1. Introduction
Musculoskeletal disorders encompass a wide range of disorders affecting various structures, such as joints, ligaments, tendons, and cartilage, often causing chronic pain and limitation. In recent years, there has been a significant increase in the prevalence of musculoskeletal disorders due to an aging population, overuse, and injuries [
1].
Various injection techniques have been explored to treat musculoskeletal complaints, including corticosteroid injections, hyaluronic acid (HA), platelet-rich plasma, and prolotherapy [
2]. There is also a growing interest in collagen injections as a potential therapeutic option [
3,
4,
5]. In fact, collagen, the main structural protein in the extracellular matrix, plays a crucial role in tissue integrity and function [
6]. Different types of collagens have specific functions in different tissue types [
7]. Experimental models using collagen have shown favorable biocompatibility, biodegradability, and weak immunogenic reactions [
8]. Porcine injections have been widely used in clinical settings, acting as natural bio-scaffolds and stimulating endogenous collagen synthesis [
4,
9]. Indeed, porcine collagen, structurally similar to human collagen, is preferred for treating musculoskeletal conditions as well: great trochanteric pain syndrome (GTPS) [
10,
11], Knee Osteoarthritis (K-OA) [
12,
13,
14], hip tendinitis [
15,
16,
17], rotator cuff disease [
18] due to its high safety and low immunogenicity [
19] compared with the other most common extraction source of collage, namely bovine collagen [
20], making it an ideal material for bio-scaffold production [
4,
5,
21,
22] and to be more compatible for humans instead of the bovine one [
19]. Indeed, Porcine collagen does not cause allergic reactions unlike bovine collagen, which causes adverse reactions in 3% of the population [
19].
Despite the promising results of collagen injections, there is a lack of specific guidelines for these injections in treating musculoskeletal complaints regarding different anatomical regions [
10,
16,
17,
22,
23], therapeutic indications [
16,
24,
25,
26], treatment protocols [
2,
4,
18,
27], injection modality [
11,
27,
28] and the combination with other treatments [
14,
24,
29,
30]. In order to address this void, a Scientific Board comprised of Italian authorities on the diagnosis and treatment of musculoskeletal disorders launched work on a consensus document. This eDelphi consensus aimed to gather experiences and opinions from Italian physicians, mainly physiatrists and rheumatologists, with expertise in diagnosing and treating musculoskeletal pain. In order to ensure informed clinical practice based on a corpus of knowledge, the aim was to set the path for future guidelines in this area.
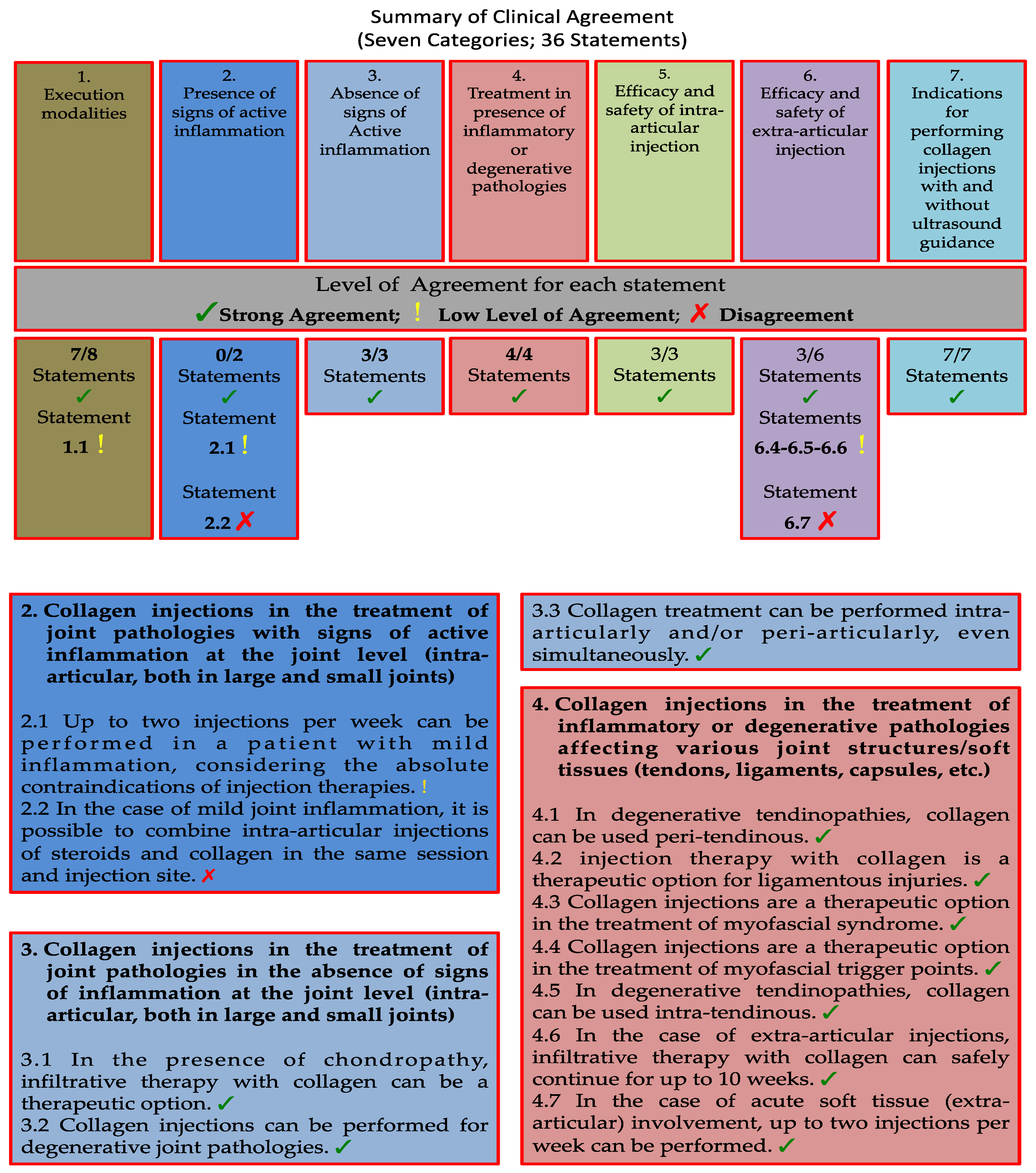
Figure 1.
Summary of Clinical Agreement. All 36 statements are represented by different colors, within their respective boxes. The percentages of strong agreement are reported below the box related to the respective category. Each statement is marked according to the legend associated with the image. Additional final considerations regarding the statements without "strong agreement" are reported in the last dark gray box.2. Materials and Methods.
Figure 1.
Summary of Clinical Agreement. All 36 statements are represented by different colors, within their respective boxes. The percentages of strong agreement are reported below the box related to the respective category. Each statement is marked according to the legend associated with the image. Additional final considerations regarding the statements without "strong agreement" are reported in the last dark gray box.2. Materials and Methods.
2.1. Delphi survey and Panel composition
In this study, we employed a two-round eDelphi process conducted through an online survey aiming to achieve consensus on the use of collagen injections in treating musculoskeletal pain, similar to a previous report by Bernetti et al. [
31], aimed at reaching consensus on the use of slow-acting drug for osteoarthritis treatment (SYSADOA) Regarding the use of collagen injections in this specific area, there are no evidence-based guidelines or recommendations. The Steering Committee (SC) comprised highly experienced physiatrists and rheumatologists (DLO, GiF, BA, CA, CC, LMG, GeF, BL, RMA) with substantial expertise in diagnosing and treating musculoskeletal pain. The SC then identified a panel of 23 Italian experts based on their extensive publication record or their significant clinical and academic backgrounds (Appendix 1). The SC agreed to structure the study into seven distinct categories and developed statements within each category (
Table 1 and
Figure 3).
The web-based eDelphi questionnaire was emailed to each panel member (
Figure 1) with a secure link using the private platform, following the Checklist for Reporting Results of Internet E-Surveys (CHERRIES) [
32].
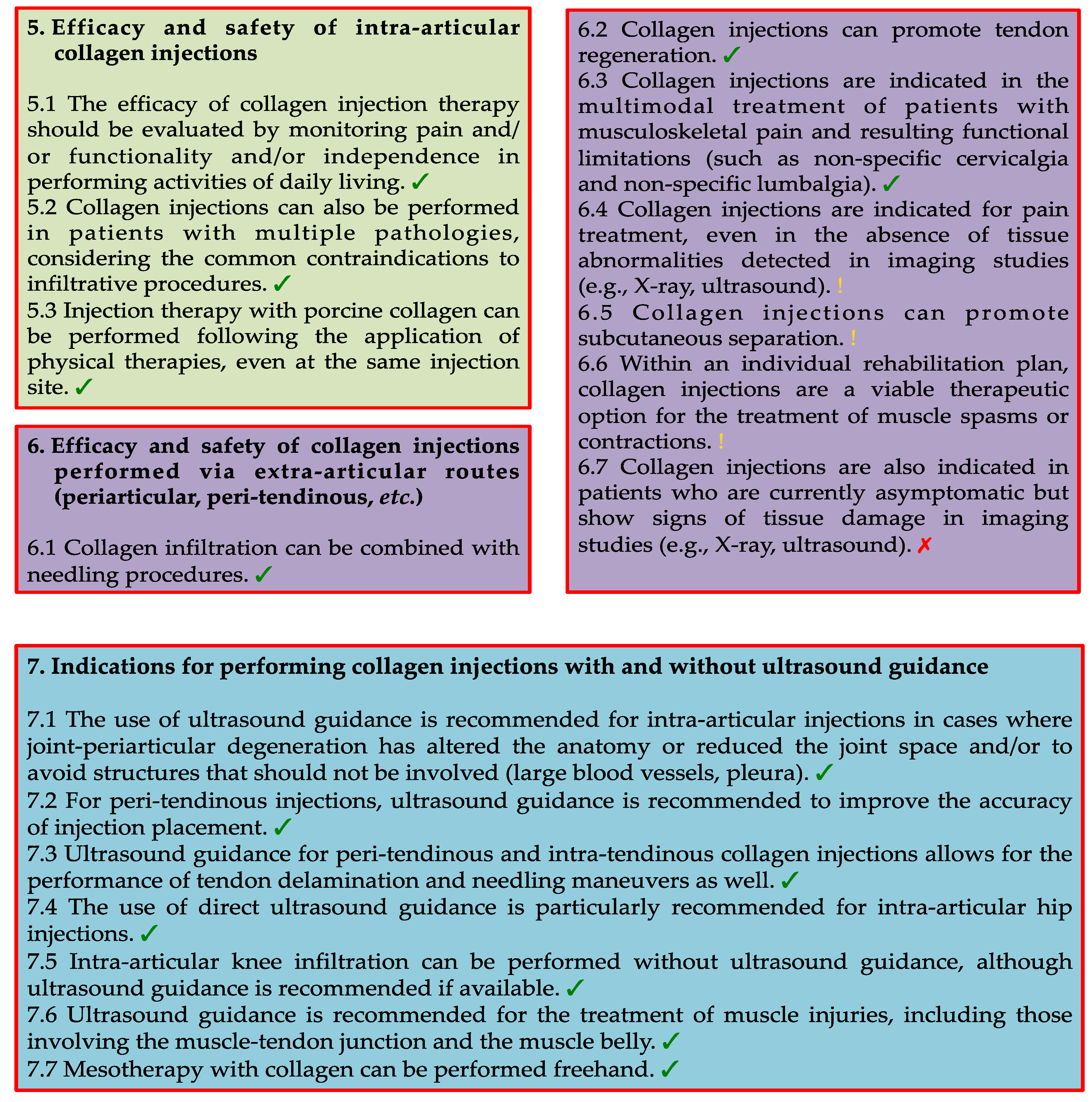
Figure 2.
Flowchart depicting the Delphi process employed to establish consensus statements regarding the use of collagen injections in physical and rehabilitation medicine. Source: Original.
Figure 2.
Flowchart depicting the Delphi process employed to establish consensus statements regarding the use of collagen injections in physical and rehabilitation medicine. Source: Original.
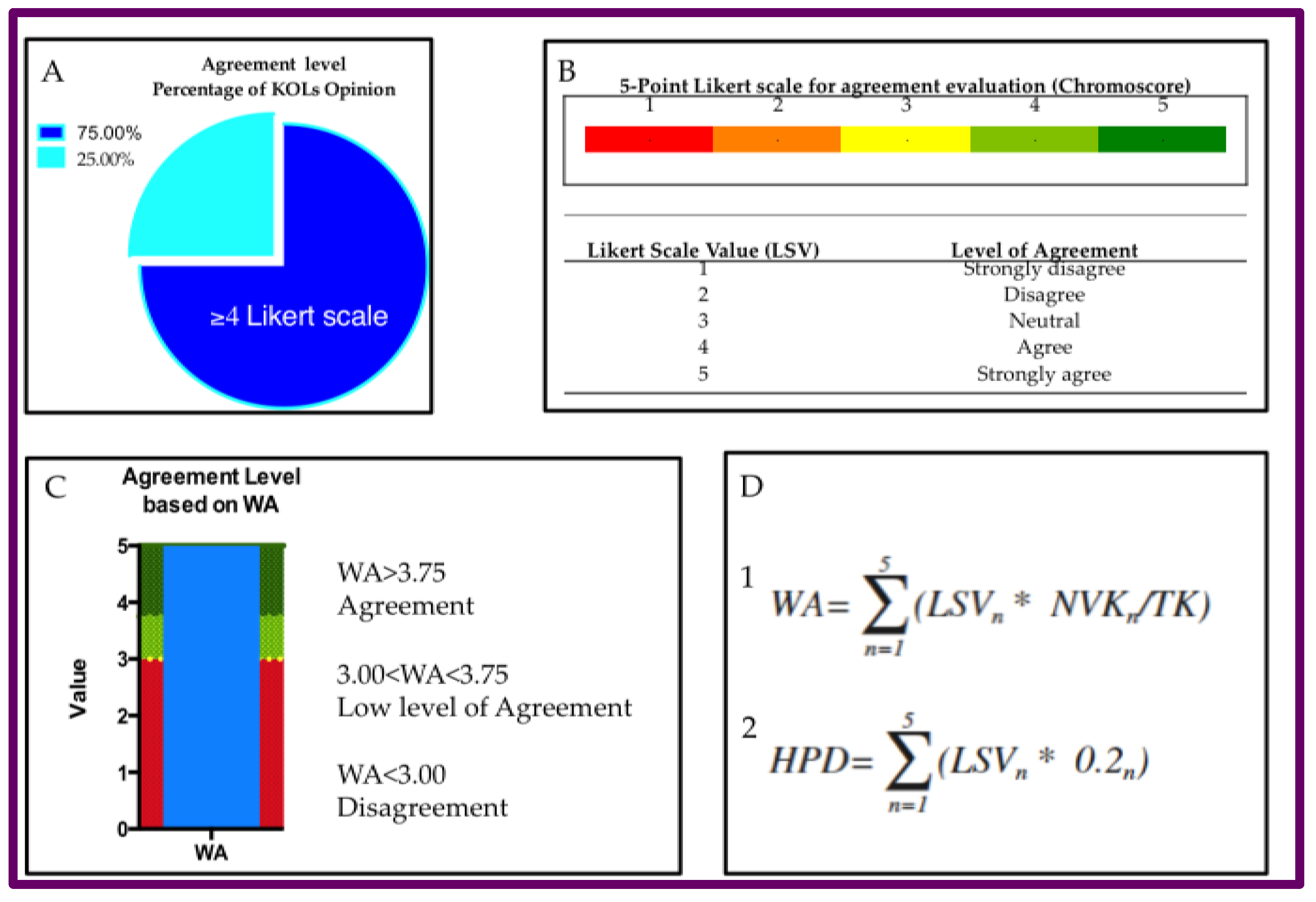
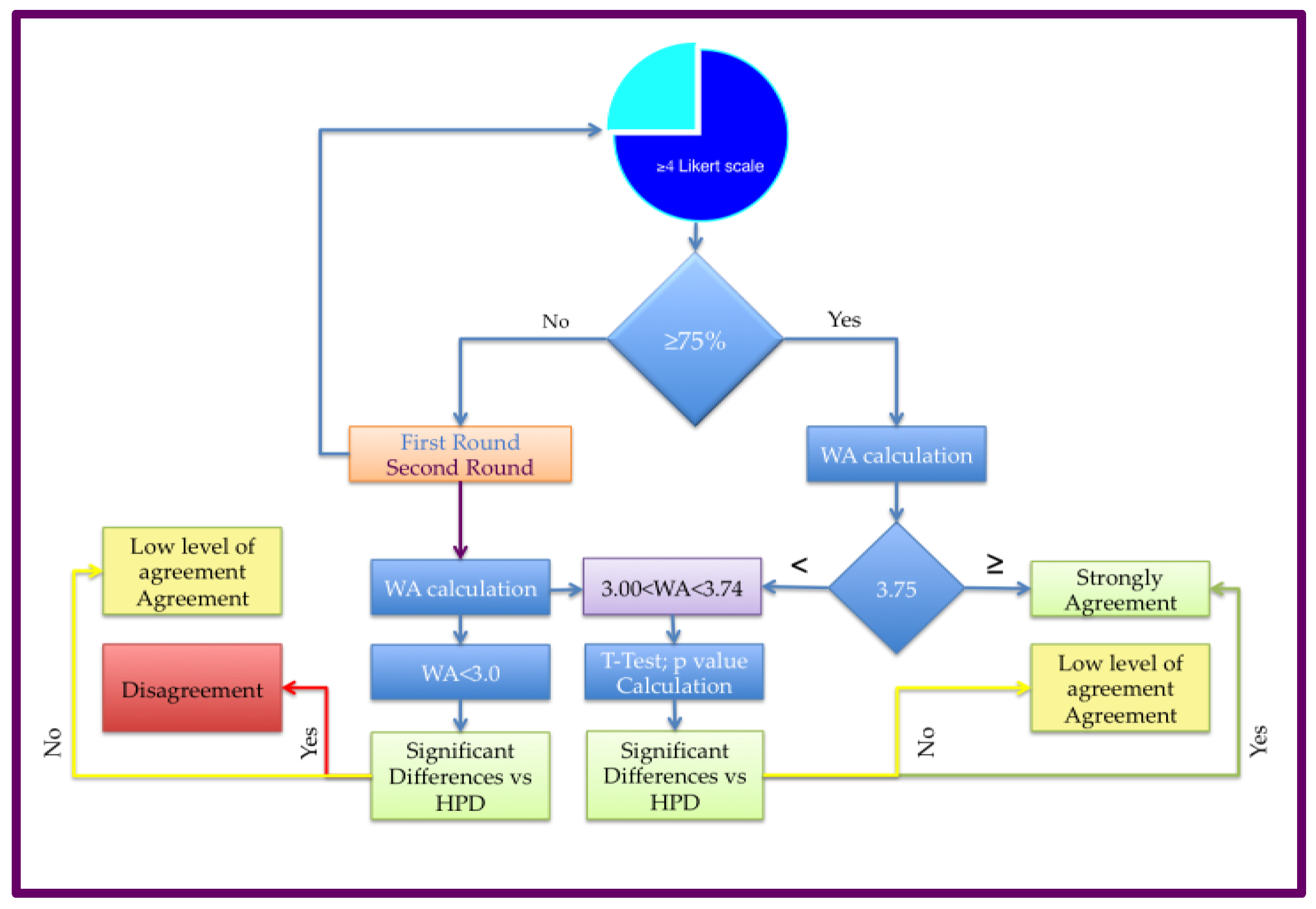
The anonymity of their responses was strictly maintained, ensuring that their identities remained undisclosed. Access to the individual responses was only granted to the independent study moderator, who was not affiliated with the SC or the expert Panel. The respondents had to rate their agreement with each statement on a 5-point Likert scale (score 1 indicated “strongly disagreement”, while score 5 indicated “strongly agreement”; the score 3 represented the “neutral position). The Likert scale was used jugging the following options for panelists: Strongly Disagreement, Disagreement, Neutral, strongly, strongly agreement (
Figure 4A). To facilitate comparisons between different statements within the same category, a Weighted Average (WA) regarding the eDelphi statement was calculated, applying the weighted average formula as well in the equation below (
Figure 4D; Equation 1). For each statement, the formula considered the product among the Frequency of Key Opinion Leaders voting (NKV) out of the Total Key Opinion Leaders (TK), for the specific value of Likert scale (LSV). The WA for each question was obtained by the sum of all products as reported in Equation 1. To understand whether the collected responses were provided randomly or following a causal distribution, a Hypothetical Parametric Distribution (HPD;
Figure 4D;Equation 2) was calculated assessing the same frequency for each LSV equal to 0.2 (Equation 2) for each statement. The WA of HPD was equal to 3.00. Quantitative analyses were performed comparing WA vs HPD by Welcoxon test. Parametric test were used in order to find statistical significant differences. The results of the comparison mentioned above were judged significant according the p values <0.05. Shaphiro-wilk tests were also performed in order to verify the parametric distribution in the three cohorts mentioned above.
Figure 3.
: All criteria tu evaluate the agreement mumbers. A) First criterion of decision for the “strongly agreement; B) Likrt Scale Values and their associated-scores; C) Grapf bar regarding the borderline value of WA; Equation used in order to obtain the WA of Delphi (equation 1) and the weighted average Hypotetical Parametric Distributions (HPD, equation 2).
Figure 3.
: All criteria tu evaluate the agreement mumbers. A) First criterion of decision for the “strongly agreement; B) Likrt Scale Values and their associated-scores; C) Grapf bar regarding the borderline value of WA; Equation used in order to obtain the WA of Delphi (equation 1) and the weighted average Hypotetical Parametric Distributions (HPD, equation 2).
Consensus was achieved on a statement when at least 75% of the respondents scored their agreement with that statement as ≥4 of LSV. Notably, the maximum of WE in this condition is equal to 3.75%. The SC define the statement as follows: “Strong Agreement”. When the stamen doesn’t reach the condition mentioned above the following flowchart was used in order to establish which type of consensus how is better to asses.
Figure 4.
Flowchart for the Agreement decision. The blu BUS indicate the decision three for the agreement. Light green BUS: Algorythm for “Strongly agreement”, Yellow BUS: Algorythm for “Low Level of Agreement” and Red BUS: “Disagreement”. Two crucial Value foe WA were found: WA>3.75 measns “Strongly agreement”, while WE<3.00 means “Disagreement.
Figure 4.
Flowchart for the Agreement decision. The blu BUS indicate the decision three for the agreement. Light green BUS: Algorythm for “Strongly agreement”, Yellow BUS: Algorythm for “Low Level of Agreement” and Red BUS: “Disagreement”. Two crucial Value foe WA were found: WA>3.75 measns “Strongly agreement”, while WE<3.00 means “Disagreement.
After the initial phase of the poll, the SC convened virtually to discuss the findings. During this session, they identified statements that did not reach a consensus but were deemed to deserve a revoting when reformulated more precisely. The revised statements were then included in the round 2 (R2) survey. Importantly, all the respondents from the round 1 (R1) survey actively participated in the R2 survey. After the second round of voting, the SC reviewed the panel responses again to assess whether the panelists' opinions were sufficiently convergent. No ethical approval was required for the present study, as it was limited to collecting clinicians' opinions and did not involve patient-specific data.
3. Results
The total of 23 invited panelists completed the eDelphi questionnaire. All panelists expressed their level of agreement within each statement (no missing items were presented). The R1 eDelphi contained 29 out of 36 statements (80.55%) were judged with “Strong agreement”. While, five statements were revised by the SC and included in the R2 eDelphi. After the second round of voting and the Wilcoxon test, the SC agreed that the panelists' opinions were sufficiently convergent. These statements (13.89%) were assessed as “Low level of Agreement” As final report result, two statements (5.56%) received a consensus for a “Disagreement”.
3.1. Category 1: Execution Modalities of Collagen Injections in Musculoskeletal Disorders
Within the topic area of the execution modalities of porcine type I collagen injections in musculoskeletal disorders, the panelists reached a consensus on 7 out 8 statements with a “Strongly Agreement”, while, the statement C1.1 received a “Low level of Agreement” (
Table 2).
STATEMENTS - 1.1 Collagen injections can be combined with therapeutic exercise and/or physiotherapy treatment and/or incorporated into an individual rehabilitation plan. - 1.2 Before infiltration, contraindications to infiltrative therapy should always be ruled out in the patient (e.g., local sepsis, compromised skin, systemic signs of infection). - 1.3 In case of initial benefit followed by recurrence of symptoms, a repeat treatment can be considered after a minimum interval of 3 months. - 1.4 Even in the absence of immediate benefit, provided there are no contraindications or adverse events, three to five injections should be performed to assess the effectiveness of the treatment. - 1.5 Intra-articular collagen injections can be combined with periarticular injections in the same session to enhance their effectiveness. - 1.6 Collagen injections can be used in patients with mild inflammation, provided the disease has been diagnosed and proper systemic therapy is administered. - 1.7 Collagen injections can be combined with a local anesthetic (e.g., lidocaine). - 1.8 Collagen injection can be combined with hyaluronic acid.
A complete consensus was reached (23/23 panelists assigned a score of 4/5 or 5/5 to their agreement level with the statements) on the association of collagen injections with therapeutic exercise and physiotherapy (
Table 2). In addition. the panelists unanimously stressed (100% agreement) the significance of excluding any contraindication to infiltrative therapy such as local sepsis and compromised skin or systemic signs of infection before proceeding with the infiltration procedure (
Table 2).
The Panel also reached a consensus (20 out of 23; 87%) on the appropriateness of repeating porcine type I collagen injections in cases where initial benefits were observed. followed by symptom recurrence. provided that at least 3 months have elapsed since the previous treatment (
Table 2). In case, the immediate benefit was not observed. The 87% (20 out of 23) of the Panelists agreed to perform three to five porcine type I collagen injections to evaluate the effectiveness of the treatment, provided there were no contraindications or adverse events (
Table 2). The possibility of combining intra-articular collagen injections with periarticular injections in the same session received a consensus rate of 83% (19 out of 23) (
Table 2). The use of porcine type I collagen injections in patients with mild inflammation provided that the disease was diagnosed and any systemic therapy was adequately administered achieved (18 out of 23; 78%) (
Table 2). The Panelists had a low level on whether porcine type I collagen injections could be combined with local anesthetic (e.g. lidocaine) (15 out of 23; 65% agreement) (
Table 2). Furthermore a low percentage of agreement (7 out of 23; 30%) was reported for the possibility of combining collagen infiltration and HA (
Table 2).
3.2. Category 2: Collagen Injections in the Treatment of Joint Pathologies with Signs of Active Inflammation at the Joint Level (Intra-Articular. Both in Large and Small Joints)
None of the statements in this area reached a 75% consensus among the panelists (
Table 3).
STATEMENTS - 2.1 Up to two injections per week can be performed in a patient with mild inflammation, considering the absolute contraindications of injection therapies. 2.2 In the case of mild joint inflammation, it is possible to combine intra-articular injections of steroids and collagen in the same session and injection site. .
Only 57% (13 out of 23) of the Panelists agreed that up to two collagen injections per week could be performed in a patient with mild inflammation once absolute contraindications of infiltrative therapies have been ruled out (
Table 3).
Originally, the statement was formulated as: "Up to two injections per week can be performed to achieve the clinical outcome in a patient with signs of inflammation"; it received 30% agreement in the first Delphi round (7 out of 23), The Panelists commented that a corticosteroid infiltration should be performed before initiating collagen injections to reduce inflammation. Some Panelists argued that corticosteroids and collagen injections could be alternated within the same week. Additionally, the Panelists stressed the importance of specifying the type of inflammation. whether it is arthritis. osteoarthrosis. or trauma. The SC reworded the statement: "Up to two injections per week can be performed in a patient with mild inflammation. taking into consideration the absolute contraindications of infiltrative therapies." but as reported above. in the second Delphi round. consensus was still not achieved (57% agreement). The statement regarding the possibility of combining intra-articular injections of steroids and porcine type I collagen in the same session and injection site in cases of mild joint inflammation did not reach agreement (10 out of 23; 43%;
Table 3).
3.3. Category 3: Collagen Injections in the Treatment of Joint Pathologies in the Absence of Signs of Inflammation at the Joint Level (Intra-Articular. Both in Large and Small Joints)
All three statements regarding the treatment of joint pathologies in the absence of signs of inflammation garnered high consensus (
Table 4). The Panel achieved high consensus (22 out of 23; 96%) on including porcine type I collagen injections as a therapeutic option for chondropathy (
Table 4). Additionally, there was high agreement (21 out of 23; 91%) on the possibility of performing porcine type I collagen injections for degenerative joint pathologies and injecting collagen both intra-articularly or extra-articularly (peri-articularly) even simultaneously (
Table 4).
STATEMENTS - 3.1 In the presence of chondropathy, infiltrative therapy with collagen can be a therapeutic option. 3.2 Collagen injections can be performed for degenerative joint pathologies. 3.3 Collagen treatment can be performed intra-articularly and/or peri-articularly, even simultaneously.
3.4. Category 4: Collagen Injections in the Treatment of Inflammatory or Degenerative Pathologies Affecting Various Joint Structures/Soft Tissues (Tendons. Ligaments. Capsules. etc.)
Almost unanimous agreement was reached (22 out of 23; 96%) on the appropriateness of peritendinous porcine type I collagen injections for treating degenerative tendinopathies (
Table 5).
STATEMENTS - 4.1 In degenerative tendinopathies, collagen can be used peri-tendinous. 4.2 injection therapy with collagen is a therapeutic option for ligamentous injuries. 4.3 Collagen injections are a therapeutic option in the treatment of myofascial syndrome. 4.4 Collagen injections are a therapeutic option in the treatment of myofascial trigger points. 4.4 Collagen injections are a therapeutic option in the treatment of myofascial trigger points. 4.5 In degenerative tendinopathies, collagen can be used intra-tendinous. 4.6 In the case of extra-articular injections, infiltrative therapy with collagen can safely continue for up to 10 weeks. 4.7 In the case of acute soft tissue (extra-articular) involvement, up to two injections per week can be performed.
Seventy-eight percent of the Panelists (18 out of 23) considered infiltrative therapy with collagen as a therapeutic option for ligamentous injuries (
Table 5). However, the use of collagen injections for the treatment of myofascial syndrome and trigger points did not reach the consensus threshold. although the percentages of agreement were relevant (74% [17 out of 23] and 70% [16 out of 23]. respectively) (
Table 5). Originally, myofascial syndrome and trigger points were included in a single statement. but this did not reach consensus in the first Delphi round (17 out of 23; 74%). The Panelists expressed that they would have rated the use of porcine type I collagen injections for myofascial syndrome and trigger points differently. As a result. the SC decided to split the statement into two separate statements. one for each condition. However. even in the second round. these statements did not reach the 75% agreement threshold (74% agreement for myofascial syndrome and 70% for trigger points-related statements. respectively). The Panelists did not reach a consensus on the use of collagen intra-tendinous in the case of degenerative tendinopathies (15 out of 23; 65%) (
Table 5). In relation to extra-articular injections, there was no consensus on the possibility of safely continuing porcine type I collagen injection therapy for up to 10 weeks (13 out of 23; 57%;
Table 5). Similarly, the possibility of performing up to two injections per week in the case of acute soft tissue involvement (12 out of 23; 52% agreement) did not reach consensus (
Table 5).
3.5. Category 5: Efficacy and Safety of Intra-Articular Collagen Injections
The Panelists unanimously agreed on the importance of evaluating the effectiveness of porcine type I collagen injection therapy by monitoring pain function and independence in performing activities of daily living (
Table 6).
STATEMENTS - 5.1 The efficacy of collagen injection therapy should be evaluated by monitoring pain and/or functionality and/or independence in performing activities of daily living. 5.2 Collagen injections can also be performed in patients with multiple pathologies, considering the common contraindications to infiltrative procedures. 5.3 Injection therapy with porcine collagen can be performed following the application of physical therapies, even at the same injection site.
They also unanimously agreed on the possibility of performing porcine type I collagen injections in patients with multiple pathologies while considering the before-mentioned common contraindications to infiltrative procedures (
Table 6).
Furthermore. 83% of the Panelists (19 out of 23) agreed on the possibility of performing porcine type I collagen injection therapy following the application of physical therapies. even at the same injection site (
Table 6).
3.6. Category 6: Efficacy and Safety of Collagen Injections Performed Via Extra-Articular Routes (Periarticular. Peri-Tendinous. etc.)
Only one statement within this category reached consensus, which was the possibility of combining porcine type I collagen injections with needling procedures (19 out of 23; 83%;
Table 7).
STATEMENTS - 6.1 Collagen infiltration can be combined with needling procedures. 6.2 Collagen injections can promote tendon regeneration. 6.3 Collagen injections are indicated in the multimodal treatment of patients with musculoskeletal pain and resulting functional limitations (such as non-specific cervicalgia and non-specific lumbalgia). 6.4 Collagen injections are indicated for pain treatment, even in the absence of tissue abnormalities detected in imaging studies (e.g., X-ray, ultrasound). 6.5 Col l agen inj e c t ions c an promot e subcutaneous separation. 6.6 Within an individual rehabilitation plan, collagen injections are a viable therapeutic option for the treatment of muscle spasms or contractions. 6.7 Collagen injections are also indicated in patients who are currently asymptomatic but show signs of tissue damage in imaging studies (e.g., X-ray, ultrasound).
The Panel disagreed with the affirmation that collagen injections can promote tendon regeneration (15 out of 23; 65%;
Table 7). Only 65% of the Panelists (15 out of 23) believed that collagen injections are indicated in the multimodal treatment of patients with musculoskeletal pain and resulting functional limitations (such as non-specific cervicalgia and non-specific low back pain) (
Table 7). The statement was revised after the first Delphi round: the original statement was "collagen injections are recommended in cases where the patient complains of musculoskeletal pain and reduced mobility (e.g.. in the cervical spine)" and received an agreement rate of 52% (12 out of 23). The statement was reworded by the SC as follows: "Collagen injections are indicated in the multimodal treatment of patients with musculoskeletal pain and resulting functional limitations (such as non-specific cervicalgia and non-specific low back pain)." but it still did not reach the threshold for agreement in the second round. The statement regarding the use of collagen injections for pain treatment even in the absence of tissue abnormalities detected in imaging studies (e.g.. X-ray. ultrasound). did not reach an agreement (13 out of 23; 57%) (
Table 7). Similarly, the use of collagen injections in asymptomatic patients who show signs of tissue damage in imaging studies did not reach consensus (8 out of 23; 35%;
Table 7). Only 52% of the Panelists (12 out of 23) agreed that collagen injections can promote subcutaneous tissue plane separation (
Table 7). The original statement. "Collagen injections are recommended in cases where the patient presents muscle spasms or contractures (e.g.. in the cervical or lumbar spine)." reached 43% agreement (10 out of 23). with one Panelist commenting that there are likely no side effects but expressing skepticism about their beneficial effects. The SC revised the statement to "Within an individual rehabilitation plan. collagen injections are a viable therapeutic option for the treatment of muscle spasms or contractions." However. the percentage of agreement achieved upon revoting during the second Delphi round was only 48% (11 out of 23).
4. Discussion
This Delphi study aimed to gather expert opinions about administering porcine type I collagen injections in musculoskeletal pain. The study was structured around seven distinct categories that spanned a broad spectrum of considerations related to porcine collagen injections. Within these categories. 37 statements were crafted. each intended to explore specific facets of the procedure. These statements addressed crucial aspects such as execution modalities. safety. efficacy. and the potential benefits of administering ultrasound-guided collagen injections. Upon completing the Delphi process. the expert Panel reached a consensus on 22 of the 37 statements. The following sections delve into this Delphi survey's key findings and implications. These results contribute significantly to understanding the current use of porcine type I collagen injections in clinical practice in musculoskeletal pain management. In addition they also provide a foundation for further research.
Category 1: Execution Modalities of Collagen Injections in Musculoskeletal Disorders
The experts unanimously believed collagen injections could be fruitfully combined with therapeutic exercise or physiotherapy and integrated into an individual rehabilitation plan. In this respect. their experience confirms the literature data showing some beneficial effects of the combination of rehabilitative treatment and collagen injections in treating lumbar pain [
33]. Supporting the advantages of combining injection therapy and physiotherapy. the combination of HA injections and physiotherapy proved effective in rehabilitating patients with moderate knee osteoarthritis [
34].
The Panelists unanimously agreed that absolute contraindications to collagen injections are generally consistent with those for any therapeutic injection procedure. including drug allergies and infections [
35].
In total. 35% of the experts disagreed with combining collagen and local anesthetic injections. and the related statement did not reach a consensus. Specific concerns were raised regarding mixing collagen and anesthetic in the same syringe. Despite the limited evidence available in the literature supporting the use of collagen and lidocaine. some members of the SC pointed out that lidocaine could be beneficial in addressing pain or alleviating discomfort resulting from the distension of a particularly constricted target space after collagen injection. Additionally, they proposed that collagen could be a valuable adjunctive therapeutic option in clinical settings where lidocaine injections are already employed such as in treating trigger points [
5]. Furthermore, it should be noted that combined injections of porcine type I collagen and lidocaine have previously been administered in a case series of patients with Morton's neuroma. with no reported side effects [
24]. Combining collagen injections with HA injections drew disagreement among 70% of the Panelists. Some Panelists felt that specific clinical experience was needed or stressed the lack of relevant literature demonstrating the efficacy of this combination. Others emphasized the importance of avoiding both infiltrations in the same session. using the same syringe. or of the same anatomical district. Some early clinical findings suggest potential benefits from combining collagen and HA injections: in in vivo studies. HA injections have shown the ability to slow osteoarthritis progression [
36] by reducing glycosaminoglycan release and pro-inflammatory molecules. such as MMP-13. MMP-3 and IL-1β [
37,
38]. Porcine type I collagen increases the expression of tissue inhibitors of metalloproteinase-1 and leads to an augmentation in collagen type I secretion in cultured human tenocytes [
9].This effect is likely favored by the inhibition of collagen degradation by MMP-1 [
9]. A non-inferiority prospective randomized controlled trial by Martin et al. observed benefits in patients treated with intra-articular injections of porcine type I collagen or sodium hyaluronate [
4]. Collagen injected peri-articularly may stabilize the extracellular matrix. as suggested by Randelli et al. al. [
9,
39]As early as a decade ago. Matsiko and colleagues [
40]demonstrated that incorporating HA into collagen scaffolds stimulated the migration and chondrogenesis of mesenchymal stem cells. Similar results have been obtained recently by Muran et al. [
41]. The potential synergistic effect of HA and collagen injections makes this a promising clinical avenue for future studies.
Category 2: Collagen Injections in the Treatment of Joint Pathologies with Signs of Active Inflammation at the Joint Level (Intra-Articular. Both in Large and Small Joints)
Forty-three percent of the experts disagreed with the possibility of performing up to two injections per week. In particular, they expressed concerns about the risk of infection. In this line of reasoning. the need to rule out infection before injection was stressed. and Panelists showed skepticism towards infiltrative therapy when signs of inflammation were present. Similarly. regarding the frequency of collagen injections. a recent review indicates that most studies involving intra-articular collagen injections for knee osteoarthritis administered the injections once a week or less frequently [
42]. This frequency may be due to the satisfactory results obtained with this regimen. but evidence of the efficacy and safety of increasing the frequency of injections is lacking. In total. 57% of the experts disagreed with combining intra-articular injections of steroids and collagen in the same session and injection site for mild joint inflammation. There are no reports that demonstrate the usefulness and the safety of this approach.
Corticosteroid injections are commonly employed to treat joint pathologies characterized by synovial inflammation. such as osteoarthritis [
30]. However. the effects of corticosteroids are short-lived. making them more suitable for short-term pain relief [
30]. On the other hand. type I collagen injections seem to have a more lasting effect on osteoarthritis than corticosteroids [
43].
Category 3: Collagen Injections in the Treatment of Joint Pathologies in the Absence of Signs of Inflammation at the Joint Level (Intra-Articular. Both in Large and Small Joints)
The Panel showed a firm consensus (96%) on including porcine type I collagen injections as a therapeutic option for chondropathy. It is noteworthy here that intra-articular collagen injections have the potential to stimulate the production of hyaline cartilage by chondrocytes thus countering cartilage erosion commonly observed in joint pathologies. including osteoarthritis [
43]. The experts reached a high consensus (91%) on the possibility of performing porcine collagen injections in the presence of degenerative joint pathologies. aligning with existing literature suggesting collagen injections' positive effects in addressing osteoarthritis symptoms and progression [
3,
4,
43,
23,
44]. Collagen injections showed efficacy in treating musculoskeletal disorders whether administered in the peri-articular [
26]or intra-articular space [
23,
44]. The Panelists' responses showed that both these routes are used in clinical practice. In conclusion. it is important to emphasize the high degree of consensus among the panelists (91%) regarding the simultaneous use of intra-articular and periarticular collagen injections.
Category 4: Collagen Injections in the Treatment of Inflammatory or Degenerative Pathologies Affecting Various Joint Structures/Soft Tissues (Tendons. Ligaments. Capsules. etc.)
The possibility of using porcine collagen injections as therapeutic options for treating myofascial syndrome and myofascial trigger points did not achieve consensus (74% and 70%. respectively). although it approached the threshold of 75% agreement. The pain associated with myofascial syndrome primarily arises from trigger points. which are small nodules. bumps. or knots within the muscle that cause pain when compressed [
5]. In a study by Nitecka-Buchta et al. [
5]. the effectiveness of two intramuscular injections. 1 week apart. of porcine type 1 collagen or lidocaine into the trigger points of the masseter muscle in patients with myofascial pain was compared. The study found that collagen injections were more effective than lidocaine in reducing masseter muscle activity and alleviating pain [
5]. However, there is controversy about the relative contribution of collagen itself and dry needling in injection therapy for trigger points. Dry needling of myofascial trigger points in the masseter muscle has been reported to provide immediate improvement in pain and jaw function. with effects lasting for at least 1 week [
45]. Other studies also support the efficacy of dry needling for treating musculoskeletal pain [
46]. Nonetheless, it is believed that while needling per se may contribute to the pain-relieving effect of collagen injections. the long-term benefits. possibly due to its regenerative potential. come primarily from collagen's biological properties. In total. 35% of the experts disagreed with using intra-tendinous porcine collagen injections in degenerative tendinopathies. One Panelist suggested that physical therapies. such as ultrasound. magnetic fields. laser or electric stimulation. should be associated with intra-tendinous collagen infiltration for a beneficial response. The efficacy of collagen injections in the treatment of tendinopathies has only recently been investigated. with studies providing valuable insights [
28,
17,
47,
48]. Kim et al. [
28] conducted the first randomized clinical trial in 2020. They found that a single ultrasound-guided intratendinous atelocollagen injection led to a decrease in tear size and functional improvement in about one out of three patients with partial-thickness rotator cuff tears [
28]. A case series reported that intratendinous porcine type I collagen injections improved function and decreased pain in chronic supraspinatus tendinopathies [
16].
Category 5: Efficacy and Safety of Intra-Articular Collagen Injections
Eighty-three percent of the Panelists agreed with the possibility of performing porcine collagen injection therapy after applying physical therapies. even at the same injection site. Evidence indicates that physical therapies. such as extracorporeal shockwave therapy (ESWT). low-level laser therapy (LLLT). and ultrasound. can enhance collagen synthesis and tendon repair after injury [
49,
50]. Similarly. LLLT. ultrasound. and the combined LLLT and ultrasound therapy have increased type I collagen synthesis in animal tendons following injury [
51]. On this basis, it can be hypothesized that these physical modalities may work as adjuvant therapies when performing collagen injections.
Limitations of the Study
This study has some limitations. To begin, despite the fact that the physicians recruited here can be considered authorities in the field of musculoskeletal pain medicine (in this regard. they are all either specialists in Physical and Rehabilitation Medicine or Rheumatologists) their precise level of expertise in collagen injection has not been quantified. For example we did not collect data on the average number of injections performed by the Panelists. Second. our research was carried out exclusively within the framework of a national (Italian) scenario. Given that physicians from different countries might provide different responses caution seems appropriate in generalizing our findings to the global population of physicians dealing with musculoskeletal pain.
5. Conclusions
This Delphi study aimed to gather consensus on the modalities of execution of porcine type I collagen injections for different anatomical districts. the frequency of injections. the possibility of combining collagen and other drugs in the same session. and several other aspects concerning collagen injections for the treatment of musculoskeletal disorders for which clear indications in the literature are lacking. We retain that this work provides insights into the current practice of porcine type I collagen injection procedures and represents an initial step toward establishing an informed clinical practice.
However, this process highlighted that collagen injection modalities are still surrounded by many uncertainties. especially in specific contexts such as the possibility of combining collagen and anesthetics. HA or steroids. the management of infiltrative therapy in the presence of inflammation. and the optimal frequency and number of collagen injections needed to achieve a therapeutic effect in different anatomical districts and conditions. In addition, there was a lack of agreement regarding the administration of collagen injections for the treatment of myofascial syndrome. trigger points. and tendinopathy. Hence, our research effectively underscores the necessity for further investigation into the clinical effectiveness of porcine type I collagen injections in alleviating pain and enhancing function in musculoskeletal disorders. Awaiting the results of clinical efficacy trials, the current study may provide clinicians involved in musculoskeletal pain management with useful albeit provisional guidance.
Author Contributions
All Authors contributed to the definition and contextualization of statements. and critically edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
An unconditioned grant from Guna supported this project.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
Editorial assistance was provided by Valeria Benedusi. PhD. Francesca Cappellini. PhD. Valentina Attanasio. and Aashni Shah (Polistudium Srl. Milan. Italy). Guna supported this assistance.
Conflicts of Interest
ODL. AB. CC. GLM. LB. and ARM declared no potential conflicts of interest with respect to the research. authorship. and/or publication of this article. FGi received fees for teaching, research, and editorial activities from Imagine Srl, GlaxoSmithKline Consumer Healthcare Srl. EdiErmes Srl. Springer Healthcare Italia Srl. Edra Sp,. Dynamicom Education Srl. Medik Srl, Polistudium Srl, Guna SpA. Keep International Srl, FGe received fee for teaching and editorial activities by MaCro Lifescience Srl. Angelini Pharma SpA. Abiogen Pharma, Imagine Srl. GlaxoSmithKline Consumer Healthcare Srl, Unipersonale, Saluber MD Srl, Doceo Srl, Gruppo Humantech, EdiErmes Srl, Summeet Srl, Asti Incentives & Congressi srl. Pharmanutra. Scuola Veneta di Medicina Generale. Maize Srl (H-Farm Innovation), Springer Healthcare Italia Srl, IBSA, Edra SpA, Affidabile Formazione per la Sanità, Carocci Editore SpA. Master Srl. Dynamicom Education Srl. Medik Srl. Giana Erminio ASD. Italfarmaco. Podartis Srl, I-Tech Medical Division, Guna, Polistudium Srl, DueCi Events & Communication, Laborest, E.C.M. 2 S.r.l., HINOVIA, Kyowa Kirin Srl, Clinical research fee by Società Aziende Chimiche Riunite Angelini Francesco A.C.R.A.F, SpA and AC received a consultancy fee from Guna.
The funder had no role in the design of the study regarding
collection, analyses and interpretation of data; writing of the manuscript and in the decision to publish the results.
Appendix A
Delphi Panel of Italian Experts and Their Region of Work
Aluena Battaglioli (Toscana); Federica Bertolucci (Toscana); Lucia Calbucci (Emilia-Romagna); Viviana Colantonio (Lombardia); Bruno Corrado (Campania); Alessandro De Sire (Calabria); Paolo di Benedetto (Friuli-Venezia Giulia); Giuseppe Falcone (Toscana); Giacomo Farì (Puglia); Paola Galligioni (Veneto); Nicole Lonoce (Toscana); Giorgio Mariani (Emilia-Romagna); Edoardo Milano (Piemonte); Annalisa Orioli (Toscana); Benedetta Panni (Lombardia); Teresa Paolucci (Abruzzo); Vincenzo Ricci (Lombardia); Paola Rodriguez (Toscana); Maria Teresa Pereira Ruiz (Liguria); Federico Salvò (Lombardia); Lucrezia Tognolo (Veneto); Francesca Uboldi (Lombardia); Nikoleta Vaso (Lombardia); Alen Zabotti (Friuli-Venezia Giulia).
References
- Cieza, A.; Causey, K.; Kamenov, K.; Hanson, S.W.; Chatterji, S.; Vos, T. Global Estimates of the Need for Rehabilitation Based on the Global Burden of Disease Study 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. The Lancet 2020, 396, 2006–2017. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.-T.; Chiang, C.-F.; Wu, C.-H.; Huang, Y.-T.; Tu, Y.-K.; Wang, T.-G. Comparative Effectiveness of Injection Therapies in Rotator Cuff Tendinopathy: A Systematic Review, Pairwise and Network Meta-Analysis of Randomized Controlled Trials. Arch. Phys. Med. Rehabil. 2019, 100, 336–349.e15. [Google Scholar] [CrossRef]
- Furuzawa-Carballeda, J.; Lima, G.; Llorente, L.; Nuñez-Álvarez, C.; Ruiz-Ordaz, B.H.; Echevarría-Zuno, S.; Hernández-Cuevas, V. Polymerized-Type I Collagen Downregulates Inflammation and Improves Clinical Outcomes in Patients with Symptomatic Knee Osteoarthritis Following Arthroscopic Lavage: A Randomized, Double-Blind, and Placebo-Controlled Clinical Trial. Sci. World J. 2012, 2012, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Martin Martin, L.S.; Massafra, U.; Bizzi, E.; Migliore, A. A Double Blind Randomized Active-Controlled Clinical Trial on the Intra-Articular Use of Md-Knee versus Sodium Hyaluronate in Patients with Knee Osteoarthritis (“Joint”). BMC Musculoskelet. Disord. 2016, 17, 94. [Google Scholar] [CrossRef]
- Nitecka-Buchta, A.; Walczynska-Dragon, K.; Batko-Kapustecka, J.; Wieckiewicz, M. Comparison between Collagen and Lidocaine Intramuscular Injections in Terms of Their Efficiency in Decreasing Myofascial Pain within Masseter Muscles: A Randomized, Single-Blind Controlled Trial. Pain Res. Manag. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Ricard-Blum, S. The Collagen Family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978–a004978. [Google Scholar] [CrossRef] [PubMed]
- Grounds, M.D. Complexity of Extracellular Matrix and Skeletal Muscle Regeneration. In Skeletal Muscle Repair and Regeneration; Advances in Muscle Research; Springer Netherlands: Dordrecht, 2008; Vol. 3, pp. 269–302; ISBN 978-1-4020-6767-9. [Google Scholar]
- Myllyharju, J. Collagens, Modifying Enzymes and Their Mutations in Humans, Flies and Worms. Trends Genet. 2004, 20, 33–43. [Google Scholar] [CrossRef]
- Randelli, F.; Menon, A.; Giai Via, A.; Mazzoleni, M.; Sciancalepore, F.; Brioschi, M.; Gagliano, N. Effect of a Collagen-Based Compound on Morpho-Functional Properties of Cultured Human Tenocytes. Cells 2018, 7, 246. [Google Scholar] [CrossRef]
- Randelli, F.; Fioruzzi, A.; Mazzoleni, M.G.; Radaelli, A.; Rahali, L.; Verga, L.; Menon, A. Efficacy of Ultrasound-Guided Injections of Type I Collagen-Based Medical Device for Greater Trochanteric Pain Syndrome: A Pilot Study. Life 2025, 15, 366. [Google Scholar] [CrossRef]
- Koszela, K.; Woldańska-Okońska, M.; Słupiński, M.; Gasik, R. The Role of Injection Collagen Therapy in Greater Trochanter Pain Syndrome. A New Therapeutic Approach? Rheumatology 2025. [Google Scholar] [CrossRef]
- Tarantino, D.; Mottola, R.; Palermi, S.; Sirico, F.; Corrado, B.; Gnasso, R. Intra-Articular Collagen Injections for Osteoarthritis: A Narrative Review. Int. J. Environ. Res. Public. Health 2023, 20, 4390. [Google Scholar] [CrossRef] [PubMed]
- Borja-Flores, A.; Macías-Hernández, S.I.; Hernández-Molina, G.; Perez-Ortiz, A.; Reyes-Martínez, E.; Belzazar-Castillo De La Torre, J.; Ávila-Jiménez, L.; Vázquez-Bello, M.C.; León-Mazón, M.A.; Furuzawa-Carballeda, J.; et al. Long-Term Effectiveness of Polymerized-Type I Collagen Intra-Articular Injections in Patients with Symptomatic Knee Osteoarthritis: Clinical and Radiographic Evaluation in a Cohort Study. Adv. Orthop. 2020, 2020, 1–9. [Google Scholar] [CrossRef]
- Lee, H.S.; Oh, K.J.; Moon, Y.W.; In, Y.; Lee, H.J.; Kwon, S.Y. Intra-Articular Injection of Type I Atelocollagen to Alleviate Knee Pain: A Double-Blind, Randomized Controlled Trial. CARTILAGE 2021, 13, 342S–350S. [Google Scholar] [CrossRef]
- Aicale, R.; Savarese, E.; Mottola, R.; Corrado, B.; Sirico, F.; Pellegrino, R.; Donati, D.; Tedeschi, R.; Ruosi, L.; Tarantino, D. Collagen Injections for Rotator Cuff Diseases: A Systematic Review. Clin. Pract. 2025, 15, 28. [Google Scholar] [CrossRef]
- Corrado, B.; Bonini, I.; Chirico, V.A.; Filippini, E.; Liguori, L.; Magliulo, G.; Mazzuoccolo, G.; Rosano, N.; Gisonni, P. Ultrasound-Guided Collagen Injections in the Treatment of Supraspinatus Tendinopathy: A Case Series Pilot Study. J. Biol. Regul. Homeost. Agents 2020, 34, 33–39, ADVANCES IN MUSCULOSKELETAL DISEASES AND INFECTIONS-SOTIMI 2019. [Google Scholar]
- Corrado, B.; Bonini, I.; Alessio Chirico, V.; Rosano, N.; Gisonni, P. Use of Injectable Collagen in Partial-Thickness Tears of the Supraspinatus Tendon: A Case Report. Oxf. Med. Case Rep. 2020, 2020, omaa103. [Google Scholar] [CrossRef]
- Aicale, R.; Savarese, E.; Mottola, R.; Corrado, B.; Sirico, F.; Pellegrino, R.; Donati, D.; Tedeschi, R.; Ruosi, L.; Tarantino, D. Collagen Injections for Rotator Cuff Diseases: A Systematic Review. Clin. Pract. 2025, 15, 28. [Google Scholar] [CrossRef] [PubMed]
- Silvipriya, K.; Kumar, K.; Bhat, A.; Kumar, B.; John, A.; Lakshmanan, P. Collagen: Animal Sources and Biomedical Application. J. Appl. Pharm. Sci. 2015, 123–127. [Google Scholar] [CrossRef]
- Ellingsworth, L.R.; DeLustro, F.; Brennan, J.E.; Sawamura, S.; McPherson, J. The Human Immune Response to Reconstituted Bovine Collagen. J. Immunol. 1986, 136, 877–882. [Google Scholar] [CrossRef]
- Brandão, R.A.C.S.; Costa, B.S.; Dellaretti, M.A.; De Carvalho, G.T.C.; Faria, M.P.; De Sousa, A.A. Efficacy and Safety of a Porcine Collagen Sponge for Cranial Neurosurgery: A Prospective Case-Control Study. World Neurosurg. 2013, 79, 544–550. [Google Scholar] [CrossRef]
- Corrado, B.; Mazzuoccolo, G.; Liguori, L.; Chirico, V.A.; Costanzo, M.; Bonini, I.; Bove, G.; Curci, L. Treatment of Lateral Epicondylitis with Collagen Injections: A Pilot Study. Muscle Ligaments Tendons J. 2019, 09, 584. [Google Scholar] [CrossRef]
- De Luca, P.; Colombini, A.; Carimati, G.; Beggio, M.; De Girolamo, L.; Volpi, P. Intra-Articular Injection of Hydrolyzed Collagen to Treat Symptoms of Knee Osteoarthritis. A Functional In Vitro Investigation and a Pilot Retrospective Clinical Study. J. Clin. Med. 2019, 8, 975. [Google Scholar] [CrossRef]
- Giarda, F.; Agostini, A.; Colonna, S.; Sciumè, L.; Meroni, A.; Beretta, G.; Dalla Costa, D. Infiltrative Type I Collagen in the Treatment of Morton’s Neuroma: A Mini-Series. J. Clin. Med. 2023, 12, 4640. [Google Scholar] [CrossRef]
- Fischer, A.A. Local Injections in Pain Management. Phys. Med. Rehabil. Clin. N. Am. 1995, 6, 851–870. [Google Scholar] [CrossRef]
- Buda, M.; Dlimi, S.; Parisi, M.; Benoni, A.; Bisinella, G.; Di Fabio, S. Subacromial Injection of Hydrolyzed Collagen in the Symptomatic Treatment of Rotator Cuff Tendinopathy: An Observational Multicentric Prospective Study on 71 Patients. JSES Int. 2023, 7, 799–804. [Google Scholar] [CrossRef]
- Damjanov, N.; Micu, M.C. Ultrasound Guided Injection with Collagen-Based Medical Device: Real-Life Evaluation of Efficacy and Safety in Hip Osteoarthritis. Med. Ultrason. 2024, 1, 26. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Kim, D.-J.; Lee, H.-J.; Kim, B.-K.; Kim, Y.-S. Atelocollagen Injection Improves Tendon Integrity in Partial-Thickness Rotator Cuff Tears: A Prospective Comparative Study. Orthop. J. Sports Med. 2020, 8, 2325967120904012. [Google Scholar] [CrossRef]
- De Pascalis, M.; Mulas, S.; Sgarbi, L. Combined Oxygen–Ozone and Porcine Injectable Collagen Therapies Boosting Efficacy in Low Back Pain and Disability. Diagnostics 2024, 14, 2411. [Google Scholar] [CrossRef] [PubMed]
- Ayhan, E. Intraarticular Injections (Corticosteroid, Hyaluronic Acid, Platelet Rich Plasma) for the Knee Osteoarthritis. World J. Orthop. 2014, 5, 351. [Google Scholar] [CrossRef]
- Bernetti, A.; Mangone, M.; Villani, C.; Alviti, F.; Valeo, M.; Grassi, M.C.; Migliore, A.; Viora, U.; Adriani, E.; Quirino, N.; et al. Appropriateness of Clinical Criteria for the Use of SYmptomatic Slow-Acting Drug for OsteoArthritis (SYSADOA). A Delphi Method Consensus Initiative among Experts in Italy. Eur. J. Phys. Rehabil. Med. 2019, 55. [Google Scholar] [CrossRef]
- Eysenbach, G. Improving the Quality of Web Surveys: The Checklist for Reporting Results of Internet E-Surveys (CHERRIES). J. Med. Internet Res. 2004, 6, e34. [Google Scholar] [CrossRef]
- Milano E COLLAGEN MEDICAL DEVICE LUMBAR NEL TRATTAMENTO COMBINATO DEL DOLORE DA INSTABILITÀ DEL RACHIDE LOMBARE 2019.
- Onu, I.; Matei, D.; Sardaru, D.-P.; Cascaval, D.; Onu, A.; Gherghel, R.; Serban, I.L.; Mocanu, G.D.; Iordan, D.A.; Murariu, G.; et al. Rehabilitation of Patients with Moderate Knee Osteoarthritis Using Hyaluronic Acid Viscosupplementation and Physiotherapy. Appl. Sci. 2022, 12, 3165. [Google Scholar] [CrossRef]
- Stephens, M.B.; Beutler, A.I.; O’Connor, F.G. Musculoskeletal Injections: A Review of the Evidence. Am. Fam. Physician 2008, 78, 971–976. [Google Scholar] [PubMed]
- Amiel, D.; Toyoguchi, T.; Kobayashi, K.; Bowden, K.; Amiel, M.E.; Healey, R.M. Long-Term Effect of Sodium Hyaluronate (Hyalgan®) on Osteoarthritis Progression in a Rabbit Model. Osteoarthritis Cartilage 2003, 11, 636–643. [Google Scholar] [CrossRef]
- Takahashi, K.; Goomer, R.S.; Harwood, F.; Kubo, T.; Hirasawa, Y.; Amiel, D. The Effects of Hyaluronan on Matrix Metalloproteinase-3 (MMP-3), Interleukin-1β(IL-1β), and Tissue Inhibitor of Metalloproteinase-1 (TIMP-1) Gene Expression during the Development of Osteoarthritis. Osteoarthritis Cartilage 1999, 7, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, N.; Takahashi, K.A.; Arai, Y.; Sakao, K.; Mazda, O.; Kishida, T.; Honjo, K.; Tanaka, S.; Kubo, T. Intra-articular Injection of Hyaluronan Restores the Aberrant Expression of Matrix Metalloproteinase-13 in Osteoarthritic Subchondral Bone. J. Orthop. Res. 2011, 29, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Randelli, F.; Sartori, P.; Carlomagno, C.; Bedoni, M.; Menon, A.; Vezzoli, E.; Sommariva, M.; Gagliano, N. The Collagen-Based Medical Device MD-Tissue Acts as a Mechanical Scaffold Influencing Morpho-Functional Properties of Cultured Human Tenocytes. Cells 2020, 9, 2641. [Google Scholar] [CrossRef] [PubMed]
- Matsiko, A.; Levingstone, T.J.; O’Brien, F.J.; Gleeson, J.P. Addition of Hyaluronic Acid Improves Cellular Infiltration and Promotes Early-Stage Chondrogenesis in a Collagen-Based Scaffold for Cartilage Tissue Engineering. J. Mech. Behav. Biomed. Mater. 2012, 11, 41–52. [Google Scholar] [CrossRef]
- Muran, A.C.; Schaffler, B.C.; Wong, A.; Neufeld, E.; Swami, P.; Pianka, M.; Grande, D. Effect of Increasing Hyaluronic Acid Content in Collagen Scaffolds on the Maintenance of Chondrogenic Phenotype in Chondrocytes and Mesenchymal Stem Cells. J. Cartil. Jt. Preserv. 2023, 3, 100099. [Google Scholar] [CrossRef]
- Tarantino, D.; Mottola, R.; Palermi, S.; Sirico, F.; Corrado, B.; Gnasso, R. Intra-Articular Collagen Injections for Osteoarthritis: A Narrative Review. Int. J. Environ. Res. Public. Health 2023, 20, 4390. [Google Scholar] [CrossRef]
- V. Reshkova, R. Rashkov, R. Nestorova EFFICACY AND SAFETY EVALUATION OF GUNA COLLAGEN MDs INJECTIONS IN KNEE OSTEOARTHRITIS − A CASE SERIES OF 30 PATIENTS. Physiol. Regul. Med. 2017.
- Volpi, P.; Zini, R.; Erschbaumer, F.; Beggio, M.; Busilacchi, A.; Carimati, G. Effectiveness of a Novel Hydrolyzed Collagen Formulation in Treating Patients with Symptomatic Knee Osteoarthritis: A Multicentric Retrospective Clinical Study. Int. Orthop. 2021, 45, 375–380. [Google Scholar] [CrossRef]
- Blasco-Bonora, P.M.; Martín-Pintado-Zugasti, A. Effects of Myofascial Trigger Point Dry Needling in Patients with Sleep Bruxism and Temporomandibular Disorders: A Prospective Case Series. Acupunct. Med. 2017, 35, 69–74. [Google Scholar] [CrossRef]
- Kalichman, L.; Vulfsons, S. Dry Needling in the Management of Musculoskeletal Pain. J. Am. Board Fam. Med. 2010, 23, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Choi, Y.S.; You, M.-W.; Kim, J.S.; Young, K.W. Sonoelastography in the Evaluation of Plantar Fasciitis Treatment: 3-Month Follow-Up After Collagen Injection. Ultrasound Q. 2016, 32, 327–332. [Google Scholar] [CrossRef]
- Rodina Nestorova, Rasho Rashkov, Tzvetanka Petranova CLINICAL AND SONOGRAPHIC ASSESSMENT OF THE EFFECTIVENESS OF GUNA COLLAGEN MDs INJECTIONS IN PATIENTS WITH PARTIAL THICKNESS TEAR OF THE ROTATOR CUFF. YSIOLOGICAL Regul. Med. 2017.
- Vetrano, M.; d’Alessandro, F.; Torrisi, M.R.; Ferretti, A.; Vulpiani, M.C.; Visco, V. Extracorporeal Shock Wave Therapy Promotes Cell Proliferation and Collagen Synthesis of Primary Cultured Human Tenocytes. Knee Surg. Sports Traumatol. Arthrosc. 2011, 19, 2159–2168. [Google Scholar] [CrossRef]
- Bosch, G.; De Mos, M.; Van Binsbergen, R.; Van Schie, H.T.M.; Van De Lest, C.H.A.; Van Weeren, P.R. The Effect of Focused Extracorporeal Shock Wave Therapy on Collagen Matrix and Gene Expression in Normal Tendons and Ligaments. Equine Vet. J. 2009, 41, 335–341. [Google Scholar] [CrossRef]
- Wood, V.T.; Pinfildi, C.E.; Neves, M.A.I.; Parizoto, N.A.; Hochman, B.; Ferreira, L.M. Collagen Changes and Realignment Induced by Low-level Laser Therapy and Low-intensity Ultrasound in the Calcaneal Tendon. Lasers Surg. Med. 2010, 42, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Pirri, C.; Fede, C.; Petrelli, L.; De Rose, E.; Biz, C.; Guidolin, D.; De Caro, R.; Stecco, C. Immediate Effects of Extracorporeal Shock Wave Therapy in Fascial Fibroblasts: An In Vitro Study. Biomedicines 2022, 10, 1732. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, F.; Akkus, O.; King, M.W. A Collagen/PLA Hybrid Scaffold Supports Tendon-derived Cell Growth for Tendon Repair and Regeneration. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 2624–2635. [Google Scholar] [CrossRef]
- Shiroud Heidari, B.; Ruan, R.; Vahabli, E.; Chen, P.; De-Juan-Pardo, E.M.; Zheng, M.; Doyle, B. Natural, Synthetic and Commercially-Available Biopolymers Used to Regenerate Tendons and Ligaments. Bioact. Mater. 2023, 19, 179–197. [Google Scholar] [CrossRef] [PubMed]
- Joines, M.; Motamedi, K.; Seeger, L.; DiFiori, J. Musculoskeletal Interventional Ultrasound. Semin. Musculoskelet. Radiol. 2007, 11, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Saha, P.; Smith, M.; Hasan, K. Accuracy of Intraarticular Injections: Blind vs. Image Guided Techniques—A Review of Literature. J. Funct. Morphol. Kinesiol. 2023, 8, 93. [Google Scholar] [CrossRef] [PubMed]
- Bardowski, E.A.; Byrd, J.W.T. Piriformis Injection: An Ultrasound-Guided Technique. Arthrosc. Tech. 2019, 8, e1457–e1461. [Google Scholar] [CrossRef]
- Bernetti, A.; Agostini, F.; Alviti, F.; Giordan, N.; Martella, F.; Santilli, V.; Paoloni, M.; Mangone, M. New Viscoelastic Hydrogel Hymovis MO.RE. Single Intra-Articular Injection for the Treatment of Knee Osteoarthritis in Sportsmen: Safety and Efficacy Study Results. Front. Pharmacol. 2021, 12, 673988. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).