Submitted:
02 July 2025
Posted:
02 July 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
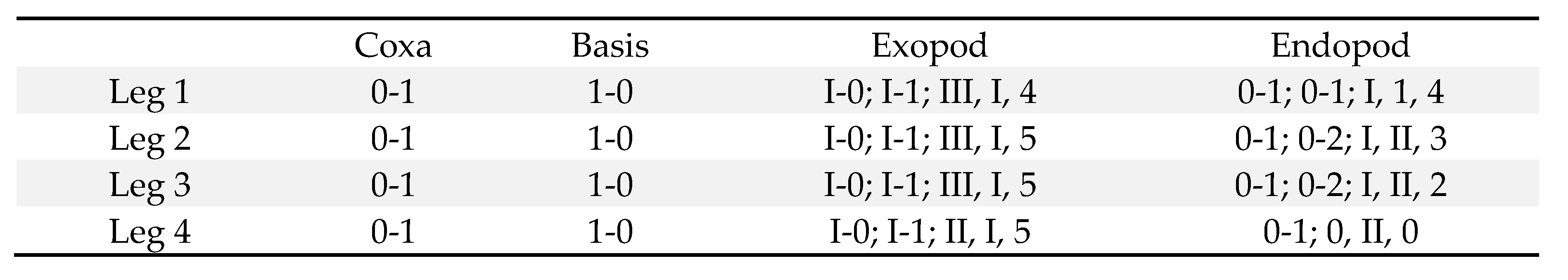
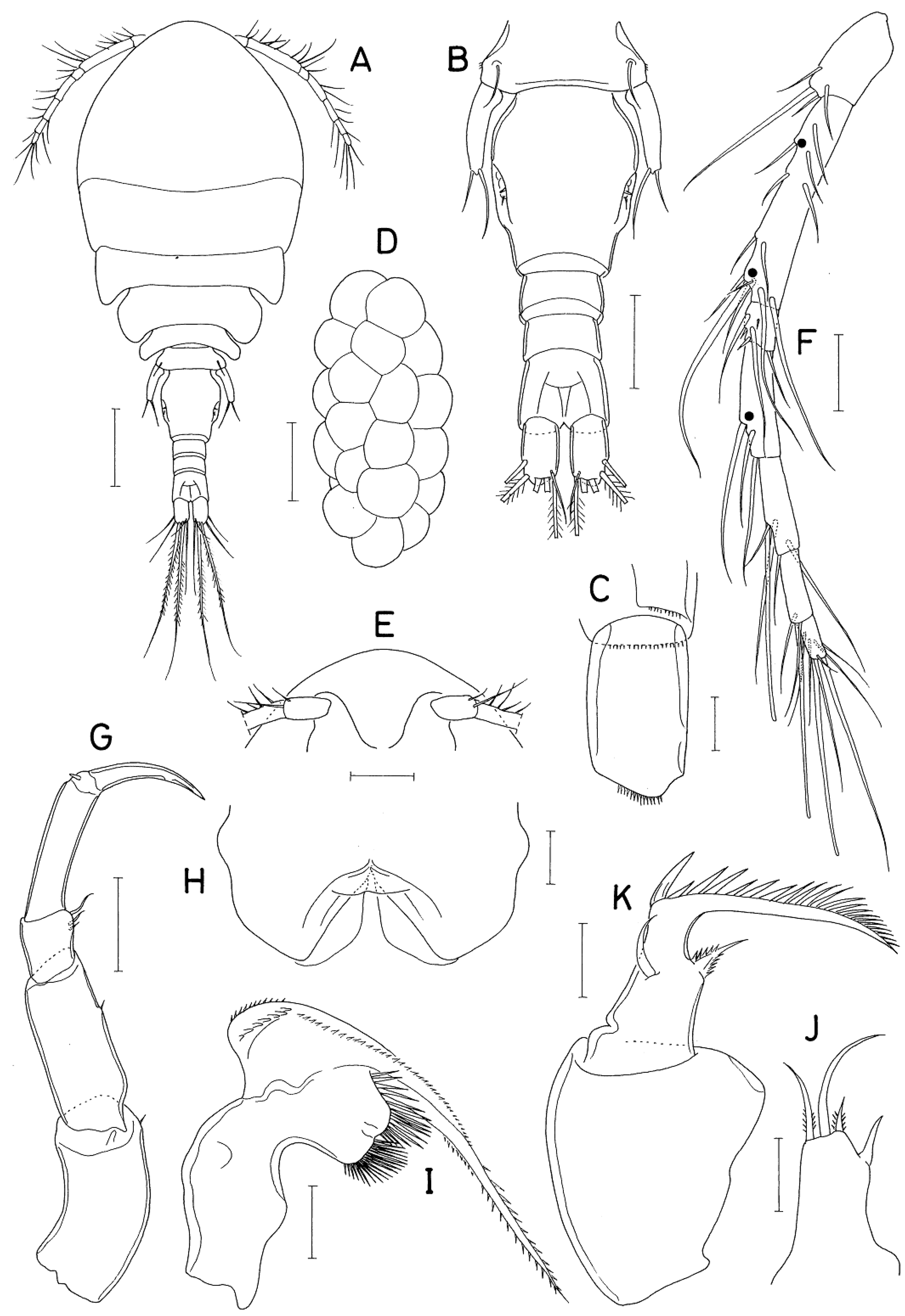
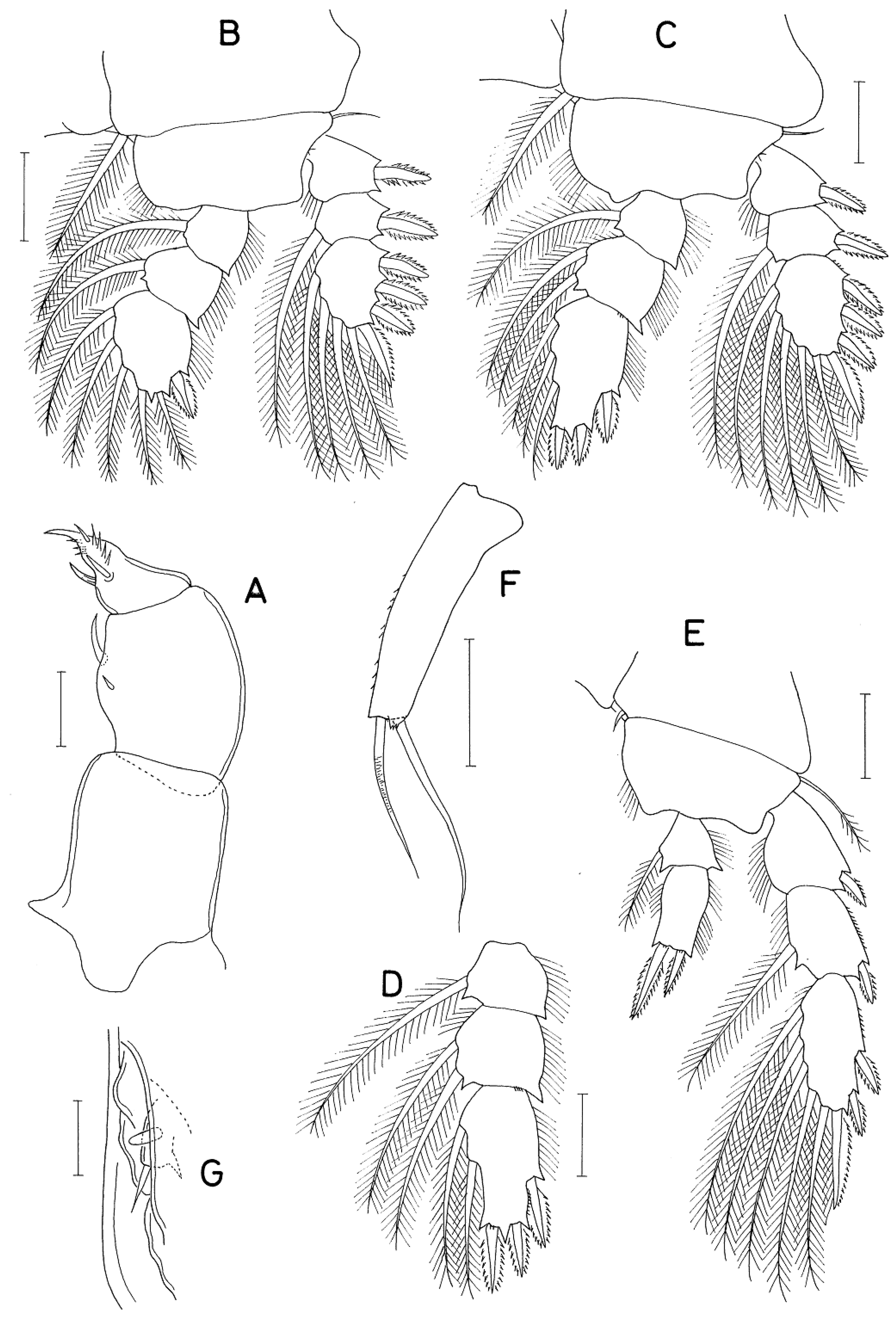
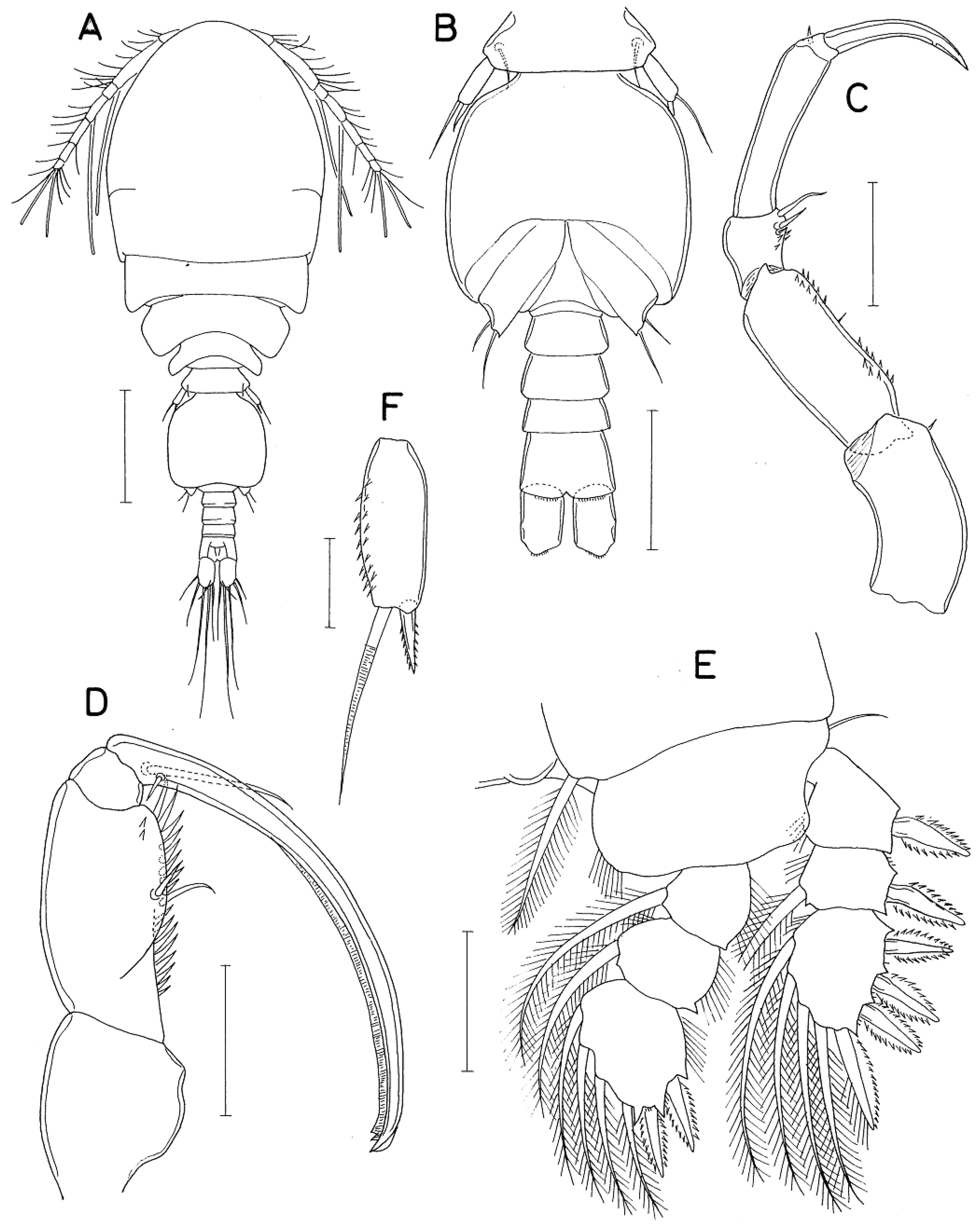
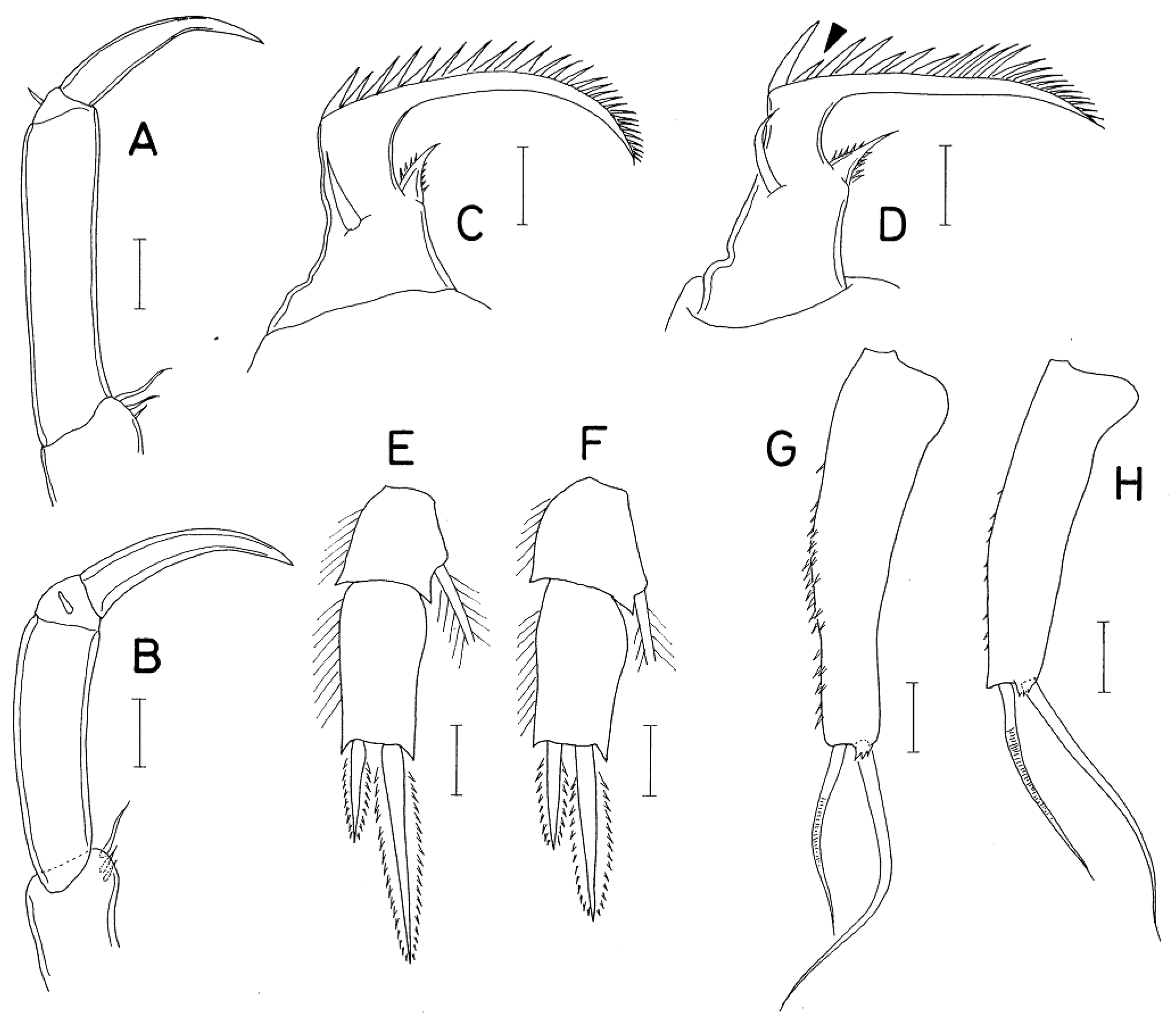
3. Taxonomic Description
4. Discussion
4.1. Species Characterization
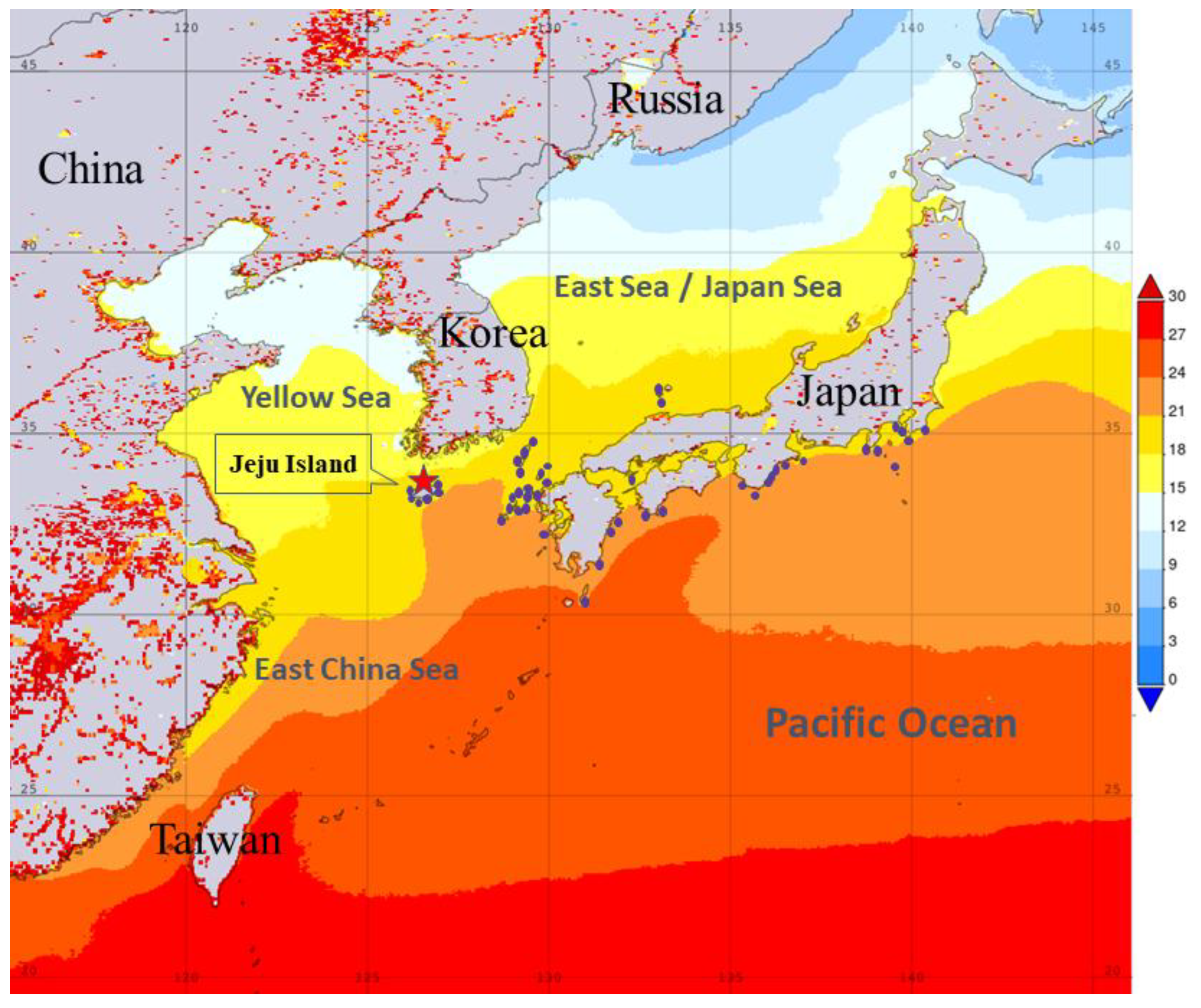
4.2. Ecological Implications of the Discovery of the Symbiotic Copepod Anchimolgus jejuicus n. sp. on the Scleractinian Coral Alveopora japonica from Jeju Island
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Walter, T.C.; Boxshall, G.A. World of Copepods Database 2025. Accessed at https://www.marinespecies.org/copepoda on 2025-04-16.
- Humes, A.G.; Boxshall, G.A. A revision of the lichomolgoid complex (Copepoda: Poecilostomatoida), with the recognition of six new families. J. Nat. Hist. 1996, 30, 175–227. [Google Scholar] [CrossRef]
- Boxshall, G.A.; Halsey, A.H. An Introduction to Copepod Diversity. The Ray Society, London, 2004, 966 pp.
- WoRMS Editorial Board. World Register of Marine Species, 2025. Available from: http://www.marinespecies.org (accessed 14 April 2025).
- Humes, A.G. Cyclopoid copepods (Lichomolgidae) from fungiid corals in New Caledonia. Zool. Anz. 1973, 190, 312–333. [Google Scholar]
- Kim, I.-H. Copepods (Crustacea) associated with marine invertebrates from New Caledonia. Korean J. Syst. Zool. 2003, Special Issue 4. 1–167.
- Kim, I.-H. Copepods (Crustacea) associated with marine invertebrates from the Moluccas. Korean J. Syst. Zool. 2007, Special Issue 6, 1–126. [Google Scholar]
- Kim, I.-H. Siphonostomatoid Copepoda (Crustacea) associated with invertebrates from tropical waters. Korean J. Syst. Zool. 2010, Special Issue 8, 1–176. [Google Scholar]
- Humes, A.G. How many copepods? Hydrobiologia 1994, 292/293, 1–7. [Google Scholar] [CrossRef]
- Denis, V.; Chen, C.A.; Song, J.I.; Woo, S. Alveopora japonica beds thriving under kelp. Coral Reefs 2013, 32, 503. [Google Scholar] [CrossRef]
- Vieira, C.; Keshavmurthy, S.; Ju, S.-J.; Hyeong, K.; Seo, I.; Kang, C.-K.; Hong, H.-K.; Chen, C.A; Choi, K.S. Population dynamics of a high-latitude coral Alveopora japonica Eguchi from Jeju Island, off the southern coast of Korea. Mar. Freshwater Res. 2016, 67, 594–604. [Google Scholar] [CrossRef]
- Lee, K.-T.; Lee, H.-M.; Subramaniam,T. ; Yang, H.-S.; Patk, S.R.; Kang, C.-K.; Keshavmurthy, S.; Choi, K.-S. Dominance of the scleractinian coral Alveopora japonica in the barren subtidal hard bottom of high-latitude Jeju Island off the south coast of Korea assessed by high-resolution underwater images. PLoS One 2022, 17, e0275244. [Google Scholar] [CrossRef]
- Noseworthy, R.G.; Hong, H.-K.; Ju, S.-J.; Yang, H.-S.; Choi, K.-S. Mollusk species associated with the scleractinian coral Alveopora japonica Eguchi, 1968 forming a coral carpet in northwestern Jeju Island. Ocean Polar Res. 2022, 44, 331–338. [Google Scholar]
- Shin, S.; Ribas-Deulofeu, L.; Subramaniam, T.; Lee, K.T.; Kang, C-K. ; Denis, V.; Choi, K.-S. The vertical distribution of Alveopora japonica provides insight into the characteristics and factors controlling population expansion at Jeju Island off the south coast of Korea. Mar. Biodiversity 2024, 54, 20. [Google Scholar] [CrossRef]
- Humes, A.G.; Gooding, R.U. A method for studying the external anatomy of copepods, Crustaceana 1964, 6, 238–240. [CrossRef]
- Huys, R.; Boxshall, G.A. Copepod evolution. Ray Society, London, 1991, 1-468. ISBN 0-903-87421-0.
- Sugihara, K.; Yamano, H. ; Choi, K-S.; Hyeong, K. Zooxanthellate scleractinian corals of Jeju Island, Republic of Korea. In: Nakano SI, Yahara T, Nakashizuka T (eds) Integrative observations and assessments. Ecological Research Monographs, Springer, Tokyo, 2014; pp. 111–130. [Google Scholar]
- Veron, J.E.N. Corals of the World. Vols. 1–3. Aust. Inst. Mar. Sci. 2000, 1410 pp.
- Dai, C.F.; Horng, S. Scleractinia fauna of Taiwan. I. The complex group. National Taiwan University, Taipei 2009, 1–172.
- Kang. J.H.; Jang, J.E.; Kim, J.H.; Kim, S.; Keshavmurthy, S.; Agostini, S.; Rimer, J.D.; Chen, C.A.; Choi, K.-S.; Park, S.R.; Lee, H.J. The origin of the subtropical coral Alveopora japonica (Scleractinia: Acroporidae) in high-latitude environments. Front. Ecol. Evol. 2020, 8, 12. [Google Scholar] [CrossRef]
- Song, J.-I. A study on the classification of the Korean Anthozoa 7. Scleractinia (Hexacorallia). Korean J. Zool. 1982, 25, 131–148. [Google Scholar]
- Denis, V.; Ribas-Deulofeu, L.; Loubeyres, M.; De Palmas, S.; Hwang, S.-J.; Woo, S.; Song, J.-I.; Chen, C.A. Recruitment of the subtropical coral Alveopora japonica in the temperate waters of Jeju Island, South Korea. Bull. Mar. Sci. 2015, 91, 85–96. [Google Scholar] [CrossRef]
- Ribas-Deulofeu, L.; Loubeyres, M.; Denis, V.; de Palmas, S.; Hwang, S.-J.; Woo, S.; Song, J.-I.; Chen, C.A. Chen. Jeju Island: a sentinel for tracking ocean warming impacts on high-latitude benthic communities. Coral Reefs 2023, 42, 1097–1112. [Google Scholar] [CrossRef]
- Matsumoto, A. ; Hashimoto, S; Arakawa, H. Short-term population dynamics of high-latitude Alveopora japonica in Tateyama Bay, Japan. Galaxea, J. Coral Reef Stud 2015, 17, 33–39. [Google Scholar]
- Preston, N.P.; Doherty, P.J. Cross-shelf patterns in the community structure of coral-dwelling Crustacea in the central region of the Great Barrier Reef. II. Cryptofauna. Mar. Ecol. Prog. Ser. 1994, 104, 27–38. [Google Scholar] [CrossRef]
- Hoeksema, B.W.; van der Meij. S.E.T.; Fransen, C.H.J.M. The mushroom coral as a habitat. J. Mar. Biol. Assoc. U. K. 2012, 92, 647–663. [Google Scholar] [CrossRef]
- Cheng, Y.R.; Mayfield, A.B.; Meng, P.-J; Dai, C.-F.; Huys, R. Copepods associated with scleractinian corals: a worldwide checklist and a case study of their impact on the reef-building coral Pocillopora damicornis (Linnaeus, 1758) (Pocilloporidae). Zootaxa 2016, 4174, 291–345. [Google Scholar] [CrossRef]
- Kim, I.-H.; Hong, J.-S. Copepods (Crustacea, Copepoda, Cyclopoida) associated with marine invertebrates from Thailand. Anim. Syst. Evol. Divers. 2014, 30, 274–318. [Google Scholar] [CrossRef]
- Cheng, Y.R. : Dai, C.F. Poecilostomatoid copepods associated with two species of widely distributed corals, Galaxea astreata (Lamarck, 1816) and Galaxea fascicularis (Linnaeus, 1767), in the South China Sea. Mar. Biodivers. 2016, 48, 1057–1072. [Google Scholar] [CrossRef]
- Kim, T.; Kim, T.; Yang, H.-S.; Choi, S K. ; Son, Y.B.; Kang, D.-H. Alveopora japonica conquering temperate reefs despite massive coral bleaching. Diversity, 2022, 14, 86. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




