Submitted:
26 June 2025
Posted:
01 July 2025
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
- is suitable for multimodal data searches in heterogeneous data sets, e.g. numbers, text, images, sound, and categorical data,
- uses a novel mathematical similarity measure superseding a single (e.g. Euclidean, Hamming) distance used in the existing methods. In this way, inaccurate measurement of similarity on a large number of heterogeneous variables is avoided,
- search is fast even on large data sets, with millions of records and thousands of variables,
- includes advanced personalised searches with multiple parameters and other features,
- facilitates multiple solutions with corresponding probabilities,
- is suitable for unsupervised clustering in multimodal heterogeneous data,
- is suitable for personalised model creation to classify or predict specific outcomes based on multimodal and heterogeneous data.
2. Mathematical Description
2.1. Database
- objects (samples) ,
- each object () is defined by criteria (variables) with values in linearly ordered domains with and ; if some value (, ) is either missing or uncertain, then its value is recorded as ∞,
- weights in with , where each () quantifies the importance of the criterion ; if for all , then all criteria are equally important; a criterion is ignore if .
| Objects/Criteria | ... | ... | ||||
| ... | ... | |||||
| ⋮ | ⋮ | ⋮ | ... | ⋮ | ... | ⋮ |
| ... | ... | |||||
| ⋮ | ⋮ | ⋮ | ... | ⋮ | ... | ⋮ |
| ... | ... | |||||
| w | ... | ... |
2.2. Distance Metrics
- Logical Boolean domain: , where .
- Logical non-Boolean domain: , where and .
- Numerical domain with natural values: , where .
- Numerical domain with rational values: , where .
- Binary code: , where the domain consists of all binary strings of length n, and for all , , ,
2.3. Tasks Specification
- Task
- 1: Calculate the distance (or similarity metric) between the new object and each object in Table 2. If the distance corresponding to is , then
- Task
- 2: Given a threshold , calculate all objects at a distance at most to x.
- Task
- 3: Calculate the probability of a new object to belong to a labelled class (e.g. low risk vs. high risk) using a threshold and Table 2.
- Task
- 4: Rank the criteria in Table 2 and calculate the marker or markers criterion/criteria, that is the most important one/ones.
- Task
- 5: Assign alternative weights to criteria.
- Task
- 6: Test the accuracy of data and method for Task 4.
2.4. Tasks Solutions
| Object/Criteria | ... | ... | Class label | ||||
| ... | ... |
- Compute the distances between each object in Table 2 and , so obtain a vector with n non-negative real components .
- For each , compute the distances taking into consideration all criteria in Table 2except: obtain the vector .
- Compute the distances between , using the formulaand sort them in increasing order. The criterion is a marker if , for every .
2.5. An Example
- : real number , e.g. age, weight, BMI etc.;
- : Boolean value , e.g. gender;
- : integer number , e.g. gene expression;
- : categorical {small, med, large }, e.g. size of tumour, body size, keywords;
- : colour {red, yellow, white, black}, e.g. colour of a spot on the body, on the heart;
- : spike sequence of e.g. encoded EEG, ECG;
- : black and white image, e.g. MRI, face image.
| 0.197 | 1 | 0.3889 | 1 | 0 | 0.4 | 0.11111111 | 3.09701111 | |
| 0.445 | 0 | 0.52321 | 0.5 | 0.33333333 | 0.6 | 0.22222222 | 2.62376556 | |
| 0.04 | 0 | 0.40079 | 0 | 0 | 0.5 | 0.22222222 | 1.16301222 | |
| 0.083 | 1 | 0.4559 | 1 | 0.66666667 | 0.45 | 0.11111111 | 3.76667778 | |
| 0.222 | 1 | 0.36223 | 0 | 0.33333333 | 0.45 | 0.22222222 | 2.58978556 | |
| 0.33 | 0 | 0.33276 | 0.5 | 0 | 0.45 | 0.11111111 | 1.72387111 | |
| 0.082 | 0 | 0.23221 | 1 | 0.33333333 | 0.45 | 0.22222222 | 2.31976556 | |
| 0.285 | 1 | 0.37846 | 0 | 0.33333333 | 0.45 | 0.22222222 | 2.66901556 | |
| 0.285 | 1 | 0.37846 | 1 | 0.33333333 | 0.45 | 0.22222222 | 3.66901556 |
| 0.04 | 0 | 0.40079 | 0 | 0 | 0.5 | 0.22222222 | 1.16301222 | |
| 0.33 | 0 | 0.33276 | 0.5 | 0 | 0.45 | 0.11111111 | 1.72387111 | |
| 0.082 | 0 | 0.23221 | 1 | 0.33333333 | 0.45 | 0.22222222 | 2.31976556 | |
| 0.222 | 1 | 0.36223 | 0 | 0.33333333 | 0.45 | 0.22222222 | 2.58978556 | |
| 0.445 | 0 | 0.52321 | 0.5 | 0.33333333 | 0.6 | 0.22222222 | 2.62376556 | |
| 0.285 | 1 | 0.37846 | 0 | 0.33333333 | 0.45 | 0.22222222 | 2.66901556 | |
| 0.197 | 1 | 0.3889 | 1 | 0 | 0.4 | 0.11111111 | 3.09701111 | |
| 0.285 | 1 | 0.37846 | 1 | 0.33333333 | 0.45 | 0.22222222 | 3.66901556 | |
| 0.083 | 1 | 0.4559 | 1 | 0.66666667 | 0.45 | 0.11111111 | 3.76667778 |
| 0.2 | 0 | 0.00089 | 0 | 1 | 0.1222210012 | 0.100001001 |
| 1.469 | 0.987 | 1.469 | 1.402 | 1.469 | 0.669 | 1.359 | 1.459 |
| 3.709 | 2.979 | 2.709 | 2.730 | 3.209 | 3.309 | 3.609 | 3.709 |
| 3.220 | 2.975 | 2.220 | 3.165 | 2.220 | 2.420 | 3.110 | 3.210 |
| 0.378 | 0.010 | 0.378 | 0.378 | 0.378 | 0.378 | 0.378 | 0.368 |
| 2.167 | 2.104 | 2.167 | 2.073 | 1.167 | 1.167 | 2.167 | 2.157 |
| 3.824 | 3.209 | 2.824 | 3.035 | 3.324 | 3.024 | 3.714 | 3.814 |
| 3.066 | 2.699 | 2.066 | 2.378 | 3.066 | 2.066 | 3.066 | 3.055 |
| 1.487 | 1.487 | 1.487 | 1.410 | 0.487 | 1.087 | 1.477 | 1.487 |
| 1.487 | 1.487 | 1.487 | 1.410 | 0.487 | 1.087 | 1.477 | 1.487 |
| Distances | 2.870 | 4.00 | 2.826 | 5.00 | 5.60 | 0.450 | 0.061 |
| Weights | 0.137 | 0.192 | 0.135 | 0.240 | 0.269 | 0.021 | 0.002 |
3. Survival Analysis in SAIN
3.1. Data and Tasks
- Table 17 in which the first column lists the patients treated for the same disease with the same method under strict conditions and the last column records the times till the patient’s deaths.
- Table 18, which includes the record of the new patient p.
- A threshold which defines the acceptable similarity between p and the relevant ’s in the Survival database (i.e. ).
3.2. Tasks Solutions
-
For Task 1,
- (a)
- Compute the set of patients that are similar up to to p:
- (b)
- Using , compute the probability that p will survive the time :
- (c)
- Compute the life expectancy of p using the formula:
- For Task 2, calculate the probability that the life expectancy of p is at least time T:
3.3. An Example
| patients | units of time | |||||||
| 0.682 | 0 | 0.06789 | 0 | 0.2 | 0.012211001 | 0.110001001 | 12.3 | |
| 0.93 | 1 | 0.98 | 0.5 | 0.6 | 0.022220012 | 0.100001001 | 15 | |
| 0.445 | 1 | 0.056 | 1 | 0.2 | 0.012121001 | 0.110101111 | 68 | |
| 0.568 | 0 | 0.00089 | 0 | 1 | 0.122221001 | 0.110011101 | 1.4 | |
| 0.263 | 0 | 0.09456 | 1 | 0 | 0.122201001 | 0.110111101 | 40.5 | |
| 0.815 | 1 | 0.78955 | 0.5 | 0.2 | 0.012122001 | 0.110001111 | 97.2 | |
| 0.567 | 1 | 0.689 | 0 | 0 | 0.122121001 | 0.111001111 | 97.2 | |
| 0.2 | 0 | 0.07833 | 1 | 0.6 | 0.112211022 | 0.100001111 | 55.7 | |
| 0.2 | 0 | 0.07833 | ∞ | 0.6 | 0.112211022 | 0.100001111 | 63.7 |
| 0.485 | 1 | 0.45679 | 1 | 0.2 | 0.1002121001 | 0.110001101 |
-
For , , that is the entire database. Then
- (a)
- ,
- (b)
- ,
- ,
- ,
- ,
- ,
- ,
- ,
- .
- (c)
- ,
- ,
- ,
- ,
- ,
- ,
- ,
- ,
We can calculate other probabilities, for example, . -
For , . Then
- (a)
- ,
- (b)
- ,
- ,
- ,
- ,
- ,
- (c)
- ,
- ,
- ,
- ,
- .
Similarly, we can calculate the probabilities , .
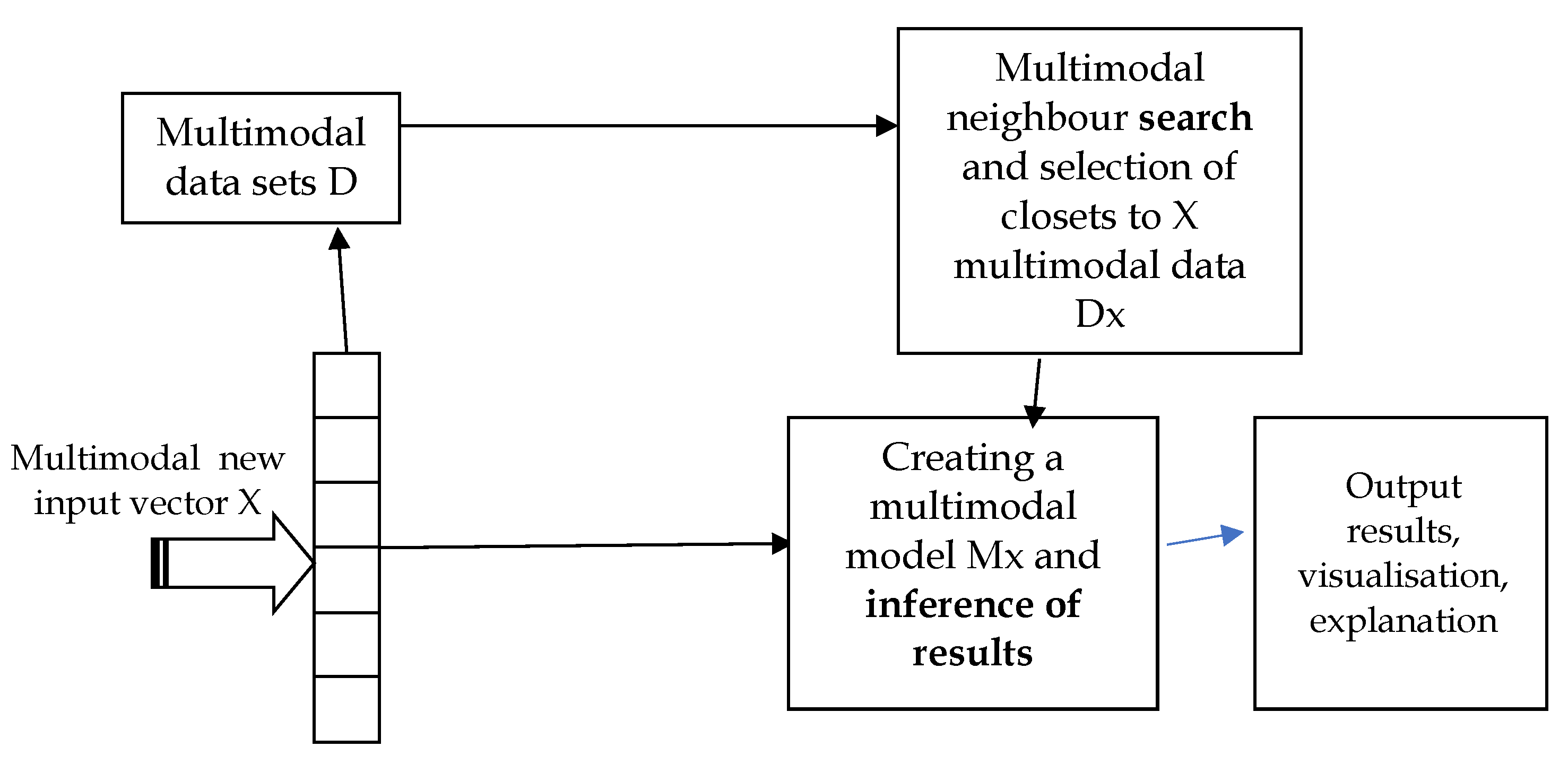
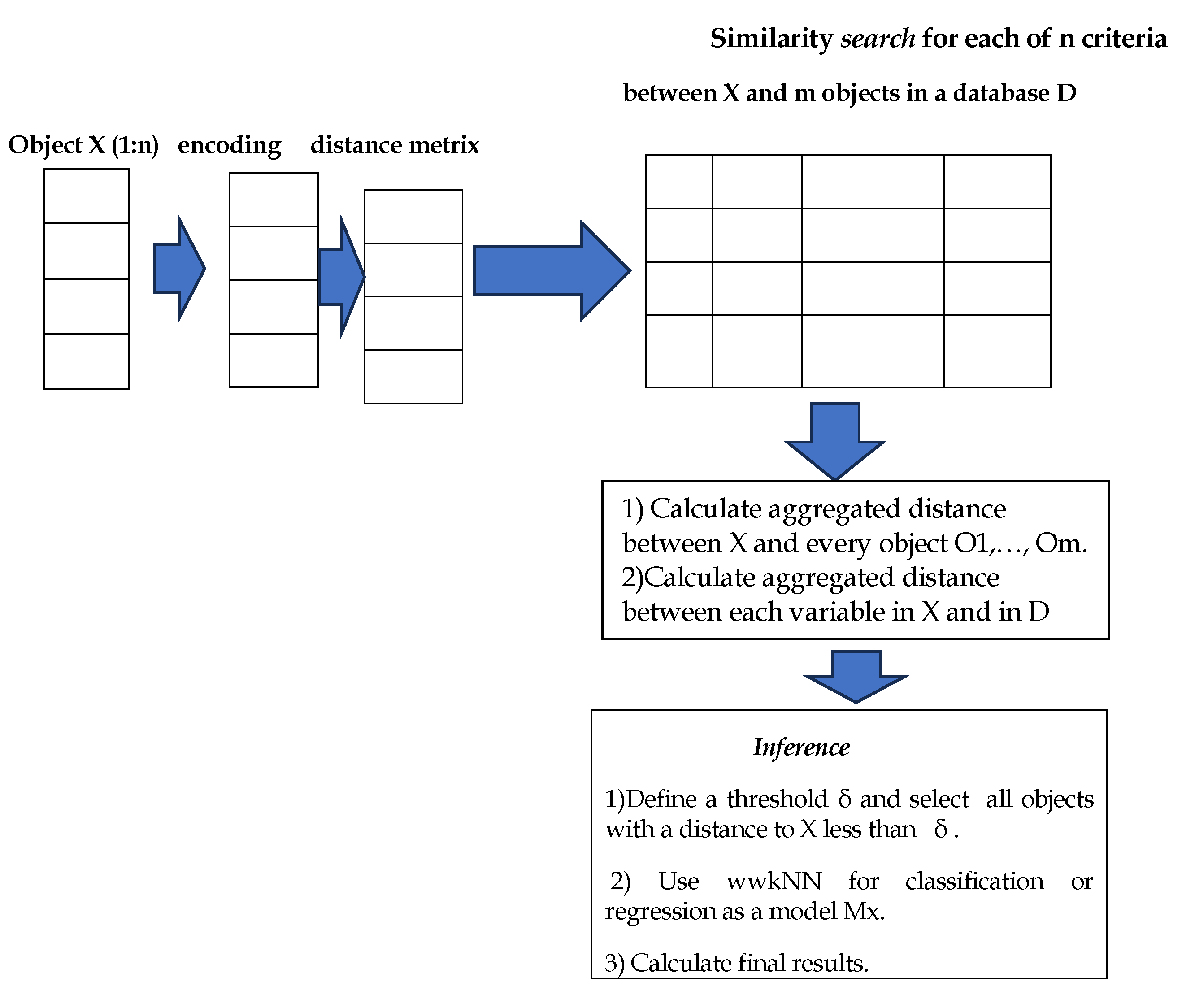
4. SAIN: A Modular Diagram and Functional Information Flow
5. Case Studies for Medical Diagnosis and Prognosis
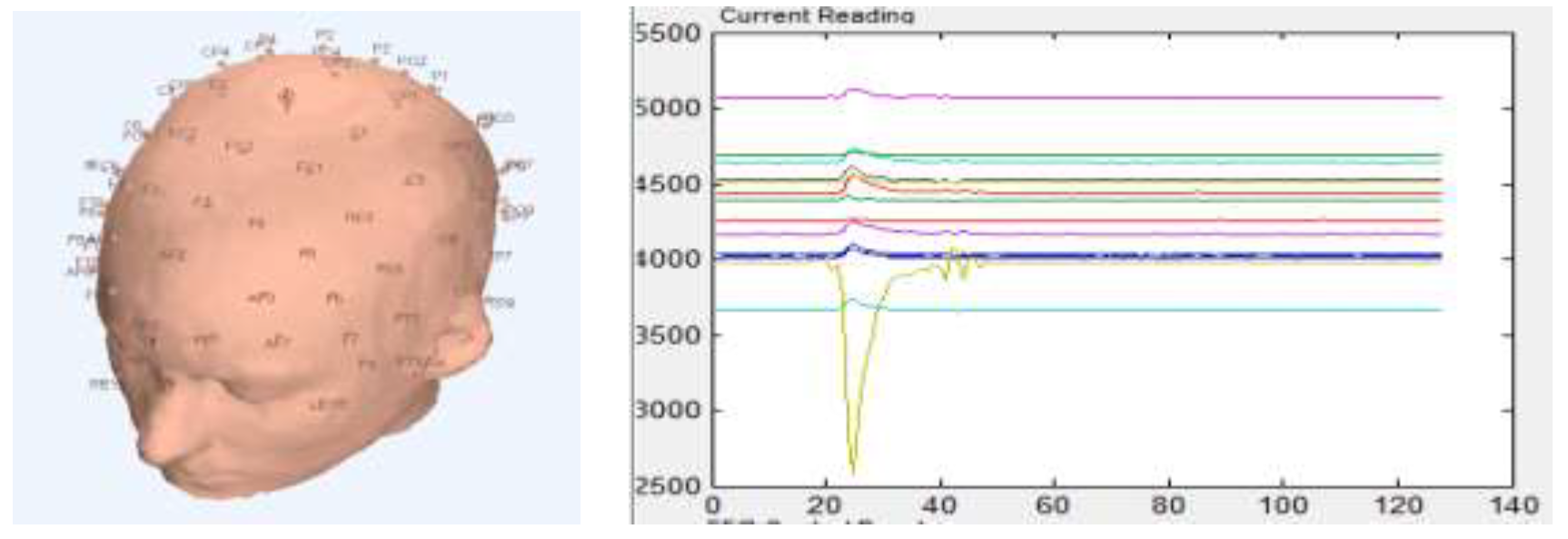
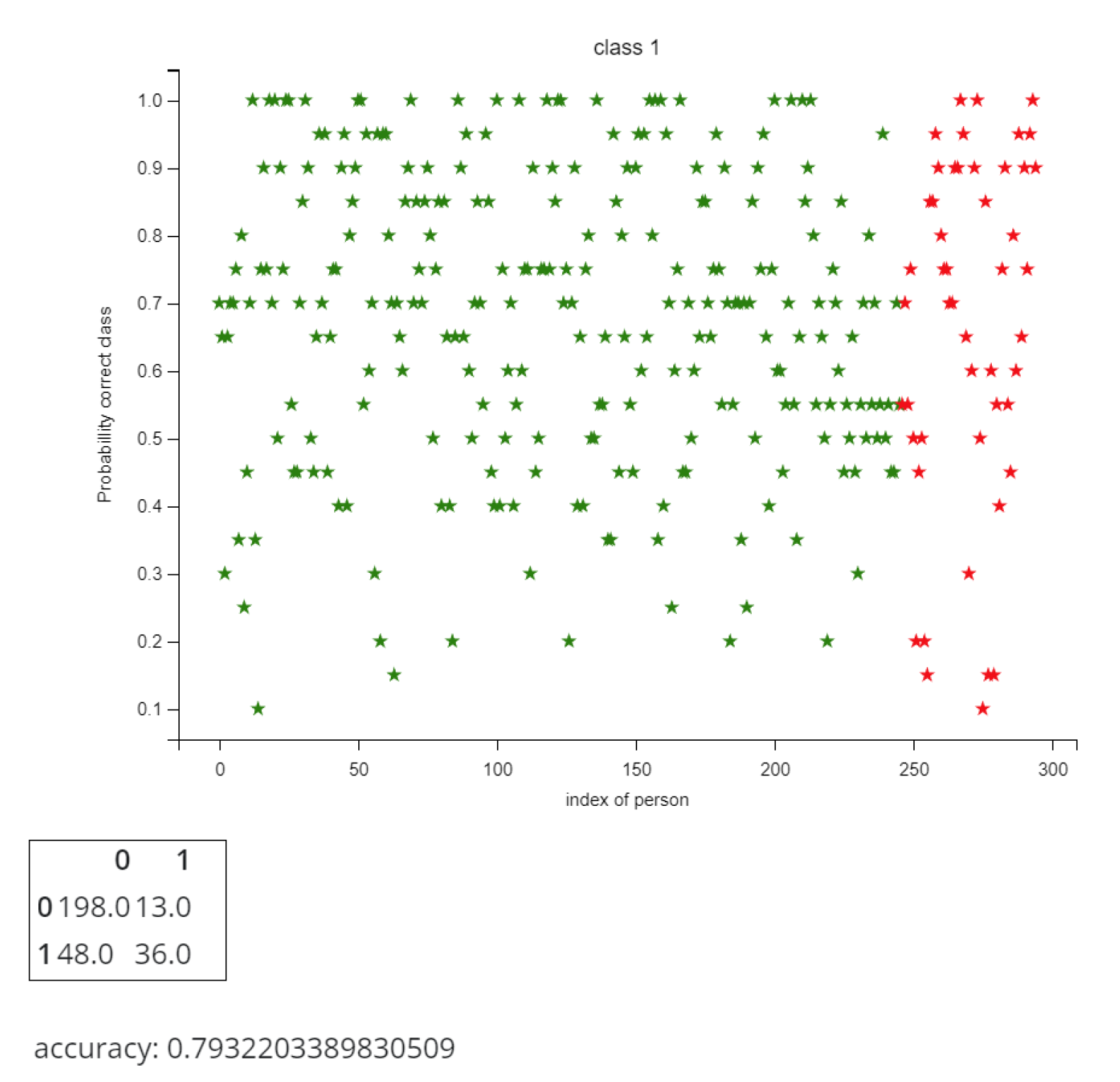
5.1. Heart Disease Diagnosis
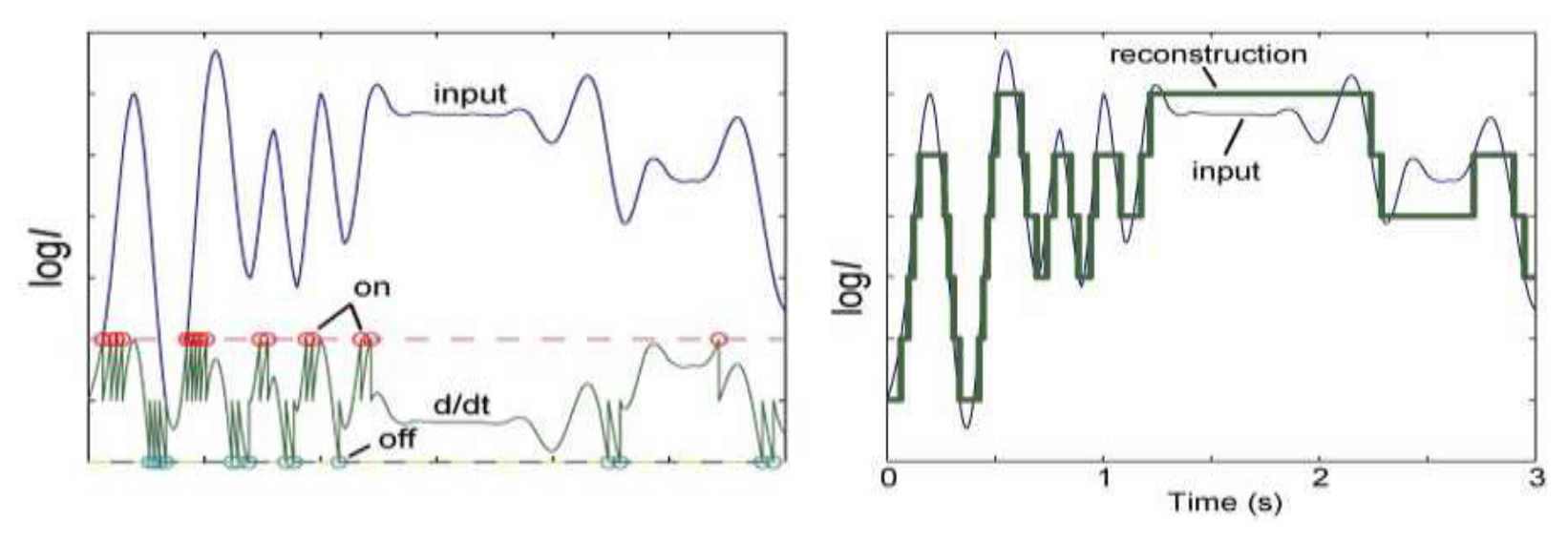
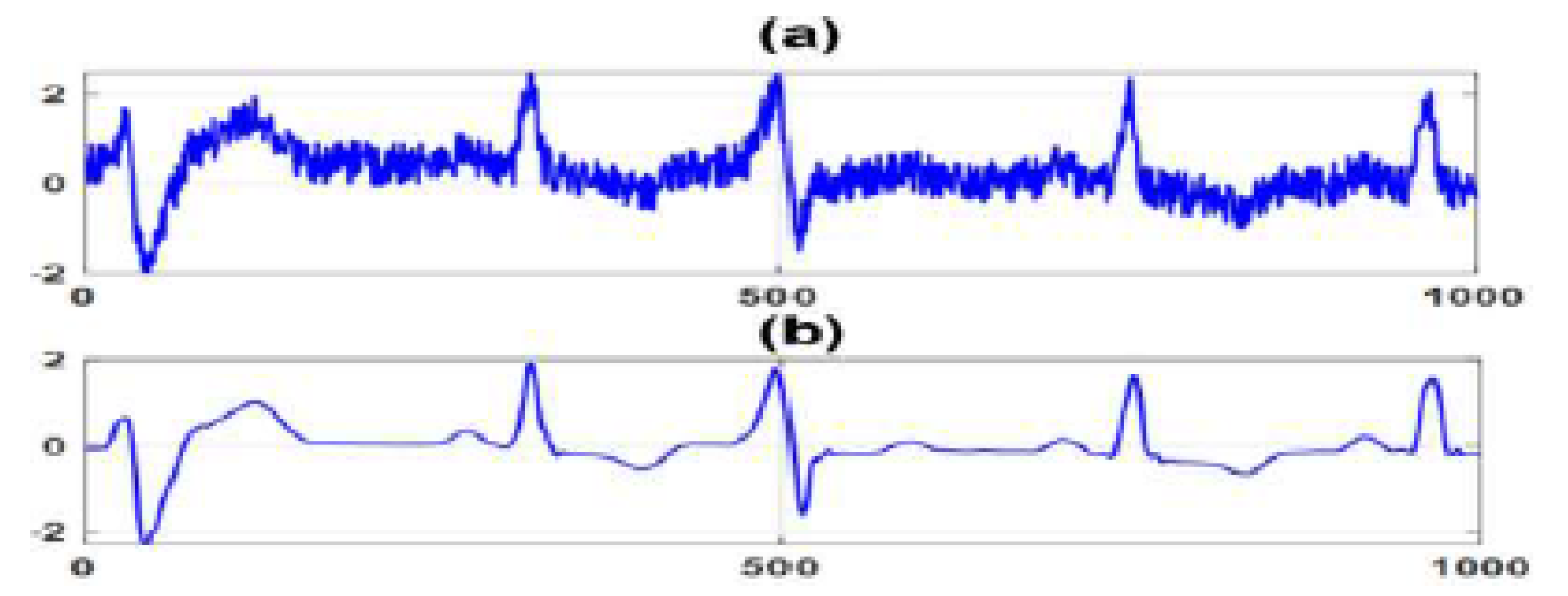
5.2. Time Series Classification
| Record | Channel 1 | Channel 2 | Channel 3 | Label |
| R1 | (1, 1, -1, 0, 1) | (0, 1, 1, 1, -1) | (1, 1, -1, -1, 0) | 1 |
| R2 | (1, 0, -1, 0, 1) | ( 0, 1, 1, 1, -1) | (1, 0, -1, -1, 1 ) | 1 |
| R3 | (1, 1, -1, 0, 1) | (0, -1, 1, 1, -1) | (1, 1, -1, 0, 1) | 2 |
| R4 | (1, 1, -1, 0, 1) | (0, -1, 1, 0, -1) | (1, 1, -1, 0, 1) | 2 |
| R5 | (1, 1, -1, 0, 0) | (0, -1, 0, 1, -1) | (1, 1, -1, 1, 1) | 3 |
| R6 | (1, -1, -1, 0, 1) | (0, -1, 1, 0, -1) | (1, 1, -1, 0, 1) | 3 |
5.3. Predicting Longevity in Cardiac Patients
- demographics, risk factors, disease states, medication and deprivation scores,
- echocardiography, cardiac ultrasound measurements,
- advanced ECG measurements,
6. Data and Software Availability
7. Conclusions
Acknowledgement
References
- AbouHassan, I.; Kasabov, N.K.; Jagtap, V.; Kulkarni, P. Spiking neural networks for predictive and explainable modelling of multimodal streaming data with a case study on financial time series and online news. Sci. Rep. 2023, 13, 18367. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.; Markou, I.; Pereira, F.C. Combining time-series and textual data for taxi demand prediction in event areas: A deep learning approach. Information Fusion 2019, 49, 120–129. [Google Scholar] [CrossRef]
- Li, J.; Liu, J.; Zhou, S.; Zhang, Q.; Kasabov, N.K. GeSeNet: A General Semantic-Guided Network With Couple Mask Ensemble for Medical Image Fusion. IEEE Transactions on Neural Networks and Learning Systems 2024, 35, 16248–16261. [Google Scholar] [CrossRef] [PubMed]
- Kasabov, N. Data Analysis and Predictive Systems and Related Methodologies, U.S. Patent 9,002,682 B2, 7 April 2015. [Google Scholar]
- Doborjeh, M.; Doborjeh, Z.; Merkin, A.; Bahrami, H.; Sumich, A.; Krishnamurthi, R.; Medvedev, O.N.; Crook-Rumsey, M.; Morgan, C.; Kirk, I.; et al. Personalised predictive modelling with brain-inspired spiking neural networks of longitudinal MRI neuroimaging data and the case study of dementia. Neural Networks 2021, 144, 522–539. [Google Scholar] [CrossRef] [PubMed]
- Budhraja, S.; Singh, B.; Doborjeh, M.; Doborjeh, Z.; Tan, S.; Lai, E.; Goh, W.; Kasabov, N. Mosaic LSM: A Liquid State Machine Approach for Multimodal Longitudinal Data Analysis. In Proceedings of the 2023 International Joint Conference on Neural Networks (IJCNN). IEEE; 2023; pp. 1–8. [Google Scholar] [CrossRef]
- Kasabov, N.K. Evolving connectionist systems, 2 ed.; Springer: London, England, 2007. [Google Scholar]
- Kasabov, N.K. Time-Space, Spiking Neural Networks and Brain-Inspired Artificial Intelligence; Springer Berlin Heidelberg, 2019. [CrossRef]
- Santomauro, D.F.e.a. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. The Lancet, 1700. [Google Scholar]
- Swaddiwudhipong, N.; Whiteside, D.J.; Hezemans, F.H.; Street, D.; Rowe, J.B.; Rittman, T. Pre-diagnostic cognitive and functional impairment in multiple sporadic neurodegenerative diseases. bioRxiv, 2022. [Google Scholar] [CrossRef]
- Calude, C.; Calude, E. A metrical method for multicriteria decision making. St. Cerc. Mat 1982, 34, 223–234. [Google Scholar]
- Calude, C.; Calude, E. On some discrete metrics. Bulletin mathématique de la Société des Sciences Mathématiques de la République Socialiste de Roumanie.
- Gleeson, S.; Liao, Y.W.; Dugo, C.; Cave, A.; Zhou, L.; Ayar, Z.; Christiansen, J.; Scott, T.; Dawson, L.; Gavin, A.; et al. ECG-derived spatial QRS-T angle is associated with ICD implantation, mortality and heart failure admissions in patients with LV systolic dysfunction. PLOS ONE 2017, 12, e0171069. [Google Scholar] [CrossRef] [PubMed]
- Kahramanli, H.; Allahverdi, N. Design of a hybrid system for the diabetes and heart diseases. Expert systems with applications 2008, 35, 82–89. [Google Scholar] [CrossRef]
- Kasabov, N.K. Time-space, Spiking Neural Networks and Brain-inspired Artificial Intelligence; Vol. 750, Springer, 2019.
| Objects/Criteria | ... | ... | Class label | ||||
| ... | ... | ||||||
| ⋮ | ⋮ | ⋮ | ... | ⋮ | ... | ⋮ | ⋮ |
| ... | ... | ||||||
| ⋮ | ⋮ | ⋮ | ... | ⋮ | ... | ⋮ | ⋮ |
| ... | ... |
| Criteria weights | ... | ... | ||||
| w | ... | ... |
| Object/Criteria | ... | ... | ||||
| x | ... | ... |
| 68.2 | 0 | 6789 | small | red | 0,1,-1,-1,1,1,0,0, 1,-1 | 1,1,0 | 1 |
| 0,0,1 | |||||||
| 0,0,1 | |||||||
| 93 | 1 | 98000 | medium | yellow | 0,-1,-1,-1,-1,0,0, 1,-1,1 | 1,0,0 | 1 |
| 0,0,1 | |||||||
| 0,0,1 | |||||||
| 44.5 | 1 | 5600 | large | red | 0,1,-1,1,-1,1,0,0, 1,-1 | 1,1,0 | 1 |
| 1,0,1 | |||||||
| 1,1,1 | |||||||
| 56.8 | 0 | 89 | small | white | 1,-1,-1,-1,-1,1,0,0, 1,-1 | 1,1,0 | 1 |
| 0,1,1 | |||||||
| 1,0,1 | |||||||
| 26.3 | 0 | 9456 | large | black | 1,-1,-1,-1,0,1,0,0, 1,-1 | 1,1,0 | 2 |
| 1,1,1 | |||||||
| 1,0,1 | |||||||
| 81.5 | 1 | 78955 | medium | red | 0, 1,-1,1,-1,-1,0,0, 1,-1 | 1,1,0 | 2 |
| 0,0,1 | |||||||
| 1,1,1 | |||||||
| 56.7 | 1 | 68900 | small | black | 1,- 1,-1,1,-1,1,0,0, 1,1 | 1,1,1 | 2 |
| 0,0,1 | |||||||
| 1,1,1 | |||||||
| 20 | 0 | 7833 | large | yellow | 1,1,-1,-1,1,1,0,-1, -1,1 | 1,0,0 | 2 |
| 0,0,1 | |||||||
| 1,1,1 | |||||||
| 20 | 0 | 7833 | ∞ | yellow | 1,1,-1,-1,1,1,0,-1, -1,1 | 1,0,0 | 2 |
| 0,0,1 | |||||||
| 1,1,1 |
| 48.5 | 1 | 45679 | large | red | 1, 0, 0, -1, 1, -1, 1, 0, 0, 1 | 1,1,0 |
| 0,0,1 | ||||||
| 1,0,1 |
| 68.2 | 0 | 6789 | 0 | FF0000 | 0122110012 | 110001001 | 1 | |
| 111111110000000000000000 | ||||||||
| 93 | 0 | 98000 | 1 | FFFF00 | 0222200121 | 110001001 | 1 | |
| 111111111111111100000000 | ||||||||
| 44.5 | 1 | 5600 | 2 | FF0000 | 0121210012 | 110101111 | 1 | |
| 111111110000000000000000 | ||||||||
| 56.8 | 0 | 89 | 0 | FFFFFF | 1222210012 | 110011101 | 1 | |
| 111111111111111111111111 | ||||||||
| 26.3 | 0 | 9456 | 2 | 000000 | 1222010012 | 110111101 | 2 | |
| 000000000000000000000000 | ||||||||
| 81.5 | 1 | 78955 | 1 | FF0000 | 0121220012 | 110001111 | 2 | |
| 111111110000000000000000 | ||||||||
| 56.7 | 1 | 68900 | 0 | 000000 | 1221210011 | 111001111 | 2 | |
| 000000000000000000000000 | ||||||||
| 20 | 0 | 7833 | 2 | FFFF00 | 1122110221 | 100001111 | 2 | |
| 111111111111111100000000 | ||||||||
| 20 | 0 | 7833 | ∞ | FFFF00 | 1122110221 | 100001111 | 2 | |
| 111111111111111100000000 |
| x | 48.5 | 1 | 45679 | 2 | FF0000 | 1002121001 | 110001101 |
| 111111110000000000000000 |
| 0.682 | 0 | 0.06789 | 0 | 0.2 | 0.0122110012 | 0.110001001 | |
| 0.93 | 1 | 0.98 | 0.5 | 0.6 | 0.0222200121 | 0.100001001 | |
| 0.445 | 1 | 0.056 | 1 | 0.2 | 0.0121210012 | 0.110101111 | |
| 0.568 | 0 | 0.00089 | 0 | 1 | 0.1222210012 | 0.110011101 | |
| 0.263 | 0 | 0.09456 | 1 | 0 | 0.1222010012 | 0.110111101 | |
| 0.815 | 1 | 0.78955 | 0.5 | 0.2 | 0.0121220012 | 0.110001111 | |
| 0.567 | 1 | 0.689 | 0 | 0 | 0.1221210011 | 0.111001111 | |
| 0.2 | 0 | 0.07833 | 1 | 0.6 | 0.1122110221 | 0.100001111 | |
| 0.2 | 0 | 0.07833 | ∞ | 0.6 | 0.1122110221 | 0.100001111 |
| x | 0.485 | 1 | 0.45679 | 1 | 0.2 | 0.1002121001 | 0.110001101 |
| Patients/Criteria | ... | ... | Units of time | ||||
| ... | ... | ||||||
| ⋮ | ⋮ | ⋮ | ... | ⋮ | ... | ⋮ | ⋮ |
| ... | ... | ||||||
| ⋮ | ⋮ | ⋮ | ... | ⋮ | ... | ⋮ | ⋮ |
| ... | ... |
| Patient/Criteria | ... | ... | ||||
| p | ... | ... |
| Distance d | ||||||||
| 0.1970 | 1 | 0.388900 | 1.0 | 0.0 | 0.08800109890 | 0.000000100 | 2.67390119890 | |
| 0.4450 | 0 | 0.523210 | 0.5 | 0.4 | 0.07799208800 | 0.010000100 | 1.95620218800 | |
| 0.0400 | 0 | 0.400790 | 0.0 | 0.0 | 0.08809109890 | 0.000100010 | 0.52898110890 | |
| 0.0830 | 1 | 0.455900 | 1.0 | 0.8 | 0.02200890110 | 0.000010000 | 3.36091890110 | |
| 0.2220 | 1 | 0.362230 | 0.0 | 0.2 | 0.02198890110 | 0.000110000 | 1.80632890110 | |
| 0.3300 | 0 | 0.332760 | 0.5 | 0.0 | 0.08809009890 | 0.000000010 | 1.25085010890 | |
| 0.0820 | 0 | 0.232210 | 1.0 | 0.2 | 0.02190890100 | 0.001000010 | 1.53711891100 | |
| 0.2850 | 1 | 0.378460 | 0.0 | 0.4 | 0.01199892200 | 0.009999990 | 2.08545891200 | |
| 0.2850 | 1 | 0.378460 | 1.0 | 0.4 | 0.01199892200 | 0.009999990 | 3.08545891200 |
| Name | Data type | Definition |
| age | integer | age in years |
| sex | binary | sex |
| cp | {1,2,3,4} | chest pain type |
| trestbps | integer | resting blood pressure |
| chol | integer | serum cholesterol in mg/dl |
| fbs | binary | fasting blood sugar > 120 mg/d |
| restecg | {0,1,2} | resting electrocardiographic results |
| thalach | I integer | maximum heart rate achieved |
| exang | binary | exercise-induced angina |
| oldpeak | float | ST depression induced by exercise relative to rest |
| slope | {1,2,3} | the slope of the peak exercise ST segment |
| ca | {0,1,2,3,} | number of major vessels colored by flourosopy |
| thal | {3,6,7} | heart status |
| num | {0,1,2,3,4} | diagnosis of heart disease |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





