1. Introduction
Cutting and forming tools as well as machine components as thrust bearings and fasteners, operating in an abrasive medium, undergo degradation after few years. This degradation is attributed to severe environmental conditions where corrosion, high-temperature oxidation and wear are concerned [
1]. One of the approaches for overcoming of this problem is deposition of wear and corrosion resistant coatings for enhancing performance, reliability, safe and lifetime of the tools and components as well friction decreases [
2].
Vanadium nitride (VN) coatings have increased attention due to their excellent mechanical, high melting point, good chemical resistance, good electrical conductivity, low friction coefficient and good wear resistance. Vanadium nitride coatings are widely utilized for applications in different fields such as: microelectronics, decorative and protective coatings, superconductors, electrical systems for hybrid vehicles load levelling battery during start up, acceleration and braking, memory storage systems and digital telecommunications systems [
3,
4].
Various Phase Vapour deposition (PVD) and Chemical vapour deposition (CVD) techniques have been used for vanadium nitride films deposition. The authors in Ref. [
5] have evaluated the influence of the thickness and nitrogen percentage on the structure, mechanical and tribological properties of VN coatings, deposited on Si and XC100 steel substrates by reactive magnetron sputtering technique. The results showed a polycrystalline structure of the deposited VN films. The increasing of the thickness led to the enhancement of the mechanical and tribological properties of VN coatings. The vanadium nitride coatings under 20 % nitrogen exhibited higher hardness (26 GPa) compared to the films under 10 % nitrogen (24 GPa). The electrochemical behaviour of the reactive magnetron-sputtered VN coatings was determined as a function of Si substrates bias voltage between 0 V and – 150 V [
6]. It was found out the VN films grown at a bias voltage of −150 V revealed greater corrosion rate and hardness. Unbalanced magnetron sputtering method was applied for hard VN coatings production to investigate the correlation between the preferred orientation and the fracture toughness. It was observed the higher fracture toughness corresponds to the coating with (200) texture [
7]. Other scientists have fabricated VN films by pulsed laser deposition (PLD) technique at room temperature and 500 °C to investigate the effect of the temperature on the thickness of the obtained VN coatings. The results indicated that the thickness of the VN coatings is improved linearly with the temperature [
8]. The investigators in Reference [
9] have exhibited the possibility for VN films formation applying cathodic arc evaporation method. The experiments were carried out by nitrogen pressure from 0.001 Pa to 3 Pa and a substrate negative bias voltage from 50 to 300 V. It was observed that the increase in nitrogen pressure and a bias voltage correspond to a high hardness (37 GPa) and a good adhesion to the substrate as well improved wear resistance of the VN coatings. Other way for production of vanadium nitride coatings is using inductively coupled plasma (ICP) assisted sputtering at different powers. Increasing ICP power, the coating microstructure changed from a porous columnar structure to a dense one. The highest hardness (28.2 GPa) and the smoothest surface (1.7 nm) were evaluated for the coatings deposited at 200 W[
10]. Chemical vapour deposition in-situ reactive deposition technique was used for VN films preparation on T12 steel substrates. The results showed a presence of dispersed uneven and abrasive micro-voids on the VN coatings surface. It was found out vanadium nitride coatings have a thickness of about 20 µm and grain size of 34.36 nm [
11]. The most common investigated properties of VN films are hardness, elastic modulus and structure at different conditions deposition. It was found out a columnar structure. Also, with the increasing nitrogen flow rate, two phases –V
2N and VN in the coatings were observed, the preferred orientation was (111) and the mechanical properties were enhanced. It has been reported the correlation between friction coefficient and the thickness of the coatings. Thicker VN films (2500 nm) have the lowest friction coefficient of 0.4 [
12,
13,
14,
15]. The researchers in [
16] have investigated the influence of nitrogen partial pressure between and substrate negative bias voltage on the hardness of VN coatings obtained by magnetron sputtering. The results exhibited higher hardness (23.3 GPa) at a bias voltage of – 200 V and a partial pressure of 0.23 Pa. Gueddaoui et al. have prepared polycrystalline vanadium nitrides films on Si substrates at nitrogen flow varying between 0 and 15 sccm by DC planar magnetron sputtering. It was understood at low nitrogen (below 4 sccm) the crystalline phase b-V
2N
1-x was appeared and over this value of nitrogen flow the crystalline phase δ – VN
1-x was existed [
17]. Mechanical and tribological performance of VN films, deposited by arc-evaporation method, depending on the surface defects were studied. The results showed a significant negative influence on tribological behaviour of the VN coatings [
18]. The coatings on based on VN were grown at various angles deposition [
19]. The results exhibited lower growth rate with raising tilt angle. Vanadium nitride coatings were produced by high power impulse magnetron sputtering (HIPIMS) and direct current magnetron sputtering technique on SiO
2 substrates. It was found out higher density and smoother surface of the VN coatings deposited by HIPIMS compared to DC magnetron sputtered VN coatings [
20]. The authors in Reference [
21] have evaluated the possibility for enhancing the wear resistance of the vanadium nitride coatings by adding of Si low quantity into the material. It was found out a significant improvement in hardness and tribological properties of the VN coating deposited by magnetron sputtering technique.
It is obvious VN films are produced by different techniques on various substrates. One of the most common method for VN layers deposition is direct current magnetron sputtering due to use of non-toxic working gases, a high degree of smoothness, uniformity and density of the deposited coatings. On the other hand, the VN films deposition on 304 L stainless steel substrates at various technological parameters has not been investigated in details. For this purpose, we deposited VN films on 304 L stainless steel substrates at a temperature of 250 oC, 300 oC and 350 oC by DC magnetron sputtering technique. Our study aims to investigate the effect of the temperature on the structure, morphology, mechanical and corrosion properties of the VN coatings, deposited on 304 L stainless steel substrates.
2. Materials and Methods
2.1. VN Films Deposition and Applied Technology
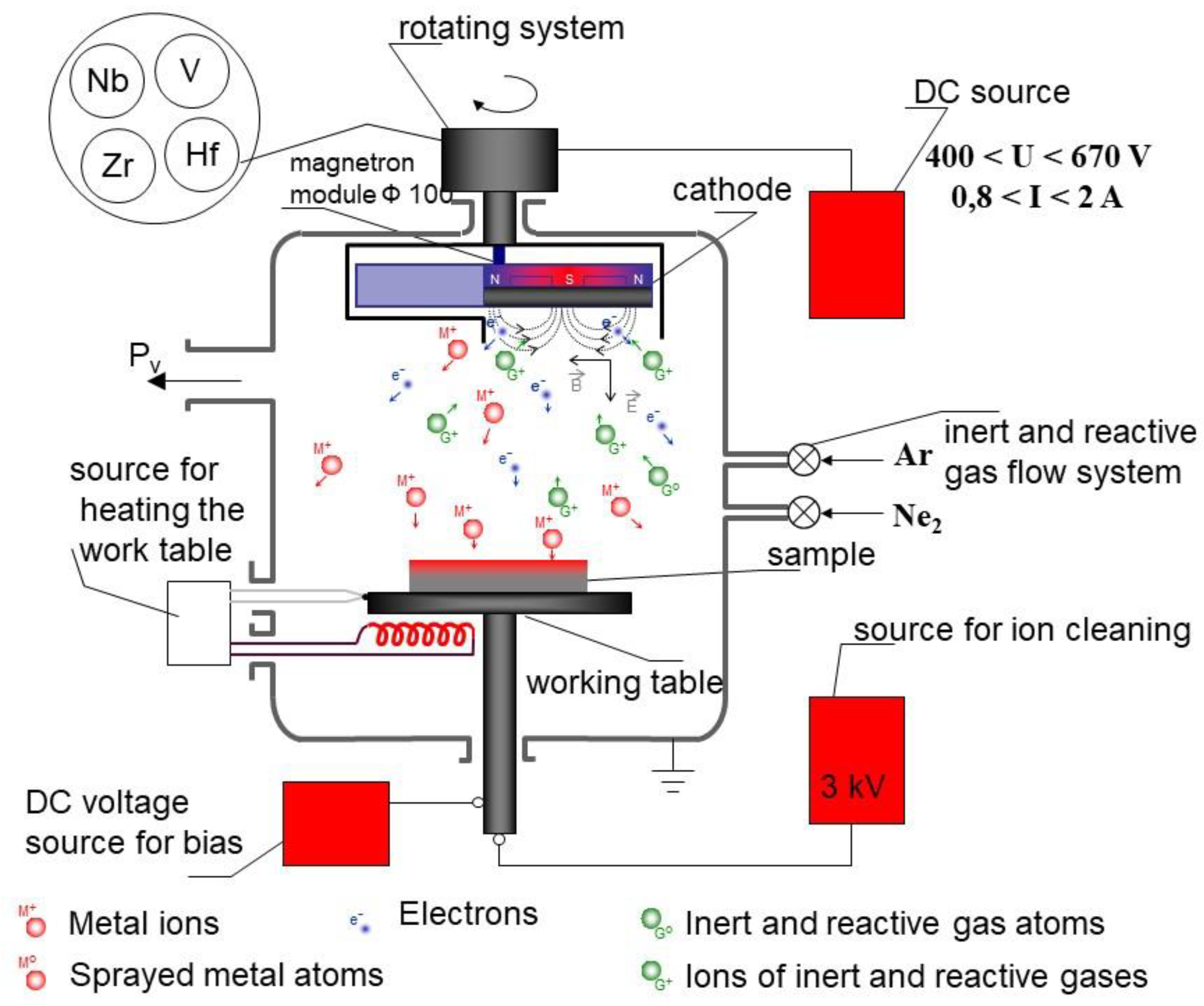
The VN coatings were deposited on 304 L stainless steel substrates with the following composition: 0.029 wt.% C; 0.3 wt.% Si; 1.6 wt% Mn; 0.026 wt.% P; 0.001 wt.% S; 0.065 wt.% N; 18.06 wt.% Cr and 8.0 wt.% Ni. The experiments were carried out at temperatures of 250 oC, 300 oC and 350 oC by direct current magnetron sputtering (DCMS). The substrates were polished mechanically and then being loaded into the deposition chamber. The diameter of the sputtered target was 100 mm as the purity of V was 99.8%. The technological regime for VN films deposition includes three main processes:
-
Cathode cleaning – This is a process of high energetic ion bombardment at which 304 L stainless steel substrates were etched by Ar positive ions for 10 minutes at a bias voltage of 1050 V to remove surface contaminations, including the oxide layers from the substrates surface. This process is performed at the following technological conditions:
A working pressure (the working gas is Ar) — before the deposition process, the vacuum chamber was evacuated to a base pressure of PAr = 8 Pa;
A discharge voltage U = 900 V;
A discharge current I = 0.1 A;
A temperature t◦ = 270 ◦C;
A cleaning time t = 10 min.
-
Deposition of intermediate pure V layer in order to improve the adhesion between the coatings and the substrate. This process is performed at the following conditions:
A working pressure PAr = 9 × 10−2 Pa;
A discharge voltage U = 460 V;
A discharge current I = 1 A;
A temperature t◦ = 250 ◦C;
Deposition time t = 3 min.
-
Deposition process of VN coatings – The production process took place in Ar-N2 atmosphere and the substrates bias voltage – 50 V.
A nitrogen pressure PAr = 2.4 × 10−2 Pa;
Pressure ratio between reactive and inert gas PN2/PAr = 2.3;
A voltage U = 575 V;
Substrates temperatures — vanadium nitride coatings were deposited at a temperature of 250 oC, 300 oC and 350 oC.
A constant current I = 1 A;
A deposition time t= 30 min.
The principle scheme of the used vacuum equipment „TITAN 22” for nitride coatings deposition is given in
Figure 1.
2.2. XRD Analysis
X-ray diffraction method was assessed to evaluate the crystalline structure of the experiments of the samples were performed by Empyrean system (Malvern Panalytical) equipped with parabolic X-ray mirror, parallel plate collimator and scintillation detector with Cu Kα radiation. The experiments were performed at 2θ from 30 to 85° with a step size of 0.05° and scan step time of 1.5 s. Phase identification was carried out using ICDD (International Centre for Date Diffraction) database to determine the phases of the films.
2.3. XPS Analysis
X-ray photoelectron spectra (XPS) studies were performed in a VG ESCALAB II electron spectrometer using AlKα radiation with an energy of 1486.6 eV. The binding energies (BE) were determined with an accuracy of ±0.1 eV. The changes in composition and chemical surrounding in the depth (5-10nm) of the films were determined on the basis of the areas and binding energies of C1s, O1s, N1s and V2p photoelectron peaks (after Shirley-type subtraction of the background) and Scofield’s photoionization cross-sections.
2.4. AFM Investigations
The surface morphology, as well the roughness of the VN layers and the initial material, were estimated by means of Atomic Force Microscopy (AFM)—MFP-3D, Asylum Research, Oxford Instruments, Santa Barbara, CA, 93117, USA. The AFM equipment was used to characterize the surface topography of the coatings. The studies were realized in non-contact mode (AC-mode) of operation, and the scanned area for all samples was 40 × 40 μm. During the measurements, Si-Tap300Al-G (standard type, budget Sensors) with a frequency of 300 kHz and an elasticity coefficient of k = 20 N/m were used. The data was analyzed and the surface roughness (root mean square deviation, RMS) was calculated by a special software—IgorPro 6.37.
2.5. Thickness
To determine the thickness of the VN layers we used an optical microscope (3D Optical profiler, Zeta-20, Zeta Instruments, Milpitas, CA, USA). All experiments were performed at room temperature. The optical images of the layers were obtained using a magnification 50x objective lens. The Zeta system scans samples over a user specified vertical (or Z) range. Vertical (Z) resolution is < 1 nm.
2.6. Mechanical Properties
The hardness and elastic modulus were determined by Nanomechanical Tester (Bruker). The Young modulus and hardness are calculated by utilizing the Oliver-Pharr method [
23]. The software program contained 4 lines with 12 indentations each (a total of 48 indentations) and spacing of 80 µm. Each indentation was made with a force of 50 mN.
2.7. Tribological Behavior
The tribological properties are assessed via dry slide examination by ball-on-flat with ball from hardened steel of UMT-2M (Bruker - CETR) tribotester. The experiments were carried out at a loading force of 2 N, for 5 and 10 min. All tests were performed at room temperature (~ 25° С) and air humidity 30-40 %.
2.8. Corrosion Studies
Reagents used in corrosion studies were: potassium hexacyanoferrates K3[Fe(CN)6] and K4[Fe(CN)6], salts for preparation of buffer solutions (mono- and dibasic potassium phosphates) and KCl, all of reagent grade (purity ≥ 98 %), all of them used without further purification. An ultrapure water (0.055 μS cm-1, Adrona B30 Bio, Vilnius, Lithuania) and reagent grade chemicals were used to prepare buffer solutions and the electrolytes for running electrochemical experiments.
The specimens under investigation were 2-3 mm thick circle-shaped lamellae with one side coated with VN, and the rest of them was initial material. Prior to the measurements, a crocodile clip was connected to each specimen. After that, the non-coated sides of each sample were insulated with a non-conductive polish and allowed to dry overnight under a fume cupboard. All electrochemical measurements were performed in a standard, non-compartmentalized electrochemical cell made of Pyrex glass with a working volume of 10–50 mL in a three-electrode configuration. The VN coated surfaces were utilized as working electrodes, whilst a Ag/AgCl, 3 M KCl and a Pt wire were reference and counter electrodes, respectively, connected to a compact potentiostat–galvanostat Autolab Vionic (Metrohm, Utrecht, The Netherlands) with embedded frequency response analyzer (FRA-module) for carrying out electrochemical impedance spectroscopy, controlled by Intello software. The corrosion resistance of the specimens has been examined as described previously [
22]. Firstly, the possibility of the vanadium based coatings to protect the substrate surface from corrosion was evaluated by the equilibrium potential (at zero current flowing through the electrochemical cell) in a 0.1 M KCl neutral aqueous solution. The protective function of the VN film against oxidative dissolution was determined by the impedance spectra (EIS) with frequencies ranged from 50 kHz to 1 Hz with 10 frequencies per decade in the same solution, where potassium hexacyanoferrates played the role of redox probe. The current variation with the applied potential (known as polarization characteristics of the coatings) was investigated by cyclic voltammetry at a scan rate of 50 mV/s in 0.1 M phosphate buffer with pH = 7.0, and were recorded over the potential region from −0.5 to 1.1 V vs. Ag/AgCl, 3 M KCl reference system. The potentials were reported against this reference electrode.
3. Results and Discussion
3.1. XRD Analysis
Figure 2 exhibits the XRD patterns of the VN coatings deposited at a temperature of 250
oC, 300
oC and 350
oC. All diffractions maximums are indexed. Phase identification was carried out with ICDD Database files PDF #35-0768 and #33-1439 for VN and V
2N crystal phases.
For all coatings, three diffraction peaks at 37.6
o, 43.7
o and 63.5
o of face-centred cubic (fcc) VN phase and reflections, corresponding to (111), (200) and (220) crystallographic planes, were observed. The XRD patterns reveal Bragg peak at 2θ = 43.7° with the highest intensity is at a temperature of 350
oC and corresponds to reflections in the (200) planes. This indicates a high degree of crystallinity. The peak intensity for VN phase in direction (200) rises with increasing of the substrate temperature up to 350
oC due to the higher energy with which Ar positive ions bombards vanadium target. Also, the grains oriented in direction (200) are dominated, as (200) peak becomes more remarkable at higher growth temperature. On the other hand, this enhances the probability one vanadium atom reacts with one nitrogen atom (V: N =1:1). We also notice the existence of the other peaks for V
2N phase at 2θ=41.7° and 65.8
o which were associated with reflections in (211) and (300) crystallographic planes for all VN samples. The presence of V
2N phase for all coatings proves that VN film is a mixture of VN and V
2N phases. As regards V
2N phase, the peak in direction (300) raises insignificantly at a higher temperature. However, increasing the deposition temperature up to 350
oC, the peak, corresponding to V
2N phase in (211) direction, gets almost invisible. This phenomenon occurs that at high temperatures V
2N phase is unstable. The same effect is observed at VN coatings deposition by HIPIMS at different growth temperatures [
24]. According to the Nelkel [
25], the peak high intensity depends on the stoichiometry of the deposited VN films. If the vanadium and nitrogen atoms have approximately equal ratio, the diffraction feature is more intensive and the quantity of the VN bonds increases. Applying a high temperature to the substrate, the atomic mobility and rearrangement are also raised. A decrease in the temperature means a small quantity of nitrogen atoms will be incorporated into the VN crystal lattice and lower thermal energy [
26].
3.2. XPS Analysis
The surface composition and chemical state of VN films, deposited at a temperature of 250
o C, 300
o C and 350
o C, were assessed by XPS measurement. The analysis shows that the chemical elements C, O, N and V are registered on the surface and their quantitative composition is presented in
Table 1.
The presence of the high concentration of oxygen on the VN films surface is probably due to contaminations or residual oxygen in the deposition vacuum chamber [
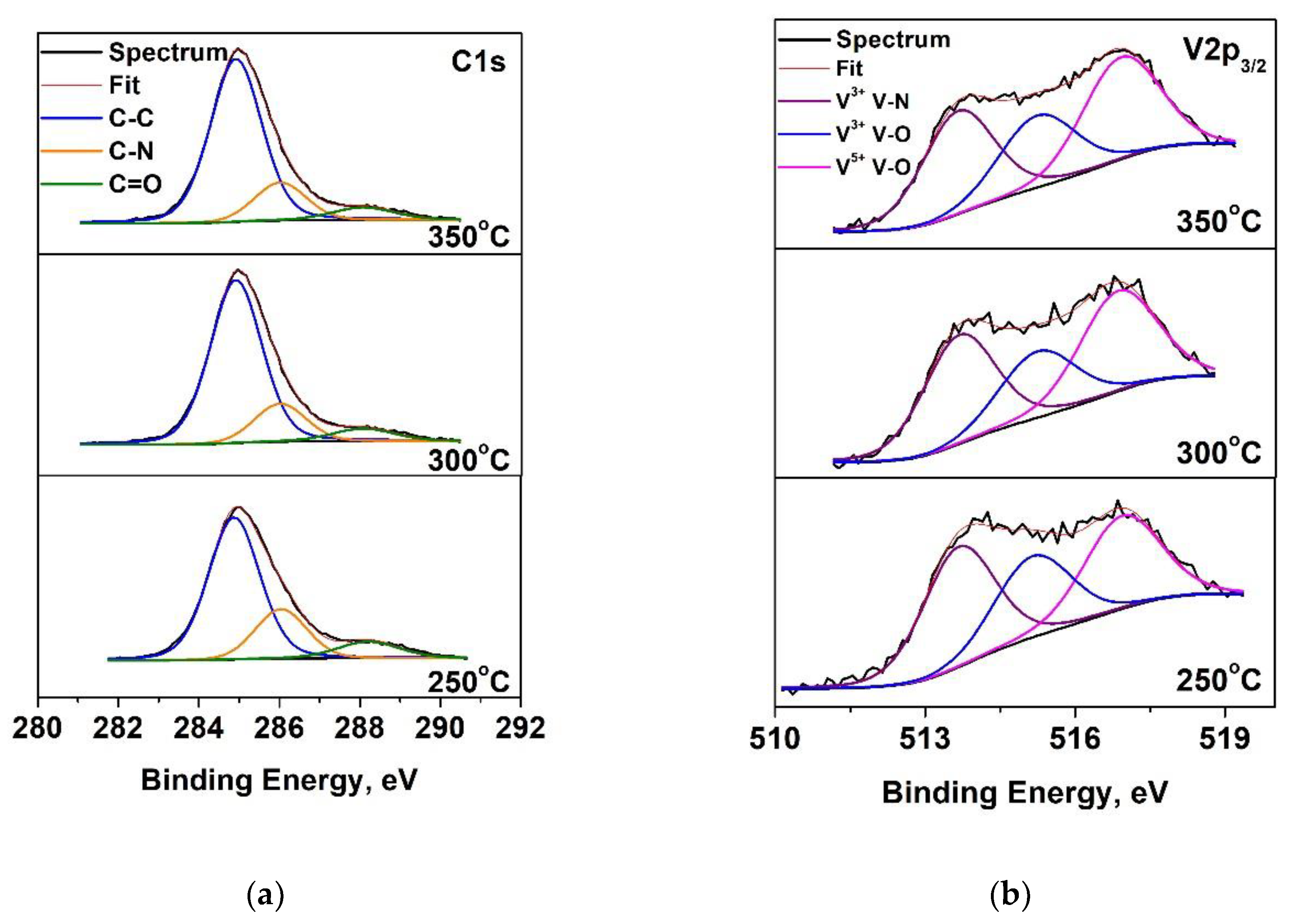
13]. The C1s spectra and the V2p3/2 spectra are shown in
Figure 3. It can be seen that the carbon spectra are decomposed into three peaks, which are attributed to the C-C bonds (adventitious carbon), C-N and C=O. The V2p spectra contains two lines - the first one V2p
3/2 is fixed at 513.7 eV and second V2p
1/2 at 521.1 eV. The V2p
1/2 peak overlaps with the satellite of the oxygen spectrum and for this reason only the V2p3/2 peak is decomposed. The deconvolution displays two oxidation states of vanadium: V
3+ in V-N (assigning to V
2N phases), V
3+ in V
2O
3 and V
5+ in V
2О
5. These results are in the accordance to the data, obtained in XRD analysis. The concentrations and binding energies of the different types of vanadium are presented in
Table 2. The data exhibits a decrease in V-N phase concentration in the VN coatings with raising of the substrate temperature. This reduction is probably due to the low quantity of vanadium and nitrogen atoms that are deposited on the substrate surface and lower rate during the deposition process [
27]. The results in our study are in a good agreement with ones, described by the authors in Reference [
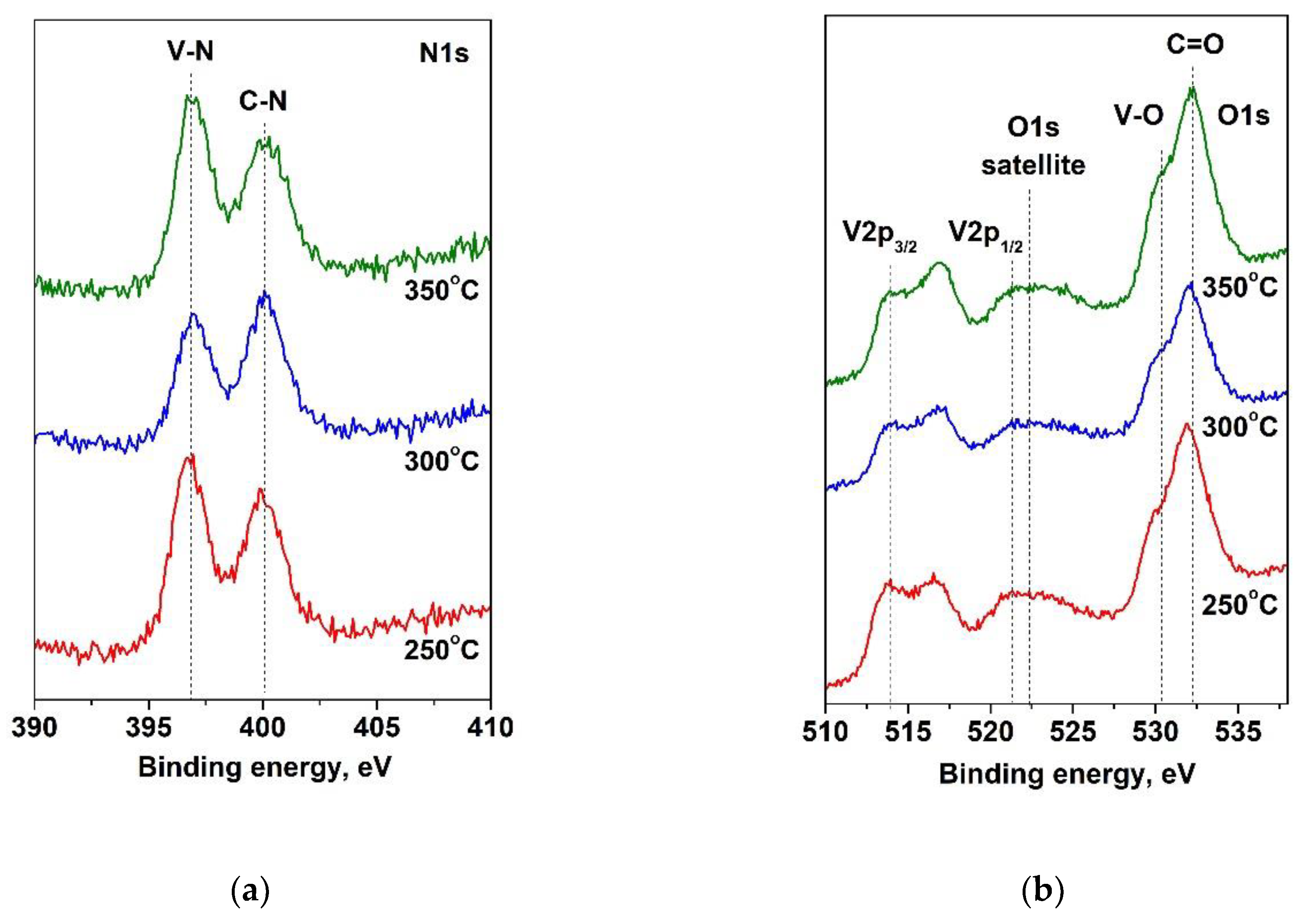
28]. The XPS spectra in the V2p, O1s and N1s levels of the vanadium layers, deposited at different substrate temperatures, are exhibited in
Figure 4. The oxygen spectra have two strong features, corresponding to oxygen bound to vanadium at 530.4 eV and a carbonyl group at 532.2 eV. In N1s spectra, the binding energies of the peaks are positioned at 396.8 eV and 400.1 eV and are assigned to V-N and C-N bonds, respectively [
29,
30]. The peak intensity, corresponded to V-N phase, increases at a temperature of 350
oC as a result of high energy of interaction between vanadium and nitrogen atoms [
31].
The XPS observations showed the existence of V-N, C-N, V-O and C=O. bonds on the surface of the VN films. It can be noted that temperature treatment has an impact on the quantity of vanadium on the surface. The highest amount of vanadium is observed at treatment of 2500C, as confirmed by the table with the different species of vanadium.
3.3. AFM Analysis
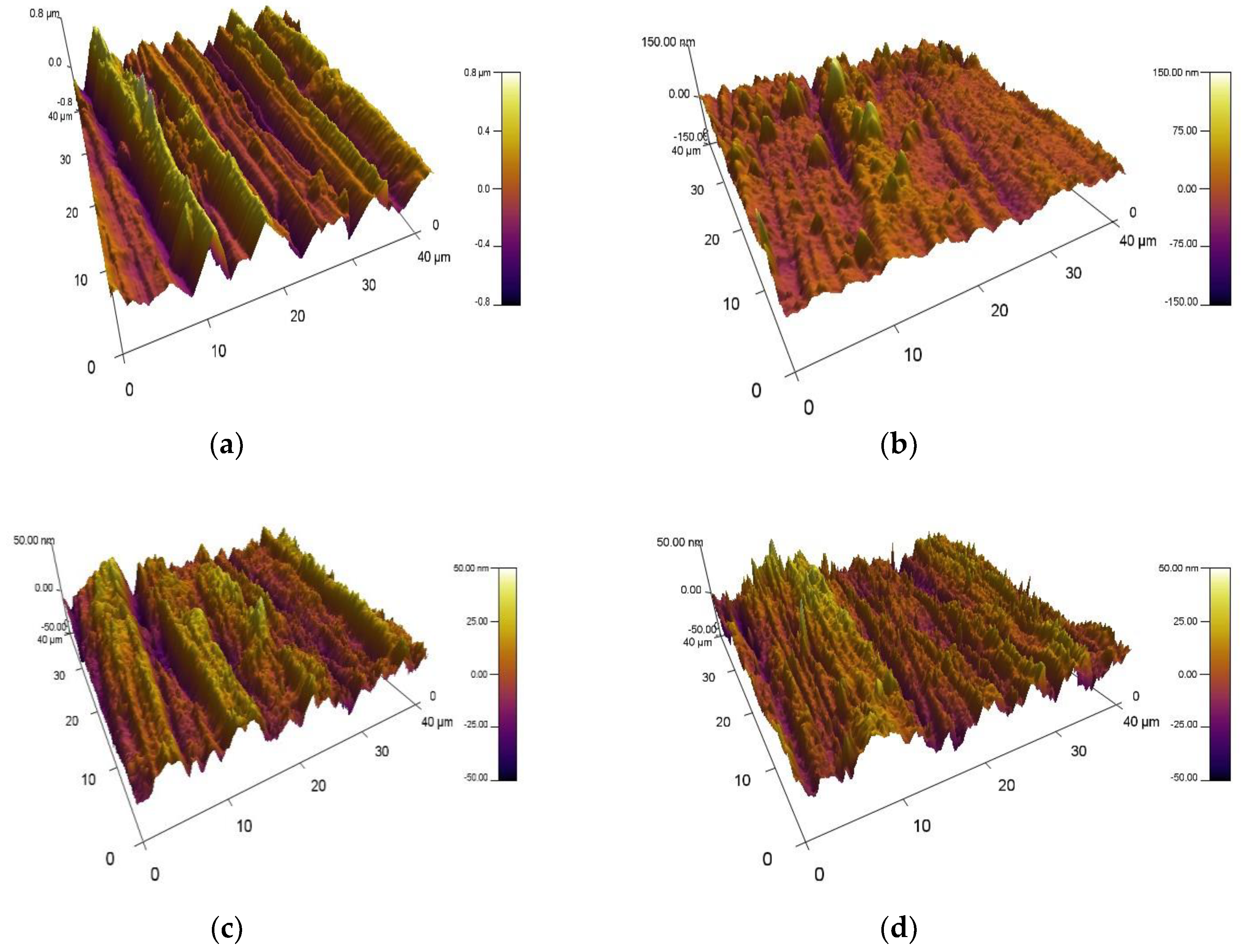
Surface roughness of VN films, deposited at different substrates temperatures on 304 L stainless steel substrates, is investigated by Atomic Force Microscopy. 3D AFM surface images of VN coatings are given in
Figure 5. It is also exhibited a surface morphology of the bare material to estimate the effect of the temperature on surface properties of the initial material.
The alteration of the substrate temperature during the magnetron sputtering process influences on the surface roughness of the as-deposited VN films. It was found out the Nano roughness of the bare substrate is 216 nm. Applying the temperature of 250 oC to the initial material, the surface of the VN film gets smoother and the roughness diminished significantly to 16 nm. Increasing the substrate temperature up to 300 oC leads to a slight increase in the surface roughness up to 18 nm and a subsequent decrease to 10 nm at a substrate temperature of 350 oC. Increasing the temperature up to 350 oC leads to a decrease in the crystallites size and lower roughness. For all specimens, it is observed a wave-like topography. The lower roughness is probably due to the small quantity of vanadium ions which bombards the substrate surface. On the other hand, the smoother surface results in the low presence of microstains. Besides, the lower value of the roughness with raising of the temperature is attributed to a good homogeneity in the film composition, redistribution of internal stresses in the layer and enhanced density of the coating.
It is possible the smoother surface contributes for increasing of the corrosion resistance of the films when they are exposed to chloride-containing solutions [
32]. The lowest surface roughness is achieved for the VN coating, produced at a temperature of 350
oC, as a result of the enough raised ion flux and bombarding energy. The high temperature is related to the higher energy, the smooth surface and a low quantity of defects on the surface of the films [
33,
34].
3.4. Thickness
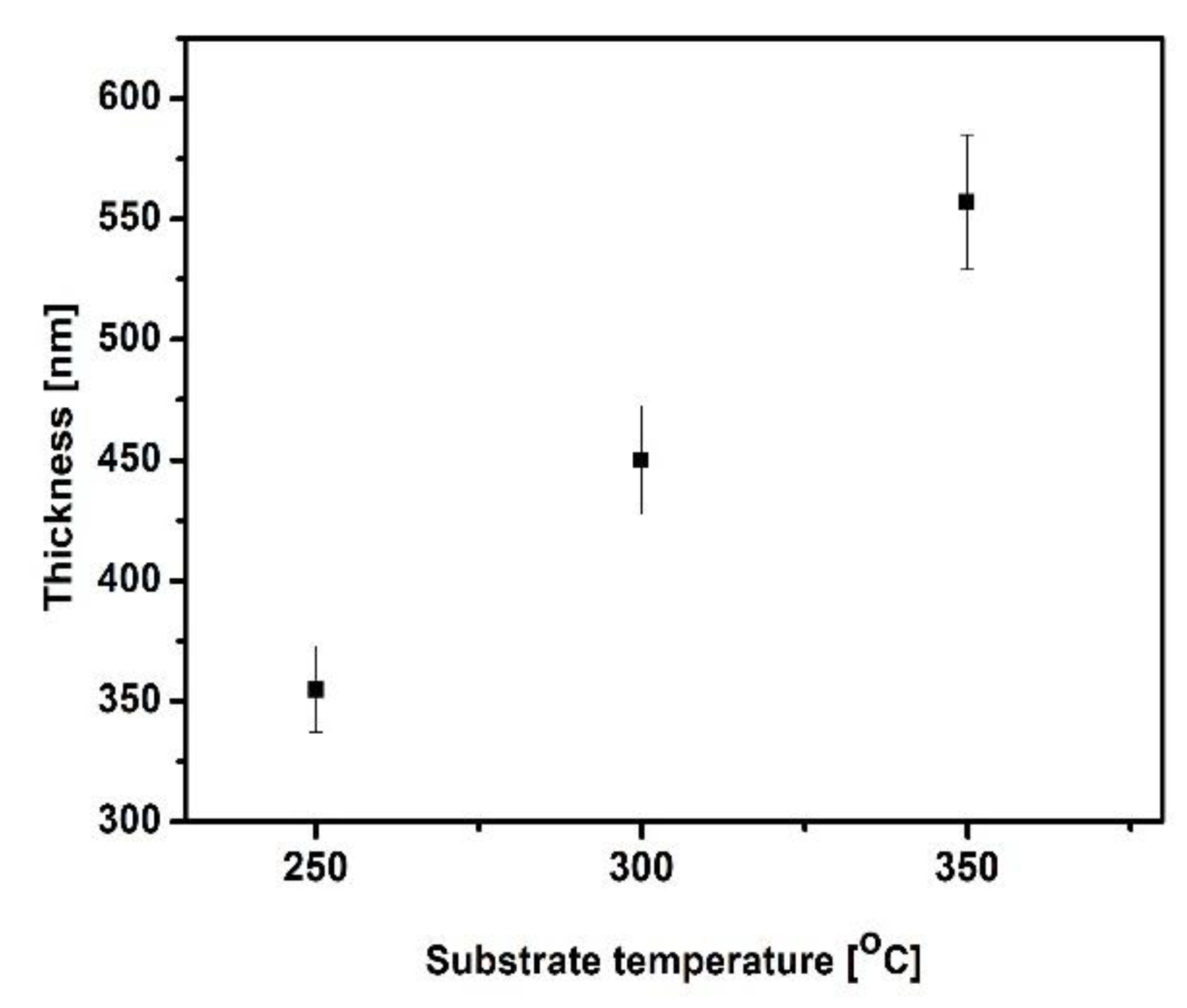
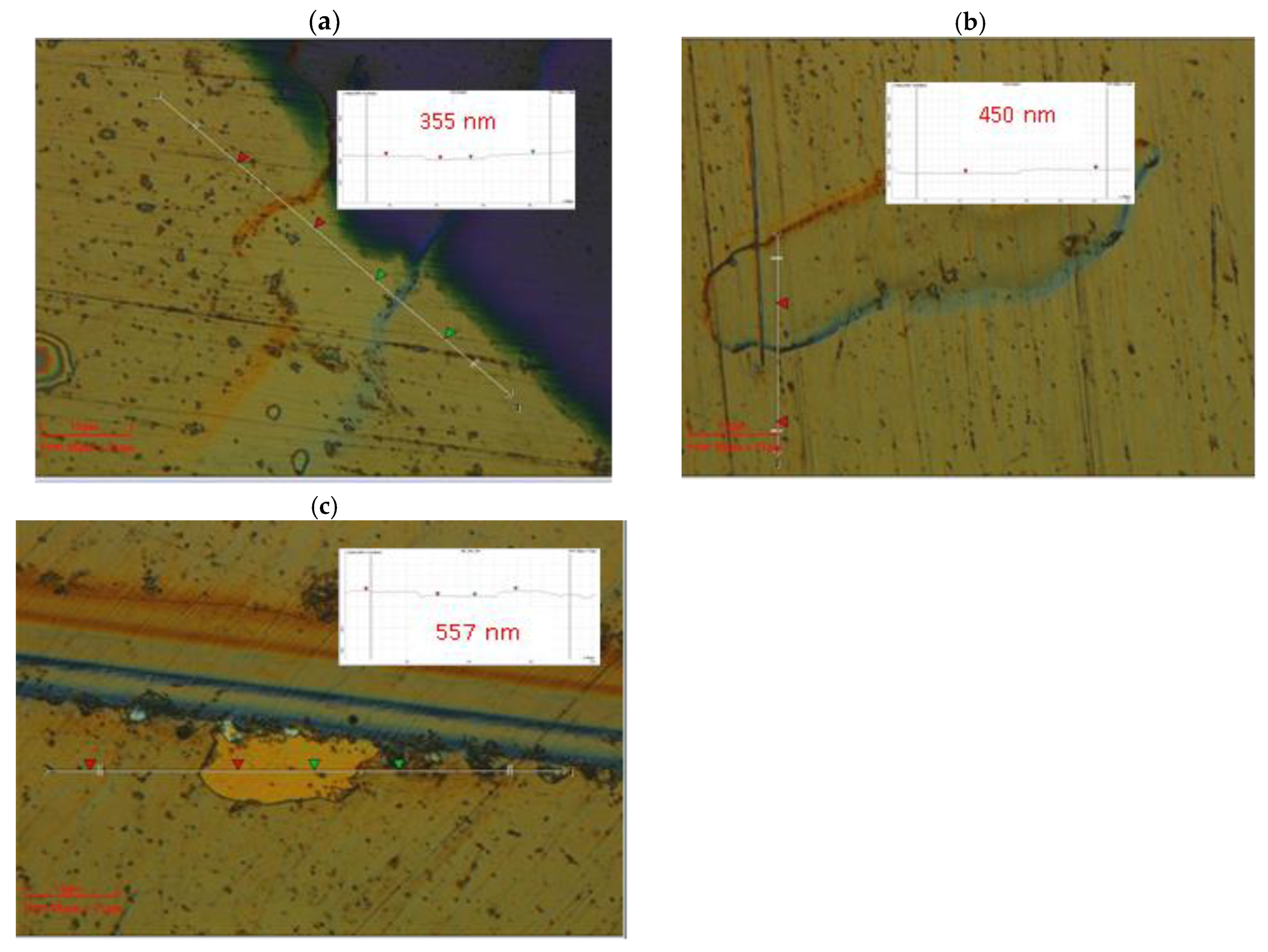
The deposition temperature is one of the most important factor, affecting on the thickness and microstructure of the as-deposited vanadium nitride coatings. The VN films thickness as a function of the substrate temperatures is displayed in
Figure 6 and the optical 2D images are given in
Figure 7.
The results exhibited a linear increase in the coatings thickness with the raising of the temperature. The VN coating, deposited at 250
oC, shows a thickness of 350 nm. Rising the temperature up to 350
oC, the thickness of the vanadium nitride sample is enhanced (557 nm). The higher temperature during the deposition leads to thicken of the layer due to the higher kinetic energy of the vanadium atoms, deposited on the surface and the adhesion improvement between the bare material and film. This phenomena can be resulted in a presence of the nucleation sites which probably enhance the thickness of the as-deposited film. [
35,
36]. The nucleation process is the initial stage in the phase transition for formation of a new phase or structure in the coating. The phase transitions require enough energy to overcome the energetic barrier of new phase formation. Also, the nucleation sites provide the surface by lowing the energetic barrier and make it easier for production of the new phase. The highest thickness (500 nm) of the VN film is probably attributed to the denser structure and low compressive residual stresses, proving a lack of defects in the film during it growth [
37]. On the other hand, the film with the highest thickness exhibited the higher intensive peak for V-N phase in (200) direction which is in a good agreement with XRD results in our study. It was found out that the increasing of the thickness is related to more quantity of V
2O
5 in the film and a good adhesion between the substrate and coating. The thicker film provides low surface roughness, the coating with a high density, reduction the friction coefficient and enhancement in the tribological properties.
3.5. Mechanical Properties
The hardness and elastic modulus of the VN films, produced at a substrate temperature of 250
oC, 300
oC and 350
oC by D.C. magnetron sputtering method, are given in
Figure 8. It can be observed diminution in the hardness and elastic modulus with the increasing of the temperature due to change in the films stresses. The highest hardness (10.6 GPa) is achieved in the film, deposited at 300
oC and the lowest value of the hardness (9.5 GPa) is obtained at a temperature of 350
oC. The enhancement in the coating hardness is related to the highest quantity of V-N bonds in the layer. According to the authors in Reference [
38], the high hardness is a result of high compressive stresses in the film and a good stoichiometry between vanadium and nitrogen ions. On the other hand, raising of the substrate temperature leads to decrease in the surface roughness and the hardness due to smaller size of the crystallites and the unevenness of the VN film. Besides, the reduction in the coatings hardness result in the presence of V
2O
5 phase which is confirmed by the XPS measurements in our study.
The modulus of elasticity characterizes the stiffness of the material to resist deformation under the tension or compression. The highest Young ‘modulus (208 GPa) is observed for the film, deposited at a temperature of 300
oC and the lowest value of the elasticity modulus (191 GPa) is achieved in the coating at a temperature of 350
oC. These values are significantly lower compared to the results, reported by Huang et al. where the elastic modulus for VN film reaches to 370 GPa [
12]. According to the researchers in Reference [
39], this distinction is probably attributed to high compressive stresses and the smooth surface of the films. Zhang et al. [
40] reported lower values of the elastic modulus due to more porous structure in the coatings. The high values of elasticity modulus correspond to the increased stiffness of the material and a small deformation [
41]. According to the authors in Reference [
42], the mechanical behavior of the vanadium coatings depends on their structure. The films with a good density and homogeneity enhance remarkably their hardness and elastic modulus [
43,
44]. In our study, vanadium nitride layer, deposited at 300
oC, is the densest and homogeneous.
The hardness and elastic modulus ratios (H/E or H
3/E
2) have an important significance for determination of the functional properties of the materials. H/E ratio evaluates the elastic deformation which is related to the ability of the film to resist loads. H
3/E
2 parameter characterizes the plasticity of the film which undergoes irreversible deformation without any change in stresses or loads.
Table 3 gives information for mechanical characteristics of the VN coatings, deposited at different temperatures.
The temperature is an essential factor for the plasticity of the metal materials. When the temperature is increased, the plastic deformation is enhanced and the material gets more malleable. This leads to a high strength of the material and make it suitable for many applications. In our study, the obtained results are very close each other but the highest values of H/E (0.051) and H
3/E
2 (0.027) ratios are obtained for the vanadium nitride coating at a temperature of 300
oC. On the other hand, the enhancement in the elasticity and plasticity is probably related to improved density of the film and the existence of the more stable V-N phase [
45]. The lower values of the H/E (0.048) and H
3/E
2 (0.023) are observed for the VN layer, produced at 250
oC and it is an indicator for non-enough resistance to a plastic deformation and susceptibility to wear. Besides, the high values of elastic deformation (H/E ratio) contribute for the improvement in the VN coatings toughness and the wear resistance. The relationship between the mechanical properties and structure of the films exists. The slight increase in the mechanical properties for VN film at 300
oC can be explained by a larger number of the crystallites in (200) direction as established by XRD analysis in our research. Besides, the enhancement in the hardness and elastic modulus can be associated to the lower concentration of the oxygen (12.2 %) on the surface of the film as it is shown from XPS results.
3.6. Friction Coefficient
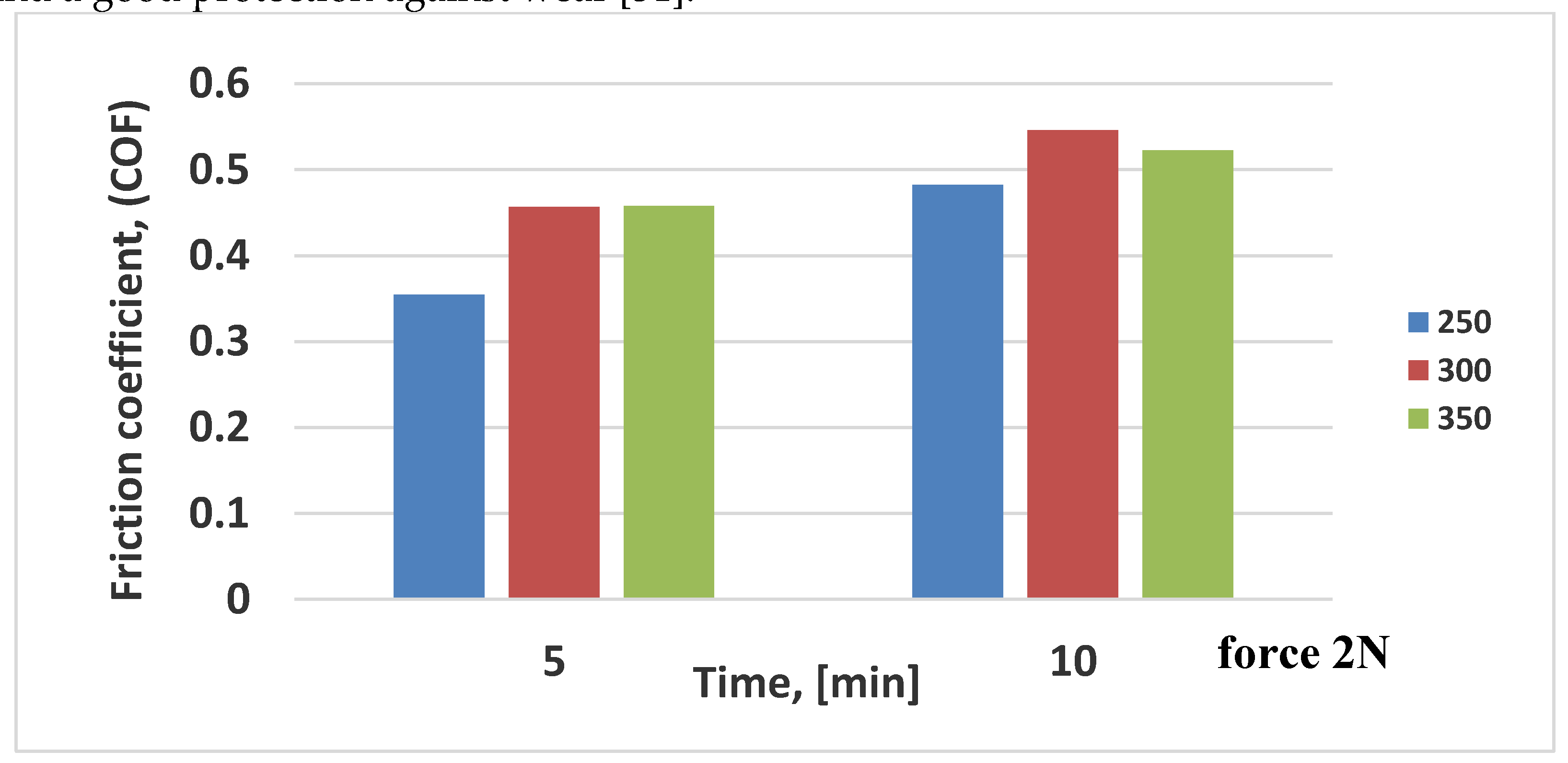
The elastic and plastic deformations are associated with the films toughness and give an information for the tribological performance of the films. The friction coefficients (COF) of VN coatings, obtained at a temperature of 250
oC, 300
oC and 350°С, are evaluated by ball-on-flat tests sliding opposite tempered ball (with Cr coating) at room temperature and different time. The results are shown in
Table 4 and are illustrated graphically in
Figure 9.
From
Table 4 it can be seen that the raising of the friction time from 5 to 10 minutes, COF increases for all coatings. The similar trend is achieved with increasing of the temperature. Raising of VN coatings friction coefficient with increase of friction time would be explained with friction mechanism at sliding, owing to material transport from the ball on the coating [
46]. The lowest COF (0.35) is measured for the VN film, obtained at 250 °С for friction time of 5 minutes and the highest value is estimated for the VN film, deposited at 300
oC for 10 minutes. The lower friction coefficient is probably due to raised density of the film and an existence of V
2O
3 and V
2O
5 which is consistent with XPS results. Similar statements were reported by the authors in Reference [
47]. According to them, the formation of the vanadium oxides is probably cause for lower friction coefficient. No essential change is observed in the friction coefficients (0.45) for the layers at 300 and 350
oC and 5 minutes’ time.
The coating COF depends on the material, from which the ball is made, as it is shown in Reference [
48]. As longer time the ball slides on the coating as much material is transferred and in this way the friction coefficient enhances. Other authors reported the similar values of friction coefficient (0.45) for aforementioned films but at a higher deposition temperature of 400°C [
49,
50]. The high values of the plastic deformation lead to the improved hardness of the vanadium nitride film and a good protection against wear [
51].
A relationship between the surface roughness and friction coefficient for the investigated films is found out. It is displayed that the friction coefficient (0.5226) increases with diminution of the surface roughness (10 nm) for the VN sample, obtained at 350
oC. Aissani et al. in [
5] show a friction coefficient of 0.61 which can be explained by a formation of unstable phase V
2N. The enhancement in the friction coefficient with raising of the temperature can be resulted in obtaining of the films` wear debris [
52]. On the other hand, the rougher a surface is, the higher friction coefficient is compared to the surfaces without asperities. The friction is directly related to the wear. When the surface is uneven, the wear is faster. This is attributed to the lower effective contact area between the mating surfaces and the wear resistance of the films decreases.
3.7. Corrosion Resistance
The protective role that the coating of VN, annealed at three different temperatures, plays over the substrate was estimated on the basis of the following electrochemical techniques: determination of the equilibrium potential at open circuit (OCP measurements); impedance studies (EIS) and polarization studies implemented by means of cyclic voltammetry (CV). The corrosion protection of the samples, ensured by the VN films, was characterized by electrochemical studies, carried out at equilibrium conditions without or with small perturbations (measurements of the open circuit potentials, OCP and impedance studies). An open circuit technique (at zero current flowing through the cell, until an equilibrium value is reached) was used to determine the corrosion potentials of the as-deposited VN films.
Table 5 shows the measured OCP values, reported vs. a Ag|AgCl, 3 M KCl reference electrode, determined in 0.1 M KCl, pH = 7.0.
For comparison, 304 L stainless steel initial material exhibits a corrosion potential of −0.652 mV [
53] that is a proof for enhancement of the vanadium nitride coated specimen`s corrosion resistance, as it can be seen from
Table 5. Taking into account that the reference electrode is 200 mV more positive than the standard hydrogen electrode (SHE), the resulting positive values of the OCPs found for VN coated samples, produced at temperatures of 250, 300 and 350
oC, undoubtedly indicate that these specimens would not corrode even in chloride ions-containing solutions, as chlorides are evaluated to be ones of the most aggressive corrosive agents.
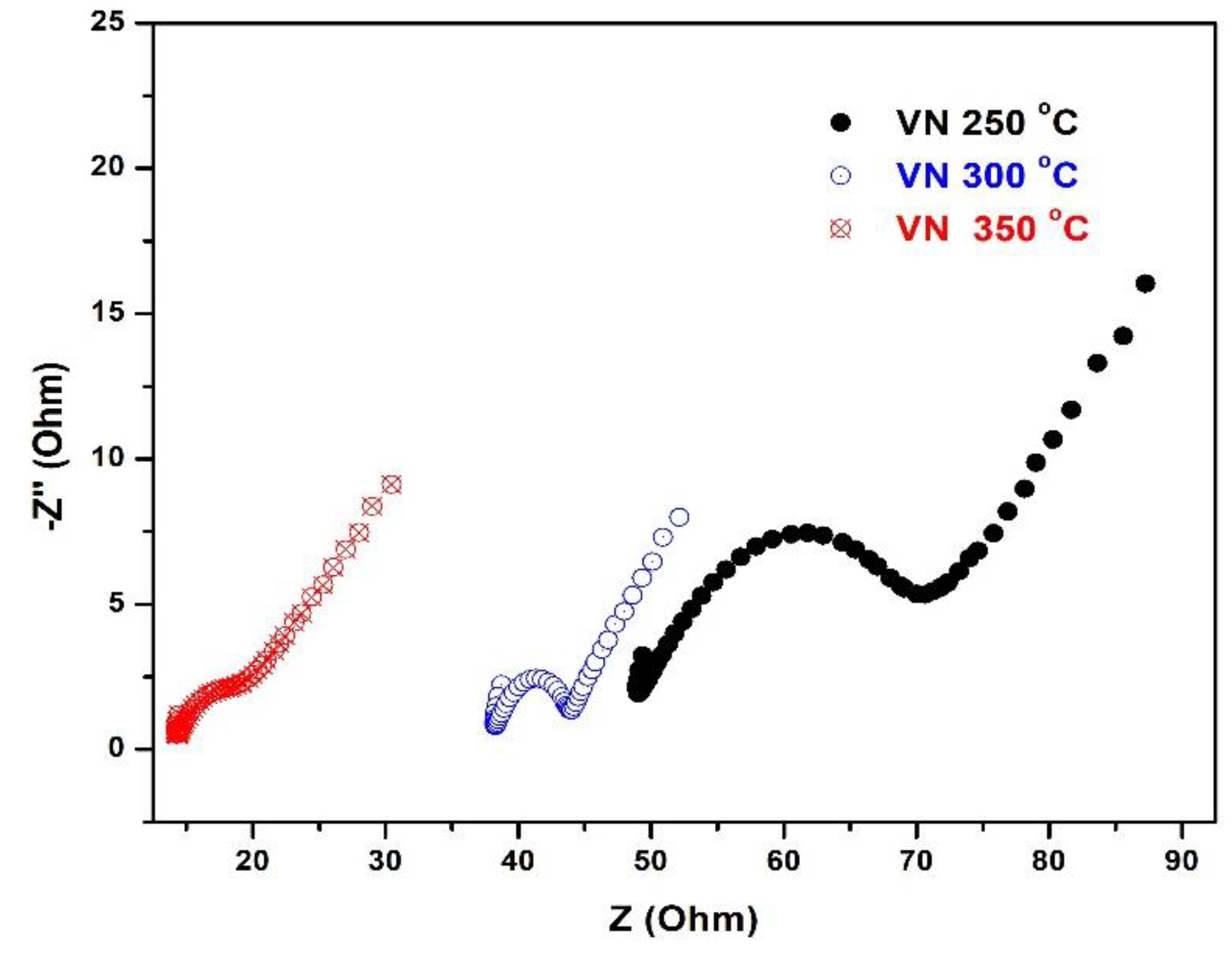
The above considered statements for the corrosion behavior of the investigated films are in a good agreement with the EIS studies, as shown in
Figure 10.
Electrochemical Impedance Spectroscopy (EIS) is a well-known qualitative method for evaluation of the corrosion behaviour at the interface of the coating and electrolyte solution containing a redox probe. Also, the data, obtained by this technique, are reliable and can predict the long-term performance of the protective coatings. As it can be seen from
Figure 10, the impedance spectra of the VN films, deposited at a temperature of 250 and 300
oC, exhibit an appearance of a semi-circle, proving the formation of a homogeneous protective layer over their metallic surface. The EIS measurements show the inability of redox particles to reach the conductive surface to reduce or oxidize over it and the semi-circle’s diameter raises with the density of the protective VN layer. On the other hand, as the temperature of the treated sample raises, the diameters of the impedance spectra semi-circles for the samples under study decrease. The vanadium nitride films, produced at 250 and 300
oC, demonstrate the best protection against corrosion, as it can be observed from the semi-circle diameter of the Nyquist plot. The specimen, obtained at 350
oC, provides much weaker protecting ability to the metal substrate due to the much smaller resistance of the charge transfer as the semi-circle diameter reduces drastically.
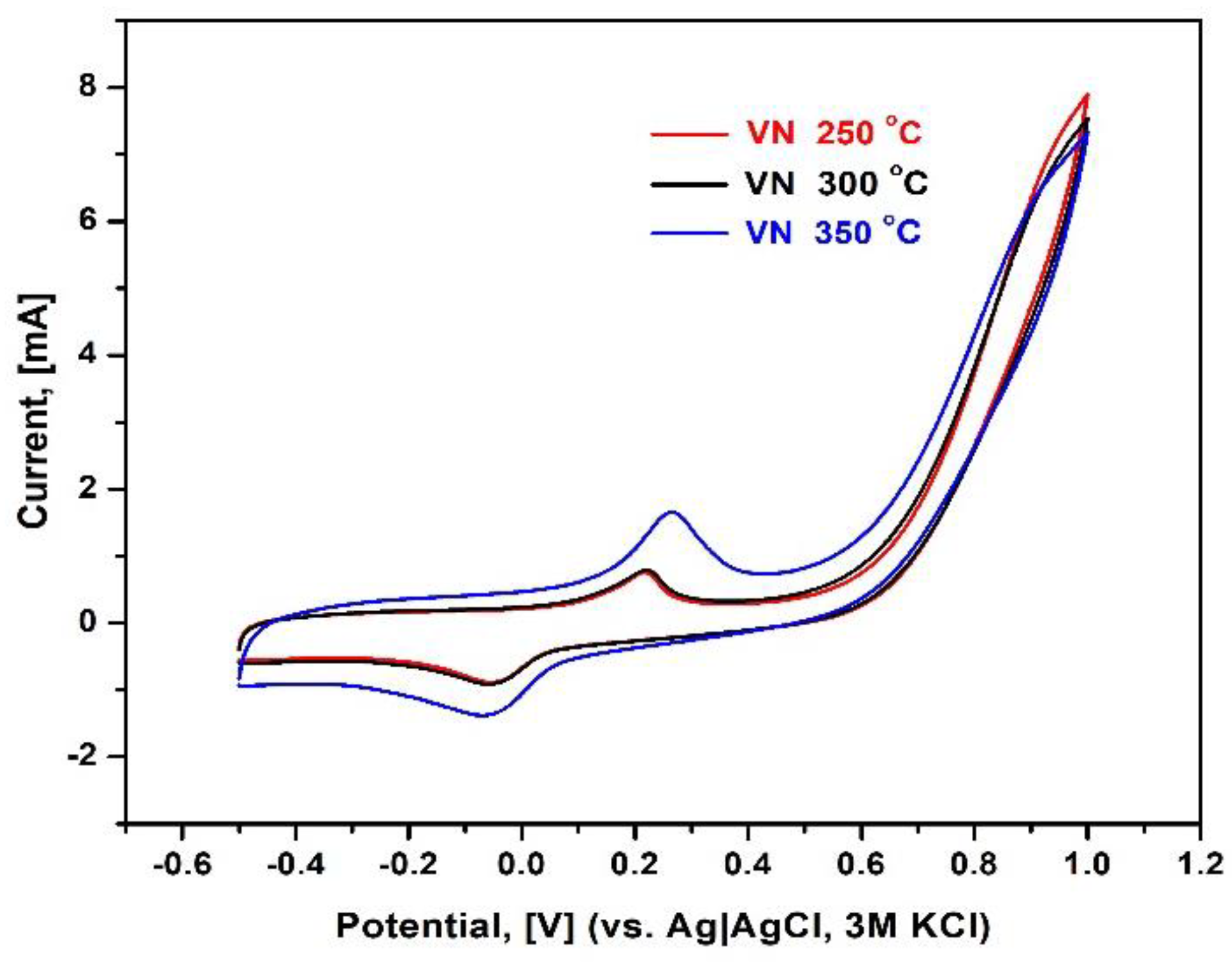
The potentiodynamic technique, which is used to detect the presence of oxidation and reduction processes at the interface electrode–electrolyte, is cyclic voltammetry (CV). CV is a direct current electrochemical technique that examines the relationship between different current values at a potential varying linearly with time from an initial to an end value and then back to the beginning one. The polarization behavior of all VN samples was followed in the absence of strong corrosion agents – e.g. chloride ions, as it can be deduced from
Figure 11.
It can be seen that all studied samples are characterized by the appearance of a pair of two well-defined peaks: the first one is observed at the forward scan at 200- 250 mV, while the peak on the backward scan raises at -100 mV, thus implying an irreversible redox process. The sample behavior at applied potentials exceeding 550 mV is described with sharp current increase and the shape of cyclic voltammograms is typical for faradaic processes – e.g. sample dissolution. No signs of such were observed, however, after removing the samples from the electrolytic solution where the corrosion tests were performed. It is plausible that a new surface compound is formed upon testing the samples in the neutral phosphate buffer. Analogously to EIS studies, the both specimens, deposited at 250 and 300 oC manifest smaller faradaic currents compared to the sample treated at a temperature of 350 oC.
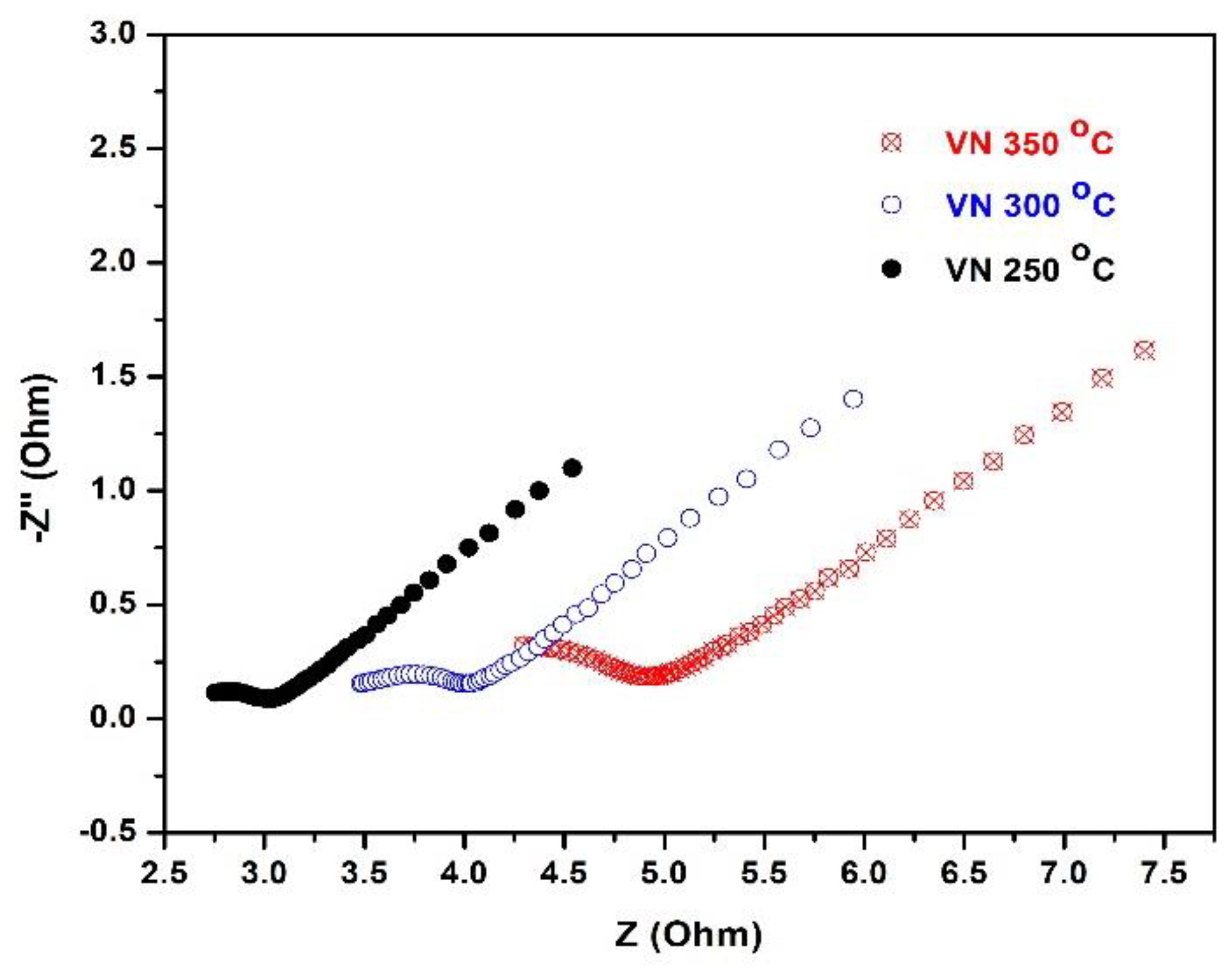
In order to investigate the consequences from the polarization tests of the examined samples, a second round of impedance studies were performed under equivalent experimental conditions (
Figure 12). The resulting EIS spectra suggest that both the shape and the charge transfer resistance changed drastically: unlike the Nyquist plots of the samples obtained with the first impedance tests that varied from sample to sample, the second ones appear very similar, with scarcely expressed semi-circles, followed by a linear tail tilted at 45
0. The last finding indicates strong diffusion control over the redox probe reaching the metal surface. The other important finding is the vastly reduced charge transfer resistance as compared with the first impedance tests: for the VN film at 250
oC it decay is the most substantial (~ 20 times), while the other two samples indicate a reduction of the charge transfer resistance of 8 and 5 times for VN coatings at 300
oC and 350
oC, respectively.
The surface roughness and adhesion of the coatings have an important role for their protection against corrosion processes. The good adhesion between coating and substrate material diminishes the roughness and improves the corrosion resistance of the films [
54]. As regards to the bare substrate, namely 304 L stainless steel, the significant value of the roughness (216 nm) is responsible for decreasing in the potential in the strong aggressive solutions.
Summarizing, the performed studies on the corrosion resistance if VN coated stainless steel indicate, that the layer of VN, deposited over the sample’s surface, definitely protects the steel from corrosion even in the presence of strong corrosion agents (e.g. chloride ions).
The obtained results in our research exhibited good possibility for deposition of nanostructured coatings with low roughness and friction coefficient. The XRD patterns demonstrated a presence of VN and V2N phases as the peak of V2N gets insignificant at higher temperature. It was proved that the rising of the substrate temperature leads to low surface roughness. The raising of the VN films thickness improves the mechanical properties, enhances crystallinity and the adhesion between the bare material and film and reduces the surface roughness. The high density and smoothness of the vanadium nitride coatings decrease the friction coefficient and contribute for enhancement of the as-deposited films` wear resistance. The electrochemical tests revealed a remarkable positive shift of the corrosion potential for all films in comparison with the initial material whose potential is negative. The best mechanical, tribological and corrosion properties showed VN films, deposited at 250 oC and 300 oC which is in a good agreement with the XPS results, obtained in our research.
4. Conclusions
In the present study, vanadium nitride coatings films were deposited on 304 L stainless steel substrates at a temperature of 250, 300 and 350 oC by DC magnetron sputtering process to investigate their effect on the structural, mechanical and tribological properties as well as corrosion behavior of the obtained coatings. It was found out a presence of (111), (200) and (220) orientations of the crystallites, corresponding to VN and V2N crystal phases, respectively. Raising of the substrate temperature up to 350oC leads to low quantity of microstrains in the layer. These results were confirmed from estimated lattice parameters whose values are close to ones published data in the literature. XPS results proved an existence of V-N bonds for all coatings as the highest quantity of the V- N phase is observed for the film, produced at 250 oC, showing the lowest friction coefficient at 5 minute. The films thickness rises with the temperature. The thicker VN coating provides better hardness, the smother surface and a good adhesion between the film and the initial material which contributes for a good wear resistance. The reduction in the friction coefficient resulted in the existence of vanadium oxides- V2O3 and V2O5, which can improve the tribological properties of the coating. The surface roughness decreases with rising of the temperature. No essential difference was observed in the mechanical properties of the VN films at various temperatures. The electrochemical measurements showed a good protection against corrosion processes for all specimens even in the strong aggressive agents. The low value of surface roughness, small friction coefficient, the good smoothness and the as-deposited VN coatings improved mechanical properties enhance the wear resistance and corrosion protection of the films and make them potential candidates for modern industrial applications.
Author Contributions
Conceptualization, S.R., M. O. and S.V.; methodology, S.R., D.D., N.I., N.D., V.S. and S.V.; formal analysis, S.R., D.D., N.I., N.D., V.S.,V.K., M.S., G.A., M.O. and S.V.; investigation, S.R., D.D., N.I., N.D., V.S.,V.K.,M.S.,G.A. ,M.O. and S.V.; writing—original draft preparation, S.R. and S.V.; writing—review and editing, S.R. and S.V. All authors have read and agreed to the published version of the manuscript. All authors have read and agreed to the published version of the manuscript.”
Funding
“This research received no external funding”.
Acknowledgments
Research equipment of Distributed Research Infrastructure INFRAMAT, part of Bulgarian National Roadmap for Research Infrastructures, supported by Bulgarian Ministry of Education and Science was used in this investigation. N.D. gratefully acknowledges the access to research infrastructure of Centre for Competence “Personalized Innovative Medicine, PERIMED-2 (BG Programme “Research, Innovation and Digitalization for Smart Transformation” 2021-2027, co-financed by EU, grant BG16RFPR002-1.014-0007).
Conflicts of Interest
“The authors declare no conflicts of interest.”
References
- Y. Wang, J-W. Lee, J-G. Duh. Mechanical strengthening in self-lubricating Can/VN multilayer coatings for improved high-temperature tribological characteristics, Surf. Coat. Technol. 2016, 303, 12-17.
- P.Eh. Hovsepian, Q. Luo, G. Robinson, M. Pittman, M. Howard, D. Doerwald, R. Tietema, W.M. Sim, A. Deeming, T. Zens. TiAlN/VN superlattice structured PVD coatings: A new alternative in machining of aluminum alloys for aerospace and automotive components, Surf. Coat. Technol. 2006, 201, 265.
- J. C. Caicedo, G. Zambrano, W. Aerators, L. Escobar-Alarcon, E. Camps. Mechanical and electrochemical characterization of vanadium nitride (VN) thin films, App. Surf. Sci. 2011, 258, 312–320.
- Bautista, J.M. Campelo, D. Luna, J. Luque, J.M. Marinas. Gas-phase selective oxidation of toluene on TiO2–sepiolite supported vanadium oxides: Influence of vanadium loading on conversion and product selectivities, Catal. Today. 2007, 128, 183-190.
- Linda Aissani, Akram Alhussein, Corinne Nouveau, Laala Ghelani, Mourad Zaabat, Influence of film thickness and Ar/N2 plasma gas on the structure and performance of sputtered vanadium nitride coatings. Surf. Coat.Technol. 2019, 378, 124948. [CrossRef]
- Liao M-J, Y. Gotoh, H. Tsuji, J. Ishikawa. Crystallographic structure and composition of vanadium nitride films deposited by direct sputtering of a compound target, J. Vac. Sci. Technol. 2004 A 22, 146–150.
- Jia-Hong Huang, Liang-Ju Wei, I-Sheng Ting. Evaluation of fracture toughness of VN hard coatings: Effect of preferred orientation, Mater. Chem. and Physics 2022, 275, 125253.
- Matei Ghimbeu, F. Sima, R.V. Ostaci, G. Socol, I.N. Mihailescu, C. Vix-Guterl. Crystalline vanadium nitride ultra-thin films obtained at room temperature by pulsed laser deposition, Surf.& Coat.Technol. 2012, 211, 158–162.
- A.Cupric, A. Gilewicz, G.Tolmachova, I.Klimenko, I. Kolodiy, R. Vasilenko, B. Warcholinski. Effect of Nitrogen Pressure and Substrate Bias Voltage on Structure and Mechanical Properties of Vacuum Arc Deposited VN Coatings, Metallur. Mater. Transact. 2023, A 54, 4438–4455.
- Sung -Yong Chun. Properties of VN Coatings Deposited by ICP Assisted Sputtering: Effect of ICP Power, J. Korean Ceramic Soc. 2017, 54, 38–42.
- Meng Gao, Xianfeng Xu, Hui Li. Investigation on preparation of vanadium nitride hard coating by in-situ method technique, Mater. Lett. 2020, 274, 128045.
- Jia-Hong, H.; Cheng-Han, L.; Ge-Ping, Y. Texture evolution of vanadium nitride thin films, Thin Solid Films 2019, 688, 137415.
- Linda Aissani, Mamoun Fellah, Ablel Hakim Chadli, Mohammed Abdul Samad, Abderrahmane Cheriet, Faiza Salhi, Corinne Nouveau, Sabine Wei, Aleksei Orbison, Akram Alhussein. Investigating the effect of nitrogen on the structural and tribo-mechanical behavior of vanadium nitride thin films deposited using R.F. magnetron sputtering, J. Mater. Scie. 2021, 56, 17319–17336.
- Zhaobing, C.; Jibin, P.; Liping, W.; Qunji, X. Synthesis of a new orthorhombic form of diamond in varying- C VN films: Microstructure, mechanical and tribological properties, Appl. Surf. Scie. 2019, 481, 767–776. [Google Scholar]
- Hongjian, G.; Bo, L; Jianyi, W.; Wenyuan, C.; Zhenyu, Z.; Wenzhen , W.; Junhong, J. Microstructures, mechanical and tribological properties of VN films deposited by PLD technique, RSC Advances 2016 , 6, 33403-33408.
- Yuexiu, Qiu, Sam, Zhang, Bo, Li, Jyh-Wei L. ; Dongliang, Z. Influence of nitrogen partial pressure and substrate bias on the mechanical properties of VN coatings, Procedia Engineering 2012, 36, 217–225. [Google Scholar]
- Gueddaoui, H.; Schmerber, G.; Abes, M.; Guemmaz, M.; Parlebas, J.C. Effects of experimental parameters on the physical properties of non-stoichiometric sputtered vanadium nitrides films, Catal. Today 2006, 113, 270–274. [Google Scholar] [CrossRef]
- Fallqvist, M.; Olsson, M. The influence of surface defects on the mechanical and tribological properties of VN-based arc-evaporated coatings, Wear 2013, 297, 1111–1119.
- Krause, B.; Kaufholz, M.; Kotapati, S.; Schneider, R.; Müller, E.; Gerthsen, D.; Wochner, P.; Baumbach, T. Angle-resolved X-ray reflectivity measurements during off-normal sputter deposition of VN, Surf. & Coat. Technol. 2015, 277, 52–57. [Google Scholar]
- Hajihoseini, H.; Gudmundsson, J. T. Vanadium and vanadium nitride thin films grown by high power impulse magnetron sputtering, J. Physics D: Applied Physics 2017, 50, 505302. [Google Scholar] [CrossRef]
- Fangfang, G.; Zhu, P.; Fanping, M. Enhancing the wear resistance of magnetron sputtered VN coating by Si addition, Wear 2016, 354–355, 32-40.
- Rabadzhiyska, S.; Dechev, D.; Ivanov, N. , Ivanova, T. ; Strijkova, V.; Katrova, V.; Rupetsov, V.; Dimcheva, N.; Valkov, S. Wear and Corrosion Resistance of ZrN Coatings Deposited on Ti6Al4V Alloy for Biomedical Applications, Coatings 2024, 14, 1434. [Google Scholar]
- W. C. Oliver, G.M. Pharr, Measurement of hardness and elastic modulus by instrumented indentation: Advances in understanding and refinements to methodology, J. Mater. Res. 2004, 19, 3–20.
- Hajihoseini, H.; Gudmundsson, T. , Vanadium and vanadium nitride thin films grown by high power impulse magnetron sputtering, J. Phys. D: Appl. Phys. 2017, 50, 505302. [Google Scholar] [CrossRef]
- Nelkel, A. ; The Physics and Chemistry of Carbides; Nitrides and Borides, Kluwer Academic Publishers, Netherlands in: R. Freer (Ed.) (1990) 279–296.
- Iordanova, P.J. Kelly, R. Mircheva, V. Antonov, Crystallography of magnetron sputtered TiN coatings on steel substrates, Vacuum 81 (2007) 830–842.
- Pelleg, J.; Zevin, L-Z.; Lungo, S.; Croitoru, N.; Reactive sputter- deposited TiN films on glass substrates, Thin Solid Films 1991 197, 117–128.
- Jinghua, L.; Fengfan, L.; Weiwei, L.; Xin, L. Effect of calcination temperature on the microstructure of vanadium nitride/nitrogen doped graphene nanocomposites as anode materials in electrochemical capacitors, Inorg. Chem. Front. 2019, 6, 164–171. [Google Scholar]
- Osonkie, V.; Chukwunenye, PL.; Cundari, T.; Kelbera, J. ; Plasma modification of vanadium oxynitride surfaces: characterization by in situ XPS experiments and DFT calculations, J. Chem. Phys. 2020, 153, 144709. [Google Scholar] [CrossRef]
- Liu, H-H,; Zhang, H-L. ;, Xu, H-B. ; Lou, T-P.; Sui, Z-T.; Zhang, Y. Hierarchically nanostructured vanadium nitride microspheres assembled with porous nanosheets fabricated by a template-free route, Ceram. Int. 2018, 44, 1583–1588. [Google Scholar]
- Chua, L.; Muhammad, Azizi, Y.; Mahdi,T.; Maslinda, K.; Mustapha, Ali T.; Norlin, N.;, Wan, Fahmin, A.; Effect of substrate roughness and PVD deposition temperatures on hardness and wear performance of AlCrN-coated WC-Co, Surf. & Coat. Technol. 2022, 436, 128304.
- Sánchez, E.; Sanchéz, M.; Ipaz, L.; Aperador, W.; Caicedo, J.; Amaya, C.; Hernández, M.; Landaverde, F.; Beltran, E.; Muñoz-Saldaña, J.; Zambrano, G. Mechanical, tribological, and electrochemical behavior of Cr1 xAlxN coatings deposited by r. f. reactive magnetron co-sputtering method, Appl. Surf. Scie. 2010, 256, 2380–2387. [Google Scholar]
- Kong, Q.; Ji, L.; Li, H.; Liu, X.; Wang, Y.; Chen, J.; Zhou, H. Influence of substrate bias voltage on the microstructure and residual stress of CrN films deposited by medium frequency magnetron sputtering, Mater. Scien. and Enginer.: B 2011, 176, 850–854. [Google Scholar] [CrossRef]
- Chang, H.; Huang, P. ; Yeh, J; Davison, A.; Tsau, C.; Yang, C. Influence of substrate bias, deposition temperature and post-deposition annealing on the structure and properties of multi-principal-component (AlCrMoSiTi)N coatings, Surf. Coat. Technol. 2008, 202, 3360-3366.
- K. Wasa, Handbook of Sputter Deposition Technology, S. Hayakawa (U.S.A., Westwood, New Jersey, 1992).
- Camacho-Espinosa, E.; Rosendo E.; Díaz, T.; Oliva, A.; Rejon, V.; Peña, J. Effects of temperature and deposition time on the RF- sputtered CdTe films preparation. Superf. vacío 2014, 27.
- Aissani, L.; Fellah, M.; Nouveau, C.; Samade, M.; Montagne, A.; Iost, A. Structural and mechanical properties of Cr–Zr–N coatings with different Zr content, Surf. Eng. 2017, 35, 1–9. [Google Scholar]
- Kiryukhantsev-Korneev, P.; Pierson, J.; Petrzhik, M.; Alnot, M.; Levashov, E.; Shtansky, D. Effect of nitrogen partial pressure on the structure, physical and mechanical properties of CrB2 and Cr–B–N films, Thin Solid Films 517 (2009) 2675–2680.
- Aissani, L.; Nouveau, C.; Walock, M.; Djebaili, H.; Djelloul, A. Influence of vanadium on structure, mechanical and tribological properties of CrN coatings. Surf. Eng. 31 (2015) 779–788.
- Zhang, S.; Yan, F.; Yang, Y.; Yan, M.; Zhang, Y.; Guo, J.; Li, H. ; Effects of sputtering gas on microstructure and tribological properties of titanium nitride films. Appl. Surf. Sci. 2019, 488, 61–69. [Google Scholar] [CrossRef]
- Zhang, B.; Qigang, H.; Zhang, J.; Han, Z.; Niu, S.; Luquan, R. Advanced bio-inspired structural materials: Local properties determine overall performance, Materials Today 2020, 41,177-197.
- Guo, H.; Lu, C.; Zhang, Z.; Liang, B.; Jia, J. Comparison of microstructures and properties of VN and VN/Ag nanocomposite films fabricated by pulsed laser deposition, Applied Physics A 2018, 124, 694.
- Pfeiler-Deutschmann, M.; Mayrhofer, P.; Chladil, K.; Penoy, M.; Michotte, C. ; Kathrein, M, Effect of wavelength modulation of arc evaporated Ti–Al–N/Ti–Al–V–N multilayer coatings on microstructure and mechanical/tribological properties. Thin Solid Films 2015, 581, 20–24. [Google Scholar] [CrossRef]
- Pfeiler, M.; Kutschej, K.; Penoy, M.; Michotte, C.; Mitterer, C.; Kathrein, M. The effect of increasing V content on structure, mechanical and tribological properties of arc evaporated Ti–Al–V–N coatings, Int. J. Refract. Metals Hard Mater. 2009, 27, 502–506. [Google Scholar] [CrossRef]
- Lv. ; Y.; Ji, L.; Liu, X.; Li, H.; Zhou, H. Influence of substrate bias voltage on structure and properties of the CrAlN films deposited by unbalanced magnetron sputtering. Appl. Phys. Lett. 2012, 258, 3864–3870. [Google Scholar]
- Gassner, G.; Mayrhofer, P.; Kutschej, K.; Mitterer, C.; Kathreinc, M. A new low friction concept for high temperatures: lubricious oxide formation on sputtered VN coatings, Tribology Letters 2004, 17, 751-756.
- Qiu, Y.; Zhang, S.; Li, B.; Wang, Y. ; Lee, Jyh-W. ; Li, F.; Zhao, D. Improvement of tribological performance of CrN coating via multilayering with VN, Surf.& Coat. Techn. 2013, 231, 357–363. [Google Scholar]
- Wiklund, U.; Casas, B.; Stavlid, N. Evaporated vanadium nitride as a friction material in dry sliding against stainless steel, Wear 2006, 261, 2–8.
- Fattah, N.; Fontalvo, G.; Gassner, G.; Mitterer, C. Influence of high-temperature oxide formation on the tribological behavior of TiN and VN coatings, Wear 2007, 262, 1152–1158.
- Cai, Z.; Pu, J.; Lu, X.; Jiang, X.; Wang, L.; Xue, Q. Improved tribological property of VN film with the design of pre-oxidized layer, Cer. Inter. 2019, 45, 6051–6057. [Google Scholar]
- Ju, H.; Yu, D.; Xu, J.; Yu, L.; Geng, Y.; Gao, T., Yi, G.; Bian, S. Microstructure, mechanical, and tribological properties of niobium vanadium carbon nitride films, J. Vac. Sci. Technol. A 2018 , 36, 1–7.
- Holmberg, K.; Mathews, A. Coatings tribology: a concept, critical aspects and future directions, Thin Solid Films 1994, 253, 173-178.
- Malik, G.; Kumar, A.; Adalati, R.; Sharma, S.; Bansal, A.; Chandra, R. Enhanced electrochemical corrosion resistance of SS(304L) alloy with nano-pyramids c-Tina layer for saline media application, J. Alloys and Metallur.Systems 2023, 3, 100028. [Google Scholar] [CrossRef]
- Toloei, A.; Stoilov, V.; Northwood, D. The Relationship Between Surface Roughness and Corrosion, Proceedings of the ASME 2013 International Mechanical Engineering Congress & Exposition IMECE 65498.
Figure 1.
A principle scheme of the direct current magnetron sputtering technology [
22].
Figure 1.
A principle scheme of the direct current magnetron sputtering technology [
22].
Figure 2.
XRD patterns of VN coatings at different temperatures.
Figure 2.
XRD patterns of VN coatings at different temperatures.
Figure 3.
Deconvolution of photoelectron spectra of: (a) C1s; (b) V2p3/2 of VN layers at various temperatures.
Figure 3.
Deconvolution of photoelectron spectra of: (a) C1s; (b) V2p3/2 of VN layers at various temperatures.
Figure 4.
Photoelectron spectra of: (a) N1s; (b) O1s and V2p of VN layers, deposited at substrate different temperatures.
Figure 4.
Photoelectron spectra of: (a) N1s; (b) O1s and V2p of VN layers, deposited at substrate different temperatures.
Figure 5.
Three- dimensional AFM images of: (a) the base material and VN coatings deposited at: (b) 250 °C; (c) 300 °C; (d) 350 °C.
Figure 5.
Three- dimensional AFM images of: (a) the base material and VN coatings deposited at: (b) 250 °C; (c) 300 °C; (d) 350 °C.
Figure 6.
Variation of the thickness with the substrate temperature.
Figure 6.
Variation of the thickness with the substrate temperature.
Figure 7.
Two-dimensional optical images of the VN coatings, deposited at: (a) 250 °C; (b) 300 °C; (c) 350 °C.
Figure 7.
Two-dimensional optical images of the VN coatings, deposited at: (a) 250 °C; (b) 300 °C; (c) 350 °C.
Figure 8.
Variation of hardness and elastic modulus of the VN films with the substrate temperature.
Figure 8.
Variation of hardness and elastic modulus of the VN films with the substrate temperature.
Figure 9.
Coefficient of friction (COF) obtained in ball-on-flat test of VN coatings (temperature deposition – 250, 300 and 350 °C sliding against hardened steel ball at room temperature).
Figure 9.
Coefficient of friction (COF) obtained in ball-on-flat test of VN coatings (temperature deposition – 250, 300 and 350 °C sliding against hardened steel ball at room temperature).
Figure 10.
Electrochemical impedance spectra (Nyquist plots) of VN coated stainless steel samples, treated at the following temperatures: 250, 300 and 350 o C.
Figure 10.
Electrochemical impedance spectra (Nyquist plots) of VN coated stainless steel samples, treated at the following temperatures: 250, 300 and 350 o C.
Figure 11.
Cyclic voltammograms of VN coated stainless steel in 0.1 M phosphate buffer, pH = 7.0; scan rate 50 mV/s, room temperature.
Figure 11.
Cyclic voltammograms of VN coated stainless steel in 0.1 M phosphate buffer, pH = 7.0; scan rate 50 mV/s, room temperature.
Figure 12.
Impedance studies of VN: electrochemical impedance spectra (Nyquist plots) of VN coated stainless steel samples treated at different temperatures. The EIS tests followed the polarization measurements.
Figure 12.
Impedance studies of VN: electrochemical impedance spectra (Nyquist plots) of VN coated stainless steel samples treated at different temperatures. The EIS tests followed the polarization measurements.
Table 1.
XPS results of quantitative composition on the surface.
Table 1.
XPS results of quantitative composition on the surface.
| VN samples |
C, at.% |
O, at.% |
V, at.% |
N, at.% |
| 250oC |
68.5 |
16.3 |
4.4 |
10.8 |
|
300oC
|
77.1 |
12.2 |
2.6 |
8.1 |
|
350oC
|
70.2 |
17.3 |
3.6 |
8.9 |
Table 2.
The percentages of V from various species.
Table 2.
The percentages of V from various species.
| Binding energy, eV |
Oxidation state |
|
Concentration, % |
|
| |
|
250oC |
300oC |
350oC |
| 513.7 |
V3+ →V-N |
40.0 |
27.3 |
32.7 |
| 515.2 |
V3+ →V-O |
38.6 |
23.7 |
37.6 |
| 517.0 |
V4+ →V-O |
36.9 |
26.0 |
37.0 |
Table 3.
Mechanical characteristics of the deposited VN films at different temperatures.
Table 3.
Mechanical characteristics of the deposited VN films at different temperatures.
| VN samples |
Hardness, GPa |
Elastic modulus, GPa |
H/E ratio |
H3/E2 ratio |
| 250oC |
9.7 ± 0.8 |
198 ± 18 |
0.048 |
0.023 |
|
300oC
|
10.6 ±1.4 |
208 ± 28 |
0.051 |
0.027 |
|
350oC
|
9.5 ± 0.6 |
191 ± 14 |
0.050 |
0.024 |
Table 4.
Friction coefficients at different time of the VN films, deposited at 304L SS substrates temperatures.
Table 4.
Friction coefficients at different time of the VN films, deposited at 304L SS substrates temperatures.
| Time, min |
VN – 250 oC |
VN – 300 oC |
VN – 350 oC |
| 5 |
0.3546 |
0.4569 |
0.4579 |
| 10 |
0.4825 |
0.5458 |
0.5226 |
Table 5.
Open circuit potentials of the VN coated stainless steel (annealed at temperatures 250, 300 and 350oC) determined in 0.1 M KCl, neutral aqueous medium.
Table 5.
Open circuit potentials of the VN coated stainless steel (annealed at temperatures 250, 300 and 350oC) determined in 0.1 M KCl, neutral aqueous medium.
| No |
Sample |
OCP, V |
OCP2 , V* |
| 1 |
VN 250 oC |
0.245 |
0.266 |
| 2 |
VN 300 oC |
0.236 |
0.266 |
| 3 |
VN 350 oC |
0.224 |
0.264 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).