Submitted:
12 June 2023
Posted:
13 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
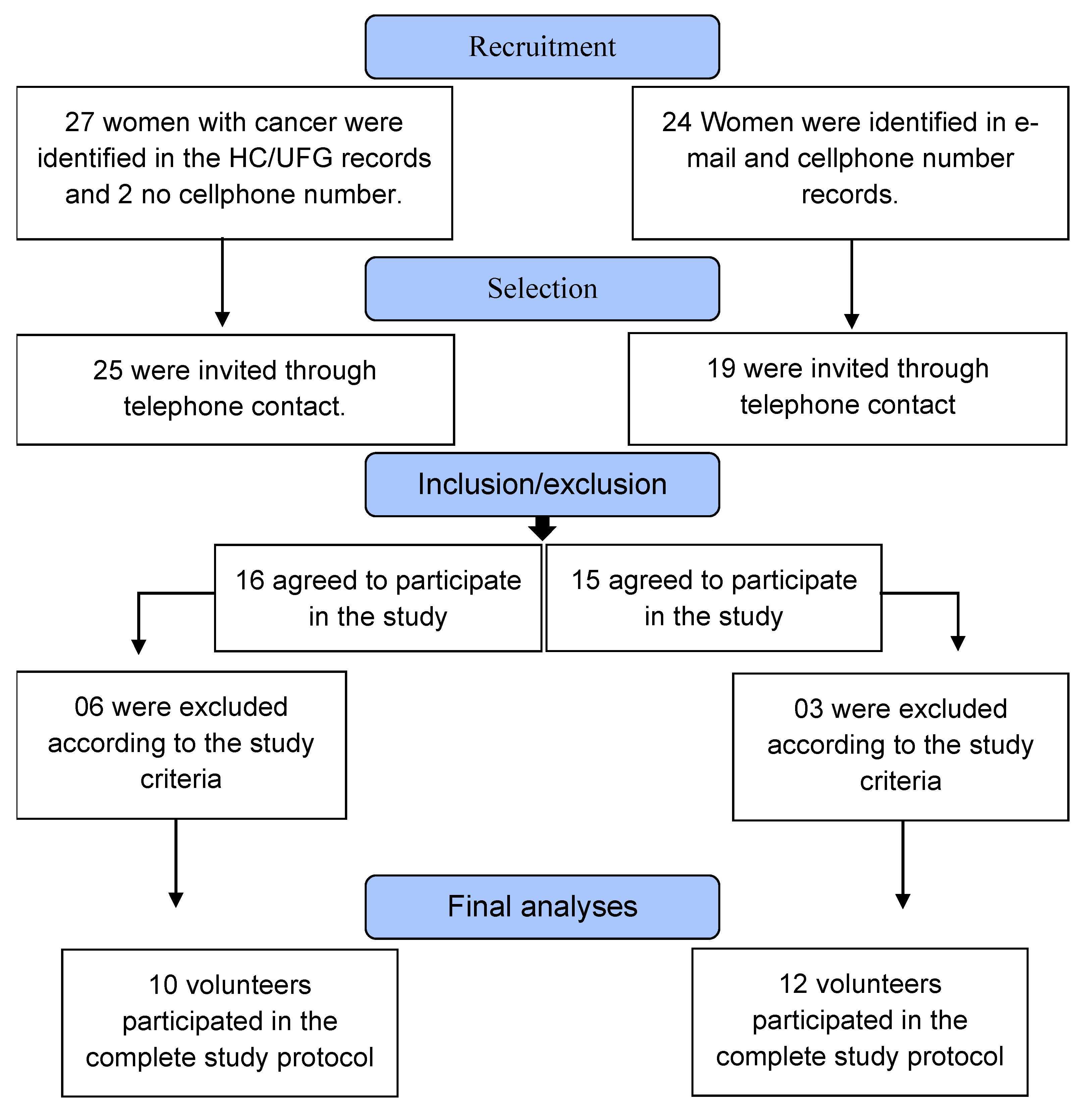
Participants
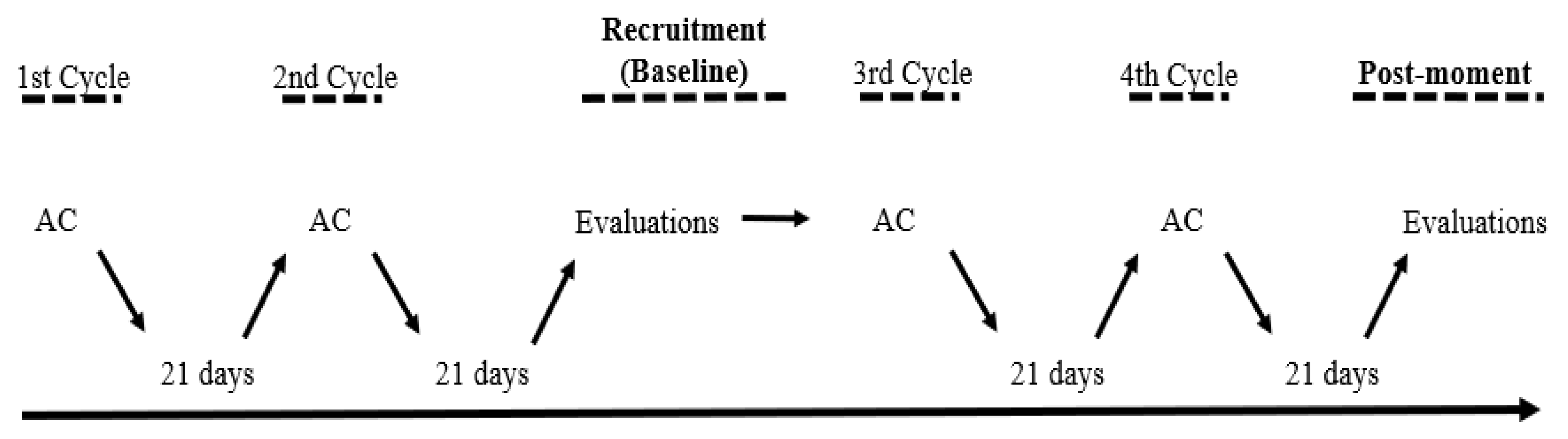
Experimental design
Anthropometric measurements
Evaluation of muscle performance
Statistical analysis
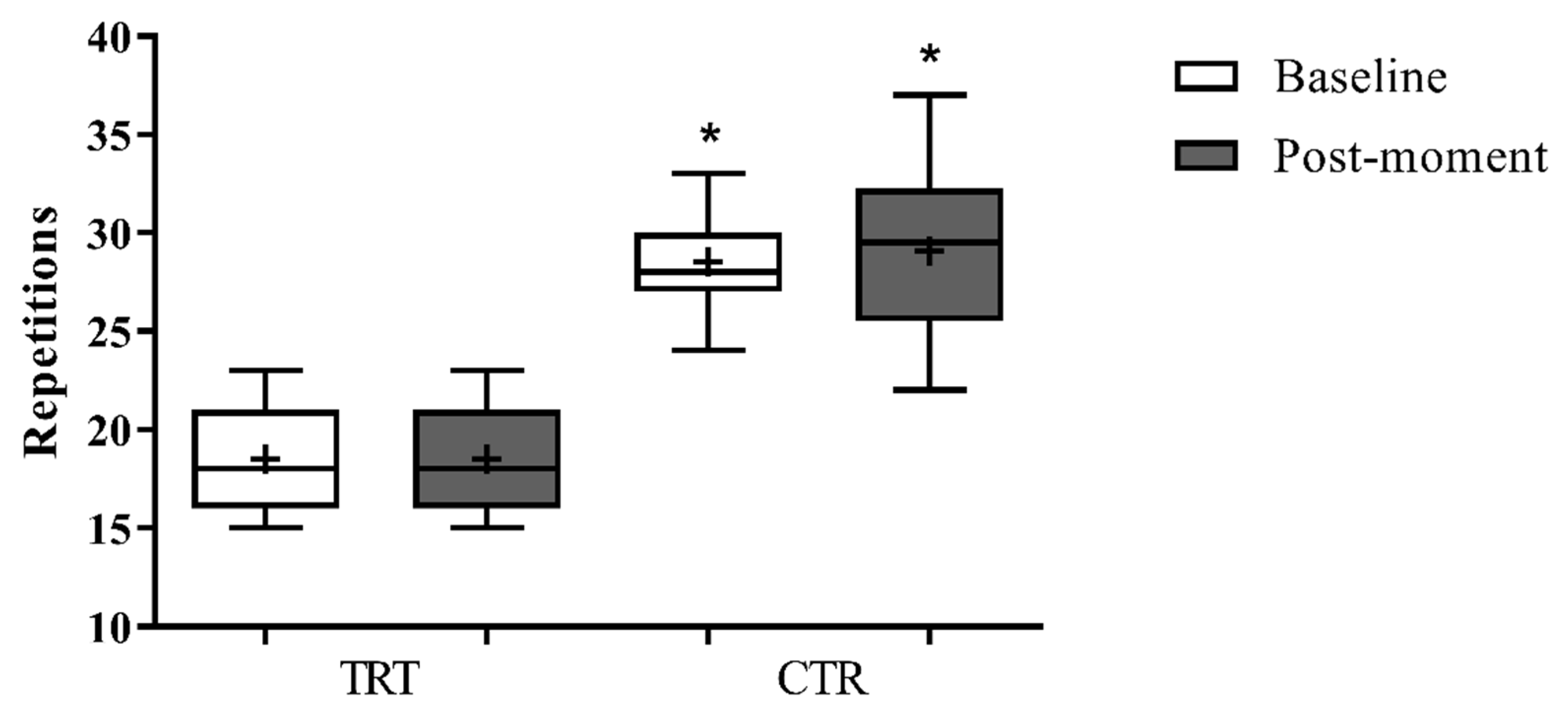
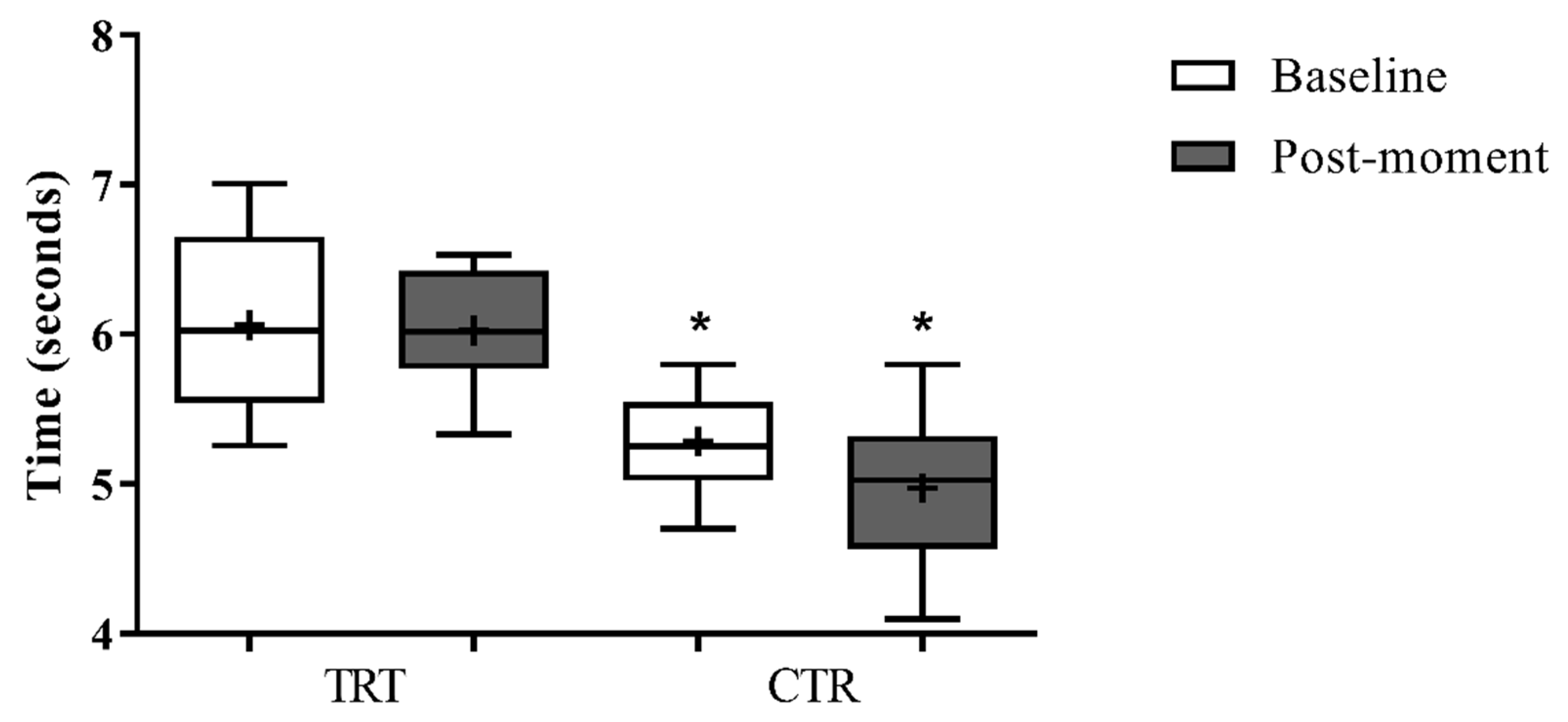
3. Results
4. Discussion
5. Conclusions
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
TERM OF CONSENT STATEMENT
-
You are being invited to participate, as a volunteer, in the research "Effects of chemotherapy treatment on indicators of muscle strength, functional capacity and biopsychosocial aspects of women with breast cancer". The objective of this research is: To analyze the effects of chemotherapy cycles on muscle strength and activation, functional capacity, quality of life, fatigue and anxiety of women with breast cancer. The research is justified because most women in treatment have symptoms related to fatigue, quality of life and anxiety, and for many, even after treatment, these symptoms are still present. Reduced ability to work and decreased abilities to perform tasks of daily living are often accompanied by burnout and tiredness, conditions typical of cancer-related fatigue.This project will be developed in the laboratories of the Faculty of Nutrition and Health and in the University Hospital. It will take a few meetings to carry out all the necessary assessments. Each meeting will be scheduled in advance and will last close to 1 hour.At the first meeting you must sign the consent statement, as well as the following questionnaires, in random order, about quality of life, fatigue and anxiety. In addition, you will perform the functional capacity tests, and in one of them will be measured the muscle activity, through electromyography.The research will last approximately 2 months, containing two analyses on days and times to be combined with the volunteers. The times of your participation will be scheduled in advance respecting the intervals mentioned above, as well as your availability. You should be at the designated locations on the scheduled days and times and inform the researchers of any discomfort you may notice.The study does not involve expenses for you and / or companions, however if there are expenses with transportation and / or food the amounts will be reimbursed by the researchers. When necessary, the amounts will be reimbursed in the form of transportation vouchers and/or food stamps. All materials and equipment required for the tests will be provided by the researchers. This study should not be applied to people with the following conditions: those with heart disease, joint disease, respiratory problems or any contraindication to exercise.With the results of the study we will be able to obtain information about the effects of chemotherapy treatment on muscle responses, functional capacity, fatigue levels, quality of life and anxiety, defining its benefits or not the population studied, this knowledge will be useful to health professionals.The information obtained in this experiment may be used as scientific research data, and may be published and disseminated, being protected the identity of the participants. You can access your results through the responsible researcher.The responsible researcher will suspend the research immediately if he perceives any risk or damage to the participant's health, both those foreseen and those not foreseen in this term. In the unlikely physical damage resulting from participation in this study, treatment will be feasible at the nearest and most appropriate site.This project was approved by the Research Ethics Committee of the Federal University of Goiás.Your permission to participate in this research is voluntary. You will be free to deny it or to at any time give it up if you wish. You may refuse to answer any questions that cause you embarrassment. In case you agree to be part of the study, after having read and being enlightened about the above information, sign at the end of this phone, which is in two ways. One of them is yours and the other will be with the responsible researcher. All sheets must be initialed by the research volunteer or guardian and by the responsible researcher. In case of doubt or complaint, you can contact the researchers Rafael Ribeiro Alves, Vitor Alves Marques, Weder Silva, or by calling the responsible researcher Carlos Alexandre Vieira – (62) 98111-3242 or the Ethics and Research Committee of the Federal University of Goiás by phone (62) 3521-1215.Goiânia,_______de ____________ de _______________________________________________________Name / signature________________________________________________Researcher - Name / signature
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- INCA. Instituto Nacional de Cancer José Alencar Gomes da Silva. Estimativa 2016: Incidência de Câncer No Brasil; 2016. doi:978-85-7318-283-5.
- Santana, silva, J. “Chorar podia emagrecer”:desejo e sacrificio na construção digital do corpo anoréxico feminino. Sociedade Brasiliera de Estudos Interdisciplinares da Comunicação 2017.
- Vieira, C.; Battaglini, C.; Ferreira-Junior, J.; Vieira, A.; Brito Vogt, M.; Freitas-Junior, R.; et al. Effects of Rest Interval on Strength Recovery in Breast Cancer Survivors. International Journal of Sports Medicine 2015, 36(07), 573–578. [CrossRef]
- Peart, O. Breast intervention and breast cancer treatment options. Radiologic technology 2015, 86(5), 535M-558M; quiz 559–562.
- Fisusi, F. A.; Akala, E. O. Drug Combinations in Breast Cancer Therapy. Pharmaceutical Nanotechnology 2019, 7(1), 3–23. [CrossRef]
- Kirjner, A.; Pinheiro, R. D. L. Interferência da Obesidade no Tratamento Quimioterápico em Mulheres com Câncer de Mama. Revista Brasileira de Cancerologia 2007, 53(3), 345–354.
- Osmani, K.; Vignes, S.; Aissi, M.; Wade, F.; Milani, P.; Lévy, B.; et al. Taxane-induced peripheral neuropathy has good long-term prognosis: A 1- to 13-year evaluation. J Neurol 2012, 249(1936–1943). [CrossRef]
- Artero, E. G.; Lee, D. C.; Ruiz, J. R.; Sui, X.; Ortega, F. B.; Church, T. S.; et al. A prospective study of muscular strength and all-cause mortality in men with hypertension. Journal of the American College of Cardiology 2011, 57(18), 1831–1837. [CrossRef]
- Prado, C. M. M.; Baracos, V. E.; McCargar, L. J.; Mourtzakis, M.; Mulder, K. E.; Reiman, T.; et al. Body composition as an independent determinant of 5-fluorouracil-based chemotherapy toxicity. Clinical Cancer Research 2007. [CrossRef]
- Jones, L. W.; Mourtzakis, M.; Peters, K. B.; Friedman, A. H.; West, M. J.; Mabe, S. K.; et al. Changes in Functional Performance Measures in Adults Undergoing Chemoradiation for Primary Malignant Glioma: A Feasibility Study. The Oncologist 2010. [CrossRef]
- Klassen, O.; Schmidt, M. E.; Ulrich, C. M.; Schneeweiss, A.; Potthoff, K.; Steindorf, K.; et al. Muscle strength in breast cancer patients receiving different treatment regimes. Journal of Cachexia, Sarcopenia and Muscle 2017. [CrossRef]
- Fayers, P. M.; Machin, D. Quality of Life: The Assessment, Analysis and Interpretation of Patient-Reported Outcomes: Second Edition; 2007. [CrossRef]
- Juniper, E. F.; Guyatt, G. H.; Epstein, R. S.; Ferrie, P. J.; Jaeschke, R.; Hiller, T. K. Evaluation of impairment of health related quality of life in asthma: Development of a questionnaire for use in clinical trials. Thorax 1992. [CrossRef]
- Bassarath, L. Conduct disorder: A biopsychosocial review. Canadian Journal of Psychiatry. 2001. [CrossRef]
- Suls, J.; Rothman, A. Evolution of the Biopsychosocial Model: Prospects and Challenges for Health Psychology. Health Psychology. 2004. [CrossRef]
- Caffo, O.; Amichetti, M.; Ferro, A.; Lucenti, A.; Valduga, F. Pain and quality of life after surgery for breast cancer. 2003, 39–48. [CrossRef]
- Galantino, M. Lou; Desai, K.; Greene, L.; Demichele, A.; Stricker, C. T.; Mao, J. J. Impact of yoga on functional outcomes in breast cancer survivors with aromatase inhibitor-associated arthralgias. Integrative Cancer Therapies 2012. [CrossRef]
- Donovan, K. A.; Jacobsen, P. B.; Andrykowski, M. A.; Winters, E. M.; Balducci, L.; Malik, U.; et al. Course of fatigue in women receiving chemotherapy and/or radiotherapy for early stage breast cancer. Journal of Pain and Symptom Management 2004. [CrossRef]
- Kilgour, R. D.; Vigano, A.; Trutschnigg, B.; Hornby, L. Cancer-related fatigue : The impact of skeletal muscle mass and strength in patients with advanced cancer. 2010, 177–185. [CrossRef]
- Taghian, N. R.; Miller, C. L.; Jammallo, L. S.; O’Toole, J.; Skolny, M. N. Lymphedema following breast cancer treatment and impact on quality of life: A review. Critical Reviews in Oncology/Hematology. 2014. [CrossRef]
- Montazeri, A. Health-related quality of life in breast cancer patients: A bibliographic review of the literature from 1974 to 2007. Journal of Experimental and Clinical Cancer Research. 2008. [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods 2007, 39(2), 175–191. [CrossRef]
- Marques, V. A.; Ferreira-Junior, J. B.; Lemos, T. V.; Moraes, R. F.; Junior, J. R. de S.; Alves, R. R.; et al. Effects of Chemotherapy Treatment on Muscle Strength, Quality of Life, Fatigue, and Anxiety in Women with Breast Cancer. International Journal of Environmental Research and Public Health 2020, 17(19), 7289. [CrossRef]
- Mosconi, P.; Cifani, S.; Crispino, S.; Fossati, R.; Apolone, G. The performance of SF-36 health survey in patients with laryngeal cancer. Head and Neck Cancer Italian Working Group. Head & neck 2000, 22(2), 175–182. [CrossRef]
- Bf, P.; Sl, D.; Mj, D.; Mc, W.; Re, S.; Sm, P. The revised Piper Fatigue Scale: Psychometric evaluation in women with breast cancer. Oncology nursing forum 1998, 25(4), 677–684.
- Biaggio, A. M. B.; Natalício, L.; Spielberger, C. D. Desenvolvimento da forma experimental em português do Inventário de Ansiedade Traço-Estado (IDATE) de Spielberger. Arquivos Brasileiros de Psicologia Aplicada 1977.
- (FAO), O. M. de la S. (OMS) y O. de las N. U. para la A. y la A.; (OMS), O. M. de la S.; (OMS), O. de las N. U. para la A. y la A. (FAO) / O. M. de la S. Segunda Conferencia Internacional sobre Nutrición Roma , 19-21 de noviembre de 2014 Documento final de la Conferencia : Declaración de Roma sobre la Nutrición. Orgaización Mundial de la Salud 2014.
- Hermens, H. J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. Journal of Electromyography and Kinesiology 2000, 10(5), 361–374. [CrossRef]
- Borg, G. Psychophysical scaling with applications in physical work and the perception of exertion. In Scandinavian Journal of Work, Environment and Health; 1990. [CrossRef]
- Borg, G. A. Psychophysical bases of perceived exertion. Medicine and science in sports and exercise 1982, 14(5), 377–381.
- Rikli, R. E.; Jones, C. J. Development and validation of a functional fitness test for community- residing older adults. Journal of Aging and Physical Activity 1999. [CrossRef]
- Swenson, K. K.; Nissen, M. J.; Henly, S. J.; Maybon, L.; Pupkes, J.; Zwicky, K.; et al. Identification of Tools to Measure Changes in Musculoskeletal Symptoms and Physical Functioning in Women With Breast Cancer Receiving Aromatase Inhibitors. Oncology Nursing Forum 2013, 40(6), 549–557. [CrossRef]
- Ciconelli, R. M.; Ferraz, M. B.; Santos, W.; Meinão, I.; Quaresma, M. R. Tradução para a língua portuguesa e validação do questionário genérico de avaliação de qualidade de vida SF-36 (Brasil SF-36). Revista Brasileira De Reumatologia. 1999, pp 143–150. doi:296502.
- Treanor, C.; Donnelly, M. A methodological review of the Short Form Health Survey 36 (SF-36) and its derivatives among breast cancer survivors. Quality of Life Research 2015, 24(2), 339–362. [CrossRef]
- Van Esch, L.; Roukema, J. A.; Van der Steeg, A. F. W.; De Vries, J. Trait anxiety predicts disease-specific health status in early-stage breast cancer patients. Quality of Life Research 2011, 20(6), 865–873. [CrossRef]
- Cohen, J. The concepts of power analysis. Statistical Power Analysis for the Behavioral Sciences. Lawrence Elbaum Associates 1988, 1–17.
- Klassen, O.; Schmidt, M. E.; Ulrich, C. M.; Schneeweiss, A.; Potthoff, K.; Steindorf, K.; et al. Muscle strength in breast cancer patients receiving different treatment regimes. Journal of cachexia, sarcopenia and muscle 2016. [CrossRef]
- Marques, V. A. Efeitos do tratamento quimioterápico sobre desempenho muscular de mulheres com câncer de mama., Universidade Federal de Goiás, 2017.
- Santos, E. C. D. O.; Galvão, L. L.; Tribess, S.; Meneguci, J.; Santos, R. G. dos; Silva, R. R.; et al. Valores normativos de força muscular em idosos. Arquivos de Ciências do Esporte 2019, 6(4). [CrossRef]
- Souza Saraiva, W.; Prestes, J.; Schwerz Funghetto, S.; Navalta, J. W.; Tibana, R. A.; da Cunha Nascimento, D. Relation Between Relative Handgrip Strength, Chronological Age and Physiological Age with Lower Functional Capacity in Older Women. Open Access Journal of Sports Medicine 2019, Volume 10, 185–190. [CrossRef]
- Lee, J. Associations Between Handgrip Strength and Disease-Specific Mortality Including Cancer, Cardiovascular, and Respiratory Diseases in Older Adults: A Meta-Analysis. Journal of Aging and Physical Activity 2019, 1–12. [CrossRef]
- McGowan, J. V.; Chung, R.; Maulik, A.; Piotrowska, I.; Walker, J. M.; Yellon, D. M. Anthracycline Chemotherapy and Cardiotoxicity. Cardiovascular Drugs and Therapy 2017. [CrossRef]
- Yang, E. J.; Kwon, Y. O. Changes in shoulder muscle activity pattern on surface electromyography after breast cancer surgery. Journal of Surgical Oncology 2018. [CrossRef]
- Perez, C.; Neves, L.; Vacari, A. luiza; Fonseca, M.; Guirro, R.; Guirro, E. Reduction in handgrip strength and electromyographic activity in women with breast cancer. Journal of Back and Musculoskeletal Rehabilitation 2017, 1(10:32), 1–6. [CrossRef]
- Pion, C. H.; Barbat-Artigas, S.; St-Jean-Pelletier, F.; Chevalier, S.; Gaudreau, P.; Gouspillou, G.; et al. Muscle strength and force development in high- and low-functioning elderly men: Influence of muscular and neural factors. Experimental Gerontology 2017, 96, 19–28. [CrossRef]
- Maeo, S.; Shan, X.; Otsuka, S.; Kanehisa, H.; Kawakami, Y. Single-joint eccentric knee extension training preferentially trains the rectus femoris within the quadriceps muscles. Translational Sports Medicine 2018. [CrossRef]
- Fernandez, C.; Firdous, S.; Jehangir, W.; Behm, B.; Mehta, Z.; Berger, A.; et al. Cancer-Related Fatigue: Perception of Effort or Task Failure? American Journal of Hospice and Palliative Medicine® 2019, 104990911984942. [CrossRef]
- Jacobsen, P. B.; Hann, D. M.; Azzarello, L. M.; Horton, J.; Balducci, L.; Lyman, G. H. Fatigue in women receiving adjuvant chemotherapy for breast cancer: Characteristics, course, and correlates. Journal of Pain and Symptom Management 1999. [CrossRef]
- Clevenger, L.; Schrepf, A.; Christensen, D.; DeGeest, K.; Bender, D.; Ahmed, A.; et al. Sleep disturbance, cytokines, and fatigue in women with ovarian cancer. Brain, Behavior, and Immunity. 2012. [CrossRef]
- Lutgendorf, S. K.; Weinrib, A. Z.; Penedo, F.; Russell, D.; DeGeest, K.; Costanzo, E. S.; et al. Interleukin-6, cortisol, and depressive symptoms in ovarian cancer patients. Journal of Clinical Oncology 2008. [CrossRef]
- Ferreira, A. S.; Bicalho, B. P.; Neves, L. F. G.; Menezes, M. T.; Silva, T. A.; Faier, T. A.; et al. Prevalência de Ansiedade e Depressão em Pacientes Oncológicos e Identificação de Variáveis Predisponentes. Revista Brasileira de Cancerologia 2019. [CrossRef]
- Villar, R. R.; Fernández, S. P.; Garea, C. C.; Pillado, M. T. S.; Barreiro, V. B.; Martín, C. G. Quality of life and anxiety in women with breast cancer before and after treatment. Revista latino-americana de enfermagem 2017. [CrossRef]
- Silva, A. V. da; Zandonade, E.; Amorim, M. H. C. Anxiety and coping in women with breast cancer in chemotherapy. Revista Latino-Americana de Enfermagem 2017. [CrossRef]
- Klapheke, A. K.; Keegan, T. H. M.; Ruskin, R.; Cress, R. D. Changes in health-related quality of life in older women after diagnosis with gynecologic cancer. Gynecologic Oncology 2019. [CrossRef]
- Hassen, A. M.; Taye, G.; Gizaw, M.; Hussien, F. M. Quality of life and associated factors among patients with breast cancer under chemotherapy at Tikur Anbessa specialized hospital, Addis Ababa, Ethiopia. PLoS ONE 2019. [CrossRef]
- Safaee, A.; Moghimi-Dehkordi, B.; Zeighami, B.; Tabatabaee, H. R.; Pourhoseingholi, M. A. Predictors of quality of life in breast cancer patients under chemotherapy. Indian Journal of Cancer 2008. [CrossRef]
- Dubashi, B.; Vidhubala, E.; Cyriac, S.; Sagar, T. Quality of life among young women with breast cancer: Study from a tertiary cancer institute in south India. Indian Journal of Cancer 2010. [CrossRef]
- Lôbo, S. A.; Fernandes, A. F. C.; Almeida, P. C. de; Carvalho, C. M. de L.; Sawada, N. O. Qualidade de vida em mulheres com neoplasias de mama em quimioterapia. Acta Paulista de Enfermagem 2014, 27(6), 554–559. [CrossRef]
| Groups* | Age (years) | Height (cm) | Body mass (kg) | BMI (kg/m2) | Treatment |
|---|---|---|---|---|---|
| TRT | 46.6 ± 9.6 | 1.54 ± 0.6 | 59.4 ± 5.9 | 24.9 ± 2.5 | AC |
| CTR | 51.6 ± 7.0 | 1.56 ± 0.3 | 63.3 ± 9.7 | 25.9 ± 3.7 | N/T |
| Group | Moment | ||||
|---|---|---|---|---|---|
| TRT | CTR | ||||
| Baseline | Post-moment | Baseline | Post-moment | ||
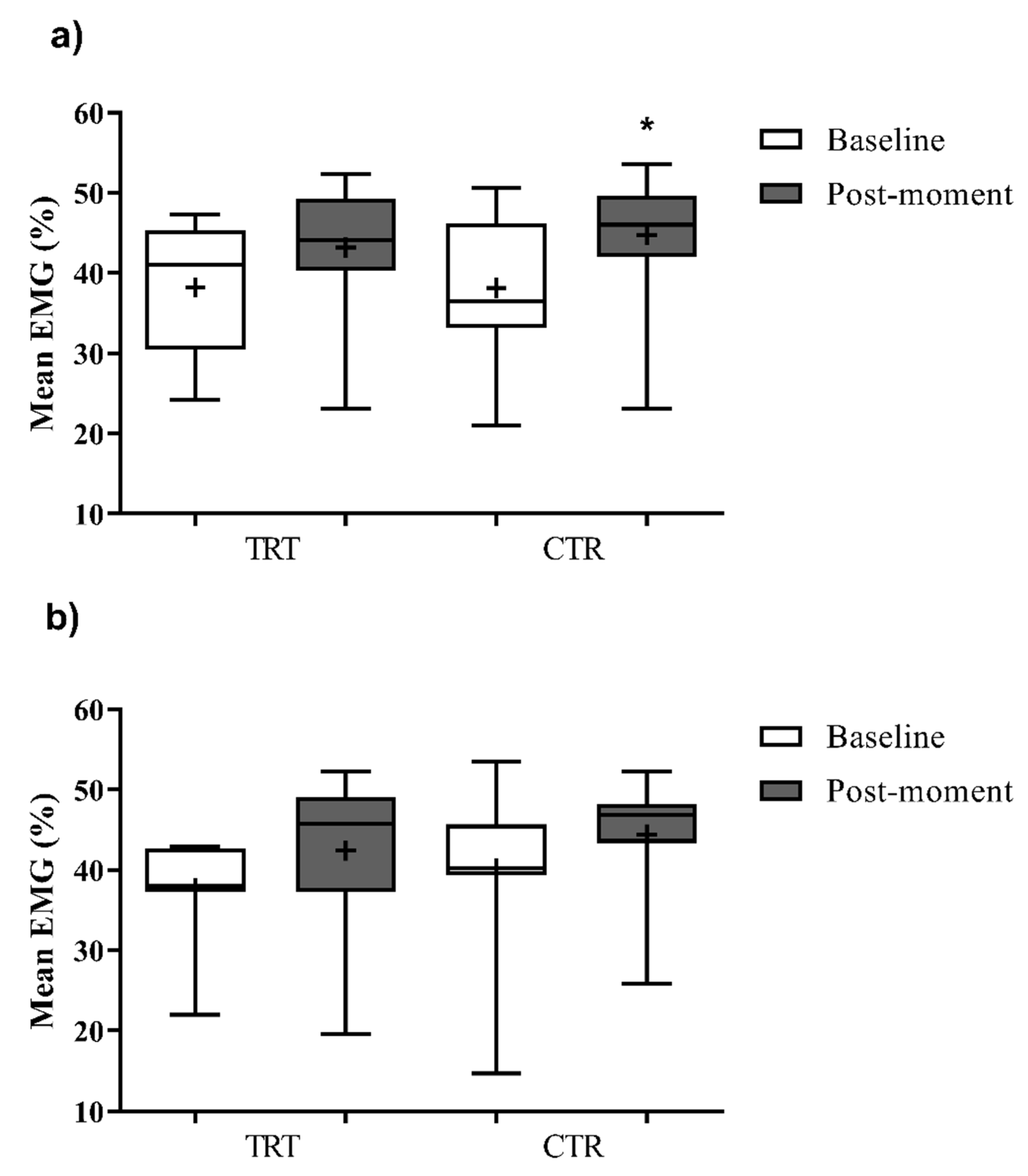
| RF | 37,7 ± 6,1 | 40,2 ± 10,2 | 42,4 ± 10,3 | 44,4 ± 7,8 | |
| VM | 38,2 ± 8,7 | 36,2 ± 13,7 | 42,4 ± 8,2 | 44,8 ± 7,8 | |
| Variable | Group TRT (n=10) | Group CRT (n=12) |
Effect size |
p* |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Post-moment | p# | Baseline | Post-moment | p# | ||||
| Mean±DP | Mean±DP | Effect size | Mean±DP | Mean±DP | Group x Moment |
||||
| Quality of life | |||||||||
| Physical functioning | 77.0 ± 16.9 § | 58.7 ± 11.6 § | 0.01# | -1.26 | 88.1 ± 7.2 | 85.9 ± 10.2 | 0.65 | -0.25 | 0.03 * |
| Role physical | 12.5 ± 13.2 | 45.7 ± 45.5 | 0.02# | 0.99 | 81.4 ± 26.3 | 81.7 ± 26.3 | 0.98 | 0.01 | 0.07 |
| Bodily pain | 63.0 ± 33.7 | 57.9 ± 17.3 | 0.64 | -0.19 | 71.6 ± 24.7 | 73.5 ± 17.6 | 0.85 | 0.09 | 0.64 |
| General health | 59.8 ± 18.9 | 67.0 ± 20.1 | 0.31 | 0.37 | 72.0 ± 11.6 | 74.8 ± 12.1 | 0.66 | 0.24 | 0.65 |
| Vitality | 53.5 ± 17.1 | 64.0 ± 24.9 | 0.06 | 0.49 | 68.1 ± 13.0 | 69.0 ± 11.4 | 0.49 | 0.07 | 0.35 |
| Social functioning | 75.1 ± 22.8 | 68.3 ± 38.4 | 0.55 | -0.22 | 81.7 ± 21.6 | 85.6 ± 14.6 | 0.70 | 0.21 | 0.49 |
| Role emotional | 40.0 ± 41.0 | 44.6 ± 41.6 | 0.78 | 0.11 | 76.1 ± 35.1 | 74.3 ± 31.2 | 0.90 | -0.05 | 0.77 |
| Mental health | 66.8 ± 22.6 | 65.4 ± 23.3 | 0.86 | -0.06 | 73.0 ± 12.5 | 69.1 ± 13.0 | 0.62 | -0.31 | 0.83 |
| General status | 56.0 ± 16.3 | 59.2 ± 21.9 | 0.62 | 0.17 | 76.6 ± 9.9 | 76.7 ± 7.5 | 0.97 | 0.01 | 0.73 |
| Domain |
Fatigue level |
Groups | |||
|---|---|---|---|---|---|
| TRT | CRT | ||||
| Baseline | Post-moment | Baseline | Post-moment | ||
| Behavioral | Absence | ------ | ------ | ------ | ------ |
| Middle | 60.0%(n=6) | 50.0%(n=5) | 100.0%(n=12) | 100.0%(n=12) | |
| Moderate | 40.0%(n=4) | 20.0%(n=2) | ------ | ------ | |
| Severe | ------ | 30.0%(n=3) | ------ | ------ | |
| Affective | Absence | ------ | ------ | ------ | ------ |
| Middle | 60.0%(n=6) | 50.0%(n=5) | 100.0%(n=12) | 100.0%(n=12) | |
| Moderate | 20.0%(n=2) | 40.0%(n=4) | ------ | ------ | |
| Severe | 20.0%(n=2) | 10.0%(n=1) | ------ | ------ | |
| Sensory | Absence | ------ | ------ | ------ | ------ |
| Middle | 70.0%(n=7) | 70.0%(n=7) | 100.0%(n=12) | 83.3%(n=7) | |
| Moderate | 30.0%(n=7) | 30.0% (n=7) | ------ | 16.7%(n=2) | |
| Severe | ------ | ------ | ------ | ------ | |
| Cognitive | Absence | ------ | ------ | ------ | ------ |
| Middle | 80.0%(n=8) | 80.0%(n=8) | 91.7%(n=11) | 100.0%(n=12) | |
| Moderate | 20.0%(n=2) | 20.0%(n=2) | 8.3%(n=1) | ------ | |
| Severe | ------ | ------ | ------ | ------ | |
| General mean | Absence | ------ | ------ | ------ | ------ |
| Middle | 60.0%(n=6) | 50.0%(n=5) | 100.0%(n=12) | 100.0%(n=12) | |
| Moderate | 40.0%(n=4) | 50.0%(n=5) | ------ | ------ | |
| Severe | ------ | ------ | ------ | ------ | |
| Domain |
Anxiety level |
Groups | |||
|---|---|---|---|---|---|
| TRT | CRT | ||||
| Baseline | Post-moment | Baseline | Post-moment | ||
| Trace | Low | 40.0%(n=4) | 30.0%(n=3) | 41.7%(n=5) | 33.3%(n=4) |
| Moderate | 60.0%(n=6) | 50.0%(n=5) | 58.3%(n=7) | 58.3%(n=7) | |
| High | ------ | 20.0%(n=2) | ------ | 8.3%(n=1) | |
| Severe | ------ | ------ | ------ | ------ | |
| State | Low | 40.0%(n=4) | 20.0%(n=2) | 33.3%(n=4) | 41.7%(n=5) |
| Moderate | 50.0%(n=5) | 70.0%(n=7) | 66.7%(n=8) | 50.0%(n=6) | |
| High | 10.0%(n=1) | 10.0%(n=1) | ------ | 8.3%(n=1) | |
| Severe | ------ | ------ | ------ | ------ | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




