1. Introduction
Regarding immunization strategies, mRNA-based vaccines can be regarded as a significant breakthrough in the ongoing efforts to protect against viral diseases. Traditional vaccines typically contain a live or attenuated virus, an inactivated pathogen, or fragments of the pathogen. In contrast, mRNA vaccines function by delivering into cells the genetic code required to produce a specific protein that elicits the immune response. This novel mechanism not only extended the timeline for vaccine development but also provided a sufficiently flexible platform to accommodate new pathogens [
1]. This potential was demonstrated when the combined mRNA that proved effective in combating COVID-19 was developed. At the core of this recent advancement is the multiple-step manufacturing process associated with the production of mRNA vaccines. These steps include: the synthesis of high-purity mRNA, the encapsulation of mRNA into a lipid nanoparticle (LNP) carrier system, and the mitigation of the likelihood of adverse events. Each step encompasses distinct technical, logistical, analytical, and practical dimensions: these facets must integrate biochemistry, molecular biology, engineering, and regulatory science [
2]. The fundamental working mechanism of mRNA vaccines is depicted in
Figure 1
The knowledge that has emerged from the development of mRNA vaccines should not be studied or retained solely within the context of COVID-19 but should be applied globally. mRNA can facilitate the development of vaccines for various infectious diseases, including influenza, Zika virus, HIV, and others. Additionally, its potential as an oncological treatment and as a platform for targeted therapy has been explored; concurrently, employing mRNA in patient-specific therapies will depend on individual patient profiles [
3]. Nonetheless, this potential can only be realized when numerous critical constraints in the manufacturing process are addressed and resolved. Other noted advantages of mRNA technology include the capacity to rapidly produce booster doses, particularly during global emergencies [
4]. However, new challenges arise when devising solutions to satisfy the increasing global demand for a product already on the market, such as the availability of advanced equipment, adequate raw materials, and a sufficiently skilled workforce. Commending what may present difficulties includes ensuring a high-quality and safe product, and simultaneously, one that fully adheres to the manufacturing standards stipulated by regulatory authorities [
5].
Some avenues for addressing these issues include implementing intelligent manufacturing, continuous manufacturing, and artificial intelligence. These innovations may contribute to developing more advanced mRNA vaccine production facilities by enhancing and optimizing processes for efficiency, quality, and economic viability [
6]. The principal factor is that mRNA vaccines can meet public health needs and potentially transform numerous medical specialties [
7]. However, for serialization to be effectively achieved, it is imperative to recognize that resolving the technical, logistical, and regulatory challenges related to the distribution and manufacturing of these products is essential. A broad spectrum of challenges continues to hinder mRNA vaccine development, ranging from production to delivery logistics as shown in
Figure 2.
Therefore, this review seeks to underscore these challenges and elucidate control measures for managing the complex dynamics associated with mRNA vaccines.
2. Inhibition and Isolation of BPS mRNA Synthesis
The first processes after mRNA creation are mRNA synthesis and mRNA purification. Each one of them has several technical and logistical issues. All of these questions should be solved when creating vaccines that use mRNA. An application of an application in the in vitro transcription process is to generate a clean and constant mRNA molecule by replicating the genetic message of the DNA template. This step involves critical optimization on some reaction conditions to obtain high yields of the desired functional mRNA, but with minimal interferences from short RNA bands and uncapped RNA [
8]. Slight differences in synthesis can radically change the activity and toxicity characteristics of the resulting substance. Acquiring the right DNA template is a limitation, as it involves RNA synthesis using T7 RNA polymerase. These reagents are costly, and all their activity has to be preserved – they become easily contaminated. As to the complete RNA, the IVT reaction to the following high yield is relatively sensitive for controlling and monitoring, as it is the process that requires strong skills to operate [
9]. In essence, other characteristics, including magnesium ions, nucleotide, and temperature, are significant and quantified for in vitro transcription. Once RNA is synthesized, it gets capped to add a 5’ capping required for the cell's lifespan and translation of the mRNA. The challenge of the technical problem is that if this cap structure is not perfect, the partially spine-capped mRNA may be less efficient in terms of functionality or may cause adverse immune reactions [
10].
The other crucial step is purification, where the effects, including nucleotides, enzymes, double-stranded RNA, and other successive synthesis interferences, are abated. Other typical chromatography methodologies are ion exchange chromatography or size exclusion chromatography, but such processes are easier said when it comes to questions of scalability. Both must be optimized to get a linear, high-yield mRNA of high purity simultaneously, as its sequence is relatively sensitive and cannot be degraded easily [
11]. These difficulties increase with translating so much mRNA, especially during a pandemic. Some techniques that are useful for bioactive compounds’ purification on a small scale may be scaled up; hence, they may not be effective on a large scale. In addition, high cost per unit and easy availability of high-grade reagents and consumables are other stiff hurdles [
12]. Refining these problems in the synthesis of mRNA and the subsequent purification is crucial for the further potential development of vaccine safety and efficacy, and the approach’s scalability. The above challenges could be addressed through optimization of enzymatic reactions and improvements in the biochemical purification processes, as well as by developing improved production methods [
13].
3. Formulation and Stability: Overcoming Delivery Hurdles
Two questions are most often asked concerning the formulation, development, and manufacturing of the mRNA vaccines: stability. As mentioned above, another step in translating the mRNA sequence into functional immunogenic molecules used for humans is applying the synthetically produced mRNA into human cells. However, mRNAs are relatively unstable because they are prone to degradation by nucleases and changes in temperature and other conditions [
14]. However, another development challenge is manufacturing high-quality mRNA, synthesizing, purifying, and encapsulating it in a formulation that protects mRNA from degradation while improving its bio distribution. As such, the main issue with the mRNA vaccines is what form the mRNA takes when encapsulated in the LNPs. Several of the LNPs employed for delivery additionally enable the uptake of the mRNA into host cells and enhance the protection of the mRNA from degradation. Structural and fundamental forms of LNPs related to the encapsulation of mRNAs and the release of the same to molecules in the body are well represented by their structures and architecture [
15]. The LNPs need to be small enough to ensure an early immune response cannot occur and neutralize the mRNA before it is internalized. The enhancement of the characteristics of the material under study is an urgent problem and is still topical at the present stage.
The nature of lipid components incorporated in the nanoparticles includes cationic lipids, phospholipids, cholesterol, and PEGylated lipids, and the right concentration of these components needs to be achieved to cause stability and function [
16]. The association of LNP has to be expandable to produce large batches at the same rate of equal convection and parity. Some challenges are noted in the formulation stage, with the stability of the mRNA in the LNPs. The mRNA-LNP complex must be stable at different temperatures for longer because the transporting and storage conditions might not be ideal. This problem becomes essential in the COVID-19 pandemic when developing mRNA vaccines stored at low temperatures of -70 °C [
17]. This was especially so where distribution and access presented implementation or practical challenges in delivering treatment in a low-resource context. In light of these challenges, researchers are always looking for ways to increase the thermal stability of mRNA-LNP formulation. This includes cryoprotectants, the ratio of the mRNA to lipid in the nanoparticle formulation, and other delivery systems, including polymers or lipids other than those in the current formulation [
18]. The idea is to generate mRNA vaccines that require comparatively less stringent storage conditions since this would overcome some of the complexities of distribution worldwide, or at least the complex distribution to remote regions or rural areas.
Moreover, there is a need to generate stable mRNA vaccines capable of having a stable conformation to uphold resistance every time there is a change in humidity, temperature, and light exposure- the main aspects in stability analysis. The studies involve estimation of the shelf-life of the vaccines and additionally furnish details concerning storage conditions and other treatments that the product requires [
19]. The FDA and the EMA, among other regulatory agencies, have put down substantial regulations for stability testing, and they must comply with them when giving out a product. The issue of changing the specific mRNA sequence and the stability of the vaccines is connected with the molecule, as well as with its delivery system. It is applied to introduce it into the cells [
20]. Overcoming them entails refining the aspects of lipid formulation; nanoparticle stability during storage and transportation, and other issues associated with scale-out and distribution. The concept of additional development will remain focused on the idea of mRNA vaccines for responding to different global problems of infectious diseases and other diseases; a significant problem arises regarding formulation and stability regarding the large-scale usage of vaccines [
21].
4. Implementing Measures to Enhance Supply in Response to Global Market Expansion
There are numerous problems regarding increasing the production of mRNA vaccines to meet the needs and address the demand around the world. Although the mRNA vaccines contain traits that can be easily manipulated and are effective in formulation, developing mRNA requires a substantial resource commitment in its production. This manufacturing transition from the small scale production as that which is done in the laboratory or pilot scale to the industrial or commercial scale production is a factor that needs to be taken into consideration if there will be any possibility of producing these mRNA vaccines in significant qualities to meet the global demand as was evidenced by the COVID 19 pandemic [
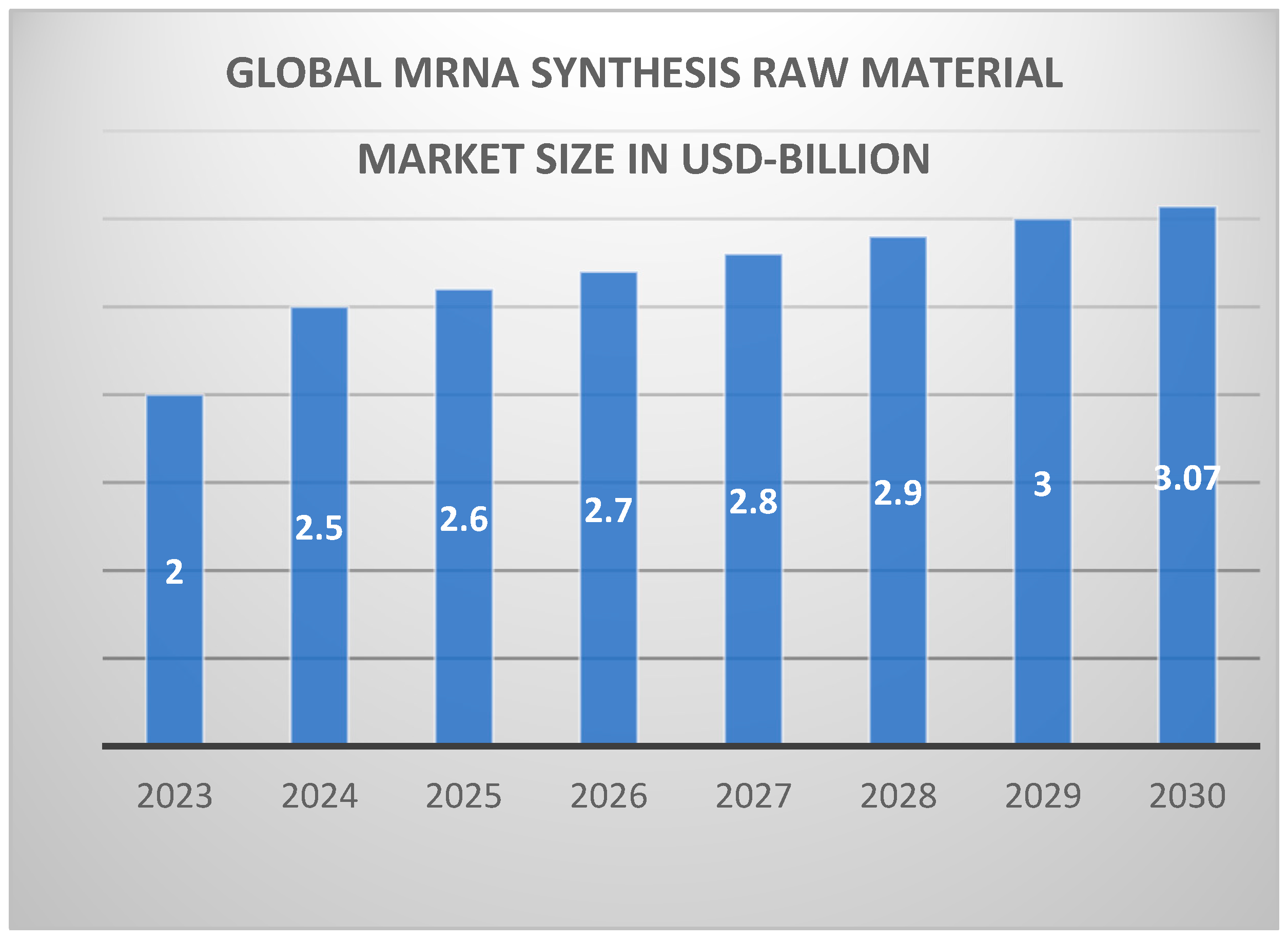
22]. The first difficulty manufacturers experience when moving to this scale is the need for large-scale equipment and infrastructure. mRNA synthesis demands large bioreactors, strict temperature and pH regulation, and large-scale IVT equipment. The increasing demand for critical raw materials further underscores the need for resilient global supply chains as shown in
Figure 3. Concerning the requirements for reproducing equipment for high-volume production, the issue of synthesizing large volumes of reagents, buffers, and raw materials must be addressed. Moreover, such equipment must be versatile to support any mRNA vaccine because different products may need different synthesis procedures. Several considerations must be taken to achieve the desired yield from the reaction level scaling from milliliters to hundred-liter levels [
23].
Another problem organizations encounter when planning to increase productivity is the unavailability of raw materials. The production of mRNA vaccines entails more subordinate materials, including nucleotides, plasmid DNA, and enzymes, which are known to be in limited supply. For instance, in the case of plasmid DNA utilized as the transcription template, only purified DNA of some recommended quality characteristics should be used [
24]. Neglecting these materials' procurement also affects the production timelines and, in the long run, the prices skyrocket. Geographically, the sources of these raw materials are global; hence, a hitch anywhere in the world distorts the production of vaccines. To produce and operate machinery in large quantities of a given product, it is necessary to ensure adequate stocks of these materials. Loss of jobs and lack of skills additionally present other core challenges to increasing the production of mRNA vaccines [
25]. This is attributable to several reasons. Firstly, those who write the products are well-trained individuals who have studied molecular biology, biochemistry, and engineering in manufacturing. This has been another major setback occasioned by the rapid increase in the production of mRNA vaccines that have inadequate human capital. To overcome this problem, more funds should be invested first of all in training new personnel, both by companies and governments. Substitute technology: They additionally assist in managing the scarcity of skilled and unskilled workers, as it offers a chance to eliminate several instances of manual labor regarding continuous, mechanical, patterned processes [
26].
Another operational factor, which has become more paramount as manufacturers move from original production to mass production, is the quality of the mRNA vaccines. However, when the production takes on more volumes, the variability of the quality of the product additionally rises. It becomes challenging to ensure that every batch that has been manufactured has fulfilled the criterion, and doing so at a large scale is almost impossible [
27]. Here, it is mandatory to establish high levels of quality assurance and simultaneously use analytical chemistry applications to identify possible contamination and, secondly, to decide the stability of the mRNA molecules and other formulation characteristics. Preliminary real-time monitors, including PAT and other sophisticated monitoring apparatus, assist in elevating an organization’s possibilities for identifying issues that may emerge in the initial stages of manufacturing. Furthermore, manufacturing capacity must be relevant to respond to needs in the global market within a short time [
28]. For instance, when COVID-19 erupted, mRNA vaccines had the noble task of manufacturing billions of doses quickly. Whereas through AM, the volatile demand, notwithstanding, has been met adequately, production in the traditional manufacturing setting never could have coped with such a spillover of demand due to flow turbulence. To avoid such pitfalls in the future, manufacturers are investing heavily in the development and erection of modular and flexible plants that could easily be adapted to produce any vaccines as deemed necessary due to the threat posed by any disease [
29].
Others are regulations, while increasing the production of mRNA vaccines to meet the demand from another challenge that must be met. At this point, there were nods with acceptance that vaccine manufacturers have specific regulatory necessities offered by agencies, including the U.S FDA and EMA [
30]. When production increases, it becomes tough to stick to such regulations later. Each facility needs an inspection, and numerous records must be provided to support that the manufacturing process complies with the health authority regarding safety, effectiveness, and quality. Of course, such rules still help to minimize risks connected to the activities of the technological companies. Still, such restrictions bring some restrictions to the ramping-up process in the new markets and niche [
31]. Moving from producing central mRNA vaccines means that issues concerning infrastructure, materials and components, human resources and workforce cadre, dimensional requirements, quality control, and compliance with global market regulations become pertinent. These challenges are, nevertheless, not far from being tackled [
32]. Thus, further bolstering research in technology and workforce in the creation of mRNA vaccines, it is relatively easy to come up with a larger number of doses for any vaccine, and at an affordable cost to many. These advantages of the rapidly developed and delivered COVID-19 vaccines should be retained for the next public health emergency and to expand the usage of mRNA in worldwide medical services [
33].
5. Navigating Regulatory Complexities and Supply Chain Limitations in the Advancement of mRNA Vaccines
Two of the challenges associated with developing and deploying mRNA vaccines are regulatory and supply-chain related. These difficulties are further compounded by the need to respond rapidly during crises with appropriate products and by the inherent complexity of the emerging mRNA technology. A critical balance in meeting all regulatory requirements for mRNA vaccines lies in accomplishing this without disrupting the supply chain, which is crucial in ensuring the global distribution of vaccines [
34]. The initial obstacle in synthesizing mRNA vaccines is the regulatory landscape, which is subject to active updates and varies according to different authorities, beginning with national bodies such as the FDA and EMA and extending to international organizations such as the WHO. These agencies dedicate time and resources to ensuring that vaccines are safe, effective, and produced using high-quality materials and processes. Nevertheless, the mRNA platform remains relatively novel within the global pharmaceutical market, and for an extended period, such products had not been developed [
35].
This usually includes pre-clinical studies, clinical trials, and submission of timelines and other miscellaneous documents showing the vaccine's safety, efficacy, and quality. There are additional complexities for mRNA vaccines, including ensuring that the encapsulated lipid nanoparticles (LNPs) are not toxic and do not provoke adverse immune responses [
36]. These actions could slow the development of the production procedures related to the vaccines and their distribution, especially where the uptake is fast. Although the requests for more rapid approval mechanisms like the EUAs during calamities are as valuable as they are, they present unknowns and additional burden to the manufacturers [
37]. Moreover, the standards differ from country to country; therefore, a product approved to be produced and manufactured in a particular region may take a lot of time or skip the approval process altogether in another area. These problems would be solved to the extent that distilling regulatory standards, besides coming up with international mechanisms for approval of vaccines, is an outstanding achievement [
38]. First of all, it is rather intricate to organize the supply chain of mRNA vaccines per se, as it speaks of the factors needed for production, equipment that is necessarily different, and other aspects concerning the transportation of the vaccines. Creating the mRNA vaccines, especially the synthesis of the vaccines, involves using high-purity materials that are nucleotides, enzymes, plasmid DNA, and lipids for nanoparticle construction [
39]. Such significant inputs can be complicated to source or delay delivery, a situation that is experienced especially when there is an influx of orders, as was witnessed with the COVID-19 vaccines. Moreover, the distribution of mRNA vaccines is also an issue in some way. For example, the Pfizer-BioNTech vaccines must be stored and transported at extremely low temperatures, limiting distribution or the cold chain, especially in low-income or specific locations with limited facilities and transportation. This implies that an appropriate supply system should exist so that vaccines do not expire during transit or while waiting for the right circumstances to establish storage conditions [
40].
Considerations, including political and/or economic factors, including restrictions in trade, geopolitics, and even available biological transport, should be taken to eliminate any factors causing delays. For instance, restrictions in movement like factory lockdowns, closure of borders, and limitations in the number of spaces available to ship vaccines during the COVID-19 pandemic [
41]. This paper notes that one of the major concerns is how regulatory hurdles and supply chain factors impair the punctual and optimal manufacturing and distribution of mRNA vaccines. Organizing efforts to simplify regulation, a robust supply chain, and building relationships are necessary to address those challenges. Eliminating these bottlenecks enables more people to be vaccinated immediately, with mRNA vaccines being a viable shield against global health challenges [
42]. Furthermore
5. Future Directions Towards Innovations and Strategic Solutions
The future of manufacturing mRNA vaccines is bright, as constant research is being conducted, and strategic solutions to existing problems are being perfected. While the mRNA applied for COVID-19 vaccine development is still under development, the breakthrough has many more implications for the future in creating vaccines for various types of viral infections and treatments for cancer, genetic diseases, and other diseases. The way ahead is steeped in significant challenges concerning production improvement and scale-up, compliance and registration, and delivery across the international environment [
43]. There are many opportunities to increase the speed of mRNA vaccine production, and one of them is the application of new technologies like automation, artificial intelligence, and continuous manufacturing. It has also started removing human operators' dependence, the possibility of errors, deviations from standard, and slower work rates. Some steps that can be readily automated include Quality assurance, mRNA synthesis, and packaging within LNPs. Automation also minimizes labor use and hastens the increase in production capabilities to ensure that the international immunization program's needs can be met [
44].
One unused concept innovation area is another area of continuous manufacturing. It is pretty different from the standard batch production type, which prolongs the duration of mRNA vaccine production and increases the production cost. This approach has a continuous and smooth flow of materials and the processes involved, and there is real-time control and optimization, which makes it efficient and consistently delivers excellent quality products. With continuous manufacturing, companies can quickly supply more mRNA vaccines while achieving increased purity and utilizing fewer resources [
45]. This could bring about a massive change in how vaccines will be manufactured and administered, most probably during pandemics. Similarly, AI and machine learning are likely to help improve the efficiency of mRNA vaccine production. These technologies can be applied in forecasting and enhancing production processes, as well as in determining the critical flow rates and/or designing improved mRNA sequences or lipid nanoparticle formulations. They could additionally use machine learning to identify likely issues in the production process and recommend changes that would enhance yield, quality, and reliability, thereby speeding up vaccine manufacture [
46].
Logistical challenges will remain a crucial consideration integral to the long-term success of vaccination with mRNA vaccines; stability and formulation of mRNA vaccines will be critical. Another is increasing the thermal stability of an mRNA-LNP formulation. Still, more work is being done to develop new stabilizers and lipid formulations that could encapsulate the mRNA to sustain the heat shock while keeping it viable at more moderate temperatures of storage and transport [
47]. For example, improvements in cryoprotants, polymers, and lipid content can increase the temperature of storage of mRNA vaccines to conditions other than the ultra-low temperatures currently needed, thereby helping to remove a significant barrier in getting vaccines to developing countries and areas with less sophisticated infrastructures and transportation. Also, new technologies, including dry formulations that will eliminate the need for a cold chain, could facilitate the expansion of mRNA worldwide. Under these types of dry formulations, several costs associated with maintaining the cold chain would be significantly cut, including the Hardship related to administering vaccines to remote regions where ultra-cold facilities are scarce would additionally be solved [
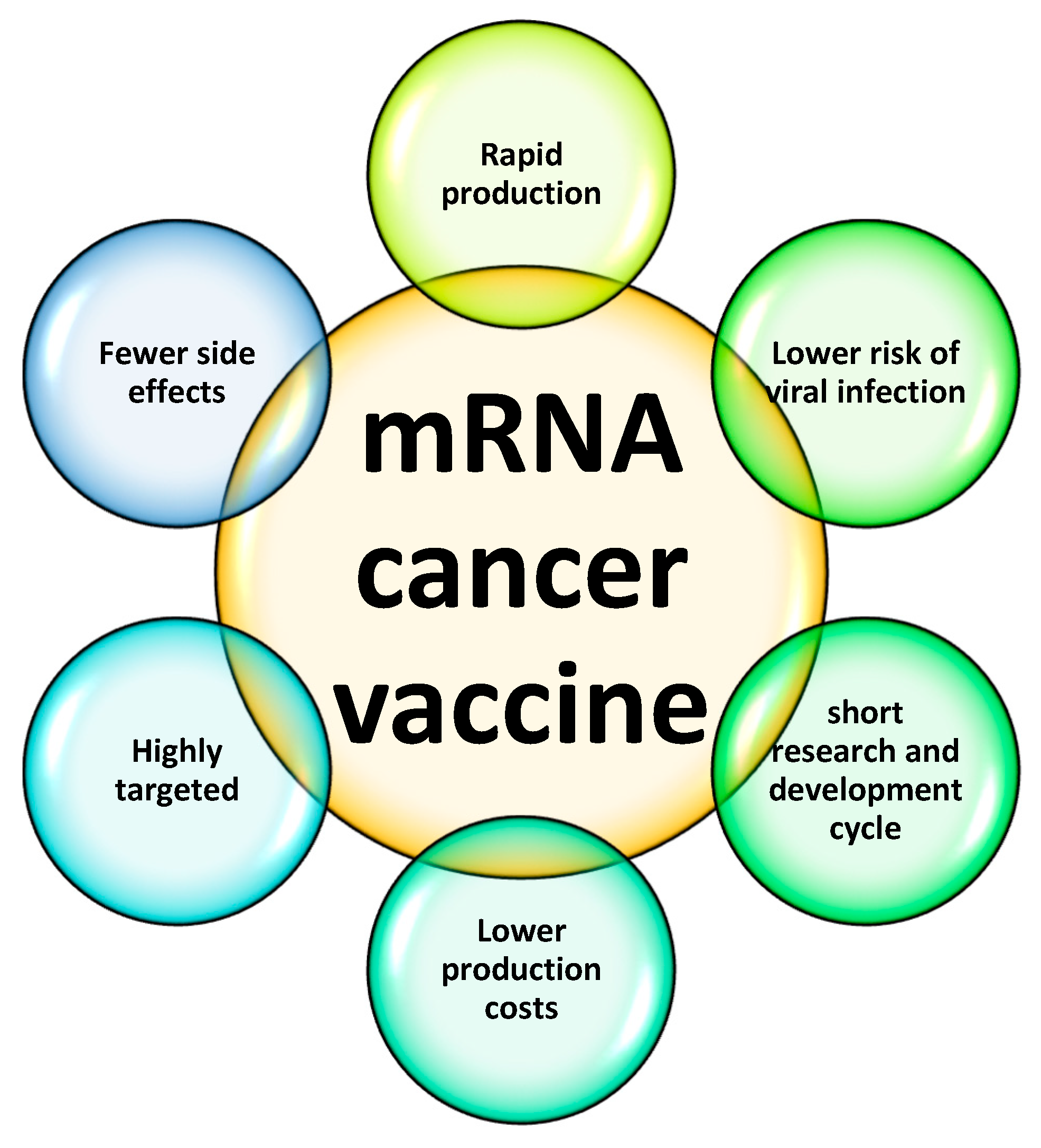
48]. Beyond infectious diseases, mRNA vaccines are being explored for oncological applications, as illustrated in the emerging therapeutic frontiers as shown in
Figure 4.
The COVID-19 mRNA vaccines must additionally be scaled up and distributed worldwide; this is why supply chain development is needed. One is expected to center the construction of more global production facilities, but it is limited to specific regions, particularly the LMICs. Such facilities may lessen the degree of reliance on large manufacturing centers and might additionally increase equality in terms of dose distribution [
49]. Moreover, such production sites would decrease transportation and storage expenses, solve the problem of supply chain constriction, and avoid the consequences of geopolitical instability. Government collaboration with other governments or international agencies and other companies could assist in supporting these localized producing factories, whereby vaccines are manufactured in areas of need. The regional production centers' development would also result in establishing more robust and adaptive supply chains that can quickly respond to newly realized health threats [
50].
It is even going to be more of a challenge as more brands of vaccines are being developed, especially the mRNA vaccines. Therefore, regulatory systems must be worked out and standardized across the globe. Although the mRNA vaccine technology has been approved faster for COVID-19, people may not always benefit from the fast-track approval pathways for other diseases or future health emergencies [
51]. Based on the current analysis, future regulators may want to adopt more liberal arrangements because the current rigid regulatory methodologies slow down the approval processes where mRNA technology is involved. These measures would be beneficial in avoiding the many pitfalls that are observed individually on a regional level each time an approval is sought for a single vaccine [
52]. A harmonization of safety and efficacy measures could be provided through cooperation with international organizations, including the World Health Organization WHO, enabling manufacturers to face fewer difficulties while going through the bodies. If the approvals were aligned better and international centers were more coordinated, the vaccines would be manufactured and delivered faster in times of need [
53].
Besides the vaccines, mRNA technology can potentially cure many other diseases, including cancer, genetic diseases, and autoimmune diseases. According to researchers, the development of protein therapeutics, gene editing tools, and personalized cancer vaccines is now possible with the help of mRNA. Designing and synthesizing mRNA is relatively easy and quick, making the technology convenient to tailor treatments [
54]. The versatility of switching between targets and promptly adapting to new health challenges makes mRNA an innovative tool for today’s medicine. The future of the manufacturing of mRNA vaccines is promising due to successive extraordinary technological advancements, the enhancement of different formulations, and efficient, effective, and smooth regulatory mechanisms to make life-saving vaccines accessible across the globe. Technological productivity will open new horizons in automation and continuous manufacturing with the help of artificial intelligence. At the same time, developments in the vaccine, their stability, and formulation will neutralize the main problems connected with distribution [
55]. The global academic landscape around mRNA vaccines has evolved rapidly, as seen in the keyword co-occurrence network generated from recent literature as shown in
Figure 5. Local production, better management of the supply chain, and better coordination of regulatory procedures will help ensure that mRNA vaccines are available to as many people around the globe as possible. With the mRNA platform evolving as a discipline, this technology is poised to deal with global health crises and reshape modern medicine.
Conclusions
Getting mRNA vaccines off the ground has provided vaccine scientists with unprecedented means to produce low-cost and highly effective vaccines to address global infectious diseases within a shorter timeframe. However, the transition from laboratory-scale production to the worldwide distribution of this technology has encountered numerous challenges and constraints that must be addressed to realize the platform's full potential. This review has concentrated on mRNA synthesis and purification, mRNA formulation, scale-up, and other regulatory and supply chain challenges essential for producing mRNA vaccines safely, effectively, and at scale. Synthesizing and purifying mRNA is notably sensitive and requires precise and high-quality reagents. These processes demand careful optimization to ensure high yields and product purity while minimizing contamination risks. Additionally, the concept of mRNA itself—particularly the encapsulation of mRNA into lipid nanoparticles—presents unique challenges associated with stability and delivery. Lipid nanoparticle technology must continue to evolve rapidly, particularly as thermal stability remains a concern, offering opportunities to overcome these barriers and make vaccines more storable and transportable.
Perhaps the most significant challenge is the ability to scale up production to meet international demand, which necessitates substantial investment in machinery and equipment, reliable sourcing of raw materials, and the development of a skilled workforce, given the capital-intensive nature of production. The COVID-19 pandemic has demonstrated that conventional, rigid industrial systems must be restructured to become more adaptable and that steady advances such as automation, continuous production, and real-time monitoring significantly reduce bottlenecks. Likewise, securing the availability of raw materials and diversifying supply chains will be instrumental in enhancing vaccine production. Further complexity arises from regulatory and supply chain issues. For this reason, regulatory agencies must adapt; mRNA vaccines represent a novel platform and warrant streamlined approval processes to expedite patient access to these therapeutic innovations. Concurrently, it is imperative to bolster global distribution capacities, operating continuously in a connected world, while minimizing geopolitical and other barriers to ensure equitable access to mRNA vaccines worldwide. The continued advancement of mRNA technology—which has so far shown early promise in combating infectious diseases—is anticipated to be profoundly transformative. Emerging improvements in the manufacturing, formulation, and regulatory processes for mRNA vaccines and therapies will strengthen existing supply chains. When these challenges are addressed and international collaboration is fostered, the world will usher in a new medical era, leveraging mRNA technology to combat future threats to global health more effectively.