Submitted:
06 July 2025
Posted:
07 July 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
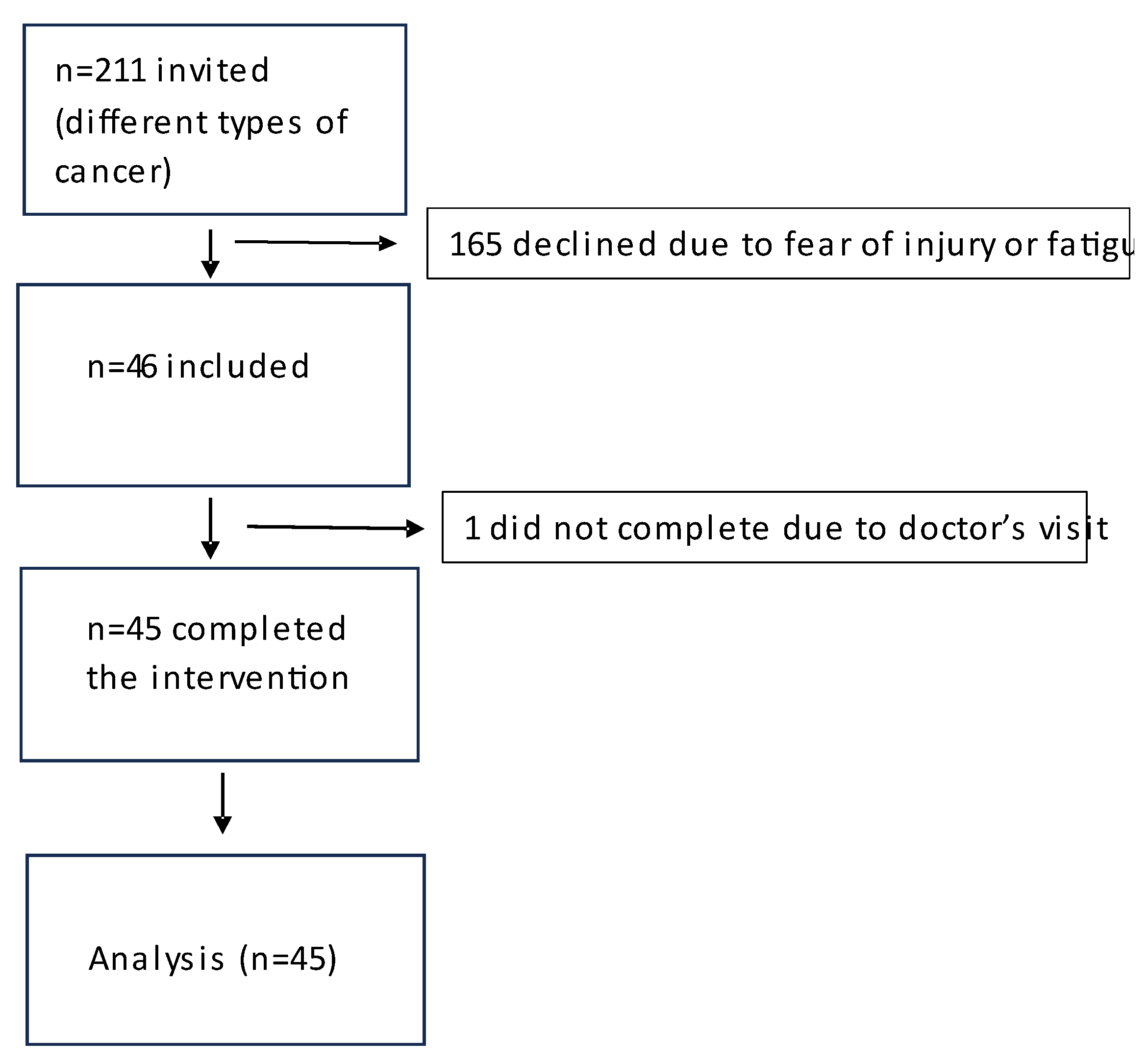
2. Materials and Methods
Intervention
Statistical Analysis
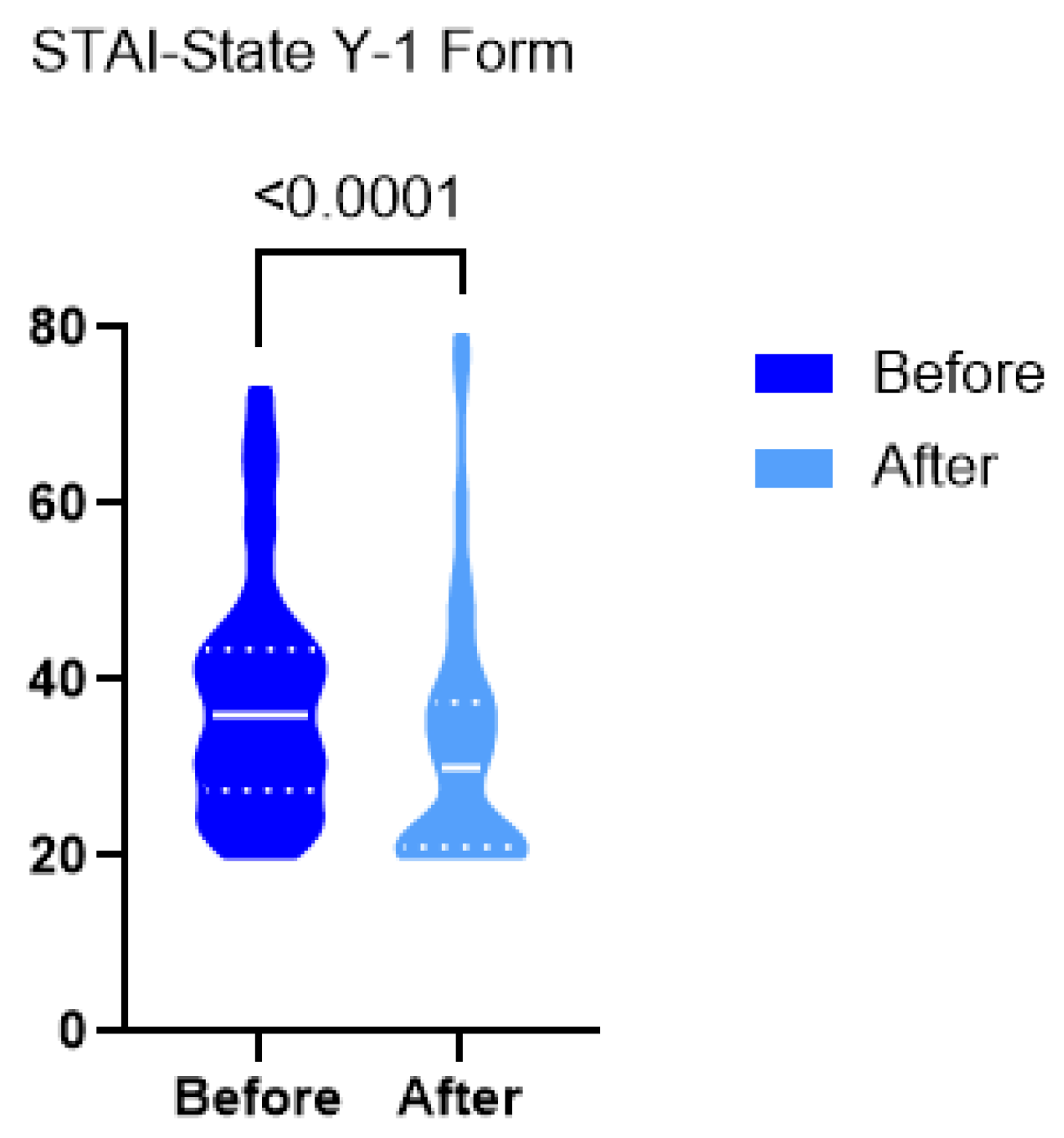
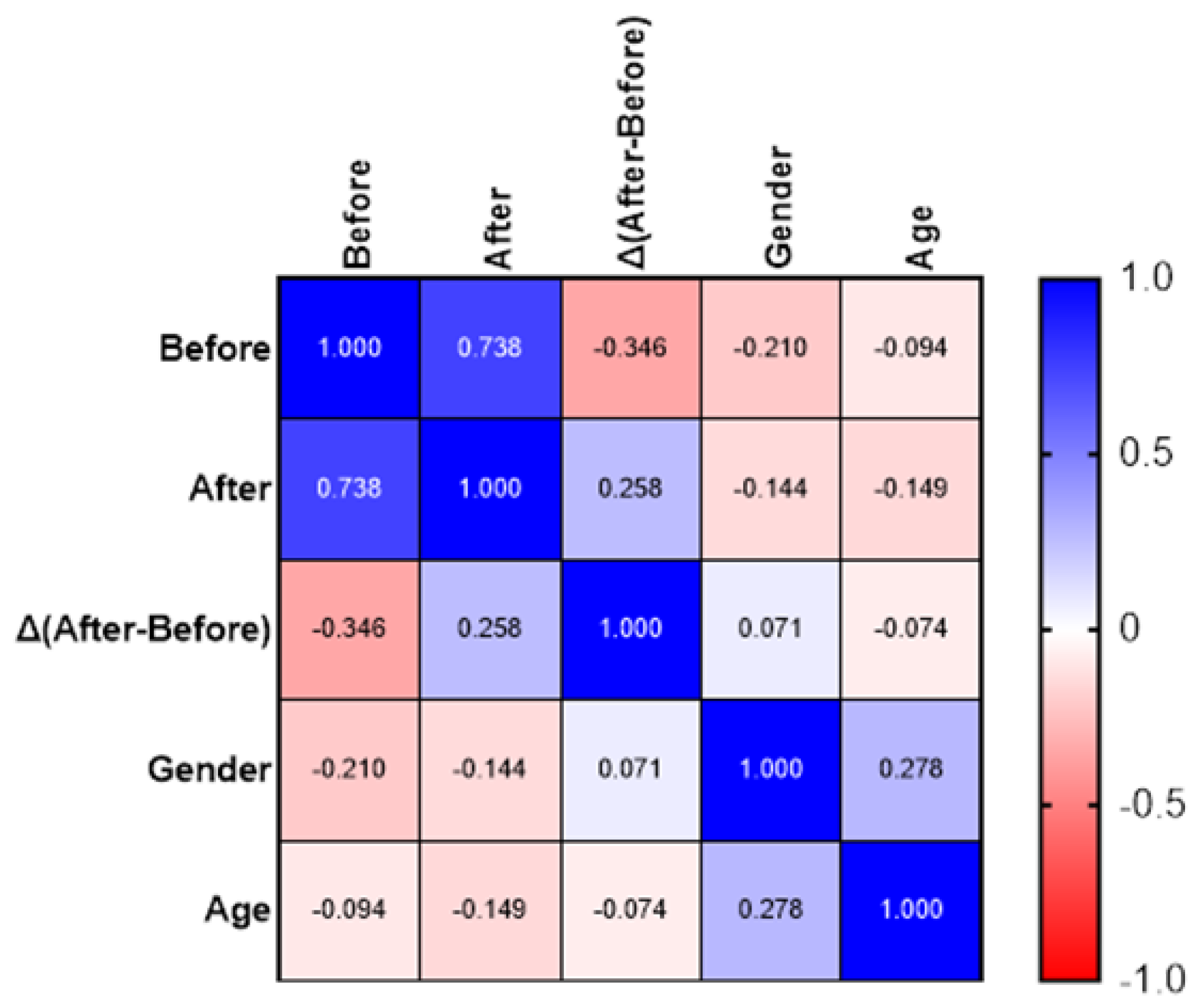
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| QoL | Quality of Life |
| STAI | State-Trait Anxiety Inventory |
| CI | Confidence Interval |
| ACSM | American College of Sports Medicine |
| ExCITE | Exercise and Cancer Integrative Therapy and Education Program |
References
- ‘Wang Y, Feng W. Cancer-related psychosocial challenges. Gen Psychiatr. 2022 Oct 6;35(5):e100871. PMID: 36311374; PMCID: PMC9540834.’. [CrossRef]
- ‘Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011;61:69–90.’.
- ‘Mayer RS, Engle J. Rehabilitation of ndividuals With Cancer. Ann Rehabil Med. 2022 Apr;46(2):60-70.’.
- ‘Brown M, Farquhar-Smith P. Pain in cancer survivors; filling in the gaps. Br J Anaesth. 2017 Oct 1;119(4):723-736.’. [CrossRef]
- ‘Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71:209-49.’. [CrossRef]
- ‘Hassanzadeh A, Heidari Z, Feizi A, Hassanzadeh Keshteli A, Roohafza H, Afshar H, Adibi P. Association of Stressful Life Events with Psychological Problems: A Large-Scale Community-Based Study Using Grouped Outcomes Latent Factor Regression with Latent Predictors. Comput Math Methods Med. 2017;2017:3457103. Epub 2017 Sep 19. Erratum in: Comput Math Methods Med. 2018 Feb 20;2018:8020962. doi: 10.1155/2018/8020962. PMID: 29312459; PMCID: PMC5625761. [CrossRef] [PubMed]
- ‘Grassi L, Caruso R, Riba MB, Lloyd-Williams M, Kissane D, Rodin G, McFarland D, Campos-Ródenas R, Zachariae R, Santini D, Ripamonti CI; ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Anxiety and depression in adult cancer patients: ESMO Clinical Practice Guideline. ESMO Open. 2023 Apr;8(2):101155. Epub 2023 Mar 14. PMID: 37087199; PMCID: PMC10163167.’. [CrossRef] [PubMed]
- Stark, D., House, A. Anxiety in cancer patients. Br J Cancer ,2000;83: 1261–1267’.
- ‘Mitchell AJ, Ferguson DW, Gill J, Paul J, Symonds P. Depression and anxiety in long-term cancer survivors compared with spouses and healthy controls: a systematic review and meta-analysis. Lancet Oncol. 2013 Jul;14(8):721-32. Epub 2013 Jun 5. PMID: 23759376. ’. [CrossRef]
- ‘Choudhury, A. Impact of Social Isolation, Physician-Patient Communication, and Self-perception on the Mental Health of Patients With Cancer and Cancer Survivors: National Survey Analysis. Interact J Med Res. 2023 Apr 7;12:e45382.’. [CrossRef]
- ‘Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvão DA, Pinto BM, Irwin ML, Wolin KY, Segal RJ, Lucia A, Schneider CM, von Gruenigen VE, Schwartz AL; American College of Sports Medicine. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010 Jul;42(7):1409-26’. [CrossRef]
- ‘Mishra SI, Scherer RW, Snyder C, Geigle PM, Berlanstein DR, Topaloglu O. Exercise interventions on health-related quality of life for people with cancer during active treatment. Cochrane Database Syst Rev. 2012 Aug 15;2012(8):CD008465. PMID: 22895974; PMCID: PMC7389071.’. [CrossRef]
- ‘Meneses-Echávez JF, González-Jiménez E, Ramírez-Vélez R. Effects of Supervised Multimodal Exercise Interventions on Cancer-Related Fatigue: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Biomed Res Int. 2015;2015:328636. Epub 2015 Jun 17. PMID: 26167483; PMCID: PMC4488083.’. [CrossRef]
- ‘Cormie P, Zopf EM, Zhang X, Schmitz KH. The Impact of Exercise on Cancer Mortality,Recurrence, and Treatment-Related Adverse Effects. Epidemiol Rev. 2017 Jan 1;39(1):71-92. PMID: 28453622.’. [CrossRef]
- ‘Gerritsen JK, Vincent AJ. Exercise improves quality of life in patients with cancer: a systematic review and meta-analysis of randomised controlled trials. Br J Sports Med. 2016 Jul;50(13):796-803. Epub 2015 Dec 30. PMID:26719503.’. [CrossRef]
- ‘Buffart LM, Kalter J, Sweegers MG, Courneya KS, Newton RU, Aaronson NK, Jacobsen PB, May AM, Galvão DA, Chinapaw MJ, Steindorf K, Irwin ML, Stuiver MM, Hayes S, Griffith KA, Lucia A, Mesters I, van Weert E, Knoop H, Goedendorp MM, Mutrie N, Daley AJ, McConnachie A, Bohus M, Thorsen L, Schulz KH, Short CE, James EL, Plotnikoff RC, Arbane G, Schmidt ME, Potthoff K, van Beurden M, Oldenburg HS, Sonke GS, van Harten WH, Garrod R, Schmitz KH, Winters-Stone KM, Velthuis MJ, Taaffe DR, van Mechelen W, Kersten MJ, Nollet F, Wenzel J, Wiskemann J, Verdonck-de Leeuw IM, Brug J. Effects and moderators of exercise on quality of life and physical function in patients with cancer: An individual patient data meta-analysis of 34 RCTs. Cancer Treat Rev. 2017 Jan;52:91-104.’. [CrossRef]
- ‘Grassi L, Caruso R, Riba MB, Lloyd-Williams M, Kissane D, Rodin G, McFarland D,Campos-Ródenas R, Zachariae R, Santini D, Ripamonti CI; ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Anxiety and depression in adult cancer patients: ESMO Clinical Practice Guideline. ESMO Open. 2023 Apr;8(2):101155.’. [CrossRef]
- ‘Schmitz KH, Potiaumpai M, Schleicher EA, Wolf LJ, Doerksen SE, Drabick JJ, Yee NS, Truica CI, Mohamed AA, Shaw BW, Farley DC. The exercise in all chemotherapy trial. Cancer. 2021 May 1;127(9):1507-1516. Epub 2020 Dec 17. PMID: 33332587.’. [CrossRef]
- ‘Moraes RF, Ferreira-Júnior JB, Marques VA, Vieira A, Lira CAB, Campos MH, Freitas-Junior R, Rahal RMS, Gentil P, Vieira CA. Resistance Training, Fatigue, Quality of Life,Anxiety in Breast Cancer Survivors. J Strength Cond Res. 2021 May 1;35(5):1350-1356.’.
- ‘Balsamo M, Cataldi F, Carlucci L, Fairfield B. Assessment of anxiety in older adults: a review of self-report measures. Clin Interv Aging. 2018 Apr 6;13:573-593. PMID: 29670342; PMCID: PMC5896683.’. [CrossRef]
- ‘Liakos, A., & Giannitsi, S. (1984). The Reliability and Validity of the Greek Version of Spielberger’s, State and Trait Anxiety Inventory. Encephalos, 21, 71-76.’.
- ‘Pandey, M., Sarita, G.P., Devi, N. et al. Distress, anxiety, and depression in cancer patients undergoing chemotherapy. World J Surg Onc 4, 68 (2006).’. [CrossRef]
- J. Dietrich, M. Prust, and J. Kaiser, ‘Chemotherapy, cognitive impairment and hippocampal toxicity’, Hippocampal Vulnerability Mol. Dis., vol. 309, pp. 224–232, Nov. 2015. [CrossRef]
- ‘Yang Y, Wen Y, Bedi C, Humphris G. The relationship between cancer patient’s fear of recurrence and chemotherapy: A systematic review and meta-analysis. J Psychosom Res. 2017 Jul;98:55-63. Epub 2017 May 3. PMID: 28554373.’. [CrossRef]
- ‘Mahdizadeh MJ, Tirgari B, Abadi O, Bahaadinbeigy K (2019) Guided imagery: reducing anxiety, depression, and selected side effects associated with chemotherapy. Clin J Oncol Nurs 23(5):87–92’.
- ‘Pitman A, Suleman S, Hyde N, Hodgkiss A. Depression and anxiety in patients with cancer. BMJ. 2018 Apr 25;361:k1415. PMID: 29695476.’. [CrossRef]
- ‘V. Bucciarelli, F. Bianco, A. Di Blasio, et al., “Cardiometabolic Profile, Physical Activity, and Quality of Life in Breast Cancer Survivors After Different Physical Exercise Protocols: A 34-Month Follow-Up Study,” Journal of Clinical Medicine 12, no. 14 (July 2023): 4795.’. [CrossRef]
- ‘Buffart LM, Kalter J, Sweegers MG, Courneya KS, Newton RU, Aaronson NK, Jacobsen PB, May AM, Galvão DA, Chinapaw MJ, Steindorf K, Irwin ML, Stuiver MM, Hayes S, Griffith KA, Lucia A, Mesters I, van Weert E, Knoop H, Goedendorp MM, Mutrie N, Daley AJ, McConnachie A, Bohus M, Thorsen L, Schulz KH, Short CE, James EL, Plotnikoff RC, Arbane G, Schmidt ME, Potthoff K, van Beurden M, Oldenburg HS, Sonke GS, van Harten WH, Garrod R, Schmitz KH, Winters-Stone KM, Velthuis MJ, Taaffe DR, van Mechelen W, Kersten MJ, Nollet F, Wenzel J, Wiskemann J, Verdonck-de Leeuw IM, Brug J. Effects and moderators of exercise on quality of life and physical function in patients with cancer: An individual patient data meta-analysis of 34 RCTs. Cancer Treat Rev. 2017 Jan;52:91-104. Epub 2016 Dec 5. PMID: 28006694.’. [CrossRef]
- ‘Ashcraft KA, Peace RM, Betof AS, Dewhirst MW, Jones LW. Efficacy and Mechanisms of Aerobic Exercise on Cancer Initiation, Progression, and Metastasis: A Critical Systematic Review of In Vivo Preclinical Data. Cancer research 2016, 76(14): 4032–4050’.
- ‘Nieman DC, Wentz LM. The compelling link between physical activity and the body’s defense system. J Sport Health Sci. 2019;8:201–17.’. [CrossRef]
- ‘Mustian KM, Sprod LK, Palesh OG, Peppone LJ, Janelsins MC, Mohile SG, Carroll J. Exercise for the management of side effects and quality of life among cancer survivors. Curr Sports Med Rep. 2009 Nov-Dec;8(6):325-30. PMID: 19904073; PMCID: PMC2875185.’. [CrossRef]
- ‘Scott JM, Zabor EC, Schwitzer E, Koelwyn GJ, Adams SC, Nilsen TS, Moskowitz CS, Matsoukas K, Iyengar NM, Dang CT, Jones LW. Efficacy of Exercise Therapy on Cardiorespiratory Fitness in Patients With Cancer: A Systematic Review and Meta-Analysis. J Clin Oncol. 2018 Aug 1;36(22):2297-2305. Epub 2018 Jun 12. PMID: 29894274; PMCID: PMC6804903.’. [CrossRef]
- ‘Loughney L, West MA, Kemp GJ, Grocott MP, Jack S. Exercise intervention in people with cancer undergoing neoadjuvant cancer treatment and surgery: A systematic review. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology 2016, 42(1): 28–38’.
- ‘Champ, C.E., Carpenter, D.J., Diaz, A.K. et al. Resistance Training for Patients with Cancer: A Conceptual Framework for Maximizing Strength, Power, Functional Mobility, and Body Composition to Optimize Health and Outcomes. Sports Med 53, 75–89 (2023).’. [CrossRef]
- ‘Henriksson A, Arving C, Johansson B, Igelström H, Nordin K. Perceived barriers to and facilitators of being physically active during adjuvant cancer treatment. Patient Educ Couns. 2016 Jul;99(7):1220-1226. Epub 2016 Jan 28. PMID: 26860549.’. [CrossRef]
- ‘Thomas, V.J., Seet-Lee, C., Marthick, M. et al. Aerobic exercise during chemotherapy infusion for cancer treatment: a novel randomised crossover safety and feasibility trial. Support Care Cancer 28, 625–632 (2020).’. [CrossRef]
- ‘Singh B, Hayes SC, Spence RR, Steele ML, Millet GY, Gergele L. Exercise and colorectal cancer: a systematic review and meta-analysis of exercise safety, feasibility and effectiveness. Int J Behav Nutr Phys Act. 2020 Sep 24;17(1):122. PMID: 32972439; PMCID: PMC7513291.’. [CrossRef]
- ‘Avancini A, Borsati A, Toniolo L, Ciurnelli C, Belluomini L, Budolfsen T, Lillelund C, Milella M, Quist M, Pilotto S. Physical activity guidelines in oncology: A systematic review of the current recommendations. Crit Rev Oncol Hematol. 2025 Jun;210:104718. Epub 2025 Apr 5. PMID: 40194715.’. [CrossRef]
- ‘Hayes, S.C., Spence, R.R., Galvao, D.A., Newton, R.U., 2009. Australian Association for Exercise and Sport Science position stand: optimising cancer outcomes through exercise. J. Sci. Med Sport 12 (4), 428–434’. [CrossRef]
- ‘Campbell, K.L., Winters-Stone, K.M., Wiskemann, J., May, A.M., Schwartz, A.L., Courneya, K.S., Zucker, D.S., Matthews, C.E., Ligibel, J.A., Gerber, L.H., Morris, G.S., Patel, A.V., Hue, T.F., Perna, F.M., Schmitz, K.H., 2019. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med Sci. Sports Exerc 51 (11), 2375–2390.’. [CrossRef]
- ‘Ligibel, J.A., Bohlke, K., May, A.M., Clinton, S.K., Demark-Wahnefried, W., Gilchrist, S. C., Irwin, M.L., Late, M., Mansfield, S., Marshall, T.F., Meyerhardt, J.A., Thomson, C. A., Wood, W.A., Alfano, C.M., 2022. Exercise, Diet, and Weight Management During Cancer Treatment: ASCO Guideline. J. Clin. Oncol. 40 (22), 2491–2507.’. [CrossRef]
- ‘D. Kerrigan, S. Keteyian, J. K. Ehrman, S. Brown, R. Filipiak, N. Martinez, D. Ihlenfeldt, J. Varga, E. M. Walker.A pilot study of aerobic exercise performed in breast cancer patients during chemotherapy infusion. Journal of Clinical Oncology 2010 28:15_suppl, e19527-e19527’. [CrossRef]
- ‘Housman B, Flores R, Lee DS (2021) Narrative review of anxiety and depression in patients with esophageal cancer: underappreciated and undertreated. J Thorac Dis 13(5):3160–3170’. [CrossRef]
- ‘Wang YH, Li JQ, Shi JF, Que JY, Liu JJ, Lappin JM, Leung J, Ravindran AV, Chen WQ, Qiao YL, Shi J, Lu L, Bao YP. Depression and anxiety in relation to cancer incidence and mortality: a systematic review and meta-analysis of cohort studies. Mol Psychiatry. 2020 Jul;25(7):1487-1499. Epub 2019 Nov 19. PMID: 31745237.’. [CrossRef]
- ‘Saviola, F., Pappaianni, E., Monti, A. et al. Trait and state anxiety are mapped differently in the human brain. Sci Rep 10, 11112 (2020). [CrossRef]
- ‘Battle DE. Diagnostic and Statistical Manual of Mental Disorders (DSM). Codas. 2013;25(2):191-2. PMID: 24413388. [CrossRef]
- ‘NCCN. Distress during cancer care. NCCN Guidelines for Patients, National Comprehensive Cancer Network. 2020. Available from https://www.nccn.org/patients/guidelines/content/PDF/distress-patient.pdf.
- ‘Üstündag, S., Zencirci, A.D. (2015) “Factors affecting the quality of life of cancer patients undergoing chemotherapy: A questionnaire study”, Asia-Pacific Journal of Oncology Nursing, 2(1), pp. 17–25. [CrossRef]
- ‘Committee on Improving the Quality of Cancer Care: Addressing the Challenges of an Aging Population; Board on Health Care Services; Institute of Medicine; Levit L, Balogh E, Nass S, et al., editors. Delivering High-Quality Cancer Care: Charting a New Course for a System in Crisis. Washington (DC): National Academies Press (US); 2013 Dec 27. 3, Patient-Centered Communication and Shared Decision Making. Available from: https://www.ncbi.nlm.nih.gov/books/NBK202146/’.
- ‘Spielberger, C. D., Gorsuch, R. L., Lushene, R., Vagg, P. R. & Jacobs, G. A. Manual for the state-trait anxiety inventory (Consulting Psychologists Press, Palo Alto, 1983).’.
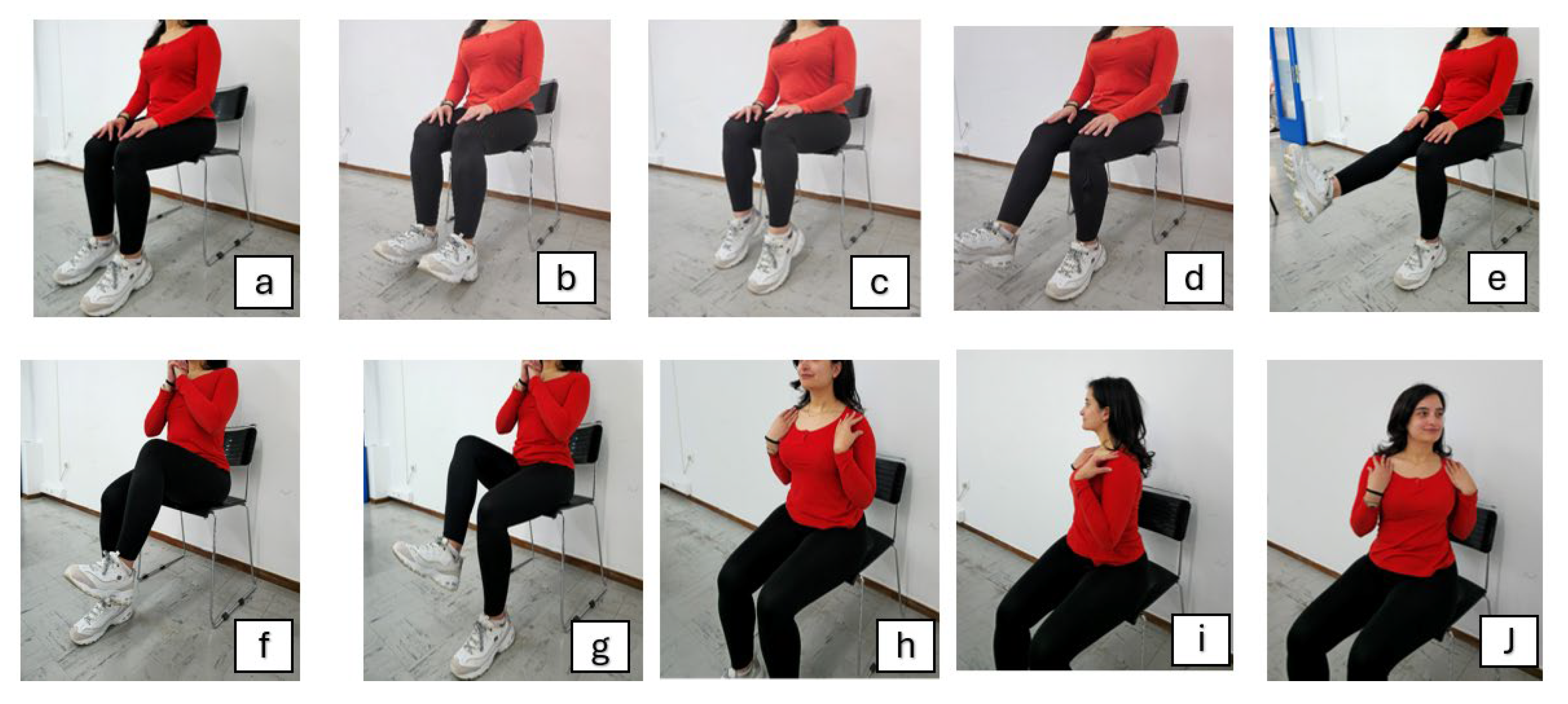

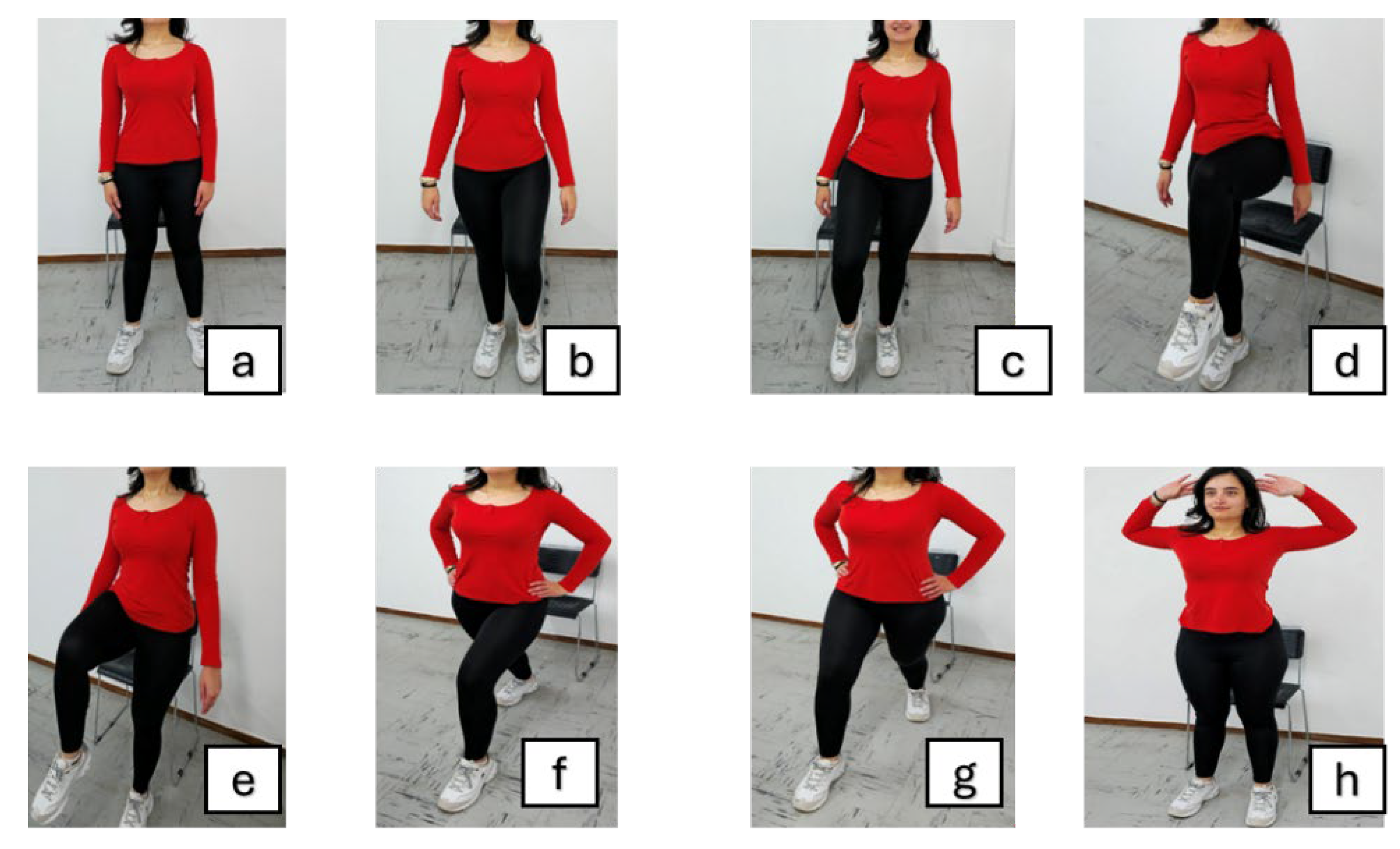

| Exercise stage | Included exercises |
|
Warm-Up [5 minutes]: |
|
| |
| |
|
Main Exercise [10 minutes]: |
|
| |
| |
| |
| |
|
Cool-Down [5 minutes]: |
|
| |
| |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





