1. Introduction
Plasmacytoma is a type of neoplasm that arises from monoclonal B cells. There are various types of plasmacytoma: solitary plasmacytoma of bone (SPB), extramedullary plasmacytoma (EMP), multiple myeloma (MM) and, lymphoplasmacytic lymphoma.
The EMP was initially described by Schridde and colleagues in the year 1905 [
1].
EMP is rare condition that is defined as localized plasma cell lesion that develop in tissues outside of bone marrow plasmacytosis [
2].
EMP is a very rare tumor, representing 3-5% of all plasma neoplasms. [
3].
EMP can occur in any part of the body, however, the head and neck region is the predominant location, with approximately 80-90% of cases occurring at this level. Nasal cavity and paranasal sinuses are most commonly involved, followed by the palatine tonsils and oral cavity. The symptomatology is not specific and may include headache, epistaxis, nasal obstruction, nasal discharge, dysphagia, and sore throat [
4,
5,
6].
EMP may result from inhalation of chemicals, viral infection, excessive irradiation, and genetic abnormalities that affect the reticuloendothelial system. [
7] .
EMP is typically diagnosed based on histological and immunochemical exams that highlight markers such as Vs38c, CD138, CD20, CD38, CD 56, CD79a, CD117, lambda light chains (LLC), kappa light chains (KLC). CD138, which can be identified as the marker that is used most frequently to identify plasma cells [
8,
9].
An EMP may be classified as primary when it arises de novo, or as secondary when it develops during the progression of multiple myeloma (MM). Extramedullary disease has the potential to progress to MM in 17–33% of cases. [
10,
11,
12]. The transformation of EMP into MM can occur in 8-31% of cases, as described in the literature [
8,
13,
14].
For the management of EMP, there are no clear guidelines, and the majority of the experience has been collected from a number of different case reports that have been published over the course of several decades. However, in the case of sinonasal EMP, radiotherapy is frequently the preferred option because the increased sensitivity of EMP to radiation. Surgical excision in association with radiotherapy, has demonstrated the most favorable survival rates. The efficacy of chemotherapy in the treatment of sinonasal EMP remains unclear. [
15].
This study includes on overview of the documented cases of extramedullary plasmacytoma of the sinonasal region from the literature published between 2000 and 2023 and a separate analysis describing our experience, presenting 3 clinical cases..
2. Materials and Methods
We performed an extensive literature search by accessing the PubMed database, which identified all English and non- English language manuscripts on sinonasal EMP published between 2000 and 2023. Our search criteria included keywords such as "sinonasal plasmacytomas," "sinus plasmacytomas," and "nasal plasmacytomas," which initially yielded 127 articles. We then refined our search by excluding non-English language studies and those involving non-human subjects, so this paper gives a complete overview of the sinonasal EMP cases reported in the last 23 years.
We included only human studies conducted in the English language that provided individual data on sinonasal plasmacytomas, encompassing details on diagnosis, treatment, follow-up, and outcomes.
We extracted outcome measures that covered various aspects, such as demographic information, tumor location, presenting symptoms, radiographic imaging, the primary mode of treatment, adjuvant therapy, instances of recurrence, metastasis, development of multiple myeloma, follow-up duration, secondary treatment for recurrence or metastasis, and overall survival. we summarize all the information in Table 1.
| Case No./Ref |
Sex/ Age |
Symptoms |
Localization |
Treatment |
Outcome on the follow-up |
Multiple myeloma |
| 1/ [16] |
M/ 20 |
No symptoms |
Nasal cavity |
surgery |
No recurrence |
No |
| 2/ [16] |
M/ 48 |
Epistaxis |
Nasal cavity |
Surgery + RT |
No recurrence |
No |
| 3/ [16] |
M/ 60 |
Epistaxis |
Nasal cavity |
RT |
Recurrence |
No |
| 4/ [17] |
M/ 61 |
Epistaxis
Facial pain
Rhinorrhea |
Nasal cavity
Maxillary sinus |
RT+CHT |
Recurrence |
No |
| 5/ [17] |
M/ 60 |
Nasal obstruction
Epistaxis
Rhinorrhea |
Nasal cavity
Ethmoid sinus |
RT |
Recurrence |
No |
| 6/ [17] |
F/ 37 |
Nasal obstruction |
Nasal cavity
Maxillary sinus |
CHT |
No recurrence |
No |
| 7/ [18] |
M/ 75 |
Epistaxis |
Nasopharynx |
RT |
No recurrence |
No |
| 8/ [19] |
F/ 44 |
Epistaxis |
Nasal cavity
Maxillary sinus |
Surgery |
No recurrence |
No |
| 9/ [20] |
M/ 32 |
Change/ loss of vision |
Sphenoid sinus |
Surgery + RT |
No recurrence |
No |
| 10/ [21] |
M/ 56 |
Nasal obstruction
Epistaxis
Facial pain and swelling |
Nasal cavity Maxillary sinus
Ethmoid sinus
Sphenoid sinus
nasopharynx |
CHT |
Died of the disease |
No |
| 11/ [22] |
F/ 67 |
Nasal obstruction
Epistaxis
Rhinorrhea |
Nasal cavity
Ethmoid sinus |
Surgery + RT |
No recurrence |
no |
| 12/ [23] |
F/ 75 |
Nasal obstruction |
Nasal cavity
Maxillary sinus |
Surgery + RT |
No recurrence |
No |
13/ [24]
|
F/ 32 |
Nasal obstruction
Epistaxis
Facial pain and swelling
Rhinorrhea
Chance/ loss of vision |
Nasal cavity
Maxillary sinus
Ethmoid sinus
Frontal sinus |
Surgery +RT |
No recurrence |
no |
| 14/ [25] |
F/ 87 |
Epistaxis |
Nasal cavity
Maxillary sinus, Sphenoid sinus
Frontal sinus
Nasopharynx |
RT |
No recurrence |
no |
| 15/ [26] |
F/ 50 |
Epistaxis
Rhinorrhea
Facial swelling |
Nasal cavity
Maxillary sinus
Ethmoid sinus |
Surgery + RT + CHT |
- |
yes |
| 16/ [27] |
M/ 24 |
Nasal obstruction
Facial swelling and pain
Rhinorrhea
proptosis |
Maxillary sinus |
Surgery +RT |
No recurrence |
no |
| 17/ [28] |
F/ 16 |
Nasal obstruction
Epistaxis |
Nasal cavity
Maxillary sinus
Ethmoid sinus
Sphenoid sinus |
Surgery + RT |
No recurrence |
No |
| 18/ [28] |
F/ 26 |
Nasal obstruction
Facial swelling |
Maxillary sinus |
Surgery |
No recurrence |
no |
19/ [28]
|
F/ 52 |
Nasal obstruction
Epistaxis |
Nasal cavity
Nasopharynx |
Surgery+ RT |
No recurrence |
no |
| 20/ [28] |
F/ 15 |
Epistaxis
Facial swelling |
Nasal cavity
Maxillary sinus
Sphenoid sinus |
Surgery +RT + CHT |
Died of the disease |
no |
| 21/ [28] |
M/ 23 |
Nasal obstruction
epistaxis |
Nasal cavity
Maxillary sinus
Ethmoid sinus |
Surgery |
No recurrence |
No |
| 22/ [28] |
F/ 15 |
Facial swelling |
Maxillary sinus |
No treatment |
- |
- |
| 23/ [29] |
M/ 51 |
Nasal obstruction
Change/ loss of vision
Headache |
Nasal cavity
Maxillary sinus |
RT +CHT |
- |
Yes |
| 24/ [30] |
M/ 31 |
Nasal obstruction
Rhinorrhea
Facial swelling |
Nasal cavity
Maxillary sinus, Ethmoid sinus, Sphenoid sinus
Nasopharynx |
RT |
No recurrence |
no |
| 25/ [30] |
M/ 60 |
Nasal obstruction
Epistaxis |
Nasal cavity
Nasopharynx |
Surgery +RT |
No recurrence |
no |
| 26/ [31] |
F/ 54 |
Change/ loss of vision
Headache |
Sphenoid sinus |
Surgery + RT |
No recurrence |
no |
| 27/ [32] |
M/ 42 |
Nasal obstruction
Headache |
Nasal cavity
Maxillary sinus |
No treatment |
|
|
| 28/ [33] |
M/ 49 |
Facial swelling and pain
proptosis |
Maxillary sinus
Frontal sinus |
Surgery + RT + CHT |
No recurrence |
no |
We used descriptive statistics to analyze the available data for statistical purposes. Descriptive analysis of characteristics including gender, tumor location, symptomatology, treatment modalities, and outcomes highlighted frequency and percentage distributions. Categorical variables were summarized with absolute and relative frequencies, while continuous variables, such as age, were characterized by mean and standard deviation. All analyses have been performed using Microsoft Excel.
Following surgical treatment, the sample was rinsed with phosphate-buffered saline (PBS), and tissue fragments were fixed in 10% neutral buffered formalin for 24 hours at room temperature. The specimens were then processed for paraffin embedding. Tissue sections of 3–4 μm thickness were mounted on glass slides and stained with Hematoxylin–Eosin (HE) to evaluate histological characteristics. Serial sections of 3–4 μm thickness were dewaxed and rehydrated. Antigen retrieval was performed by incubating the sections in a microwave oven using an appropriate buffer. To block endogenous peroxidase activity, the sections were treated with 3% hydrogen peroxide (H₂O₂) in methanol.
After a blocking step to minimize nonspecific binding, the sections were incubated overnight at 4°C with one of the primary antibodies listed in Table 1. The next day, the sections were washed with phosphate-buffered saline (PBS) at pH 7.4–7.6 and subjected to immune signal amplification using the Dako Envision™+ Dual Link System–Horseradish Peroxidase (HRP) (Dako, Carpinteria, USA), according to the manufacturer's instructions. Finally, the slides were counterstained with Mayer’s Hematoxylin.
For each antibody, a negative control was included by substituting the primary antibody with 10 mM PBS at pH 7.4–7.6. Color development was carried out using 3,3’-Diaminobenzidine (DAB) tetrahydrochloride (Sigma-Aldrich) in the presence of hydrogen peroxide (H₂O₂) (Merck). Nuclear counterstaining was performed with Mayer’s Hematoxylin. Finally, the sections were mounted using DPX mounting medium (Sigma-Aldrich).
For the IHC study, we used the following primary antibodies for both positive and differential diagnosis: (anti-human CD20cy, clone L26,Dako,dilution 1:100;anti-human, CD79-α, clone JCB117, Dakoz,dilution 1:50; anti-S100,rabbit polyclonal,Dako,dilution 1:50,antigen retrieval:citrate buffer,pH 6; anti-EMA,mouse monoclonal,Dako,dilution 1:50,antigen retrieval:citrate buffer,pH 6; anti-synaptophysin,mouse monoclonal,Dako,dilution 1:20,antigen retrieval:citrate buffer,pH 6; anti-CK AE1/AE3,mouse monoclonal,Dako,dilution 1:50,antigen retrieval:citrate buffer,pH 6; anti-CD45/LCA/CLA,mouse monoclonal,Dako,dilution 1:50,antigen retrieval:citrate buffer,pH 6;anti-human; CD138 (Syndecan-1),rabbit monoclonal,clone A23248,ABclonal,dilution 1:100,antigen retrieval:citrate buffer,pH 6)
3. Results
3.1. Literature Data Analysis
There were 28 cases of SN-EMP included in this study (Table 1). The average age at diagnosis was 45.07 years old (SD ± 19.70). The majority of patients were aged between 40 and 70 years, with 15 (53.57%) male and 13 (46.42%) female.
Among the cohort of 28 patients, tumor localization predominantly affected the nasal cavity and paranasal sinuses, occurring in 22 (78.57%) of patients. Only 3 (13.63%) of nasal cavity tumors were localized to that area, while 19 (86,37%) exhibited extension beyond it. In the same way, paranasal sinuses were implicated in 22 (78.57%) of cases, with only 5 (22.72%) cases limited to the paranasal sinuses – 3 cases confined to the maxillary sinus and 2 to the sphenoid sinus. Nasopharyngeal involvement was noted in 7 (25%) of patients; however, only 2 (28.57%) were limited to the nasopharynx, while 5 (71.42%) exhibited extension beyond this region.
The most common treatment in the reviewed literature was surgery and radiotherapy- 35.71%, followed by radiotherapy alone -17.86%. Surgery alone represented 14.29% of the cases, radiotherapy with chemotherapy, and association of radiotherapy, surgery, and chemotherapy were used 10.71% of the time. Chemotherapy alone was rare - 3.57%, and 7.14% received no treatment.
The most frequent symptomatology was unilateral epistaxis, followed by nasal obstruction.
At follow-up, 67.89% were disease-free and showed no tumor recurrence, 10.71% relapsed, and 7.14% died. No follow-up data was available for the 2 (7.14%) patients with multiple myeloma and also for the 2 (7.14%) of the cases that refused the treatment.
3.2. Presentation and Analysis of Our Cohor
The patients were diagnosed with EMP by meeting the following criteria: the presence of one or more extramedullary plasma cell tumors, normal plasma cell density in the bone marrow with minimal morphological alterations or plasma cell infiltration below 10%, absence of radiological evidence of osteolysis, absence of hypercalcemia or renal failure, and low or absent serum M-protein levels.
1.4. CASE REPORTS
CASE 1
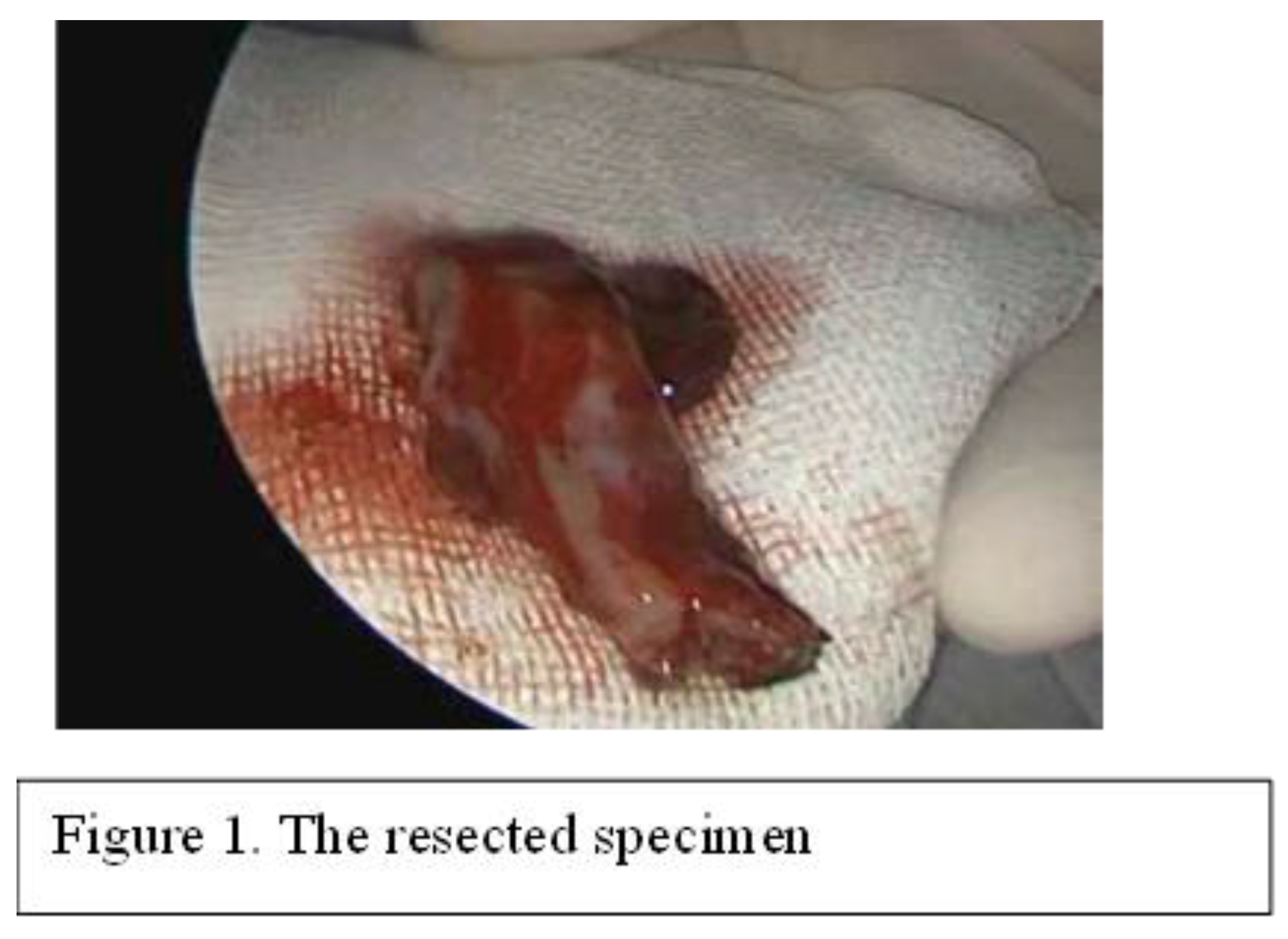
43-year-old male patient presents for bilateral nasal obstruction, posterior rhinorrhea, and recurrent epistaxis, symptoms that have begun 3 months ago and have progressively worsened. The ENT clinical examination and nasal endoscopy reveal an infiltrating, vegetative mass on the left nasal fossa and nasopharynx, measuring approximately 2 cm, reddish in color, mildly tender, and bleeding easily, without any pathological secretions. No lymphadenopathy was found, and his blood test results, including hemoglobin levels, were within the normal limits. The images obtained from the MRI scan of the head revealed a mass of soft tissue in the nasopharynx and left nasal fossa. The mass did not cause any bone destruction. It was decided to surgically remove the mass, so, under general anesthesia and endoscopic control, the tumor was extirpated “en bloc” (Figure 1)
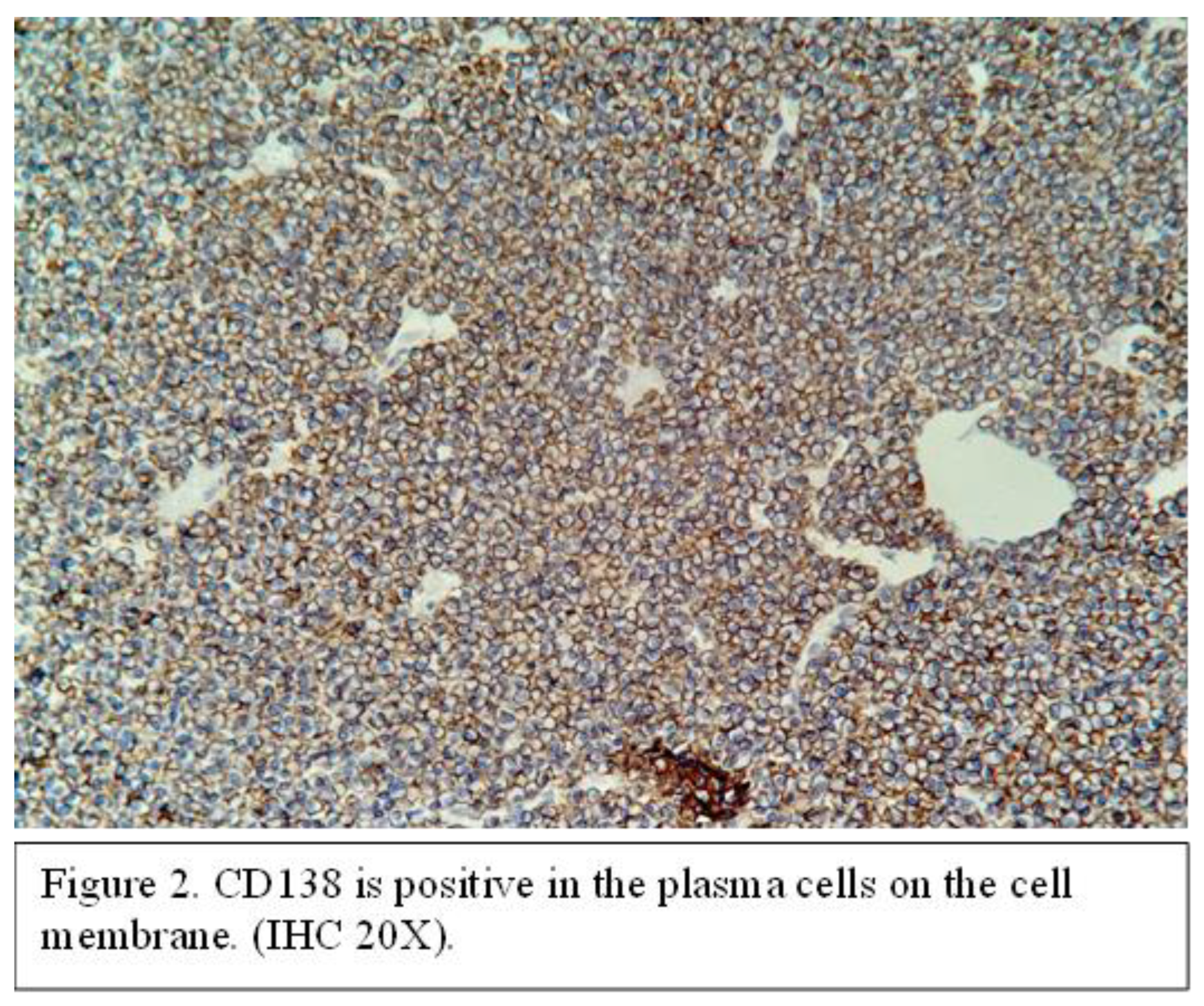
The resected tumor specimen has been sent for histopathological examination. Pathological examination revealed diffuse tumor infiltration resembling plasmacytoid cells, accompanied by hemorrhagic areas and focal ulceration of the overlying epithelium. At the immunochemistry examination, the tumor cells expressed strong immunoreactivity with CD38 and CD138 (Figure 2).
This established the diagnosis of EMP of the nasopharynx. The patient declined all forms of oncological therapy, including radiotherapy treatment. One-year postoperative, the patient underwent control imaging (MRI of the head and neck) and ENT exam, which did not reveal signs of recurrence.
CASE 2
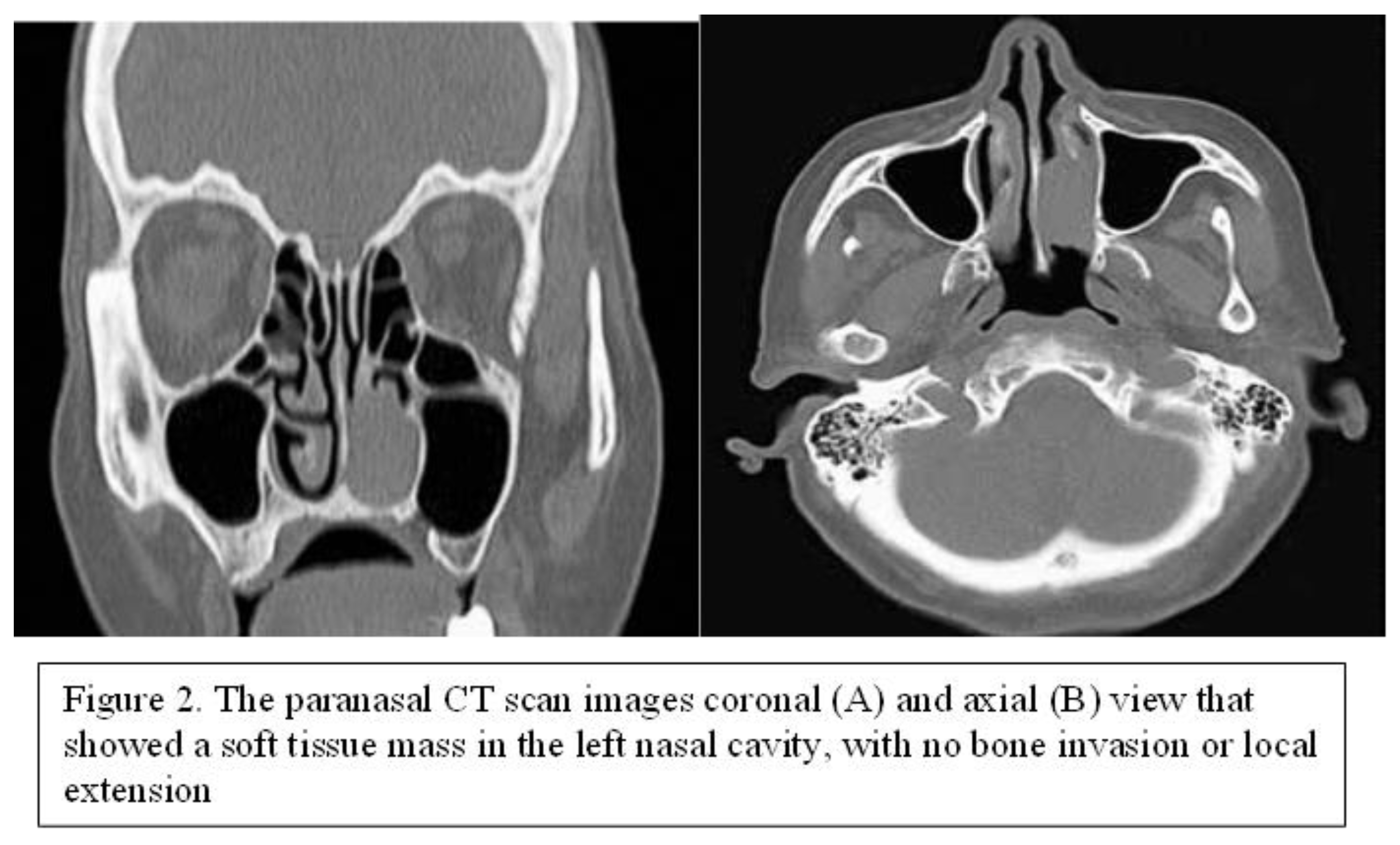
79-year-old female patient, presented with progressive left nasal obstruction and repeated episodes of left unilateral epistaxis at minimal effort. The symptomatology of the patient started 2 years before the presentation. The patient did not have major comorbidities and routine blood examinations were within normal limits. ENT clinical examination and nasal endoscopy revealed a sessile, reddish mass, slightly bleeding upon instrumental palpation, located in the left nasal fossa, which almost completely obstructed it. The CT scan of the paranasal sinuses revealed a soft tissue mass in the left nasal cavity, arising from the left inferior turbinate, without bone invasion or local extension (Figure 3).
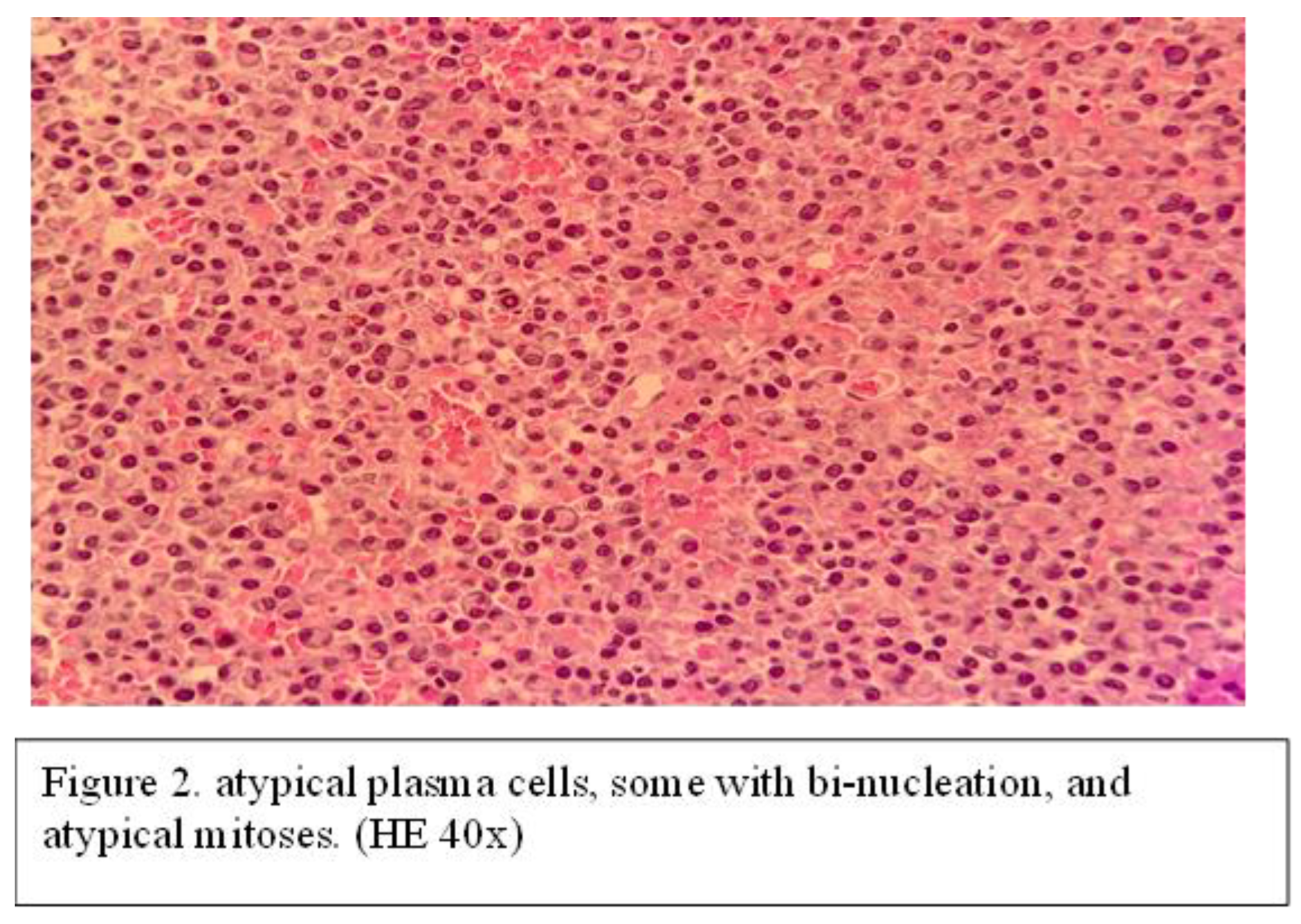
It was decided to remove the mass surgically. Consequently, the tumor was extirpated "en bloc" under endoscopic control and general anesthesia and then sent for histopathological examination. Histopathological examination shows diffused plasmacytoid tumor cells in the stroma along with areas of necrosis and hemorrhage and cells with bi-nucleation, and atypical mitoses (Figure 4).
Immunohistochemical examination evidenced positive expressions of CD79a, CD138, and CD56. This led to the diagnosis of EMP in the left nasal cavity. After the diagnosis was established, for better disease control, the patient underwent local radiotherapy (40 Gy over a period of 3 weeks). On follow up after 2 years of completed oncological care, nasal endoscopy and CT scan examen of the head showed no signs of recurrence.
CASE 3
A 47-year-old male patient presented with unilateral (right) nasal obstruction for over one year. The patient had no comorbidities, and blood tests are within normal limits. ENT examination and nasal endoscopy revealed a mass in the right nasal cavity that completely obstructed the ipsilateral osteomeatal complex and purulent secretions. The mass was bleeding on instrumental palpation. CT examination of the paranasal sinuses revealed a soft tissue mass that obstructed the entire right nasal fossa, extended into the right ethmoid sinus, and opacified the right maxillary sinus. The patient underwent endoscopically guided surgery under general anesthesia for tumor removal. The specimen was sent for histopathological that showed compact mass of cells with abundant eosinophilic cytoplasm and eccentrically located nuclei and some cells with a spindled morphology (Figure 5).
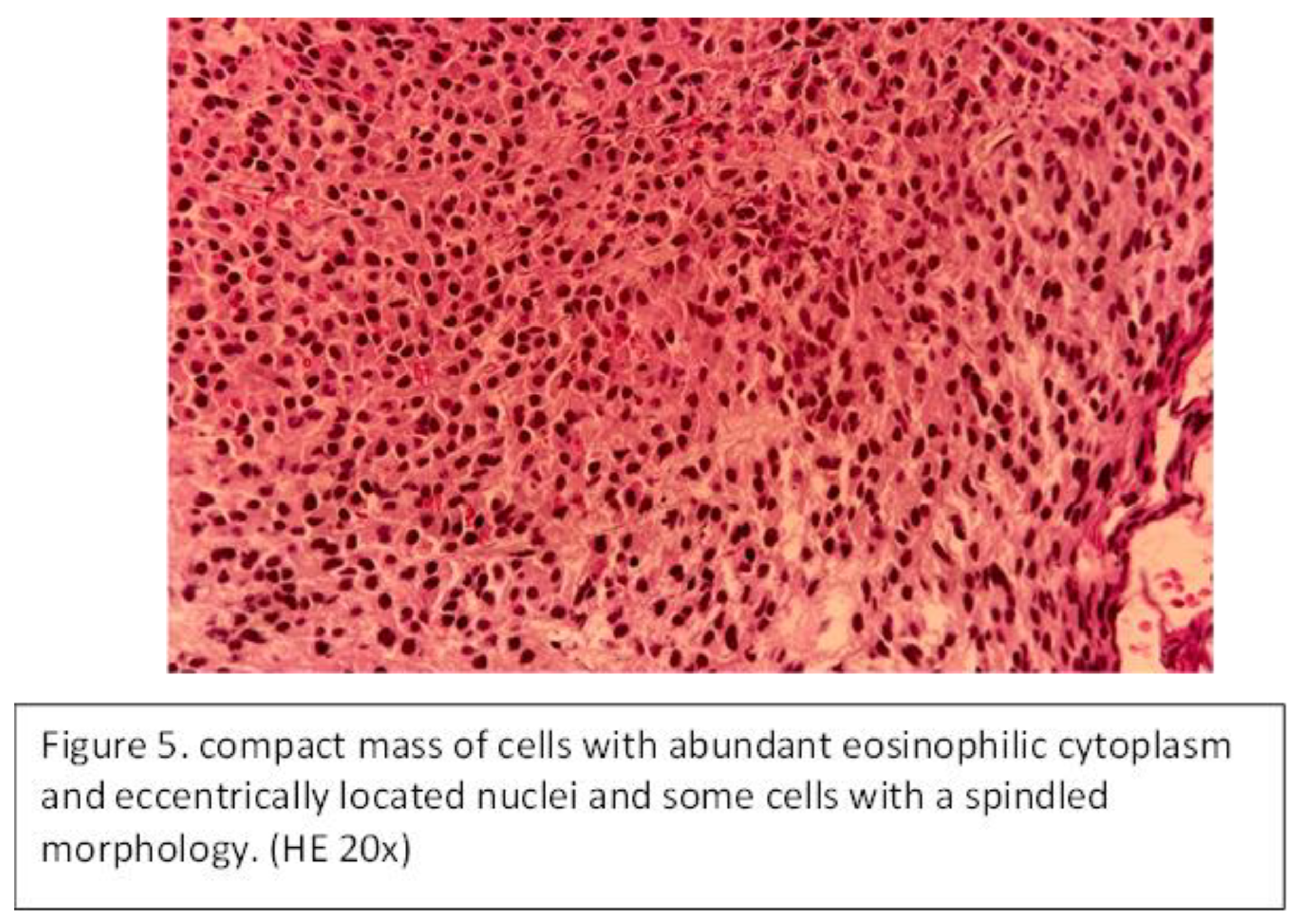
The immunochemistry examination identified that the tumor cells were positive for CD138. The patient also underwent oncological treatment—radiotherapy with 44 Gy over a period of 1 month. The endoscopic exam and CT scan of the paranasal sinuses showed no signs of relapse at the 18-month follow-up.
In all of our three patients presented in the study, we performed also an extensive evaluation to establish if they associated also multiple myeloma (serum protein electrophoresis and bone marrow examination). These investigations were negative in all the patients.
4. Discussion
The published literature on EMP of the nasal cavity and paranasal sinuses is limited to case reports or small series due to its rarity. The present study includes our experience with three patients and a literature review of 28 cases.
EMP originates predominantly in the head and neck region, primarily affecting the elderly, with a peak incidence in the 6th and 7th decades of life. 44% of the EMP of the head and neck are located in the nasal cavity/ paranasal sinuses while 18% involve the oropharynx [
34]. It appears to have a male predilection (male/female ratio = 3:1) and it was first described by Schridde in 1905 [
1,
35]. In the case of the patients that we presented in the study, EMP was more frequent in males, with a male-to-female ratio of 2:1 and a median age of 56.3 years.
In the head and neck region, about 80%-90% of EMP cases are located in the sinonasal cavity [
36].
In the cohort of 28 patients analyzed for sinonasal EMP, tumor localization showed an individual pattern of frequent involvement of the nasal cavity and paranasal sinuses, with a tendency for extension beyond a singular anatomical site in most cases. In 22 patients, we observed that tumors involving the nasal cavity were the most frequent. The majority of cases (19 out of 22) showed extension of the tumor beyond the nasal cavity, and only three cases indicate a localized disease process limited to the nasal cavity.
Paranasal sinus involvement was similarly common, observed in 22 of the 28 patients. In this group, three lesions were restricted exclusively to the maxillary sinus, while two were limited to the sphenoid sinus. The remaining cases indicated either multi-sinus involvement or extension beyond the paranasal sinuses into the nasal cavity or adjacent regions.
In seven cases, the nasopharynx was determined to be the primary location of involvement. Additionally, in three cases, the tumor was limited only to the nasopharyngeal region, while the other four cases presented evidence of dissemination beyond the nasopharynx.
Symptomatology is insidious, and this might lead to a delayed presentation at the doctor, resulting in a late diagnosis by the physician and advanced stages of the disease. The symptoms refer to the location of the tumor more than its characteristics. Some of the presentation symptoms include localized edema, unilateral nasal obstruction, recurrent unilateral epistaxis, rhinorrhea or adenopathy, facial pain, proptosis, perforated nasal septum, and nasal pyramidal dysmorphism.
The most frequent manifestations of disease, as reported in both the literature and our cases, are nasal obstruction and recurrent epistaxis [
36].
In a group of 20 patients with sinonasal EMP, Kapadia and colleagues [
37] noticed the following most common symptoms: tumor or local edema in 80%, nasal obstruction in 35%, epistaxis in 35%, localized pain in 20%, proptosis in 15%, rhinorrhea in 10%, regional lymphadenopathy in 10%, and paralysis of the VI cranial nerve in 5% of cases.
In our analyses, the results are similar to the data reported by other. All of our three patients, presented with unilateral nasal obstruction, and two of them associated also epistaxis. In the 28 cases that we study from the literature, unilateral epistaxis was the most common symptomatology, followed by nasal obstruction.
Paraclinical exams such as nasal endoscopy and radiological investigations are nonspecific; they are used to assess the lesion. The MRI examination may reveal a mildly heterogeneous signal that appears hyperintense on T2 and intermediate on T1, while various levels of intensity can also be observed on the craniofacial CT examination after the administration of contrast substance. Current international guidelines indicate that CT and MRI are appropriate for initial staging and the whole-body PET-CT, is helpful for evaluating disease at any other sites, and it also helps in monitoring the patient's response to treatment [
38].
Histopathological examination reveals dense, homogeneous proliferation of plasma cells arranged in cords, solid masses, or chains, accompanied by nuclear atypia characterized by round or oval nuclei, vesicular nuclear chromatin, and mitotic activity [
9,
39,
40]. The immunochemical analysis indicates a uniform infiltration of monoclonal plasma cells, typically expressing CD138 and/or CD38 on their surface[
41] . In our cases, one of the patients had both expressions CD38 and CD138, while the others two had only the CD 138.
Up to 20-30% of cases of EMP have the potential to progress to MM and it is still impossible to determine based on the paraclinical investigations which cases of EMP.
To diagnose EMP and differentiate it from MM, histopathological and immunohistochemical examinations must be performed, along with the fulfillment of specific criteria [
42,
43]: (1) The presence of one or more extramedullary plasma cell tumors; (2) Bone marrow smear with a normal ratio of plasma cells in the bone marrow, including no observed plasma cell morphology or a plasma cell ratio of less than 10%; (3) Absence of radiological evidence indicating osteolysis; (4) Absence of hypercalcemia or renal insufficiency; (5) Absence or minimal M-protein serum concentration.
After confirmation of EMP, additional investigations must be conducted, including bone marrow evaluation, serum protein electrophoresis, complete blood count, renal function evaluation (eGFR), and skeletal examination to exclude MM [
17].
In our cases bone marrow evaluation and laboratory serological tests evidence the absence of bone marrow involvement, no hypercalcemia or renal failure and the immunoglobulin electrophoresis within normal parameters, which confirmed the absence of MM. From the 28 of the cases, 2 of them (7.14%) developed to MM.
The differential diagnosis of nasal EMP includes pathologies like lymphoma, melanoma, inverted papilloma and sinonasal fungal material, and also with rhinoscleroma, olfactory neuroblastoma and, pituitary adenoma [
30,
44]. From a histopathological perspective, the distinction between EMP and multiple myeloma is the most difficult. However, the absence of bone lysis, normal protein electrophoresis, and the absence of anemia are the distinguishing features between the two tumor types [
45,
46] .
The treatment of nasal EMP requires the development of a treatment plan in association with the oncology team. This plan should be personalized based on the histological type of the tumor, its stage, the feasibility of complete resection, the patient's medical condition, the risks of treatment, and comorbidities. The surgical team's expertise in managing potential intraoperative complications, the patient's preferences, and the available reconstructive options should also be taken into account.
Endoscopic nasal surgery is a safe and minimally invasive procedure utilized for both diagnostic purposes—such as biopsy for histopathological and immunohistochemical examination—and therapeutic intervention through the effective resection of tumor with safe borders, when this is possible [
47,
48].
EMP is a radiosensitive tumor, so, the first recommended therapeutic method is radiotherapy when the surgery cannot be done. Radiotherapy offers a local control rate of 90-100%. Regarding the optimal recommended dose there is no consensus, in the literature, effective control rates range from 35 to 80 Gy[
49,
50].
Polymodal treatment, which include extensive surgery followed by radiotherapy are also described in the literature and is considered to offer the most effective therapeutic results [
39,
47,
51]. Chemotherapy may be indicated for patients with refractory and/or relapsed disease and in instances of progression to multiple myeloma [
52].
Alexiou et al. [
34] found that in the treatment of head and neck EMP, the combination of radiation and surgery resulted in an increase in 5-year survival of approximately 50% and they recommended surgery followed by RT for EMP when complete resection is difficult to achieve.
Bachar et al. [
13] in a study of 68 patients with head and neck EMP analyzed the rates of local recurrence, regional recurrence, and progression to MM after RT alone and after simple surgery. They found that surgery without RT decreased the recurrence rate at 5 years of follow-up from 82% to 75%. These authors recommend that RT be considered primary therapy and that postoperative RT be applied to patients with involved surgical margins but is not necessary for those who underwent complete surgical excision with negative margins.
In our cases, all patients underwent tumor resection by surgery, and two of them also received radiotherapy. All of three patients did not show any relapse at the follow up (12–24 months), and additional tests ruled out MM.
In the reviewed literature, 10 patients (35.71%) underwent combined surgery and radiotherapy. Radiotherapy was administered exclusively in 5 cases, constituting 17.86% of the total. 4 patients (14.29%) underwent surgery exclusively, another 3 cases (10.71%) received radiotherapy combined with chemotherapy, and 3 patients (10.71%) received a combination of surgery, radiotherapy, and chemotherapy. There was one patient who received chemotherapy on its own (3.57%), and 2 patients (7.14%) did not receive any treatment at all. At follow-up, 67.86% of patients were alive and disease-free. Recurrence was observed in 10.71% of the patients, while 7.14% died due the disease. It is not mentioned in the articles that follow-up was performed on 2 patients (7.14%) who were also diagnosed with multiple myeloma respectively and on the 2 (7.14) that refused any type of treatment.
Two patients with recurrence received only radiotherapy, while another underwent combination of radiotherapy and chemotherapy. One patient who died to the disease underwent surgery and chemotherapy, while the other received a combination of radiotherapy, surgery, and chemotherapy.
Among the analyzed cases, patients who underwent both surgery and radiotherapy did not experience any recurrence of the tumor during the follow-up period. This highlights that this method is more effective than other alternative treatment protocols.
5. Conclusions
Sinonasal extramedullary plasmacytoma (EMP) is a rare entity that presents with non-specific clinical manifestations, such as unilateral nasal obstruction and epistaxis, leading to a delay in patient presentation and diagnosis.
Histopathological and immunohistochemical examinations are essential for diagnosis, and in conjunction with the results of other laboratory investigations—normal bone marrow smear, absence of osteolysis, no hypercalcemia or renal insufficiency, and absence or minimal M-protein serum concentration—evaluate systemic involvement to exclude multiple myeloma.
Due to the rarity of EMP, the nonspecific presentation, and nonspecific radiological findings, we consider that treatment strategies should be individualized. Based on our experience which includes three patients and a review of 28 cases from the literature, we considered that radiotherapy in association with surgery when complete excision of the tumor with safe margins is possible is the preferred therapeutic modality, but also, radiotherapy alone may also be considered.
The follow-up is essential in cases of EMP due to the potential for relapse and development of multiple myeloma. Multidisciplinary management involving otolaryngologists, oncologists, radiation therapists, and pathologists is essential to improve outcomes in patients with sinonasal EMP.
Author Contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used “Conceptualization, X.X. and Y.Y.; methodology, X.X.; software, X.X.; validation, X.X., Y.Y. and Z.Z.; formal analysis, X.X.; investigation, X.X.; resources, X.X.; data curation, X.X.; writing—original draft preparation, X.X.; writing—review and editing, X.X.; visualization, X.X.; supervision, X.X.; project administration, X.X.; funding acquisition, Y.Y. All authors have read and agreed to the published version of the manuscript.” Please turn to the CRediT taxonomy for the term explanation. Authorship must be limited to those who have contributed substantially to the work reported.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| Full Term |
Abbreviation |
| Extramedullary plasmacytoma |
EMP |
| Multiple myeloma |
MM |
| Solitary plasmacytoma of bone |
SPB |
| Radiotherapy |
RT |
| Chemotherapy |
CHT |
| Magnetic resonance imaging |
MRI |
| Computed tomography |
CT |
| Cluster of differentiation |
CD |
| Kappa light chains |
KLC |
| Lambda light chains |
LLC |
| Endoscopic nasal surgery |
ENS |
| Complete blood count |
CBC |
| Estimated glomerular filtration rate |
eGFR |
| Sinonasal extramedullary plasmacytoma |
SN-EMP |
References
- Schridde, H. Weitere Untersuchungen uber die kornelungen der Plasmazellen. Central-bl f allg Path Anat. 1905;16:433–5.
- Venkatesulu, B.; Mallick, S.; Giridhar, P.; Upadhyay, A.D.; Rath, G.K. Pattern of care and impact of prognostic factors on the outcome of head and neck extramedullary plasmacytoma: a systematic review and individual patient data analysis of 315 cases. Eur. Arch. Oto-Rhino-Laryngology 2017, 275, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Hamilos, G. Solitary bone plasmacytoma and extramedullary plasmacytoma. Curr. Treat. Options Oncol. 2002, 3, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Caers J, Paiva B, Zamagni E, Leleu X, Bladé J, Kristinsson SY, et al. Diagnosis, treatment, and response assessment in solitary plasmacytoma: updated recommendations from a European Expert Panel. J Hematol Oncol. 2018 Jan;11(1):10.
- Tanrivermis Sayit A, Elmali M, Gün S. Evaluation of Extramedullary Plasmacytoma of the Larynx with Radiologic and Histopathological Findings. Radiologia. 2020.
- Agarwal, A. Neuroimaging of Plasmacytoma. Neuroradiol. J. 2014, 27, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Gholizadeh, N.; Mehdipour, M.; Rohani, B.; Esmaeili, V. Extramedullary Plasmacytoma of the Oral Cavity in a Young Man: a Case Report. J Dent (Shiraz). 2016 Jun;17(2):155–8.
- Strojan, P.; Šoba, E.; Lamovec, J.; Munda, A. Extramedullary plasmacytoma: clinical and histopathologic study. Int. J. Radiat. Oncol. 2002, 53, 692–701. [Google Scholar] [CrossRef] [PubMed]
- Trojan S, Rika ES. EXTRAMEDULLARY PLASMACYTOMA : CLINICAL AND HISTOPATHOLOGIC STUDY ˇ OBA, M. D.,* J ANEZ L AMOVEC, M. D., P H. D., †. 2002;53(3):692–701.
- Aznab, M.; Khazaei, M. Multifocal Extramedullary and Multiple Solitary Bone Plasmacytoma: A Case Report and Review of the Literature. Int. J. Cancer Manag. 2019, 12. [Google Scholar] [CrossRef]
- Report, C. Multiple Solitary Plasmacytoma of Chest Wall and Scalp with Extramedullary Solitary Plasmacytoma of Orbit Occurring Simultaneously : A Rare Case Report. 2019. [Google Scholar]
- Ahnach, M.; Marouan, S.; Rachid, M.; Madani, A.; Quessar, A.; Benchekroun, S.; Quachouh, M. Extramedullary plasmocytoma relapsing at differents sites: an unusual presentation. Pan Afr. Med J. 2013, 2, 34. [Google Scholar] [CrossRef] [PubMed]
- Bachar, G.; Goldstein, D.; Brown, D.; Tsang, R.; Lockwood, G.; Perez-Ordonez, B.; Irish, J. Solitary extramedullary plasmacytoma of the head and neck—Long-term outcome analysis of 68 cases. Head Neck 2008, 30, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Tournier-Rangeard, L.; Lapeyre, M.; Graff-Caillaud, P.; Mege, A.; Dolivet, G.; Toussaint, B.; Charra-Brunaud, C.; Hoffstetter, S.; Marchal, C.; Peiffert, D. Radiotherapy for solitary extramedullary plasmacytoma in the head-and-neck region: A dose greater than 45 Gy to the target volume improves the local control. Int. J. Radiat. Oncol. 2006, 64, 1013–1017. [Google Scholar] [CrossRef] [PubMed]
- Wen, G.; Wang, W.; Zhang, Y.; Niu, S.; Li, Q.; Li, Y. Management of extramedullary plasmacytoma: Role of radiotherapy and prognostic factor analysis in 55 patients. Chin. J. Cancer Res. 2017, 29, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.J.; Azarpira, N.; Khademi, B.; Abedi, E.; Hakimzadeh, A.; Valibeigi, B. Extramedullary Plasmacytoma of the Nasal Cavity Report of Three Cases With Review of the Literature. Iran. Red Crescent Med J. 2013, 15, 363–6. [Google Scholar] [CrossRef] [PubMed]
- Cantone, E.; Di Lullo, A.M.; Marano, L.; Guadagno, E.; Mansueto, G.; Capriglione, P.; Catalano, L.; Iengo, M. Strategy for the treatment and follow-up of sinonasal solitary extramedullary plasmacytoma: a case series. J. Med Case Rep. 2017, 11, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Natt RS, O’Sullivan G. An extraosseous plasmacytoma of the nasopharynx. J Clin Med Res. 2009 Jun;1(2):121–2.
- Helman, S.N.; Filip, P.; Iacob, C.; Colley, P. Bilateral sinonasal extramedulary plasmacytoma treated with radiotherapy and a medial maxillectomy with a Denker's procedure. Am. J. Otolaryngol. 2017, 38, 360–362. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Kim, Y.Z.; Lee, E.H.; Kim, K.H. Endoscopic Endonasal Transsphenoidal Resection of Solitary Extramedullary Plasmacytoma in the Sphenoid Sinus with Destruction of Skull Base. J. Korean Neurosurg. Soc. 2009, 46, 156–60. [Google Scholar] [CrossRef] [PubMed]
- Report, C. Extramedullary plasmacytoma in the maxillary sinus. 2008;49(11):310–1.
- Attanasio, G.; Viccaro, M.; Barbaro, M.; De Seta, E.; Filipo, R. Extramedullary plasmacytoma of paranasal sinuses. A combined therapeutic strategy.. 2006, 26, 118–120. [Google Scholar]
- Shreif JA, Goumas PD, Mastronikolis N, Naxakis SS. Extramedullary plasmacytoma of the nasal cavity. Otolaryngology - Head and Neck Surgery [Internet]. 2001;124(1):119–20. Available from: https://www.sciencedirect.com/science/article/pii/S0194599801701714.
- Lomeo, P.E.; McDonald, J.E.; Finneman, J. ; Shoreline Extramedullary plasmacytoma of the nasal sinus cavities. Am. J. Otolaryngol. 2007, 28, 50–51. [Google Scholar] [CrossRef] [PubMed]
- Erkal, H.; Han, Ü.; Ulubay, H. Extramedullary plasmacytoma presenting as a large lesion protruding from the nasal cavity with massive hemorrhage. J. Neuroradiol. 2006, 33, 143–144. [Google Scholar] [CrossRef] [PubMed]
- Kalayoglu-Besisik, S.; Yonal, I.; Hindilerden, F.; Agan, M.; Sargin, D. Plasmacytoma of the Nasolacrimal Duct Simulating Dacryocystitis: An Uncommon Presentation for Extramedullary Relapse of Multiple Myeloma. Case Rep. Oncol. 2012, 5, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Ghazizadeh, M.; Amlashi, H.A.; Mehrparvar, G. Radioresistant Extramedullary Plasmacytoma of the Maxillary Sinus: A Case Report and review article. Iran J Otorhinolaryngol. 2015 Jul;27(81):313–8.
- Araújo R de P, Gomes EF, Menezes DB de, Ferreira LM de BM, Rios AS do N. Rare nasosinusal tumors: case series and literature review. Braz J Otorhinolaryngol. 2008;74(2):307–14.
- Pantazidou, G.; Papaioannou, I.; Karagkouni, E.; Fragkakis, I.; Korovessis, P. Sinonasal Extramedullary Plasmacytoma With Rare Osteolytic Lesions. Cureus 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Hazarika, P.; Balakrishnan, R.; Singh, R.; Pujary, K.; Aziz, B. Solitary Extramedullary Plasmacytoma of the Sinonasal Region. Indian J. Otolaryngol. Head Neck Surg. 2011, 63, 33–35. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Qi, X.; Wu, X.; Luo, C.; Lu, Y. Solitary Intracranial Plasmacytoma Located in the Spheno-Clival Region Mimicking Chordoma: A Case Report. J. Int. Med Res. 2010, 38, 1868–1875. [Google Scholar] [CrossRef] [PubMed]
- Meziane M, Boulaadas M, Essakalli L, Kzadri M, Harmouch A. Solitary plasmocytoma: ghost tumour? Int J Oral Maxillofac Surg. 2012 Jan;41(1):17–9.
- Çakir, E.; Karaarslan, G.; Usul, H.; Baykal, S.; Arslan, E. Solitary plasmacytoma with intracranial intraorbital and, paranasal sinus extension. J. Clin. Neurosci. 2003, 10, 266–268. [Google Scholar] [CrossRef] [PubMed]
- Alexiou, C.; Kau, R.J.; Dietzfelbinger, H.; Kremer, M.; Spiess, J.C.; Schratzenstaller, B.; Arnold, W. Extramedullary plasmacytoma: tumor occurrence and therapeutic concepts. Cancer. 1999, 85, 2305–14. [Google Scholar] [CrossRef]
- McQUISTON, R.J.; Jones, D.E. Extramedullary Plasmacytoma of the Nasal Cavity and Lateral Wall of the Nose. Arch. Otolaryngol. Neck Surg. 1959, 69, 457–458. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, K.S.; Khandelwal, N.; Virmani, V.; Das, A.; Panda, N. Solitary Extramedullary Plasmacytoma of the Nasal Tract: An Unusual Cause of Epistaxis. Ear, Nose Throat J. 2013, 92, E51–E54. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, S.B.; Desai, U.; Cheng, V.S. Extramedullary Plasmacytoma of the Head and Neck. Medicine 1982, 61, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Ching, A.S.-C.; Khoo, J.B.-K.; Chong, V.F.-H. CT and MR Imaging of Solitary Extramedullary Plasmacytoma of the Nasal Tract. 2002, 23, 1632–1636.
- Hu, H.; Hu, X.; Hu, G.; Li, D.; Cai, J. Diagnosis and management of extramedullary plasmacytoma in nasal cavity: Clinical experience and literature review. Medicine 2023, 102, e32647. [Google Scholar] [CrossRef] [PubMed]
- Ooi, G.C.; Chim, J.C.-S.; Au, W.-Y.; Khong, P.-L. Radiologic Manifestations of Primary Solitary Extramedullary and Multiple Solitary Plasmacytomas. Am. J. Roentgenol. 2006, 186, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Soutar R, Lucraft H, Jackson G, Reece A, Bird J, Low E, et al. Guidelines on the diagnosis and management of solitary plasmacytoma of bone and solitary extramedullary plasmacytoma. Br J Haematol. 2004 Mar;124(6):717–26.
- Galieni, P.; Cavo, M.; Pulsoni, A.; Avvisati, G.; Bigazzi, C.; Neri, S.; Caliceti, U.; Benni, M.; Ronconi, S.; Lauria, F. Clinical outcome of extramedullary plasmacytoma. Haematologica. 2000, 85, 47–51. [Google Scholar] [PubMed]
- Rajkumar, S.V.; Dimopoulos, M.A.; Palumbo, A.; Blade, J.; Merlini, G.; Mateos, M.-V.; Kumar, S.; Hillengass, J.; Kastritis, E.; Richardson, P.; et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014, 15, e538–e548. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S.; Jawad, S.; Kowa, X.-Y. Head and neck manifestations of extramedullary plasmacytomas and their differential diagnoses: a pictorial review. Br. J. Radiol. 2025, 98, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Raghuram, S.; Faizal, B.; Sanjeevan, K.; Eapen, M.; Nair, I.R.; Philip, A.; Pavithran, K. Recurrent extramedullary plasmacytomas without multiple myeloma: A case report with review of the literature. Cancer Treat. Res. Commun. 2022, 31, 100550. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.; Doig, A.; Soutar, R. Solitary plasmacytoma and multiple myeloma: adhesion molecule and chemokine receptor expression patterns. Br. J. Haematol. 2007, 137, 486–487. [Google Scholar] [CrossRef] [PubMed]
- Tojima, I.; Ogawa, T.; Kouzaki, H.; Seno, S.; Shibayama, M.; Shimizu, T. Endoscopic resection of malignant sinonasal tumours with or without chemotherapy and radiotherapy. J. Laryngol. Otol. 2012, 126, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Rawal RB, Farzal Z, Federspiel JJ, Sreenath SB, Thorp BD, Zanation AM. Endoscopic Resection of Sinonasal Malignancy: A Systematic Review and Meta-analysis. Otolaryngol Head Neck Surg. 2016 Sep;155(3):376–86.
- D'AGuillo, C.; Soni, R.S.; Gordhan, C.; Liu, J.K.; Baredes, S.; Eloy, J.A. Sinonasal extramedullary plasmacytoma: a systematic review of 175 patients. Int. Forum Allergy Rhinol. 2013, 4, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Mishra, U.P.; Verma, A.K.; Chaurasia, J.K. Solitary Extramedullary Plasmacytoma of Nasal Cavity. Indian J. Otolaryngol. Head Neck Surg. 2023, 75, 4060–4065. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, L.; Zhu, Y.; Diao, W.; Li, W.; Gao, Z.; Chen, X. Extramedullary Plasmacytoma: Long-Term Clinical Outcomes in a Single-Center in China and Literature Review. Ear, Nose Throat J. 2020, 100, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Cantone, E.; Borzillo, V.; Di Lullo, A.M.; Marano, L.; Guadagno, E.; Mansueto, G.; Di Franco, R.; Cammarota, F.; Catalano, L.; Muto, P.; et al. Cyberknife® system: a new therapeutic strategy for sinonasal solitary extramedullary plasmacytomae. J Biol Regul Homeost Agents. 2017, 31, 763–768. [Google Scholar] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).