Submitted:
30 June 2025
Posted:
01 July 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
2.1. Composition of Fatty Acids in Deer Tallow
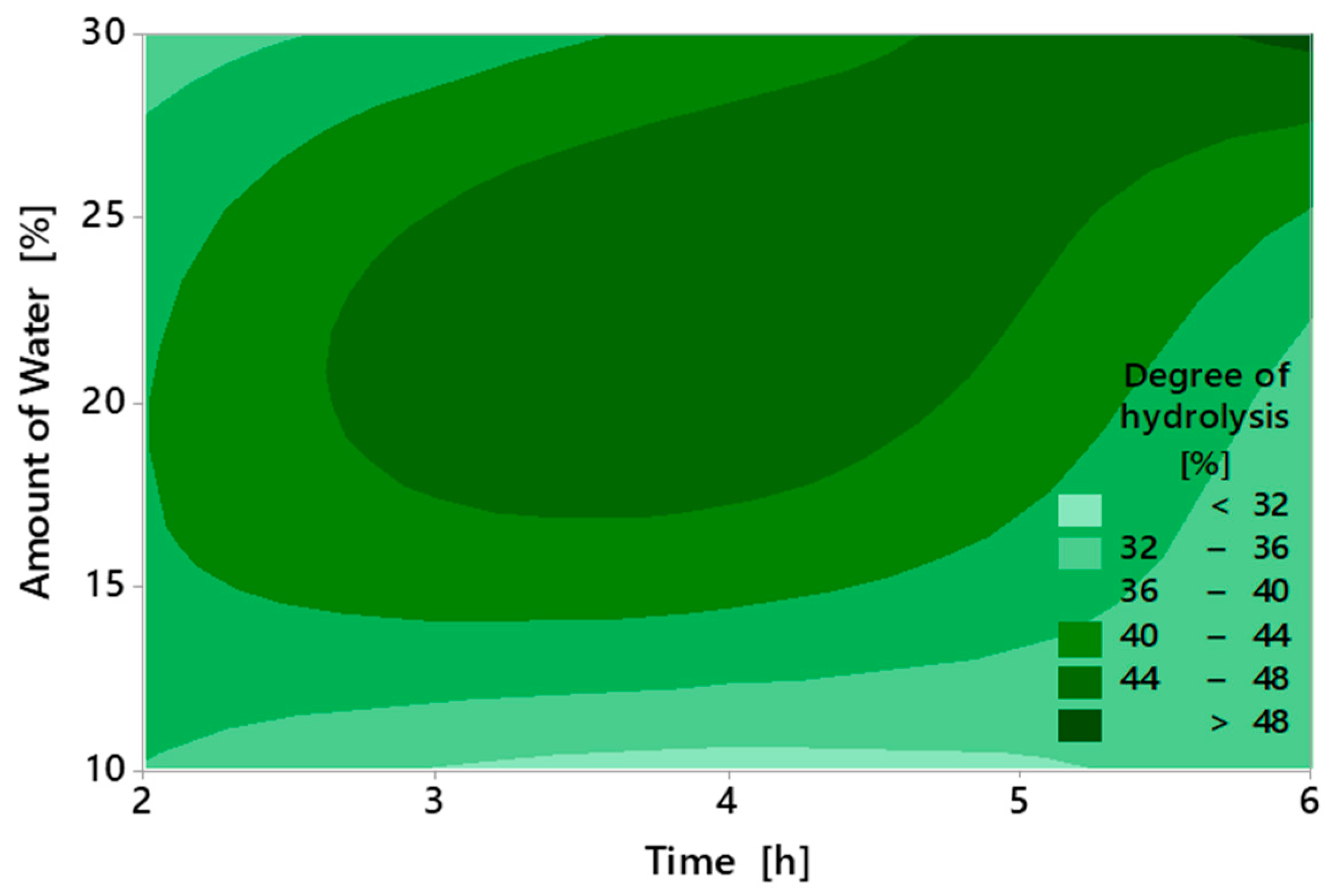
2.2. Degree of Hydrolysis and Acid Value
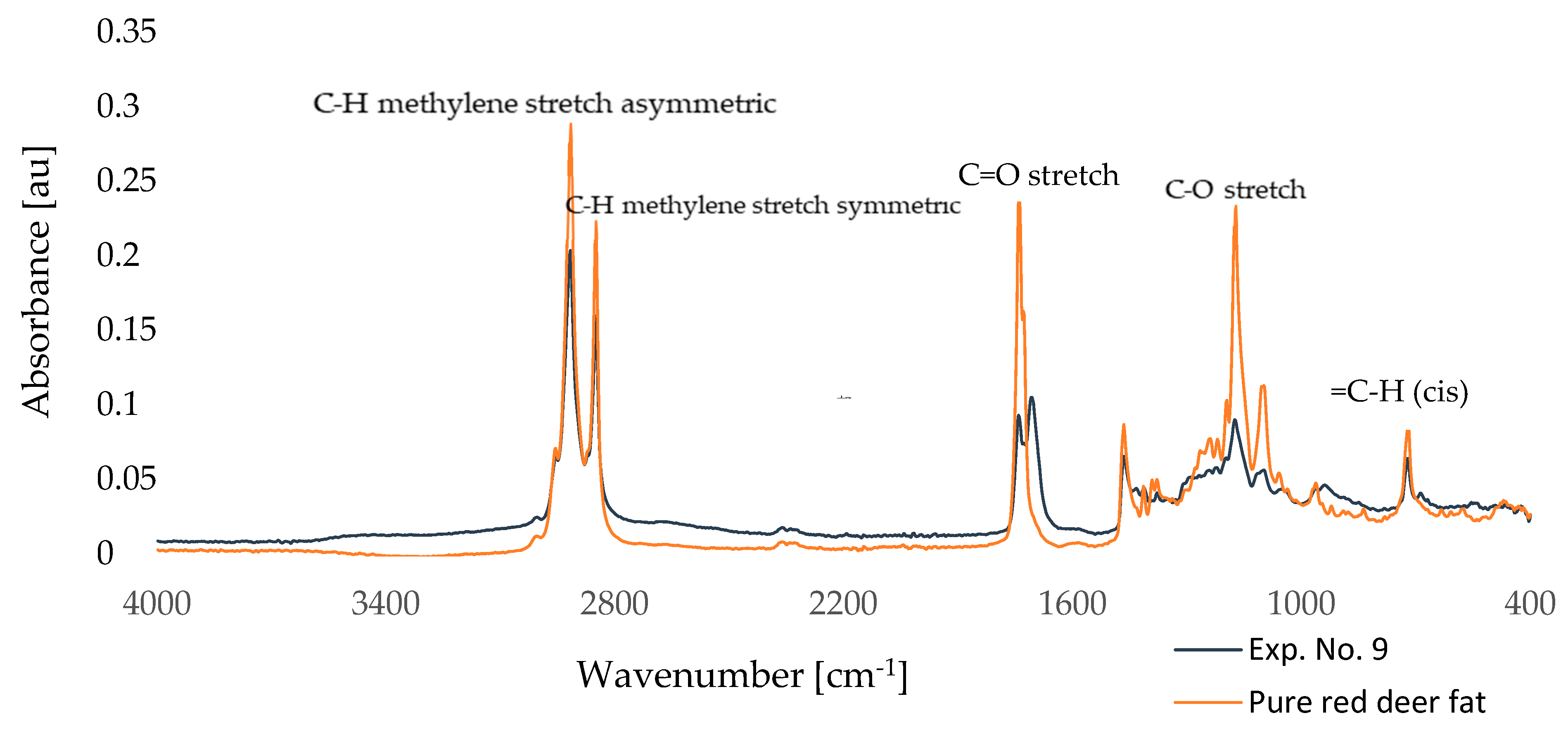
2.3. Vibrational Characterization of Functional Groups
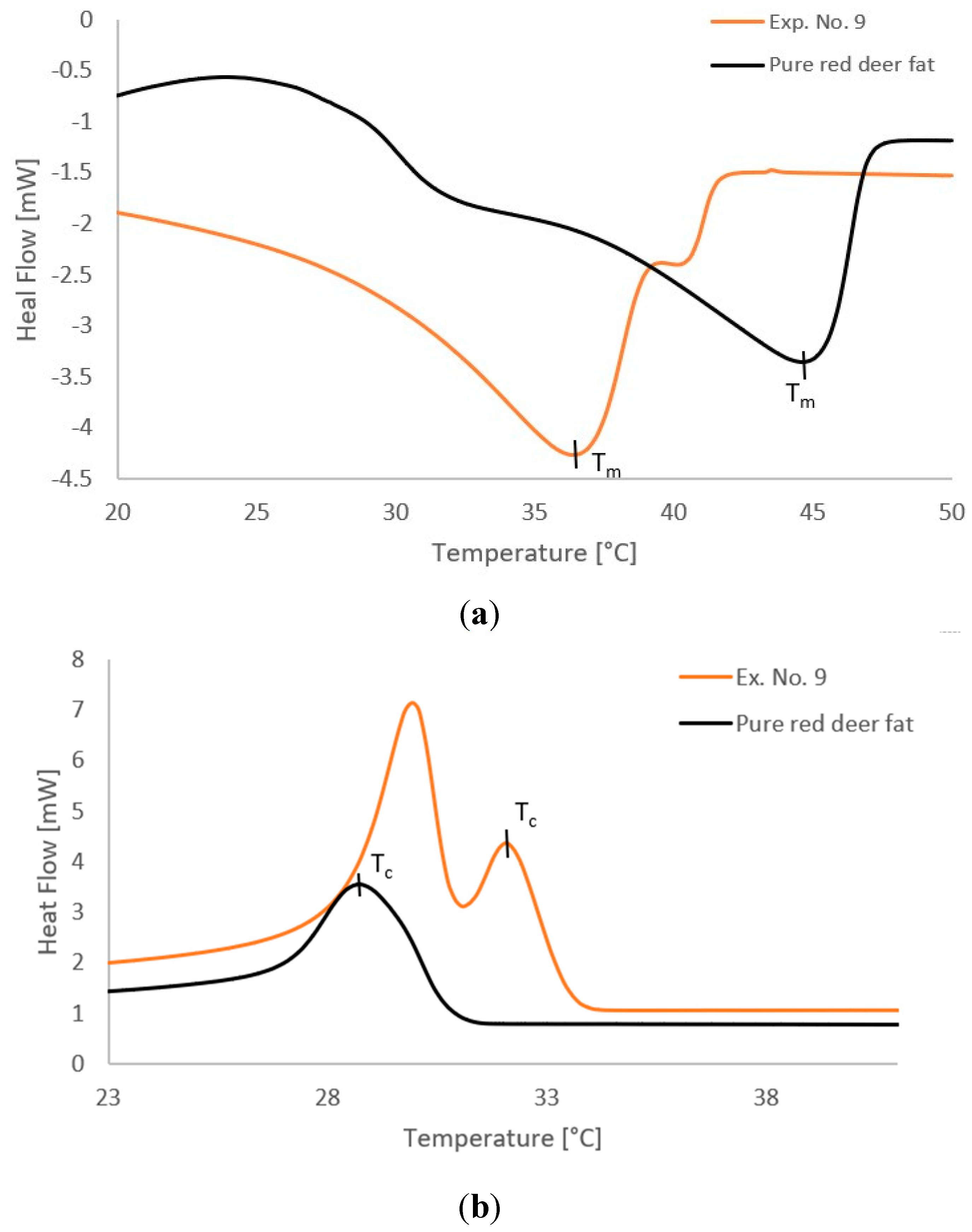
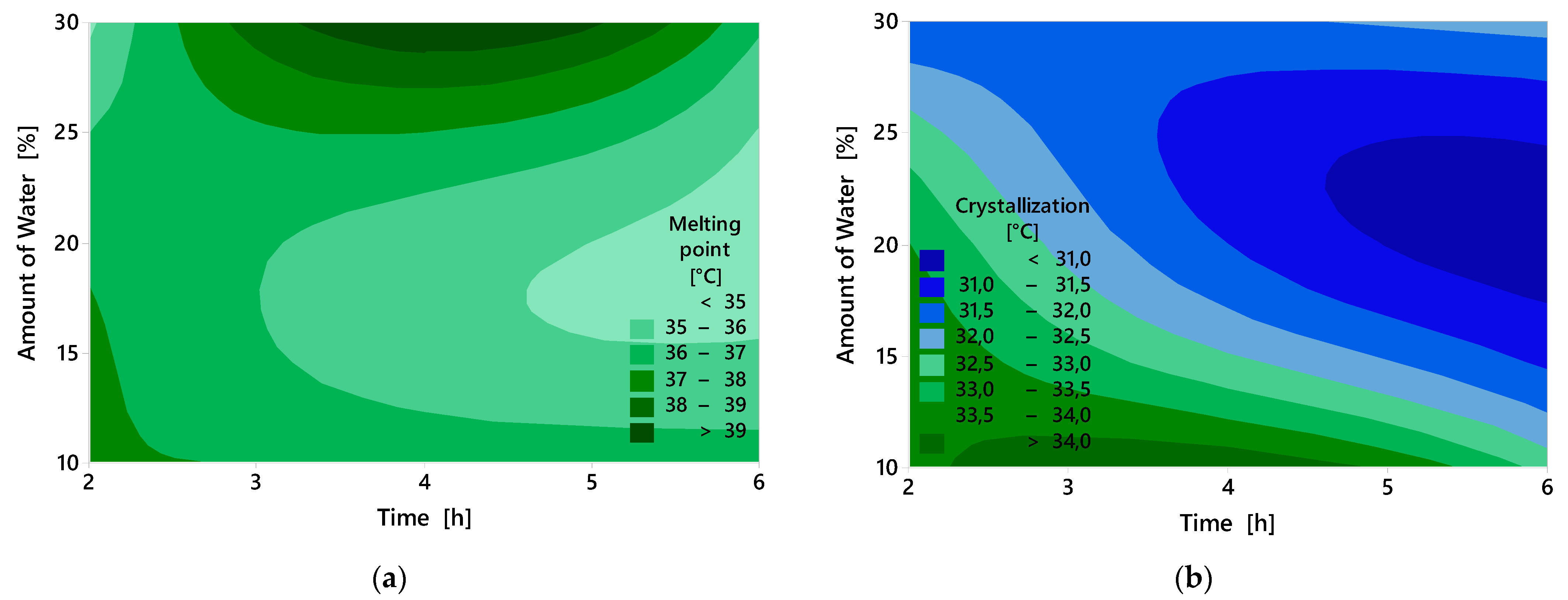
2.4. Thermal Properties
2.5. Texture Properties
| Degree of Freedom | Sum of Squares | Mean Squares | p-Value | |
|---|---|---|---|---|
| Regression equation | Hardness = 52.78 – 0.338 A – 2.41 B | |||
| Regression | 2 | 207.98 | 103.99 | 0.220 |
| Factor A: Amount of water [%] | 1 | 68.68 | 68.68 | 0.297 |
| Factor B: Time [h] | 1 | 139.30 | 139.30 | 0.155 |
| Error | 6 | 316.43 | 52.74 | |
| Total | 8 | 524.41 | ||
| Regression equation | Spreadability = 42.74 – 0.309 A – 2.11 B | |||
| Regression | 2 | 164.48 | 82.24 | 0.181 |
| Factor A: Amount of water [%] | 1 | 57.29 | 57.29 | 0.252 |
| Factor B: Time [h] | 1 | 107.19 | 107.19 | 0.134 |
| Error | 6 | 214.17 | 35.70 | |
| Total | 8 | 378.65 | ||
| Regression equation | Stickiness = -1.72 + 0.1000 A + 0.325 B | |||
| Regression | 2 | 8.535 | 4.2675 | 0.054 |
| Factor A: Amount of water [%] | 1 | 6.000 | 6.0000 | 0.039 * |
| Factor B: Time [h] | 1 | 2.535 | 2.5350 | 0.137 |
| Error | 6 | 5.181 | 0.8634 | |
| Total | 8 | 13.716 | ||
| Regression equation | Adhesiveness = -0.0922 + 0.00633 A+ 0.0092 B | |||
| Regression | 2 | 0.026083 | 0.013042 | 0.133 |
| Factor A: Amount of water [%] | 1 | 0.024067 | 0.024067 | 0.061 |
| Factor B: Time [h] | 1 | 0.002017 | 0.002017 | 0.530 |
| Error | 6 | 0.027206 | 0.004534 | |
| Total | 8 | 0.053289 | ||
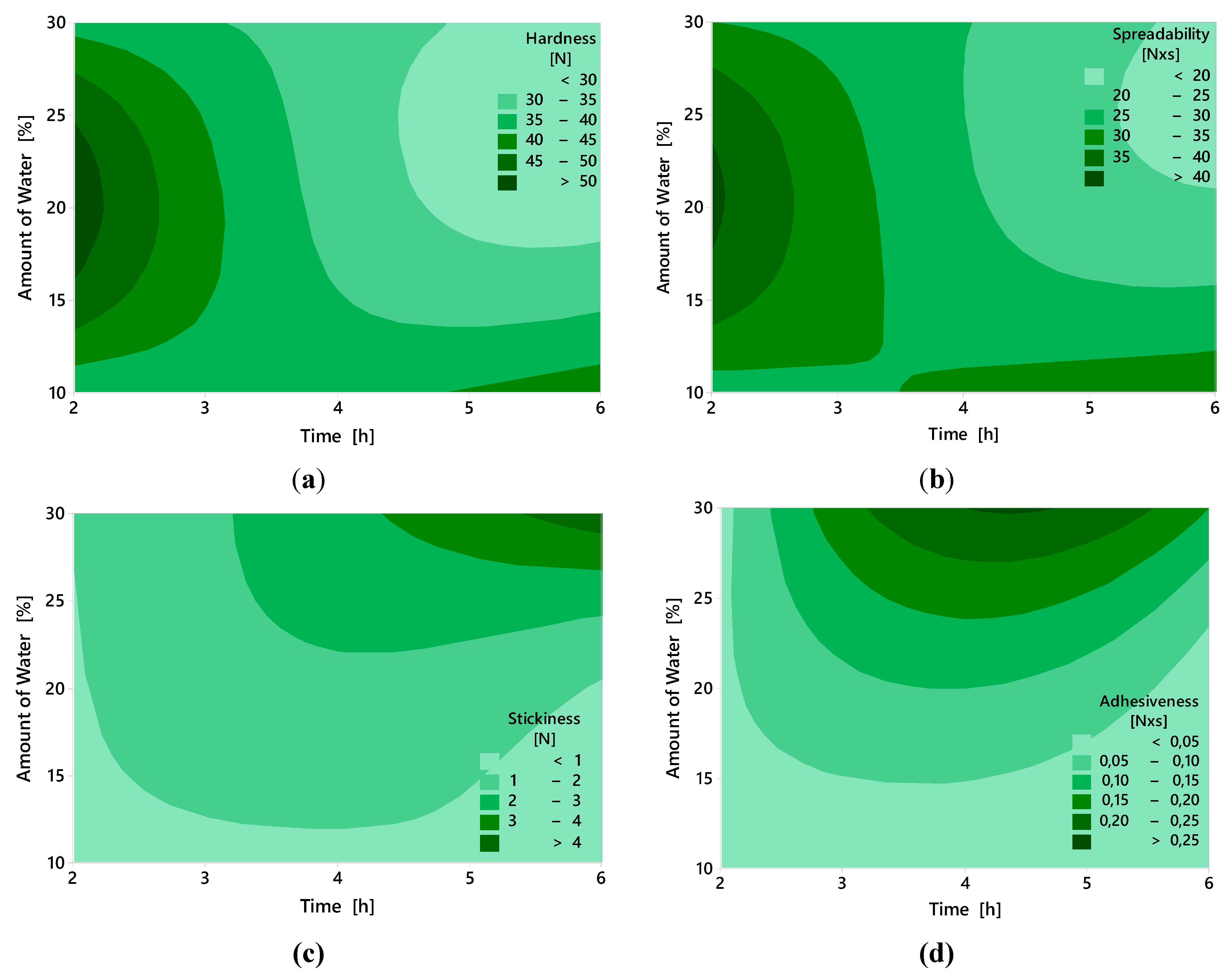
2.6. Color Parameters
2.7. The Relevance for Practice and the Limitations of the Study
2.7.1. Optimal Conditions for Enzymatic Modification and Utilization of Modified Fats
2.7.2. Limitations of the Study
2.7.3. Future Perspectives
3. Materials and Methods
3.1. Raw Material
3.2. Experiment Design and Statistical Analysis
3.3. Preparation and Enzymatic Modification of Red Deer Fat
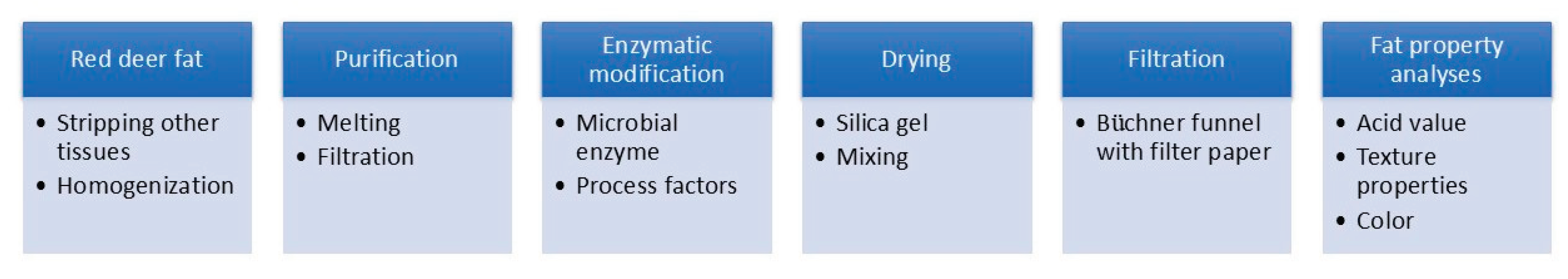
3.4. Analytical Part
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AV | Acid value |
| DH | Degree of hydrolysis |
| DOE | Design of the experiment |
| DSC | Differential scanning calorimetry |
| FTIR | Fourier transform infrared spectroscopy |
| IV | Iodine value |
| MUFA | Monounsaturated fatty acid |
| PUFA | Polyunsaturated fatty acid |
| PV | Peroxide value |
| SFA | Saturated fatty acid |
| SV | Saponification value |
| Tc | Crystallization temperature |
| Tm | Melting temperature |
References
- Razmaitė, V.; Pileckas, V.; Šiukščius, A.; Juškienė, V. Fatty Acid Composition of Meat and Edible Offal from Free-Living Red Deer (Cervus elaphus). Foods 2020, 9, 923. [CrossRef]
- Demartini, E.; Vecchiato, D.; Tempesta, T.; Gaviglio, A.; Viganò, R. Consumer Preferences for Red Deer Meat: A Discrete Choice Analysis Considering Attitudes towards Wild Game Meat and Hunting. Meat Sci. 2018, 146, 168–179. [CrossRef]
- Mattiello, S. Welfare Issues of Modern Deer Farming. Ital. J. Anim. Sci. 2016, 8, 205–217. [CrossRef]
- Wiklund, E.; Manley, T.R.; Littlejohn, R.P.; Stevenson-Barry, J.M. Fatty Acid Composition and Sensory Quality of Musculus longissimus and Carcass Parameters in Red Deer (Cervus elaphus) Grazed on Natural Pasture or Fed a Commercial Feed Mixture. J. Sci. Food Agric. 2003, 83(5), 419–424. [CrossRef]
- Polak, T.; Rajar, A.; Gašperlin, L.; Žlender, B. Cholesterol Concentration and Fatty Acid Profile of Red Deer (Cervus elaphus) Meat. Meat Sci. 2008, 80, 864–869. [CrossRef]
- Prates, J.A.M. The Role of Meat Lipids in Nutrition and Health: Balancing Benefits and Risks. Nutrients 2025, 17. [CrossRef]
- Toldrá, F. Lawrie’s Meat Science, 8th ed.; Elsevier: Amsterdam, 2017. Available online: https://app.knovel.com/hotlink/toc/id:kpLMSE0011/lawries-meat-science/lawries-meat-science.
- Bartoň, L.; Bureš, D.; Kotrba, R.; Sales, J. Comparison of meat quality between eland (Taurotragus oryx) and cattle (Bos taurus) raised under similar conditions. Meat Sci. 2014, 96, 346–352. [CrossRef]
- Phillip, L.E.; Oresanya, T.F.; St. Jacques, J. Fatty acid profile, carcass traits and growth rate of red deer fed diets varying in the ratio of concentrate: dried and pelleted roughage, and raised for venison production. Small Rumin. Res. 2007, 71, 215–221. [CrossRef]
- Demartini, E.; Vecchiato, D.; Tempesta, T.; Gaviglio, A.; Viganò, R. Consumer preferences for red deer meat: a discrete choice analysis considering attitudes towards wild game meat and hunting. Meat Sci. 2018, 146, 168–179. [CrossRef]
- Adenuga, B.M.; Biltes, R.; Villa, C.; Costa, J.; Spychaj, A.; Montowska, M.; Mafra, I. Unravelling red deer (Cervus elaphus) meat adulteration in gourmet foods by quantitative real-time PCR. Food Control 2025, 168, 110872. [CrossRef]
- Atanassova, V.; Apelt, J.; Reich, F.; Klein, G. Microbiological quality of freshly shot game in Germany. Meat Sci. 2008, 78, 414–419. [CrossRef]
- Wiklund, E.; Manley, T.R.; Littlejohn, R.P.; Stevenson-Barry, J.M. Fatty acid composition and sensory quality of Musculus longissimus and carcass parameters in red deer (Cervus elaphus) grazed on natural pasture or fed a commercial feed mixture. J. Sci. Food Agric. 2003, 83, 419–424. [CrossRef]
- Shahidi, F. Bailey's Industrial Oil and Fat Products, Volumes 1–6, 6th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005. Available online: https://app.knovel.com/hotlink/toc/id:kpBIOFPVE1/baileys-industrial-oil/baileys-industrial-oil.
- Talbot, G. Reducing Saturated Fats in Foods; Woodhead Publishing: Cambridge, UK, 2011. Available online: https://app.knovel.com/hotlink/toc/id:kpRSFF0003/reducing-saturated-fats/reducing-saturated-fats.
- Truong, T.L.; Bhandari, C.; Prakash, B.; Sangeeta. Dairy Fat Products and Functionality – Fundamental Science and Technology; Springer Nature: Cham, Switzerland, 2020. Available online: https://app.knovel.com/hotlink/toc/id:kpDFPFFST6/dairy-fat-products-functionality/dairy-fat-products-functionality.
- Marangoni, A.; Wright, A. Physical Properties of Fats and Oils. In: Akoh, C., Ed. Handbook of Functional Lipids; CRC Press: Boca Raton, FL, USA, 2005; pp. 135–162. Available online: . [CrossRef]
- Shahidi, F., Ed. Bailey's Industrial Oil and Fat Products; Wiley: Hoboken, NJ, USA, 2005. Available online: . [CrossRef]
- Bornscheuer, U.T. Enzymes in Lipid Modification. Annu. Rev. Food Sci. Technol. 2018, 9, 85–103. [CrossRef]
- Bustos-Baena, A.-S.; Quintana-Castro, R.; Sánchez-Otero, M.G.; Espinosa-Luna, G.; Mendoza-López, M.R.; Peña-Montes, C.; Oliart-Ros, R.M. Enantioselectivity Enhancement of a Geobacillus thermoleovorans CCR11 Lipase by Rational Design. Catalysts 2025, 15, 20168. [CrossRef]
- Filho, D.G.; Silva, A.G.; Guidini, C.Z. Lipases: Sources, Immobilization Methods, and Industrial Applications. Appl. Microbiol. Biotechnol. 2019, 103, 7399–7423.. [CrossRef]
- Reis, P.; Holmberg, K.; Watzke, H.; Leser, M.E.; Miller, R. Lipases at Interfaces: A Review. Adv. Colloid Interface Sci. 2009, 147–148, 237–250.. [CrossRef]
- Kontkanen, H.; Rokka, S.; Kemppinen, A.; et al. Enzymatic and physical modification of milk fat: A review. Int. Dairy J. 2011, 21, 3–13. [CrossRef]
- Hasan, M.Y.; Saari, N.; Ismail, A.; Ghazali, H.M. Enzymatic Modification to Produce Health-Promoting Lipids from Fish Oils: Recent Trends. J. Funct. Foods 2020, 71, 104025.. [CrossRef]
- Zhou, J.; Lee, Y.-Y.; Mao, Y.; Wang, Y.; Zhang, Z. Future of Structured Lipids: Enzymatic Synthesis and Their New Applications in Food Systems. Foods 2022, 11, 2400. [CrossRef]
- Mitsou, E.; Theocharí, I.; Gad, E.; et al. Enzymatic Modification of Triglycerides in Conventional and Surfactant-Free microemulsions and in Olive Oil. Colloids Surf. A Physicochem. Eng. Asp. 2022, 647, 129170. [CrossRef]
- Meng, Z.; Geng, W.; Wang, X.; Liu, Y. Fat Crystal Migration and Aggregation and Polymorphism Evolution during the Formation of Granular Crystals in Beef Tallow and Palm Oil. J. Agric. Food Chem. 2013, 61, 12676–12682.. [CrossRef]
- Arita-Merino, N.; van Valenberg, H.; Gilbert, E.P.; Scholten, E. Quantitative Phase Analysis of Complex Fats during Crystallization. Cryst. Growth Des. 2020, 20, 5193–5202.. [CrossRef]
- Macridachis, J.; Bayés-García, L.; Calvet, T. Polymorphic Crystallization and Transformation Pathways of 1,2-Dipalmitoyl-3-Oleoyl-rac-Glycerol (PPO) during a Liquid–Solid–Liquid Journey. J. Therm. Anal. Calorim. 2025, 150, 187–199.. [CrossRef]
- Smith, K.W.; Bhaggan, K.; Talbot, G.; van Malssen, K.F. Crystallization of Fats: Influence of Minor Components and Additives. J. Am. Oil Chem. Soc. 2011, 88, 1085–1101.. [CrossRef]
- Meng, Z.; Liu, Y.-F.; Jin, Q.-Z.; Huang, J.-H.; Song, Z.-H.; Wang, F.-Y.; Wang, X.-G. Characterization of Graininess Formed in All Beef Tallow-Based Shortening. J. Agric. Food Chem. 2010, 58, 11463–11470.. [CrossRef]
- Liu, S.; Li, D.; He, X.; Li, H.; Li, X.; Liao, Z.; Wang, Z. Study on Crystallization Kinetics of Dry Fractionation Products of Beef Tallow. Int. J. Food Eng. 2021, 17, 945–958.. [CrossRef]
- Feiner, G. The Protein and Fat Content of Meat. In Meat Products Handbook: Practical Science and Technology; CRC Press: Boca Raton, FL, USA, 2006; pp. 3–32..
- Guillén, M.D.; Cabo, N. Infrared Spectroscopy in the Study of Edible Oils and Fats. J. Sci. Food Agric. 1997, 75, 1–11. . [CrossRef]
- Rohman, A. Application of FTIR spectroscopy and chemometrics for authentication of meat and meat products: A review. J. Adv. Vet. Anim. Res. 2019, 6, 1–9. [CrossRef]
- Yang, H.; Irudayaraj, J.; Paradkar, M.M. Discriminant Analysis of Edible Oils and Fats by FTIR, FT-NIR and FT-Raman Spectroscopy. Food Chem. 2005, 93, 25–32.. [CrossRef]
- Shenk, J.S.; Westerhaus, M.O. Analysis of Agriculture and Food Products by Near Infrared Reflectance Spectroscopy. NIRSystems Inc.: Silver Spring, MD, USA, 1995..
- Rohman, A.; Ghazali, M.A.B.; Windarsih, A.; Irnawati, I.; Riyanto, S.; Yusof, F.M.; Mustafa, S. Comprehensive Review on Application of FTIR Spectroscopy Coupled with Chemometrics for Authentication Analysis of Fats and Oils in Food Products. Molecules 2020, 25, 5485. [CrossRef]
- Guaratini, T.; Gianeti, M.D.; Campos, P.M.B.G.M. Stability of cosmetic formulations containing esters of Vitamins E and A: Chemical and physical aspects. Int. J. Pharm. 2006, 327, 12–16. [CrossRef]
- Badruddoza, A.Z.M.; Yeoh, T.; Shah, J.C.; Walsh, T. Assessing and Predicting Physical Stability of Emulsion-Based Topical Semisolid Products: A Review. J. Pharm. Sci. 2023, 112, 1772–1793.. [CrossRef]
- Ly, B.C.K.; Dyer, E.B.; Feig, J.L.; Chien, A.L.; Del Bino, S. Research Techniques Made Simple: Cutaneous Colorimetry: A Reliable Technique for Objective Skin Color Measurement. J. Investig. Dermatol. 2020, 140, 3–12.e1. [CrossRef]
- Brudzyńska, P.; Kurzawa, M.; Sionkowska, A.; Grisel, M. Antioxidant Activity of Plant-Derived Colorants for Potential Cosmetic Application. Cosmetics 2022, 9, 81. [CrossRef]
- Zhou, S.; Zhou, J.; Du, Y.; Cheng, J.; Wang, Y.; et al. Texture and Volatile Profiles of Beef Tallow Substitute Produced by a Pilot-Scale Continuous Enzymatic Interesterification. Food Chem. 2023, 429, 136980. [CrossRef]
- Kowalska, M.; Woźniak, M.; Zbikowska, A.; Ivanišová, E.; Molik, A. Quality of Emulsions Containing Fat Blends Modified by Enzymatic Catalysis. Catalysts 2021, 11, 453. [CrossRef]
- Starčević, M.; Glamočlija, N.; Baltić, B.; Glišić, M.; Laudanović, M.; Krstić, M.; Bošković Cabrol, M. Nutritional Value of Wild-Harvested Game Meat of Fallow Deer (Dama dama), Red Deer (Cervus elaphus), and Roe Deer (Capreolus capreolus). Acta Vet. 2025, 75, 63–81. [CrossRef]
- Lorenzo, J.M.; Maggiolino, A.; Gallego, L.; et al. Effect of age on nutritional properties of Iberian wild red deer meat. J. Sci. Food Agric. 2019, 99, 1561–1567. [CrossRef]
- Teng, D.; Le, R.; Yuan, F.; Yang, J.; He, L.; Gao, Y. Optimization of enzymatic hydrolysis of chicken fat in emulsion by response surface methodology. J. Am. Oil Chem. Soc. 2009, 86, 485–494. [CrossRef]
- Alahmad, K.; Noman, A.; Xia, W.; Jiang, Q.; Xu, Y. Influence of the Enzymatic Hydrolysis Using Flavourzyme Enzyme on Functional, Secondary Structure, and Antioxidant Characteristics of Protein Hydrolysates Produced from Bighead Carp (Hypophthalmichthys nobilis). Molecules 2023, 28, 519. [CrossRef]
- Kapral-Piotrowska, J.; Strawa, J. W.; Jakimiuk, K.; Wiater, A.; Tomczyk, M.; Gruszecki, W. I.; Pawlikowska-Pawlęga, B. Investigation of the membrane localization and interaction of selected flavonoids by NMR and FTIR spectroscopy. Int. J. Mol. Sci., 2023, 24(20). [CrossRef]
- Sato, E. T.; Machado, N.; Araújo, D. R.; Paulino, L. C.; Martinho, H. Fourier transform infrared absorption (FTIR) on dry stratum corneum, corneocyte-lipid interfaces: experimental and vibrational spectroscopy calculations. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 249, 119218. [CrossRef]
- Monnier, G.; Frahm, E.; Luo, B.; Missal, K. Developing FTIR Microspectroscopy for the analysis of animal-tissue residues on stone tools. J. Archaeol. Method Th. 2018, 25, 1-44. [CrossRef]
- Novotná, T.; Mokrejš, P.; Pavlačková, J.; Gál, R. Study of Processing Conditions during Enzymatic Hydrolysis of Deer By-Product Tallow for Targeted Changes at the Molecular Level and Properties of Modified Fats. Int. J. Mol. Sci. 2024, 25, 4002. [CrossRef]
- Salimon, J.; Abdullah, B.M.; Salih, N. Hydrolysis optimization and characterization study of preparing fatty acids from Jatropha curcas seed oil. Chem. Cent. J. 2011, 5, 67. [CrossRef]
- Meng, Z.; Geng, W.; Wang, X.; Liu, Y. Fat Crystal Migration and Aggregation and Polymorphism Evolution during the Formation of Granular Crystals in Beef Tallow and Palm Oil. J. Agric. Food Chem. 2013, 61, 12676–12682. [CrossRef]
- Pruchnik, H.; Włoch, A.; Gładkowski, W.; Grudniewska, A.; Chojnacka, A.; Krzemiński, M.; Rudzińska, M. Effect of Distigmasterol-Modified Acylglycerols on the Fluidity and Phase Transition of Lipid Model Membranes. Membranes 2022, 12, 11054. [CrossRef]
- Nusantoro, B.P.; Xanthina, M.; Kadivar, S.; Yanty, N.A.M.; Dewettinck, K. Enzymatic Interesterification of Lauric Fat Blends Formulated by Grouping Triacylglycerol Melting Points. J. Am. Oil Chem. Soc. 2016, 93, 1051–1062. [CrossRef]
- Zou, S.; Zhou, J.; Du, Y.; Cheng, J.; Wang, Y.; Zhang, Z. Texture and Volatile Profiles of Beef Tallow Substitute Produced by a Pilot-Scale Continuous Enzymatic Interesterification. Food Chem. 2023, 429, 136980. [CrossRef]
- Paravina, R.D.; Ghinea, R.; Herrera, L.J.; et al. Color Difference Thresholds in Dentistry. J. Esthet. Restor. Dent. 2015, 27(S1). [CrossRef]
- ISO 1442:2023. Meat and Meat Products — Determination of Moisture Content — Reference Method; ISO: Geneva, Switzerland, 2023. Available online: https://www.iso.org/standard/82664.html.
- ISO 5983-1:2005. Animal Feeding Stuffs — Determination of Nitrogen Content and Calculation of Crude Protein Content — Part 1: Kjeldahl Method; ISO: Geneva, Switzerland, 2005. Available online: https://www.iso.org/standard/39145.html.
- ISO 1443:1973. Meat and Meat Products — Determination of Total Fat Content; ISO: Geneva, Switzerland, 1973.
- ISO 6884:2008. Animal and Vegetable Fats and Oils — Determination of Ash; ISO: Geneva, Switzerland, 2008. Available online: https://www.iso.org/standard/51415.html.
- ISO 660:2020. Animal and Vegetable Fats and Oils — Determination of Acid Value and Acidity; ISO: Geneva, Switzerland, 2020. Available online: https://www.iso.org/standard/75594.html.
- ISO 3657:2023. Animal and Vegetable Fats and Oils — Determination of Saponification Value; ISO: Geneva, Switzerland, 2023. Available online: https://www.iso.org/standard/85171.html.
- ISO 3960:2017. Animal and Vegetable Fats and Oils — Determination of Peroxide Value — Iodometric (Visual) Endpoint Determination; ISO: Geneva, Switzerland, 2017. Available online: https://www.iso.org/standard/71268.html.
- ISO 3961:2018. Animal and Vegetable Fats and Oils — Determination of Iodine Value; ISO: Geneva, Switzerland, 2018. Available online: https://www.iso.org/standard/71868.html.
- Grömping, U. R Package DoE.base for Factorial Experiments. J. Stat. Softw. 2018, 85(5). [CrossRef]
- Antony, J. Design of Experiments for Engineers and Scientists, 2nd ed.; Elsevier: London, UK, 2014; pp. 33–85.
- Ma, X.; Zhan, P.; Tian, H.; Wei, Z.; Wang, P. Effects of Different Enzymatic Hydrolyses of Mutton Tallow on the Aroma Characteristics of the Maillard Reaction of Xylose–Cysteine Based on GC-MS, E-Nose, and Statistical Analysis. Eur. J. Lipid Sci. Technol. 2020, 122, 1900212. [CrossRef]
- Ye, Y.; Ye, S.; Wanyan, Z.; Ping, H.; Xu, Z.; He, S.; Cao, X.; Chen, X.; Hu, W.; Wei, Z. Producing Beef Flavors in Hydrolyzed Soybean Meal-Based Maillard Reaction Products Participated with Beef Tallow Hydrolysates. Food Chem. 2022, 378, 132119. [CrossRef]
- ISO 12966-1:2014. Animal and Vegetable Fats and Oils — Gas Chromatography of Fatty Acid Methyl Esters — Part 1: Guidelines on Modern Gas Chromatography of Fatty Acid Methyl Esters; ISO: Geneva, Switzerland, 2014. Available online: https://www.iso.org/standard/52294.html.
- ISO 12966-2:2017. Animal and Vegetable Fats and Oils — Gas Chromatography of Fatty Acid Methyl Esters — Part 2: Preparation of Methyl Esters of Fatty Acids; ISO: Geneva, Switzerland, 2017. Available online: https://www.iso.org/standard/72142.html.
- ISO 12966-3:2016. Animal and Vegetable Fats and Oils — Gas Chromatography of Fatty Acid Methyl Esters — Part 3: Preparation of Methyl Esters Using Sodium Methoxide; ISO: Geneva, Switzerland, 2016. Available online: https://www.iso.org/standard/70249.html.
- ISO 12966-4:2015. Animal and Vegetable Fats and Oils — Gas Chromatography of Fatty Acid Methyl Esters — Part 4: Determination by Capillary Gas Chromatography; ISO: Geneva, Switzerland, 2015. Available online: https://www.iso.org/standard/63503.html.
- AOCS. Official Methods and Recommended Practices of the American Oil Chemists’ Society; AOCS: Champaign, IL, 1998.
- Gao, Y.; Mao, J.; Meng, Z. Tracing Distribution and Interface Behavior of Water Droplets in W/O Emulsions with Fat Crystals. Food Res. Int. 2023, 163, 112215. [CrossRef]
- ISO 6321:2021. Animal and Vegetable Fats and Oils — Determination of Melting Point in Open Capillary Tubes (Slip Point); ISO: Geneva, Switzerland, 2021. Available online: https://www.iso.org/standard/81472.html.
- Glibowski, P.; Zarzycki, P.; Krzepkowska, M. The Rheological and Instrumental Textural Properties of Selected Table Fats. Int. J. Food Prop. 2008, 11, 678–686. [CrossRef]
- Chudy, S.; Bilska, A.; Kowalski, R.; Teichert, J. Color of milk and milk products in CIE L*a*b* space. Med. Weter. 2020, 76, 6327-2020. [CrossRef]
| Fatty acid | Amount [%] | Fatty acid | Amount [%] | Fatty acid | Amount [%] |
|---|---|---|---|---|---|
| C10:0 | 0.245 ± 0.036 | C15:1 | 6.362 ± 0.001 | C18: 2(n-6) | 21.133 ± 0.057 |
| C12:0 | 1.305 ± 0.039 | C16:0 | 35.551 ± 0.042 | C18:3(n-3) | 0.416 ± 0.041 |
| C14:0 | 7.122 ± 0.011 | C16:1 | 0.349 ± 0.040 | ||
| Unknown | 0.410 ± 0.008 | C17:0 | 0.993 ± 0.003 | Σ SFA | 66.64 |
| C14:1 | 0.956 ± 0.002 | C18:0 | 20.898 ± 0.045 | Σ MUFA | 11.05 |
| C15:0 | 0.528 ± 0.002 | C18:1 | 3.734 ± 0.024 | Σ PUFA | 21.55 |
| Exp. No. | Factor A [%] |
Factor B [h] |
Acid value [mg/g] |
Degree of hydrolysis [%] |
|---|---|---|---|---|
| 1 | 10 | 2 | 84.29 ± 1.25 | 35.79 |
| 2 | 10 | 4 | 71.66 ± 2.41 | 30.43 |
| 3 | 10 | 6 | 81.98 ± 0.77 | 34.82 |
| 4 | 20 | 2 | 93.85 ± 0.95 | 39.85 |
| 5 | 20 | 4 | 109.23 ± 1.08 | 46.39 |
| 6 | 20 | 6 | 79.76 ± 0.22 | 33.87 |
| 7 | 30 | 2 | 79.74 ± 0.88 | 33.86 |
| 8 | 30 | 4 | 97.68 ± 0.14 | 41.48 |
| 9 | 30 | 6 | 115.54 ± 1.38 | 49.07 |
| 10* | 20 | 4 | 3.41 ± 0.34 | 1.45 |
| Pure red deer fat | ̶ | ̶ | 2.04 ± 0.09 | ̶ |
| Degree of Freedom | Sum of Squares | Mean Squares | p-Value | |
|---|---|---|---|---|
| Regression equation | DH = 27.85 + 0.389 A + 0.69 B | |||
| Regression | 2 | 102.40 | 51.20 | 0.305 |
| Factor A: Amount of water [%] | 1 | 91.03 | 91.03 | 0.159 |
| Factor B: Time [h] | 1 | 11.37 | 11.37 | 0.590 |
| Error | 6 | 211.16 | 35.19 | |
| Total | 8 | 313.56 | ||
| Reference value [cm−1] | 700–900 | 1000–1200 | 1700–1750 | 2830–2850 | 2900–2950 |
|---|---|---|---|---|---|
|
Molecular action |
=C–H (cis) | C–O stretch | C=O stretch | C–H methylene stretch symmetric | C–H methylene stretch asymmetric |
| Wavenumber [cm-1] | 721 | 1173 | 1739 | 2850 | 2916 |
| Pure red deer fat | 0.088 | 0.234 | 0.245 | 0.224 | 0.289 |
| 1 | 0.075 | 0.110 | 0.104 | 0.154 | 0.192 |
| 2 | 0.079 | 0.119 | 0.117 | 0.162 | 0.203 |
| 3 | 0.073 | 0.108 | 0.108 | 0.159 | 0.199 |
| 4 | 0.076 | 0.102 | 0.098 | 0.158 | 0.198 |
| 5 | 0.071 | 0.094 | 0.091 | 0.153 | 0.191 |
| 6 | 0.078 | 0.114 | 0.115 | 0.163 | 0.207 |
| 7 | 0.101 | 0.122 | 0.118 | 0.183 | 0.230 |
| 8 | 0.071 | 0.104 | 0.099 | 0.175 | 0.225 |
| 9 | 0.056 | 0.080 | 0.086 | 0.149 | 0.192 |
| 10 * | 0.090 | 0.232 | 0.243 | 0.221 | 0.286 |
| Exp. No. | Melting point Capillary [°C] |
DSC – Melting [°C] |
Melting Enthalpy [mJ] |
DSC – Crystallization [°C] | Crystallization Enthalpy [mJ] |
|---|---|---|---|---|---|
| 1 | 36.98 ± 0.31 | 37.01 ± 0.07 | −200.9 ± 1.8 | 33.36 ± 0.26 | 52.7 ± 1.4 |
| 2 | 35.10 ± 0.28 | 36.53 ± 0.17 | −174.9 ± 1.0 | 34.29 ± 0.11 | 58.0 ± 1.5 |
| 3 | 36.03 ± 0.19 | 36.42 ± 0.07 | −172.7 ± 1.8 | 32.82 ± 0.01 | 60.9 ± 0.8 |
| 4 | 37.23 ± 0.08 | 36.75 ± 0.07 | −175.7 ± 0.5 | 33.11 ± 0.23 | 44.8 ± 0.4 |
| 5 | 36.48 ± 0.19 | 35.54 ± 0.02 | −213.8 ± 1.5 | 31.64 ± 0.08 | 53.7 ± 0.3 |
| 6 | 34.45 ± 0.09 | 34.53 ± 0.11 | −177.9 ± 0.9 | 30.77 ± 0.02 | 73.0 ± 0.3 |
| 7 | 36.90 ± 0.16 | 35.04 ± 0.14 | −192.0 ± 0.1 | 30.53 ± 0.02 | 49.0 ± 0.5 |
| 8 | 38.00 ± 0.25 | 39.77 ± 0.09 | −248.1 ± 0.7 | 32.01 ± 0.06 | 54.9 ± 0.2 |
| 9 | 37.13 ± 0.22 | 36.31 ± 0.08 | −246.8 ± 0.3 | 32.22 ± 0.03 | 30.4 ± 0.3 |
| 10* | 44.63 ± 0.18 | 45.07 ± 0.10 | −290.6 ± 1.4 | 29.87 ± 0.02 | 89.7 ± 0.8 |
| Pure red deer tallow | 44.68 ± 0.33 | 44.52 ± 0.11 | −288.1 ± 0.7 | 28.84 ± 0.24 | 84.2 ± 0.5 |
| Degree of Freedom | Sum of Squares | Mean Squares | p-Value | |
|---|---|---|---|---|
| Regression equation | Crystallization = 35.29 – 0.0900 A – 0.250 B | |||
| Regression | 2 | 6.360 | 3.1800 | 0.096 |
| Factor A: Amount of water [%] | 1 | 4.860 | 4.8600 | 0.059 |
| Factor B: Time [h] | 1 | 1.500 | 1.5000 | 0.243 |
| Error | 6 | 5.369 | 0.8948 | |
| Total | 8 | 11.729 | ||
| Regression equation | Melting point = 36.77 + 0.0083 A – 0.117 B | |||
| Regression | 2 | 0.3683 | 0.18417 | 0.945 |
| Factor A: Amount of water [%] | 1 | 0.0417 | 0.04167 | 0.913 |
| Factor B: Time [h] | 1 | 0.3267 | 0.32667 | 0.761 |
| Error | 6 | 19.3117 | 3.21861 | |
| Total | 8 | 19.6800 | ||
| Exp. No. | Hardness [N] |
Spreadability [N·m] |
Stickiness [N] |
Adhesiveness [N·m] |
|---|---|---|---|---|
| 1 | 35.350 ± 2.159 | 27.111 ± 4.575 | 0.585 ± 0.195 | 0.014 ± 0.008 |
| 2 | 38.149 ± 2.336 | 30.903 ± 1.826 | 0.804 ± 0.237 | 0.020 ± 0.007 |
| 3 | 43.120 ± 3.611 | 33.900 ± 1.190 | 0.860 ± 0.228 | 0.024 ± 0.006 |
| 4 | 52.847 ± 3.394 | 40.967 ± 3.525 | 0.935 ± 0.074 | 0.044 ± 0.020 |
| 5 | 33.376 ± 5.446 | 25.931 ± 5.992 | 1.781 ± 0.674 | 0.101 ± 0.093 |
| 6 | 28.186 ± 5.401 | 20.740 ± 3.046 | 0.921 ± 0.479 | 0.022 ± 0.008 |
| 7 | 37.845 ± 3.215 | 30.046 ± 1.558 | 1.025 ± 0.506 | 0.027 ± 0.009 |
| 8 | 32.564 ± 1.238 | 25.191 ± 0.702 | 2.684 ± 1.097 | 0.249 ± 0.222 |
| 9 | 25.829 ± 1.284 | 18.129 ± 0.637 | 4.642 ± 0.986 | 0.154 ± 0.037 |
| 10* | 23.691 ± 2.070 | 18.452 ± 2.376 | 1.510 ± 0.291 | 0.052 ± 0.039 |
| Pure red deer fat | 21.384 ± 2.886 | 16.304 ± 2.353 | 1.604 ± 0.470 | 0.074 ± 0.062 |
| Exp. No. | L* | a* | b* | ∆E* |
|---|---|---|---|---|
| 1 | 90.13 ± 0.29 | –3.91 ± 0.03 | 13.02 ± 0.12 | 2.90 |
| 2 | 87.17 ± 0.93 | –2.65 ± 0.05 | 13.94 ± 0.35 | 5.55 |
| 3 | 88.80 ± 0.15 | –3.58 ± 0.02 | 9.11 ± 0.10 | 2.85 |
| 4 | 88.43 ± 0.17 | –3.51 ± 0.06 | 12.52 ± 0.19 | 3.66 |
| 5 | 89.07 ± 0.60 | –3.76 ± 0.07 | 10.67 ± 0.34 | 2.00 |
| 6 | 86.47 ± 0.50 | –2.82 ± 0.10 | 14.20 ± 0.60 | 5.40 |
| 7 | 85.52 ± 0.27 | –0.88 ± 0.10 | 15.42 ± 0.24 | 7.61 |
| 8 | 89.07 ± 0.67 | –4.16 ± 0.07 | 13.41 ± 0.69 | 3.28 |
| 9 | 89.98 ± 0.94 | –3.89 ± 0.20 | 12.33 ± 0.63 | 2.21 |
| 10* | 89.17 ± 0.97 | –3.19 ± 0.14 | 7.33 ± 0.17 | 3.43 |
| Pure red deer fat | 91.56 ± 0.24 | –3.70 ± 0.08 | 11.38 ± 0.16 | ̶ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





