1. Introduction
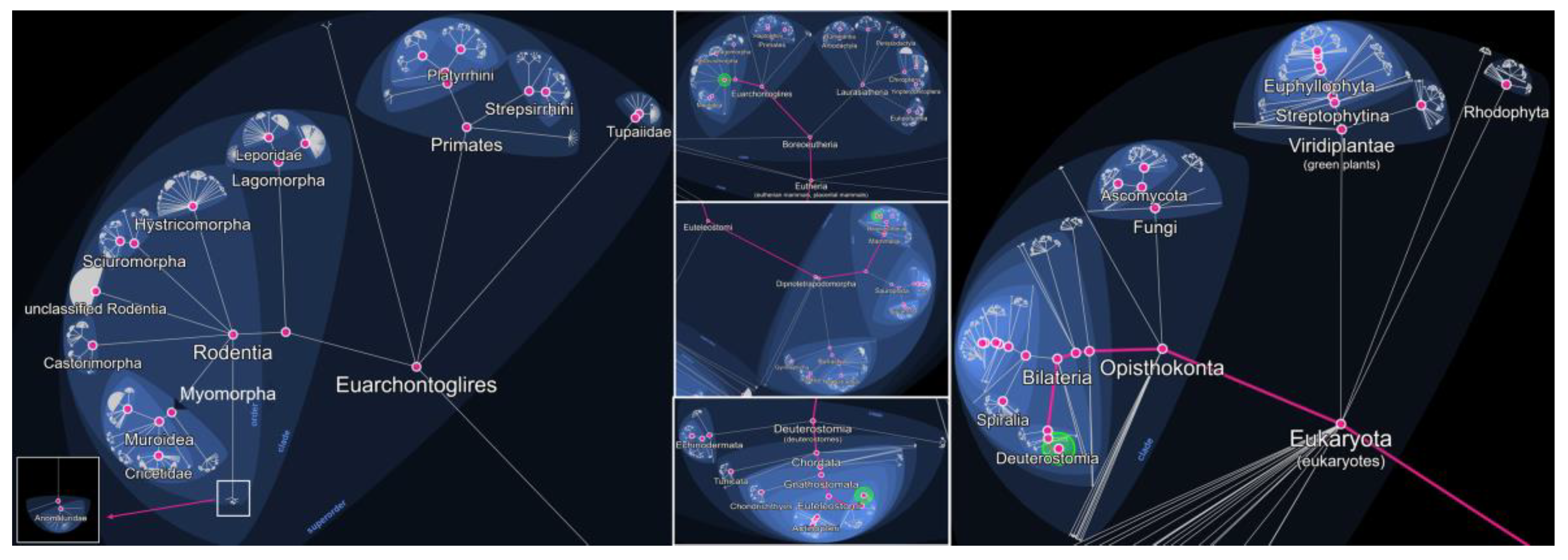
Rodents are among the most widespread and adaptable mammals on Earth, inhabiting a diverse range of ecosystems, from rural environments to densely populated urban centers. Rodentia is an order within the class Mammalia, subclass Theria (live-bearing mammals), and infraclass Eutheria (placental mammals) (
Figure 1). It belongs to the superorder Euarchontoglires, which also includes primates, lagomorphs (such as rabbits and hares), and treeshrews. Within Rodentia, over 2,000 recognized species are grouped into several suborders (
Figure 1), including Myomorpha (which comprises the family Muridae, including true rats, true mice, and gerbils) (
Figure 2), Sciuromorpha (squirrels and chipmunks), Hystricomorpha (porcupines and guinea pigs), and others. This order represents the largest and most diverse group of mammals, accounting for approximately 40% of all known mammalian species. Rodents are unified by a distinct dental formula, most notably a single pair of continuously growing incisors in both the upper and lower jaws, which they use for gnawing and feeding [
1,
2,
3].
Their proximity to human habitats, high reproductive rates, and capacity to carry a wide array of pathogens make them key reservoirs for zoonotic diseases. As urbanization, environmental disruption, and climate change continue to reshape rodent populations and their interactions with humans, the risk of transmission of rodent-borne pathogens is increasing. These dynamics have significant implications for public health, particularly in resource-limited settings where surveillance and control measures may be inadequate. Understanding the role of rodents in the ecology of infectious diseases is essential for developing effective prevention and mitigation strategies [
4,
5,
6].
Rodents are hosts to numerous zoonotic parasites that pose significant public health threats. Among the protozoan parasites,
Toxoplasma gondii is particularly concerning, as it causes toxoplasmosis, which can have a severe impact on immunocompromised individuals and pregnant women.
Trypanosoma cruzi, responsible for Chagas disease, is transmitted through triatomine bugs and can lead to severe cardiac and digestive complications. Other protozoa, such as
Leishmania spp.,
Giardia intestinalis, and
Cryptosporidium spp., also infect rodents and can be transmitted to humans through contaminated water, leading to gastrointestinal and systemic diseases [
7,
8].
Several helminths found in rodents also pose zoonotic risks.
Hymenolepis diminuta and
Hymenolepis nana are tapeworms that can infect humans via contaminated food or water.
Trichinella spiralis causes trichinellosis, a severe disease associated with the consumption of undercooked, infected meat.
Angiostrongylus cantonensis, known as the rat lungworm, is responsible for eosinophilic meningitis in humans, while
Capillaria hepatica can cause hepatic disease. Additionally,
Baylisascaris procyonis, a nematode found in rodents, has been associated with severe neurological disorders in accidental human hosts [
9,
10,
11].
Ectoparasites that infest rodents also contribute to the spread of zoonotic diseases. The oriental rat flea (
Xenopsylla cheopis) is the primary vector of
Yersinia pestis, the causative agent of plague, while Rickettsia typhi, transmitted by fleas, leads to murine typhus. The tropical rat mite (
Ornithonyssus bacoti) can cause dermatitis in humans, which may lead to secondary infections. Various tick species (
Ixodes spp.) and lice can also transmit diseases such as Lyme disease and relapsing fever [
12,
13,
14].
The presence of these zoonotic parasites in rodents highlights the importance of vector control and public health interventions. Poor sanitation, urbanization, and inadequate waste management contribute to the growth of rodent populations, thereby increasing the risk of disease transmission. Surveillance, rodent control programs, and hygiene measures are crucial in preventing outbreaks and mitigating the impact of rodent-associated parasitic infections on human health (
Table 1) [
15,
16].
2. Protozoa
2.1. Toxoplasma gondii
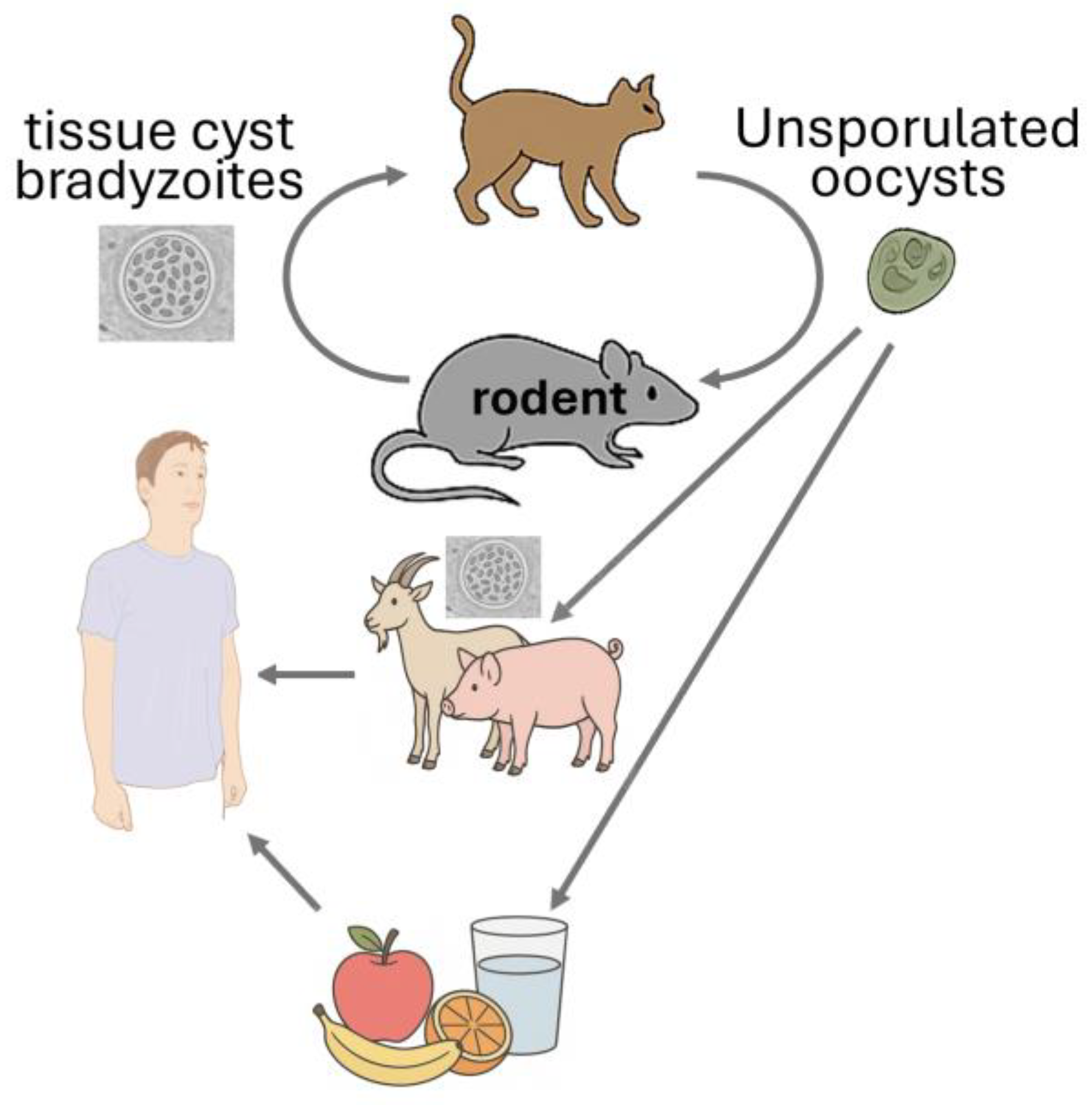
Toxoplasma gondii is a highly significant protozoan parasite that infects rodents and plays a crucial role in its life cycle (
Figure 2). Rodents serve as intermediate hosts, harbouring the parasite in tissue cysts, particularly in the brain and muscles. This infection can alter rodent behaviour, making them more susceptible to predation by felines, the definitive hosts. This behavioural alteration is believed to result from the parasite’s interference with neural signalling in the amygdala, a small, almond-shaped brain structure located deep within the temporal lobes. The amygdala is central to the processing of fear, anxiety, aggression, memory, and decision-making. When its normal function is disrupted, rodents may fail to respond appropriately to threats, increasing their risk of predation by cats. This manipulation enhances the likelihood of the parasite completing its life cycle (
Table 1), representing a remarkable example of evolutionary adaptation. A meta-analysis identified that the overall seroprevalence was calculated at 6% (95%CI=6-7%), with the highest amount observed in Africa (24%) and South America (18%) [
17]. Research conducted in Egypt found that 18% (44 out of 244) of examined rodents tested positive for
T. gondii DNA using PCR methods [
18]. A study in Croatia and Slovenia detected
T. gondii DNA in 2.94% of rodents across five species, including
Rattus rattus and
Mus musculus, collected from waste disposal sites [
19].
From a public health perspective, rodents play a critical role in environmental contamination. Although they are not definitive hosts and do not shed oocysts directly, their presence in human habitats contributes to the maintenance of T. gondii transmission cycles. They may serve as prey for cats, which then shed infectious oocysts in their faeces, contaminating soil, water, and food sources. Consequently, controlling rodent populations and improving hygiene practices are essential measures for reducing human exposure and preventing toxoplasmosis[
20,
21].
2.2. Trypanosoma cruzi
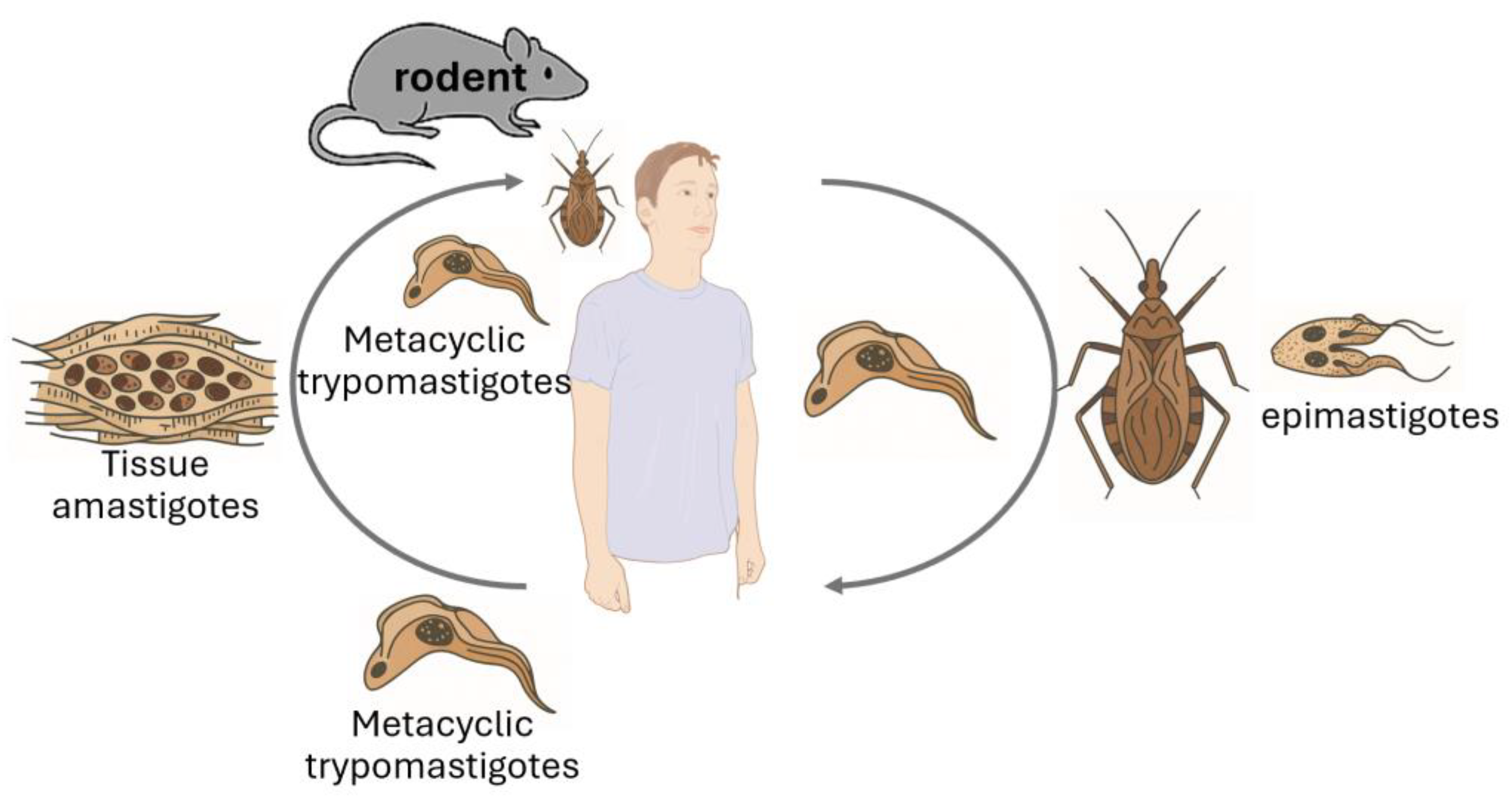
Trypanosoma species are protozoan parasites with significant implications for both animal and human health [
22,
23] (
Table 1). Rodents serve as natural reservoirs for various
Trypanosoma species, facilitating their maintenance and transmission within ecosystems (
Figure 3).
Trypanosoma cruzi, the causative agent of Chagas disease, utilizes rodents as reservoir hosts, playing a crucial role in the epidemiology of this disease. Given the global distribution of these parasites, monitoring rodent populations is essential for understanding transmission dynamics and mitigating public health risks [
24,
25].
Several studies have investigated the prevalence of
Trypanosoma infections in rodent populations. A study conducted in San Luis province, Argentina, examined wild rodents for
T. cruzi infection. Out of 25 isolates obtained, 44% were identified as
T. cruzi, while the remaining 56% were classified as
T. cruzi-like organisms. This research highlights the role of wild rodents in maintaining the transmission cycle of
T. cruzi in areas with low endemicity [
26]. Research in a rural community in Chile assessed
T. cruzi infection in synanthropic and wild rodents. The study found that 83.1% of the rodents were infected with
T. cruzi, with parasite loads comparable to those observed in human cases. This high prevalence underscores the potential risk of zoonotic transmission in rural settings [
27]. Meanwhile, an analysis in New Mexico, USA, identified an 11% infection rate of
T. cruzi among 1,428 rodents tested, underscoring the role of rodents in the transmission dynamics of Chagas disease [
28].
These studies highlight the importance of rodent surveillance in controlling
Trypanosoma infections. Rodents contribute to the environmental spread of these parasites, which can potentially lead to zoonotic outbreaks. Understanding their prevalence in different regions helps implement targeted vector control measures, reducing the risk of human and animal infections [
29].
2.3. Leishmania spp.
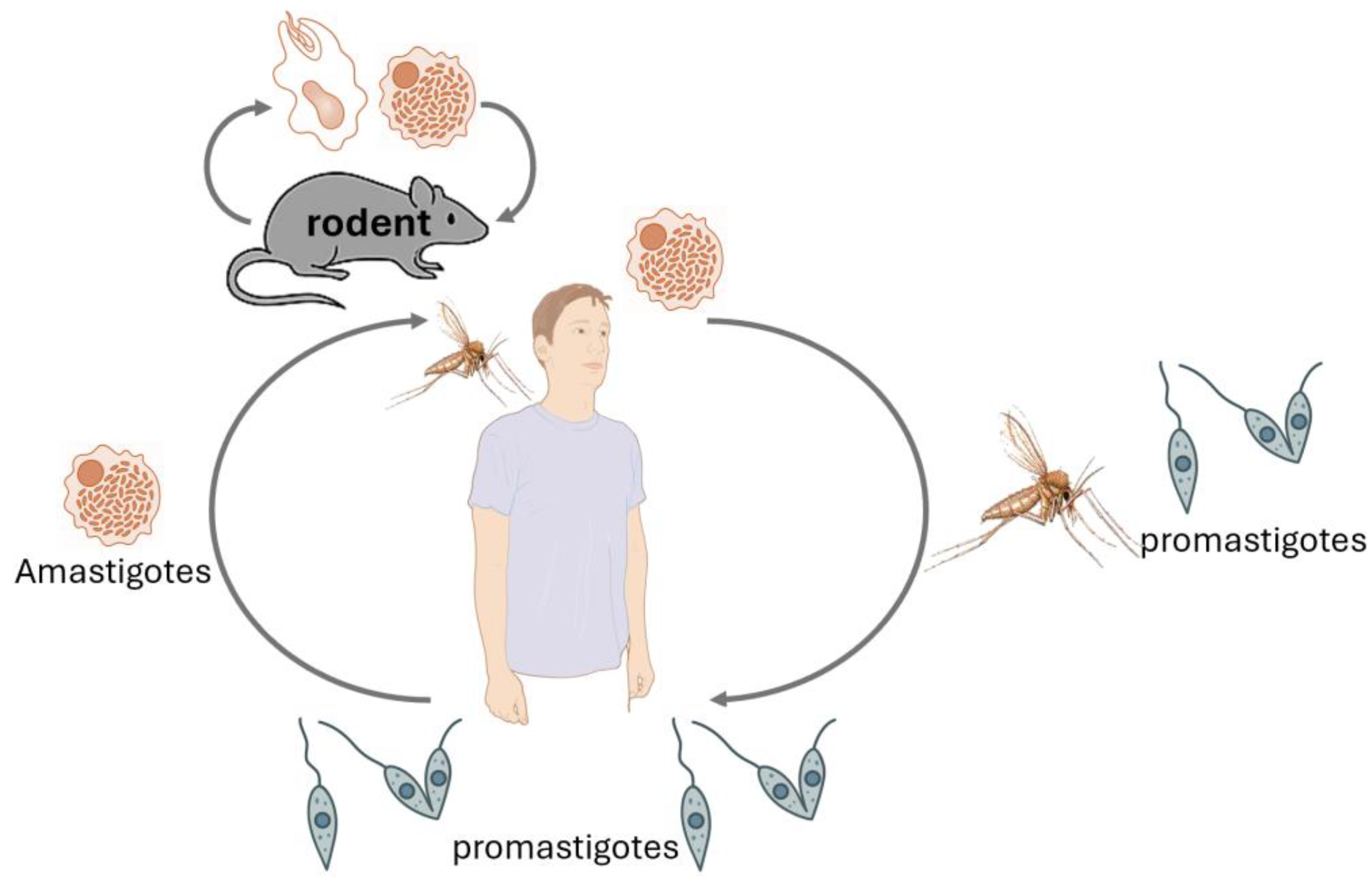
Leishmania species are protozoan parasites responsible for leishmaniasis, a disease affecting humans and animals [
30,
31,
32,
33] (
Table 1), still highly prevalent worldwide and concerning, especially the visceral form associated with significant fatal outcomes (
Table 2). Rodents play a crucial role as reservoir hosts in the transmission cycle of various
Leishmania species (
Figure 4). Their close association with human habitats facilitates the spread of the parasite to sand fly vectors, which subsequently transmit the infection to humans (
Figure 4) and other animals, especially dogs (
Figure 5). Understanding the prevalence of
Leishmania in rodent populations is essential for developing effective control strategies and reducing the incidence of leishmaniasis [
34,
35].
Several studies have investigated the prevalence of
Leishmania infections in rodents. Research in Barcelona, Spain, revealed a 33.3% prevalence of
Leishmania infection among Norway rats (
Rattus norvegicus) inhabiting the city's sewer system [
36]. This high infection rate suggests that urban rodent populations can serve as significant reservoirs for the parasite, potentially impacting public health. A study conducted in Greece found that 54.55% of examined rodents had been exposed to
Leishmania spp., as evidenced by serological tests [
37]. This indicates a substantial exposure rate among rodent populations, underscoring their role in the epidemiology of leishmaniasis in the region. In northeastern Brazil, a longitudinal study reported persistent
Leishmania (Viannia) braziliensis infections in wild rodent populations over a 13-month period. Notably, a median of 48% of exposed sandflies became infected after feeding on these rodents, highlighting the rodents' significant role in maintaining the transmission cycle of leishmaniasis [
38].
These studies emphasize the importance of monitoring rodent populations as part of integrated leishmaniasis control programs [
36,
37,
38]. Public health initiatives can more effectively reduce the transmission of
Leishmania to humans by identifying and targeting rodent reservoirs. Surveillance, vector control, and habitat management are key strategies in limiting the spread of this parasitic disease.
2.4. Giardia intestinalis
Giardia intestinalis (also known as
G. duodenalis or
G. lamblia) is a flagellated protozoan parasite that causes giardiasis, an enteric disease characterized by diarrhea, malabsorption [
39], and abdominal discomfort, which is still prevalent, especially in low and middle-income countries [
40,
41]. Rodents are significant reservoirs for
Giardia, and multiple genotypes (assemblages) have been identified in these hosts, some of which are zoonotic [
42]. The parasite is transmitted via the fecal-oral route, primarily through the ingestion of cysts in contaminated water, food, or environments (
Table 1) [
43].
Studies have demonstrated a high prevalence of
G. intestinalis in wild and synanthropic rodent populations [
44,
45]. In urban areas, rodents such as
Rattus norvegicus and
Mus musculus frequently harbor
Giardia cysts, contributing to environmental contamination [
46,
47]. In agricultural and peri-urban settings, wild rodent species have also been implicated in maintaining
Giardia in the environment, potentially impacting livestock and human populations [
47]. In a study in Qatar,
Giardia spp. were detected in 4.1% of rodents by microscopy, predominantly in young animals. However, PCR confirmed that these were not Giardia intestinalis, suggesting the presence of non-zoonotic species, such as
G. muris or
G. microti [
48].
Molecular studies have revealed that rodents carry both host-specific and zoonotic
Giardia assemblages, including Assemblages A and B, which are commonly found in humans. This suggests the potential for direct or indirect zoonotic transmission from rodents to humans, particularly in settings with poor sanitation or inadequate water treatment. Additionally, rodents may act as sentinels for environmental contamination with
Giardia, underscoring their importance in public health surveillance [
47,
49].
A study in Hungary found that by parasitological examination, cysts in 58.3% of asymptomatic Norway rats and 27.6% of chinchillas were identified [
44]. Additionally,
Giardia infection was detected in three degus (prevalence: 16.7%) using the flotation technique. PCR analysis targeting three genetic markers yielded a positivity rate of 3.2%, while flotation revealed a higher prevalence of 21.9%. DNA sequencing was successfully performed on PCR products from five samples. Phylogenetic analysis of partial beta-giardin gene sequences indicated the presence of assemblages B and G in rats [
44].
In a systematic review and meta-analysis of five million animals,
G. intestinalis was detected in 19.3% of rodents, with assemblages A and B identified, indicating potential zoonotic transmission from wild rodents to humans in the study area [
50].
The presence of
Giardia in rodent populations underscores the need for integrated monitoring approaches. Improvements in water sanitation, waste disposal, and rodent control are essential strategies to reduce the risk of giardiasis outbreaks in human communities. Surveillance programs that include genotyping of
Giardia isolates from rodents, humans, and water sources can help elucidate transmission pathways and inform effective public health interventions [
51,
52,
53].
2.5. Cryptosporidium spp.
Cryptosporidium spp. are apicomplexan protozoan parasites that cause cryptosporidiosis, an intestinal disease marked by watery diarrhea, dehydration, and weight loss [
54,
55]. While several
Cryptosporidium species infect humans, rodents have emerged as essential reservoirs for both rodent-adapted and zoonotic species, such as
C. parvum,
C. muris, and
C. hominis [
56,
57]. Transmission occurs through ingestion of oocysts, which are excreted in feces and can contaminate water, food, or fomites (
Table 1).
Rodent populations in both urban and rural environments frequently harbor
Cryptosporidium spp., with varying infection rates depending on species, location, and season. Studies from urban centers in Asia and Latin America have reported
Cryptosporidium prevalence in rats ranging from 5% to over 25%, with higher rates often associated with inadequate sanitation infrastructure. In agricultural contexts, wild rodents can contaminate water supplies and crops, increasing the risk of transmission to livestock and humans [
58,
59].
Molecular analyses have revealed that rodents not only carry rodent-specific species (e.g.,
C. muris,
C. andersoni) but also zoonotic types, particularly
C. parvum, which poses a significant public health concern. The presence of zoonotic genotypes in rodent feces, particularly in areas with high human population densities, suggests a role for rodents in environmental contamination and potential outbreaks. Oocysts are resistant to conventional chlorination, making their presence in water systems particularly problematic [
60,
61,
62].
A recent systematic review found that
Cryptosporidium spp. were detected in 8.8% of rodents, with several novel genotypes identified, suggesting wild rodents may serve as reservoirs for diverse and potentially zoonotic
Cryptosporidium species [
63].
Rodents may serve as both amplifiers and indicators of environmental
Cryptosporidium contamination. In developing regions, cryptosporidiosis is a major contributor to childhood diarrheal disease, and the role of rodents in sustaining transmission cycles should not be underestimated. Control measures focusing on rodent exclusion from food and water sources, combined with improved water treatment and public education, are critical components of prevention strategies [
64,
65,
66].
Given the protozoan's resilience in the environment and the diversity of
Cryptosporidium species harbored by rodents, comprehensive surveillance, including genotyping and environmental monitoring, is necessary to understand and mitigate the zoonotic risks associated with rodent populations [
67,
68].
3. Helminths
3.1. Hymenolepis spp.
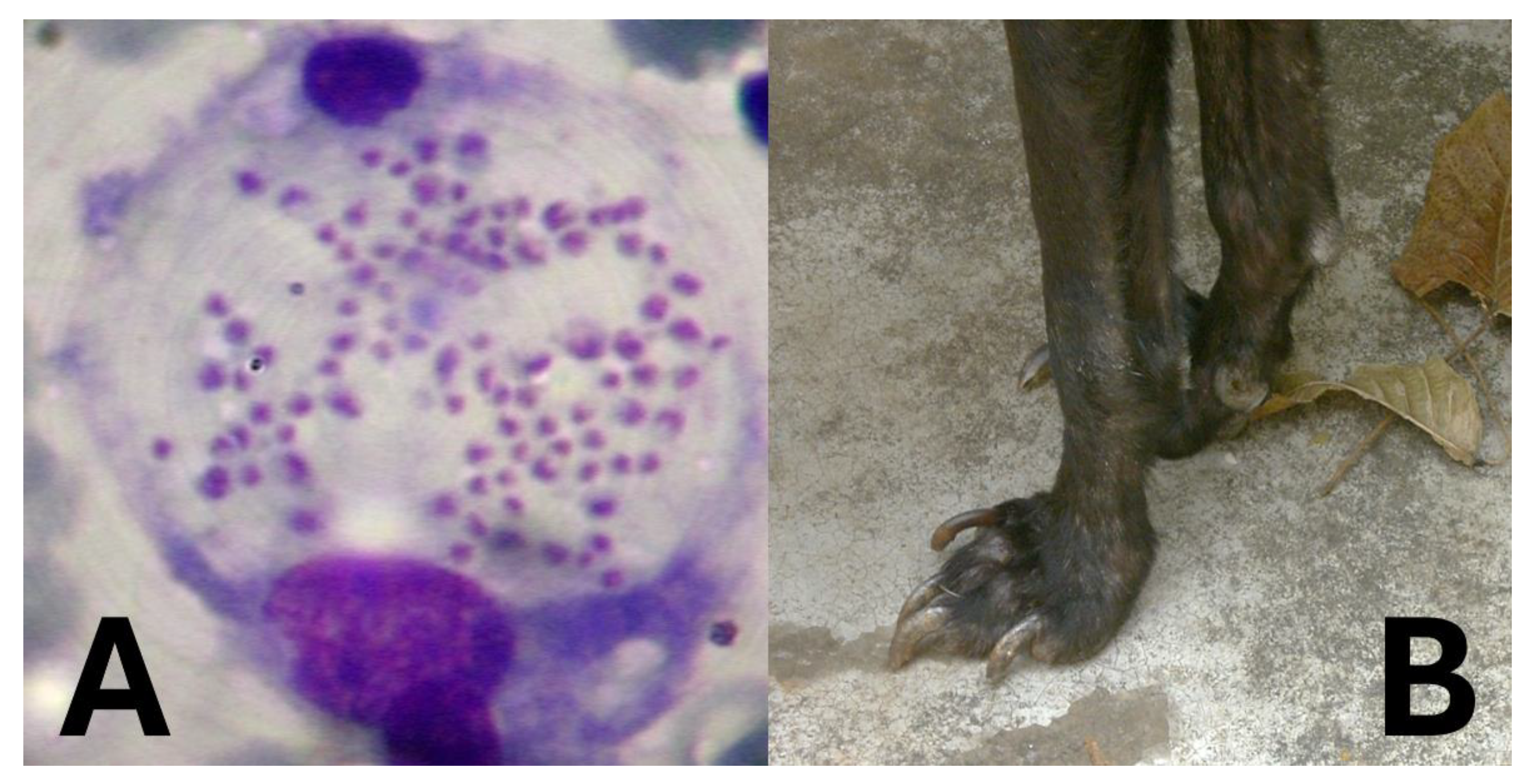
Hymenolepis spp. are cestode parasites commonly found in rodents, with two species,
Hymenolepis (H.) diminuta and
H. nana (
Figure 6), having zoonotic potential [
15,
69]. These tapeworms are frequently used as indicators of environmental contamination and can pose health risks to humans, particularly in areas with poor sanitation and high rodent activity (
Table 1) [
70].
H. diminuta, the rat tapeworm, primarily infects rodents as definitive hosts and requires arthropods (such as beetles or fleas) as intermediate hosts for its life cycle. Rodents acquire the infection by ingesting infected arthropods, while accidental ingestion of contaminated food can lead to human infection, particularly in children [
71,
72]. Although
H. diminuta infections in humans are relatively rare, they are documented in both urban and rural settings, and their presence is considered a marker of poor hygiene and rodent infestation. In rodent populations, prevalence can vary widely, from 5% to over 50%, depending on ecological conditions and the presence of intermediate hosts [
73,
74].
In a systematic review and meta-analysis, it was found that
H. diminuta (21.2%) and
H. nana (13.4%) were prevalent in rodents, indicating significant zoonotic potential and highlighting public health concerns in the studied urban environments [
75].
H. nana, also known as the dwarf tapeworm, is more epidemiologically significant due to its direct life cycle and ability to autoinfect the host. Unlike
H. diminuta,
H. nana does not require an intermediate host, although it can use arthropods facultatively [
76,
77]. This unique feature enables it to rapidly amplify within rodent hosts—and humans—resulting in high worm burdens and persistent infections.
H. nana is considered the most common cestode infection in humans globally, particularly in children, and is closely associated with rodent exposure and fecal-oral transmission. Rodents such as
Rattus norvegicus and
Mus musculus are important reservoirs, and studies in endemic regions have reported infection rates exceeding 60% in rodent populations [
78,
79].
Rodents infected with
Hymenolepis spp. contribute to environmental contamination by shedding eggs in feces, which can persist under favourable conditions. Human infection may lead to symptoms such as abdominal pain, diarrhea, anorexia, and irritability, although many cases remain asymptomatic. The autoinfective nature of
H. nana poses additional challenges in control, as reinfection can occur without further environmental exposure [
71,
80].
Public health interventions should prioritize rodent control, improved sanitation, and health education to minimize human exposure to diseases. Regular monitoring of rodent populations for
Hymenolepis spp. can help identify areas at risk and inform integrated parasite management strategies. Deworming programs in at-risk populations, especially school-aged children, may be necessary in endemic regions with high human-rodent contact [
15,
81].
3.2. Trichinella spiralis
Trichinella spiralis is a parasitic nematode responsible for trichinellosis (trichinosis), a zoonotic disease acquired through the consumption of raw or undercooked meat containing infective larvae. Rodents play a key role in the sylvatic and synanthropic transmission cycles of
T. spiralis, acting as both reservoirs and amplifiers of the parasite (
Table 1) [
82,
83].
Infected rodents harbor encysted larvae in their striated muscles. Transmission occurs when carnivorous or omnivorous animals, including other rodents, consume infected tissue, perpetuating the parasite’s life cycle. Rodent cannibalism, predation, or scavenging behavior facilitates intra- and inter-species transmission. Their interaction with pigs, particularly in unregulated farming systems, creates a key epidemiological interface that should not be underestimated. This ecological dynamic also creates a link between wild rodent populations and domestic or peridomestic animals, such as pigs, which are the principal source of human infections [
84,
85].
Epidemiological studies have demonstrated that
T. spiralis can circulate silently in rodent populations across various habitats. In agricultural settings, rodents foraging in or around pig pens and food storage areas increase the likelihood of transmitting infection to swine, especially in backyard or poorly regulated farming systems. Surveys in rural areas of Asia, Eastern Europe (
Table 3), and Latin America have reported
T. spiralis prevalence in wild and synanthropic rodents ranging from 1% to over 10%, depending on trapping location and diagnostic method [
86,
87]. Although direct transmission of T. spiralis from rodents to humans is rare, cultural practices involving the consumption of rodent meat have been implicated in certain outbreaks. More commonly, rodents contribute indirectly by infecting pigs, which are later consumed by humans.
Although human trichinellosis is primarily associated with the consumption of pork, outbreaks have been linked to the ingestion of infected wild game or rodent meat in particular cultural contexts. The role of rodents in maintaining
Trichinella spp. in the environment highlights the need for continued vigilance, particularly in regions with overlapping wildlife-livestock-human interfaces. Infected rodents can also serve as sentinels for environmental contamination and provide early warning of potential risks to animal and human health [
88,
89].
Control of
T. spiralis requires an integrated One Health approach that includes rodent control in pig-rearing facilities, proper cooking of meat, routine veterinary inspection of pork, and public awareness campaigns about the risks of consuming undercooked animal products. Surveillance of rodent populations, especially in endemic areas, can support efforts to interrupt the zoonotic transmission cycle and prevent human trichinellosis outbreaks [
90,
91].
3.3. Angiostrongylus cantonensis
Angiostrongylus cantonensis, commonly known as the rat lungworm, is a neurotropic nematode that causes eosinophilic meningitis in humans. Rodents, particularly
Rattus norvegicus and
Rattus rattus, serves as the definitive hosts of this parasite, while mollusks such as snails and slugs act as intermediate hosts (
Table 1). Rodents acquire infection by consuming infected gastropods, and in turn, they harbor adult worms in the pulmonary arteries. First-stage larvae (L1) are subsequently shed in rodent feces, contaminating the environment and facilitating the parasite’s life cycle.[
92,
93].
The global distribution of
A. cantonensis has expanded significantly in recent decades, primarily due to the movement of infected rats and gastropods via trade, travel, and climate change-driven ecological shifts. Originally endemic to Southeast Asia and the Pacific Islands, the parasite has now been reported in parts of Africa, the Americas, and the Caribbean. Rodents play a central role in this geographic spread, serving as mobile reservoirs that sustain local transmission cycles [
94,
95].
Numerous studies have confirmed high prevalence rates of
A. cantonensis in rodent populations across endemic areas. For instance, surveys in urban and rural sites in Southeast Asia have shown infection rates of 20–60% in
Rattus species. At the same time, emerging reports in Brazil and the southern United States highlight the parasite’s introduction and establishment in new ecological niches. The presence of
A. cantonensis in synanthropic rodents increases the risk of human exposure, especially in regions with poor sanitation and high gastropod populations [
96,
97,
98].
Human infection occurs through accidental ingestion of infective third-stage larvae (L3), typically by consuming raw or undercooked snails, slugs, freshwater shrimp, or contaminated vegetables. While rodents do not directly transmit the parasite to humans, their presence is crucial for sustaining the life cycle and contributing to environmental contamination with L1 larvae. Human angiostrongyliasis manifests with severe neurological symptoms, including headache, stiff neck, and eosinophilic meningitis, and in some cases may lead to long-term sequelae or death [
99,
100,
101].
Public health efforts to control
A. cantonensis must include rodent population management, reduction of gastropod intermediate hosts, and education on safe food handling and consumption practices. Integrated surveillance systems that include rodent monitoring can help identify risk areas and facilitate timely interventions. In newly affected regions, early detection in rodent hosts can serve as a sentinel indicator for the parasite’s emergence and guide public health responses [
102,
103].
3.4. Capillaria hepatica
Capillaria hepatica (syn.
Calodium hepaticum) is a zoonotic nematode that infects the liver of mammals, particularly rodents, which serve as the primary reservoir hosts. This parasite causes hepatic capillariasis, a rare but potentially severe disease in humans, characterized by granulomatous hepatitis and hepatic dysfunction. Unlike most helminths,
C. hepatica requires host death and decomposition or predation for egg dissemination, making its life cycle unique among zoonotic parasites (
Table 1) [
104,
105].
Rodents, especially
Rattus norvegicus and
Mus musculus, as well as various wild species, are frequently infected with
C. hepatica and play a central role in maintaining its sylvatic and synanthropic transmission cycles. The parasite’s eggs are deposited in the liver parenchyma but are only released into the environment following the host's death, scavenging, or cannibalism by other organisms. Once in the environment, the eggs must embryonate under favorable conditions before becoming infective to new hosts via ingestion [
15,
106,
107].
In rodent populations, prevalence rates can be surprisingly high, with studies from urban and peri-urban environments in South America, Africa, and Asia reporting infection rates ranging from 5% to over 30%, depending on ecological and environmental conditions. The high density and rapid turnover of rodent populations, especially in cities with poor waste management, facilitate the sustained transmission of
C. hepatica [
104,
105].
Human infections are rare but likely underreported, as diagnosis requires liver biopsy or histopathological examination, and clinical presentation often mimics other hepatic diseases such as tuberculosis, neoplasms, or viral hepatitis. Documented cases have mainly involved children and individuals with close contact to rodents or poor hygiene conditions. Symptoms may include hepatomegaly, fever, eosinophilia, and elevated liver enzymes, occasionally progressing to liver fibrosis or cirrhosis [
108].
The public health significance of
C. hepatica lies not only in its potential to cause human disease but also in its role as an indicator of environmental rodent infestation and fecal contamination. Control efforts should prioritize rodent management, improved sanitation, and education on minimizing exposure to contaminated soil or rodent carcasses. Monitoring rodent populations in high-risk areas, particularly those in proximity to human dwellings or food storage sites, can support early detection and prevention strategies for hepatic capillariasis [
109].
3.5. Baylisascaris procyonis
Baylisascaris procyonis is a large ascarid nematode primarily parasitizing raccoons (
Procyon lotor) as definitive hosts, but rodents and other small mammals serve as critical paratenic (transport) hosts. In paratenic hosts such as rodents, the ingested infective eggs hatch and larvae migrate through tissues, particularly the central nervous system (CNS), causing visceral, ocular, and neural larva migrans (
Table 1). Though human infections are rare, they are often severe or fatal, making
B. procyonis a parasite of significant public health concern [
110,
111].
Rodents, including species such as
Peromyscus spp.,
Microtus spp., and
Mus musculus, are frequently involved in the sylvatic life cycle of
B. procyonis. After ingesting embryonated eggs from raccoon feces-contaminated environments, larvae migrate to the CNS, where they can cause behavioral changes, such as disorientation or ataxia. These neurological impairments make the rodents more susceptible to predation by raccoons, thereby completing the life cycle [
112,
113].
Although rodents are not the definitive hosts, their role as reservoirs of infective larvae is epidemiologically critical. Infected rodents can harbor hundreds of migrating larvae, creating a substantial risk to predators, including raccoons, domestic animals, and humans. Human infections occur through accidental ingestion of embryonated eggs from contaminated soil, water, or fomites. Children are particularly vulnerable due to geophagia and poor hygiene [
114,
115].
Environmental contamination with raccoon feces in peri-urban and suburban areas has become increasingly common, especially in North America, where raccoon populations have expanded into human-inhabited environments. Studies have shown that up to 70–80% of raccoons in some regions are infected with
B. procyonis, and eggs can persist in the environment for years under favorable conditions. This high prevalence among raccoons and the frequent exposure of rodents to raccoon latrines amplify transmission risk [
113,
116].
Due to the severe neuropathology caused by larval migration, human
Baylisascaris infections are often devastating. Clinical manifestations include eosinophilic meningoencephalitis, coma, and death. Survivors may experience permanent neurological deficits. No specific antemortem diagnostic test exists, and treatment is limited, especially in late-stage disease [
117,
118].
Preventive strategies should focus on reducing environmental exposure to raccoon feces, discouraging the feeding of raccoons, and minimizing human contact with contaminated areas. Rodent control also plays an indirect but valuable role by reducing the availability of infected paratenic hosts and limiting parasite propagation in ecosystems. Public health education about the risks of
B. procyonis is critical in areas where raccoons and humans coexist closely [
115,
119].
4. Ectoparasites
4.1. Xenopsylla cheopis
Xenopsylla cheopis, commonly known as the oriental rat flea, is one of the most medically essential ectoparasites associated with rodents. It is the primary vector of
Yersinia pestis, the etiologic agent of plague, and also transmits
Rickettsia rickettsii, the causative agent of Rocky Mountain spotted fever. This flea species predominantly parasitizes commensal rodents such as
Rattus norvegicus and
Rattus rattus, which are abundant in urban and peri-urban environments globally (
Table 1) [
13,
14].
Adult
X. cheopis feed on the blood of rodents, but they may also bite humans when rodent hosts are scarce or when human-rodent contact increases, particularly during outbreaks or in poorly maintained dwellings. The flea becomes infected with
Y. pestis after feeding on a bacteremic rodent and subsequently transmits the bacterium to new hosts by regurgitating infected blood during subsequent feeding attempts. Blockage of the flea's proventriculus enhances transmission efficiency [
120,
121].
Historically,
X. cheopis played a central role in the global spread of plague during the third pandemic and remains a threat in endemic regions across Africa, Asia, and the Americas. Surveillance studies in these regions consistently report high flea indices (i.e., average number of fleas per rodent) in areas where plague persists. For example, in Madagascar and the Democratic Republic of the Congo,
X. cheopis is frequently recovered from peridomestic rodents during plague outbreaks [
120,
121,
122].
Besides being a competent vector of plague,
X. cheopis is also a competent vector of
R. typhi, which causes murine typhus—a febrile illness characterized by headache, rash, and systemic symptoms. While murine typhus is generally less lethal than plague, its burden in endemic areas is underappreciated, and outbreaks often go undiagnosed due to nonspecific clinical features [
123,
124].
Environmental factors, including poor sanitation, high rodent densities, and warm climates, favor the proliferation of
X. cheopis. Its life cycle involves egg deposition in rodent nests and burrows, with larval development occurring in organic debris. Urbanization and unregulated waste management contribute to the persistence of flea populations and increase the risk of human exposure [
125,
126].
Control strategies against
X. cheopis include integrated rodent and flea management. Insecticide application targeting flea habitats, combined with rodent control measures and housing improvements, is effective in reducing flea infestations and interrupting transmission cycles. Public health efforts must also emphasize early detection of plague and murine typhus cases, prompt treatment, and risk communication in endemic regions [
13,
127].
Given its central role in the epidemiology of plague and other flea-borne diseases,
X. cheopis remains a priority target for vector surveillance and control programs, especially in areas where human and rodent populations intersect closely [
127,
128].
4.2. Ornithonyssus bacoti
Ornithonyssus bacoti, commonly known as the tropical rat mite, is a hematophagous ectoparasite primarily associated with commensal rodents, particularly
Rattus norvegicus and
Rattus rattus. Although it is not an actual flea or tick, this mite can bite humans, resulting in a condition known as rat mite dermatitis. In specific contexts,
O. bacoti may also act as a mechanical or potential biological vector for various pathogens, underscoring its relevance in rodent-borne zoonoses (
Table 1) [
129,
130].
Unlike fleas or lice,
O. bacoti does not live permanently on its rodent host. Instead, it resides in nests, cracks, walls, or bedding materials, emerging periodically to feed on blood. When rodent hosts die or migrate, mites may seek alternative hosts, including humans, for blood meals. Human exposure typically occurs in urban dwellings, warehouses, or laboratories infested with rodents, and outbreaks are often associated with rodent control campaigns that displace mite populations [
130,
131].
Infestation by
O. bacoti in humans causes intense pruritus, erythematous papules, and sometimes vesicular or pustular lesions, often misdiagnosed as scabies, bedbug bites, or allergic dermatitis. The dermatitis may persist for weeks and can lead to secondary bacterial infections due to scratching. Clusters of cases are often reported among residents of infested buildings or personnel working in rodent facilities [
132,
133].
Beyond dermatologic effects,
O. bacoti has been experimentally implicated in the transmission of several zoonotic pathogens, including
Rickettsia akari,
Coxiella burnetii,
Borrelia spp., and
Haemophilus influenzae. While the exact epidemiological significance of these associations in natural settings remains to be fully clarified, the potential for pathogen transmission reinforces the need for surveillance and control in rodent-infested areas [
134,
135].
Prevalence studies have shown high infestation rates of
O. bacoti in rodent populations from urban environments in North America, Asia, and parts of Europe. In some cities, more than 50% of captured rats harbor the mite, especially in poorly maintained structures with abundant rodent nesting sites. The mite’s resilience and ability to survive without feeding for extended periods further complicate eradication efforts [
129,
136].
Effective management of
O. bacoti infestations requires an integrated approach, including rodent eradication, environmental decontamination, and application of acaricides in infested areas. Importantly, rodent removal must be accompanied by concurrent mite control to prevent the dispersal of mites and minimize the risk of human bites. Education and training of public health workers, pest control professionals, and clinicians are essential to recognize and manage mite infestations and implement appropriate interventions [
129,
137].
As urban rodent populations expand and infestations increase in frequency,
O. bacoti represents a growing ectoparasitic concern with potential dermatological and vector-borne implications for human health [
136].
4.3. Ixodes spp.
Ixodes spp. are hard ticks of significant medical and veterinary importance, serving as vectors for numerous zoonotic pathogens. Several species, including Ixodes scapularis, Ixodes ricinus, and Ixodes persulcatus, parasitize rodents during their immature stages, playing a crucial role in the transmission of bacterial, viral, and protozoal pathogens to humans and other animals (
Table 1). Rodents serve as essential hosts for the larval and nymphal stages of these ticks, contributing to the maintenance and amplification of vector-borne disease cycles [
138,
139].
Among the most prominent diseases associated with
Ixodes spp. is Lyme borreliosis, caused by
Borrelia burgdorferi sensu lato. Rodents, particularly species such as
Peromyscus leucopus (white-footed mouse) and
Apodemus flavicollis, act as competent reservoirs for
Borrelia, infecting feeding ticks that later transmit the pathogen to larger vertebrates, including humans. Additionally,
Ixodes ticks transmit other pathogens such as
Anaplasma phagocytophilum (human granulocytic anaplasmosis),
Babesia microti (babesiosis), Tick-borne encephalitis virus (TBEV), and
Borrelia miyamotoi [
140,
141,
142,
143,
144].
The geographic distribution of
Ixodes spp. is expanding globally, driven by climate change, altered land use, and changes in host population dynamics. As temperature and humidity influence tick survival and development, warming climates have facilitated the northward and altitudinal expansion of key
Ixodes species in North America, Europe, and Asia. In parallel, growing rodent populations in peri-urban environments have increased opportunities for human exposure to infected ticks [
142,
143].
Surveillance studies consistently identify rodents as critical amplifiers of tick populations and associated pathogens. High infestation rates of larvae and nymphs are frequently observed on wild rodent species, and the overlap of rodent habitats with human recreational or residential areas increases the risk of spillover. For example, studies in temperate woodlands in Europe and North America have documented co-infection of rodents with multiple pathogens and simultaneous infestation by
Ixodes spp., highlighting their role in the ecology of tick-borne disease complexes [
145,
146].
Public health strategies to reduce the risk of tick-borne diseases must consider the pivotal role of rodents. Integrated approaches involving tick control, habitat management, personal protective measures (e.g., repellents and protective clothing), and public education are essential. Rodent population control in endemic areas, combined with environmental modifications such as vegetation management, can reduce tick abundance and interrupt transmission cycles [
147,
148].
Given the diversity of
Ixodes-borne pathogens and the increasing incidence of tick-borne diseases, ongoing monitoring of rodent–tick–pathogen interactions is vital. Understanding the ecological drivers of these complex systems is key to predicting and mitigating future public health threats posed by
Ixodes spp [
149,
150].
5. Climate Change and Anthropogenic Activities Leading to the Emergence of Rodent-Borne Zoonoses
Climate change and anthropogenic activities are key drivers in the emergence and spread of rodent-borne zoonoses. Rising global temperatures, altered precipitation patterns, and habitat disruptions are influencing rodent population dynamics, leading to increased human exposure to zoonotic pathogens. Warmer climates can expand the geographical range of rodent species, enabling them to thrive in new environments and increasing the risk of pathogen spillover. Additionally, changes in seasonal rainfall can impact food availability, driving rodents into urban and peri-urban areas where contact with humans becomes more frequent, facilitating disease transmission [
5,
151,
152].
Anthropogenic activities such as deforestation, agricultural expansion, and urbanization exacerbate the risks associated with rodent-borne zoonoses. Habitat destruction forces rodents to migrate, often bringing them into closer proximity to human settlements, livestock, and domestic animals. This increases the likelihood of disease outbreaks, particularly for zoonotic parasites such as
Toxoplasma gondii,
Leishmania spp., and
Trypanosoma cruzi. Intensive farming and irrigation projects create favourable conditions for rodent proliferation, leading to higher parasite transmission rates [
5,
153,
154].
Climate-induced shifts in vector populations, such as fleas, ticks, and mites, further compound the risk by altering transmission cycles. Integrated surveillance, environmental management, and rodent control strategies are essential in mitigating the impact of climate change and human activities on the emergence of rodent-borne zoonotic diseases [
155,
156,
157].
5.1. Habitat Modification and Behavioral Shifts in Rodents
Climate change is significantly reshaping rodent habitats and behaviours, increasing the risk of zoonotic disease transmission. Rising temperatures and shifting precipitation patterns modify ecosystems, forcing rodents to adapt by migrating to new areas, including human settlements. Changes in vegetation cycles affect food resource availability, often triggering population booms in rodent communities. Increased densities intensify intraspecies competition and may lead to heightened aggression, fostering the transmission of pathogens within and across rodent populations. These ecological alterations are closely linked to the transmission of rodent-borne zoonoses, including hantavirus infections, leptospirosis, and
Toxoplasma gondii, underscoring the urgency of sustained ecological surveillance and disease control programs [
154,
158,
159].
5.2. Impact of Extreme Weather Events
Extreme weather events, such as hurricanes, floods, droughts, and wildfires, significantly influence the emergence of rodent-borne zoonotic diseases [
160,
161,
162]. Flooding can displace rodent populations, forcing them into human-inhabited areas, thereby increasing direct contact and contamination of water and food sources with pathogens such as
Leptospira spp., which significantly raises the risk of leptospirosis outbreaks. Droughts reduce natural food availability, driving rodents to forage in urban environments and heightening the risk of disease spillover. Wildfires destroy habitats, prompting rodent migration and altering predator-prey dynamics, potentially increasing rodent densities [
163]. These disturbances amplify the transmission of zoonotic pathogens, emphasizing the need for proactive disease surveillance and environmental management in disaster-prone regions [
164,
165,
166].
5.3. Alteration of Pathogen Dynamics
Climate change alters pathogen dynamics by influencing the survival, replication, and transmission of zoonotic agents carried by rodents. Rising temperatures and humidity levels can enhance the persistence of bacterial, viral, and parasitic pathogens in the environment, increasing their transmission potential. Warmer conditions may also accelerate the life cycles of vectors, such as fleas and ticks, facilitating the spread of diseases like plague and Lyme disease. Additionally, altered rodent immunity and stress from environmental changes can lead to increased pathogen shedding rates. These shifts contribute to the emergence and re-emergence of rodent-borne zoonoses, necessitating improved monitoring and adaptive public health interventions [
156,
167,
168].
6. Public Health Implications and Mitigation Strategies
Rodent-borne zoonotic diseases pose significant public health challenges due to their ability to spread rapidly in human populations, often leading to outbreaks with severe consequences. These diseases, including hantavirus, leptospirosis, toxoplasmosis, and plague, can cause severe morbidity and mortality, particularly in vulnerable communities with poor sanitation and limited healthcare access. The increasing interaction between rodents and human populations, driven by urbanization, climate change, and habitat destruction, further exacerbates disease risks. Additionally, rodents serve as reservoirs for emerging pathogens, making them a persistent threat in both rural and urban environments. Public health responses must address not only immediate outbreaks but also the underlying environmental and ecological factors that drive transmission [
169,
170,
171].
Mitigation strategies require an integrated, multidisciplinary approach that includes surveillance, rodent control, and public awareness. Improved sanitation, waste management, and secure food storage help reduce rodent access to human settlements. Vector control programs targeting fleas, ticks, and mites also minimize transmission risks. Early detection systems and research on rodent-pathogen interactions are essential for predicting and managing outbreaks. Additionally, educating communities on hygiene practices and safe food handling can reduce exposure to rodent-borne pathogens. Collaboration among governments, researchers, and health agencies is crucial for developing effective policies to prevent and mitigate the impact of rodent-borne zoonotic diseases [
131,
156,
172].
6.1. Surveillance and Early Warning System
Surveillance and early warning systems are essential for the timely detection and control of rodent-borne zoonotic diseases. Continuous monitoring of rodent populations, their pathogens, and environmental factors enables health authorities to identify emerging risks before outbreaks occur. Molecular diagnostics, serological testing, and ecological surveillance help track the circulation of pathogens in rodent reservoirs. Early detection allows for rapid intervention, such as targeted rodent control, vaccination campaigns, and public health alerts. Integrating these systems with climate and environmental data enhances predictive modelling, enabling proactive measures. Strengthening global surveillance networks improves preparedness, reducing the public health and economic burden of rodent-borne zoonotic diseases [
173,
174,
175].
6.2. Rodent Control and Habitat Management
Rodent control and habitat management are crucial strategies for reducing the risk of rodent-borne zoonotic diseases. Effective rodent control involves monitoring the population, using traps and rodenticides, and implementing biological control measures to minimize infestations. Habitat management focuses on reducing food and shelter availability for rodents by improving waste disposal, securing food storage, and eliminating breeding sites. Urban planning, incorporating rodent-proof infrastructure and sustainable land-use practices, can further limit human-rodent interactions. Integrating these strategies with public awareness campaigns ensures long-term success in controlling rodent populations and reducing the spread of zoonotic pathogens, ultimately protecting public health [
176,
177,
178].
6.3. Climate Change Adaptation and Mitigation
Climate change adaptation and mitigation play a crucial role in controlling rodent-borne zoonotic diseases by addressing the environmental factors that drive disease emergence and transmission. Adaptation strategies include strengthening surveillance systems, improving urban planning to minimize rodent habitats, and enhancing public health infrastructure to respond to outbreaks. Mitigation efforts focus on reducing greenhouse gas emissions, preserving ecosystems, and promoting sustainable agricultural practices to prevent habitat disruption. Integrated pest management and ecological restoration help regulate rodent populations naturally. By implementing climate-resilient health policies and environmental strategies, ensures that communities are not only protected from current zoonotic threats but are also better prepared for future climate-related challenges [
179,
180,
181].
7. Limitations
The variability and heterogeneity of available data across geographic regions, rodent species, and diagnostic methodologies limit the scope of this review. Many studies focus on specific parasites or localities, hindering broader generalization. Additionally, underreporting and lack of standardized surveillance in resource-limited settings may lead to an underestimation of true prevalence and public health impact. The review also relies heavily on published literature, which may introduce publication bias and overlook unpublished data or grey literature relevant to rodent-borne parasitic diseases.
8. Conclusions
Rodent-borne parasitic diseases pose a significant and escalating threat to global public health. This review highlights the diverse protozoan, helminthic, and ectoparasitic pathogens carried by rodents that can be transmitted to humans through various ecological and environmental pathways. The interactions between rodents, their parasites, and human populations are intensifying due to urbanization, climate change, and habitat disruption. The emergence and re-emergence of diseases such as toxoplasmosis, leishmaniasis, giardiasis, cryptosporidiosis, and trichinellosis underscore the need for a One Health approach, integrating surveillance, rodent control, environmental management, and public education. Surveillance systems must be strengthened to detect zoonotic threats early, while multisectoral collaboration is essential for effective intervention. Reducing the burden of rodent-borne parasitic diseases requires proactive public health strategies, particularly in vulnerable communities. As global environmental conditions continue to shift, coordinated and sustained efforts are essential to mitigate the burden of rodent-associated parasitic infections. Comprehensive monitoring, prevention, and control strategies must be applied not only in endemic regions but also in areas where disease emergence is anticipated due to ecological change.
Author Contributions
Conceptualization, A.J.R.-M., A.A.S. and S.B.; methodology, A.A.S. and S.B.; software, A.A.S., R.P. and S.B.; validation, A.A.S. and S.B.; formal analysis, A.A.S. and S.B.; investigation, A.A.S. and S.B.; resources, A.A.S. and S.B.; data curation, A.A.S. and S.B.; writing—original draft preparation, A.A.S., R.P., S.T., P.M.D., A.J.R.-M. and S.B.; writing—review and editing, A.A.S., R.P., S.T., P.M.D., A.J.R.-M. and S.B.; visualization, A.A.S., R.P., S.T., P.M.D., A.J.R.-M. and S.B.; supervision, A.A.S.:; project administration, A.A.S.; funding acquisition, A.A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgements
This article has been registered in the Research Proposal Registration of the Coordination of Scientific Integrity and Surveillance of Universidad Cientifica del Sur, Lima, Peru.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Lundwall, Å.; Persson, M.; Hansson, K.; Jonsson, M. Identification of the major rabbit and guinea pig semen coagulum proteins and description of the diversity of the REST gene locus in the mammalian clade Glires. PLoS One 2020, 15, e0240607. [Google Scholar] [CrossRef] [PubMed]
- Maxeiner, S.; Benseler, F.; Brose, N.; Krasteva-Christ, G. Of Humans and Gerbils- Independent Diversification of Neuroligin-4 Into X- and Y-Specific Genes in Primates and Rodents. Front Mol Neurosci 2022, 15, 838262. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.; Smiley, K.O.; Lonstein, J.S. Parental Behavior in Rodents. Adv Neurobiol 2022, 27, 1–53. [Google Scholar] [CrossRef]
- Hardgrove, E.; Zimmerman, D.M.; von Fricken, M.E.; Deem, S. A scoping review of rodent-borne pathogen presence, exposure, and transmission at zoological institutions. Prev Vet Med 2021, 193, 105345. [Google Scholar] [CrossRef]
- Islam, M.M.; Farag, E.; Hassan, M.M.; Jaffrey, S.S.; Atta, M.; Al-Marri, A.M.; Al-Zeyara, A.M.; Al Romaihi, H.; Bansal, D.; Mkhize-Kwitshana, Z.L. Rodent-borne zoonoses in Qatar: A possible One-Health framework for the intervention of future epidemic. One Health 2023, 16, 100517. [Google Scholar] [CrossRef] [PubMed]
- Morand, S.; Blasdell, K.; Bordes, F.; Buchy, P.; Carcy, B.; Chaisiri, K.; Chaval, Y.; Claude, J.; Cosson, J.F.; Desquesnes, M. , et al. Changing landscapes of Southeast Asia and rodent-borne diseases: decreased diversity but increased transmission risks. Ecol Appl 2019, 29, e01886. [Google Scholar] [CrossRef]
- Moratal, S.; Dea-Ayuela, M.A.; Cardells, J.; Marco-Hirs, N.M.; Puigcercós, S.; Lizana, V.; López-Ramon, J. Potential Risk of Three Zoonotic Protozoa (Cryptosporidium spp., Giardia duodenalis, and Toxoplasma gondii) Transmission from Fish Consumption. Foods 2020, 9. [Google Scholar] [CrossRef]
- Robertson, L.J.; Clark, C.G.; Debenham, J.J.; Dubey, J.P.; Kváč, M.; Li, J.; Ponce-Gordo, F.; Ryan, U.; Schares, G.; Su, C. , et al. Are molecular tools clarifying or confusing our understanding of the public health threat from zoonotic enteric protozoa in wildlife? Int J Parasitol Parasites Wildl 2019, 9, 323–341. [Google Scholar] [CrossRef]
- Galán-Puchades, M.T.; Gosálvez, C.; Trelis, M.; Gómez-Samblás, M.; Solano-Parada, J.; Osuna, A.; Sáez-Durán, S.; Bueno-Marí, R.; Fuentes, M.V. Parasite Fauna and Coinfections in Urban Rats Naturally Infected by the Zoonotic Parasite Angiostrongylus cantonensis. Pathogens 2023, 13. [Google Scholar] [CrossRef]
- Paller, V.G.V.; Fornesa, R.N.; Fernandez, D.A.P.; Estaño, L.A. Rats and their helminth parasites: Potential zoonosis threats of land use change in the northeastern sub-watersheds of Mount Makiling, Laguna, Philippines. Helminthologia 2024, 61, 30–39. [Google Scholar] [CrossRef]
- Tijjani, M.; Majid, R.A.; Abdullahi, S.A.; Unyah, N.Z. Detection of rodent-borne parasitic pathogens of wild rats in Serdang, Selangor, Malaysia: A potential threat to human health. Int J Parasitol Parasites Wildl 2020, 11, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Frye, M.J.; Firth, C.; Bhat, M.; Firth, M.A.; Che, X.; Lee, D.; Williams, S.H.; Lipkin, W.I. Preliminary Survey of Ectoparasites and Associated Pathogens from Norway Rats in New York City. J Med Entomol 2015, 52, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Rajamannar, V.; Govindarajan, R.; Kumar, A.; Samuel, P.P. A review of public health important fleas (Insecta, Siphonaptera) and flea-borne diseases in India. J Vector Borne Dis 2022, 59, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Wells, L.E.; Elston, D.M. What's eating you? oriental rat flea (Xenopsylla cheopis). Cutis 2020, 106, 124–126. [Google Scholar] [CrossRef]
- Dini, F.M.; Mazzoni Tondi, C.; Galuppi, R. Helminthofauna Diversity in Synanthropic Rodents of the Emilia-Romagna Region (Italy): Implications for Public Health and Rodent Control. Vet Sci 2024, 11. [Google Scholar] [CrossRef]
- Ellwanger, J.H.; Veiga, A.B.G.; Kaminski, V.L.; Valverde-Villegas, J.M.; Freitas, A.W.Q.; Chies, J.A.B. Control and prevention of infectious diseases from a One Health perspective. Genet Mol Biol 2021, 44, e20200256. [Google Scholar] [CrossRef]
- Galeh, T.M.; Sarvi, S.; Montazeri, M.; Moosazadeh, M.; Nakhaei, M.; Shariatzadeh, S.A.; Daryani, A. Global Status of Toxoplasma gondii Seroprevalence in Rodents: A Systematic Review and Meta-Analysis. Front Vet Sci 2020, 7, 461. [Google Scholar] [CrossRef]
- Ijaz, M.; Khan, A.U.; Ullah, S.; Khan, A.; Ibenmoussa, S.; Sitotaw, B.; Dawoud, T.M.; Khan, A.; Iqbal, F. Toxoplasma gondii infection affects the complete blood count and disturbs the markers of oxidative stress from the vital organs of wild rodents. Sci Rep 2024, 14, 22716. [Google Scholar] [CrossRef]
- Ivovic, V.; Potusek, S.; Buzan, E. Prevalence and genotype identification of Toxoplasma gondii in suburban rodents collected at waste disposal sites. Parasite 2019, 26, 27. [Google Scholar] [CrossRef]
- Bastien, M.; Vaniscotte, A.; Combes, B.; Umhang, G.; Germain, E.; Gouley, V.; Pierlet, A.; Quintaine, T.; Forin-Wiart, M.A.; Villena, I. , et al. High density of fox and cat faeces in kitchen gardens and resulting rodent exposure to Echinococcus multilocularis and Toxoplasma gondii. Folia Parasitol (Praha) 2018, 65. [Google Scholar] [CrossRef]
- Dubey, J.P.; Murata, F.H.A.; Cerqueira-Cézar, C.K.; Kwok, O.C.H.; Su, C. Epidemiological Significance of Toxoplasma Gondii Infections in Wild Rodents: 2009-2020. J Parasitol 2021, 107, 182–204. [Google Scholar] [CrossRef] [PubMed]
- Franco-Paredes, C.; Villamil-Gómez, W.E.; Schultz, J.; Henao-Martínez, A.F.; Parra-Henao, G.; Rassi, A., Jr.; Rodríguez-Morales, A.J.; Suarez, J.A. A deadly feast: Elucidating the burden of orally acquired acute Chagas disease in Latin America - Public health and travel medicine importance. Travel Med Infect Dis 2020, 36, 101565. [Google Scholar] [CrossRef]
- Madigan, R.; Majoy, S.; Ritter, K.; Luis Concepción, J.; Márquez, M.E.; Silva, S.C.; Zao, C.L.; Pérez Alvarez, A.; Rodriguez-Morales, A.J.; Mogollón-Mendoza, A.C. , et al. Investigation of a combination of amiodarone and itraconazole for treatment of American trypanosomiasis (Chagas disease) in dogs. J Am Vet Med Assoc 2019, 255, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Botto-Mahan, C.; Rojo, G.; Sandoval-Rodríguez, A.; Peña, F.; Ortiz, S.; Solari, A. Temporal variation in Trypanosoma cruzi lineages from the native rodent Octodon degus in semiarid Chile. Acta Trop 2015, 151, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Cortazar, I.; Cecilia Amaya Guardia, K.; Torres-Castro, M.; Acosta-Viana, K.; Guzmán-Marín, E.; Israel Chan-Pérez, J.; Ortega-Pacheco, A.; Rodríguez-Vivas, R.I.; Medina-Pinto, R.; Jiménez-Coello, M. Frequency of Trypanosoma cruzi Infection in Synanthropic and Wild Rodents Captured in a Rural Community in Southeast of Mexico. Vet Med Int 2018, 2018, 8059613. [Google Scholar] [CrossRef]
- Brigada, A.M.; Doña, R.; Caviedes-Vidal, E.; Moretti, E.; Basso, B. American tripanosomiasis: a study on the prevalence of Trypanosoma cruzi and Trypanosoma cruzi-like organisms in wild rodents in San Luis province, Argentina. Rev Soc Bras Med Trop 2010, 43, 249–253. [Google Scholar] [CrossRef]
- Yefi-Quinteros, E.; Muñoz-San Martín, C.; Bacigalupo, A.; Correa, J.P.; Cattan, P.E. Trypanosoma cruzi load in synanthropic rodents from rural areas in Chile. Parasit Vectors 2018, 11, 171. [Google Scholar] [CrossRef]
- Ghersi, B.M.; Peterson, A.C.; Gibson, N.L.; Dash, A.; Elmayan, A.; Schwartzenburg, H.; Tu, W.; Riegel, C.; Herrera, C.; Blum, M.J. In the heart of the city: Trypanosoma cruzi infection prevalence in rodents across New Orleans. Parasit Vectors 2020, 13, 577. [Google Scholar] [CrossRef]
- Mendoza, A.P.; Muñoz-Maceda, A.; Ghersi, B.M.; De La Puente, M.; Zariquiey, C.; Cavero, N.; Murillo, Y.; Sebastian, M.; Ibañez, Y.; Parker, P.G. , et al. Diversity and prevalence of zoonotic infections at the animal-human interface of primate trafficking in Peru. PLoS One 2024, 19, e0287893. [Google Scholar] [CrossRef]
- Cardenas, R.; Sandoval, C.M.; Rodriguez-Morales, A.J.; Bendezu, H.; Gonzalez, A.; Briceno, A.; De-La-Paz-Pineda, J.; Rojas, E.M.; Scorza, J.V. Epidemiology of American tegumentary leishmaniasis in domestic dogs in an endemic zone of western Venezuela. Bull Soc Pathol Exot 2006, 99, 355–358. [Google Scholar]
- Cardenas, R.; Sandoval, C.M.; Rodriguez-Morales, A.J.; Franco-Paredes, C. Impact of climate variability in the occurrence of leishmaniasis in northeastern Colombia. Am J Trop Med Hyg 2006, 75, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Ocampo, E.; Villamizar-Pena, R.; Cortes-Bonilla, I.; Garcia-Zuluaga, L.M.; Holguin-Rivera, Y.; Ospina-Arzuaga, H.D.; Cardona-Trujllo, M.C.; Trejos-Mendoza, A.E.; Perez-Vargas, S.; Arteaga-Livias, K. , et al. Human visceral leishmaniasis prevalence by different diagnostic methods in Latin America: a systematic review and meta-analysis. Infez Med 2021, 29, 199–208. [Google Scholar] [PubMed]
- Paniz-Mondolfi, A.E.; Talhari, C.; Garcia Bustos, M.F.; Rosales, T.; Villamil-Gomez, W.E.; Marquez, M.; Perez Alvarez, A.M.; Talamo Sanchez, A.I.; Rodriguez-Morales, A.J. American cutaneous leishmaniasis in infancy and childhood. Int J Dermatol 2017, 56, 1328–1341. [Google Scholar] [CrossRef]
- Kassahun, A.; Sadlova, J.; Dvorak, V.; Kostalova, T.; Rohousova, I.; Frynta, D.; Aghova, T.; Yasur-Landau, D.; Lemma, W.; Hailu, A. , et al. Detection of Leishmania donovani and L. tropica in Ethiopian wild rodents. Acta Trop 2015, 145, 39–44. [Google Scholar] [CrossRef]
- Kato, H. Epidemiology of Leishmaniasis: Risk factors for its pathology and infection. Parasitol Int 2025, 105, 102999. [Google Scholar] [CrossRef]
- Galán-Puchades, M.T.; Gómez-Samblás, M.; Suárez-Morán, J.M.; Osuna, A.; Sanxis-Furió, J.; Pascual, J.; Bueno-Marí, R.; Franco, S.; Peracho, V.; Montalvo, T. , et al. Leishmaniasis in Norway Rats in Sewers, Barcelona, Spain. Emerg Infect Dis 2019, 25, 1222–1224. [Google Scholar] [CrossRef]
- Tsakmakidis, Ι.; Angelopoulou, K.; Dovas, C.I.; Dokianakis, Ε.; Tamvakis, A.; Symeonidou, I.; Antoniou, Μ.; Diakou, A. Leishmania infection in rodents in Greece. Trop Med Int Health 2017, 22, 1523–1532. [Google Scholar] [CrossRef] [PubMed]
- Marinho-Júnior, J.F.; Monteiro, J.; Sales de Carvalho, A.W.; de Carvalho, F.G.; de Paiva Cavalcanti, M.; Shaw, J.; Courtenay, O.; Brandão-Filho, S.P. High levels of infectiousness of asymptomatic Leishmania (Viannia) braziliensis infections in wild rodents highlights their importance in the epidemiology of American Tegumentary Leishmaniasis in Brazil. PLoS Negl Trop Dis 2023, 17, e0010996. [Google Scholar] [CrossRef]
- Durán, C.; Hidalgo, G.; Aguilera, W.; Rodriguez-Morales, A.J.; Albano, C.; Cortez, J.; Jiménez, S.; Díaz, M.; Incani, R.N. Giardia lamblia infection is associated with lower body mass index values. J Infect Dev Ctries 2010, 4, 417–418. [Google Scholar] [CrossRef]
- Escobedo, A.A.; Rodriguez-Morales, A.J.; Trujillo, A.M.; Sánchez-Duque, J.A. Introductory Chapter: Giardiasis - Still a Globally Relevant Protozoan and Zoonotic Disease. In Giardiasis, Rodriguez-Morales, A.J., Ed. IntechOpen: Rijeka, 2017; 10.5772/intechopen.70900.
- Rodríguez-Morales, A.J.; Granados-Álvarez, S.; Escudero-Quintero, H.; Vera-Polania, F.; Mondragon-Cardona, A.; Díaz-Quijano, F.A.; Sosa-Valencia, L.; Lozada-Riascos, C.O.; Escobedo, A.A.; Liseth, O. , et al. Estimating and mapping the incidence of giardiasis in Colombia, 2009-2013. Int J Infect Dis 2016, 49, 204–209. [Google Scholar] [CrossRef]
- Ramírez-Ocampo, S.; Cotte-Alzate, J.D.; Escobedo Á, A.; Rodríguez-Morales, A.J. Prevalence of zoonotic and non-zoonotic genotypes of Giardia intestinalis in cats: a systematic review and meta-analysis. Infez Med 2017, 25, 326–338. [Google Scholar] [PubMed]
- Rodriguez-Morales, A.J.; Franco-Paredes, C. Giardia infections. In Encyclopedia of global health, Zhang, Y., Ed. SAGE Publications, Inc.: 2008; Vol. 4, pp. 739–739.
- Tuska-Szalay, B.; Sipos, D.; Czabán, D.; Kalmár, Z.; Keve, G.; Szekeres, S.; Kelemen, B.S.; Sándor, A.D.; Hornok, S. Pet and wild rodents as hosts of Giardia duodenalis in Central Europe, Hungary. Acta Vet Hung 2025, 73, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.X.; Zheng, W.B.; Ma, J.G.; Yao, Q.X.; Zou, Y.; Bubu, C.J.; Zhao, Q.; Zhu, X.Q. Occurrence and multilocus genotyping of Giardia intestinalis assemblage C and D in farmed raccoon dogs, Nyctereutes procyonoides, in China. Parasit Vectors 2016, 9, 471. [Google Scholar] [CrossRef] [PubMed]
- Asghari, A.; Motazedian, M.H.; Asgari, Q.; Shamsi, L.; Sarkari, B.; Shahabi, S.; Mohammadi-Ghalehbin, B. Occurrence, genetic characterization, and zoonotic importance of Giardia duodenalis in various species of rodents (Mus musculus, Rattus norvegicus, and Rattus rattus). Comp Immunol Microbiol Infect Dis 2022, 85, 101812. [Google Scholar] [CrossRef]
- Egan, S.; Barbosa, A.D.; Feng, Y.; Xiao, L.; Ryan, U. Critters and contamination: Zoonotic protozoans in urban rodents and water quality. Water Res 2024, 251, 121165. [Google Scholar] [CrossRef]
- Islam, M.M.; Farag, E.; Hassan, M.M.; Enan, K.A.; Mohammadi, A.; Aldiqs, A.K.; Alhussain, H.; Al Musalmani, E.; Al-Zeyara, A.A.; Al-Romaihi, H. , et al. Rodent-borne parasites in Qatar: A possible risk at the human-animal-ecosystem interface. One Health 2024, 18, 100708. [Google Scholar] [CrossRef]
- Ryan, U.; Zahedi, A. Molecular epidemiology of giardiasis from a veterinary perspective. Adv Parasitol 2019, 106, 209–254. [Google Scholar] [CrossRef]
- Hatam-Nahavandi, K.; Ahmadpour, E.; Badri, M.; Eslahi, A.V.; Anvari, D.; Carmena, D.; Xiao, L. Global prevalence of Giardia infection in nonhuman mammalian hosts: A systematic review and meta-analysis of five million animals. PLoS Negl Trop Dis 2025, 19, e0013021. [Google Scholar] [CrossRef]
- Coelho, C.H.; Durigan, M.; Leal, D.A.G.; Schneider, A.B.; Franco, R.M.B.; Singer, S.M. Giardiasis as a neglected disease in Brazil: Systematic review of 20 years of publications. PLoS Negl Trop Dis 2017, 11, e0006005. [Google Scholar] [CrossRef]
- Roshidi, N.; Mohd Hassan, N.H.; Abdul Hadi, A.; Arifin, N. Current state of infection and prevalence of giardiasis in Malaysia: a review of 20 years of research. PeerJ 2021, 9, e12483. [Google Scholar] [CrossRef]
- Tsui, C.K.; Miller, R.; Uyaguari-Diaz, M.; Tang, P.; Chauve, C.; Hsiao, W.; Isaac-Renton, J.; Prystajecky, N. Beaver Fever: Whole-Genome Characterization of Waterborne Outbreak and Sporadic Isolates To Study the Zoonotic Transmission of Giardiasis. mSphere 2018, 3. [Google Scholar] [CrossRef] [PubMed]
- Silva-Ramos, C.R.; Noriega, J.; Fajardo, R.F.; Chala-Quintero, S.M.; Del Pilar Pulido-Villamarín, A.; Pérez-Torres, J.; Castañeda-Salazar, R.; Cuervo, C. Molecular Detection and Genotyping of Cryptosporidium spp. Isolates from Bats in Colombia. Acta Parasitol 2023, 68, 676–682. [Google Scholar] [CrossRef]
- Triviño-Valencia, J.; Nati-Castillo, A.; Cabeza, N.Y.; Lora-Suarez, F.; Gómez-Marín, J. Molecular confirmation of Cryptosporidium and Cyclospora species in children with acute diarrhoea in Quindio region, Colombia. Gut Pathog 2025, 17, 14. [Google Scholar] [CrossRef]
- Jones, K.R.; Tardieu, L. Giardia and Cryptosporidium in Neo-Tropical Rodents and Marsupials: Is There Any Zoonotic Potential? Life (Basel) 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Fu, Y.; Li, J.; Zhang, L. Public health and ecological significance of rodents in Cryptosporidium infections. One Health 2022, 14, 100364. [Google Scholar] [CrossRef]
- Carrera-Játiva, P.D.; Acosta-Jamett, G.; Muñoz, P. Molecular detection of Cryptosporidium parvum in wild rodents (Phyllotis darwini) inhabiting protected and rural transitional areas in north-central Chile. Int J Parasitol Parasites Wildl 2024, 24, 100971. [Google Scholar] [CrossRef] [PubMed]
- Hancke, D.; Suárez, O.V. A review of the diversity of Cryptosporidium in Rattus norvegicus, R. rattus and Mus musculus: What we know and challenges for the future. Acta Trop 2022, 226, 106244. [Google Scholar] [CrossRef]
- Ayinmode, A.B.; Obebe, O.O.; Falohun, O.O. Molecular detection of Cryptosporidium species in street-sampled dog faeces in Ibadan, Nigeria. Vet Parasitol Reg Stud Reports 2018, 14, 54–58. [Google Scholar] [CrossRef]
- García-Livia, K.; Martín-Alonso, A.; Foronda, P. Diversity of Cryptosporidium spp. in wild rodents from the Canary Islands, Spain. Parasit Vectors 2020, 13, 445. [Google Scholar] [CrossRef]
- Tan, T.K.; Low, V.L.; Ng, W.H.; Ibrahim, J.; Wang, D.; Tan, C.H.; Chellappan, S.; Lim, Y.A.L. Occurrence of zoonotic Cryptosporidium and Giardia duodenalis species/genotypes in urban rodents. Parasitol Int 2019, 69, 110–113. [Google Scholar] [CrossRef]
- Taghipour, A.; Olfatifar, M.; Foroutan, M.; Bahadory, S.; Malih, N.; Norouzi, M. Global prevalence of Cryptosporidium infection in rodents: A systematic review and meta-analysis. Prev Vet Med 2020, 182, 105119. [Google Scholar] [CrossRef] [PubMed]
- El-Alfy, E.S.; Nishikawa, Y. Cryptosporidium species and cryptosporidiosis in Japan: a literature review and insights into the role played by animals in its transmission. J Vet Med Sci 2020, 82, 1051–1067. [Google Scholar] [CrossRef] [PubMed]
- Golomazou, E.; Malandrakis, E.E.; Panagiotaki, P.; Karanis, P. Cryptosporidium in fish: Implications for aquaculture and beyond. Water Res 2021, 201, 117357. [Google Scholar] [CrossRef] [PubMed]
- Golomazou, E.; Mamedova, S.; Eslahi, A.V.; Karanis, P. Cryptosporidium and agriculture: A review. Sci Total Environ 2024, 916, 170057. [Google Scholar] [CrossRef]
- García-Livia, K.; Fernández-Álvarez, Á.; Feliu, C.; Miquel, J.; Quilichini, Y.; Foronda, P. Cryptosporidium spp. in wild murids (Rodentia) from Corsica, France. Parasitol Res 2022, 121, 345–354. [Google Scholar] [CrossRef]
- Li, X.; Atwill, E.R. Diverse Genotypes and Species of Cryptosporidium in Wild Rodent Species from the West Coast of the USA and Implications for Raw Produce Safety and Microbial Water Quality. Microorganisms 2021, 9. [Google Scholar] [CrossRef]
- Quintero, K.; Durán, C.; Duri, D.; Medina, F.; Garcia, J.; Hidalgo, G.; Nakal, S.; Echeverria-Ortega, M.; Albano, C.; Incani, R.N. , et al. Household social determinants of ascariasis and trichuriasis in North Central Venezuela. Int Health 2012, 4, 103–110. [Google Scholar] [CrossRef]
- Fitte, B.; Robles, M.R.; Dellarupe, A.; Unzaga, J.M.; Navone, G.T. Hymenolepis diminuta and Rodentolepis nana (Hymenolepididae: Cyclophyllidea) in urban rodents of Gran La Plata: association with socio-environmental conditions. J Helminthol 2018, 92, 549–553. [Google Scholar] [CrossRef]
- Ito, A.; Budke, C.M. Perspectives on intestinal tapeworm infections: An evaluation of direct and indirect life-cycles with a special emphasis on species of Hymenolepis. Curr Res Parasitol Vector Borne Dis 2021, 1, 100023. [Google Scholar] [CrossRef]
- Panti-May, J.A.; Rodríguez-Vivas, R.I.; García-Prieto, L.; Servián, A.; Costa, F. Worldwide overview of human infections with Hymenolepis diminuta. Parasitol Res 2020, 119, 1997–2004. [Google Scholar] [CrossRef]
- Mane, P.; Sangwan, J. Hymenolepis diminuta infection in a young boy from rural part of Northern India. J Family Med Prim Care 2016, 5, 166–167. [Google Scholar] [CrossRef] [PubMed]
- Horáková, B.; Čadková, Z.; Száková, J.; Jankovská, I. The identification of risk and essential elements along the strobila of the rat tapeworm Hymenolepis diminuta. J Helminthol 2017, 91, 555–560. [Google Scholar] [CrossRef]
- Islam, M.M.; Farag, E.; Hassan, M.M.; Bansal, D.; Awaidy, S.A.; Abubakar, A.; Al-Rumaihi, H.; Mkhize-Kwitshana, Z. Helminth Parasites among Rodents in the Middle East Countries: A Systematic Review and Meta-Analysis. Animals (Basel) 2020, 10. [Google Scholar] [CrossRef]
- Fitte, B.; Cavia, R.; Robles, M.D.R.; Dellarupe, A.; Unzaga, J.M.; Navone, G.T. Predictors of parasite and pathogen infections in urban rodents of central Argentina. J Helminthol 2021, 95, e71. [Google Scholar] [CrossRef]
- Galán-Puchades, M.T.; Sanxis-Furió, J.; Pascual, J.; Bueno-Marí, R.; Franco, S.; Peracho, V.; Montalvo, T.; Fuentes, M.V. First survey on zoonotic helminthosis in urban brown rats (Rattus norvegicus) in Spain and associated public health considerations. Vet Parasitol 2018, 259, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Brar, S.K.; Singla, N.; Singla, L.D. Comparative Comprehensive Analysis on Natural Infections of Hymenolepis Diminuta and Hymenolepis Nana in Commensal Rodents. Helminthologia 2021, 58, 248–262. [Google Scholar] [CrossRef]
- Yang, D.; Zhao, W.; Zhang, Y.; Liu, A. Prevalence of Hymenolepis nana and H. diminuta from Brown Rats (Rattus norvegicus) in Heilongjiang Province, China. Korean J Parasitol 2017, 55, 351–355. [Google Scholar] [CrossRef]
- Coello Peralta, R.D.; Salazar Mazamba, M.L.; Pazmiño Gómez, B.J.; Cushicóndor Collaguazo, D.M.; Gómez Landires, E.A.; Ramallo, G. Hymenolepiasis Caused by Hymenolepis nana in Humans and Natural Infection in Rodents in a Marginal Urban Sector of Guayaquil, Ecuador. Am J Case Rep 2023, 24, e939476. [Google Scholar] [CrossRef] [PubMed]
- Haldeman, M.S.; Nolan, M.S.; Ng'habi, K.R.N. Human hookworm infection: Is effective control possible? A review of hookworm control efforts and future directions. Acta Trop 2020, 201, 105214. [Google Scholar] [CrossRef]
- Bilska-Zając, E.; Korpysa-Dzirba, W.; Bełcik, A.; Karamon, J.; Sroka, J.; Cencek, T. Scheme of Effective Epidemiological Investigations in Trichinella Outbreaks on Pig Farms. Foods 2023, 12. [Google Scholar] [CrossRef]
- Echeverry, D.M.; Henríquez, A.; Oyarzún-Ruiz, P.; Silva-de la Fuente, M.C.; Ortega, R.; Sandoval, D.; Landaeta-Aqueveque, C. First record of Trichinella in Leopardus guigna (Carnivora, Felidae) and Galictis cuja (Carnivora, Mustelidae): new hosts in Chile. PeerJ 2021, 9, e11601. [Google Scholar] [CrossRef] [PubMed]
- Akibekov, O.S.; Syzdykova, A.S.; Lider, L.A.; Zhumalin, A.K.; Zhagipar, F.S.; Gajimuradova, A.M.; Borovikov, S.N.; Suranshiyev, Z.A.; Ashimov, S.A. Trichinellosis dissemination among wild carnivores in the Republic of Kazakhstan: A 10-year study. Vet World 2023, 16, 1840–1848. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.; Interisano, M.; Deksne, G.; Pozio, E. The subnivium, a haven for Trichinella larvae in host carcasses. Int J Parasitol Parasites Wildl 2019, 8, 229–233. [Google Scholar] [CrossRef]
- Espinoza-Rojas, H.; Lobos-Chávez, F.; Silva-de la Fuente, M.C.; Echeverry, D.M.; Muñoz-Galaz, J.; Yáñez-Crisóstomo, C.; Oyarzún-Ruiz, P.; Ortega, R.; Sandoval, D.; Henríquez, A. , et al. Survey of Trichinella in American minks (Neovison vison Schreber, 1777) and wild rodents (Muridae and Cricetidae) in Chile. Zoonoses Public Health 2021, 68, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Garbarino, C.; Interisano, M.; Chiatante, A.; Marucci, G.; Merli, E.; Arrigoni, N.; Cammi, G.; Ricchi, M.; Tonanzi, D.; Tamba, M. , et al. Trichinella spiralis a new alien parasite in Italy and the increased risk of infection for domestic and wild swine. Vet Parasitol 2017, 246, 1–4. [Google Scholar] [CrossRef]
- Crisóstomo-Jorquera, V.; Landaeta-Aqueveque, C. The genus Trichinella and its presence in wildlife worldwide: A review. Transbound Emerg Dis 2022, 69, e1269–e1279. [Google Scholar] [CrossRef]
- Malone, C.J.; Oksanen, A.; Mukaratirwa, S.; Sharma, R.; Jenkins, E. From wildlife to humans: The global distribution of Trichinella species and genotypes in wildlife and wildlife-associated human trichinellosis. Int J Parasitol Parasites Wildl 2024, 24, 100934. [Google Scholar] [CrossRef]
- Diaz, J.H.; Warren, R.J.; Oster, M.J. The Disease Ecology, Epidemiology, Clinical Manifestations, and Management of Trichinellosis Linked to Consumption of Wild Animal Meat. Wilderness Environ Med 2020, 31, 235–244. [Google Scholar] [CrossRef]
- Zhang, X.Z.; Wang, Z.Q.; Cui, J. Epidemiology of trichinellosis in the People's Republic of China during 2009-2020. Acta Trop 2022, 229, 106388. [Google Scholar] [CrossRef]
- Galán-Puchades, M.T.; Gómez-Samblás, M.; Osuna, A.; Sáez-Durán, S.; Bueno-Marí, R.; Fuentes, M.V. Update on the First Finding of the Rat Lungworm, Angiostrongylus cantonensis, in Rattus spp. in Continental Europe, Valencia, Spain, 2022. Pathogens 2023, 12. [Google Scholar] [CrossRef]
- Gottdenker, N.L.; Nascimento Ramos, R.A.; Hakimi, H.; McHale, B.; Rivera, S.; Miller, B.M.; Howerth, E.W.; Burrell, C.E.; Stilwell, J.M.; McManamon, R. , et al. Angiostrongylus cantonensis Infection in Brown Rats (Rattus norvegicus), Atlanta, Georgia, USA, 2019-2022. Emerg Infect Dis 2023, 29, 2167–2170. [Google Scholar] [CrossRef] [PubMed]
- Flerlage, T.; Qvarnstrom, Y.; Noh, J.; Devincenzo, J.P.; Madni, A.; Bagga, B.; Hysmith, N.D. Angiostrongylus cantonensis Eosinophilic Meningitis in an Infant, Tennessee, USA. Emerg Infect Dis 2017, 23, 1756–1758. [Google Scholar] [CrossRef]
- Lv, S.; Zhou, X.N.; Andrews, J.R. Eosinophilic Meningitis Caused by Angiostrongylus cantonensis. ACS Chem Neurosci 2017, 8, 1815–1816. [Google Scholar] [CrossRef]
- de Almeida, L.R.; de Souza Joaquim, J.; Botelho, L.M.; Vidigal, T.; Ecco, R.; de Souza Trindade, G.; Paglia, A.P.; Pereira, C.A.J.; Dos Santos Lima, W. Parasitism in Rattus rattus and sympatric Achatina fulica by Angiostrongylus cantonensis in an urban park in southeast Brazil. Parasitol Res 2023, 122, 347–352. [Google Scholar] [CrossRef]
- Hancke, D.; Guzman, N.; Tripodi, M.; Muschetto, E.; Suárez, O.V. Reaching new lands: Updating the distribution of Angiostrongylus cantonensis in South America with the first record in Argentina. Zoonoses Public Health 2024, 71, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Souza, F.N.; Aguiar Santos, M.; Almeida Alves, D.; Cecília Vieira de Melo, L.; Jessé Gonçalves da Mota, D.; Cristina Pertile, A.; Gava, R.; Luiz Silva Pinto, P.; Eyre, M.T.; Graco Zeppelini, C. , et al. Angiostrongylus cantonensis in urban populations of terrestrial gastropods and rats in an impoverished region of Brazil. Parasitology 2021, 148, 994–1002. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, J.P.; Shenoy, A.S.; Goel, N.A. Eosinophilic meningitis due to A. Cantonensis revealed at autopsy. Indian J Pathol Microbiol 2022, 65, 420–421. [Google Scholar] [CrossRef]
- Liu, E.W.; Schwartz, B.S.; Hysmith, N.D.; DeVincenzo, J.P.; Larson, D.T.; Maves, R.C.; Palazzi, D.L.; Meyer, C.; Custodio, H.T.; Braza, M.M. , et al. Rat Lungworm Infection Associated with Central Nervous System Disease - Eight U.S. States, January 2011-January 2017. MMWR Morb Mortal Wkly Rep 2018, 67, 825–828. [Google Scholar] [CrossRef]
- Rivory, P.; Lee, R.; Šlapeta, J. Rat lungworm (Angiostrongylus cantonensis) active larval emergence from deceased bubble pond snails (Bullastra lessoni) into water. Parasitology 2023, 150, 700–704. [Google Scholar] [CrossRef]
- Martin-Carrillo, N.; Baz-González, E.; García-Livia, K.; Amaro-Ramos, V.; Abreu-Acosta, N.; Miquel, J.; Abreu-Yanes, E.; Pino-Vera, R.; Feliu, C.; Foronda, P. Data on New Intermediate and Accidental Hosts Naturally Infected with Angiostrongylus cantonensis in La Gomera and Gran Canaria (Canary Islands, Spain). Animals (Basel) 2023, 13. [Google Scholar] [CrossRef]
- Underwood, E.B.; Walker, M.J.; Darden, T.L.; Kingsley-Smith, P.R. Frequency of Occurrence of the Rat Lungworm Parasite in the Invasive Island Apple Snail in South Carolina, USA. J Aquat Anim Health 2019, 31, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Bolukbas, C.S.; Demirtas, S.; Gurler, A.T.; Inal, S.; Acici, M.; Umur, S. Molecular characterization of Calodium hepaticum in grey dwarf hamster (Cricetulus migratorius). Parasitol Int 2020, 78, 102133. [Google Scholar] [CrossRef] [PubMed]
- Miterpáková, M.; Hurníková, Z.; Komorová, P.; Stanko, M.; Chovancová, G.; Syrota, Y. Micromammals as a reservoir for the zoonotic nematode Calodium hepaticum (syn. Capillaria hepatica) in recreational areas of Slovakia. Curr Res Parasitol Vector Borne Dis 2024, 6, 100214. [Google Scholar] [CrossRef]
- Dini, F.M.; Caffara, M.; Magri, A.; Cantori, A.; Luci, V.; Monno, A.; Galuppi, R. Sentinels in the shadows: Exploring Toxoplasma gondii and other Sarcocystidae parasites in synanthropic rodents and their public health implications. Int J Parasitol Parasites Wildl 2024, 24, 100939. [Google Scholar] [CrossRef]
- Gravinatti, M.L.; Barbosa, C.M.; Soares, R.M.; Gregori, F. Synanthropic rodents as virus reservoirs and transmitters. Rev Soc Bras Med Trop 2020, 53, e20190486. [Google Scholar] [CrossRef] [PubMed]
- Dubey, A.; Bagchi, A.; Sharma, D.; Dey, A.; Nandy, K.; Sharma, R. Hepatic Capillariasis- Drug Targets. Infect Disord Drug Targets 2018, 18, 3–10. [Google Scholar] [CrossRef]
- Abalaka, S.E.; Ejeh, S.A. Histopathological evaluation of Capillaria hepatica (Bancroft, 1893) in Cricetomys gambianus (Waterhouse, 1840). J Parasit Dis 2025, 49, 186–192. [Google Scholar] [CrossRef]
- Al-Sabi, M.N.S.; Chriél, M.; Hansen, M.S.; Enemark, H.L. Baylisascaris procyonis in wild raccoons (Procyon lotor) in Denmark. Vet Parasitol Reg Stud Reports 2015, 1-2, 55–58. [Google Scholar] [CrossRef]
- Ogdee, J.L.; Henke, S.E.; Wester, D.B.; Fedynich, A.M. Permeability and Viability of Baylisascaris procyonis Eggs in Southern Texas Soils. J Parasitol 2016, 102, 608–612. [Google Scholar] [CrossRef]
- Sapp, S.G.; Weinstein, S.B.; McMahan, C.S.; Yabsley, M.J. Variable Infection Dynamics in Four Peromyscus Species Following Experimental Inoculation with Baylisascaris procyonis. J Parasitol 2016, 102, 538–544. [Google Scholar] [CrossRef]
- Weinstein, S.B. Introduced Rats and an Endemic Roundworm: Does Rattus rattus Contribute to Baylisascaris procyonis Transmission in California? J Parasitol 2017, 103, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Sapp, S.G.H.; Elsemore, D.A.; Hanna, R.; Yabsley, M.J. Experimental comparison of Baylisascaris procyonis definitive host competence between domestic dogs and raccoons (Procyon lotor). Parasitology 2020, 147, 1344–1351. [Google Scholar] [CrossRef] [PubMed]
- Umhang, G.; Frantz, A.C.; Ferté, H.; Fournier Chambrillon, C.; Gautrelet, M.; Gritti, T.; Thenon, N.; Le Loc'h, G.; Isère-Laoué, E.; Egal, F. , et al. Surveys on Baylisascaris procyonis in two of the three French wild raccoon populations. Int J Parasitol Parasites Wildl 2024, 23, 100928. [Google Scholar] [CrossRef] [PubMed]
- Straif-Bourgeois, S.; Cloherty, E.; Balsamo, G.; Gee, L.; Riegel, C. Prevalence of Baylisascaris procyonis in Raccoons Trapped in New Orleans, Louisiana, 2014-2017. Vector Borne Zoonotic Dis 2020, 20, 22–26. [Google Scholar] [CrossRef]
- Dzikowiec, M.; Góralska, K.; Błaszkowska, J. Neuroinvasions caused by parasites. Ann Parasitol 2017, 63, 243–253. [Google Scholar] [CrossRef]
- Hoberg, E.P.; Burek-Huntington, K.; Beckmen, K.; Camp, L.E.; Nadler, S.A. Transuterine infection by Baylisascaris transfuga: Neurological migration and fatal debilitation in sibling moose calves (Alces alces gigas) from Alaska. Int J Parasitol Parasites Wildl 2018, 7, 280–288. [Google Scholar] [CrossRef]
- Rentería-Solís, Z.; Birka, S.; Schmäschke, R.; Król, N.; Obiegala, A. First detection of Baylisascaris procyonis in wild raccoons (Procyon lotor) from Leipzig, Saxony, Eastern Germany. Parasitol Res 2018, 117, 3289–3292. [Google Scholar] [CrossRef]
- Bland, D.M.; Jarrett, C.O.; Bosio, C.F.; Hinnebusch, B.J. Infectious blood source alters early foregut infection and regurgitative transmission of Yersinia pestis by rodent fleas. PLoS Pathog 2018, 14, e1006859. [Google Scholar] [CrossRef]
- Lemon, A.; Cherzan, N.; Vadyvaloo, V. Influence of Temperature on Development of Yersinia pestis Foregut Blockage in Xenopsylla cheopis (Siphonaptera: Pulicidae) and Oropsylla montana (Siphonaptera: Ceratophyllidae). J Med Entomol 2020, 57, 1997–2007. [Google Scholar] [CrossRef]
- Rodriguez-Morales, A.J.; Escalera-Antezana, J.P.; Alvarado-Arnez, L.E. Is Plague Globally Reemerging? Infectio 2019, 23, 7–9. [Google Scholar] [CrossRef]
- Blanton, L.S. Murine Typhus: A Review of a Reemerging Flea-Borne Rickettsiosis with Potential for Neurologic Manifestations and Sequalae. Infect Dis Rep 2023, 15, 700–716. [Google Scholar] [CrossRef] [PubMed]
- Caravedo Martinez, M.A.; Ramírez-Hernández, A.; Blanton, L.S. Manifestations and Management of Flea-Borne Rickettsioses. Res Rep Trop Med 2021, 12, 1–14. [Google Scholar] [CrossRef]
- McKee, C.D.; Osikowicz, L.M.; Schwedhelm, T.R.; Maes, S.E.; Enscore, R.E.; Gage, K.L.; Kosoy, M.Y. Acquisition of Bartonella elizabethae by Experimentally Exposed Oriental Rat Fleas (Xenopsylla cheopis; Siphonaptera, Pulicidae) and Excretion of Bartonella DNA in Flea Feces. J Med Entomol 2018, 55, 1292–1298. [Google Scholar] [CrossRef]
- Rahelinirina, S.; Razafiarimanga, Z.N.; Rajerison, M.; Djedanem, M.; Handschumacher, P.; Jambou, R. Impact of Sanitation on Rodent Pullulation and Plague Status in an Informal Settlement on the Outskirts of Mahajanga (Madagascar). Pathogens 2024, 13. [Google Scholar] [CrossRef] [PubMed]
- Rasoamalala, F.; Gostic, K.; Parany, M.J.; Rahelinirina, S.; Rahajandraibe, S.; Gorgé, O.; Valade, E.; Harimalala, M.; Rajerison, M.; Ramasindrazana, B. Population dynamics of plague vector fleas in an endemic focus: implications for plague surveillance. J Med Entomol 2024, 61, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Eneku, W.; Erima, B.; Byaruhanga Maranda, A.; Cleary Gillian, N.; Atim, G.; Tugume, T.; Ukuli Aquino, Q.; Kibuuka, H.; Mworozi, E.; Douglas, C. , et al. Molecular detection and characterization of Rickettsia felis, R. asembonensis, and Yersinia pestis from peri-domestic fleas in Uganda. Infect Ecol Epidemiol 2025, 15, 2473159. [Google Scholar] [CrossRef]
- Clancy, B.M.; Theriault, B.R.; Schoenberger, J.M.; Bowers, C.J.; Mitchell, C.M.; Langan, G.P.; Ostdiek, A.M.; Luchins, K.R. Identification and Control of an Ornithonyssus Bacoti Infestation in a Rodent Vivarium by Using Molecular Diagnostic Techniques. Comp Med 2022, 72, 113–121. [Google Scholar] [CrossRef]
- Yin, P.W.; Guo, X.G.; Jin, D.C.; Fan, R.; Zhao, C.F.; Zhang, Z.W.; Huang, X.B.; Mao, K.Y. Distribution and Host Selection of Tropical Rat Mite, Ornithonyssus bacoti, in Yunnan Province of Southwest China. Animals (Basel) 2021, 11. [Google Scholar] [CrossRef]
- Islam, M.M.; Farag, E.; Eltom, K.; Hassan, M.M.; Bansal, D.; Schaffner, F.; Medlock, J.M.; Al-Romaihi, H.; Mkhize-Kwitshana, Z. Rodent Ectoparasites in the Middle East: A Systematic Review and Meta-Analysis. Pathogens 2021, 10. [Google Scholar] [CrossRef]
- Sarathep, P.; Phonkaew, W. A lost world disease: Copra itch outbreak caused by Tyrophagus longior mite. IDCases 2018, 12, 58–63. [Google Scholar] [CrossRef]
- Thomas, C.; Castillo Valladares, H.; Berger, T.G.; Chang, A.Y. Scabies, Bedbug, and Body Lice Infestations: A Review. Jama 2024. [Google Scholar] [CrossRef] [PubMed]
- Eikenbary, B.; Devaraju, P.; Chakkravarthi, A.; Sihag, K.K.; Nathan, T.; Thangaraj, G.; Srinivasan, L.; Kumar, A. A molecular survey of zoonotic pathogens of public health importance in rodents/shrews and their ectoparasites trapped in Puducherry, India. Trans R Soc Trop Med Hyg 2024, 118, 616–624. [Google Scholar] [CrossRef]
- Moraga-Fernández, A.; Muñoz-Hernández, C.; Sánchez-Sánchez, M.; Fernández de Mera, I.G.; de la Fuente, J. Exploring the diversity of tick-borne pathogens: The case of bacteria (Anaplasma, Rickettsia, Coxiella and Borrelia) protozoa (Babesia and Theileria) and viruses (Orthonairovirus, tick-borne encephalitis virus and louping ill virus) in the European continent. Vet Microbiol 2023, 286, 109892. [Google Scholar] [CrossRef]
- Sargison, N.D.; Chaudhry, U.; Costa-Junior, L.; Kutcher, J.R.; Li, K.; Sargison, F.A.; Zahid, O. The diagnosis and vector potential of Ornithonyssus bacoti tropical rat mites in northern Europe. Vet Parasitol Reg Stud Reports 2025, 58, 101204. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, Y.; Fang, J.; Wang, J.; Zhou, Y. A rapid diagnosis and treatment of Ornithonyssus bacoti infection. Parasitol Res 2023, 122, 1567–1572. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.S.; Kahl, O.; Lane, R.S.; Levin, M.L.; Tsao, J.I. Diapause in ticks of the medically important Ixodes ricinus species complex. Ticks Tick Borne Dis 2016, 7, 992–1003. [Google Scholar] [CrossRef]
- Larson, R.T.; Lee, X.; Zembsch, T.; Bron, G.M.; Paskewitz, S.M. Immature Ixodes scapularis (Acari: Ixodidae) Collected From Peromyscus leucopus (Rodentia: Cricetidae) and Peromyscus maniculatus (Rodentia: Cricetidae) Nests in Northern Wisconsin. J Med Entomol 2020, 57, 304–307. [Google Scholar] [CrossRef]
- Asman, M.; Witecka, J.; Solarz, K.; Zwonik, A.; Szilman, P. Occurrence of Borrelia burgdorferi sensu lato, Anaplasma phagocytophilum and Babesia microti in Ixodes ricinus ticks collected from selected areas of Opolskie Province in south-west Poland. Ann Agric Environ Med 2019, 26, 544–547. [Google Scholar] [CrossRef]
- Werden, L.; Lindsay, L.R.; Barker, I.K.; Bowman, J.; Gonzales, E.K.; Jardine, C.M. Prevalence of Anaplasma phagocytophilum and Babesia microti in Ixodes scapularis from a Newly Established Lyme Disease Endemic Area, the Thousand Islands Region of Ontario, Canada. Vector Borne Zoonotic Dis 2015, 15, 627–629. [Google Scholar] [CrossRef]
- Acosta-España, J.D.; Herrera-Yela, A.; Altamirano-Jara, J.B.; Bonilla-Aldana, D.K.; Rodriguez-Morales, A.J. The epidemiology and clinical manifestations of anaplasmosis in humans: A systematic review of case reports. J Infect Public Health 2025, 18, 102765. [Google Scholar] [CrossRef]
- Bonilla-Aldana, D.K.; Castaño-Betancourt, K.J.; Ortega-Martínez, J.M.; Ulloque-Badaracco, J.R.; Hernandez-Bustamante, E.A.; Benites-Zapata, V.A.; Rodriguez-Morales, A.J. Prevalence of zoonotic and non-zoonotic Rickettsia in horses: A systematic review and meta-analysis. New Microbes New Infect 2023, 51, 101068. [Google Scholar] [CrossRef] [PubMed]
- Bonilla-Aldana, D.K.; Gutiérrez-Grajales, E.J.; Martínez-Arboleda, J.P.; Reina-Mora, M.A.; Trejos-Mendoza, A.E.; Pérez-Vargas, S.; Valencia-Mejía, L.; Marín-Arboleda, L.F.; Osorio-Navia, D.; Chacón-Peña, M. , et al. Seroprevalence canine survey for selected vector-borne pathogens and its relationship with poverty in metropolitan Pereira, Colombia, 2020. Parasite Epidemiol Control 2022, 17, e00249. [Google Scholar] [CrossRef] [PubMed]
- Cayol, C.; Koskela, E.; Mappes, T.; Siukkola, A.; Kallio, E.R. Temporal dynamics of the tick Ixodes ricinus in northern Europe: epidemiological implications. Parasit Vectors 2017, 10, 166. [Google Scholar] [CrossRef] [PubMed]
- Karbowiak, G.; Biernat, B. The role of particular tick developmental stages in the circulation of tick-borne pathogens affecting humans in Central Europe. 2. Tick-borne encephalitis virus. Ann Parasitol 2016, 62, 3–9. [Google Scholar] [CrossRef]
- Eisen, L. Control of ixodid ticks and prevention of tick-borne diseases in the United States: The prospect of a new Lyme disease vaccine and the continuing problem with tick exposure on residential properties. Ticks Tick Borne Dis 2021, 12, 101649. [Google Scholar] [CrossRef]
- Eisen, L.; Dolan, M.C. Evidence for Personal Protective Measures to Reduce Human Contact With Blacklegged Ticks and for Environmentally Based Control Methods to Suppress Host-Seeking Blacklegged Ticks and Reduce Infection with Lyme Disease Spirochetes in Tick Vectors and Rodent Reservoirs. J Med Entomol 2016, 53, 1063–1092. [Google Scholar] [CrossRef]
- Cafiso, A.; Olivieri, E.; Floriano, A.M.; Chiappa, G.; Serra, V.; Sassera, D.; Bazzocchi, C. Investigation of Tick-Borne Pathogens in Ixodes ricinus in a Peri-Urban Park in Lombardy (Italy) Reveals the Presence of Emerging Pathogens. Pathogens 2021, 10. [Google Scholar] [CrossRef]
- Vikentjeva, M.; Geller, J.; Bragina, O. Ticks and Tick-Borne Pathogens in Popular Recreational Areas in Tallinn, Estonia: The Underestimated Risk of Tick-Borne Diseases. Microorganisms 2024, 12. [Google Scholar] [CrossRef]
- Ecke, F.; Han, B.A.; Hörnfeldt, B.; Khalil, H.; Magnusson, M.; Singh, N.J.; Ostfeld, R.S. Population fluctuations and synanthropy explain transmission risk in rodent-borne zoonoses. Nat Commun 2022, 13, 7532. [Google Scholar] [CrossRef]
- Mistrick, J.; Kipp, E.J.; Weinberg, S.I.; Adams, C.C.; Larsen, P.A.; Craft, M.E. Microbiome diversity and zoonotic bacterial pathogen prevalence in Peromyscus mice from agricultural landscapes and synanthropic habitat. Mol Ecol 2024, 33, e17309. [Google Scholar] [CrossRef]
- Nunes, D.O.; Fehlberg, H.F.; Carneiro, L.O.; Oliveira, K.M.M.; Bovendorp, R.S.; Ribeiro, C.M.; Albuquerque, G.R.; Oliveira, T.; Sevá, A.D.P. Synanthropic Rodents as Bioindicator of Human Pathogens in a Tourist Area of Brazil. Ecohealth 2025. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Soto, M.F.; Gaona, O.; Vigueras-Galván, A.L.; Suzán, G.; Falcón, L.I.; Vázquez-Domínguez, E. Prevalence and transmission of the most relevant zoonotic and vector-borne pathogens in the Yucatan peninsula: A review. PLoS Negl Trop Dis 2024, 18, e0012286. [Google Scholar] [CrossRef] [PubMed]
- Antúnez, M.P.; Marín Montesinos, J.C.; Corduneanu, A.; Obregón, D.; Moutailler, S.; Cabezas-Cruz, A. Tick-borne viruses and their risk to public health in the Caribbean: Spotlight on bats as reservoirs in Cuba. Heliyon 2024, 10, e26118. [Google Scholar] [CrossRef] [PubMed]
- Harrison, A.; Robb, G.N.; Alagaili, A.N.; Hastriter, M.W.; Apanaskevich, D.A.; Ueckermann, E.A.; Bennett, N.C. Ectoparasite fauna of rodents collected from two wildlife research centres in Saudi Arabia with discussion on the implications for disease transmission. Acta Trop 2015, 147, 1–5. [Google Scholar] [CrossRef]
- Wang, H.R.; Liu, T.; Gao, X.; Wang, H.B.; Xiao, J.H. Impact of climate change on the global circulation of West Nile virus and adaptation responses: a scoping review. Infect Dis Poverty 2024, 13, 38. [Google Scholar] [CrossRef]
- Esson, C.; Samelius, G.; Strand, T.M.; Lundkvist, Å.; Michaux, J.R.; Råsbäck, T.; Wahab, T.; Mijiddorj, T.N.; Berger, L.; Skerratt, L.F. , et al. The prevalence of rodent-borne zoonotic pathogens in the South Gobi desert region of Mongolia. Infect Ecol Epidemiol 2023, 13, 2270258. [Google Scholar] [CrossRef]
- Nazari, N.; Shojaee, S.; Mohebali, M.; Teimouri, A.; Ghadiri, K.; Raeghi, S.; Shiee, M.R.; Azarakhsh, Y.; Bozorgomid, A. Toxoplasma gondii And Neospora caninum In Brain Tissue Of Rodents In North-West Iran. Vet Med (Auckl) 2019, 10, 223–227. [Google Scholar] [CrossRef]
- Acosta-Espana, J.D.; Romero-Alvarez, D.; Luna, C.; Rodriguez-Morales, A.J. Infectious disease outbreaks in the wake of natural flood disasters: global patterns and local implications. Infez Med 2024, 32, 451–462. [Google Scholar] [CrossRef]
- Patwary, M.M.; Rodriguez-Morales, A.J. Deadly Flood and Landslides amid COVID-19 Crisis: A Public Health Concern for the World's Largest Refugee Camp in Bangladesh. Prehosp Disaster Med 2022, 37, 292–293. [Google Scholar] [CrossRef]
- Sutiningsih, D.; Sari, D.P.; Permatasari, C.D.; Azzahra, N.A.; Rodriguez-Morales, A.J.; Yuliawati, S.; Maharani, N.E. Geospatial Analysis of Abiotic and Biotic Conditions Associated with Leptospirosis in the Klaten Regency, Central Java, Indonesia. Trop Med Infect Dis 2024, 9. [Google Scholar] [CrossRef]
- Bonilla-Aldana, D.K.; Suarez, J.A.; Franco-Paredes, C.; Vilcarromero, S.; Mattar, S.; Gomez-Marin, J.E.; Villamil-Gomez, W.E.; Ruiz-Saenz, J.; Cardona-Ospina, J.A.; Idarraga-Bedoya, S.E. , et al. Brazil burning! What is the potential impact of the Amazon wildfires on vector-borne and zoonotic emerging diseases? - A statement from an international experts meeting. Travel Med Infect Dis 2019, 31, 101474. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowska, J.; Menéndez Orellana, A.E.; Kilian, W.; Moryl, A.; Cielecka, N.; Michałowska, K.; Policht-Latawiec, A.; Michalski, A.; Bednarek, A.; Włóka, A. Between flood and drought: How cities are facing water surplus and scarcity. J Environ Manage 2023, 345, 118557. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, E.M.; Felton, D. A Review of Climate-Driven Threats to Recreational Water Users in Hawaii. Wilderness Environ Med 2025, 36, 61–66. [Google Scholar] [CrossRef]
- Mirsaeidi, M.; Motahari, H.; Taghizadeh Khamesi, M.; Sharifi, A.; Campos, M.; Schraufnagel, D.E. Climate Change and Respiratory Infections. Ann Am Thorac Soc 2016, 13, 1223–1230. [Google Scholar] [CrossRef]
- Bron, G.M.; Malavé, C.M.; Boulerice, J.T.; Osorio, J.E.; Rocke, T.E. Plague-Positive Mouse Fleas on Mice Before Plague Induced Die-Offs in Black-Tailed and White-Tailed Prairie Dogs. Vector Borne Zoonotic Dis 2019, 19, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Mojahed, N.; Mohammadkhani, M.A.; Mohamadkhani, A. Climate Crises and Developing Vector-Borne Diseases: A Narrative Review. Iran J Public Health 2022, 51, 2664–2673. [Google Scholar] [CrossRef]
- Douglas, K.O.; Payne, K.; Sabino-Santos, G., Jr.; Agard, J. Influence of Climatic Factors on Human Hantavirus Infections in Latin America and the Caribbean: A Systematic Review. Pathogens 2021, 11. [Google Scholar] [CrossRef]
- Nimo-Paintsil, S.C.; Fichet-Calvet, E.; Borremans, B.; Letizia, A.G.; Mohareb, E.; Bonney, J.H.K.; Obiri-Danso, K.; Ampofo, W.K.; Schoepp, R.J.; Kronmann, K.C. Rodent-borne infections in rural Ghanaian farming communities. PLoS One 2019, 14, e0215224. [Google Scholar] [CrossRef]
- Rabiee, M.H.; Mahmoudi, A.; Siahsarvie, R.; Kryštufek, B.; Mostafavi, E. Rodent-borne diseases and their public health importance in Iran. PLoS Negl Trop Dis 2018, 12, e0006256. [Google Scholar] [CrossRef]
- Mawanda, P.; Rwego, I.; Kisakye, J.J.; Sheil, D. Rodents as potential hosts and reservoirs of parasites along the edge of a Central African forest: Bwindi impenetrable national park, South Western Uganda. Afr Health Sci 2020, 20, 1168–1178. [Google Scholar] [CrossRef]
- Sanker, V.; Vellekkat, F.; Dave, T. Nipah Virus Outbreaks in Kerala: An Impending Doom? Health Sci Rep 2024, 7, e70195. [Google Scholar] [CrossRef] [PubMed]
- Tambo, E.; Adetunde, O.T.; Olalubi, O.A. Re-emerging Lassa fever outbreaks in Nigeria: Re-enforcing "One Health" community surveillance and emergency response practice. Infect Dis Poverty 2018, 7, 37. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, W.; Lv, C. Modern technologies and solutions to enhance surveillance and response systems for emerging zoonotic diseases. Sci One Health 2024, 3, 100061. [Google Scholar] [CrossRef]
- Dutto, M.; Di Domenico, D.; Rubbiani, M. Use of anticoagulant rodenticides in outdoor urban areas: considerations and proposals for the protection of public health and non-target species. Ann Ig 2018, 30, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Krijger, I.M.; Gort, G.; Belmain, S.R.; Groot Koerkamp, P.W.G.; Shafali, R.B.; Meerburg, B.G. Efficacy of Management and Monitoring Methods to Prevent Post-Harvest Losses Caused by Rodents. Animals (Basel) 2020, 10. [Google Scholar] [CrossRef]
- Rahelinirina, S.; Scobie, K.; Ramasindrazana, B.; Andrianaivoarimanana, V.; Rasoamalala, F.; Randriantseheno, L.N.; Rakotoniaina, J.S.; Gorgé, O.; Lambin, X.; Valade, E. , et al. Rodent control to fight plague: field assessment of methods based on rat density reduction. Integr Zool 2021, 16, 868–885. [Google Scholar] [CrossRef] [PubMed]
- Akello, W. Climate Change and Veterinary Medicine: A Call to Action for a Healthier Planet. F1000Res 2024, 13, 1360. [Google Scholar] [CrossRef]
- Roberts, C.M.; O'Leary, B.C.; Hawkins, J.P. Climate change mitigation and nature conservation both require higher protected area targets. Philos Trans R Soc Lond B Biol Sci 2020, 375, 20190121. [Google Scholar] [CrossRef]
- Rupasinghe, R.; Chomel, B.B.; Martínez-López, B. Climate change and zoonoses: A review of the current status, knowledge gaps, and future trends. Acta Trop 2022, 226, 106225. [Google Scholar] [CrossRef]
- Lambert, R. Parasitology: identification of helminths; London: Butter-worth & Co. (Publishers ) Ltd: 1969; pp. vii + 64 pp.
Figure 1.
Taxonomic location of the order Rodentia and its suborders. Constructed using LifeMap,
https://lifemap.cnrs.fr/.
Figure 1.
Taxonomic location of the order Rodentia and its suborders. Constructed using LifeMap,
https://lifemap.cnrs.fr/.
Figure 2.
African grass rat (Arvicanthis niloticus). Species of rodent in the family Murinae. Photo taken by AJRM at the Serengeti National Park, Tanzania, December 2024, in close proximity to humans and other wild animals.
Figure 2.
African grass rat (Arvicanthis niloticus). Species of rodent in the family Murinae. Photo taken by AJRM at the Serengeti National Park, Tanzania, December 2024, in close proximity to humans and other wild animals.
Figure 3.
Life cycle of toxoplasmosis. Toxoplasma gondii infects members of the Felidae family, with domestic cats serving as the definitive hosts. They shed unsporulated oocysts in feces, which become infective after sporulating in the environment. Rodents, and other animals, act as intermediate hosts by ingesting contaminated soil, water, or vegetation. In these hosts, oocysts release tachyzoites that develop into tissue cysts. Cats become infected by consuming these cysts or ingesting sporulated oocysts directly. Humans acquire infection through undercooked meat, contaminated food or water, blood transfusion, organ transplant, or congenitally. Infected humans may carry tissue cysts in muscles, brain, and eyes for life. Diagnosis is mainly serological, though molecular methods like PCR can detect congenital infection.
Figure 3.
Life cycle of toxoplasmosis. Toxoplasma gondii infects members of the Felidae family, with domestic cats serving as the definitive hosts. They shed unsporulated oocysts in feces, which become infective after sporulating in the environment. Rodents, and other animals, act as intermediate hosts by ingesting contaminated soil, water, or vegetation. In these hosts, oocysts release tachyzoites that develop into tissue cysts. Cats become infected by consuming these cysts or ingesting sporulated oocysts directly. Humans acquire infection through undercooked meat, contaminated food or water, blood transfusion, organ transplant, or congenitally. Infected humans may carry tissue cysts in muscles, brain, and eyes for life. Diagnosis is mainly serological, though molecular methods like PCR can detect congenital infection.
Figure 4.
Life cycle of American trypanosomiasis. An infected triatomine transmits Trypanosoma cruzi by releasing trypomastigotes in its feces near the bite site. These enter the host through the wound or mucous membranes. Once inside, the parasites invade nearby cells, transform into amastigotes, and multiply by binary fission. They then convert back into trypomastigotes, which circulate in the blood and infect new cells. This cycle contributes to disease symptoms. Bloodstream trypomastigotes do not replicate and must invade cells or be ingested by another vector to continue development. When a triatomine feeds on an infected host, includen rodents, it ingests the parasites, which transform into epimastigotes in its midgut, multiply, and differentiate into infective metacyclic trypomastigotes in the hindgut. Other transmission routes include blood transfusion, organ transplant, congenital infection, and consumption of contaminated food or drink.
Figure 4.
Life cycle of American trypanosomiasis. An infected triatomine transmits Trypanosoma cruzi by releasing trypomastigotes in its feces near the bite site. These enter the host through the wound or mucous membranes. Once inside, the parasites invade nearby cells, transform into amastigotes, and multiply by binary fission. They then convert back into trypomastigotes, which circulate in the blood and infect new cells. This cycle contributes to disease symptoms. Bloodstream trypomastigotes do not replicate and must invade cells or be ingested by another vector to continue development. When a triatomine feeds on an infected host, includen rodents, it ingests the parasites, which transform into epimastigotes in its midgut, multiply, and differentiate into infective metacyclic trypomastigotes in the hindgut. Other transmission routes include blood transfusion, organ transplant, congenital infection, and consumption of contaminated food or drink.
Figure 5.
Life cycle of leishmaniasis. Leishmaniasis is transmitted through the bite of infected female phlebotomine sandflies, which inject promastigotes into the skin during feeding. These promastigotes are engulfed by macrophages and other phagocytic cells, where they transform into amastigotes. Inside these cells, they multiply and spread to other mononuclear phagocytes. The progression to cutaneous or visceral disease depends on parasite and host factors. Sandflies become infected by ingesting infected cells during a blood meal. In the sandfly, amastigotes convert back into promastigotes, develop in the gut (hindgut for Viannia, midgut for Leishmania), and migrate to the proboscis to continue the cycle. Female phlebotomine sandflies may also bite different animals transmitting Leishmania promastigotes, including rodents.
Figure 5.
Life cycle of leishmaniasis. Leishmaniasis is transmitted through the bite of infected female phlebotomine sandflies, which inject promastigotes into the skin during feeding. These promastigotes are engulfed by macrophages and other phagocytic cells, where they transform into amastigotes. Inside these cells, they multiply and spread to other mononuclear phagocytes. The progression to cutaneous or visceral disease depends on parasite and host factors. Sandflies become infected by ingesting infected cells during a blood meal. In the sandfly, amastigotes convert back into promastigotes, develop in the gut (hindgut for Viannia, midgut for Leishmania), and migrate to the proboscis to continue the cycle. Female phlebotomine sandflies may also bite different animals transmitting Leishmania promastigotes, including rodents.
Figure 6.
Amastigotes of Leishmania (A) and onychogryphosis in a dog with visceral leishmaniasis (B). Photos taken by AJRM.
Figure 6.
Amastigotes of Leishmania (A) and onychogryphosis in a dog with visceral leishmaniasis (B). Photos taken by AJRM.
Table 1.
Main parasites associated with rodents and their features.
Table 1.
Main parasites associated with rodents and their features.
| Parasite |
Parasitological Features |
Clinical Impacts |
Forms of Transmission |
Preventive Measures |
| Toxoplasma gondii |
Intracellular protozoan; form tissue cysts |
Toxoplasmosis; severe among immunocompromised individuals and pregnancy complications |
Ingestion of oocysts in contaminated food, water, or cat faeces |
Proper cooking of meat; hygiene in handling cat faeces |
| Trypanosoma cruzi |
Flagellated protozoan; transmitted by triatomine bugs |
Chagas disease; cardiomyopathy and digestive disorders |
Bite of infected triatomine bug; contaminated food or transfusions |
Vector control; improved housing and screening |
|
Leishmania spp. |
Protozoan; transmitted by sandflies |
Leishmaniasis; cutaneous, mucosal, and visceral forms |
Bite of infected sandflies |
Use of insect repellent; vector control |
| Giardia intestinalis |
Flagellated protozoan; cause intestinal infection |
Giardiasis; chronic diarrhea and malabsorption |
Ingestion of cysts in contaminated water or food |
Safe drinking water; proper sanitation |
| Cryptosporidium spp. |
Apicomplexan parasite; waterborne transmission |
Cryptosporidiosis; severe diarrhea, especially in immunocompromised patients |
Ingestion of oocysts in contaminated water or direct contact |
Water treatment and sanitation |
| Hymenolepis diminuta |
Cestode; requires an intermediate arthropod host |
Mild gastrointestinal symptoms |
Ingestion of infected arthropods |
Avoiding ingestion of contaminated arthropods |
| Hymenolepis nana |
Cestode; directly infective to humans |
Gastrointestinal discomfort and autoinfection |
Ingestion of eggs in contaminated food or autoinfection |
Proper hygiene and sanitation |
| Trichinella spiralis |
Nematode; infects muscle tissue |
Trichinellosis; muscle pain, fever, and organ damage |
Consumption of undercooked infected meat |
Cooking meat thoroughly |
| Angiostrongylus cantonensis |
Nematode; affects CNS |
Eosinophilic meningitis; neurological symptoms |
Ingestion of larvae in raw or undercooked snails and slugs |
Avoiding raw snails/slugs; proper food handling |
| Capillaria hepatica |
Nematode; liver infection |
Hepatic capillariasis; liver dysfunction |
Ingestion of eggs from contaminated soil or food |
Avoiding consumption of contaminated food |
| Baylisascaris procyonis |
Nematode; severe neurotropic potential |
Neural larva migrans; severe neurological damage |
Accidental ingestion of eggs from infected raccoons or rodents |
Rodent control; avoiding contaminated environments |
| Xenopsylla cheopis |
Flea; vector of Yersinia pestis |
Plague; bubonic, septicemic, and pneumonic forms |
Bite of infected fleas |
Flea control; rodent management |
| Ornithonyssus bacoti |
Mite; causes dermatitis |
Mite dermatitis; skin irritation and secondary infections |
Direct contact with rodents or contaminated environments |
Rodent control; personal protection |
|
Ixodes spp. |
Tick; transmits bacterial infections |
Lyme disease and other tick-borne illnesses |
Bite of infected ticks |
Use of tick repellents; habitat control |
| Country |
Cases |
Population Estimates* |
Incidence Rate** |
| Sudan |
3571 |
50,040,000 |
7.14 |
| Ethiopia |
1482 |
128,690,000 |
1.15 |
| Brazil |
1461 |
211,140,000 |
0.69 |
| Kenya |
1252 |
55,340,000 |
2.26 |
| South Sudan |
778 |
11,480,000 |
6.78 |
| Somalia |
712 |
18,360,000 |
3.88 |
| India |
538 |
1,438,000,000 |
0.04 |
| Eritrea |
376 |
3,470,000 |
10.84 |
| Yemen |
240 |
39,390,000 |
0.61 |
| Uganda |
195 |
48,660,000 |
0.40 |
| Country |
2018 |
2019 |
2020 |
2021 |
2022 |
| Number |
Rate |
Number |
Rate |
Number |
Rate |
Number |
Rate |
Number |
Rate |
| Austria |
2 |
0.02 |
1 |
0.01 |
6 |
0.07 |
10 |
0.11 |
2 |
0.02 |
| Belgium |
0 |
NRC |
NDR |
NRC |
NDR |
NRC |
0 |
NRC |
0 |
NRC |
| Bulgaria |
45 |
0.64 |
55 |
0.79 |
13 |
0.19 |
29 |
0.42 |
9 |
0.13 |
| Croatia |
0 |
0.00 |
3 |
0.07 |
0 |
0.00 |
17 |
0.42 |
NDR |
NRC |
| Cyprus |
0 |
0.00 |
0 |
0.00 |
0 |
0.00 |
0 |
0.00 |
0 |
0.00 |
| Czechia |
0 |
0.00 |
0 |
0.00 |
0 |
0.00 |
0 |
0.00 |
0 |
0.00 |
| Denmark |
NDR |
NRC |
NDR |
NRC |
NDR |
NRC |
NDR |
NRC |
NDR |
NRC |
| Estonia |
0 |
0.00 |
0 |
0.00 |
0 |
0.00 |
0 |
0.00 |
1 |
0.08 |
| Finland |
0 |
0.00 |
0 |
0.00 |
0 |
0.00 |
0 |
0.00 |
0 |
0.00 |
| France |
0 |
0.00 |
2 |
0.00 |
1 |
0.00 |
2 |
0.00 |
15 |
0.02 |
| Germany |
0 |
0.00 |
3 |
0.00 |
1 |
0.00 |
2 |
0.00 |
0 |
0.00 |
| Greece |
0 |
0.00 |
0 |
0.00 |
0 |
0.00 |
0 |
0.00 |
0 |
0.00 |
| Hungary |
2 |
0.02 |
0 |
0.00 |
0 |
0.00 |
0 |
0.00 |
0 |
0.00 |
| Iceland |
0 |
0.00 |
0 |
0.00 |
0 |
0.00 |
0 |
0.00 |
0 |
0.00 |
| Ireland |
0 |
0.00 |
0 |
0.00 |
0 |
0.00 |
0 |
0.00 |
0 |
0.00 |
| Italy |
2 |
0.00 |
10 |
0.02 |
79 |
0.13 |
0 |
0.00 |
4 |
0.01 |
| Latvia |
1 |
0.05 |
1 |
0.05 |
1 |
0.05 |
7 |
0.37 |
3 |
0.16 |
| Liechtenstein |
NDR |
NRC |
NDR |
NRC |
NDR |
NRC |
0 |
0.00 |
0 |
0.00 |
| Lithuania |
0 |
0.00 |
0 |
0.00 |
0 |
0.00 |
1 |
0.04 |
0 |
0.00 |
| Luxembourg |
0 |
0.00 |
0 |
0.00 |
0 |
0.00 |
0 |
0.00 |
0 |
0.00 |
| Malta |
0 |
0.00 |
0 |
0.00 |
0 |
0.00 |
0 |
0.00 |
0 |
0.00 |
| Netherlands |
0 |
0.00 |
1 |
0.01 |
0 |
0.00 |
0 |
0.00 |
0 |
0.00 |
| Norway |
0 |
0.00 |
0 |
0.00 |
0 |
0.00 |
0 |
0.00 |
0 |
0.00 |
| Poland |
2 |
0.01 |
2 |
0.01 |
11 |
0.03 |
2 |
0.01 |
1 |
0.00 |
| Portugal |
0 |
0.00 |
1 |
0.01 |
0 |
0.00 |
0 |
0.00 |
0 |
0.00 |
| Romania |
10 |
0.05 |
6 |
0.03 |
4 |
0.02 |
6 |
0.03 |
4 |
0.02 |
| Slovakia |
0 |
0.00 |
0 |
0.00 |
0 |
0.00 |
0 |
0.00 |
0 |
0.00 |
| Slovenia |
0 |
0.00 |
0 |
0.00 |
0 |
0.00 |
0 |
0.00 |
0 |
0.00 |
| Spain |
2 |
0.00 |
12 |
0.03 |
1 |
NRC |
1 |
NRC |
0 |
0.00 |
| Sweden |
0 |
0.00 |
0 |
0.00 |
0 |
0.00 |
0 |
0.00 |
0 |
0.00 |
EU/EEA
(30 countries)
|
66 |
0.02 |
97 |
0.02 |
117 |
0.03 |
77 |
0.02 |
39 |
0.01 |
| United Kingdom |
0 |
0.00 |
0 |
0.00 |
NA |
NA |
NA |
NA |
NA |
NA |
EU/EEA
(31 countries)
|
66 |
0.01 |
97 |
0.02 |
117 |
0.03 |
NA |
NA |
NA |
NA |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).