomic ratios-were conducted to compare fuel quality, combustion behavior, and energy yield between raw and extracted biomass across multiple HTC temperatures. In addition, comprehensive combustion analysis was performed to elucidate the thermal degradation characteristics and stability of the produced hydrochars. To provide a robust statistical framework for interpreting the experimental data, regression analysis was applied.
This research introduces a dual-valorization pathway whereby a single feedstock serves two complementary functions: extraction of high-value bioactive compounds, followed by conversion of the residual biomass into solid biofuels. Such an integrated approach not only improves the overall resource efficiency but also contributes to sustainable waste management and circular bioeconomy objectives. To our knowledge, this is the first comprehensive study to explore the HTC-based upgrading of Johnsongrass following pharmaceutical extraction. The findings provide novel insights into how waste materials from biorefinery processes can be repurposed as renewable energy sources, reinforcing the synergy between bioenergy production and sustainable agricultural residue utilization.
2. Materials and Methods
2.1. Biomass Collection
The plant material was collected from a ruderal site at Kumodraž, southern suburbs of Belgrade, Serbia (coordinates: 44.747938 N, 20.495694 E). The collected material was identified by Assistant Professor Dr. Miloš Zbiljić from the Department of Botany, Faculty of Pharmacy, University of Belgrade. The voucher specimen was deposited in the Herbarium of the Department of Botany, Faculty of Pharmacy, University of Belgrade (HFF), under the number 4323.
Plant material was cut into smaller pieces and dried naturally in a shaded and well-ventilated place. Dried plant material was finally ground into a coarse powder with a laboratory grinder and stored in airtight containers until extraction.
2.2. Plant Material Extraction
A part of the dried plant material was successively extracted with solvents of increasing polarity (n-hexane, methanol; both Fischer Chemicals, UK) in a Soxhlet-type all-glass apparatus for continuous extraction until exhausted. Two test samples were obtained using this method: extracted (ES) and non-extracted sorghum (S).
2.3. HTC Experiments
For the HTC experiments we were used a laboratory autoclave reactor (CARL Roth, Model II, Germany) equipped with a cooling system, temperature and pressure controller. For each experiment, 10 g of biomass (S and ES) was combined with 150 mL of deionized water in autoclave (1:15 ratio). Mixtures were heated at different carbonization temperatures (180, 200, 220, 240 and 260 °C) with a residence time of 60 min at constant heating rate (4°C/min). After cooling the autoclave, the solid-liquid mixtures were separated by filtration, washed with deionized water and dried at 105 °C for 24h to ensure a consistent weight. The obtained hydrochars were labelled: S180, S200, S220, S240, S260 and ES180, ES200, ES220, ES240, ES260.
2.4. Characterization
Elemental analysis (C, H, N, S) of investigated samples was performed by elemental analyzer (Vario EL II CHNS) while the content of O was calculated by subtraction of C, H, S, N and ash content (%) from 100%.
The moisture and ash contents and the volatile matters (VM) were determined in accordance with standard ASTM D176284 (2007) procedure while fixed carbon (FC) was calculated by their subtraction from 100%.
2.5. Statistical Analysis
To optimize the hydrothermal carbonization (HTC) process conditions based on hydrochar characteristics, regression analysis was performed. The regression models were developed using the built-in data analysis tools available in Microsoft Excel 2010 (Version 14.0.6023.1000, 32-bit, Microsoft Office Professional Plus 2010, Microsoft Corp., Redmond, WA, USA).
2.6. Thermogravimetric Analysis
The combustion properties of investigated samples were analyzed by TG-DTA analysis using a Netzsch STA 409 EP Analyzer (Selb, Germany) in the temperature range 50-700 °C with heating rates 10 °C/min.
3. Results and Discussions
3.1. Physicochemical Properties
3.1.1. Elemental Analysis
Table 1 presents the physicochemical properties of the S, ES and corresponded hydrochars. The outcomes of elemental analysis (
Table 1) showed that C content increased from 41.11 to 60.47 % for S and from 41.72 to 63.49 % for ES, while the content of H and O decreased during HTC process of S and ES. This is likely due to dehydration and decarboxylation reactions that occur during high-temperature carbonization, leading to the densification of carbon and the removal of oxygen [
17]. The nitrogen content in the hydrochar slightly increased, from 2.04 to 2.20% in S and from 0.63 to 1.20% in ES. This increase is likely due to the hydrolysis of carbohydrates in the raw materials during HTC, which led to a higher concentration of reducing sugars. These sugars compounds subsequently reacted with proteins through the Maillard reaction, resulting in the formation of nitrogen-containing heterocyclic compounds [
18,
19]. It is also important to emphasize that, with increasing HTC severity, a marked decrease in N content was observed in the produced hydrochars. This is a highly advantageous characteristic for fuel applications, as the reduced levels of N significantly minimize the emission of nitrogen oxides during direct combustion. In this context, HTC not only enhances the energy density of biomass but also yields cleaner solid fuels with lower environmental impact, making the process particularly attractive for sustainable energy systems [
20]. Due to the low N contents in S and ES hydrochars, NOx emissions are expected to be in the range of emission limits [
21].
The elemental composition of the samples was further analyzed and visualized using the Van Krevelen diagram. The Van Krevelen diagram was used to show the degree of coalification of S and ES during HTC synthesis (
Figure 1). The figure shows that the H/C and O/C atomic ratio decreases in hydrochar indicating a high degree of S and ES coalification. This is a result of the removal of a significant amount of oxygen from the S and ES during dehydration and decarboxylation process, which subsequently enhances the carbonization level of the hydrochar [
22]. The decrease in the O/C ratio was more notable than the reduction in the H/C ratio, highlighting the substantial effect of applied hydrothermal temperature on the removal of oxygen.
The Van Krevelen diagram also provides information about the hydrochar classifications obtained following various types of fossil fuels. As can be seen from the diagram (
Figure 1), S and ES-derived hydrochars were spread in the range of biomass, peat, and lignite. Moreover, the carbonization process conducted at 260 °C effectively transforms biomass into valuable carbon-rich material based on its elemental composition, categorized in the lignite region, while S 260 is almost on the border between lignite and coal. It is important to note that hydrochars produced from ES are different from those obtained from untreated biomass, likely because of the presence of digested organic structure after extraction of S.
3.1.2. Proximate Analysis
According to proximate analysis, VM content decreases significantly (from 93.25 to 65.19 % in S and 95.15 to 60.82 % in ES). In contrast, FC content significantly increases in single hydrochar during HTC synthesis of S and ES (
Table 1). This can be attributed to a series of reactions in the high-temperature aqueous solution, which leads to the expulsion of volatile substances and the compression of carbon [
23]. Reducing the volatile matter content in biomass when converting it to hydrochar enhances its suitability as a solid fuel, as it leads to a more stable combustion process, improved energy output, and a reduced tendency for unwanted emission during burning. At the same time, the ash content showed a minor decrease at lower temperatures but increased at higher temperatures, with the total ash content in the hydrochar remaining lower than in S and ES. This indicates that inorganic components from raw materials may have been leached into process water [
17]. Based on the observed reductions in VM, increases in FC content, and more favorable ash behavior during hydrothermal carbonization, ES demonstrates superior fuel properties compared to S, indicating its greater potential as a solid biofuel.
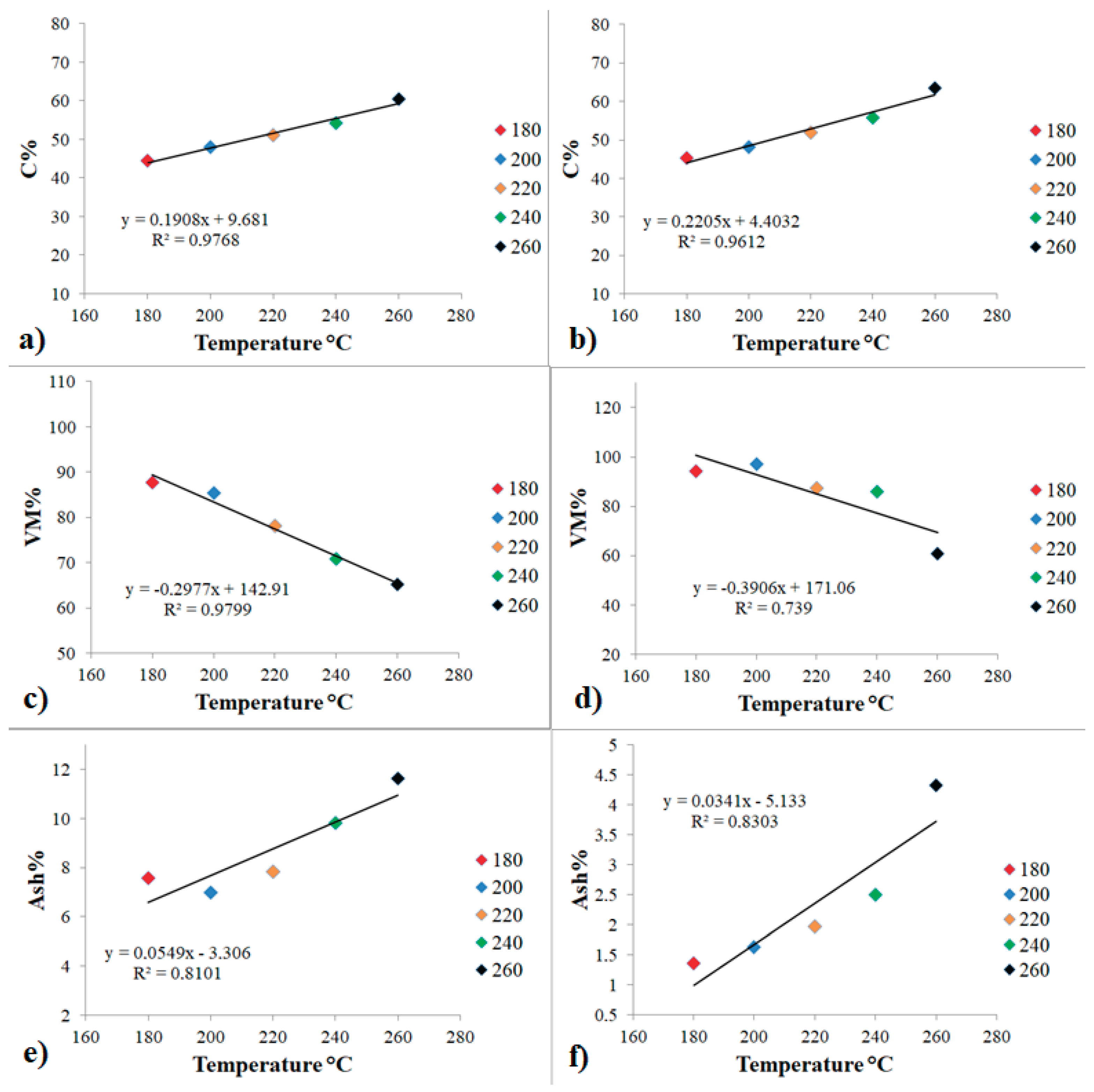
3.2. Statistical Analysis
To rigorously elucidate the influence of HTC temperature on the C%, VM% and Ash% of S and ES hydrochars, regression analysis was utilized (
Figure 2). This approach enables the quantification of linear relationships between process temperature and hydrochar properties, facilitating statistical validation of correlations and providing predictive insight into process-performance linkages. The use of regression modeling thus supports the identification of key parameters driving material transformation during HTC and offers a robust framework for optimizing process conditions to enhance fuel quality.
Statistical analysis revealed a strong linear correlation between HTC temperature and key fuel-related parameters, particularly carbon content, with coefficients of determination exceeding 0.90. This relationship suggests that increasing the HTC process temperature facilitates enhanced carbon enrichment, thereby improving the fuel quality of hydrochars [
24]. In parallel, a substantial decrease in volatile matter content (VM%) was observed with rising temperatures, a trend consistent with the intensification of devolatilization reactions during HTC [
25]. A pronounced correlation between temperature and VM% was identified in hydrochars derived from S series, whereas those produced from extracted materials (ES) exhibited a more moderate association (R² = 0.739), potentially reflecting intrinsic differences in feedstock reactivity. Ash content displayed a positive correlation with HTC temperature, likely resulting from the concentration of inorganic residues as labile organic matter is thermally degraded. These trends are in line with previous findings on the HTC of Paulownia leaves, further supporting the conclusion that elevated process temperatures contribute to the enhancement of hydrochar fuel properties through targeted physicochemical transformation [
26].
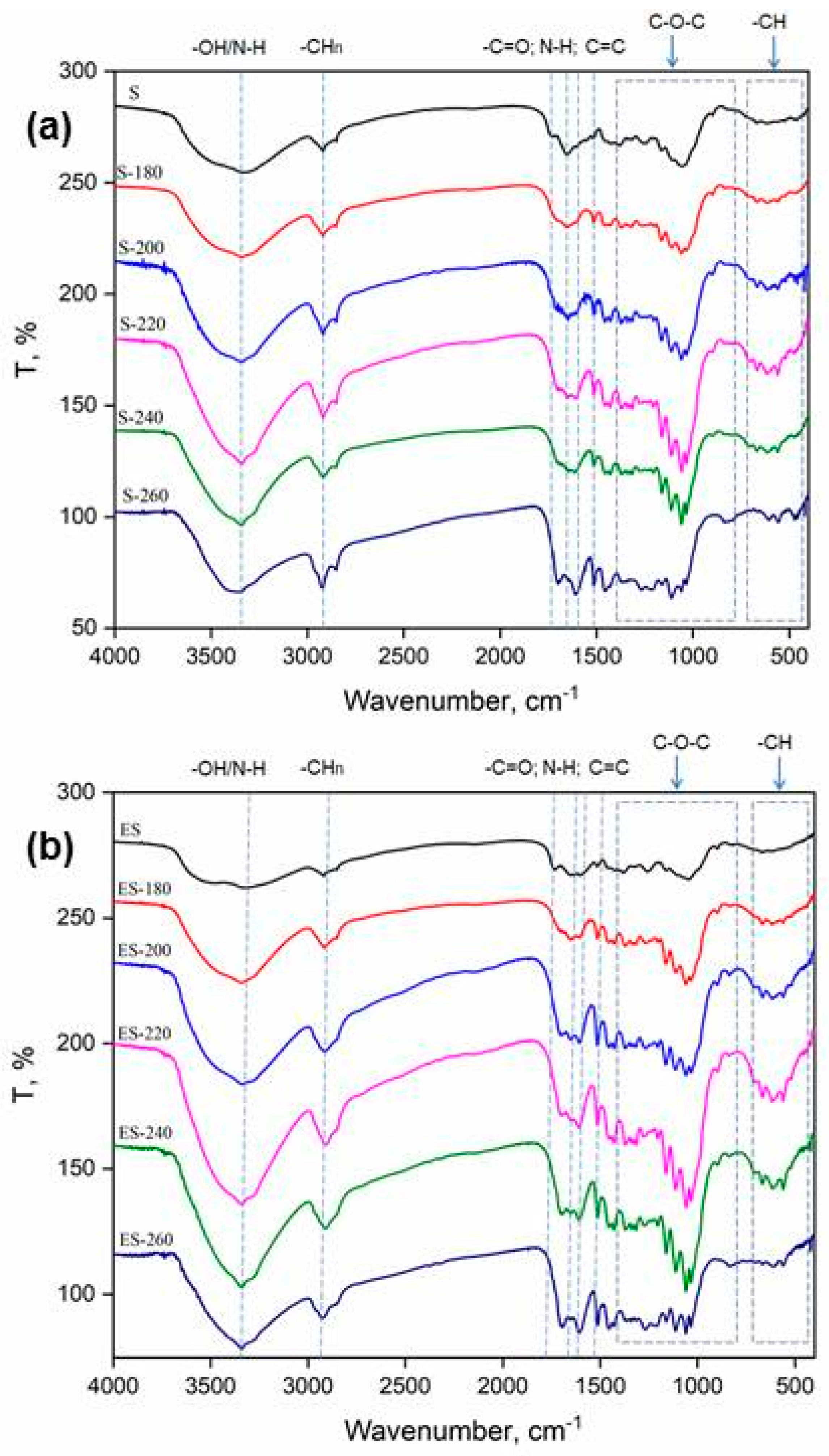
3.3. Chemical Structure
FTIR spectra are essential for examining the alterations in surface functional groups during the transformation of S and ES into their hydrochar.
Table 2 summarizes the information about the position of functional groups in the FTIR spectrum.
As can be seen from
Figure 3, several significant changes occur upon extraction and the HTC process which indicate an improvement in the fuel properties of the material. The observed peak between 3700–3200 cm⁻¹ originates from the overlapping of O–H and N–H stretching vibrations from S and ES [
27]. Notably, the broad absorption band in the 3300–3400 cm⁻¹ region is primarily attributed to N–H stretching vibrations, which indicates the formation of amine or amide groups during the HTC process. Distinct absorption bands around 1650 cm⁻¹ and 1540 cm⁻¹, along with a broad and increasingly intense band near 3300 cm⁻¹ at higher HTC treatment temperatures, strongly indicate the formation of amide structures. The band near 3300 cm⁻¹ corresponds to N–H stretching vibrations, while those at 1650 cm⁻¹ and 1540 cm⁻¹ correspond to amide I (C=O stretching) and amide II (N–H bending) modes, respectively [
27]. In biomass containing nitrogenous compounds such as proteins, HTC promotes Maillard-type reactions between reducing sugars and amino acids. These reactions lead to the formation of nitrogen-enriched aromatic structures, including amides, heterocycles, and melanoidin-like polymers. Consequently, the observed FTIR features reflect the progressive nitrogen functionalization of the carbon matrix with increasing temperature, highlighting not only the structural transformation but also the development of surface functionalities that enhance the material’s reactivity and adsorption capacity in aqueous environments. At 260 °C, the decrease in intensity of the 3300 cm⁻¹ band suggests thermal degradation or transformation of N–H-containing groups into more stable aromatic or heterocyclic nitrogen structures, accompanied by a reduction in hydrogen bonding and surface polarity [
31]. This phenomenon is particularly noticeable in the ES sample, where the removal of nonpolar components from the biomass enables a more pronounced expression of the remaining polar groups. As a result, the ES sample exhibits a stronger manifestation of nitrogen-containing functional groups, further contributing to its properties.
The stretching vibration peak between 3000 and 2800 cm
-1 assigned to aliphatic -CH
n structure [
28] showed specific differences after extraction and HTC process. Notably, the peak intensity is higher in the raw S compared to the ES, which can be attributed to the removal of lipids and other organic components during extraction. On the other hand, during the HTC process of both materials (S and ES), as the temperature increases, the intensity of this peak rises, suggesting that the generation of aliphatic compounds takes place during the HTC process [
18]. The spectral behavior remains similar up to 240°C; however, at 260°C, a significant difference is observed in the spectra. The ES 260 spectrum exhibits a marked decrease in intensity, indicating a more pronounced formation of aromatic, energy-rich structures at this temperature. This observation aligns with the results from the Van Krevelen diagram (
Figure 1), indicating that extraction results in shift toward the down-left movement of ES on the diagram to region characteristic for lignin.
The stretching vibration peak at 1737 and 1654 cm
-1 assigned to C=O and C=C from hemicellulose is visible only in S spectra [
24]. Following extraction, there is a marked reduction in peak intensity, which strongly suggests the partial degradation of hemicellulose as a result of the extraction process. As the temperature increases during the HTC process, this peak shifts and clusters in the spectral range around 1700 cm
−1 indicating formation of carboxyl, carbonyl or ester groups at both materials [
18].
The peak in the spectral range at 1526 cm
-1 assigned to C=C vibration was slightly enhanced during the HTC process, implying a higher degree of aromaticity in obtained hydrochars [
29].
Furthermore, the peaks in the range of 1220 - 945 cm
−1 are attributed to C–O–C aliphatic ethers. Following HTC treatment, the intensity of the peaks decreases, likely due to the high-temperature process that promotes hydrolysis and dehydration of carbohydrates, leading to the breakdown of compounds [
18,
22]. This reduction is particularly evident at 260°C for both S and ES samples, indicating that dehydration and subsequent degradation of carbohydrates facilitate the formation of more aromatic, energy-dense structures. The resulting transformation into aromatic compounds and carbon-rich structures enhances the material's thermal stability, contributing to an overall increase in energy density.
Finally, the differences in the peaks in the spectral range between 700-500 cm
−1, which correspond to C–H stretching vibrations on the aromatic nucleus [
30], indicate changes in aromatic structure caused by the HTC process.
3.4. Apparent Changes Analysis
A noticeable difference in color between samples S and ES is observed, which can be attributed to the extraction process (
Figure 4). As the HTC temperature increases, both samples exhibit a gradual color transition from brown to black. Interestingly, at a constant hydrothermal temperature of 240 °C, only minor changes in the color of the hydrochar are observed, regardless of further processing (
Figure 5). This behavior is observed consistently in both materials. It is mainly attributed to the Maillard reaction taking place during the HTC process, which results in the formation of low-molecular-weight melanoidins, ultimately giving the hydrochar its characteristic black color [
33]. The Maillard reaction was previously confirmed by the FTIR analysis in this study, where changes in functional groups correspond well with this reaction mechanism. Additionally, the structural morphology of both samples becomes increasingly uniform as the treatment temperature rises, indicating enhanced homogeneity at higher thermal conditions.
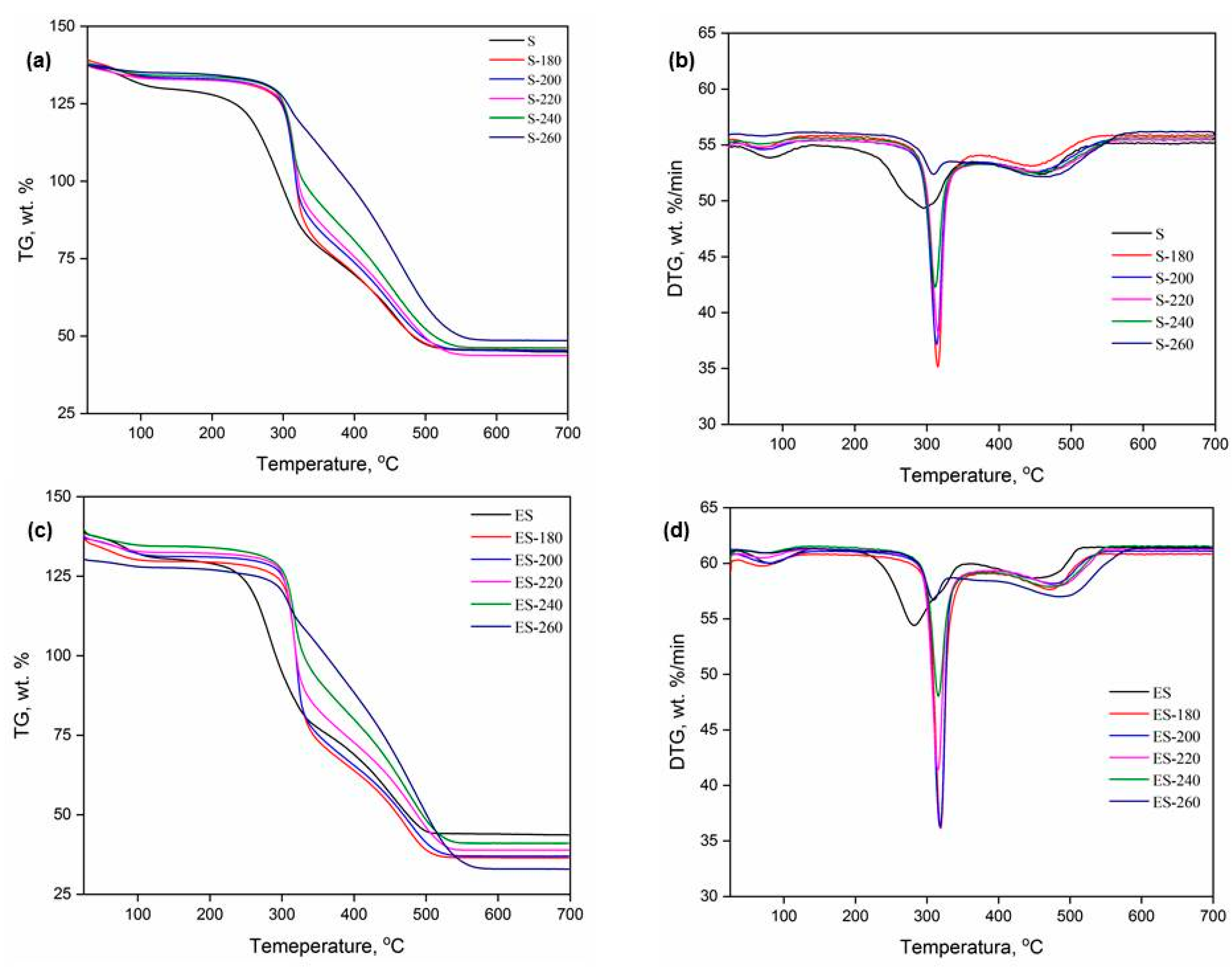
3.5. Combustion Analysis
The TG and DTG curves of S, ES, and their corresponding hydrochars (
Figure 5) reveal distinct thermal behaviours that are in line with lignocellulosic degradation pathways and the chemical evolution induced by HTC treatment. Three main decomposition regions can be identified, consistent with literature data on lignocellulosic materials [
7,
34,
35].
In the first region (up to ~150 °C), weight loss is associated with moisture evaporation and the release of light volatile compounds. This is typical for both S and ES and hydrochars and aligns with findings from multiple studies where a small DTG peak or endothermic signal is attributed to physically bound water and low molecular weight volatiles [
36].
In the second thermal region (~240–340 °C), the S exhibits a broad DTG peak around 300 °C, suggesting the decomposition of diverse biomass constituents—mainly hemicellulose and partially cellulose. With HTC treatment at 180 °C, the peak increases in intensity but remains broad, reflecting the initial thermal degradation of hemicellulose. As the HTC temperature increases to 220 and 260 °C, the same peak becomes weaker and narrower, indicating the progressive removal of hemicellulose and early-stage carbonization and cross-linking. This confirms that hemicellulose is the first to degrade, particularly during HTC at ~180 °C, and that its degradation contributes to changes in the DTG peak intensity and shape [
37]. In the ES sample, this DTG peak is narrower and slightly shifted to lower temperatures, indicating earlier decomposition onset and a more uniform decomposition profile. This behavior results from the absence of low-temperature extractive compounds and a cleaner carbohydrate matrix, as observed in similar systems in the literature [
17,
37]. Thus, extractive removal modifies thermal decomposition behavior, leading to sharper and more defined DTG peaks at slightly lower temperatures. Hydrochars derived from S and ES show similar thermal patterns in this second region. Initially, the DTG peak becomes sharper and more intense, suggesting the formation of more reactive and homogeneous carbon structures. At higher HTC temperatures, the same peak diminishes, which is attributed to further crosslinking and the formation of stable aromatic networks. This behavior illustrates that HTC leads to gradual thermal stabilization and carbon densification, as observed in other hydrochar systems [
23,
38,
39].
In the third region (>400 °C), S and ES samples show broader and later DTG peaks due to lignin and partially decomposed cellulose, which are more thermally stable. In hydrochars, this peak shifts to lower temperatures or decreases in intensity, indicating that lignin is partially depolymerized during HTC, and more aromatic, carbon-rich structures are formed that combust more uniformly and at lower [
36,
37,
40]. These changes reflect the progressive transformation of biomass into more stable, carbonized structures.
Moreover, the narrower and more unified DTG profiles of hydrochars indicate reduced moisture retention and enhanced hydrophobicity, confirming literature data that HTC improves biomass's physicochemical stability and thermal behavior [
36]. These changes correspond to a more homogenous, hydrophobic, and combustion-stable product.
The detailed combustion characteristic parameters derived from TG and DTG profiles are summarized in
Table 3. The raw samples, S and ES, exhibited relatively low ignition temperatures (Ti = 203 °C and 192 °C, respectively), which were significantly lower than those observed for their corresponding hydrochars. An increase in the hydrothermal carbonization (HTC) temperature led to a noticeable rise in Ti of the produced hydrochars. Specifically, Ti increased from 218 °C (S 180) to 244 °C (S 260), and from 206 °C (ES 180) to 231 °C (ES 260). This increase in ignition temperature is attributed to the transformation and reduction of volatile matter content in the raw materials and in hydrochars produced at lower HTC temperatures [
41,
42]. The lower ignition temperature (Ti) observed for ES compared to S can be attributed to changes in the surface characteristics of the extracted material.
The temperature corresponding to the maximum combustion rate (Tm) showed a notable rise with increasing HTC temperature, ranging from approximately 290 °C to 310 °C in S samples and from 280 °C to 310 °C in ES samples. This trend suggests a longer and more controlled combustion process for hydrochars treated at elevated temperatures. Burnout temperature (Tb) similarly increased with HTC temperature, with ES samples reaching up to 570 °C, surpassing the maximum Tb values of raw sorghum (around 490 °C). This progression indicates enhanced thermal resistance and completeness of combustion for hydrochars derived from higher HTC conditions and after solvent extraction.
For a deep understand the combustion performance of the S and ES and hydrochars, activation energy (Ea) was calculated using the Kissnger method [
43]:
where, β is heating rate [K/min], T
max is peak temperature [K] corresponding to the maximum mass loss rate, Ea is activation energy [J/mol], R is universal gas constant 8.314 J/mol.
Activation energy (Ea) exhibited a slight increase as HTC temperature rose, with ES samples generally demonstrating higher Ea values than S samples, implying a greater energy requirement for combustion. Meanwhile, the maximum weight loss rate (DTGmax) decreased with increasing HTC temperature, consistent with a more gradual combustion profile.
In summary, organic solvent extraction coupled with elevated HTC temperatures significantly improves the thermal stability and combustion characteristics of sorghum-derived hydrochars. The observed reduction in ignition temperature combined with higher burnout temperatures and activation energies suggests the formation of more thermally robust materials with potential for efficient energy recovery in combustion applications.
4. Conclusions
This study comprehensively evaluated the effects of organic solvent extraction and hydrothermal carbonization (HTC) temperature on the physicochemical properties, combustion behavior, and energy characteristics of Jonhsongrassbiomass. The results demonstrate that solvent extraction significantly modifies the biomass matrix by removing extractives and enriching carbon content, which in turn enhances the fuel properties of the produced hydrochars. Increasing HTC temperature led to progressive carbon densification, reduction of volatile matter, and improvement in combustion stability, as evidenced by increased ignition and burnout temperatures, elevated activation energies, and more uniform thermal degradation profiles. The reduction in nitrogen contents with increasing HTC severity further underscores the environmental benefits of the process by potentially lowering NOx emissions during combustion.
Overall, the integration of organic solvent extraction with optimized HTC processing offers a promising approach to producing high-quality, energy-dense, and environmentally friendly solid biofuels from lignocellulosic biomass. These findings provide valuable insights into the design of sustainable bioenergy systems, advancing the efficient conversion of agricultural residues into cleaner and more efficient renewable fuels.
Author Contributions
Conceptualization, M.S. and J.P.; methodology, M.E. and J:P.; software, M.K..; validation, M.E., J.P. and J.D.; formal analysis, J.D. and M.K.; investigation, M.S.; resources, J.D., M.O. and D.S.; data curation, M.K. and M.O.; writing—original draft preparation, M.S.; writing—review and editing, J.P.; visualization, J.P and M.K.; supervision, M.S.; project administration, M.E.; funding acquisition, J.P.
Funding
Please add: This research received no external funding
Data Availability Statement
We encourage all authors of articles published in MDPI journals to share their research data. In this section, please provide details regarding where data supporting reported results can be found, including links to publicly archived datasets analyzed or generated during the study. Where no new data were created, or where data is unavailable due to privacy or ethical restrictions, a statement is still required. Suggested Data Availability Statements are available in section “MDPI Research Data Policies” at
https://www.mdpi.com/ethics.
Acknowledgments
The authors are grateful to the Ministry of Science, Technological Development and Innovation of the Republic of Serbia for its financial support (contract no. 451-03-136/2025-03/200023 and 451-03-136/2025-03/200168). This research was supported (in part) by the Science Fund of the Republic of Serbia, contract number: 7752847 - Value-Added Products from Maize, Wheat and Sunflower Waste as Raw Materials for Pharmaceutical and Food Industry (PhAgroWaste); Principal Investigator Prof. dr Zoran Maksimović, University of Belgrade - Faculty of Pharmacy.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kanbar, A.; Flubacher, N.; Hermuth, J.; Kosová, K.; Horn, T.; Nick, P. Mining Sorghum Biodiversity—Potential of Dual-Purpose Hybrids for Bio-Economy. Diversity 2021, 13, 192. [CrossRef]
- POWO. Plants of the World Online; Facilitated by the Royal Botanic Gardens, Kew. Published online: https://powo.science.kew.org/ (accessed on 25 June 2025).Author 1, A.; Author 2, B. Book Title, 3rd ed.; Publisher: Publisher Location, Country, 2008; pp. 154–196.
- Peerzada, A.M.; Ali, H.H.; Hanif, Z.; Bajwa, A.A.; Kebaso, L.; Frimpong, D.; Iqbal, N.; Namubiru, H.; Hashim, S.; Rasool, G.; et al. Eco-Biology, Impact, and Management of Sorghum halepense (L.) Pers. Biol. Invasions 2023, 25, 955–973. [CrossRef]
- Klein, P.; Smith, C.M. Invasive Johnsongrass, a Threat to Native Grasslands and Agriculture. Biologia 2021, 76, 413–420. [CrossRef]
- Ceseski, A.; Al-Khatib, K.; Dahlberg, J.A. Biology and Management of Johnsongrass (Sorghum halepense); University of California Agriculture and Natural Resources: Oakland, CA, USA, 2017; Publication 8569. [CrossRef]
- Tyagi, V.C.; Vijayakumar, S.; Bagavathiannan, M.; Govindasamy, P.; Chander, S. Harnessing Johnsongrass (Sorghum halepense): Turning a Weed into a Resource. Indian J. Weed Sci. 2025, 57, 16–26. [CrossRef]
- Su, H.; Zhou, X.; Zheng, R.; Zhou, Z.; Zhang, Y.; Zhu, G.; Yu, C.; Hantoko, D.; Zan, M. Hydrothermal Carbonization of Food Waste after Oil Extraction Pre-Treatment: Study on Hydrochar Fuel Characteristics, Combustion Behavior, and Removal of Sodium and Potassium. Sci. Total Environ. 2021, 754, 142192. [CrossRef]
- Pech-Rodríguez, W.J.; Meléndez-González, P.C.; Hernández-López, J.M.; Suarez-Velázquez, G.G.; Sarabia-Castillo, C.R.; Calles-Arriaga, C.A. Pharmaceutical Wastewater and Sludge Valorization: A Review on Innovative Strategies for Energy Recovery and Waste Treatment. Energies 2024, 17, 5043. [CrossRef]
- Petrović, J.; Ercegović, M.; Simić, M.; Koprivica, M.; Dimitrijević, J.; Jovanović, A.; Janković Pantić, J. Hydrothermal Carbonization of Waste Biomass: A Review of Hydrochar Preparation and Environmental Application. Processes 2024, 12, 207. [CrossRef]
- Potrč, S.; Petrovič, A.; Egieya, J.M.; Čuček, L. Valorization of Biomass Through Anaerobic Digestion and Hydrothermal Carbonization: Integrated Process Flowsheet and Supply Chain Network Optimization. Energies 2025, 18, 334. [CrossRef]
- Shahnawazi, A.A.; Merckel, R.; Carvalho, L.; Schwede, S. Hydrothermal Carbonisation of Waste-Activated Sludge: Possible Route to Improve Sludge Management in Municipal Wastewater Treatment Facilities. Bioresour. Technol. 2025, 432, 132655. [CrossRef]
- Güleç, S.; Yılmaz, M. Progress in Lignocellulosic Biomass Valorization for Biofuels and Value-Added Chemical Production in the EU: A Focus on Thermochemical Conversion Processes. Biofuels Bioprod. Bioref. 2024, 18, 123–145. [CrossRef]
- Bao, C.; Cao, Y.; Zhao, L.; Li, X.; Zhang, J.; Mao, C. Biofuel Production from Phytoremediated Biomass via Various Conversion Routes: A Review. Energies 2025, 18, 822. [CrossRef]
- Saba, S.; Sulaiman, S.; Sulaiman, F. Evaluation of Fuel and Combustion Properties of Hydrochar Derived from Co-Hydrothermal Carbonization of Biomass and Plastic. Biomass Bioenergy 2023, 173, 106672. [CrossRef]
- Seray, S.; Azargohar, R.; Borugadda, V.B.; Dalai, A.K. Energy Recovery from Agro-Forest Wastes through Hydrothermal Carbonization Coupled with Hydrothermal Co-Gasification: Effects of Succinic Acid on Hydrochars and H2 Production. Chemosphere 2023, 337, 139390. [CrossRef]
- Cavali, M.; Junior, N.L.; de Sena, J.D.; Woiciechowski, A.L.; Soccol, C.R.; Filho, P.B.; Bayard, R.; Benbelkacem, H.; de Castilhos Junior, A.B. A Review on Hydrothermal Carbonization of Potential Biomass Wastes, Characterization and Environmental Applications of Hydrochar, and Biorefinery Perspectives of the Process. Sci. Total Environ. 2023, 857, 159627. [CrossRef]
- Petrović, J.; Perišić, N.; Maksimović Dragišić, J.; Maksimović, V.; Kragović, M.; Stojanović, M.; Laušević, M.; Mihajlović, M. Hydrothermal Conversion of Grape Pomace: Detailed Characterization of Obtained Hydrochar and Liquid Phase. J. Anal. Appl. Pyrolysis 2016, 118, 267–277.
- Wu, S.; Wang, Q.; Wu, D.; Cui, D.; Wu, C.; Bai, J.; Xu, F.; Liu, B.; Shan, Z.; Zhang, J. Influence of Temperature and Process Water Circulation on Hydrothermal Carbonization of Food Waste for Sustainable Fuel Production. J. Energy Inst. 2024, 107, 101459. [CrossRef]
- Kraszkiewicz, A.; Przywara, A. Impact of Ignition Technique on Pollutants Emission during the Combustion of Selected Solid Biofuels. Energies 2020, 13, 2664. [CrossRef]
- Petrović, J.; Simić, M.; Mihajlović, M.; Koprivica, M.; Kojić, M.; Nuić, I. Upgrading Fuel Potentials of Waste Biomass via Hydrothermal Carbonization. Hem. Ind. 2021, 75, 297–305. [CrossRef]
- Akarsu, K.; Duman, G.; Yilmazer, A.; Keskin, T.; Azbar, N.; Yanik, J. Sustainable Valorization of Food Wastes into Solid Fuel by Hydrothermal Carbonization. Bioresour. Technol. 2019, 292, 121959. [CrossRef]
- Wu, S.; Wang, Q.; Cui, D.; Wang, X.; Wu, D.; Bai, J.; Xu, F.; Wang, Z.; Zhang, J. Analysis of Fuel Properties of Hydrochar Derived from Food Waste and Biomass: Evaluating Varied Mixing Techniques Pre/Post-Hydrothermal Carbonization. J. Clean. Prod. 2023, 430, 139660. [CrossRef]
- Periyavaram, S.; Uppala, L.; Sivaprakash, S.; Reddy, H.P. Thermal Behaviour of Hydrochar Derived from Hydrothermal Carbonization of Food Waste Using Leachate as Moisture Source: Kinetic and Thermodynamic Analysis. Bioresour. Technol. 2023, 373, 128734. [CrossRef]
- Chen, C.; Liang, W.; Fan, F.; Wang, C. The Effect of Temperature on the Properties of Hydrochars Obtained by Hydrothermal Carbonization of Waste Camellia oleifera Shells. ACS Omega 2021, 6, 16546–16552. [CrossRef]
- Portilla-Amaguana, A.; Barraza-Burgos, J.; Guerrero-Perez, J.; Bourgadda, V.B.; Dalai, K. Hydrothermal Carbonization of Green Harvesting Residues from Sugar Cane: Effect of Temperature and Water/GHR Ratio on Mass and Energy Yield. ACS Omega 2024, 9, 26325–26335. [CrossRef]
- Koprivica, M.; Petrović, J.; Ercegović, M.; Simić, M.; Milojković, J.; Šoštarić, T.; Dimitrijević, J. Improvement of Combustible Characteristics of Paulownia Leaves via Hydrothermal Carbonization. Biomass Convers. Biorefin. 2024, 14, 3975–3985. [CrossRef]
- Afolabi, O.O.D.; Ohail, M.; Thomas, C.L.P. Characterization of Solid Fuel Chars Recovered from Microwave Hydrothermal Carbonization of Human Biowaste. Energy 2017, 134, 74–89. [CrossRef]
- Wu, S.; Wang, Q.; Fang, M.; Wu, D.; Cui, D.; Pan, S.; Bai, J.; Xu, F.; Wang, Z. Hydrothermal Carbonization of Food Waste for Sustainable Biofuel Production: Advancements, Challenges, and Future Prospects. Sci. Total Environ. 2023, 897, 165327. [CrossRef]
- Wu, S.; Wang, Q.; Cui, D.; Wu, D.; Bai, J.; Qin, H.; Xu, F.; Wang, Z. Insights into the Chemical Structure Evolution and Carbonisation Mechanism of Biomass during Hydrothermal Treatment. J. Energy Inst. 2023, 108, 101257. [CrossRef]
- Zhang, L.; Wang, Q.; Xu, F.; Wang, Z.; Zhang, G. Insights into the Evolution of Chemical Structures in Hydrochars from Hydrothermal Carbonization of PVC. J. Energy Inst. 2022, 105, 323–333. [CrossRef]
- Hunt, P.A. Why Does a Reduction in Hydrogen Bonding Lead to an Increase in Viscosity for the 1-Butyl-2,3-Dimethyl-Imidazolium-Based Ionic Liquids. J. Phys. Chem. B 2007, 111, 4844–4850. [CrossRef]
- Wądrzyk, M.; Katerla, J.; Janus, R.; Lewandowski, M.; Plata, M.; Korzeniowski, Ł. High-Energy-Density Hydrochar and Bio-Oil from Hydrothermal Processing of Spent Coffee Grounds—Experimental Investigation. Energies 2024, 17, 6446. [CrossRef]
- Wang, T.; Zhai, Y.; Li, H.; Zhu, Y.; Li, S.; Peng, C.; Wang, B.; Wang, Z.; Xi, Y.; Wang, S.; Li, C. Co-Hydrothermal Carbonization of Food Waste-Woody Biomass Blend towards Biofuel Pellets Production. Bioresour. Technol. 2018, 267, 371–377. [CrossRef]
- Peng, C.; Zhai, Y.; Zhu, Y.; Xu, B.; Wang, T.; Li, C.; Zeng, G. Production of Char from Sewage Sludge Employing Hydrothermal Carbonization: Char Properties, Combustion Behavior and Thermal Characteristics. Fuel 2016, 176, 110–118. [CrossRef]
- Cui, D.; Zhang, B.; Liu, Y.; Wu, S.; Wang, X.; Wang, Q.; Zhang, X.; Fattahi, M.; Zhang, J. Hydrochar from Co-Hydrothermal Carbonization of Sewage Sludge and Sunflower Stover: Synergistic Effects and Combustion Characteristics. J. Anal. Appl. Pyrolysis 2024, 183, 106777. [CrossRef]
- Poomsawat, S.; Poomsawat, W. Analysis of Hydrochar Fuel Characterization and Combustion Behavior Derived from Aquatic Biomass via Hydrothermal Carbonization Process. Case Stud. Therm. Eng. 2021, 27, 101255. [CrossRef]
- Mikusińska, J.; Szkadłubowicz, K.; Prus, Z.; Kuźnia, M.; Gajek, M.; Wilk, M. Fuel Properties Characterization of Hydrochars Derived from Agricultural Digestate. Renew. Energy 2025, 244, 122639. [CrossRef]
- Xie, X.; Peng, C.; Song, X.; Peng, N.; Gai, C. Pyrolysis Kinetics of the Hydrothermal Carbons Derived from Microwave-Assisted Hydrothermal Carbonization of Food Waste Digestate. Energy 2022, 245, 123269. [CrossRef]
- Fu, M.; Mo, C.; Li, H.; Zhang, Y.; Huang, W.; Wong, M.H. Comparison of Physicochemical Properties of Biochars and Hydrochars Produced from Food Wastes. J. Clean. Prod. 2019, 236, 117599. [CrossRef]
- Czerwińska, K.; Mikusińska, J.; Błoniarz, A.; Śliz, M.; Wilk, M. The Effect of Residence Time during the Hydrothermal Carbonization Process of Sewage Sludge on the Properties of Hydrochar. Energies 2024, 17, 3380. [CrossRef]
- Wang, T.; Zhai, Y.; Zhu, Y.; Peng, C.; Xu, B.; Wang, T.; Li, C.; Zeng, G. Influence of Temperature on Nitrogen Fate during Hydrothermal Carbonization of Food Waste. Bioresour. Technol. 2018, 247, 182–189. [CrossRef]
- Pala, M.; Kantarli, I.C.; Buyukisik, H.B. Hydrothermal Carbonization and Torrefaction of Grape Pomace: A Comparative Evaluation. Bioresour. Technol. 2014, 161, 255–262. [CrossRef]
- Kissinger, H.E.; Reaction kinetics in differential thermal analysis. Analytical Chemistry 1957, 29, 1702-1706. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).