Submitted:
30 June 2025
Posted:
01 July 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Raw Materials and Additives
2.2. Plackett-Burman Screening Design
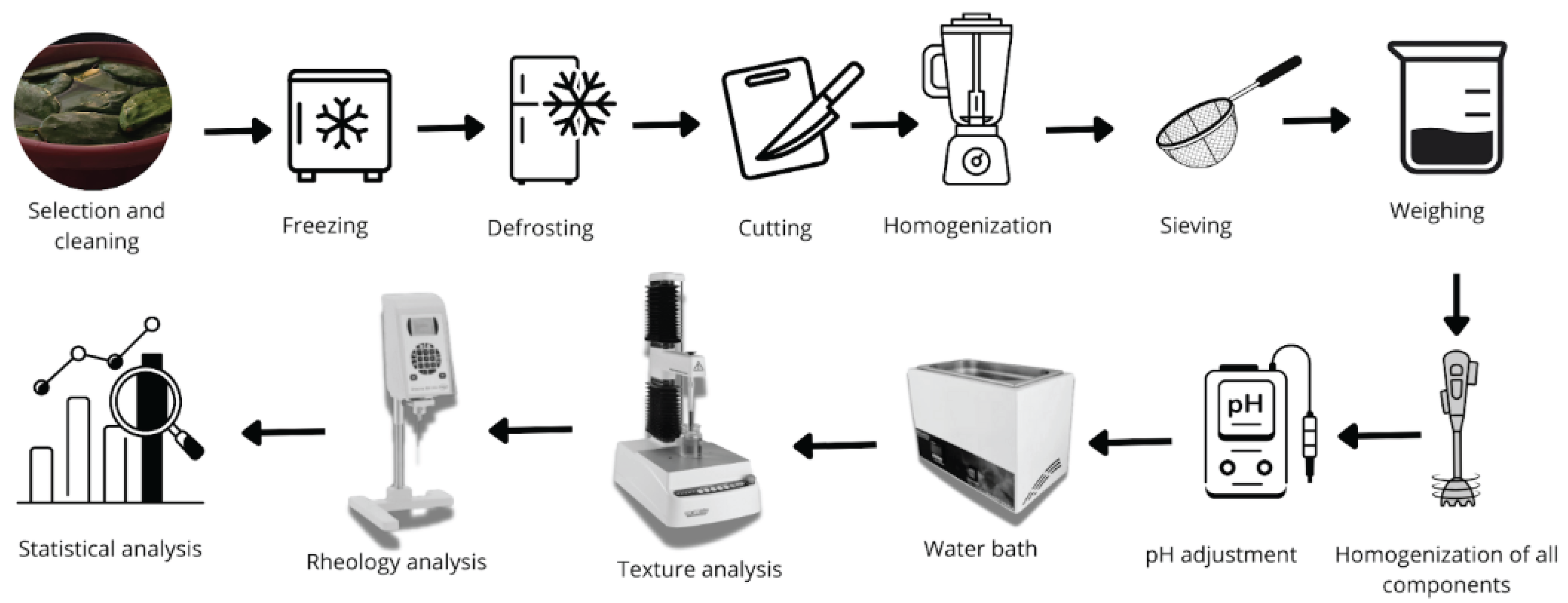
2.3. Gels Development
2.4. Rheological Behavior
2.5. Instrumental Texture
2.6. Statistical Analysis
3. Results and Discussions
3.1. Proximate Composition
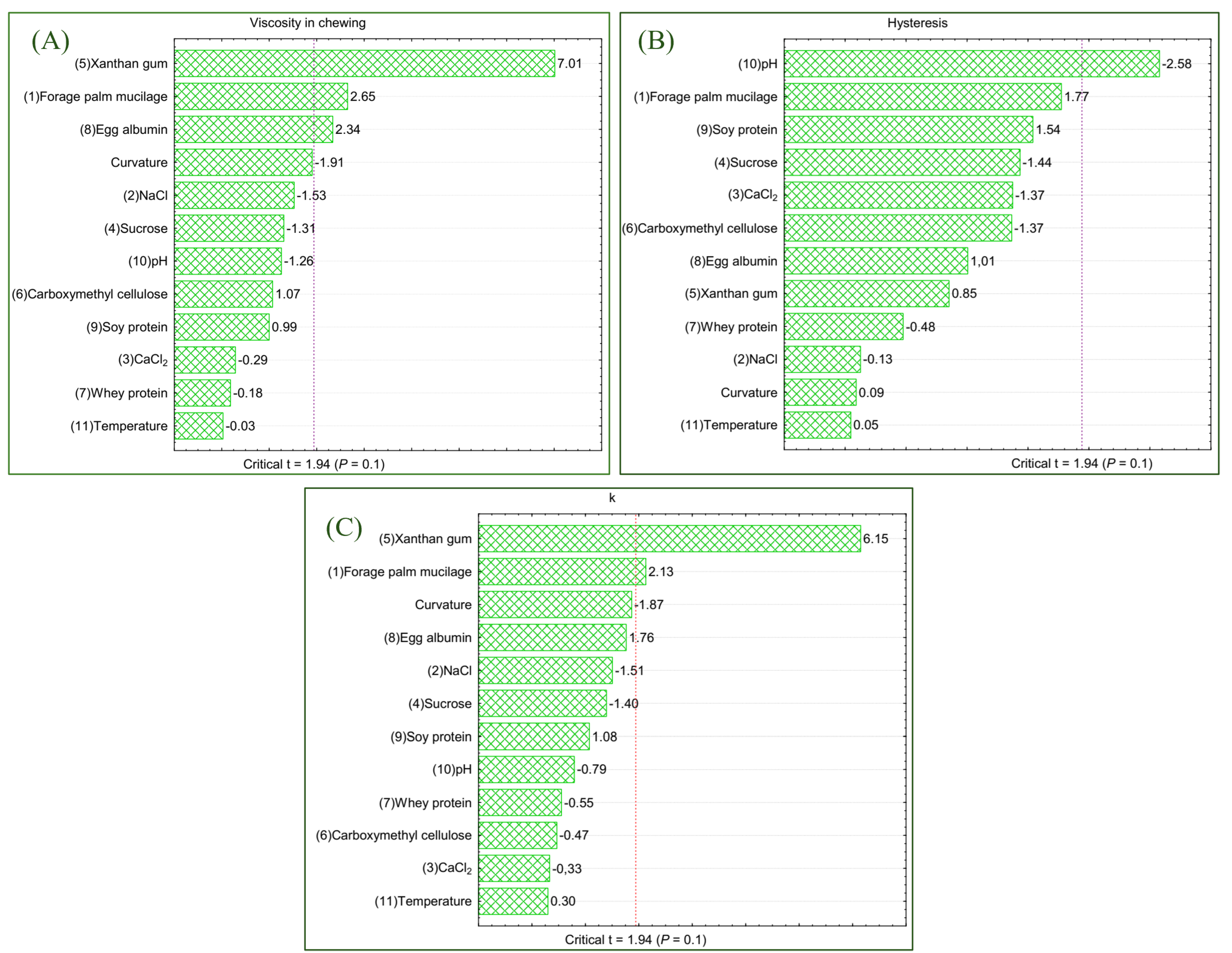
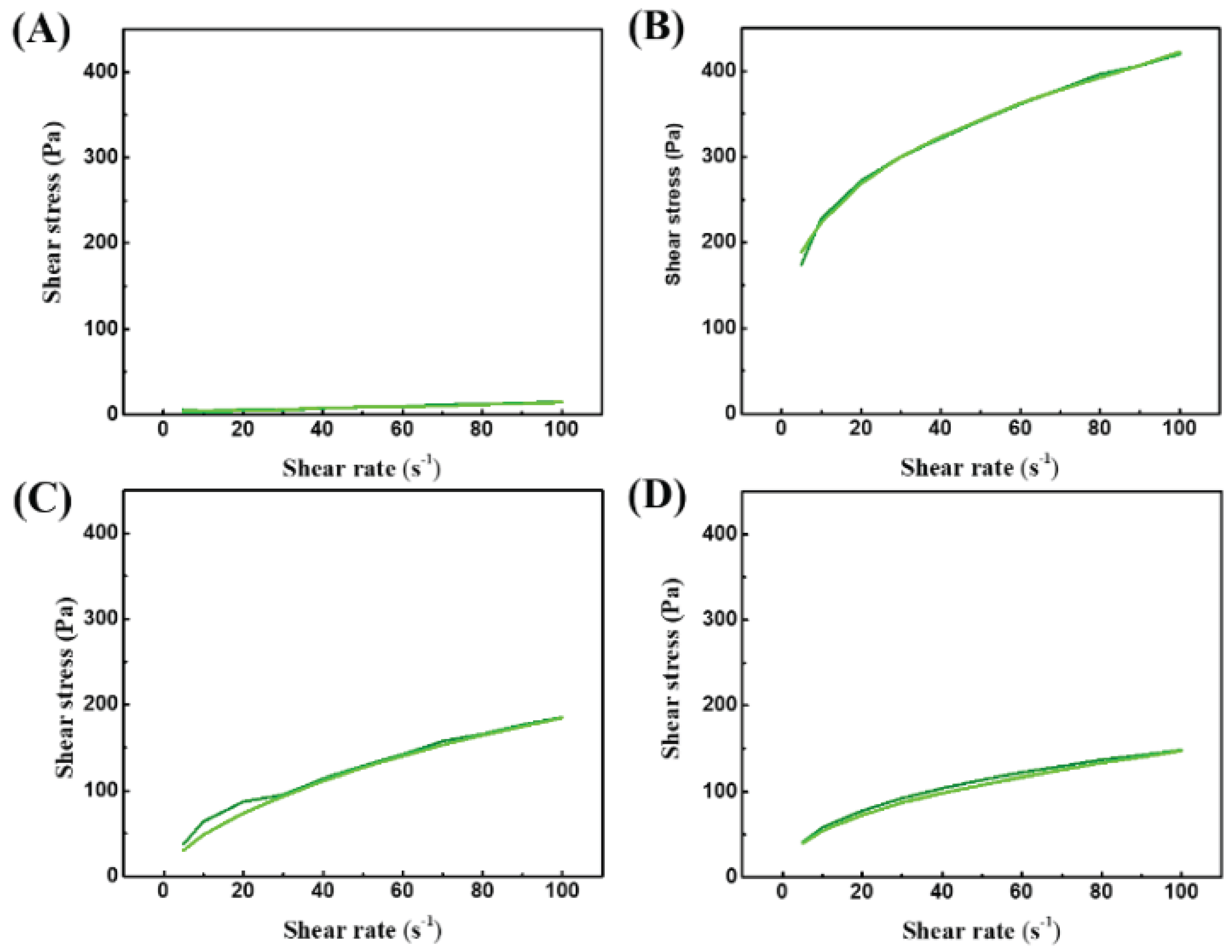
3.2. Rheological Behavior
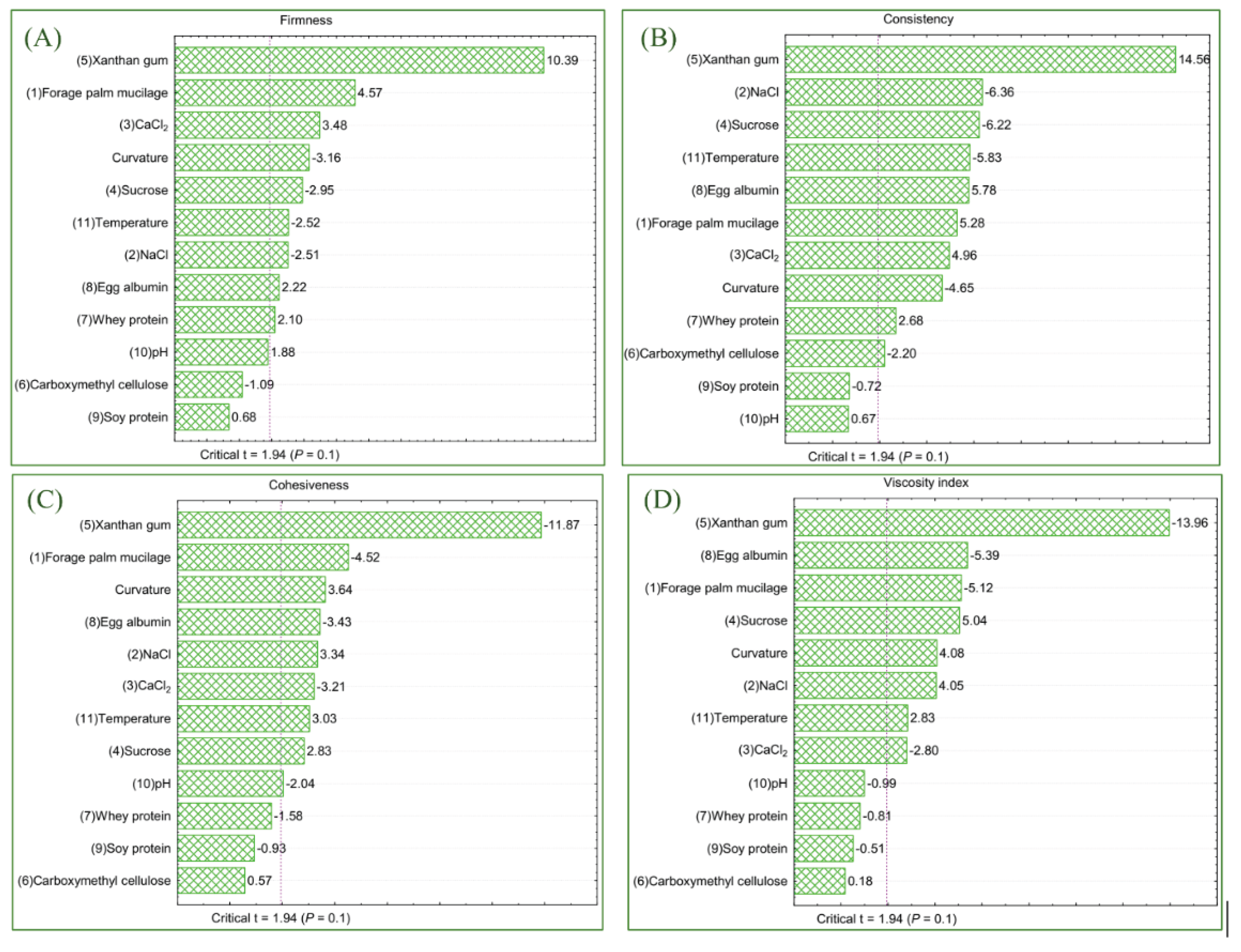
3.3. Instrumental Texture
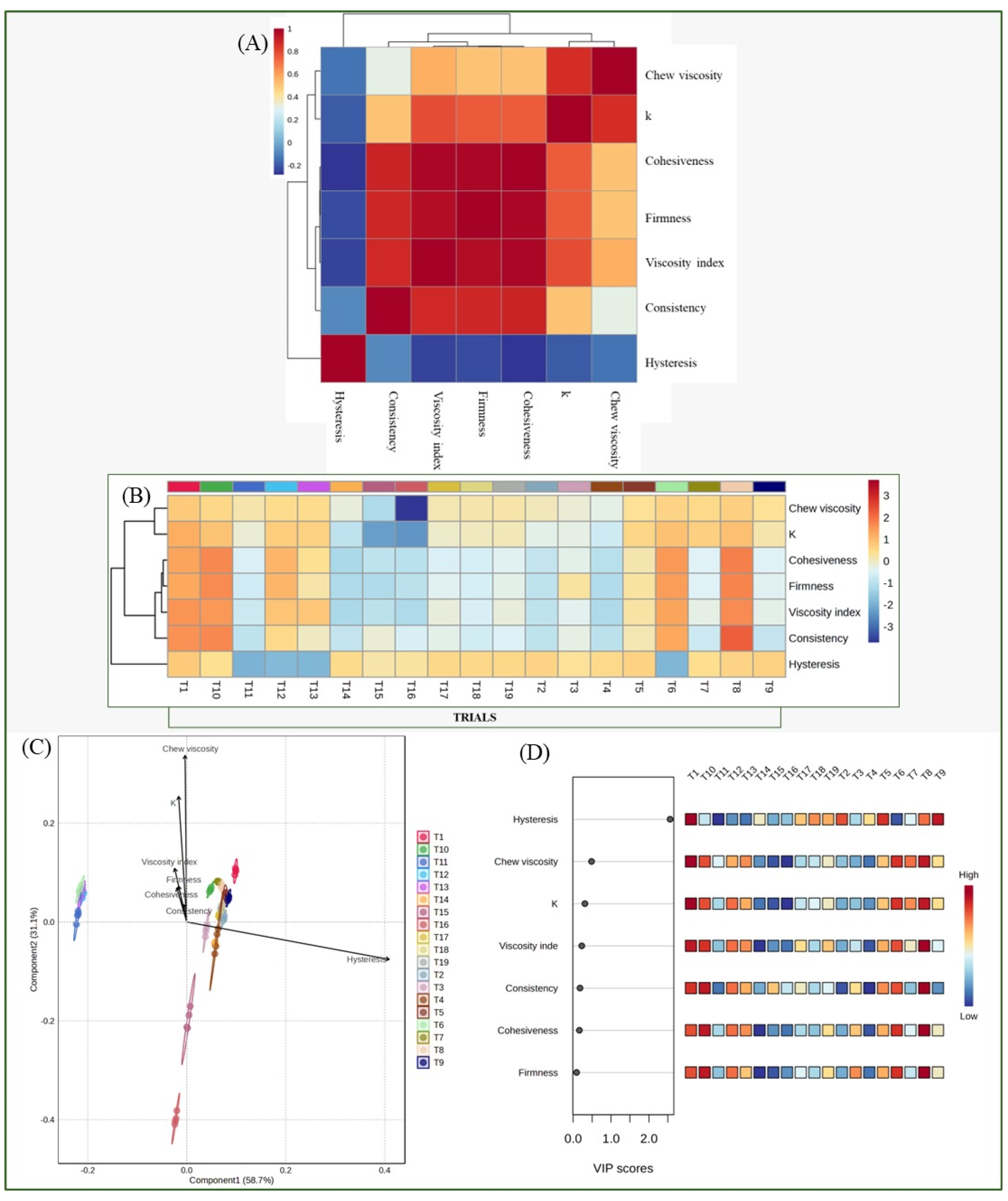
3.4. Chemometric Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | analysis of variance |
| CMC | carboxymethyl cellulose |
| FPM | forage palm mucilage |
| SPI | soy protein isolate |
| XG | xanthan gum |
References
- Di Bella, G.; Lo Vecchio, G.; Albergamo, A.; Nava, V.; Bartolomeo, G.; Macrì, A.; Bacchetta, L.; Lo Turco, V.; Potortì, A. G. Chemical characterization of Sicilian dried nopal [Opuntia ficus-indica (L.) Mill.]. J. Food Compos. Anal. 2022, 106, 104307. [Google Scholar] [CrossRef]
- Santos, D. C.; Silva, M. C.; Alves, F. A. L.; Freitas, E. V. Botânica e cultivares. In Palma forrageira: do plantio à colheita; Donato, S. L. R., Borém, A., Rodrigues, M. G. V., Eds.; Epamig: Brasil, 2020; pp. 21–41. [Google Scholar]
- Silva, L. E. P.; Moreira, S. R.; Neves, N. A.; Aguiar, E. V.; Capriles, V. D.; Amaral, T. N.; Schmiele, M. Use of integral forage palm flour as an innovative ingredient in new fettuccine-type pasta: Thermomechanical and technological properties, and sensory acceptance. Foods 2024, 13, 2683. [Google Scholar] [CrossRef] [PubMed]
- Macêdo, A. J. S.; Cesar Neto, J. M.; Oliveira, L. B.; Edvan, R. L.; Santos, E. M. The cactus pear culture, origin, introduction, expansion, utilities and future perspectives: Literature review. Braz. J. Dev. 2020, 6, 62967–62987. [Google Scholar] [CrossRef]
- Souza, G. F. A.; Pereira, M. M. L.; Silva, A. A. P.; Capuzzo, V. M. S.; Machado, F. Opuntia ficus-indica mucilage: A sustainable bio-additive for cementitious materials. Constr. Build. Mater. 2024, 456, 139254. [Google Scholar] [CrossRef]
- Tosif, M. M.; Bains, A.; Goksen, G.; Rehman, M. Z.; Ali, N.; Karabulut, G.; Chawla, P. A comparative study on utilization of different plant-derived nano-mucilage as a fat replacer in yogurt: Product optimization, physicochemical attributes, shelf-life evaluation, and consumer perception with market orientation. Food Chem. X 2024, 24, 101920. [Google Scholar] [CrossRef]
- Santana, R. D. C.; Tavares, M. B.; Coimbra, J. S. D. R.; Martins, M. A.; Sousa, R. D. C. D. Effect of extraction conditions of chia (Salvia hispanica L.) mucilage on its chemical, rheological, and emulsifying properties. ACS Food Sci. Technol. 2025, 5, 687–694. [Google Scholar] [CrossRef]
- Teotônio, D. O.; Rodrigues, S. M.; Leoro, M. G. V.; Pereira, P. A. P.; Schmiele, M. Potentialities of using cryoprotectants in gluten-free frozen dough and microwave baking as an emerging technology. Res. Soc. Dev. 2021, 10, e12410615674. [Google Scholar] [CrossRef]
- Lozano, E.; Padilla, K.; Salcedo, J.; Arrieta, A.; Andrade-Pizarro, R. Effects of yam (Dioscorea rotundata) mucilage on the physical, rheological and stability characteristics of ice cream. Polymers 2022, 14, 3142. [Google Scholar] [CrossRef]
- Felisberto, M. H. F.; Wahanik, A. L.; Gomes-Ruffi, C. R.; Clerici, M. T. P. S.; Chang, Y. K.; Steel, C. J. Use of chia (Salvia hispanica L.) mucilage gel to reduce fat in pound cakes. LWT-Food Sci. Technol. 2015, 63, 1049–1055. [Google Scholar] [CrossRef]
- Schmiele, M.; Mascarenhas, M. C. C. N.; Barretto, A. C. S.; Pollonio, M. A. R. Dietary fiber as fat substitute in emulsified and cooked meat model system. LWT-Food Sci. Technol. 2015, 61, 105–111. [Google Scholar] [CrossRef]
- Beikzadeh, S.; Khezerlou, A.; Jafari, S.; Pilevar, Z.; Mortazavian, A. Seed mucilages as the functional ingredients for biodegradable films and edible coatings in the food industry. Adv. Colloid Interface Sci. 2020, 280, 102164. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, H. J. M.; Souza, S. M.; Carvalho, C. W. P.; Nabeshima, E. H.; Schmiele, M. Cocoyam is an unconventional, innovative, and sustainable source of starch with potential use in sleek and functional biodegradable films. Starch-Stärke, 2: version, 2400. [Google Scholar] [CrossRef]
- Rooyen, B. V.; Wit, M.; Osthoff, G.; Niekerk, V. Cactus pear mucilage (Opuntia spp.) as a novel functional biopolymer: Mucilage extraction, rheology and biofilm development. Polymers 2024, 16, 1993. [Google Scholar] [CrossRef] [PubMed]
- Capitani, M.; Corzo-Ríos, L.; Chel-Guerrero, L.; Betancur-Ancona, D.; Nolasco, S.; Tomás, M. Rheological properties of aqueous dispersions of chia (Salvia hispanica L.) mucilage. J. Food Eng. 2015, 149, 70–77. [Google Scholar] [CrossRef]
- Geethalaxmi, M.; Sunil, C. K.; Venkatachalapathy, N. Tamarind seed polysaccharides, proteins, and mucilage: extraction, modification of properties, and their application in food. Sustainable Food Technol. 2024, 2, 1670–1685. [Google Scholar] [CrossRef]
- Lopes, A. C.; Ribas, M. F.; Tonial, I. B.; Lucchetta, L. Chia mucilage application (Salvia hispanica L.) in biscuit processing. Braz. J. Dev. 2020, 6, 17997–18008. [Google Scholar] [CrossRef]
- Cakmak, H.; Ilyasoglu-Buyukkestelli, H.; Sogut, E.; Ozyurt, V. H.; Gumus-Bonacina, C. E.; Simsek, S. A review on recent advances of plant mucilages and their applications in food industry: Extraction, functional properties and health benefits. Food Hydrocoll. Health 2023, 3, 100131. [Google Scholar] [CrossRef]
- Aftab, K.; Hameed, S.; Umbreen, H.; Ali, S.; Rizwan, M.; Alkahtani, S.; Abdel-Daim, M. M. Physicochemical and functional potential of hydrocolloids extracted from some Solanaceae plants. J. Chem. 2020, 563945. [Google Scholar] [CrossRef]
- Goksen, G.; Demir, D.; Dhama, K.; Kumar, M.; Shao, P.; Xie, F.; Echegaray, N.; Lorenzo, J. M. Mucilage polysaccharide as a plant secretion: Potential trends in food and biomedical applications. Int. J. Biol. Macromol. 2023, 230, 123146. [Google Scholar] [CrossRef]
- Guo, J.; Gu, X.; Meng, Z. Customized 3D printing to build plant-based meats: Spirulina platensis protein-based Pickering emulsion gels as fat analogs. Innov. Food Sci. Emerg. Technol. 2024, 94, 103679. [Google Scholar] [CrossRef]
- Caulier, S.; Doets, E.; Noort, M. An exploratory consumer study of 3D printed food perception in a real-life military setting. Food Qual. Prefer. 2020, 86, 104001. [Google Scholar] [CrossRef]
- Nadagouda, M. N.; Ginn, M.; Rastogi, V. A review of 3D printing techniques for environmental applications. Curr. Opin. Chem. Eng. 2020, 28, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, M.; Clerici, M. T. P. S.; Silva, L. R.; Nolasco, M. V. F. M. 3D food printing: A review of history, functionality and challenges in product development. Res. Soc. Dev. 2025, 14, e1714147902. [Google Scholar] [CrossRef]
- Yang, X.; Li, A.; Li, D.; Guo, Y.; Sun, L. Applications of mixed polysaccharide-protein systems in fabricating multi-structures of binary food gels—A review. Trends Food Sci. Technol. 2021, 109, 197–210. [Google Scholar] [CrossRef]
- Wang, P.; Liao, Q.; Zhang, H. Polysaccharide-based double-network hydrogels: Polysaccharide effect, strengthening mechanisms, and applications. Biomacromolecules 2023, 24, 5479–5510. [Google Scholar] [CrossRef]
- Guan, C.; Wang, C.; Fu, S. Food protein nanofibril gels: From conditions, types and properties to applications. Foods 2024, 13, 2173. [Google Scholar] [CrossRef]
- Tang, Q.; Roos, Y. H.; Miao, S. Structure, gelation mechanism of plant proteins versus dairy proteins and evolving modification strategies. Trends Food Sci. Technol. 2024, 147, 104464. [Google Scholar] [CrossRef]
- Li, M.; Feng, L.; Xu, Y.; Nie, M.; Li, D.; Zhou, C.; Dai, Z.; Zhang, Z.; Zhang, M. Rheological property, β-carotene stability and 3D printing characteristic of whey protein isolate emulsion gels by adding different polysaccharides. Food Chem. 2023, 414, 135702. [Google Scholar] [CrossRef]
- Kazemi-Taskooh, Z.; Varidi, M. How can plant-based protein–polysaccharide interactions affect the properties of binary hydrogels? (A review). Food Funct. 2023, 14, 5891–5909. [Google Scholar] [CrossRef]
- Makshakova, O. N.; Zuev, Y. F. Interaction-induced structural transformations in polysaccharide and protein-polysaccharide gels as functional basis for novel soft-matter: A case of carrageenans. Gels 2022, 8, 287. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 22nd ed.; Association of Official Analysis Chemists International: Gaithersburg, USA, 2023. [Google Scholar]
- Zhai, W.; Bao, S.; Han, R.; Wang, C.; He, J.; Zhong, F.; Xia, Y. Impact of reduced sucrose content on processed cheese: Sensory, textural, and storage stability analysis. Food Biosci. 2024, 60, 104405. [Google Scholar] [CrossRef]
- Sapper, M.; Talens, P.; Chiralt, A. Improving functional properties of cassava starch-based films by incorporating xanthan, gellan, or pullulan gums. Int. J. Polym. Sci. 2019, 367164. [Google Scholar] [CrossRef]
- Yar, M. S.; Ibeogu, I. H.; Bako, H. K.; Alnadari, F.; Bilal, M.; Rehman, F.; Zhu, J.; Zhou, T.; Zhao, Z.; Li, C. A novel carboxymethyl cellulose/gum xanthan and citric acid-based film that enhances the precision of blackcurrant anthocyanin-induced color detection for beef spoilage tracking. Food Chem. 2024, 461, 140905. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zheng, B.; Huang, L.; Zhang, Y.; Zeng, H. Saltiness perception mechanism and salt reduction strategies in food. Trends Food Sci. Technol. 2024, 148, 104521. [Google Scholar] [CrossRef]
- Alavi, F.; Tian, Z.; Chen, L.; Emam-Djomeh, Z. Effect of CaCl2 on the stability and rheological properties of foams and high-sugar aerated systems produced by preheated egg white protein. Food Hydrocoll. 2020, 106, 105887. [Google Scholar] [CrossRef]
- Sepúlveda, F.; Oyarzun-Ampuero, F.; Matiacevich, S.; Ortiz-Viedma, J.; Lemus-Mondaca, R.; Char, C. Use of whey protein concentrates to encapsulate hydrophobic natural antimicrobials to improve their incorporation into high-moisture foods enhancing their antimicrobial activity. Innovative Food Sci. Emerg. Technol. 2024, 94, 103687. [Google Scholar] [CrossRef]
- Yang, Y.; Meng, J.; Lei, L.; Huang, D.; Liu, C.; Gu, D.; Ito, Y. Different behavior of bovine serum albumin as foaming agent in foam enrichment of Rhodamine 6G and Evans blue. Sep. Purif. Technol. 2020, 252, 117509. [Google Scholar] [CrossRef]
- Liang, G.; Chen, W.; Zhang, X.; Zeng, M.; Qin, F.; He, Z.; Goff, H. D.; Chen, J.; Wang, Z. Incorporating soy protein hydrolysate and temperature-induced gelling polysaccharide as partial egg replacements for enhanced texture in sponge cake. Food Biosci. 2024, 57, 103574. [Google Scholar] [CrossRef]
- Rodrigues, M. I.; Iemma, A. F. (Eds.) Experiment Design and Process Optimization; CRC Press: USA, 2014. [Google Scholar]
- Riquelme, N. , Savignones, C., López, A., Zúñiga, R. N., & Arancibia, C. (2023). Effect of gelling agent type on the physical properties of nanoemulsion-based gels. Colloids and Interfaces. [CrossRef]
- Massaretto, I. L.; Meza, S. L. R.; Schmiele, M.; Marquez, U. M. L.; Sinnecker, P. Nutritional characterization and effect of cooking on phenolic compounds, antioxidant capacity and sensory acceptability of commercial wild rice (Zizania aquatica L.). Biocatal. Agric. Biotechnol. 2023, 50, 102705. [Google Scholar] [CrossRef]
- Silva, I. L.; Silva, L. A. O.; Coelho, L. C. B. B. The Brazilian Caatinga biome and its biotechnological potential. In Advances in Applied Science and Technology; Teodor, R., Ed.; BP International: Índia, 2019. [Google Scholar]
- Giura, L. , Urtasun, L., Belarra, A., Ansorena, D., & Astiasarán, I. (2021). Exploring tools for designing dysphagia-friendly foods: A review. Foods, 10(6), 1334. [Google Scholar] [CrossRef]
- Costa, R.S. , Costa, R. O.C. ( 10, e32310515068. [CrossRef]
- Mathias, T.R.S. , Andrade, K.C.S., Rosa, C.L.S., & Silva, B.A. (2013). Rheological evaluation of different commercial yoghurts. A. ( 16, 12–20. [CrossRef]
- Yu, J. , Wang, Y., Li, D., & Wang, L.-J. (2022). Freeze–thaw stability and rheological properties of soy protein isolate emulsion gels induced by NaCl. Food Hydrocolloids, 1071. [Google Scholar] [CrossRef]
- Kumar, P. , Kumar, B., Gihar, S., & Kumar, D. (2024). Review on emerging trends and challenges in the modification of xanthan gum for various applications. ( 538, 109070. [CrossRef] [PubMed]
- Meydanju, N. , Pirsa, S., & Farzi, J. (2022). Biodegradable film based on lemon peel powder containing xanthan gum and TiO2–Ag nanoparticles: Investigation of physicochemical and antibacterial properties. Polymer Testing, 106, 107445. [Google Scholar] [CrossRef]
- Pirsa, S. , & Hafezi, K. (2023). Hydrocolloids: Structure, preparation method, and application in food industry. Food Chemistry, 399, 133967. [Google Scholar] [CrossRef]
- Zheng, Z. , Sun, Z., Li, M., Yang, J., Yang, Y., Liang, H., Xiang, H., Meng, J., Zhou, X., Liu, L., Wu, Z., & Yang, S. (2024). An update review on biopolymer xanthan gum: Properties, modifications, nanoagrochemicals, and its versatile applications in sustainable agriculture. International Journal of Biological Macromolecules, 281, 136562. [Google Scholar] [CrossRef]
- Hassanisaadi, M. , Vatankhah, M., Kennedy, J.F., Rabiei, A., & Riseh, R.S. (2025). Advancements in xanthan gum: A macromolecule for encapsulating plant probiotic bacteria with enhanced properties. Carbohydrate Polymers, 348, 122801. [Google Scholar] [CrossRef]
- Han, C. , Feng, G., Yin, S., Wang, G., Wang, J., Wan, Z., Guo, J., & Yang, X. (2024). Stabilization of oil-water interface by non-interfacial adsorbed native starch granules using depletion attraction. Food Hydrocolloids, 1103. [Google Scholar] [CrossRef]
- Hou, J. , Liu, Y., Jiang, Z., Chuang, R., Zhang, H., Li, H., Xia, N., Ma, Y., Zheng, L., Rayan, A. M., Ghamry, M., & Qin, D. (2025). Charge density of carboxymethyl cellulose affects depletion attraction-stabilized egg yolk Pickering emulsion gels: Rheological and interfacial properties. Food Hydrocolloids, 159, 110612. [Google Scholar] [CrossRef]
- Jia, W. , Cui, B., Ye, T., Lin, L., Zheng, H., Yan, X., Li, Y., Wang, L., Liu, S., & Li, B. (2014). Phase behavior of ovalbumin and carboxymethylcellulose composite system. ( 109, 64–70. [CrossRef] [PubMed]
- Medeiros, V. P. B. , Oliveira, K. Á. R., Queiroga, T. S., & Souza, E. L. (2024). Development and application of mucilage and bioactive compounds from Cactaceae to formulate novel and sustainable edible films and coatings to preserve fruits and vegetables—A review. Foods. [CrossRef]
- Goto, T. , Shimamoto, S., Takaya, M., Sato, S., Takahashi, K., Nishimura, K., Morii, Y., Kunishige, K., Ohtsuka, A., & Ijiri, D. (2021). Impact on genetic differences among various chicken breeds on free amino acid contents of egg yolk and albumen. ( 11, 2270. [CrossRef] [PubMed]
- Muhedaner, M. , Bako, H.K., Zhou, G., & Ye, K. (2025). Impact of egg white protein on mycoprotein gel: Insights into rheological properties, protein structure and molecular interactions. Food Chemistry, 463, 141366. [Google Scholar] [CrossRef]
- Zhang, M. , Mei, L., Wu, Y., Jin, G., & Bao, D. (2024). Impact of ethanol extract of propolis on heat-induced egg white protein gels: Formation and properties. Food Hydrocolloids, 149, 109590. [Google Scholar] [CrossRef]
- Lopez, C.G. , & Richtering, W. (2021). Oscillatory rheology of carboxymethyl cellulose gels: Influence of concentration and pH. Carbohydrate Polymers, 267, 118117. [Google Scholar] [CrossRef]
- Shibaev, A.V. , Muravlev, D.A., Muravleva, A.K., Matveev, V.V., Chalykh, A.E., & Philippova, O.E. (2020). pH-dependent gelation of a stiff anionic polysaccharide in the presence of metal ions. Polymers. [CrossRef]
- Lu, F. , Chi, Y., & Chi, Y. (2024). Preparation of high internal phase emulsions based on high-temperature glycation-modified egg white protein: Structural characteristics, stability, and β-carotene bioavailability under multi-parameter regulation. International Journal of Biological Macromolecules, 283, 137870. [Google Scholar] [CrossRef]
- Zhao, Y.-R. , Peng, N., Wang, C., Li, Y.-Q., Liang, Y., Guo, Z.-W., Sun, A.-Y., & Ren, X. (2024). Preparation and characterization of pea protein isolate-egg white protein composite gels. Food Hydrocolloids, 1094. [Google Scholar] [CrossRef]
- Schmiele, M. , Araújo, T.L., Gurgueira, M.D., & Chang, Y.K. (2015). Determination of the concentration of different solvents systems in the protein solubilization of meat analogue. Ciência Rural, 1125. [Google Scholar] [CrossRef]
- Lima, C.T. , Lima, N.G., Rodrigues, S.M., Neves, N.A., Meza, S.L.R., & Schmiele, M. (2022). Experimental optimization tool for the development of muffins with high technological and nutritional value using whole flours of rice, red sorghum and carioca bean. ( 11, e34111133337. [CrossRef]
- Souza, E.C. , Cordeiro, D.A., Silva, B.S., Neves, N.A., & Schmiele, M. (2022). Development of muffin with the incorporation of olive pomace flour, extra virgin olive oil and hydrolyzed soy protein. ( 11, e58511226012. [CrossRef]
- Wang, X. , Fei, W., Shen, M., Wen, H., Chen, F., & Xie, J. (2024). Texture, swallowing and digestibility characteristics of a low-GI dysphagia food as affected by addition of dietary fiber and anthocyanins. Food Research International, 1152. [Google Scholar] [CrossRef]
- Cofelice, M. , Messia, M.C., Marconi, E., Cuomo, F., & Lopez, F. (2023). Effect of the xanthan gum on the rheological properties of alginate hydrogels. ( 142, 108768. [CrossRef]
- Amiri, M.S. , Mohammadzadeh, V., Yazdi, M.E.T., Barani, M., Rahdar, A., & Kyzas, G.Z. (2021). Plant-based gums and mucilages applications in pharmacology and nanomedicine: A review. Molecules, 26(6), 1770. [Google Scholar] [CrossRef]
- Chu, L. , Yang, L., Li, J., Lin, L., & Zheng, G. (2019). Effect of *Smilax china* L. starch on the gel properties and interactions of calcium sulfate-induced soy protein isolate gel. International Journal of Biological Macromolecules. [CrossRef]
- Damodaran, S. , & Parkin, K.L. (Eds.). (2017). Fennema’s Food Chemistry, B: CRC Press.
- Wong, D.W.S. (2018). Mechanism and Theory in Food Chemistry, S: Springer Cham.
- Garcia, J. , Moura, M., & Aouada, F. (2019). Effect of pH, ionic concentration and species on the absorption of water by hydrogel bionanocomposits constituted from CMC/PAAm/Laponite RDS. Química Nova. [CrossRef]
- Benítez, E. I. , Genovese, D. B., & Lozano, J. E. (2009). Effect of typical sugars on the viscosity and colloidal stability of apple juice. E. ( 23, 519–525. [CrossRef]
- Monteiro, S. S. , Queiroz, J. V. S. A., Gomes, H. M., Santos, L., Moreira, J. C. F., Gelain, D. P., Fook, M. V. L., Lisboa, H. M., & Pasquali, M. A. B. (2025). Characterization of mucilage from *Opuntia cochenillifera* cladodes: Rheological behavior, cytotoxicity, and antioxidant potential. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 707, 135824. [Google Scholar] [CrossRef]
- Kumawat, K. L. , Raina, S. K., Kumar, D., Verma, M. K., Singh, D., Mir, J. I., Sultan, S. M., & Sharma, O. C. (2024). Association of reproductive phenology with air temperature in almond (*Prunus dulcis* [Mill.] D.A. Webb) cultivars under northwestern Himalayan conditions. Applied Fruit Science. [CrossRef]
- Ray, A. , Ghosh, S., Ray, A., & Aswatha, S. M. (2015). An analysis of the influence of growth periods on potential functional and biochemical properties and thermal analysis of freeze-dried Aloe vera L. gel. Industrial Crops and Products. [CrossRef]
| Trials | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 25 | 0.05 | 0.05 | 0.2 | 1.00 | 0.05 | 0.2 | 2.0 | 2.0 | 3.0 | 75 |
| 2 | 25 | 3.00 | 0.05 | 0.2 | 0.05 | 1.00 | 0.2 | 0.2 | 2.0 | 8.0 | 25 |
| 3 | 25 | 3.00 | 3.00 | 0.2 | 0.05 | 0.05 | 2.0 | 0.2 | 0.2 | 8.0 | 75 |
| 4 | 25 | 3.00 | 3.00 | 15.0 | 0.05 | 0.05 | 0.2 | 2.0 | 0.2 | 3.0 | 75 |
| 5 | 1 | 3.00 | 3.00 | 15.0 | 1.00 | 0.05 | 0.2 | 0.2 | 2.0 | 3.0 | 25 |
| 6 | 25 | 0.05 | 3.00 | 15.0 | 1.00 | 1.00 | 0.2 | 0.2 | 0.2 | 8.0 | 25 |
| 7 | 1 | 3.00 | 0.05 | 15.0 | 1.00 | 1.00 | 2.0 | 0.2 | 0.2 | 3.0 | 75 |
| 8 | 25 | 0.05 | 3.00 | 0.2 | 1.00 | 1.00 | 2.0 | 2.0 | 0.2 | 3.0 | 25 |
| 9 | 25 | 3.00 | 0.05 | 15.0 | 0.05 | 1.00 | 2.0 | 2.0 | 2.0 | 3.0 | 25 |
| 10 | 1 | 3.00 | 3.00 | 0.2 | 1.00 | 0.05 | 2.0 | 2.0 | 2.0 | 8.0 | 25 |
| 11 | 1 | 0.05 | 3.00 | 15.0 | 0.05 | 1.00 | 0.2 | 2.0 | 2.0 | 8.0 | 75 |
| 12 | 25 | 0.05 | 0.05 | 15.0 | 1.00 | 0.05 | 2.0 | 0.2 | 2.0 | 8.0 | 75 |
| 13 | 1 | 3.00 | 0.05 | 0.2 | 1.00 | 1.00 | 0.2 | 2.0 | 0.2 | 8.0 | 75 |
| 14 | 1 | 0.05 | 3.00 | 0.2 | 0.05 | 1.00 | 2.0 | 0.2 | 2.0 | 3.0 | 75 |
| 15 | 1 | 0.05 | 0.05 | 15.0 | 0.05 | 0.05 | 2.0 | 2.0 | 0.2 | 8.0 | 25 |
| 16 | 13 | 1.52 | 1.52 | 7.6 | 0.51 | 0.51 | 1.1 | 1.1 | 1.1 | 5.5 | 50 |
| 17 | 13 | 1.52 | 1.52 | 7.6 | 0.51 | 0.51 | 1.1 | 1.1 | 1.1 | 5.5 | 50 |
| 18 | 13 | 1.52 | 1.52 | 7.6 | 0.51 | 0.51 | 1.1 | 1.1 | 1.1 | 5.5 | 50 |
| 19 | 13 | 1.52 | 1.52 | 7.6 | 0.51 | 0.51 | 1.1 | 1.1 | 1.1 | 5.5 | 50 |
| Trials | Viscosity in chewing - 10 (Pa· s) | Hysteresis (Pa) | Consistency index (k) | Flow behavior index (n) |
|---|---|---|---|---|
| 1 | 36.16 ± 2.85 | 3950 | 225.64 ± 33.62 | 0.21 ± 0.03 |
| 2 | 4.51 ± 0.24 | 580 | 11.15 ± 0.81 | 0.55 ± 0.07 |
| 3 | 2.35 ± 0.35 | 48 | 12.74 ± 2.72 | 0.26 ± 0.04 |
| 4 | 1.61 ± <0.01 | 256 | 6.54 ± 3.98 | 0.29 ± 0.10 |
| 5 | 10.73 ± 0.97 | 708 | 59.48 ± 6.50 | 0.28 ± 0.05 |
| 6 | 21.53 ± 2.40 | -590 | 115.57 ± 1.15 | 0.30 ± 0.02 |
| 7 | 16.40 ± 1.88 | 109 | 72.90 ± 2.26 | 0.38 ± 0.04 |
| 8 | 27.75 ± 1.24 | 528 | 122.42 ± 3.56 | 0.31 ± 0.02 |
| 9 | 10.67 ± 0.98 | 992 | 28.53 ± 3.24 | 0.50 ± 0.03 |
| 10 | 18.57 ± 0.45 | 80 | 112.05 ± 2.67 | 0.17 ± 0.04 |
| 11 | 6.32 ± 1.44 | -1138 | 15.48 ± 2.60 | 0.48 ± 0.15 |
| 12 | 14.00 ± 0.29 | -361 | 81.34 ± 3.35 | 0.24 ± 0.03 |
| 13 | 14.47 ± 1.73 | -575 | 69.74 ± 4.41 | 0.21 ± 0.14 |
| 14 | 2.09 ± 0.26 | 199 | 3.31 ± 0.07 | 0.71 ± 0.07 |
| 15 | 0.104 ± <0.01 | 14 | 0.31 ± 0.20 | 0.43 ± 0.13 |
| 16 | 0.05 ± <0.01 | 21 | 44.21 ± 1.01 | 0.31 ± 0.03 |
| 17 | 5.25 ± 0.21 | 294 | 18.49 ± 1.30 | 0.41 ± 0.03 |
| 18 | 6.14 ± 0.27 | 387 | 21.42 ± 1.00 | 0.41 ± 0.02 |
| 19 | 6.82 ± 0.27 | 368 | 23.99 ± 2.28 | 0.37 ± 0.02 |
| Trial | Firmness (N) | Consistency (N·s) | Cohesiveness (N) | Viscosity index (N·s) |
|---|---|---|---|---|
| 1 | 0.349 ± 0.014 | 2.783 ± 0.176 | 0.138 ± 0.006 | 0.734 ± 0.092 |
| 2 | 0.129 ± 0.002 | 1.300 ± 0.044 | 0.055 ± 0.001 | 0.088 ± 0.010 |
| 3 | 0.223 ± 0.009 | 1.598 ± 0.121 | 0.069 ± 0.003 | 0.145 ± 0.016 |
| 4 | 0.118 ± 0.003 | 1.286 ± 0.087 | 0.051 ± 0.002 | 0.071 ± 0.010 |
| 5 | 0.221 ± 0.016 | 1.801 ± 0.199 | 0.084 ± 0.002 | 0.237 ± 0.026 |
| 6 | 0.373 ± 0.016 | 2.582 ± 0.387 | 0.146 ± 0.003 | 0.616 ± 0.084 |
| 7 | 0.164 ± 0.015 | 1.397 ± 0.128 | 0.067 ± 0.004 | 0.160 ± 0.032 |
| 8 | 0.434 ± 0.022 | 3.397 ± 0.262 | 0.167 ± 0.004 | 0.810 ± 0.047 |
| 9 | 0.167 ± 0.001 | 1.325 ± 0.058 | 0.067 ± 0.001 | 0.149 ± 0.018 |
| 10 | 0.414 ± 0.020 | 2.898 ± 0.320 | 0.159 ± 0.004 | 0.692 ± 0.090 |
| 11 | 0.145 ± 0.001 | 1.313 ± 0.132 | 0.065 ± 0.003 | 0.106 ± 0.011 |
| 12 | 0.320 ± 0.010 | 2.023 ± 0.279 | 0.123 ± 0.005 | 0.402 ± 0.073 |
| 13 | 0.218 ± 0.027 | 1.754 ± 0.319 | 0.092 ± 0.003 | 0.399 ± 0.065 |
| 14 | 0.115 ± 0.001 | 1.338 ± 0.131 | 0.047 ± 0.005 | 0.067 ± 0.007 |
| 15 | 0.116 ± 0.001 | 1.664 ± 0.146 | 0.054 ± 0.002 | 0.085 ± 0.008 |
| 16 | 0.126 ± 0.011 | 1.466 ± 0.044 | 0.052 ± <0.001 | 0.070 ± 0.005 |
| 17 | 0.165 ± 0.002 | 1.571 ± 0.174 | 0.065 ± 0.003 | 0.163 ± 0.012 |
| 18 | 0.151 ± 0.004 | 1.425 ± 0.035 | 0.062 ± 0.001 | 0.127 ± 0.018 |
| 19 | 0.170 ± 0.006 | 1.508 ± 0.078 | 0.068 ± <0.001 | 0.165 ± 0.012 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





