1. Introduction
Transient-receptor potential (TRP) channels are non-selective cation channels that have numerous biological functions, incuding sensing temperatur and pain, regulating secretion, and neurotransmission [
1]. TRP channels are considered promising targets for the treatment of human diseases [
2].
TRPM3 channels are involved in temperature and pain sensation, the regulation of peptide secretion, muscle contraction, gene transcription, and tumorigenesis [
3,
4]. Analysis of transgenic mice models revealed an important role of TRPM3 in heat sensation and the development of inflammatory heat sensitization [
5,
6]. TRPM3 channels have been proposed as molecular markers for chronic fatigue syndrome/myalgic encephalomyelitis [
7]. Furthermore, analysis of mutated TRPM3 channels revealed that TRPM3 plays an important role in the development of neuronal disorders, including epileptic encephalopathies [
8] and the development of an inherited form of early-onset cataract [
9]. TRPM8 was originally identified as the menthol receptor in an expression cloning approach [
10]. TRPM8 is activated by cold temperatures and plays an important role in thermosensation [
11,
12,
13]. Other functions of TRPM8 include the development of migraine and tumorigenesis [
14,
15].
Stimulation of TRPM3 or TRPM8 channels induces an intracellular signaling pathway that leads to a change in the gene expression pattern of the cells via the activation of stimulus-responsive transcription factors [
3,
16,
17]. These transcription factors bind to the regulatory region of delayed response genes and stimulate transcription of these genes. The gene products of these delayed response genes are then responsible for the biochemical and physiological changes observed after cellular stimulation. In a search for delayed response genes of TRPM3 and TRPM8-induced signaling, we analyzed the gene encoding prostaglandin endoperoxide synthase-2, also known as cyclooxygenase-2. Prostaglandin endoperoxide synthase-2 (EC 1.14.99.1) catalyzes the biosynthesis of prostaglandin H
2 from arachidonate. Prostaglandins are important cellular mediators that play critical roles in infection, inflammation and cancer, acting in a paracrine and autocrine manner. Prostaglandin endoperoxide synthase-2, the enzyme that catalyzes the rate-limiting reaction in prostaglandin biosynthesis, is therefore a therapeutic target for anti-inflammatory medications, e.g. non-steroidal anti-inflammatory compounds. Prostaglandin endoperoxide synthase-2 is expressed at very low levels in various tissues but can be rapidly induced by inflammatory insults such as inflammatory cytokines and lipopolysaccharide, but also by numerous others signaling molecules, e.g. tumor promoters and hormones [
18]. In contrast, the prostaglandin endoperoxide synthase-1 isoenzyme is constitutively expressed.
Here, we analyzed the promoter of prostaglandin endoperoxide synthase-2 after stimulation of either TRPM3 or TRPM8 channels. We show that the activity of the prostaglandin endoperoxide synthase-2 promoter increases after stimulation of TRPM3 or TRPM8 channels with their cognate ligands. We identified the cAMP response element as an important genetic landmark linking signaling of the TRPM3 and TRPM8 channels to prostaglandin endoperoxide synthase-2 promoter activation. Overall, this is the first report that links TRP channel stimulation to the regulation of the prostaglandin endoperoxide synthase-2 gene.
2. Results
2.1. Prostaglandin Endoperoxide Synthase-2 Catalyzes the Biosynthesis of Prostaglandin H2 (PGH2)
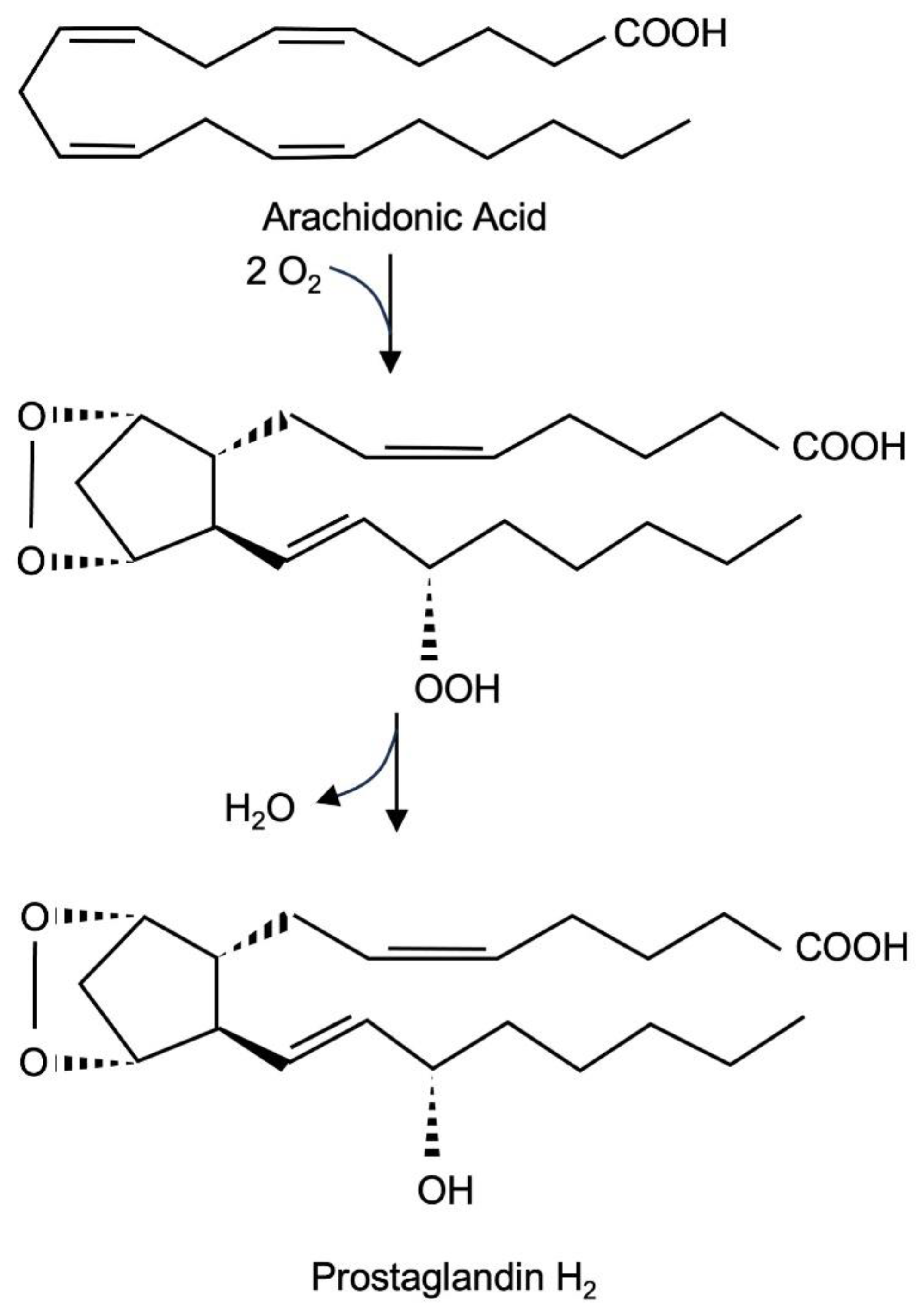
Prostaglandin endoperoxide synthase-2 is a bifunctional enzyme. First, it catalyzes the conversion of arachidonate into the hydroperoxide prostaglandin G2 (PGG2) using its cyclooxygenase activity, which results in the incorporation of two molecules of O2 into arachidonic acid. Second, the enzyme uses its hydroperoxide activity to catalyze a two-electron reduction of the hydroperoxide moiety of PGG2 to alcohol, resulting in the formation of PGH2. There are two prostaglandin endoperoxide synthase isoenzymes. Prostaglandin endoperoxide synthase-1 is constitutively expressed, while the expression of prostaglandin endoperoxide synthase-2 is induced by a variety of extracellular signaling molecules. The biological activity of prostaglandin endoperoxide synthase-2 to catalyze the biosynthesis of prostaglandins is associated with the induction of inflammation, pain and fever. It is therefore obvious that research is trying to clarify how the expression of the enzyme is regulated and how the enzyme can be pharmacologically inhibited.
Figure 1.
Biosynthesis of prostaglandin PGH2 from arachidonate. The isoenzymes prostaglandin endoperoxide synthase 1 and 2 catalyze the reaction in two steps, with prostaglandin PGG2 serving as an intermediate.
Figure 1.
Biosynthesis of prostaglandin PGH2 from arachidonate. The isoenzymes prostaglandin endoperoxide synthase 1 and 2 catalyze the reaction in two steps, with prostaglandin PGG2 serving as an intermediate.
2.2. TRPM3 Channel Stimulation Activates the Prostaglandin Endoperoxide Synthase-2 Promoter
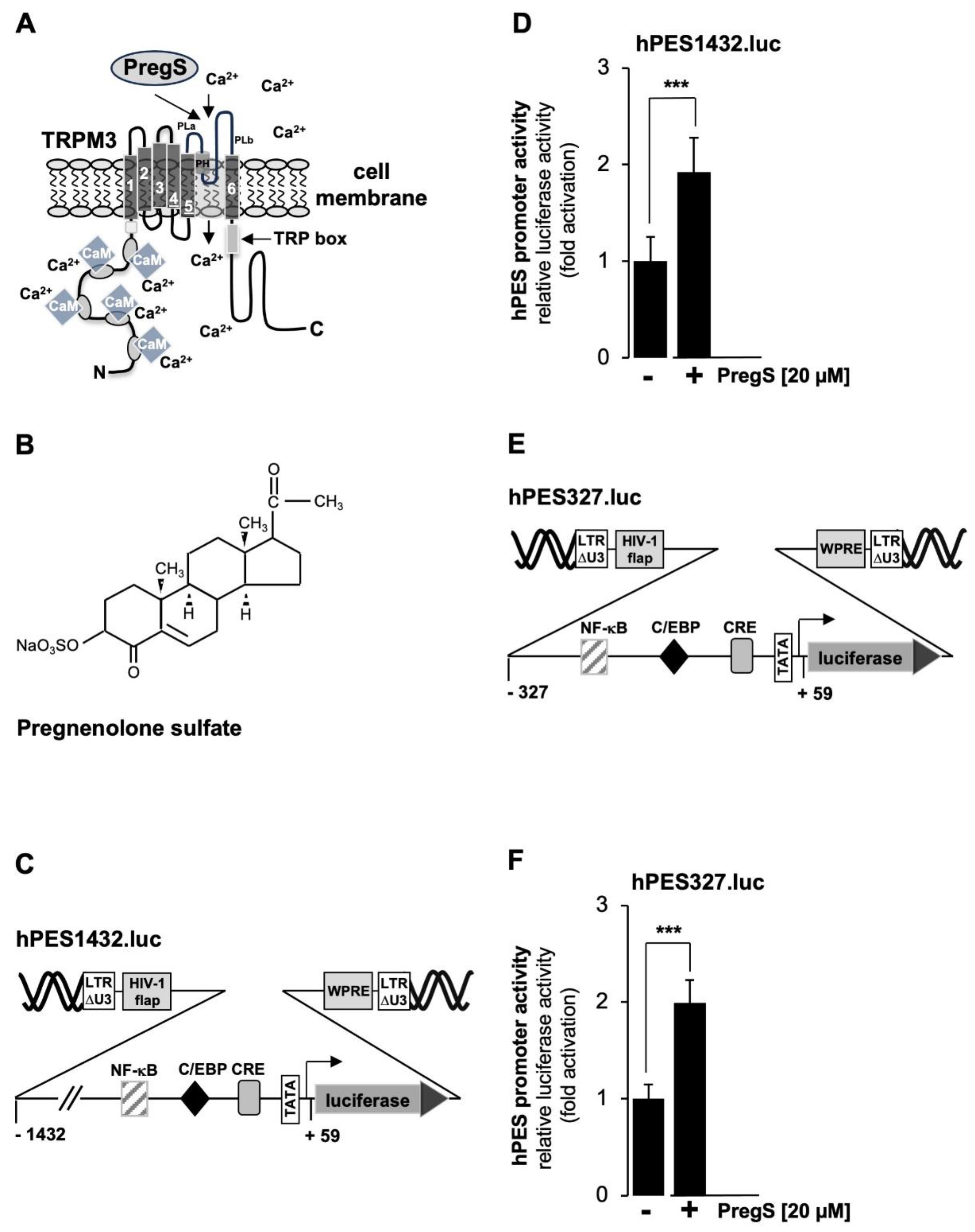
As a cellular model system to analyze TRPM3 signaling, we used HEK293 cells that contained a tetracycline-inducible TRPM3 expression unit (T-REx-TRPM3 cells). TRPM3 expression could therefore be induced by adding tetracycline to the culture medium.
Figure 2A shows the modular structure of TRPM3 channels and reveals the architecture common to all TRP channels, including 6 transmembrane domains and a cytoplasmic location of both N- and C-termini. The ion pore of TRPM3 involves the loop between the fifth and sixth transmembrane domains. TRPM3 channels are activated by pregnenolone sulfate (PregS) (
Figure 2B), resulting in an influx of Ca
2+ or Na
+ ions into the cytosol of the cells. It has been suggested that pregnenolone sulfate binds to the pore region of the channel [
19].
Stimulation of TRPM3 channels results in the transient activation of stimulus-responsive transcription factors, which in turn bind to the regulatory region of delayed response genes. The expression of these delayed-response genes is responsible for altering the biochemistry of the cells due to TRPM3 channel stimulation. We wondered whether the prostaglandin endoperoxide synthase-2 gene is one of the TRPM3-activated delayed- response genes, since expression of this gene is regulated by numerous extracellular signaling molecules. Furthermore, it has been demonstrated that an increase in the intracellular Ca
2+ ion concentration and activation of protein kinase C (PKC) and extracellular signal-regulated protein kinase ERK1/2 activates the promoter of the prostaglandin endoperoxide synthase-2 gene [
20,
21,
22,
23,
24,
25,
26,
27,
28,
29]. An increase in the intracellular Ca
2+ ion concentration and the activation of PKC and ERK1/2 are also characteristics of the TRPM3-induced signaling cascade [
30,
31,
32].
We used lentiviral gene transfer to integrate the reporter gene into the genome of the cells. Thus, the reporter gene was embedded into a nucleosomal context. Integration of reporter genes into the chromatin is important because transcriptional regulation requires not only the binding of transcription factors to DNA, but also a nucleosomal structure typical for eukaryotic genes. The transient transfection of reporter genes used by many researchers leads to inefficiently packed plasmids into chromatin with incomplete nucleosomal organization [
33,
34]. Furthermore, these reporter genes have a prokaryotic-like gene organization with the hallmark of a non-restrictive transcriptional ground state, whereas eukaryotic genes are embedded into a chromatin-structured restrictive ground state. Implanting reporter genes into chromatin ensures that they are packed into an ordered nucleosomal structure.
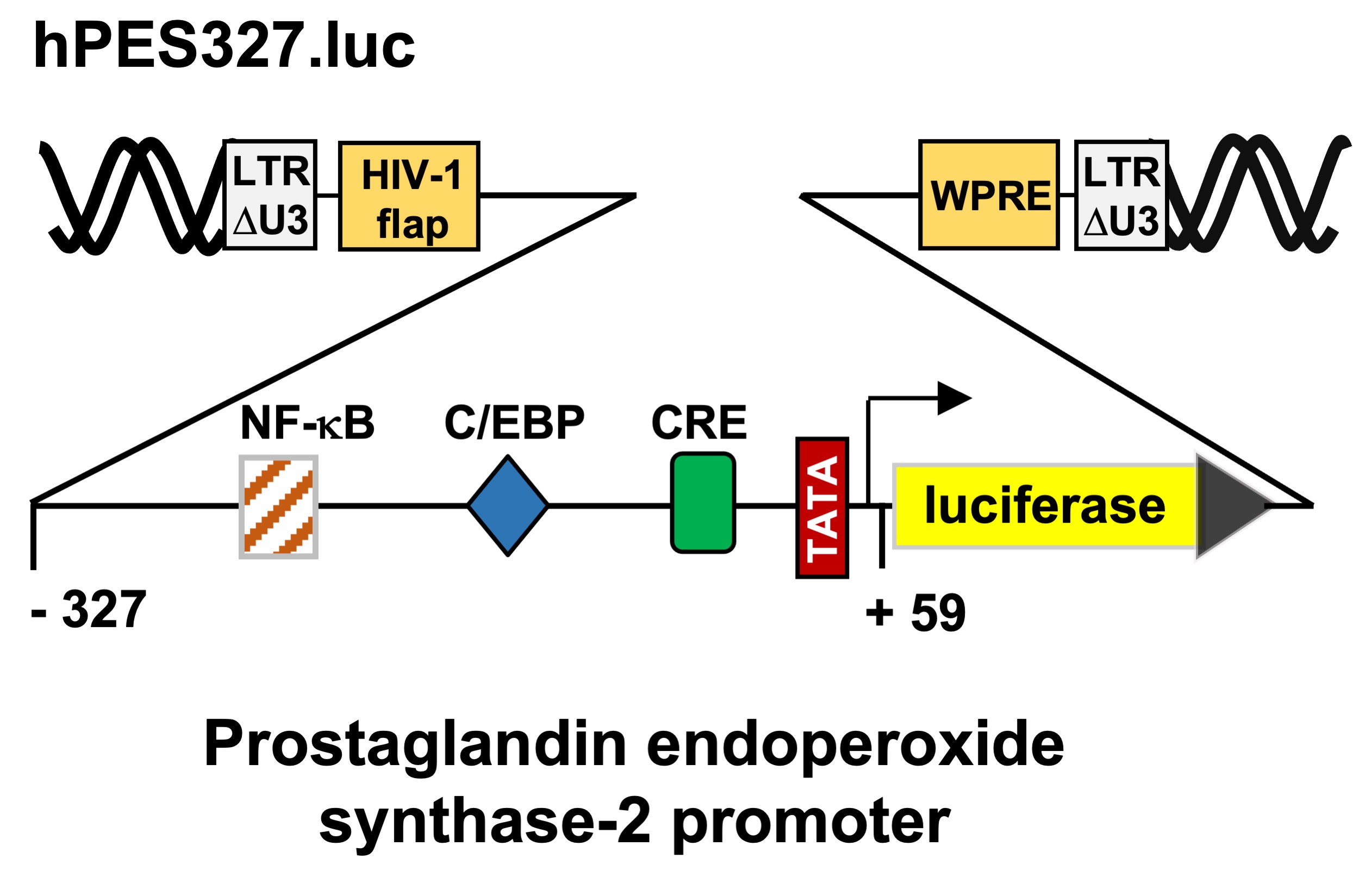
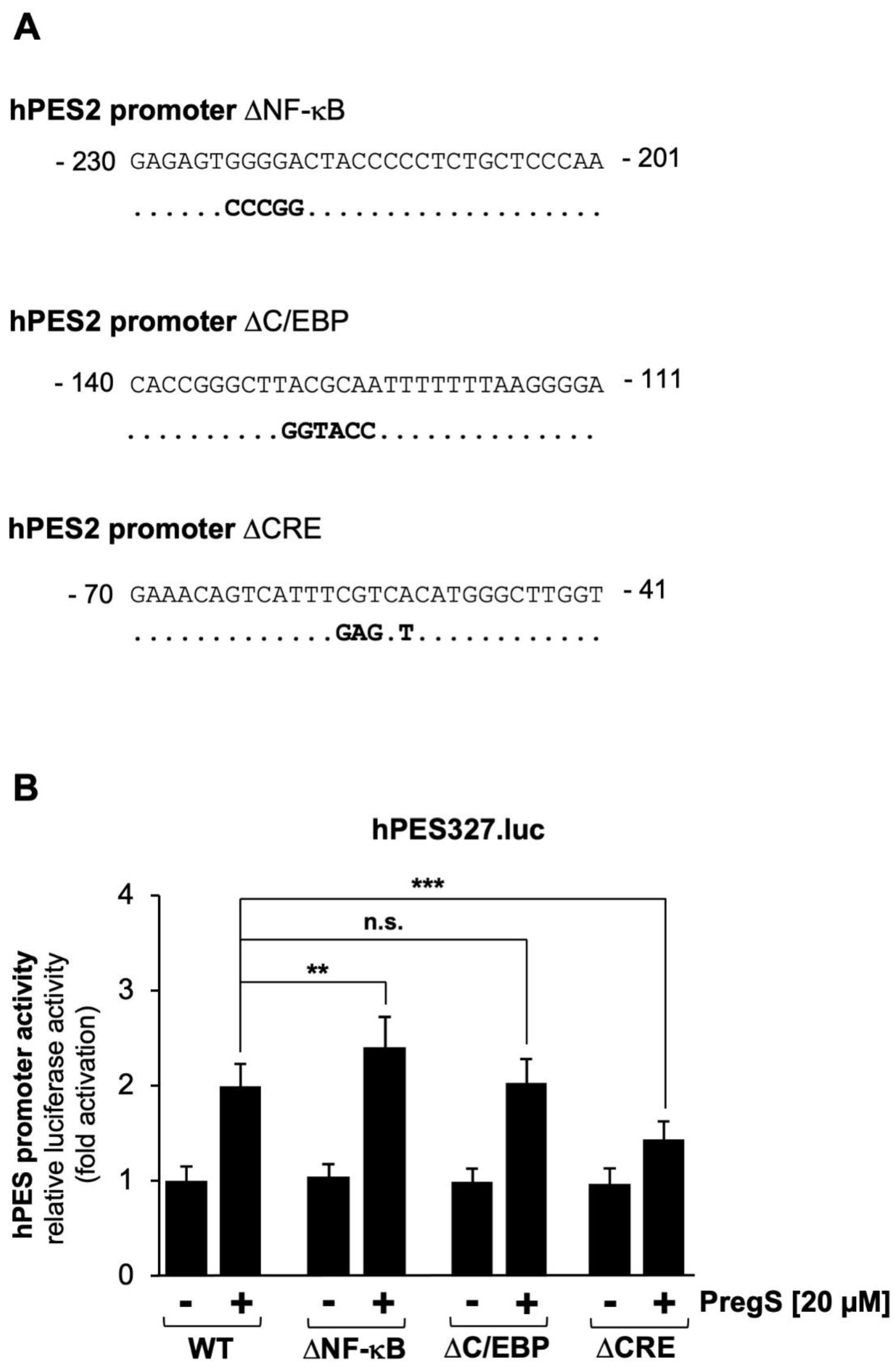
Figure 2C shows a schematic representation of the integrated provirus encoding a luciferase reporter gene under the control of 1432 nucleotides of the 5´upstream region of the human prostaglandin endoperoxide synthase-2 gene. T-REx-TRPM3 cells were infected with a recombinant lentivirus containing the prostaglandin endoperoxide synthase-2 promoter/luciferase reporter gene, cultured in serum-reduced medium in the presence of tetracycline to induce TRPM3 expression, and then stimulated with prognenolone sulfate for 24 hours. Cells were harvested, cell extracts were prepared and analyzed for luciferase activities and protein concentrations. The results show that stimulation of TRPM3 channels increased the promoter activity of the prostaglandin endoperoxide synthase-2 gene by 1.92-fold. The proximal prostaglandin endoperoxide synthase-2 promoter contains three landmark transcription factor binding sites, identified as binding sites for NF-κB, C/EBP (also termed NF-IL6 [
35]), and CREB (cAMP-response element binding protein). We wondered whether the proximal promoter of the prostaglandin synthase-2 gene is sufficient to confer responsiveness to TRPM3 stimulation. We therefore analyzed a reporter gene under the control of 327 nucleotides of the prostaglandin endoperoxide synthase-2 promoter (
Figure 2E). The results show that TRPM3 stimulation triggered a 1.99-fold upregulation of prostaglandin endoperoxide synthase-2 promoter activity (
Figure 2F), suggesting that the genetic elements required for stimulus-transcription coupling are located in the proximal promoter region.
2.3. Pharmacological Inhibition of TRPM3 Channels Impairs Pregnenolone Sulfate-Induced Upregulation of Prostaglandin Endoperoxide Synthase-2 Promoter Activity
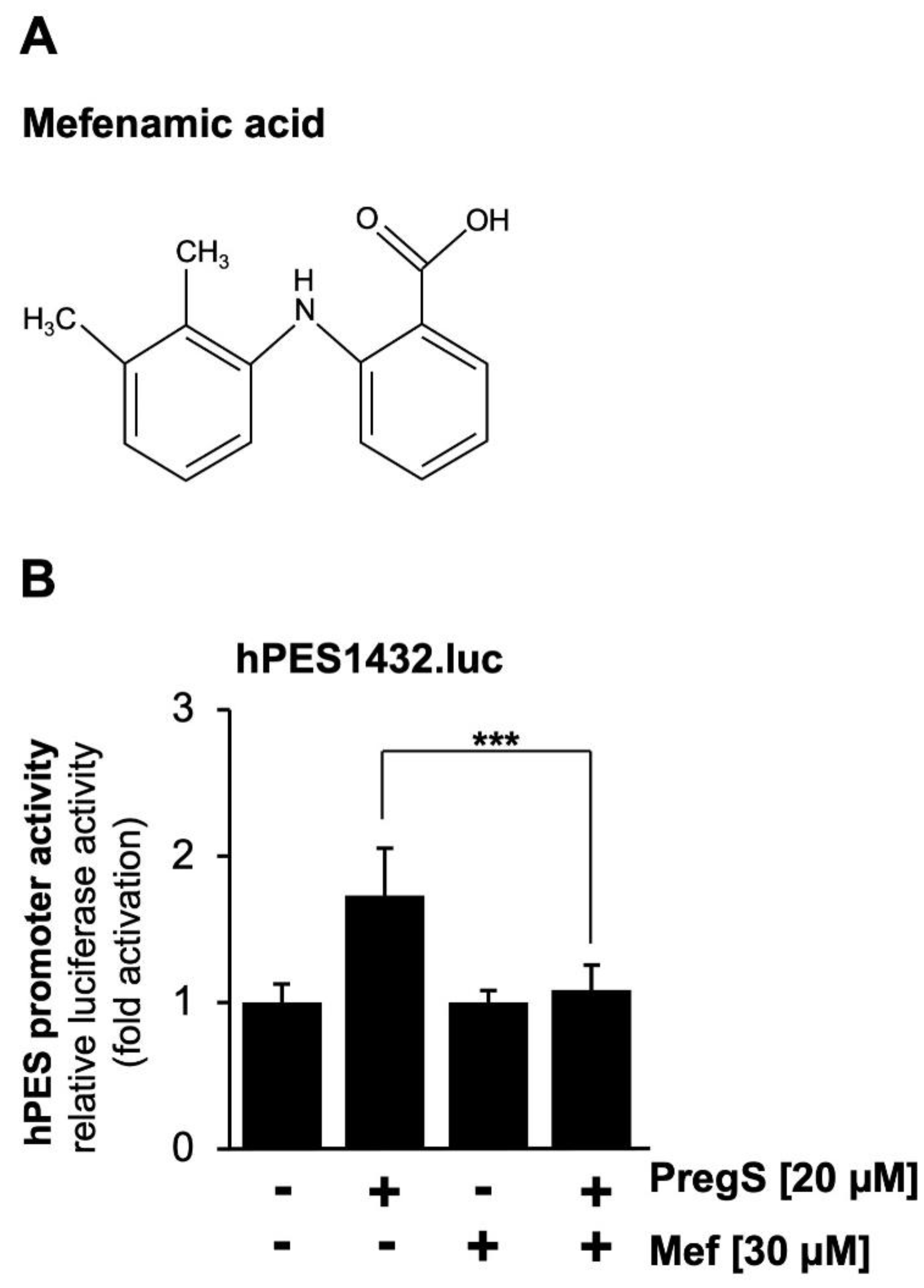
We used a pharmacological approach to confirm that pregnenolone sulfate-induced activation of the prostaglandin endoperoxide synthase-2 promoter is dependent on TRPM3. We incubated the cells with mefenamic acid, an anthranilic acid derivative of non-steroid anti-inflammatory drugs, which selectively inhibits TRPM3-mediated Ca
2+ influx into the cells and TRPM3-induced gene transcription [
36,
37,
38]. The chemical structure of mefenamic acid is shown in
Figure 3A. The results show that incubation of the cells with mefenamic acid greatly reduced pregnenolone sulfate-induced stimulation of the prostaglandin endoperoxide synthase-2 promoter (
Figure 3B).
2.4. Mutational Analysis of the Human Prostaglandin Endoperoxide Synthase-2 Promoter Identified the cAMP Response Element (CRE) as the Pregnenolone Sulfate-Responsive Element
The proximal promoter of the human prostaglandin endoperoxide synthase-2 gene contains three key genetic elements, binding sites for the transcription factors NF-κB and C/EBP, and a cyclic AMP response element (CRE), a binding site for the transcription factor CREB and related basic region leucine zipper transcription factors. Mutations were introduced into these elements, as shown in
Figure 4A, and analyzed for their responsiveness to pregnenolone sulfate.
Figure 4B shows that only mutations introduced into the CRE impaired pregnenolone sulfate-induced activation of the prostaglandin endoperoxide synthase-2 promoter in HEK293 cells expressing TRPM3 channels. Transcription of the reporter gene was reduced by 50 %. Mutations introduced into the NF-κB and C/EBP binding sites did not reduce the responsiveness of the prostaglandin endoperoxide synthase-2 promoter to the TRPM3-induced signaling cascade.
2.5. TRPM8 Channel Stimulation Activates the Prostaglandin Endoperoxide Synthase-2 Promoter
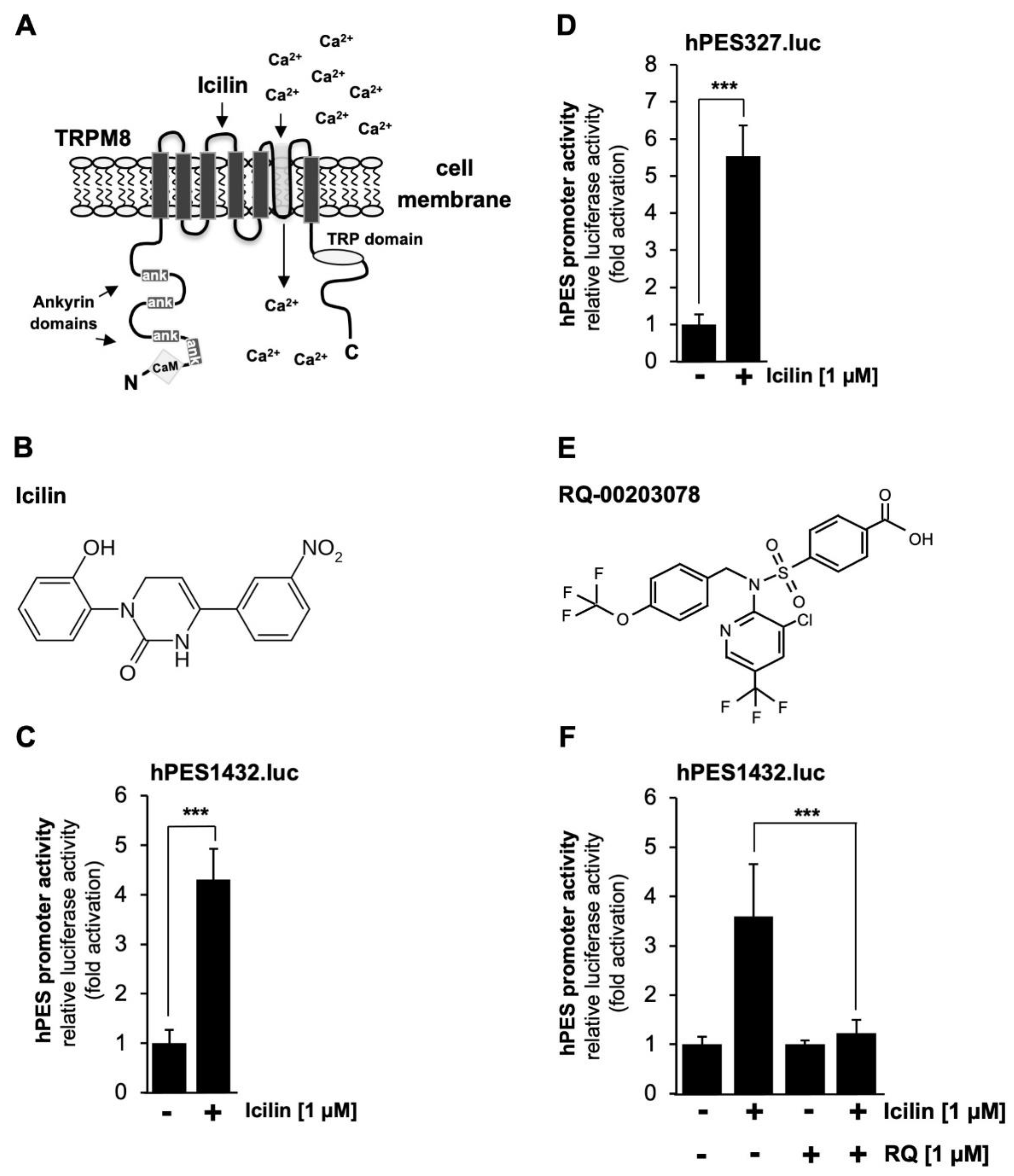
Next, we analyzed whether the prostaglandin endoperoxide synthase-2 gene could also function as delayed-response gene of the signaling pathway induced after stimulation of TRPM8 channels. TRPM8 has the typical architecture of TRP channels with 6 transmembrane domains and both N- and C-termini in the cytoplasm as shown in
Figure 5A. The figure also shows the TRP box, ankyrin repeats and putative calmodulin binding sites. TRPM8 channels are activated following incubation of the cells with plant-derived “cooling agents” such as menthol or eucalyptol. In our experiments, we used the synthetic super-cooling agent icilin to stimulate TRPM8 (
Figure 5B). The results show that icilin significantly induced transcription of the reporter gene under the control of either 1432 (
Figure 5C) or 327 nucleotides (
Figure 5D) of the 5´region of the human prostaglandin endoperoxide synthase-2 gene. The stimulation was in the range of 4.3-fold (hPES1432.luc) and 5.55-fold (hPES327.luc), respectively. These data also show that the genetic elements responsible for coupling TRPM8 stimulation with prostaglandin endoperoxide synthase-2 gene activation reside in the proximal portion of the prostaglandin endoperoxide synthase-2 promoter.
2.6. Pharmacological Inhibition of TRPM8 Channels Impairs Icilin-Induced Upregulation of Prostaglandin Endoperoxide Synthase-2 Promoter
Recently, we showed that the compound RQ-00203078 specifically inhibits TRPM8-mediated transcriptional regulation [
39]. This compound, shown in
Figure 5E, also inhibits menthol-induced Ca
2+ influx and whole-cell current as well as icilin-induced rise of the intracellular Ca
2+ concentration in TRPM8-expressing HEK293 cells [
40,
41],
https://www.alomone.com/p/rq-00203078/R-170?b=33803. We therefore used this compound to confirm that icilin-induced upregulation of reporter gene transcription relies on the presence of functional TRPM8 channels. The results show that incubation of the cells with RQ-00203078 almost completely blocked transcriptional activation of the prostaglandin endoperoxide synthase-2 promoter in icilin-stimulated HEK293-M8 cells (
Figure 5F). The stimulation of reporter gene transcription was inhibited by more than 91 %.
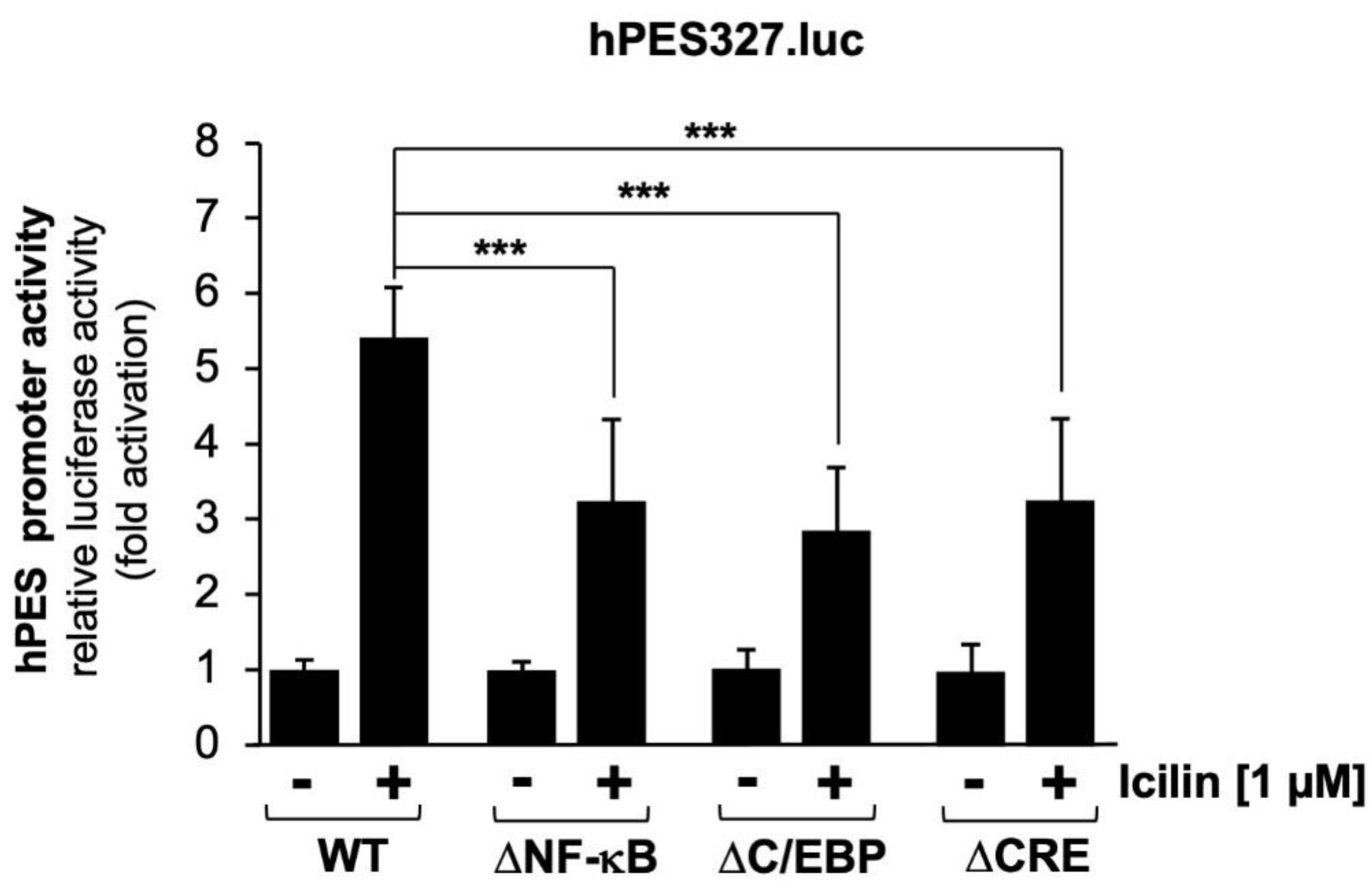
2.7. Mutational Analysis of the Human Prostaglandin Endoperoxide Synthase-2 Promoter Identified Multiple Genetic Elements as Icilin-Responsive Elements
Next, we analyzed the prostaglandin endoperoxide synthase-2 promoter mutants.
Figure 6 shows that mutations of the NF-κB and C/EBP binding sites, as well as mutations introduced into the CRE, impaired the activation of the prostaglandin endoperoxide synthase-2 promoter by icilin. Quantification revealed a reduction in reporter gene transcription of the order of 48,9 % (ΔNF-κB), 58.2 % (ΔC/EBP), and 48.6 % (ΔCRE), respectively.
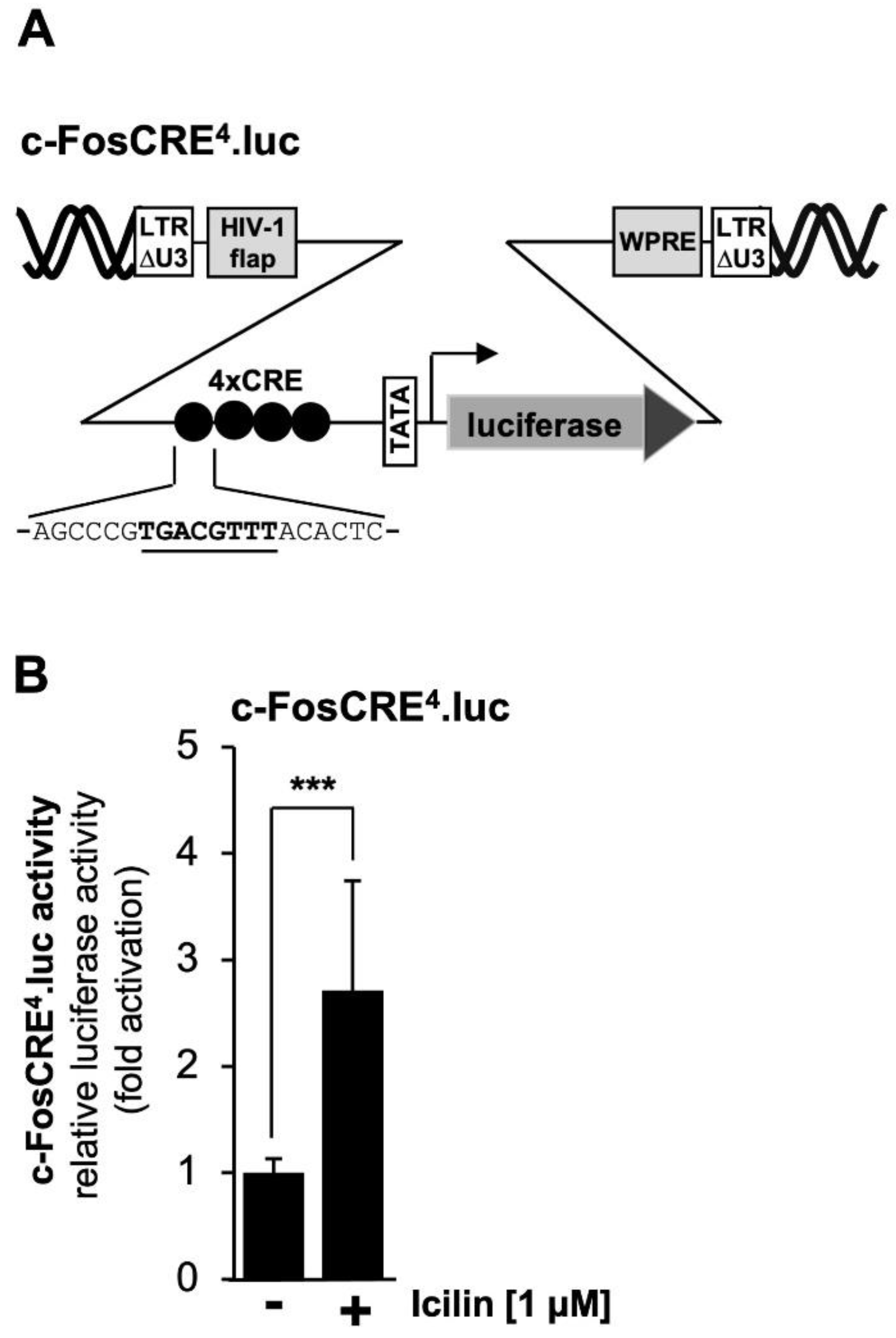
2.8. TRPM8 Channel Stimulation Activates a CRE-Containing Reporter Gene
Mutational analysis of the prostaglandin endoperoxide synthase-2 promoter revealed that the CRE is important for TRPM3 and TRPM8 signaling. It has already been shown that stimulation of TRPM3 channels activates a reporter gene under control of four copies of the canonical CRE sequence 5´-TGACGTCA-3´ [
42,
43,
44], depicted in
Figure 7A.
Figure 7B shows that stimulation of TRPM8 channels with icilin also activated transcription of a reporter gene controlled by four CREs. Transcription was stimulated by the factor of 2.71-fold.
2.9. Activation of CRE-Containing Genes Following Stimulation of TRPM3 or TRPM8 Channels
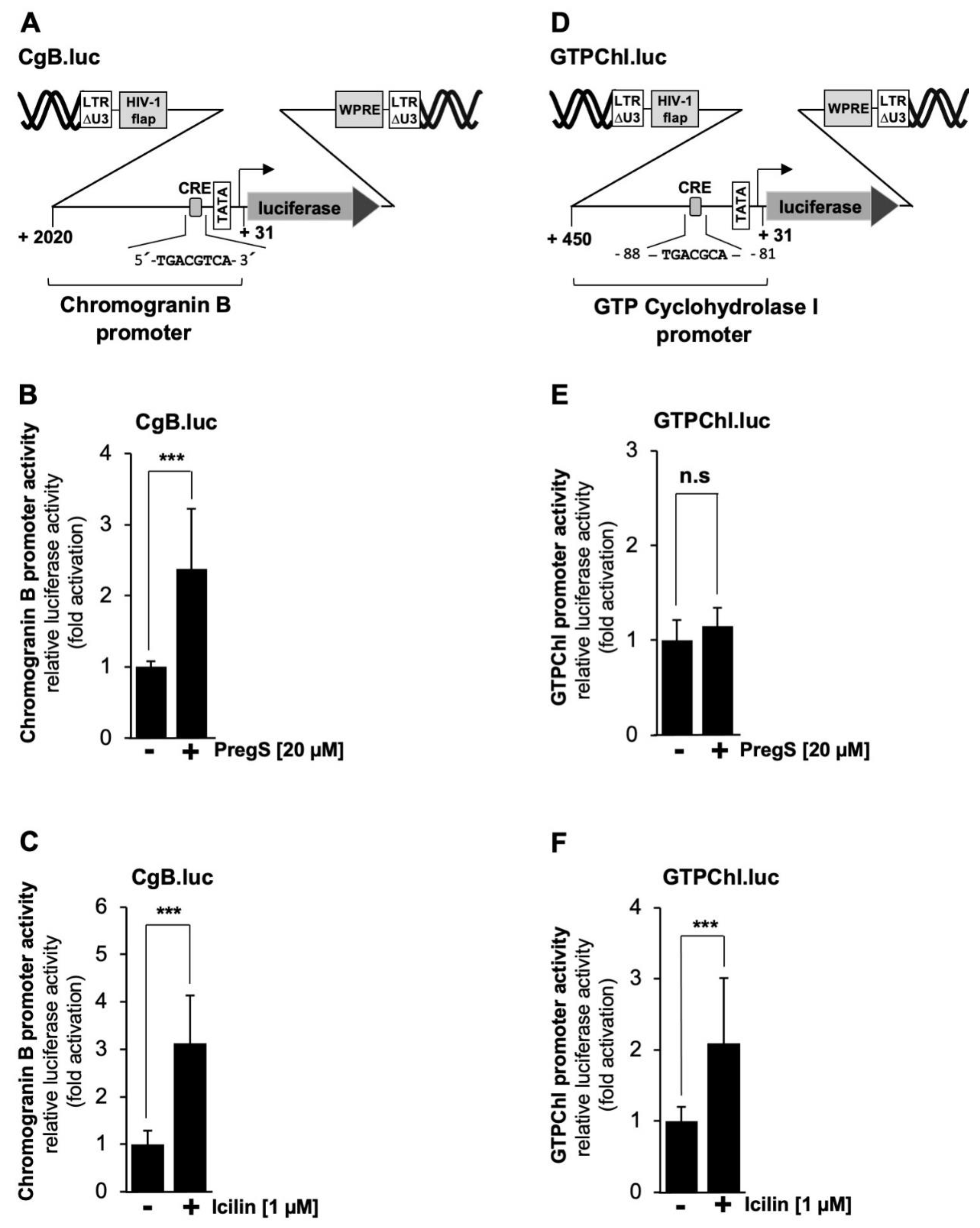
Next, we analyzed the chromogranin B promoter, which contains one copy of a canonical CRE (
Figure 8A). It has been demonstrated that transcription of the chromogranin B gene is regulated by this element [
45].
Figure 8B and C show that stimulation of TRPM3 or TRPM8 channels induced transcription of the reporter gene under the control of the chromogranin B promoter by 2.4-fold and 3.1-fold, respectively. The promoter of the GTP cyclohydrolyase gene contains an atypical version of the CRE, encompassing the sequence 5´-TGACGCAA-3 (
Figure 8D). Nevertheless, this promoter was shown to respond to a constitutively active mutant of CREB [
46].
Figure 8E shows that stimulation of TRPM3 channels had no effect on the promoter activity of this gene while stimulation of TRPM8 channels activated a reporter gene controlled by the GTP cyclohydrolase I promoter by a factor of 2.1-fold (
Figure 8F).
3. Discussion
Stimulation of TRPM3 and TRPM8 channels induces a signaling cascade that leads to the activation of the stimulus-responsive transcription factors AP-1, CREB, Elk-1, and Egr-1. These proteins bind to the regulatory regions of delayed-response genes and activate transcription of these genes. To understand the intracellular functions of TRPM3 and TRPM8, the identification of these delayed response genes is essential. In the search for delayed-response genes activated following stimulation of TRPM3 or TRPM8 channels, we analyzed the gene encoding prostaglandin endoperoxide synthase-2. Under basal conditions, the expression of prostaglandin endoperoxide synthase-2 is undetectable in many tissues. However, stimulation of the cells with cytokines, growth factors or tumor promoters triggers a strong transcriptional response, frequently involving protein kinase C and extracellular signal-regulated protein kinase ERK1/2 [
20,
21,
22,
23,
25,
27,
28,
29]. An increase in intracellular Ca
2+ has also been shown to stimulate prostaglandin endoperoxide synthase-2 gene expression [
23,
26,
29]. Since the TRPM3-induced intracellular signaling pathway has been shown to include an increase in the intracellular Ca
2+ concentration, the activation of PKC and ERK1/2 [
30,
32,
47], we hypothesized that the prostaglandin endoperoxide synthase-2 promoter would respond to TRPM3 and TRPM8 stimulation. The experiments shown in this study identified the prostaglandin endoperoxide synthase-2 gene as a delayed-response gene of the pregnenolone sulfate/TRPM3 and the icilin/TRPM8-induced signaling pathways. The essential role of both channels in stimulus-transcription coupling was confirmed in experiments with specific inhibitors against TRPM3 and TRPM8.
At the level of gene regulation, we have shown in this study that the CRE within the proximal prostaglandin endoperoxide synthase-2 promoter was important for coupling TRP channel activation with the prostaglandin endoperoxide synthase-2 promoter. The CRE has also been identified as essential for ultraviolet B irradiation-induction transcription of the prostaglandin endoperoxide synthase-2 gene [
48]. Likewise, the upregulation of prostaglandin endoperoxide synthase-2 gene transcription by nitric oxide or platelet-derived growth factor stimulation requires the CRE as
cis-acting element [
49,
50].
Several transcription factors have been proposed to bind to the CRE of the prostaglandin endoperoxide synthase-2 promoter. While the canonical CRE serves as a docking site for the transcription factor CREB and the related protein ATF-1, non-canonical CREs found in the prostaglandin endoperoxide synthase-2 promoter and the GTP cyclohydrolase I promoter can also bind additional basic region leucin zipper transcription factors. The GTP cyclohydrolase I promoter, for instance, was shown to be activated by constitutively active mutants of CREB, ATF-2 and c-Jun [
46], but stimulation experiments with a cAMP analogue showed that it is not CREB, but rather the basic region leucine zipper transcription factors ATF-4 and C/EBPβ, which mediate the response to elevated cAMP [
51]. Nevertheless, the GTP cyclohydrolase I promoter was not activated by the stimulation of TRPM3 channels, as shown in this study, suggesting that the GTP cyclohydrolase I promoter and the prostaglandin endoperoxide synthase 2 promoter are differently regulated. Regarding the prostaglandin endoperoxide synthase 2 promoter, the involvement of CREB and ATF-1 in the regulation of promoter activation induced by ultraviolet B radiation has been demonstrated [
48]. Furthermore, in an analysis of transgenic mice expressing A-CREB, an inhibitor of CREB, it was shown that CREB is an important transcription factor for the expression of prostaglandin endoperoxide synthase-2 in a model of status epilepticus [
52].
In contrast, other investigators have suggested that c-Jun interacts with the CRE of the prostaglandin endoperoxide synthase-2 promoter in EGF-treated human epidermoid carcinoma cells [
53]. CREB, ATF-2 and c-Jun stimulate transcriptional activation of the prostaglandin endoperoxide synthase-2 promoter via the CRE in nitric oxide-stimulated hypopharyngeal squamous cell carcinoma [
50]. c-Jun was identified – in addition to CREB – as a transcription factor that is activated as a result of TRPM3 or TRPM8 stimulation [
31,
42,
54]. For the signaling pathway induced by pregnenolone sulfate-induced stimulation of TRPM3 channels, other
cis-acting elements of the prostaglandin endoperoxide synthase-2 promoter, such as C/EBP and NF-κB binding sites did not play a role in stimulus transcription coupling. This is consistent with previous results showing that TRPM3 signaling does not activate NF-κB-induced gene transcription [
55]. The binding sites for NF-κB and C/EBP are important for the induction of the prostaglandin endoperoxide synthase-2 promoter after stimulation of the cells with lipopolysaccharide [
56,
57,
58]. Likewise, both NF-κB and C/EBP sites mediate prostaglandin endoperoxide synthase-2 promoter activation after stimulation of gonadotroph cells with gonadotropin releasing hormone [
26].
Analysis of the TRPM8-induced signaling pathway revealed that mutations within the C/EBP and NF-κB binding sites, as well as mutations within the CRE, reduced icilin-mediated stimulation of the prostaglandin endoperoxide synthase-2 promoter. It seems to be a common theme in the regulation of the prostaglandin endoperoxide synthase-2 gene that no single
cis-acting element mediates stimulus-transcription coupling, but rather that two or three of the landmark
cis-acting elements are required. For example, the binding sites for NF-κB, C/EBP, and the CRE are involved as
cis-acting response elements for various stimuli, e.g. NO donors, phorbol esters, bradykinin, and interleukin-1β [
50,
59]. The C/EBP site and the CRE are essential for the stimulation of the prostaglandin endoperoxide synthase-2 promoter by a combined treatment with lipopolysaccharide and 12-
O-tetradecanoylphorbol-13-acetate, while inactivation of only one of these
cis-acting elements had little effect on promoter activity [
56]. Similarly, transforming growth factor α-induced stimulation of the prostaglandin endoperoxide synthase-2 promoter requires the C/EBP site and the CRE [
21].
4. Materials and Methods
4.1. Cell Culture and Reagents
HEK293 cells containing the human TRPM3 coding region under the control of a tetracycline-responsive promoter (T-REx-TRPM3 cells) were kindly provided by David Beech and Yasser Majeed, University of Leeds, UK and cultured as described [
60]. TRPM3 expression was induced by adding tetracycline (1 μg/ml, Sigma-Aldrich # T7680, dissolved in water) to the culture medium, which contained 0.05 % fetal calf serum, for 24 hours before stimulation with the TRPM3 ligand pregnenolone sulfate (PubChem CID: 105074). Stimulation with pregnenolone sulfate (20 μM, Sigma # P162, dissolved in DMSO) was carried out for 24 hours in DMEM with 0.05 % fetal bovine serum. Mefenamic acid (2-(2,3-dimethylphenyl)aminobenzoic acid, PubChem CID: 4044) was obtained from Santa Cruz, Heidelberg, Germany (No. sc-205380), dissolved in DMSO and used at a concentration of 30 μM [
38]. HEK293 cells expressing TRPM8 channels (HEK293-M8 cells) have been described elsewhere [
61]. Cells were incubated in DMEM with 0.05 % fetal calf serum for 24 hours before stimulation with the super-cooling compound icilin (1 μM, Santa Cruz, Heidelberg, Germany, No. sc-201557). HEK293-M8 cells were incubated with the compound RQ-00203078 (PubChem CID: 49783953), which was dissolved in DMSO, at a concentration of 1 μM. The compound was a kind gift of Alomone labs, Jerusalem. T-REx-TRPM3 cells and HEK293-M8 cells were preincubated with its cognate inhibitors for 3 hours and then stimulated in the presence of the inhibitor with either pregnenolone sulfate or icilin.
4.2. Lentiviral Infection
Viral particles were produced by triple transfection of HEK293-TN cells with packaging plasmid Δ8.91, encoding the viral proteins gag, pol, and rev, pCMV-G, which codes for the VSV envelope glycoprotein, and the transfer vector as described [
62].
4.3. Reporter Assays
Reporter plasmids phPES2(-1,432/+59), phPES2(-327/+59), KBM, ILM, and CRM, containing the luciferase ORF under the control of the human prostaglandin endoperoxide synthase-2 promoter reporter, were kindly provided by Hiroyasu Inoue, Department of Food Science and Nutrition, Faculty of Human Life and Environment, Nara Woman´s University, Nara, Japan [
56,
57,
63]. Plasmids were cut with EcoRV and NheI and the fragments were inserted into a lentiviral transfer vector upstream of the luciferase coding region. The plasmids were designated pFWhPES2.luc(-1432), pFWhPES2.luc(-327), pFWhPES2.lucΔκB, pFWhPES2.lucΔC/EBP, and pFWhPES2.lucΔCRE, respectively. The plasmid p0.613hGCH1-GL3 was kindly provided by Gregory Kapatos, Wayne State University, Department of Psychiatry, Detroit, Michigan, USA [
64]. To construct the lentiviral transfer vector pFWGTPChI.luc, we cloned a fragment of plasmid p0.613hGCH1-GL3, which comprised the sequences from -450 to + 31 of the human GTP cyclohydrolase I gene, into a lentiviral transfer vector, upstream of the luciferase coding region. The lentiviral transfer vectors pFWCgB.luc, containing the sequence -2020/+31 of the human chromogranin B promoter upstream of the luciferase ORF, and pFWCRE
4.luc have already been described [
42,
65]. Infected cells were kept in a medium containing 0.05% fetal calf serum for 24 hours before stimulation. Reporter lysis buffer (Promega, Mannheim, Germany) was used to prepare cell extracts, which were used to determine luciferase activities and protein concentrations. Luciferase activities were measured using a luminometer (Berthold Detection Systems, Huntsville, Alabama, USA). Protein concentrations of the extracts were determined using a BCA protein assay kit.
4.4. Statistics
For the statistical analyses, we used the two-tailed Student's t-test. Statistical probability was expressed as ***p <0.001. Values were considered as significant if p <0.05.
5. Conclusions
Stimulation of the TRPM3 and TRPM8 channels induces intracellular signaling cascades that ultimately alter the gene expression pattern of the cells. Identification of these genes will help to further understand the biological functions of these TRP channels. We recently identified the gene encoding the pro-inflammatory cytokine interleukin-8 (IL-8), also known as CXCL8, as a delayed-response gene to TRPM3 stimulation [
55]. IL-8 acts as a chemoattractant and neutrophil activator, and expression and secretion of IL-8 has been associated to inflammatory disorders. TRPM3 stimulation has been shown to induce the secretion of calcitonin-gene related peptide (CGRP) in skin nerve terminals stimulated with pregnenolone sulfate [
66]. CGRP is a neuropeptide synthesized and released by nociceptors that acts as a neuromodulator inducing local vascular and inflammatory effects. Analysis of the TRPV1-induced signaling pathway revealed that CGRP gene transcription is activated by CREB via a conserved CRE (sequence 5′-TGACGTCA-3′) [
67]. Since it has been shown that CREB is activated following stimulation of TRPM3 or TRPM8 channels [42-44, this study], it is very likely that TRPM3 and TRPM8 stimulation leads to the biosynthesis of CGRP via activation of CREB. This study shows that the prostaglandin endoperoxide synthase-2 gene promoter is activated after stimulation of TRPM3 or TRPM8 channels, suggesting that stimulation of these TRP channels leads to the biosynthesis of the prostaglandin endoperoxide synthase-2 enzyme, which catalyzes the biosynthesis of prostaglandins. In summary, the results of this study establish a link between the stimulation of TRPM3 or TRPM8 channels and the biosynthesis of pro-inflammatory mediators via the regulation of prostaglandin endoperoxide synthase-2.
Author Contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used “Conceptualization, G.T..; methodology, G.T.; software, O.G.R.; validation, N.B., O.G.R and G.T.; formal analysis, N.B. and G.T.; investigation, N.B. and G.T.; resources, G.T.; data curation, N.B. and G.T.; writing—original draft preparation, G.T.; writing—review and editing, N.B., O.G.R. and G.T.; visualization, N.B. and O.G.R.; supervision, G.T.; project administration, G.T.; funding acquisition, G.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research in the lab was supported by the Saarland University, Germany (LOM-T201000492).
Acknowledgments
We thank Hiroyasu Inoue and Gregory Kapatos for their kind gifts of plasmids, Sabine Plant for excellent technical support and Libby Guethlein for critical reading of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CRE |
cyclic AMP response element |
| CREB |
cyclic AMP response element binding protein |
| hPES |
human prostaglandin synthase |
| PregS |
pregnenolone sulfate |
| TRP |
transient receptor potential |
References
- Samanta, A.; Hughes, T.E.T.; Moiseenkova-Bell, V.Y. Transient receptor potential (TRP) channels. Subcell. Biochem. 2018, 87, 141-165.
- Nilius, B.; Szallasi, A. Transient Receptor Potential Channels as Drug Targets: From the Science of Basic Research to the Art of Medicine. Pharmacol. Rev. 2014, 66, 676–814. [CrossRef]
- Thiel, G.; Rubil, S.; Lesch, A.; Guethlein, L.A.; Rössler, O.G. Transient receptor potential TRPM3 channels: Pharmacology, signaling, and biological functions. Pharmacol. Res. 2017, 124, 92–99. [CrossRef]
- Held, K.; Tóth, B.I. TRPM3 in Brain (Patho)Physiology. Front. Cell Dev. Biol. 2021, 9. [CrossRef]
- Vriens, J.; Owsianik, G.; Hofmann, T.; Philipp, S.E.; Stab, J.; Chen, X.; Benoit, M.; Xue, F.; Janssens, A.; Kerselaers, S.; et al. TRPM3 Is a Nociceptor Channel Involved in the Detection of Noxious Heat. Neuron 2011, 70, 482–494. [CrossRef]
- Vandewauw, I.; De Clercq, K.; Mulier, M.; Held, K.; Pinto, S.; Van Ranst, N.; Segal, A.; Voet, T.; Vennekens, R.; Zimmermann, K.; et al. A TRP channel trio mediates acute noxious heat sensing. Nat. 2018, 555, 662–666. Correction in 2018, 559, E7. [CrossRef]
- Cabanas, H.; Muraki, K.; Balinas, C.; Eaton-Fitch, N.; Staines, D.; Marshall-Gradisnik, S. Validation of impaired Transient Receptor Potential Melastatin 3 ion channel activity in natural killer cells from Chronic Fatigue Syndrome/ Myalgic Encephalomyelitis patients. Mol. Med. 2019, 25, 14. [CrossRef]
- Roelens, R.; Peigneur, A.N.F.; Voets, T.; Vriens, J. Neurodevelopmental disorders caused by variants in TRPM3. Biochim. et Biophys. Acta (BBA) - Mol. Cell Res. 2024, 1871, 119709. [CrossRef]
- Zhou, Y.; Bennett, T.M.; Shiels, A. Mutation of the TRPM3 cation channel underlies progressive cataract development and lens calcification associated with pro-fibrotic and immune cell responses. FASEB J. 2021, 35, e21288–e21288. [CrossRef]
- McKemy, D.D.; Neuhausser, W.M.; Julius, D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 2002, 416, 52–58. [CrossRef]
- Bautista, D.M.; Siemens, J.; Glazer, J.M.; Tsuruda, P.R.; Basbaum, A.I.; Stucky, C.L.; Jordt, S.-E.; Julius, D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 2007, 448, 204–208. [CrossRef]
- Colburn, R.W.; Lubin, M.L.; Stone, D.J. Jr.; Wang, Y.; Lawrence, D.; D´Andrea, M.R.; Brandt, M.R.; Liu, Y.; Flores, C.M.; Qin, N. Attenuated cold sensitivity in TRPM8 null mice. Neuron 2007, 54, 379-386.
- Dhaka, A.; Murray, A.N.; Mathur, J.; Earley, T.J.; Petrus, M.J.; Patapoutian, A. TRPM8 Is Required for Cold Sensation in Mice. Neuron 2007, 54, 371–378. [CrossRef]
- I Caceres, A.; Liu, B.; Jabba, S.V.; Achanta, S.; Morris, J.B.; Jordt, S. Transient Receptor Potential Cation Channel Subfamily M Member 8 channels mediate the anti-inflammatory effects of eucalyptol. Br. J. Pharmacol. 2017, 174, 867–879. [CrossRef]
- Hantute-Ghesquier, A.; Haustrate, A.; Prevarskaya, N.; Lehen´kyi, V. TRPM family channels in cancer. Pharmaceuticals 2018, 11, 58.
- Thiel, G.; Rössler, O.G. Stimulus–Transcription Coupling of TRPM3 Channels: A Signaling Pathway from the Plasma Membrane to the Nucleus. Biomolecules 2025, 15, 521. [CrossRef]
- Ulrich, M.; Wissenbach, U.; Thiel, G. The super-cooling compound icilin stimulates c-Fos and Egr-1 expression and activity involving TRPM8 channel activation, Ca2+ ion influx and activation of the ternary complex factor Elk-1. Biochem. Pharmacol. 2020, 177, 113936. [CrossRef]
- Kang, Y.-J.; Mbonye, U.R.; DeLong, C.J.; Wada, M.; Smith, W.L. Regulation of intracellular cyclooxygenase levels by gene transcription and protein degradation. Prog. Lipid Res. 2007, 46, 108–125. [CrossRef]
- Yin, Y.; Park, C.-G.; Feng, S.; Guan, Z.; Lee, H.-J.; Zhang, F.; Sharma, K.; Borgnia, M.J.; Im, W.; Lee, S.-Y. Molecular basis of neurosteroid and anticonvulsant regulation of TRPM3. Nat. Struct. Mol. Biol. 2025, 32, 1–13. [CrossRef]
- Peri, K.G.; Almazan, G.; Varma, D.R.; Chemtob, S. A role for protein kinase C alpha in stimulation of prostaglandin G/H synthase-2 transcription by 14,15-epoxyeicosatrienoic acid. Biochem. Biophys. Res. Comm. 1998, 244, 96-101.
- Matsuura, H.; Sakaue, M.; Subbaramaiah, K.; Kamitani, H.; Eling, T.E.; Dannenberg, A.J.; Tanabe, T.; Inoue, H.; Arata, J.; Jetten, A.M. Regulation of Cyclooxygenase-2 by Interferon γ and Transforming Growth Factor α in Normal Human Epidermal Keratinocytes and Squamous Carcinoma Cells. J. Biol. Chem. 1999, 274, 29138–29148. [CrossRef]
- McGinty, A.; Foschi, M.; Chang, Y.W.; Han, J.; Dunn, M.J.; Sorokin, A. Induction of prostaglandin endoperoxide synthase-2 by mitogen-activated protein kinase cascades. Biochem. J. 2000, 352, 419-424.
- Guo, Y.-S.; Hellmich, M.R.; Wen, X.D.; Townsend, C.M. Activator Protein-1 Transcription Factor Mediates Bombesin-stimulated Cyclooxygenase-2 Expression in Intestinal Epithelial Cells. 2001, 276, 22941–22947. [CrossRef]
- Ogata, S.; Kubota, Y.; Satoh, S.; Ito, S.; Takeuchi, H.; Ashizuka, M.; Shirasuna, K. Ca2+ stimulates COX-2 expression through calcium-sensing receptor in fibroblasts. Biochem. Biophys. Res. Commun. 2006, 351, 808–814. [CrossRef]
- Alique, M.; Herrero, J.F.; Lucio-Cazana, J. All-trans retinoic acid induce COX-2 and prostaglandin E2 synthesis in SH-SY5Y human neuroblastoma cells: involvement of retinoid acid receptor and extracellular-regulated protein kinase 1/2. J. Neuroinflamm. 2007, 4, 1.
- Naidich, M.; Shterntal, B.; Furman, R.; Pawson, A.J.; Jabbour, H.N.; Morgan, K.; Millar, R.P.; Jia, J.; Tomic, M.; Stojilkovic, S.; et al. Elucidation of Mechanisms of the Reciprocal Cross Talk between Gonadotropin-Releasing Hormone and Prostaglandin Receptors. Endocrinology 2010, 151, 2700–2712. [CrossRef]
- Wang, J.-Y.; Chen, B.-K.; Wang, Y.-S.; Tsai, Y.-T.; Chang, W.-C.; Hou, M.-F.; Wu, Y.-C.; Chang, W.-C. Involvement of store-operated calcium signaling in EGF-mediated COX-2 gene activation in cancer cells. Cell. Signal. 2012, 24, 162–169. [CrossRef]
- Wouters, E.; A Hudson, C.; A McArdle, C.; Bernal, A.L. Central role for protein kinase C in oxytocin and epidermal growth factor stimulated cyclooxygenase 2 expression in human myometrial cells. BMC Res. Notes 2014, 7, 357–357. [CrossRef]
- Wong, J.-H.; Ho, K.-H.; Nam, S.; Hsu, W.-L.; Lin, C.-H.; Chang, C.-M.; Wang, J.-Y.; Chang, W.-C. Store-operated Ca2+ Entry Facilitates the Lipopolysaccharide-induced Cyclooxygenase-2 Expression in Gastric Cancer Cells. Sci. Rep. 2017, 7, 1–10. [CrossRef]
- Mayer, S.I.; Müller, I.; Mannebach, S.; Endo, T.; Thiel, G. Signal Transduction of Pregnenolone Sulfate in Insulinoma Cells. Activation of Egr-1 expression involving TRPM3, voltage-gated calcium channels, ERK, and ternary complex factors. J. Biol. Chem. 2011, 286, 10084–10096. [CrossRef]
- Lesch, A.; Hui, X.; Lipp, P.; Thiel, G. Transient receptor potential melastatin-3 (TRPM3)-induced activation of AP-1 requires Ca2+ ions and the transcription factors c-Jun, ATF2, and ternary complex factor. Mol. Pharmacol. 2015, 87, 617-628.
- Lesch, A.; Rössler, O.G.; Thiel, G. Extracellular Signal-Regulated Protein Kinase, c-Jun N-Terminal Protein Kinase, and Calcineurin Regulate Transient Receptor Potential M3 (TRPM3) Induced Activation of AP-1. J. Cell. Biochem. 2017, 118, 2409–2419. [CrossRef]
- Jeong, S.; Stein, A. Micrococcal nuclease digestion of nuclei reveals extended nucleosome ladders having anomalous DNA lengths for chromatin assembled on non-replicating plasmids in transfected cells. Nucleic Acids Res. 1994, 22, 370–375. [CrossRef]
- Smith, C.L.; Hager, G.L. Transcriptional Regulation of Mammalian Genes in Vivo. J. Biol. Chem. 1997, 272, 27493–27496. [CrossRef]
- Akira, S.; Isshiki, H.; Sugita, T.; Tanabe, O.; Kinoshita, S.; Nishio, Y.; Nakajima, T.; Hirano, T.; Kishimoto, T. A nuclear factor for IL-6 expression (NF-IL6) is a member of a C/EBP family.. EMBO J. 1990, 9, 1897–1906. [CrossRef]
- Klose, C.; Straub, I.; Riehle, M.; Ranta, F.; Krautwurst, D.; Ullrich, S.; Meyerhof, W.; Harteneck, C. Fenamates as TRP channel blockers: mefenamic acid selectively blocks TRPM3. Br. J. Pharmacol. 2011, 162, 1757–1769. [CrossRef]
- Straub, I.; Krügel, U.; Mohr, F.; Teichert, J.; Rizun, O.; Konrad, M.; Oberwinkler, J.; Schaefer, M. Flavanones That Selectively Inhibit TRPM3 Attenuate Thermal Nociception In Vivo. Mol. Pharmacol. 2013, 84, 736–750. [CrossRef]
- Lesch, A.; Rubil, S.; Thiel, G. Activation and inhibition of transient receptor potential TRPM3-induced gene transcription. Br. J. Pharmacol. 2014, 171, 2645–2658. [CrossRef]
- Thiel, G.; Backes, T.M.; Welck, J.; Steinhausen, S.; Fischer, A.-L.; Langfermann, D.S.; Ulrich, M.; Wissenbach, U.; Rössler, O.G. Pharmacological inhibition of TRPM8-induced gene transcription. Biochem. Pharmacol. 2019, 170, 113678. [CrossRef]
- Okamoto, Y.; Ohkubo, T.; Ikebe, T; Yamazaki, J. Blockade of TRPM8 activity reduces the invasion potential of oral squamous carcinoma cell lines. Int. J. Oncol. 2012, 40, 1431–1440. [CrossRef]
- Ohmi, M.; Shishido, Y.; Inoue, T.; Ando, K.; Fujiuchi, A.; Yamada, A.; Watanabe, S.; Kawamura, K. Identification of a novel 2-pyridyl-benzensulfonamide derivative, RQ-00203078, as a selective and orally active TRPM8 antagonist. Bioorganic Med. Chem. Lett. 2014, 24, 5364–5368. [CrossRef]
- Müller, I.; Rössler, O.G.; Thiel, G. Pregnenolone Sulfate Activates Basic Region Leucine Zipper Transcription Factors in Insulinoma Cells: Role of Voltage-Gated Ca2+ Channels and Transient Receptor Potential Melastatin 3 Channels. Mol. Pharmacol. 2011, 80, 1179–1189. [CrossRef]
- Rubil, S.; Rössler, O.G.; Thiel, G. CREB, AP-1, ternary complex factors and MAP kinases connect transient receptor potential melastatin-3 (TRPM3) channel stimulation with increased c-Fos expression. Brit. J. Pharmacol. 2016, 173, 305-318.
- Thiel, G.; Rössler, O.G. TRPM3-Induced Gene Transcription Is under Epigenetic Control. Pharmaceuticals 2022, 15, 846. [CrossRef]
- Jüngling, S.; Cibelli, G.; Czardybon, M.; Gerdes, H.; Thiel, G. Differential Regulation of Chromogranin B and Synapsin I Gene Promoter Activity by cAMP and cAMP-Dependent Protein Kinase. Eur. J. Biochem. 1994, 226, 925–935. [CrossRef]
- Al Sarraj, J.; Vinson, C.; Han, J.; Thiel, G. Regulation of GTP cyclohydrolase I gene transcription by basic region leucine zipper transcription factors. J. Cell. Biochem. 2005, 96, 1003–1020. [CrossRef]
- Loviscach, L.; Backes, T.M.; Langfermann, D.S.; Ulrich, M.; Thiel, G. Zn2+ ions inhibit gene transcription following stimulation of the Ca2+ channels Cav1.2 and TRPM3. Metallomics 2020, 12, 1735–1747. [CrossRef]
- Tang, Q.; Chen, W.; Gonzales, M.S.; Finch, J.; Inoue, H.; Bowden, G.T. Role of cyclic AMP responsive element in the UVB induction of cyclooxygenase-2 transcription in human keratinocytes. Oncogene 2001, 20, 5164–5172. [CrossRef]
- Xie, W.; Herschman, H.R. Transcriptional Regulation of Prostaglandin Synthase 2 Gene Expression by Platelet-derived Growth Factor and Serum. J. Biol. Chem. 1996, 271, 31742–31748. [CrossRef]
- Park, S.-W.; Sung, M.-W.; Heo, D.-S.; Inoue, H.; Shim, S.-H.; Kim, K.-H. Nitric oxide upregulates the cyclooxygenase-2 expression through the cAMP-response element in its promoter in several cancer cell lines. Oncogene 2005, 24, 6689–6698. [CrossRef]
- Kapatos, G.; Stegenga, S.L.; Hirayama, K. Identification and Characterization of Basal and Cyclic AMP Response Elements in the Promoter of the Rat GTP Cyclohydrolase I Gene. 2000, 275, 5947–5957. [CrossRef]
- Lee, B.; Dziema, H.; Lee, K.H.; Choi, Y.-S.; Obrietan, K. CRE-mediated transcription and COX-2 expression in the pilocarpine model of status epilepticus. Neurobiol. Dis. 2007, 25, 80–91. [CrossRef]
- Chen, L.-C.; Chen, B.-K.; Chang, W.-C. Activating Protein 1-Mediated Cyclooxygenase-2 Expression Is Independent of N-Terminal Phosphorylation of c-Jun. Mol. Pharmacol. 2005, 67, 2057–2069. [CrossRef]
- Thiel, G.; Rössler, O.G. Signal Transduction of Transient Receptor Potential TRPM8 Channels: Role of PIP5K, Gq-Proteins, and c-Jun. Molecules 2024, 29, 2602. [CrossRef]
- Rubil; S. Lesch, A.; Mukaida, N.; Thiel, G. Stimulation of transient receptor potential M3 (TRPM3) increases interleukin-8 gene promoter activity involving AP-1 and extracellular signal-regulated protein kinase. Cytokine 2018, 103, 133-141.
- Inoue, H.; Yokoyama, C.; Hara, S.; Tone, Y.; Tanabe, T. Transcriptional Regulation of Human Prostaglandin-endoperoxide Synthase-2 Gene by Lipopolysaccharide and Phorbol Ester in Vascular Endothelial Cells. Involvement of both nuclear factor for interleukin-6 expression site and cAMP response element. J. Biol. Chem. 1995, 270, 24965–24971. [CrossRef]
- Inoue, H.; Umesono, K.; Nishimori, T.; Hirata, Y.; Tanabe, T. Glucocorticoid-mediated suppression of the promoter activity of the cyclooxygenase-2 gene is modulated by expression of its receptor in vascular endothelial cells. Biochem. Biophys. Res. Comm. 1999, 254, 293-298.
- Font-Nieves, M.; Sans-Fons, M.G.; Gorina, R.; Bonfill-Teixidor, E.; SalasPerdomo, A.; Marquez-Kisinousky, L. Induction of COX-2 Enzyme and Down-regulation of COX-1 Expression by Lipopolysaccharide (LPS) Control Prostaglandin E2 Production in Astrocytes. J. Biol. Chem. 2012, 287, 6454–6468. [CrossRef]
- Nie, M.; Pang, L.; Inoue, H.; Knox, A.J. Transcriptional regulation of cyclooxygenase 2 by bradykinin and interleukin-1b in human airway smooth muscle cells: Involvement of different promoter elements, transcription factors, and histone H4 acetylation. Mol. Cell. Biol. 2003, 23, 9233-9244.
- Naylor, J.; Milligan, C.J.; Zeng, F.; Jones, C.; Beech, D.J. Production of a specific extracellular inhibitor of TRPM3 channels. Br. J. Pharmacol. 2008, 155, 567–573. [CrossRef]
- Bödding, M.; Wissenbach, U.; Flockerzi, V. Characterisation of TRPM8 as a pharmacophore receptor. Cell Calcium 2007, 42, 618–628. [CrossRef]
- Rössler, O.G.; Thiel, G. Regulation of gene transcription following stimulation of Gαq-coupled designer receptors. In: Designer Receptors Exclusively Activated by Designer Drugs; Thiel, G., Ed.; Springer: New York, NY, USA; Humana Press, Neuromethods: Totowa, NJ, USA, 2015; Volume 108, pp. 49–60.
- Inoue, H.; Nanayama, T.; Hara, S.; Yokoyama, C.; Tanabe, T. The cyclic AMP response element plays an essential role in the expression of the human prostaglandin-endoperoxide synthase 2 gene in differentiated U937 monocytic cells. FEBS Lett. 1994, 350, 51–54. [CrossRef]
- Hirayama, K.; Shimoji, M.; Swick, L.; Meyer, A.; Kapatos, G. Characterization of GTP cyclohydrolase I gene expression in the human neuroblastoma SKN-BE(2)M17: enhanced transcription in response to cAMP is conferred by the proximal promoter. J. Neurochem. 2001, 79, 576–587. [CrossRef]
- Mayer, S.I.; Dexheimer, V.; Nishida, E.; Kitajima, S.; Thiel, G. Expression of the Transcriptional Repressor ATF3 in Gonadotrophs Is Regulated by Egr-1, CREB, and ATF2 after Gonadotropin-Releasing Hormone Receptor Stimulation. Endocrinology 2008, 149, 6311–6325. [CrossRef]
- Held, K.; Kichko, T.; De Clercq, K.; Klaassen, H.; Van Bree, R.; Vanherck, J.-C.; Marchand, A.; Reeh, P.W.; Chaltin, P.; Voets, T.; et al. Activation of TRPM3 by a potent synthetic ligand reveals a role in peptide release. Proc. Natl. Acad. Sci. 2015, 112, 201419845–72. [CrossRef]
- Nakanishi, M.; Hata, K.; Nagayama, T.; Sakurai, T.; Nishisho, T.; Wakabayashi, H.; Hiraga, T.; Ebisu, S.; Yoneda, T.; Heldin, C.-H. Acid Activation of Trpv1 Leads to an Up-Regulation of Calcitonin Gene-related Peptide Expression in Dorsal Root Ganglion Neurons via the CaMK-CREB Cascade: A Potential Mechanism of Inflammatory Pain. Mol. Biol. Cell 2010, 21, 2568–2577. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).