1. Introduction
Recent randomized controlled trials (RCTs), including the DISTAL and ESCAPE-MeVO trials, have not demonstrated a clear clinical benefit of mechanical thrombectomy (MT) for medium vessel occlusion (MeVO) in acute ischemic stroke (AIS)1,2. However, these studies have notable limitations, particularly with respect to device selection bias. In the ESCAPE-MeVO trial, the Solitaire device was mandated as the first-line treatment. In the DISTAL trial, a combined technique was employed in approximately 65% of cases, whereas stent retrievers and the A Direct Aspiration First Pass Technique (ADAPT) alone were each used in about 16% of cases. Notably, small-bore aspiration catheters were not utilized in either trial.
In the present study, we investigate the clinical outcomes associated with small-bore aspiration catheters compared to other first-line approaches. Our objective is to identify effective treatment strategies for MeVO by quantitatively assessing the influence of device selection on clinical outcomes.
2. Materials and Methods
This cohort study was conducted in accordance with the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines [
6]. For this retrospective analysis, all patients had previously provided general consent for the anonymized use of their clinical data in future research. Institutional review board approval was obtained from the Kobe City Medical Center General Hospital Institutional Review Board (approval number: zn250612, date: May 13, 2025) prior to the initiation of the study. Informed consent was obtained using an opt-out approach via the hospital’s website. Patients who declined participation were excluded from the study.
2.1. Study Design
We conducted a retrospective analysis of consecutive patients with AIS who underwent MT at a single center between March 2023 and April 2025. The study period was defined based on the clinical introduction of small-bore aspiration catheters, which became available at our institution in March 2023.
Patients were eligible for inclusion if they were 18 years or older and had AIS caused by occlusion of large or medium-sized vessels confirmed by computed tomography angiography (CTA) or magnetic resonance angiography (MRA). MeVO were defined as occlusion of the M2 or M3 segments of the middle cerebral artery, the A1 or A2 segments of the anterior cerebral artery, or the P1 or P2 segments of the posterior cerebral artery.
Patients were treated within 6 hours from last known well, or between 6 and 24 hours if advanced neuroimaging demonstrated the presence of salvageable brain tissue. Institutional review board approval was obtained prior to study initiation. Informed consent was provided through an opt-out process posted on our institution’s website; no patients opted out and thus none were excluded.
2.2. Treatments
Endovascular treatment was performed at the discretion of the treating physician using any approved device or combination of devices. All patients received standard-of-care therapy in accordance with the Japanese guidelines for the management of acute stroke. Treatment included intravenous thrombolysis with alteplase when indicated, stroke unit care, early rehabilitation, etiological investigations, secondary stroke prevention, and management of vascular risk factors.
2.3. Clinical Assessment and Outcomes
Demographic data, medical history, laboratory findings, and stroke characteristics, including symptom severity, were collected at the time of presentation. The primary outcome was the occurrence of device-related adverse events. Technical success of endovascular thrombectomy (EVT) was assessed using the expanded Thrombolysis in Cerebral Infarction (eTICI) scale (range, 0–3; higher scores indicate greater reperfusion). As no consensus exists regarding the definition of successful reperfusion for MeVO, both eTICI ≥2b67 and eTICI ≥2b50 were evaluated. For safety outcome, device-related adverse events were evaluated, which included 1) embolization in previously unaffected territory of treated vessel, 2) intracranial hemorrhage of treated vessel.
2.4. Statistical Analysis
Statistical analyses were performed using JMP version 18 (JMP Statistical Discovery LLC). A p-value of <0.05 was considered statistically significant. Multivariate logistic regression analysis adjusted for age and sex was used to assess whether the use of ADAPT as the first-line technique was independently associated with the incidence of device-related adverse events compared to other approaches.
3. Results
3.1. Patients
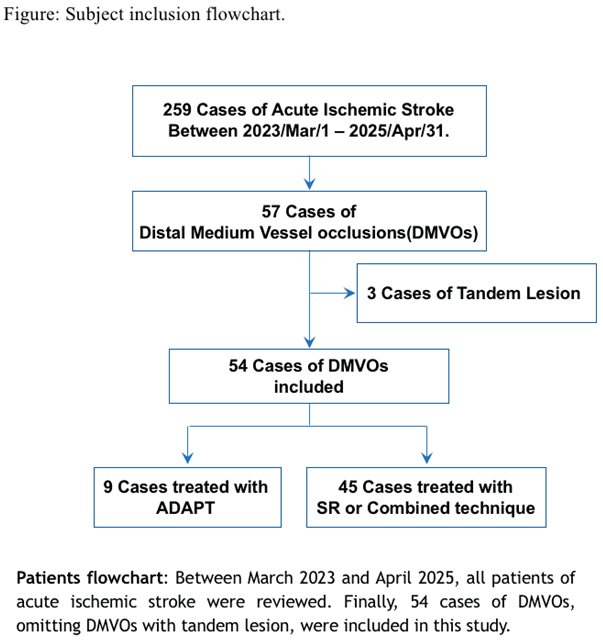
Between March 2023 and April 2025, a total of 259 patients underwent MT for AIS at a single institution. Of these, 57 cases were identified as MeVO, including 3 cases with tandem lesions. Ultimately, 54 patients with isolated MeVO were included in the present analysis. Patients were categorized into two groups according to the first-line technique used: the ADAPT group and the stent retriever (SR) or combined technique group (Figure).
The median age was 80 years (interquartile range [IQR], 77.5–86) in the ADAPT group and 82 years (IQR, 74.5–87) in the SR/Combined group. Female patients accounted for 66.7% in the ADAPT group and 60.0% in the SR/Combined group.
The median National Institutes of Health Stroke Scale (NIHSS) score at admission was 5.5 (IQR, 0.25–16) in the ADAPT group and 10.5 (IQR, 5–20.5) in the SR/Combined group. Regarding pre-stroke functional status, 44 patients (81.5%) had a modified Rankin Scale (mRS) score of 0 to 2, while 10 patients (18.5%) had an mRS score of 3 or 4.
The median time from last known well to initial diagnostic imaging was 241 minutes (IQR, 97.5–649) in the ADAPT group and 196 minutes (IQR, 63.8–459.3) in the SR/Combined group. Overall, 64.8% of patients presented within 6 hours from symptom onset.
The most common occlusion site identified on baseline CT or MR angiography was the M2 segment of the middle cerebral artery (85.2%), followed by the A2 segment of the anterior cerebral artery (5.6%) and the P1 segment of the posterior cerebral artery (5.6%). Intravenous thrombolysis was administered in 40.7% of cases.
There were no statistically significant differences between the ADAPT and SR/Combined groups in terms of demographics, stroke severity, occlusion site, or time metrics (
Table 1).
3.2. Efficacy Outcomes
Successful reperfusion, defined as an eTICI score of ≥2b50, was achieved in 100% of cases, and eTICI ≥2b67 in 77.8% of cases in the ADAPT group. In contrast, in the SR/combined group, eTICI ≥2b50 was achieved in 80.0% of cases, and eTICI ≥2b67 in 71.1%.
Multivariate logistic regression analyses adjusted for age and sex were performed separately for eTICI ≥2b50 and eTICI ≥2b67. The ADAPT group demonstrated a significantly higher rate of successful reperfusion for eTICI ≥2b50 compared to the SR or combined technique group. However, there was no significant difference between groups in the rate of reperfusion defined as eTICI ≥2b67 (
Table 2).
3.3. Safety Outcomes
Device-related adverse events were analyzed using age-adjusted logistic regression. The incidence of device-related adverse events was significantly lower in the ADAPT group compared with the SR or combined technique group (odds ratio, 1.818 × 10⁻⁹; 95% confidence interval, not estimable – 0.766; p = 0.0293;
Table 3).
Details of device-related adverse events are presented in
Table 4. The incidence of adverse events was 0% in the ADAPT group, 21.4% in the SR group, and 25.8% in the combined technique group. Notably, all cases of arterial perforation occurred exclusively in the combined technique group.
Information on the specific aspiration catheters used in the ADAPT group, along with detailed patient data, is provided in
Table 5. Except for one case of MeVO with dominant M2 occlusion, 88.9% of ADAPT as 1
st line was performed with small-bore aspiration catheter.
4. Discussion
Recent randomized controlled trials, such as DISTAL and ESCAPE-MeVO, investigating the efficacy of MT for MeVO in AIS, have failed to demonstrate a definitive clinical benefit1,2. However, these studies have several methodological limitations, particularly concerning device selection. For instance, the ESCAPE-MeVO trial mandated the Solitaire device as the first-line approach, while in the DISTAL trial, a combined technique was predominantly used (approximately 65% of cases), with stent retrievers and the ADAPT used alone in only about 16% of cases each. Notably, small-bore aspiration catheters were not incorporated into the protocol of either trial.
Small-bore aspiration catheters (e.g., AXS Vecta 46, RED 43) are specifically designed to access more distal cerebral vessels. The RED 43 has an outer diameter of 0.060 inches (1.52 mm), whereas the AXS Vecta 46 has an outer diameter of 0.056 inches (1.43 mm) at the tip. In contrast, conventional intermediate aspiration catheters (e.g., AXS Catalyst 6, RED 62, RED 72), previously employed in MeVO studies, have tip diameters ranging from 1.80 mm to 2.16 mm.
Anatomically, the diameter of M2 vessels has been reported to range from 1.1 to 2.1 mm (MRA based study3), M3 from 1.1 to 1.5 mm (CTA based study/ cadaveric studies3–5), A1 from 1.7 to 1.9 mm (DSA based study6), A2 from 1.6 to 1.7 mm (DSA based study6), P1 1.8 ± 0.54 mm (CTA based study7), P2 1.7 ± 0.3 mm (cadaveric studies8), and P3 1.7 ± 0.2 mm (cadaveric studies8). These anatomical considerations suggest that small-bore aspiration catheters are well-suited for medium-sized vessels, allowing for distal advancement without excessive stress on the intraluminal vascular wall. Moreover, the improved flexibility of these catheter tips facilitates distal navigability.
Additionally, the vascular distance and tortuosity along the access route are generally greater and more pronounced in MeVO compared to large vessel occlusion (LVO). These anatomical challenges hinder successful reperfusion, particularly in MT using stent retrievers 3,9. Vessel tortuosity not only impairs reperfusion rates but also increases the risk of complications. Specifically, tortuosity of the M1 segment has been associated with a higher incidence of intracranial hemorrhage (ICH) following MT with stent retrievers for M2 occlusion10. According to a report from the Japanese Registry of Neuroendovascular Therapy (JR-NET), which analyzed 2,394 MeVO cases, the overall incidence of ICH after MT was 12.6%, with symptomatic ICH accounting for 31.5% of these cases ⁹. Importantly, ICH after endovascular therapy for MeVO was associated with worse clinical outcomes, regardless of whether the hemorrhage was symptomatic or asymptomatic 11. Notably, small-bore aspiration catheters were not utilized in this JR-NET study.
The efficacy of RED 43 and AXS Vecta 46 has previously been reported in individual single-arm retrospective studies12,13. However, to our knowledge, this is the first retrospective comparative study to comprehensively evaluate both devices collectively as small-bore aspiration catheters in the treatment of medium vessel occlusion.
Our findings demonstrate a low incidence of ICH and device-related complications, suggesting that MT using small-bore aspiration catheters may offer a safer and more anatomically tailored strategy for treating MeVO. Taken together with prior studies, our data support the clinical value of this emerging technique and highlight its potential to overcome some of the limitations observed in earlier randomized trials.
5. Conclusions
Small-bore aspiration catheters using the ADAPT were associated with high reperfusion success and fewer device-related complications compared to SR or combined techniques. Small bore aspiration catheter was not enrolled in the previous RCT negative for the effectiveness of MT for MeVO. Our findings promise the potential role of ADAPT by small bore aspiration catheter as an effective first-line strategy for MeVO.
Limitation
Several limitations of this study should be considered. First, the retrospective design introduces inherent methodological constraints, especially selection bias. Second, safety and efficacy outcomes were not assessed through centralized adjudication. Third, vessel diameter was not independently verified, raising the possibility that some included cases may have fallen outside the defined MeVO range (0.75–2 mm). Finally, although this study suggests the safety and efficacy of small-bore aspiration catheters for MeVO, the absence of a control group treated with medical therapy alone limits our ability to determine the definitive role of MT in this patient population.
Author Contributions
Conceptualization, T.S.; methodology, T.S.; software, T.S.; validation, T.O., N.O., M.K., M.G., R.F., N.F., S.H., K.M., T.I., Y.Y., N.N., K.O., T.T., M.T., H.T., M.Y. and T.O.; formal analysis, T.S.; investigation, T.S.; resources, R.F., N.O.; data curation, R.F., N.O., T.S.; writing—original draft preparation, T.S.; writing—review and editing, T.S., T.O., N.O., M.K., M.G., S.H; visualization, T.S.; supervision, T.S.; project administration, T.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Kobe City Medical Center General Hospital (approval number: zn250612 and date of approval: May 13, 2025).
Informed Consent Statement
Informed consent was obtained using an opt-out approach, as approved by the institutional review board. Information regarding the study was made publicly available on the hospital’s website, and patients who declined participation were excluded from the study.
Data Availability Statement
The data are not publicly available due to privacy or ethical restrictions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Psychogios M, Brehm A, Ribo M et al. Endovascular Treatment for Stroke Due to Occlusion of Medium or Distal Vessels. N Engl J Med. 2025, 392, 1374–1384. [Google Scholar] [CrossRef] [PubMed]
- Goyal M, Ospel JM, Ganesh A et al. Endovascular Treatment of Stroke Due to Medium-Vessel Occlusion. N Engl J Med. 2025, 392, 1385–1395. [Google Scholar] [CrossRef] [PubMed]
- Saver JL, Chapot R, Agid R et al. Thrombectomy for Distal, Medium Vessel Occlusions: A Consensus Statement on Present Knowledge and Promising Directions. Stroke. 2020, 51, 2872–2884. [Google Scholar] [CrossRef] [PubMed]
- Cilliers K, Page BJ. Anatomy of the Middle Cerebral Artery: Cortical Branches, Branching Pattern and Anomalies. Turk Neurosurg. 2017, 27, 671–681. [Google Scholar] [CrossRef]
- Rai AT, Hogg JP, Cline B, Hobbs G, Virginia W. Cerebrovascular geometry in the anterior circulation: an analysis of diameter, length and the vessel taper. J Neurointerv Surg. 2013, 5, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Halama D, Merkel H, Werdehausen R et al. Reference Values of Cerebral Artery Diameters of the Anterior Circulation by Digital Subtraction Angiography : A Retrospective Study. Diagnostics (Basel). 2022, 12, 2471. [Google Scholar] [CrossRef]
- Vitosevic F, Rasulic L, Medenica SM. Morphological Characteristics of the Posterior Cerebral Circulation: An Analysis Based on Non-Invasive Imaging. Turk Neurosurg. 2019, 29, 625–630. [Google Scholar] [CrossRef]
- Cilliers K, Page BJ. Variation and Anomalies of the Posterior Cerebral Artery: Review and Pilot Study. Turk Neurosurg. 2019, 29, 1–8. [Google Scholar] [CrossRef]
- Schwaiger BJ, Gersing AS, Zimmer C, Prothmann S. The Curved MCA : Influence of Vessel Anatomy on Recanalization Results of Mechanical Thrombectomy after Acute Ischemic Stroke. AJNR Am J Neuroradiol. 2015, 35, 971–976. [Google Scholar]
- Nishii R, Fujiwara S, Yamamoto Y, et al. Association between M1 tortuosity and intracranial hemorrhage after mechanical thrombectomy using a stent retriever for M2 occlusions. Interv Neuroradiol. 2025, Published. [Google Scholar] [CrossRef]
- Fujiwara S, Uchida K, Ohta T, et al. Impact of Intracranial Hemorrhage After Endovascular Treatment for Medium Vessel Occlusion. Neurosurgery. 2025, 96, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Goyal N, Khattar NK, Peterson J, et al. The RED 43 catheter for aspiration thrombectomy of medium vessel occlusion ( MeVO ): a multicenter experience. J Neurointerv Surg 2025, 1–6. [CrossRef]
- Choi JW, Qiao Y, Mehta TI, et al. The Vecta 46 intermediate catheter for mechanical thrombectomy of medium vessel occlusion : A single-center experience. Interv Neuroradiol 2025. [CrossRef]
Table 1.
Baseline Characteristics of the Participants.
Table 1.
Baseline Characteristics of the Participants.
| |
ADAPT
(n = 9)
|
SR or Combined
(n = 45)
|
P value |
| Characteristics |
|
|
|
| Age |
|
|
|
|
Median(IQR) – yr
|
80 (77.5 - 86) |
82 (74.5 – 87) |
0.57 |
| Female sex – no.(%) |
6 (66.7%) |
27 (60%) |
>0.99 |
Modified Rankin Scale score
before stroke – no./total no.
|
|
|
0.92 |
|
0 or 1
|
6 |
34 |
|
|
2
|
1 |
3 |
|
|
3 or 4
|
2 |
8 |
|
Median NIHSS score at
admission(IQR)
|
9 (4.5 – 22) |
10.5 (4.3 – 17) |
0.63 |
| Occlusion location – no.(%) |
|
|
0.81 |
|
M2 segment
|
8 |
38 |
|
|
M3 segment
|
0 |
0 |
|
|
A1 segment
|
0 |
1 |
|
|
A2 segment
|
1 |
2 |
|
|
P1 segment
|
0 |
3 |
|
|
P2 segment
|
0 |
1 |
|
Intravenous thrombolysis
therapy – No.(%)
|
5 (55.6%) |
18 (40.0%) |
0.48 |
| Median ASPECTS score at admission(IQR) |
10 (7.5 - 10) |
10 (9.0 - 10) |
0.76 |
| Interval between time that participant was last seen to be well and imaging (IQR) - min |
95 (81.2 – 381.8) |
240 (82.5 – 542.5) |
0.36 |
| Interval between time that participant was last seen to be well and arterial puncture (IQR) - min |
187.5 (131.5 – 476.3) |
240 (120 – 581) |
0.76 |
Table 2.
Multivariate logistic regression analysis for eTICI≥2b50 and eTICI≥2b67, adjusted by Age and Sex.
Table 2.
Multivariate logistic regression analysis for eTICI≥2b50 and eTICI≥2b67, adjusted by Age and Sex.
| |
ADAPT
(n = 9)
|
SR or Combined
(n = 45)
|
OR |
95%CI |
P value |
| eTICI≥2b50 |
100%(9/9) |
80.0%(36/45) |
4.56x108
|
1.0578 – ne |
0.045 |
| eTIC≥2b67 |
77.8%(7/9) |
71.1%(32/45) |
1.5496 |
0.3130 – 11.5601 |
0.609 |
Table 3.
Multivariate logistic regression analysis for Device-Related Adverse Events.
Table 3.
Multivariate logistic regression analysis for Device-Related Adverse Events.
| Variable |
OR |
95%CI |
P value |
| Age |
0.986 |
0.9261 – 1.0551 |
0.673 |
| Sex (F vs M) |
0.361 |
0.0876 – 1.3869 |
0.138 |
| ADAPT vs SR /Combined |
1.82x10-9
|
ne – 0.7662 |
0.029* |
Table 4.
Detailed Device-Related Adverse Events by 1st line strategy.
Table 4.
Detailed Device-Related Adverse Events by 1st line strategy.
| |
ADAPT
(n=9)
|
SR
(n =14)
|
Combined technique
(n=31)
|
| Total number of adverse events related to device(%) |
0(0%) |
3(21.4%) |
8(25.8%) |
| Embolization in previously unaffected territory |
0 |
1 |
1 |
| Intracranial hemorrhage |
|
|
|
| Subarachnoid hemorrhage |
0 |
2 |
4 |
| Arterial perforation |
0 |
0 |
3 |
Table 5.
Aspiration catheter used in ADAPT and demographics.
Table 5.
Aspiration catheter used in ADAPT and demographics.
| Age |
SEX |
Occluded Artery |
Firstline device |
| 76 |
M |
A2 |
Vecta46 |
| 84 |
F |
M2 |
Vecta46 |
| 79 |
F |
M2 |
Vecta46 |
| 75 |
F |
Dominant M2 |
RED62 |
| 89 |
F |
M2 |
RED43 |
| 79 |
M |
M2 |
RED43 |
| 88 |
F |
M2 |
RED43 |
| 84 |
M |
M2 |
RED43 |
| 80 |
F |
M2 |
RED43 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).