1. Introduction
Severe burn injuries are often associated with complex long-term sequelae, making inpatient rehabilitation essential for optimizing recovery [
1]. The goals of burn rehabilitation are multifaceted ranging from the restoration of physical function (improving mobility, self-care independence, and activities of daily living) to addressing psychosocial needs [
2,
3]. Rehabilitation begins early—often alongside acute care—and continues through scar maturation, to help patients regain independence and quality of life [
4]. This involves not only physical reconditioning and management of pain and scarring, but also psychological support to cope with trauma and adapt to life after injury [
5,
6]. Post-traumatic stress disorder (PTSD) is a frequent psychiatric diagnosis in burn survivors, reflecting the extreme trauma of the injury and treatment experience [
7]. Meta-analyses estimate that roughly one in five burn survivors meets the diagnostic criteria for PTSD within the first couple of years after injury [
8]. However, rates can vary depending on assessment and timing, with some studies reporting even higher early post-burn PTSD symptom prevalence. This high prevalence underscores the clinical significance of PTSD in the burn population. Importantly, the presence of PTSD may adversely impact rehabilitation outcomes: for example, approximately 45% of burn patients experience significant psychological distress in the first two years post-injury, and severe PTSD shortly after discharge is associated with poorer functional, psychological, and social recovery outcomes [
9]. Moreover, it’s been shown in facial burns that even small and well-healing burn wounds can result in persistent psychological distress and reduced quality of life over the medium and long term [
10]. PTSD in burn survivors is therefore not only common but also potentially detrimental to the overall rehabilitation trajectory and quality of life.
A growing body of evidence suggests that PTSD and related psychological factors can exacerbate the physical symptom burden following burns through psychosomatic interactions [
9,
11,
12]. Adult burn survivors often endure persistent physical symptoms during inpatient rehabilitation, including skin-related issues such as chronically dry skin and abnormal sensitivity to temperature changes, as well as neuropathic and musculoskeletal complaints ranging from pruritus to joint and residual burn pain [
13]. Many of these symptoms are direct consequences of the burn injury – for instance, burned skin loses sweat and oil glands, thus requiring regular moisturizing. Pruritus and pain are especially prevalent problems that can persist or even worsen during rehabilitation [
14,
15]. Mechanistically, PTSD may amplify these somatic symptoms via sustained stress responses and hyperarousal [
6,
14]. Chronic psychological stress is known to heighten the perception of pain and other sensations; indeed, anxiety and trauma-related disorders can lower the threshold for pain and itch, leading patients to experience these symptoms more intensely [
16,
17]. There is convincing evidence of an entangled, bidirectional relationship between chronic pain and PTSD in trauma survivors, and emerging data indicate that PTSD symptoms are also associated with worse post-burn pruritus severity [
12,
18,
19]. In addition, individuals with PTSD may exhibit hypervigilance to bodily discomfort and engage in avoidant behaviors, such as reduced participation in therapy or movement due to fear of pain, which can further contribute to issues like joint stiffness and pain [
20,
21]. Through these psychosomatic pathways, PTSD has the potential to increase the physical symptom burden during burn rehabilitation significantly.
The present study aimed to explore the association between clinically diagnosed PTSD and the burden of physical skin symptoms during inpatient rehabilitation after severe burn injury. Given the complex and potentially bidirectional relationship between PTSD and physical symptoms, we analyze this heterogeneous, multi-center sample to determine whether PTSD contributes to greater physical symptom burden in the rehabilitation setting, thereby providing insights that could inform holistic burn care and targeted interventions for this vulnerable subgroup.
2. Materials and Methods
2.1. Study Design
We performed subgroup analysis embedded within a previously published multicenter, prospective non-inferiority trial, which evaluated the effectiveness of a burn-specific rehabilitation program structured around the International Classification of Functioning, Disability, and Health (ICF) framework [
5]. The original trial, conducted at two centers offering multidisciplinary inpatient care for burn injuries, systematically assessed physical and psychological outcomes over time [
22,
23]. Included patients had varying intervals between their burn injury and the start of rehabilitation, providing a diverse range of recovery timelines. The present analysis presents unpublished data, specifically focusing on differences in physical skin complaints between patients with a diagnosis of PTSD and those without.
2.2. Patient and Burn Characteristics
Demographic, clinical, and injury-related variables were assessed for all patients enrolled in the study. Demographic information included age, sex, and body mass index (BMI). Injury characteristics encompassed burn etiology (e.g., flame, scald, contact, chemical, electrical), total body surface area (TBSA) affected, and depth of injury categorized into superficial partial, deep partial, and full-thickness burns. The Abbreviated Burn Severity Index (ABSI) was calculated as a composite measure of burn severity. Additional clinical variables included the presence of inhalation injury, length of acute hospital stay, and duration of inpatient rehabilitation. These variables were extracted from standardized medical documentation completed at the time of admission to rehabilitation.
2.3. PTSD Classification
PTSD diagnosis was based on the Impact of Event Scale-Revised (IES-R) score, a validated and widely used screening tool for post-traumatic stress symptoms [
24], followed by a clinical assessment by an experienced psychologist. Based on this evaluation, patients were classified into two groups: those with a clinical diagnosis of PTSD and those without.
2.4. Physical Complaints Assessment
Physical skin symptoms were assessed at the beginning (T1) and end of burn-specific inpatient rehabilitation (T2), as well as 3 months (T3) and 12 months (T4) after discharge from rehabilitation using a standardized checklist. The complaints analyzed in this study included skin dryness (xerosis) in the area of the burn or skin graft donor site, abnormal sensitivity to temperature (heat and cold), numbness, skin tightness, and increased propensity to sweat. Skin tightness describes the subjective experience of restricted or stiff skin. Sensitivity to heat or cold was defined as a heightened reaction to environmental temperature stimuli. Each of these symptoms was recorded in binary form (present or absent) and analyzed independently.
2.5. Statistical Analysis
All statistical analyses were conducted using R version 4.3.2 (R Foundation for Statistical Computing, Vienna, Austria). Descriptive statistics were used to summarize patient characteristics and symptom frequencies within each PTSD classification group. Categorical variables, including the presence of individual complaints, were expressed as counts and percentages. Continuous or ordinal data were summarized using medians and interquartile ranges. Continuous variables were assessed for normality using summary statistics and visual inspection; non-normally distributed variables were compared using the Mann–Whitney U test to evaluate differences in medians between groups. Categorical variables were analyzed using Fisher’s exact test due to small sample sizes or low expected cell counts. Welch’s two-sample t-test was applied to compare group means, which does not assume equal variances. A two-tailed p-value of less than 0.05 was considered statistically significant.
3. Results
3.1. Demographics and Injury Characteristics
A total of 103 patients undergoing inpatient burn rehabilitation were included, of whom 43 (41.7%) had a clinical diagnosis of PTSD. Patients with PTSD were slightly older than those without (median age 48 [33–56] vs. 42 years [39–56]), though this difference was not statistically significant (p=0.39). The mean body mass index (BMI) was similar between groups (27.2 ± 4.7 kg/m
2 vs. 27.1 ± 5.8 kg/m
2; p=0.91). While all patients without PTSD were male (n=60), the PTSD group included four female patients (p=0.03). Injury mechanism did not significantly differ between groups, with flame burns being the most common cause in both (28.3% vs. 32.6%; p=0.67). Regarding TBSA burned, and its subcategories (superficial partial, deep partial, and full thickness), the prevalence of partial thickness burns did not differ between groups (8% vs. 6%, p=0.31), while full thickness burns were higher in the PTSD group (2% vs. 0%, p=0.03). Median Abbreviated Burn Severity Index (ABSI) scores were higher in the PTSD group (5 vs. 6, p=0.04). There was no statistically significant difference regarding inhalation injury between the two groups (8.3% vs. 20.9%, p=0.08). In addition, the median length of acute hospital stay and inpatient rehabilitation duration did not differ between the two groups (29 vs. 23 days, p=0.16, and 5 vs. 3 weeks, p=0.09, respectively). A detailed overview of patient and injury characteristics is provided in
Table 1.
3.2. Physical Symptom Burden of the Entire Cohort
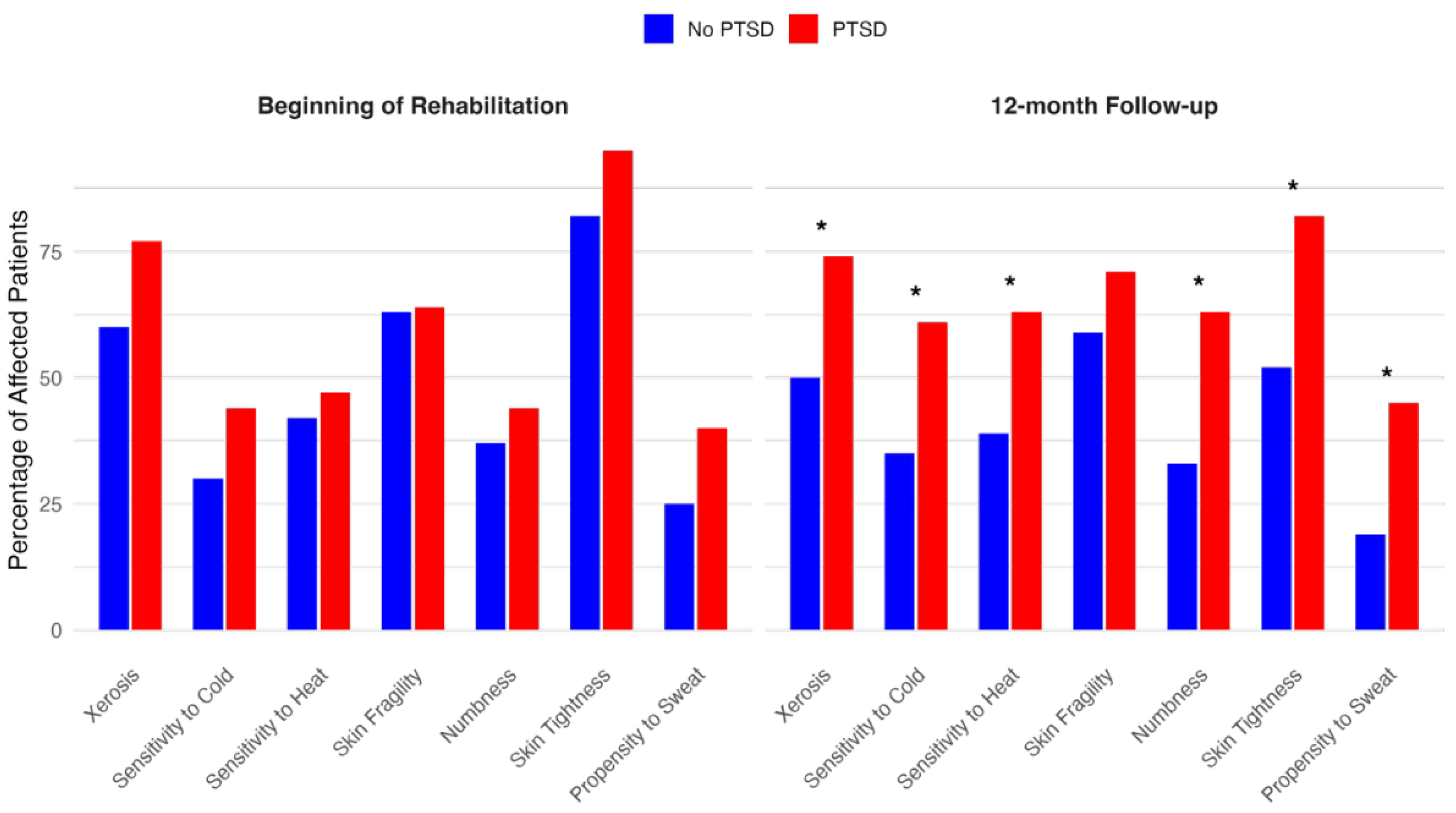
At the beginning of inpatient rehabilitation, patients exhibited a substantial burden of physical symptoms related to their burn injuries. The most frequently reported complaint was skin tightness, affecting 87% of patients, followed by xerosis (67%) and skin fragility (63%). Sensory disturbances were also common, with 44% of patients reporting sensitivity to heat, 40% reporting numbness, and 36% experiencing sensitivity to cold. A smaller proportion of patients (31%) noted an increased propensity to sweat. Patients continued to report a high burden of physical symptoms throughout the 12-month follow-up period. At T4, 60% of patients still experienced xerosis, 46% reported cold sensitivity, and 49% noted sensitivity to heat. Skin fragility remained prevalent in 64% of patients, while 46% continued to report numbness. Although skin tightness showed a gradual decrease from 87% at baseline to 64% at T4, it remained the most common complaint. Propensity to sweat was again the least frequently reported symptom, affecting 29% of patients at T4.
3.2. Impact of PTSD Status
Across all symptom categories, patients with PTSD consistently had a higher symptom burden than those without PTSD, both at the onset of inpatient rehabilitation and at all follow-up time points. While overall symptom prevalence tended to decrease over time, significant residual burden remained at T4, particularly among PTSD patients.
Figure 1 shows a comparison of physical symptom burden between T1 and T4 in both groups. While this trend was observed for all assessed symptoms, statistically significant differences particularly emerged at the 12-month follow-up (T4) in several domains. Xerosis was reported by 74% of patients with PTSD versus 50% of those without (p=0.03), and sensitivity to cold was present in 61% of patients with PTSD compared to 35% in those without (p=0.02). Similarly, sensitivity to heat was more frequent among patients with PTSD (63% vs. 39%, p=0.03). Notably, numbness and skin tightness also showed significant between-group differences at multiple timepoints: at T3, numbness was reported by 58% of PTSD patients versus 33% without (p=0.01), and at T4, this difference persisted (63% vs. 33%, p=0.006). Skin tightness at T3 and T4 was also more prevalent in the PTSD group (87% vs. 69%, p=0.04; and 82% vs. 52%, p=0.004, respectively). Additionally, a significantly greater proportion of patients with PTSD had an increased propensity to sweat at T4 (45% vs. 19%, p=0.01). An overview of the physical symptom burden over time, stratified by PTSD status, is presented in
Table 2.
Bar charts displaying the percentage of patients affected by each reported physical symptom at two timepoints: beginning of inpatient rehabilitation and at 12-month follow-up. Percentages are based on available patient data at each time point (PTSD group: beginning of rehabilitation, n=43, 12-month follow-up, n=38; no PTSD group: beginning of rehabilitation, n=60, 12-month follow-up, n=54). An asterisk (*) indicates a statistically significant difference between the PTSD and No PTSD groups at p<0.05.
4. Discussion
This study aimed to investigate whether PTSD is associated with an increased burden of physical symptoms following severe burn injuries. A key finding was that patients with PTSD consistently had higher rates of physical skin symptoms across all measured domains, including xerosis, temperature sensitivity, numbness, skin tightness, and increased propensity to sweat. Notably, this increased symptom burden persisted even one year after rehabilitation, underscoring the long-term impact PTSD may have on burn recovery.Our analysis revealed that PTSD was associated with greater burn severity, particularly regarding full-thickness burns and overall injury burden. While total TBSA burned did not significantly differ between groups, patients with PTSD had a significantly higher proportion of full-thickness burns and elevated ABSI scores, both of which reflect more extensive and complex injuries. These findings are consistent with the hypothesis that more severe and disfiguring injuries may increase the risk of PTSD, either by intensifying the traumatic experience itself or by contributing to persistent somatic and functional impairments that serve as ongoing psychological stressors. Multiple studies confirm that larger TBSA, deeper burns, and longer hospitalizations are associated with greater risk of depression, anxiety, PTSD, and impaired quality of life in both adults and children. However, recent evidence also shows that even small burns can result in substantial psychological morbidity [
10].
In addition, we found an increased physical symptom burden in patients with PTSD. These findings align well with prior research demonstrating heightened somatic symptoms in patients with PTSD. Multiple studies demonstrate that burn survivors with PTSD or significant posttraumatic stress symptoms experience higher rates and greater severity of somatic symptoms, including pain, pruritus, and neuropathic pain, compared to those without PTSD, with these symptoms often co-occurring and remaining elevated up to 18 months post-injury [
11,
12,
14,
25,
26]. Recent large database analyses also confirm that PTSD in burn patients is associated with increased risk of insomnia and that PTSD is a significant contributor to overall morbidity in this population [
25]. Further, our findings are consistent with prior research showing that burn survivors with PTSD report abnormal temperature sensitivity, and that the interplay between psychological and physical sequelae is a key determinant of long-term outcomes after burn injury [
11,
12,
14,
26]. Further, cognitive factors such as catastrophizing, negative appraisals, and maladaptive coping strategies mediate the relationship between PTSD and persistent pain or somatic complaints, reinforcing the role of neuropsychological pathways in symptom amplification [
11,
19,
27].
PTSD-related hypervigilance and fear-avoidance behaviors may intensify symptom reporting and limit patients' active engagement in rehabilitation therapies, perpetuating physical impairments like joint stiffness and reduced functional capacity. Additionally, PTSD-driven psychological distress might hinder therapeutic participation, leading to prolonged and intensified physical symptoms throughout the rehabilitation process [
28].
The present study reinforces psychosomatic models suggesting PTSD can amplify physical complaints through complex neuropsychological pathways. Multiple studies in burn populations demonstrate a bidirectional and entangled relationship between PTSD symptom clusters, particularly hyperarousal, intrusions, and emotional numbing, and increased pain interference and somatic symptoms, independent of pain intensity itself. These findings are consistent with psychosomatic models, which suggest that psychological distress can modulate physical symptom perception and chronicity via neurobiological mechanisms [
11,
12,
19,
26]. Recent work in psychoneuroimmunoendocrinology (PNIE) further elucidates that PTSD is associated with dysregulation across the central and autonomic nervous systems, immune and endocrine axes, and the gut-brain axis. PTSD is characterized by disruptions in the central and autonomic nervous systems, including altered stress reactivity, heart rate variability, and hypothalamic-pituitary-adrenal (HPA) axis function [
29]. There is also consistent evidence of immune system dysregulation, with a pro-inflammatory state and elevated cytokines such as interleukin-6 and tumor necrosis factor-α, as well as altered neuroendocrine markers, including low basal cortisol and glucocorticoid receptor sensitivity [
30,
31,
32,
33,
34]. Additionally, recent studies highlight the role of the gut-brain axis, with gut microbiota imbalances contributing to neuroinflammation and further modulating stress responses and somatic symptoms [
29,
30,
35]. These interconnected pathways help explain the amplification and persistence of somatic symptoms in PTSD, especially in populations with significant physical trauma, such as burn survivors [
29,
30,
36].
While our findings demonstrate a robust association between PTSD and increased physical symptom burden, the directionality of this relationship remains complex and likely bidirectional. On one hand, persistent somatic complaints following burn injuries may act as continuous reminders of the traumatic event, thereby contributing to the onset or maintenance of PTSD symptoms [
12,
18,
19,
37]. On the other hand, PTSD-related neurobiological alterations, including heightened stress reactivity, hypervigilance, and maladaptive coping mechanisms, may amplify the perception and persistence of physical symptoms [
27,
36,
38]. This highlights the need for future longitudinal studies incorporating repeated assessments of psychological and physical symptoms over time, ideally beginning in the acute post-injury phase. Such designs would allow for a more precise delineation of temporal relationships and mediating factors. Additionally, the integration of biomarker-based assessments – capturing neuroendocrine, immunological, and autonomic dysregulation – may provide mechanistic insights into how PTSD and somatic symptoms interact and mutually reinforce each other in the context of burn recovery.
Clinically, our results emphasize the critical importance of early psychological assessments and systematic PTSD screening in burn rehabilitation settings. Integrated care models that concurrently address psychological and somatic symptoms could substantially improve patient outcomes. Routine tracking of physical symptoms in patients identified with PTSD would facilitate targeted interventions, potentially mitigating chronic symptom burden and enhancing overall rehabilitation efficacy, as early psychological distress is a strong predictor of long-term morbidity [
39,
40]. Future research should incorporate formal PTSD diagnoses using validated psychometric instruments to enhance diagnostic precision and comparability across studies. Intervention trials examining the efficacy of psychotherapeutic support in reducing physical symptom burdens during rehabilitation could further validate the integration of psychological care into burn treatment protocols [
41].
Key strengths of this study include its multicenter, prospective design, rigorous standardization of symptom tracking, and clinically meaningful one-year follow-up period. However, several limitations should be considered. PTSD diagnosis was based on preexisting clinical diagnosis rather than standardized psychiatric assessment tools, potentially influencing diagnostic accuracy. Additionally, self-reported physical symptoms may introduce reporting bias. Furthermore, the cohort's demographic, predominantly male and characterized by occupational burn injuries, may limit generalizability. Lastly, the absence of adjustment for confounding factors such as pre-existing chronic pain or depression necessitates cautious interpretation of our findings.
5. Conclusions
This multicenter prospective cohort study demonstrates that patients with PTSD experience a significantly higher and sustained burden of physical skin symptoms following severe burn injuries compared to patients without PTSD. The persistent presence of symptoms such as xerosis, temperature sensitivity, numbness, and skin tightness at one-year post-rehabilitation underscores the clinical relevance of PTSD as a factor influencing long-term physical recovery outcomes. These findings highlight the importance of incorporating systematic PTSD screening and integrated psychotherapeutic interventions into burn rehabilitation protocols, aiming for comprehensive care that simultaneously addresses psychological trauma and somatic sequelae to optimize patient recovery and enhance quality of life.
Author Contributions
Conceptualization, F.K., M.A., and L.H.; methodology, F.K., M.A., H.N., A.S., A.P., and L.H.; software, F.K., A.S.; validation, F.K., M.A., G.H., and L.H.; formal analysis, F.K., M.A.; investigation, F.K., M.A., H.N., A.S., K.Z.; resources, U.K., H.Z., and L.H.; data curation, F.K., A.S., T.N., A.P.; writing—original draft preparation, F.K., M.A., H.N., A.S., T.N., A.C.P., H.Z., G.H., L.H.; writing—review and editing, F.K., M.A., H.N., A.S., T.N., A.C.P., H.Z., G.H., U.K., L.H.; visualization, F.K.; supervision, H.Z., U.K., and L.H.; project administration, A.S., L.H.; funding acquisition, A.S., L.H.; All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Funding Source: German Statutory Accident Insurance (Deutsche Gesetzliche Unfallversicherung, DGUV, grant number FR-268).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Rhineland-Palatinate, Germany, IRB protocol 837.236.16 (10555).
Informed Consent Statement
Written informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| PTSD |
Post-Traumatic Stress Disorder |
| ICF |
International Classification of Functioning, Disability, and Health |
| IES-R |
Impact of Event Scale-Revised |
| BMI |
Body Mass Index |
| TBSA |
Total Body Surface Area |
| ABSI |
Abbreviated Burn Severity Index |
| LOS |
Length of Stay |
| IQR |
Interquartile Range |
| SD |
Standard Deviation |
| HPA |
Hypothalamic-Pituitary-Adrenal (axis) |
| PNIE |
Psychoneuroimmunoendocrinology |
| DGUV |
Deutsche Gesetzliche Unfallversicherung |
References
- Jawad, A.M.; Kadhum, M.; Evans, J.; Cubitt, J.J.; Martin, N. Recovery of functional independence following major burn: A systematic review. Burns 2024, 50, 1406–1423. [Google Scholar] [CrossRef] [PubMed]
- Young, A.W.; Dewey, W.S.; King, B.T. Rehabilitation of Burn Injuries: An Update. Phys Med Rehabil Clin N Am 2019, 30, 111–132. [Google Scholar] [CrossRef] [PubMed]
- Klimitz FJ, R.E. , Stolle A, Hundeshagen G, Kneser U, Harhaus L, Neubauer H. Evaluation Parameters for Assessing the Effectiveness of Burn Rehabilitation: A Systematic Review. SM Physical Medicine & Rehabilitation. [CrossRef]
- Hundeshagen, G.; Suman, O.E.; Branski, L.K. Rehabilitation in the Acute Versus Outpatient Setting. Clin Plast Surg 2017, 44, 729–735. [Google Scholar] [CrossRef]
- Harhaus, L.; Ziegenthaler, H.; Neubauer, H.; Klimitz, F.J.; Strupat, M.; Ripper, S.; Kneser, U.; Stolle, A. A prospective multicenter non-inferiority trial to evaluate a new burn rehabilitation program based on the International Classification of Functioning, Disability and Health (ICF). Burns 2025, 51, 107461. [Google Scholar] [CrossRef]
- Panayi, A.C.; Heyland, D.K.; Stoppe, C.; Jeschke, M.G.; Didzun, O.; Matar, D.; Tapking, C.; Palackic, A.; Bliesener, B.; Harhaus, L.; et al. The long-term intercorrelation between post-burn pain, anxiety, and depression: a post hoc analysis of the "RE-ENERGIZE" double-blind, randomized, multicenter placebo-controlled trial. Crit Care 2024, 28, 95. [Google Scholar] [CrossRef]
- Nosanov, L.B.; Prindeze, N.J.; Schneider, D.M.; Clemente, L.E.; Parrish, K.R.; Travis, T.E.; Shupp, J.W.; Johnson, L.S. Prevalence and risk factors for acute stress disorder and posttraumatic stress disorder after burn injury. Am J Surg 2022, 223, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Boersma-van Dam, E.; Shepherd, L.; van de Schoot, R.; Engelhard, I.M.; Van Loey, N.E.E. The prevalence of posttraumatic stress disorder symptomatology and diagnosis in burn survivors: a systematic review and meta-analysis. Health Psychol Rev 2024, 1–27. [Google Scholar] [CrossRef]
- Giannoni-Pastor, A.; Eiroa-Orosa, F.J.; Fidel Kinori, S.G.; Arguello, J.M.; Casas, M. Prevalence and Predictors of Posttraumatic Stress Symptomatology Among Burn Survivors: A Systematic Review and Meta-Analysis. J Burn Care Res 2016, 37, e79–e89. [Google Scholar] [CrossRef]
- Palackic, A.; Franco-Mesa, C.; Beck, I.; Nolte, S.; Tapking, C.; Panayi, A.C.; Stolle, A.; Haug, V.; Hirche, C.; Kneser, U.; et al. The Impact of Facial Burns on Short- and Long-Term Quality of Life and Psychological Distress-A Prospective Matched Cohort Study. J Clin Med 2023, 12. [Google Scholar] [CrossRef]
- Van Loey, N.E.; Klein-König, I.; de Jong, A.E.E.; Hofland, H.W.C.; Vandermeulen, E.; Engelhard, I.M. Catastrophizing, pain and traumatic stress symptoms following burns: A prospective study. Eur J Pain 2018, 22, 1151–1159. [Google Scholar] [CrossRef]
- Bhalla, A.; Bamer, A.M.; Temes, C.; Roaten, K.; Carrougher, G.J.; Schneider, J.C.; Stoddard, F.J.; Stewart, B.; Gibran, N.S.; Wiechman, S.A. Posttraumatic Stress Disorder Symptom Clusters as Predictors of Pain Interference in Burn Survivors: A Burn Model System National Database Study. J Burn Care Res 2023, 44, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Madison, C.; Flott, G.; Brownson, E.G.; Sibbett, S.; Seek, C.; Carrougher, G.J.; Ryan, C.M.; Kowalske, K.; Gibran, N.S.; et al. Temperature Sensitivity After Burn Injury: A Burn Model System National Database Hot Topic. J Burn Care Res 2021, 42, 1110–1119. [Google Scholar] [CrossRef] [PubMed]
- Van Loey, N.E.E.; de Jong, A.E.E.; Hofland, H.W.C.; van Laarhoven, A.I.M. Role of burn severity and posttraumatic stress symptoms in the co-occurrence of itch and neuropathic pain after burns: A longitudinal study. Front Med (Lausanne) 2022, 9, 997183. [Google Scholar] [CrossRef]
- Carrougher, G.J.; Martinez, E.M.; McMullen, K.S.; Fauerbach, J.A.; Holavanahalli, R.K.; Herndon, D.N.; Wiechman, S.A.; Engrav, L.H.; Gibran, N.S. Pruritus in adult burn survivors: postburn prevalence and risk factors associated with increased intensity. J Burn Care Res 2013, 34, 94–101. [Google Scholar] [CrossRef]
- Vieira, J.S.; de Souza, G.R.; Kalil-Cutti, B.; Giusti-Paiva, A.; Vilela, F.C. Post-traumatic stress disorder increases pain sensitivity by reducing descending noradrenergic and serotoninergic modulation. Behav Brain Res 2021, 411, 113367. [Google Scholar] [CrossRef]
- Vaegter, H.B.; Andersen, T.E.; Harvold, M.; Andersen, P.G.; Graven-Nielsen, T. Increased Pain Sensitivity in Accident-related Chronic Pain Patients With Comorbid Posttraumatic Stress. Clin J Pain 2018, 34, 313–321. [Google Scholar] [CrossRef]
- Ravn, S.L.; Hartvigsen, J.; Hansen, M.; Sterling, M.; Andersen, T.E. Do post-traumatic pain and post-traumatic stress symptomatology mutually maintain each other? A systematic review of cross-lagged studies. Pain 2018, 159, 2159–2169. [Google Scholar] [CrossRef]
- de Vries, V.; de Jong, A.E.E.; Hofland, H.W.C.; Van Loey, N.E. Pain and Posttraumatic Stress Symptom Clusters: A Cross-Lagged Study. Front Psychol 2021, 12, 669231. [Google Scholar] [CrossRef]
- López-Martínez, A.E.; Ramírez-Maestre, C.; Esteve, R. An examination of the structural link between post-traumatic stress symptoms and chronic pain in the framework of fear-avoidance models. Eur J Pain 2014, 18, 1129–1138. [Google Scholar] [CrossRef]
- Devlin, A.; Casey, S.; Williams, S.; Giummarra, M.J. Association of fear-avoidance and self-efficacy on pain disability in individuals with co-morbid post-traumatic stress and chronic pain. J Health Psychol 2022, 27, 188–198. [Google Scholar] [CrossRef]
- Neubauer, H.; Stolle, A.; Ripper, S.; Klimitz, F.; Ziegenthaler, H.; Strupat, M.; Kneser, U.; Harhaus, L. Evaluation of an International Classification of Functioning, Disability and Health-based rehabilitation for thermal burn injuries: a prospective non-randomized design. Trials 2019, 20, 752. [Google Scholar] [CrossRef] [PubMed]
- Klimitz, F.J.; Neubauer, H.; Stolle, A.; Ripper, S.; Daeschler, S.C.; Aman, M.; Boecker, A.; Thomas, B.; Kneser, U.; Harhaus, L. Objective Burn Scar Assessment in Clinical Practice Using the Cutometer©: Introduction and Validation of a Standardized Measurement Protocol. J Burn Care Res 2023, 44, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Wawer, E.; Viprey, M.; Floccard, B.; Saoud, M.; Subtil, F.; Wafa, H.; Rheims, E.; Rimmelé, T.; Poulet, E. Early Detection of Patients at Risk of Developing a Post-Traumatic Stress Disorder After an ICU Stay. Crit Care Med 2020, 48, 1572–1579. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, N.; Campbell, M.S.; Dabaghi, E.; Prasai, A.; Ben-Aissa, A.; Ozhathil, D.; Jay, J.; Song, J.; Golovko, G.; Wolf, S.; et al. Post-traumatic stress disorder in burn patients - A large database analysis. Burns 2024, 50, 561–568. [Google Scholar] [CrossRef]
- Corry, N.H.; Klick, B.; Fauerbach, J.A. Posttraumatic stress disorder and pain impact functioning and disability after major burn injury. J Burn Care Res 2010, 31, 13–25. [Google Scholar] [CrossRef]
- Su, Y.J. Predicting DSM-5 PTSD symptomatology 6 months to 2 years after burn: The role of early psychological risk factors. Burns 2024, 50, 1898–1907. [Google Scholar] [CrossRef]
- Paggiaro, A.O.; Paggiaro, P.B.S.; Fernandes, R.A.Q.; Freitas, N.O.; Carvalho, V.F.; Gemperli, R. Posttraumatic stress disorder in burn patient: A systematic review. J Plast Reconstr Aesthet Surg 2022, 75, 1586–1595. [Google Scholar] [CrossRef]
- Fraile-Martinez, O.; García-Montero, C.; Álvarez-Mon, M.; Casanova-Martín, C.; Fernández-Faber, D.; Presa, M.; Lahera, G.; Lopez-Gonzalez, L.; Díaz-Pedrero, R.; Saz, J.V.; et al. Grasping Posttraumatic Stress Disorder From the Perspective of Psychoneuroimmunoendocrinology: Etiopathogenic Mechanisms and Relevance for Integrative Management. Biol Psychiatry 2025. [CrossRef]
- Pivac, N.; Vuic, B.; Sagud, M.; Nedic Erjavec, G.; Nikolac Perkovic, M.; Konjevod, M.; Tudor, L.; Svob Strac, D.; Uzun, S.; Kozumplik, O.; et al. PTSD, Immune System, and Inflammation. Adv Exp Med Biol 2023, 1411, 225–262. [Google Scholar] [CrossRef]
- Sbisa, A.M.; Madden, K.; Toben, C.; McFarlane, A.C.; Dell, L.; Lawrence-Wood, E. Potential peripheral biomarkers associated with the emergence and presence of posttraumatic stress disorder symptomatology: A systematic review. Psychoneuroendocrinology 2023, 147, 105954. [Google Scholar] [CrossRef]
- Katrinli, S.; Oliveira, N.C.S.; Felger, J.C.; Michopoulos, V.; Smith, A.K. The role of the immune system in posttraumatic stress disorder. Transl Psychiatry 2022, 12, 313. [Google Scholar] [CrossRef] [PubMed]
- von Majewski, K.; Kraus, O.; Rhein, C.; Lieb, M.; Erim, Y.; Rohleder, N. Acute stress responses of autonomous nervous system, HPA axis, and inflammatory system in posttraumatic stress disorder. Transl Psychiatry 2023, 13, 36. [Google Scholar] [CrossRef] [PubMed]
- Olff, M.; van Zuiden, M. Neuroendocrine and neuroimmune markers in PTSD: pre-, peri- and post-trauma glucocorticoid and inflammatory dysregulation. Curr Opin Psychol 2017, 14, 132–137. [Google Scholar] [CrossRef]
- Ke, S.; Hartmann, J.; Ressler, K.J.; Liu, Y.Y.; Koenen, K.C. The emerging role of the gut microbiome in posttraumatic stress disorder. Brain Behav Immun 2023, 114, 360–370. [Google Scholar] [CrossRef]
- Mellon, S.H.; Gautam, A.; Hammamieh, R.; Jett, M.; Wolkowitz, O.M. Metabolism, Metabolomics, and Inflammation in Posttraumatic Stress Disorder. Biol Psychiatry 2018, 83, 866–875. [Google Scholar] [CrossRef]
- Graham, K.; Lawrence-Wood, E.; McFarlane, A. Longitudinal Relationship Between Posttraumatic Stress Symptoms and Physical Symptoms in Military Veterans. Psychosom Med 2022, 84, 1034–1040. [Google Scholar] [CrossRef]
- Choi, J.J.; Martins, J.S.; Hwang, S.; Sinha, R.; Seo, D. Neural correlates linking trauma and physical symptoms. Psychiatry Res Neuroimaging 2022, 327, 111560. [Google Scholar] [CrossRef]
- Dahl, O.; Wickman, M.; Björnhagen, V.; Friberg, M.; Wengström, Y. Early assessment and identification of posttraumatic stress disorder, satisfaction with appearance and coping in patients with burns. Burns 2016, 42, 1678–1685. [Google Scholar] [CrossRef]
- Carmean, M.; Grigorian, A.; Stefan, J.; Godes, N.; Burton, K.; Joe, V.C. What Happens After a Positive Screen for Depression and Posttraumatic Stress Disorder in the Outpatient Burn Clinic? J Burn Care Res 2019, 40, 590–594. [Google Scholar] [CrossRef]
- Wang, S.; Cannata, B.; Vallurupalli, M.; Yenikomshian, H.A.; Gillenwater, J.; Stoycos, S.A. A Scoping Review of PTSD and Depression in Adult Burn Patients: A Call for Standardized Screening and Intervention Research. J Burn Care Res 2024, 45, 1402–1412. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).