1. Introduction
Contrary to normal (“forward”) osmosis, reverse
osmosis moves water against the osmolarity gradient, from the salt water side
to the fresh water side. It allows the production of fresh water from salt
water, and is the most widely used desalination method worldwide (Greenlee,
2009).
Reverse osmosis is normally pressure-based. A
different type of reverse osmosis takes place in narrow tubes (micrometer
scale) that also moves water in the direction opposite to normal osmosis and
therefore allows the production of fresh water. This effect relies on the
separation of charges that occurs in adsorbed water. It is amplified by
pressure, but the pressure itself is not the basis of the effect.
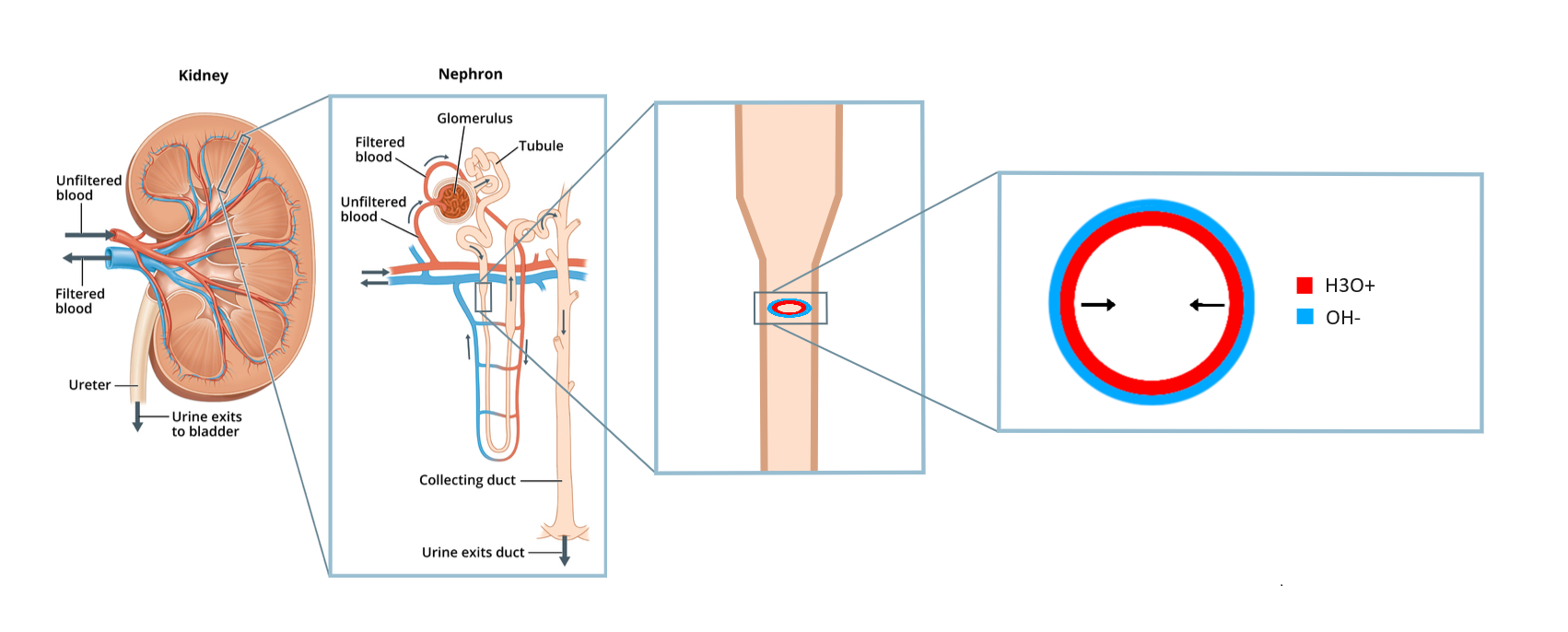
In the kidney, the narrow tube effect naturally
takes place in the thin segment of the nephron. It is the reason why the
nephron has evolved a narrow segment. The kidney can control the effect by
regulating the glomerular filtration pressure.
2. Method
In this article, the reverse osmosis effect in narrow tubes will be introduced. It will be explained how the effect is used to concentrate urine in the kidney. Before that, it will also explain the role of adsorbed water in osmosis, as it is the foundation for both models. The article presents new models, and uses simple proofs to defend that they are correct.
3. The role of Adsorbed Water in the Mechanism of Osmosis
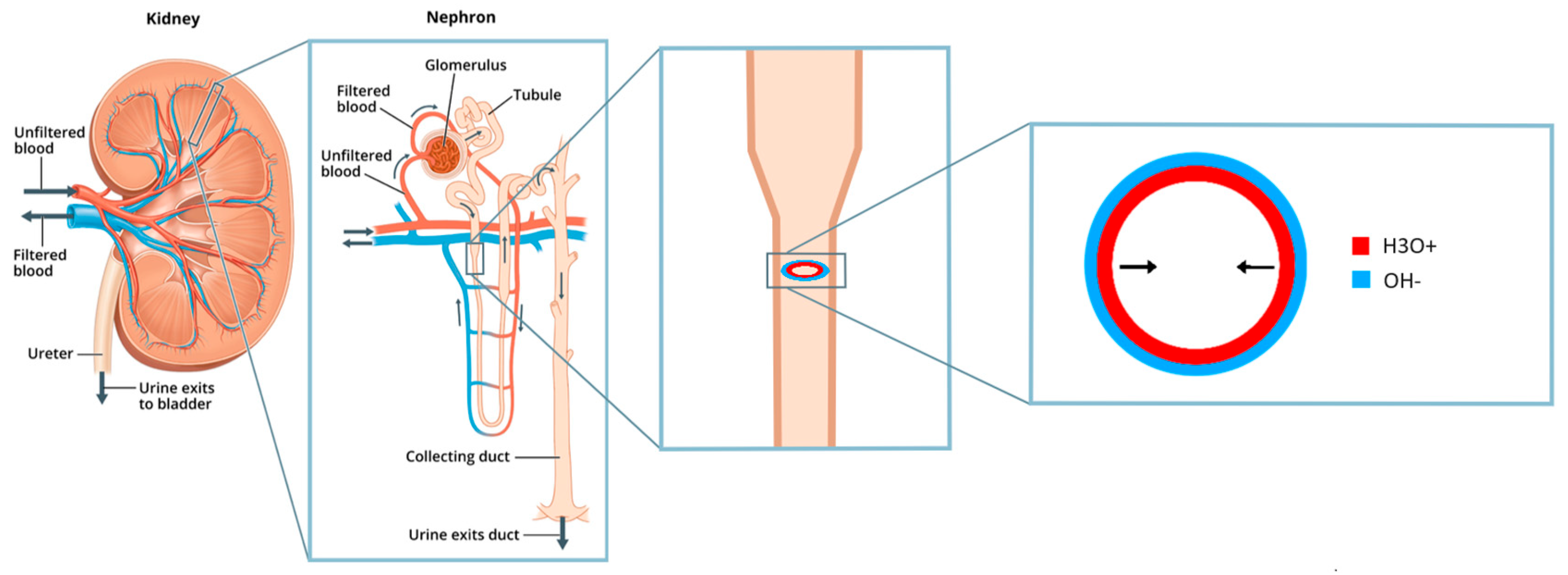
3.1. Separation of Charges in Adsorbed Water
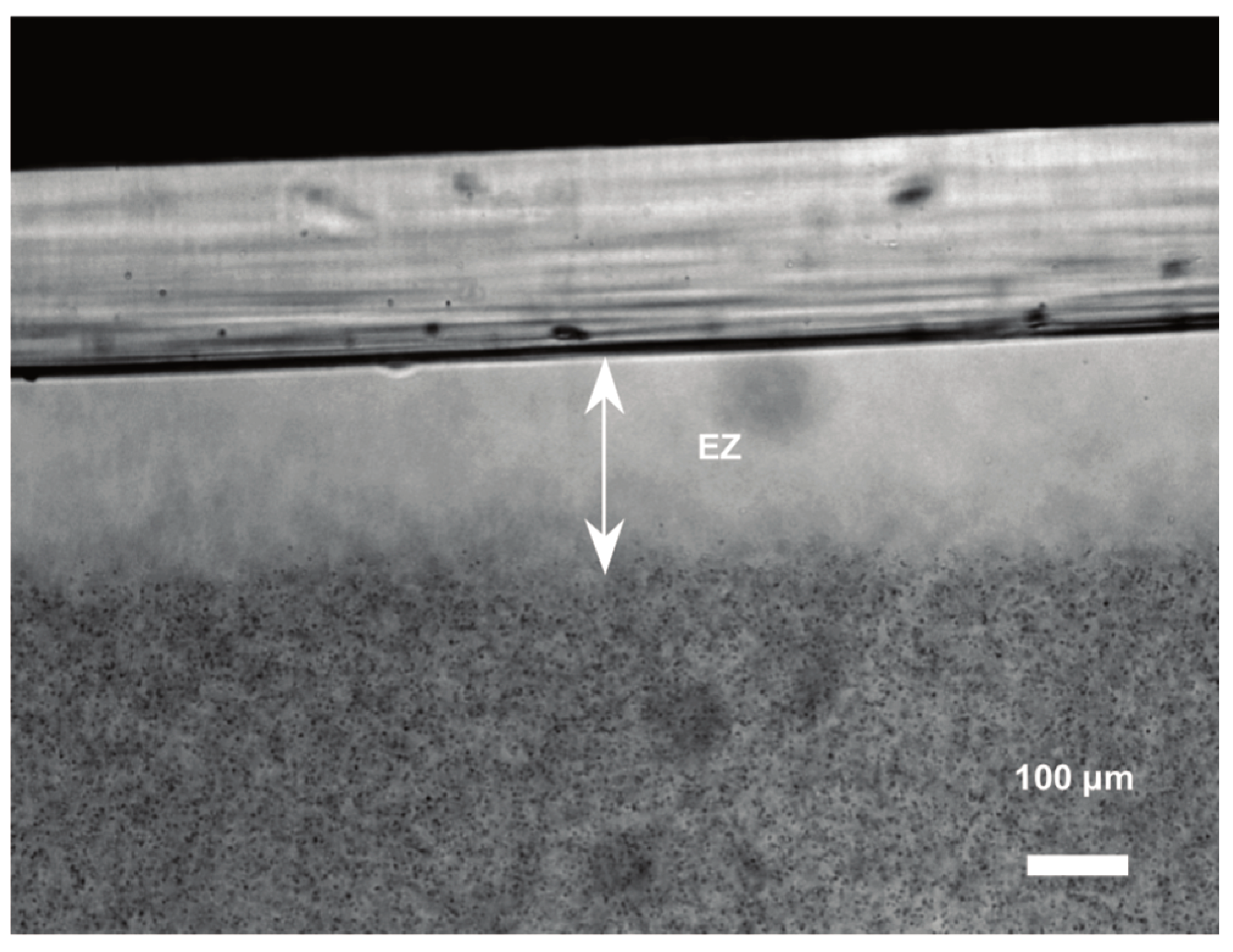
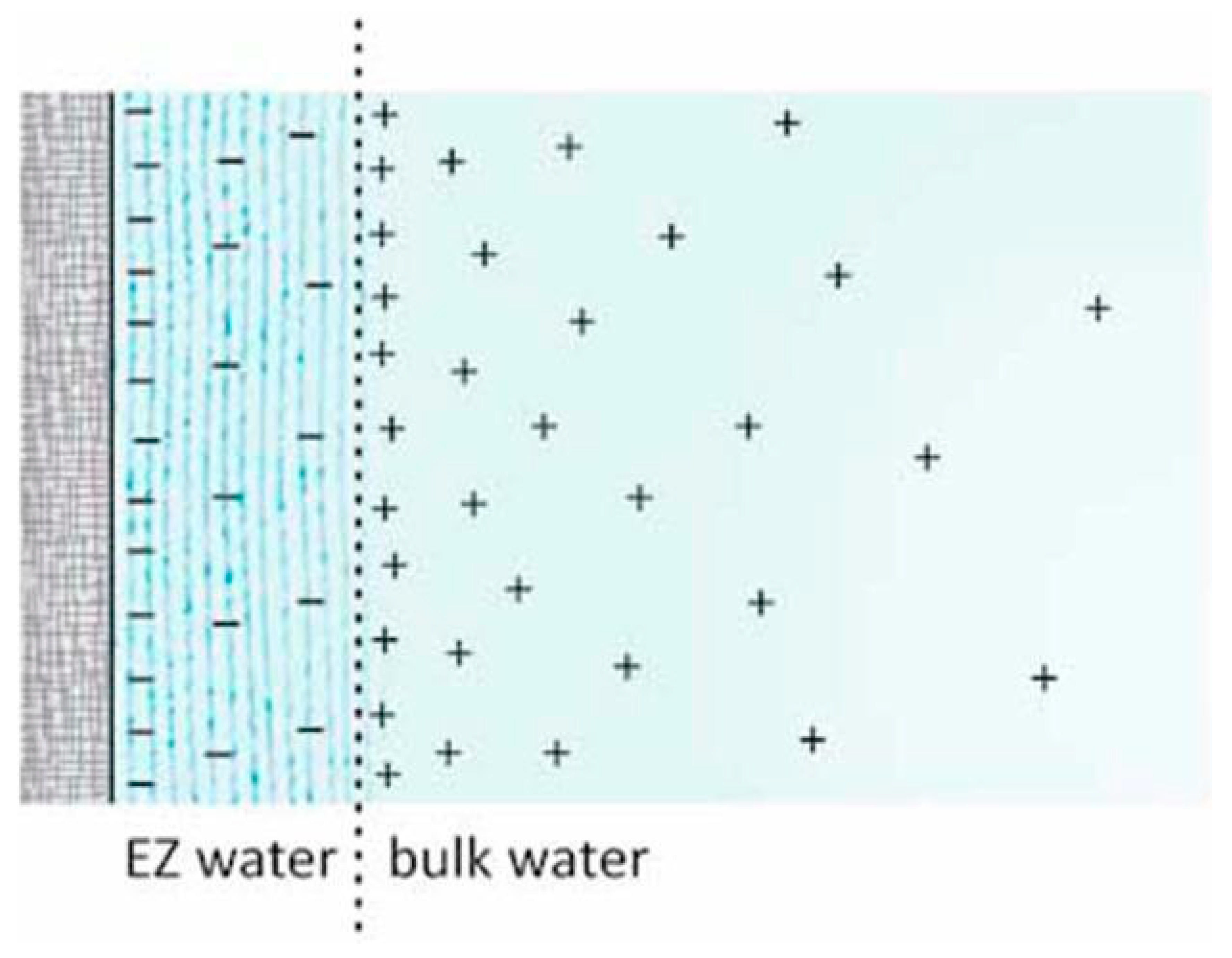
Zhao and Pollack (2009) discovered that the driving force for osmosis is the thin layer of adsorbed water that forms at hydrophilic surfaces (
Figure 1). This adsorbed phase of water has the unexpected property that it separates charges. It releases protons into the surrounding water, giving it a positive charge. The adsorbate itself gains a negative charge, from the hydroxide ions it is left with (
Figure 2).
The force behind the charge polarization effect is analogous to the force in semiconductor junctions: it is diffusion based on thermal energy. Water in the adsorbed phase (a semi-solid phase that is halfway between the liquid and solid phase) auto-ionizes similarly to liquid water. Contrary to water in the liquid phase the hydroxide ions are locked into the semi-solid adsorbate that is anchored to the hydrophilic surface. The more mobile hydrogen ion will diffuse outwards to a larger extent than the hydroxide ion, causing the charge separation effect.
3.2. The Role of Protons in the Mechanism of Osmosis
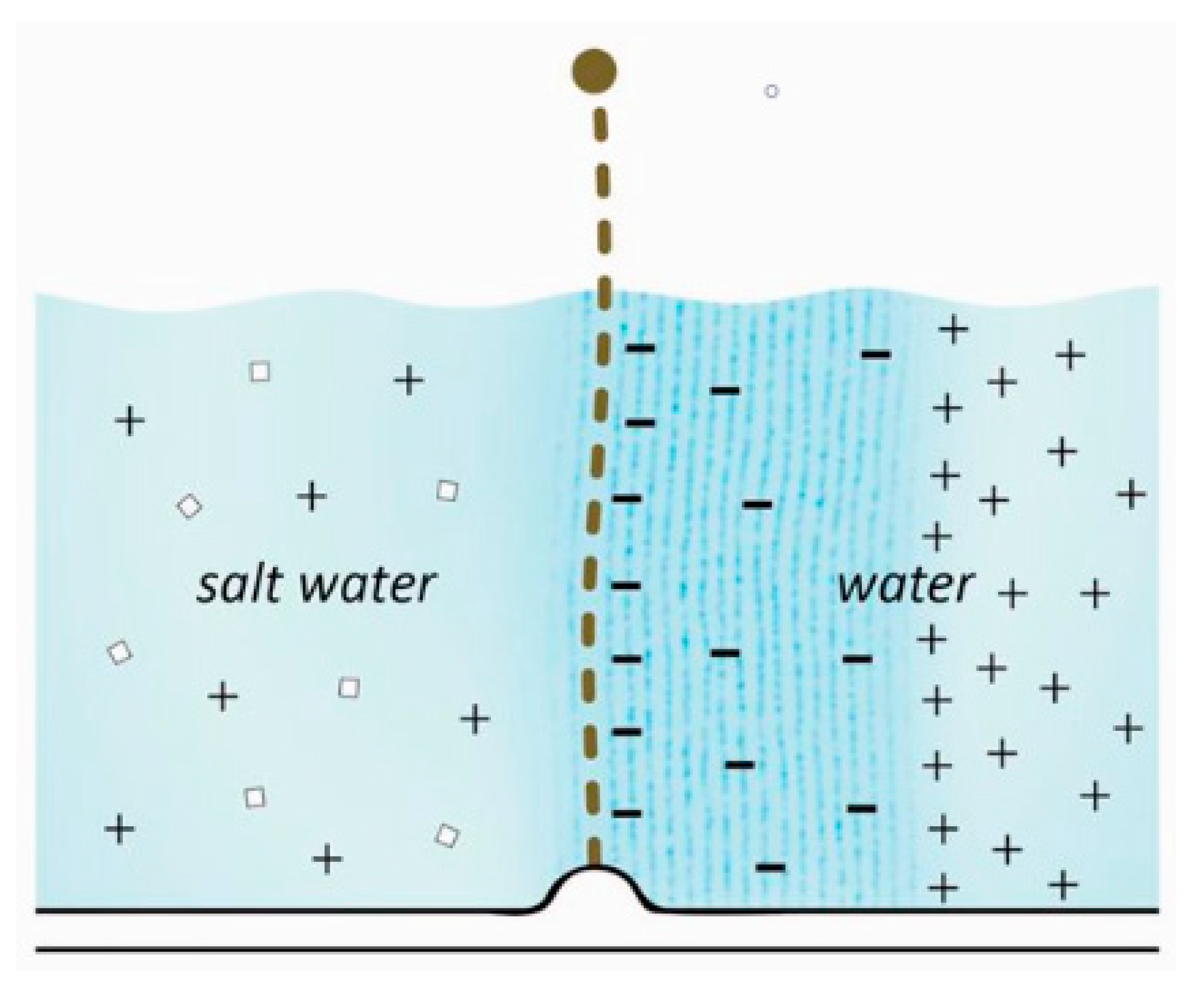
Osmosis occurs when there is an asymmetry between the thickness of the adsorbate on either side of a membrane (
Figure 3). By simple random diffusion, more protons will transfer from the side that has more protons, naturally. The combined negative mass of adsorbate on the membrane, acts to electrostatically limit the diffusion.
The transfer of protons changes the pH of the water on either side of the membrane. The compartment water moves from becomes more alkaline, and the compartment water moves to becomes more acidic (Zhao and Pollack, 2009).
3.3. The Role of Electrons in the Mechanism of Osmosis
It is well known from electrical engineering that an alkaline compartment and an acidic compartment can be turned into a battery (Weng, 2019). In the alkaline compartment, hydroxide ions can oxidize to release electrons, water and dioxide (the technical name for molecular oxygen, O2, is used in this article). In the acidic compartment, hydrogen ions and dioxide can accept the electrons to form water.
The oxygen evolution and reduction reactions in an acid-base battery are chemically the equations 4 OH- --> 2 H2O + O2 + 4 e- and 4 H+ + 4 e- + O2 --> 2 H2O (Weng, 2019). The dioxide can be supplied from an external source such as the atmosphere.
It can be reasoned: the pH gradient that develops during osmosis should cause the same chemical reactions as in an acid-base battery. Hydroxide ions in the alkaline side should start to oxidize and release electrons, and the electrons should transfer to the acidic side, and reduce dioxide to form water.
This article presents the model that osmosis does not move the water molecule as a whole. Rather, it breaks down water on one side, and produces water on the other.
That osmosis is the chemical breakdown and production of water, is a new theory.
Such a model is very simple, and fits very elegantly with known theory such as how acid-base batteries work. The model has never been suggested before. And, it fits perfectly with the model for the kidney that this article will present.
A simple lab experiment that could be used to test the theory that dioxide is required for osmosis is suggested in Nygren (2020).
3.4. Forward Osmosis, Reverse Osmosis, and Narrow Tubes
Salt impairs the formation of the adsorbed phase in the same way it impairs the solid phase by freezing point depression (a colligative property), and an increase in osmolarity on one side will decrease the amount of adsorbate at the membrane on that side. The asymmetry will cause osmosis, as described in the previous section.
Pressure acts in an opposite way. It increases the thickness of the adsorbate (Ypma, 2015), and therefore generates an osmotic transfer of water in the reverse direction to osmolarity-based osmosis, away from the pressure.
In narrow tubes, the proximity of the adsorbate around the circumference of the tube and across the diameter causes an increase in the net positive charge of the lumen of the tube. This repulsion effect is another factor in how the protons distribute around a membrane. The effect has been closely studied for its role in surface-induced flow in narrow tubes (O’Rourke, 2011; Li, 2020), and the same effect can also cause protons to transfer across the tube membrane.
The repulsion effect in narrow tubes provides a method for reverse osmosis that can operate at very low pressures, such as that within the filtration system of the kidney. Since pressure increases the amount of adsorbate, it also increases the repulsion effect in the lumen of narrow tubes. This allows the kidney to regulate the strength of the urine concentration mechanism by regulating the filtration pressure.
4. The Kidney Operates by Reverse Osmosis, not Forward Osmosis
4.1. The Loop of Henle is a Narrow Tube that Forms a Loop
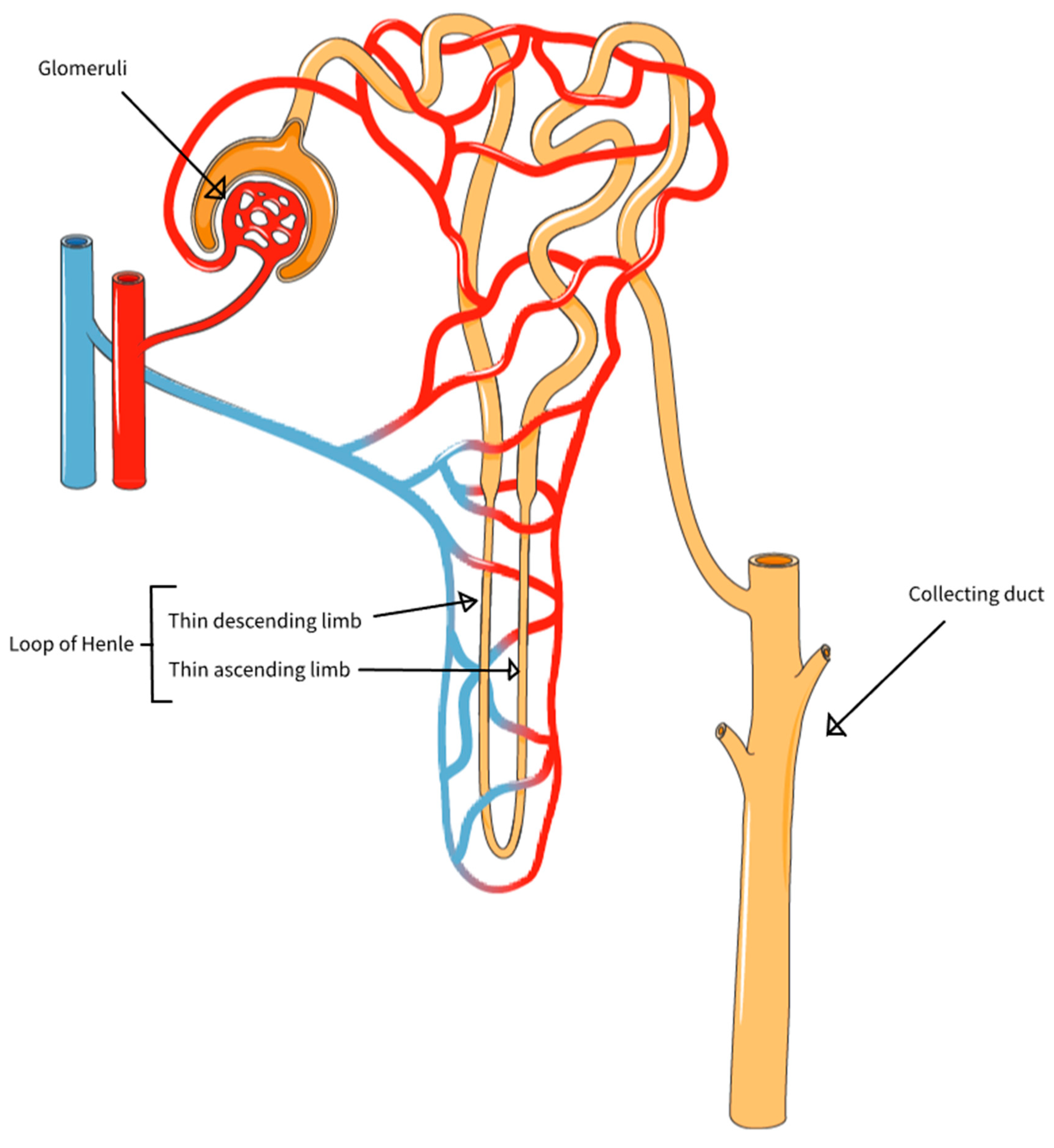
The nephrons of the kidney have an intricate and well defined structure. Each nephron consists of a glomerulus and its subjoined tubule, this tubule being differentiated into a proximal segment, thin segment, and distal segment (Smith, 1951). The glomerulus filters blood into the tube system of the nephron. The filtrate passes through the proximal segment with microvilli, that like the microvilli in the intestine is specialized for absorption. It then passes into the thin segment that forms a loop: the loop of Henle. After that, it continues into the distal segment and from there it drains into a central “collecting duct” that empties into the ureter via the renal papilla (
Figure 4).
The loop of Henle stands out both in that it forms a loop, and also in that it is a thin segment with a narrow diameter. This thin segment of the tubule is well suited to generate the repulsion effect described in section 3.4. The repulsion effect can be predicted to rely on pressure, as pressure increases the amount of adsorbate (see section 3.4) and more adsorbate means more protons released.
4.2. Evidence that Filtration Pressure Controls Urine Concentration
If the thin segment exists to generate the repulsion effect in this “electrostatic reverse osmosis” and this effect relies on pressure, then some evidence would likely have been discovered and documented throughout history. Such is also the case. It was noted by Berliner (1958) that a reduction of glomerular filtration rate in excess of 30 to 35 percent would produce marked impairment in the ability to concentrate urine. The positive correlation between glomerular filtration rate and urine osmolarity was also documented by Boybo (1996).
4.3. The History of Ideas of Kidney Function, from Forward Osmosis to Reverse Osmosis
“I still do not like it: it seems extravagant and physiologically complicated—though so is the whole glomerular filtration—tubular reabsorption pattern. . . . Least of all, however, do I like to see the squamous epithelium of the thin segment freely permeable to water (if not to sodium also) in the descending limb, only to acquire water impermeability and active sodium transport at the tip of the loop for no better reason, apparently, than the circumstance that it has turned a corner. But I suppose that I can get used to that, too.’’ Homer Smith, 1958 (via Gottschalk, 1987)
Historically, the loop of Henle motivated a physiological and evolutionary explanation. Why was it narrower than the other segments, why did it have a thin epithelium, and why did it make a loop turn? The interest in these questions grew in the 1950s when cryoscopic studies (Wirz and Kuhn, 1951) and later on micropuncture studies (Gottschalk and Mylle, 1959) showed that the kidney had an osmolarity gradient. The osmolarity was shown to gradually increase from the cortex (outer layer) and into the medulla, with a very high osmolarity of upwards of 1200 mOsm close to the renal papilla. Kuhn (1942; 1951) noted how the loop of Henle was anatomically similar to countercurrent systems in chemistry and engineering, and suggested the countercurrent multiplication model: that the kidney used forward osmosis to concentrate urine, and that the loop of Henle had evolved to move particles such as sodium ions into the renal medulla from the upwards segment, drawing water out of the downwards segment by forward osmosis.
Strictly speaking, there are two ways to achieve the osmolarity gradient in the kidney medulla: add salt, or remove water. The forward osmosis model suggested the gradient was the result of addition of salt, pumped out in the upwards segment. The reverse osmosis model (“electrostatic reverse osmosis”), suggests the gradient is a result of a removal of water.
In this article, it is suggested that Kuhn was wrong with his countercurrent multiplication theory, and that the scientific consensus in kidney science has been wrong for 70 years. The reverse osmosis effect in narrow tubes is instead suggested as how the kidney concentrates urine. In this new model, the true reason for the loop of Henle is perhaps even more directly tied to the mechanism of osmosis: the loop of Henle serves to recycle the dioxide that is released inside the nephron, and that has to be moved back again via the nephron capillaries to sustain the osmosis.
5. The Role of the Loop of Henle Is to Recirculate Dioxide
The role of the thin segment in the tubule is the narrow tube type of reverse osmosis, where protons transfer over the epithelium because of electrostatic repulsion and combine with dioxide within the capillary beside it. The reason the thin segment makes a loop is to sustain the supply of dioxide. The loop of Henle works together with the capillary next to it and with the collecting duct to recirculate dioxide. The following sections will go through every step of this process.
5.1. The Dioxide Recirculation Sustains the Concentration of Urine
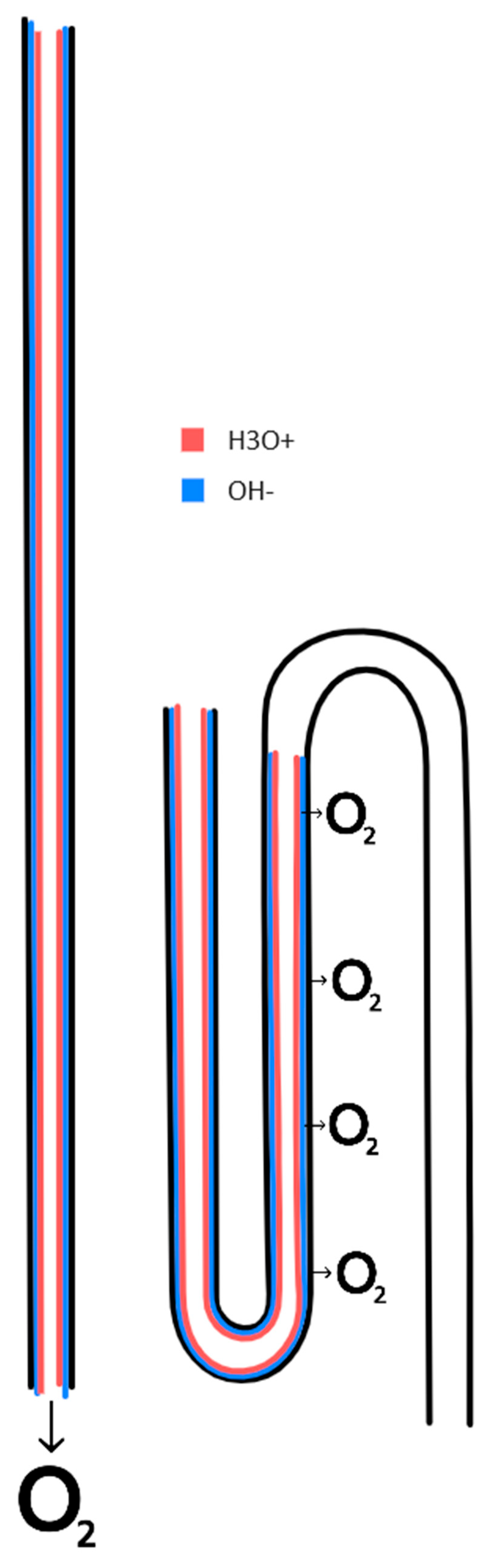
To understand what it means that the loop of Henle recirculates dioxide, it is good to consider what happens without a loop. As was described in section 3.3, dioxide is required for osmosis. The nephron capillaries provide the dioxide, and it accepts protons and electrons to form water. Inside the nephron, dioxide is released at the same time. If the dioxide that is released into the nephron is not somehow recycled, it will be excreted with the urine (
Figure 5). What the loop of Henle does is that it allows this dioxide to be returned to the beginning of the loop, so that it can be reused. Thus, more water molecules can be reabsorbed osmotically.
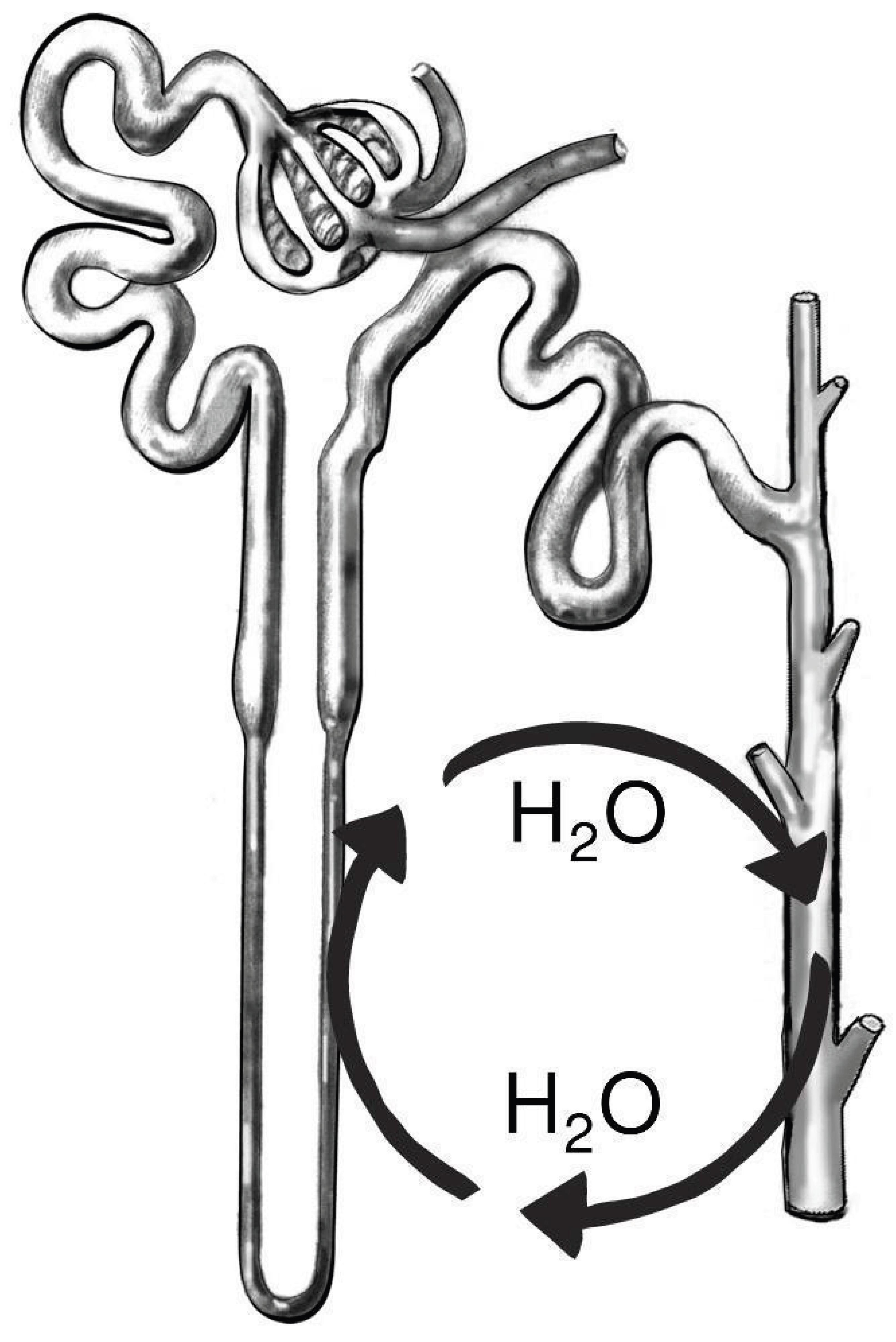
The capillaries around the loop of Henle (called “vasa recta”) have fluid flow in the opposite direction to that inside the loop of Henle (
Figure 6). Thus, dioxide reabsorbed from the ascending limb of the loop of Henle, will move with the blood in an opposite loop, to end up at the descending limb. It is in the descending limb that the reabsorption of water takes place.
To repeat the theory from section 3.3, the dioxide in the nephron capillaries accepts protons and electrons to form water as 4 H+ + 4 e- + O2 --> 2 H2O. And inside the nephron, hydrogen ions transfer from the adsorbate (by the repulsion effect), and hydroxide ions break down as 4 OH- --> 2 H2O + O2 + 4 e-. The dioxide that is released inside the nephron, is what is recirculated back to the capillaries on the outside.
The article will elaborate on how the loop of Henle recycles dioxide. But first, some math will be used to show why the recirculation is needed to start with.
5.2. Math for Why Dioxide Recirculation is Needed
It is often estimated that 25 moles of dioxide is absorbed from the lungs each day. The kidney receives 1/5th of cardiac output, thus, 5 moles of dioxide. This can sustain a reabsorption of 10 moles of water. To repeat the theory in section 3.3, 1 O2 is required to reabsorb 2 H2O.
Water is 55.5 moles per liter. 10 moles of dioxide is only enough to reabsorb around 2 deciliters of water. To compare with a normal urine production of 1.5 liters per day, concentrated to 1000 mOsm, ~4 liters of water had to be reabsorbed. This is based on that the blood osmolarity is around 275 mOsm. If you remove half the water, and then remove half the water again, you are at 275*2*2 = 1100 mOsm. Thus you need to start with 6 liters, and reabsorb 4.5 liters. That is 4.5/0.2 = 22 times more water than there is dioxide for, unless the dioxide is reused.
5.3. How the Loop of Henle Recycles Dioxide
Dioxide is released into the nephron in the thin descending limb when water is reabsorbed by reverse osmosis (and transported out of the kidney via the ascending vasa recta). This dioxide has to be returned into the medulla and the blood stream, or it will be excreted in the urine (
Figure 5).
The mechanism for reabsorbing the dioxide, is a bit counterintuitive at first, and requires multiple steps. To reabsorb the dioxide, the kidney uses osmosis all over again, and moves water back into the ascending thin limb of the loop of Henle (facilitated by the continuous lumen diameter increase along the ascending limb, see Koepsell and Schnerman, 1972). This seemingly counterproductive arrangement, where there is no net osmotic transfer of water, works together with yet another part of the kidney, the collecting duct system. It facilitates the non-osmotic reabsorption of water from the collecting duct system. The reverse osmosis in the thin descending limb dehydrates the medulla, and favors the diffusion of water from the collecting duct tree. This concentrates the urine before it leaves through the papilla. The water reabsorbed from the collecting duct, is in a 1:1 relationship with the water that is osmotically transferred into the thin ascending limb. The water loops around from the collecting duct to the ascending thin limb and back again (
Figure 7). This “second loop” facilitates the recirculation of dioxide via the vasa recta (that moves in the direction opposite to the loop of Henle. ) Thus, there is one loop for dioxide, and one for water.
To use the math example from section 5.2, to produce 1.5 liters of urine with an osmolarity of 1100 mOsm, from blood at 275 mOsm, 4.5 liters of water had to be reabsorbed from the thin descending limb, 4.5 liters had to be moved into the thin ascending limb again, and 4.5 liters had to be moved out of the collecting duct. In total, 4.5 liters was moved out of the kidney and back into the blood circulation via the renal vein.
The complex machinery described in this section exists only to recirculate the dioxide. And the recirculation is needed because only minimal quantities of water could be reabsorbed otherwise, thus the ability to concentrate urine would be insignificant. Terrestrial animals therefore evolved the loop of Henle system, to conserve water.
6. Regulation and Dysfunction of the Urine Concentration Mechanism
6.1. The Kidney Adjusts the Narrow Tube Osmosis Effect with Tubuloglomerular Feedback
“I thought it was a kind of interstitial connective tissue, but emphasized its resemblance to the elements of organic muscle tissue. Virchow declared them to be muscle fiber cells; Frerichs left their origins uncertain” - Jakob Henle, Zur Anatomie der Niere, 1862
Tubuloglomerular feedback is subordinated vasopressin and operates reflexively to decisions by the hypothalamus. Vasopressin is ultimately the control mechanism for urine concentration, thereby subordinating the kidney under the hypophysis and the brain. The role of vasopressin is to increase the resistance in the collecting duct tree by contracting fibroblast-like cells in the kidney medulla (Hughes, 1995; 2006; Gilmer, 2018), and also to increase water permeability of the collecting duct epithelium by aquaporin channels. The tubuloglomerular autoregulatory system responds to an increase in water reabsorption from the collecting duct by sensing a decrease in filtrate osmolarity at the macula densa region of the thick ascending limb, and upregulates the reverse osmosis mechanism in the thin descending limb of the loop of Henle by increasing the glomerular filtration pressure through renin and angiotensin. Or, rather, it senses only osmolarity increase (concentrating urine more than what is required by vasopressin’s effect), and release of renin is the default and it gets downregulated whenever macula densa senses that the urine is concentrated more than it should be, operating by negative feedback. This reflex autoregulatory mechanism can also maintain a steady filtration pressure (and therefore urine concentration effect) even as systemic pressure varies depending on activity.
6.2. Short-Circuiting the Dioxide Recirculation, Why Hyperglycemia Causes Medullary Hypoxia
Normally in osmosis, the oxygen evolution 4 OH- --> 2 H2O + O2 + 4 e- releases electrons whilst also producing dioxide, and in the kidney, this dioxide is recirculated back via the vasa recta. The oxidation reaction can also take place with a carbohydrate such as glucose as a reactant and will in that case not produce dioxide but instead carbon dioxide. Chemically, as CH2O + 4 OH- —> 3 H2O + CO2 + 4 e-. This has two effects. First, the urine concentration mechanism fails because the cathode for osmosis cannot be reused. And second, the total dioxide in the medulla decreases, leading to cell death and medullary necrosis.
7. Discussion
This article argues that the kidney operates by a form of reverse osmosis within the thin segment of the nephron. The kidney has evolved the loop structure of the thin segment to recirculate dioxide (via the vasa recta) that is required in the cathode reaction of osmosis. The osmotic effect in the thin segment works together with the non-osmotic reabsorption of water from the collecting duct to recirculate dioxide while also excreting concentrated urine. Advances in the understanding of the physical mechanism behind osmosis, the inherent charge separation in adsorbed phase of water, has made it possible to discover the true nature of the nephron of the metanephros. It was impossible to conceive of a similar explanation before discovering that the adsorbed water at the membrane is what drives osmosis, thus the “upside down” explanation of countercurrent multiplication was the only alternative. Reverse osmosis was also not as widely understood by the 1950s and that may be another reason forward osmosis was initially assumed, although it would still have been impossible to explain how reverse osmosis could operate at such low pressures.
References
- Berliner, R. W., Levinsky, N. G., Davidson, D. G., & Eden, M. (1958). Dilution and concentration of the urine and the action of antidiuretic hormone. In The American Journal of Medicine (Vol. 24, Issue 5, pp. 730–744). Elsevier BV. [CrossRef]
- Bouby, N., Ahloulay, M., Nsegbe, E., Déchaux, M., Schmitt, F., & Bankir, L. (1996). Vasopressin increases glomerular filtration rate in conscious rats through its antidiuretic action. Journal of the American Society of Nephrology : JASN, 7(6), 842–851. [CrossRef]
- Chai, B., Yoo, H., & Pollack, G. H. (2009). Effect of Radiant Energy on Near-Surface Water. In The Journal of Physical Chemistry B (Vol. 113, Issue 42, pp. 13953–13958). American Chemical Society (ACS). [CrossRef]
- Gilmer, G. G., Deshpande, V. G., Chou, C. L., & Knepper, M. (2018). Flow resistance along the rat renal tubule. American journal of physiology. Renal physiology, 315(5), F1398–F1405. [CrossRef]
- Gottschalk, C. W., & Mylle, M. (1959). Micropuncture study of the mammalian urinary concentrating mechanism: evidence for the countercurrent hypothesis. In American Journal of Physiology-Legacy Content (Vol. 196, Issue 4, pp. 927–936). American Physiological Society. [CrossRef]
- Gottschalk C., W. (1987). History of the urinary concentrating mechanism. Kidney international, 31(2), 507–511. [CrossRef]
- Greenlee, L. F., Lawler, D. F., Freeman, B. D., Marrot, B., & Moulin, P. (2009). Reverse osmosis desalination: Water sources, technology, and today’s challenges. In Water Research (Vol. 43, Issue 9, pp. 2317–2348). Elsevier BV. [CrossRef]
- Hargitay, B., & Kuhn, W. (1951). Das Multipikationsprinzip als Grundlage der Harnkonzentrierung in der Niere. Zeitschrift für Elektrochemie und angewandte physikalische Chemie, 55(6), 539-558.
- Henle, J., 1862. Zur Anatomie der Niere. Göttingen.
- Hughes AK, Barry WH, Kohan DE. Identification of a contractile function for renal medullary interstitial cells. J Clin Invest. 1995 Jul;96(1):411-6. [CrossRef] [PubMed] [PubMed Central]
- Hughes, A. K., & Kohan, D. E. (2006). Mechanism of Vasopressin-Induced Contraction of Renal Medullary Interstitial Cells. Nephron Physiology, 103(3), p119–p124. [CrossRef]
- Koepsell, H., Kriz, W., & Schnermann, J. (1972). Pattern of luminal diameter changes along the descending and ascending thin limbs of the loop of Henle in the inner medullary zone of the rat kidney. Zeitschrift fur Anatomie und Entwicklungsgeschichte, 138(3), 321–328. [CrossRef]
- Kuhn, W., & Ryffel, K. (1942). Herstellung konzentrierter Lösungen aus verdünnten durch bloße Membran Wirkung. Ein Modellversuch zur Funktion der Niere. In Hoppe-Seyler’s Zeitschrift für physiologische Chemie (Vol. 276, Issues 4–6, pp. 145–178). Walter de Gruyter GmbH. [CrossRef]
- Li, Z., & Pollack, G. H. (2020). Surface-induced flow: A natural microscopic engine using infrared energy as fuel. In Science Advances (Vol. 6, Issue 19). American Association for the Advancement of Science (AAAS). [CrossRef]
- Nygren, Johan. (2020). Osmosis consumes and produces water? Simple experiment to test it. [CrossRef]
- O’Rourke, C., Klyuzhin, I., Park, J. S., & Pollack, G. H. (2011). Unexpected water flow through Nafion-tube punctures. In Physical Review E (Vol. 83, Issue 5). American Physical Society (APS). [CrossRef]
- Pollack, G., 2013. The Fourth Phase Of Water. Seattle: Ebner and Sons.
- Smith, H. W. (1953). From Fish to Philosopher. Little, Brown and Company.
- Weng, G.-M., Li, C.-Y. V., & Chan, K.-Y. (2019). An Acid–Base Battery with Oxygen Electrodes: A Laboratory Demonstration of Electrochemical Power Sources. In Journal of Chemical Education (Vol. 96, Issue 8, pp. 1701–1706). American Chemical Society (ACS). [CrossRef]
- Wirz, H., Hargitay, B., & Kuhn, W. (1951). Lokalisation des Konzentrierungsprozesses in der Niere durch direkte Kryoskopie. Helv Physiol Pharmacol Acta, 9, 196–207.
- Ypma, R. E., & Pollack, G. H. (2015). Effect of hyperbaric oxygen conditions on the ordering of interfacial water. Undersea & hyperbaric medicine : journal of the Undersea and Hyperbaric Medical Society, Inc, 42(3), 257–264.
- Zhao, Q., Ovchinnikova, K., Chai, B., Yoo, H., Magula, J., & Pollack, G. H. (2009). Role of proton gradients in the mechanism of osmosis. The journal of physical chemistry. B, 113(31), 10708–10714. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).