1. Introduction
Oral isotretinoin treatment induces a series of clinical changes that may compromise therapeutic adherence.
One of the primary effects of oral isotretinoin treatment is the alteration of skin hydration due to increased transepidermal water loss (TEWL) and disruption of the stratum corneum’s permeability. These effects can trigger signs such as erythema, xerosis, and desquamation, which may be accompanied by pruritus. Side effects such as dryness and desquamation of the skin and mucous membranes (like cheilitis, xerosis, xerostomia, and dry eyes) are very frequent; secondary to a sebum- suppressive effect and epidermal cohesion. Dry skin and desquamation are frequently observed in a dose-dependent manner.[
1]
Isotretinoin-induced alterations in facial skin surface lipids are characterized primarily by an increased proportion of cholesterol (10%-25%), and decreased proportion of wax esters and squalene. [
2] Since isotretinoin significantly diminishes the production and release of sebum, predictable quantitative alterations in the ratios of epidermal surface lipids are directly associated with its application. [
3] This implies a reduction in comedogenesis but, it also induces skin fragility, intraepidermal separation, and desquamative changes, it can impact the level of skin hydration. [
4,
5]
While these side effects may reduce compliance and patient satisfaction, studies have shown that the use of cosmetic adjuvant treatment can increase patient adherence to isotretinoin treatment. [
6,
7] Currently, a skin barrier repair product (Biretix Isorepair) is available on the market, formulated with active ingredients such as ceramides, hyaluronic acid, vitamin E, and SCA® Growth Factor Technology and Browsellia serrata (anti-inflammatory botanical extract, soothes the skin. [
8]
Ceramides are natural lipids present in the skin that play a crucial role in maintaining the skin barrier function. They help retain moisture and protect against external factors that may cause irritation. Authors such as Danby et al. demonstrated that a cream containing ceramides vs placebo facilitated skin barrier restoration and protected the skin from dryness and irritation in patients with dry, eczema-prone skin. As previously commented the skin of patients under oral isotretinoin treatment has these characteristics, therefore should also benefit from its use. [
9] In addition, Tempark et al, concluded that a ceramide and niacinamide-containing moisturizer in combination with anti-acne medication can significantly improve acne lesions and decrease cutaneous irritations, compared with hydrophilic cream. [
10] Applying external ceramides within moisturizers has demonstrated positive outcomes in reducing lesions, enhancing ceramide composition, and fortifying the skin barrier. [
11]
Hyaluronic acid is an active with specifically proven efficacy in oral isotretinoin patients, as published by Herane et al, where evaluated the effect of a cream containing hyaluronic acid as adjuvant treatment in 66 patients on oral isotretinoin. The data analysis revealed superior efficacy in mitigating xerosis compared to the placebo control (64.5% versus 40.6%, p < 0.05) as well as significant improvements in desquamation, xerosis, pruritic sensation and erythema. Additionally, a significant increase in hydration from baseline was observed in the active group (41.88 vs. 47.44, p < 0.01). [
7]
Vitmamin E (Tocopheryl acetate) topical application offers significant benefits in alleviating cutaneous irritation. This potent antioxidant exhibits notable anti-inflammatory properties, as well as emollience activity. [
12] The lipid-soluble properties of vitamin E, (tocopherols), allow it to reach deeper layers of the skin via sebaceous gland secretions, to reside within cell membranes and protect them from oxidative stress through its antioxidant properties. [
13]
SCA Growth factor technology® has proven to induce regenerative, antioxidant, emollient and moisturizing effects. [
14,
15,
16,
17] SCA can also improve fibroblast growth and has demonstrated the ability to accelerate skin regeneration induced by physical aggression. [
18,
19]
The objective of this clinical experience was to evaluate the use of this specific moisturizer (Biretix isorepair®) formulated with ceramides, hyaluronic acid, vitamin E and SCA® Growth factor technology in patients under oral isotretinoin treatment, to determine the impact on hydration, side-effects and patient satisfaction.
2. Materials and Methods
2.1. Study Design
An prospective, open-label clinical study was contucted in 20 patients with mild to moderate acne vulgaris who were candidates for oral isotretinoin therapy. All participants iniciated treatment with low-dose oral isotretinoin (<0.5 mg/kg/day, fixed dose of 20 mg/day) wich was maintained throughout the 12 weeks follow-up period. From baseline, in accordance with standard clinical practice, all patients were advised to implement adjuvant skin hydration measures. Follow-up visits were scheduled at 6 weeks and 12 weeks (i.e., at approximately 1.5 and 3 months), in accordance with clinical practice.
2.2. Investigational Products
Ten participants received the investigational moisturizer (Biretix® Isorepair) while the remaining ten used a commercially available standard moisturizer crformulated for acne-prone skin (from different recognized brands). All moisturizers were applied twice daily (morning and evening). In addition, all participants used a mild facial cleanser twice daily and applied a broad-spectrum sunscreen during daytime hours. No additional cosmetic products were used during the study period.
2.3. Evaluations
Assessments were performed at baseline (T0), at 45 days (T45), and at 90 days (T90) after treatment initiation. At each time point, the following evaluations were conducted: (1) clinical examination, (2) instrumental skin hydration measurement, and (3) a subjective patient-reported outcome questionnaire. Additionally, standardized clinical photographs were obtained from five participants, with prior informed consent.
Skin hydration was assessed using a digital skin moisture analyzer (Brrnoo®, China), which provides a semi-quantitative estimate of stratum corneum hydration. The device reports hydration levels as a percentage, with reference values between 35–55%, according to the manufacturer’s specifications.
Age, sex and previous history of sensitive skin or atopic dermatitis were registered at the basal visit. Additionally, during all visits, the following data were assessed:
- -
PGA (Patient global assessment): clear (0), almost clear (1), mild (2), moderate (3), severe (4)
- -
IGA (Investigator global assessment): 0: healthy clear skin with no evidence of acne vulgaris; 1: almost clear; rare noninflammatory lesions present; rare noninflamed re-solving papules (may be hyperpigmented but not pink-red); 2: some noninflammatory lesions present; few inflammatory lesions (papules/pustules only; no nodulocystic le-sions); 3: noninflammatory lesions predominate; multiple inflammatory lesions present; several to many comedones and papules/pustules; one small nodulocystic lesion; 4: in-flammatory lesions predominate; many comedones and papules/pustules; may or may not be a few nodulocystic lesions; 5:highly inflammatory lesions predominate; variable number of comedones; many papules/pustules and nodulocystic lesions. [
20]
- -
Number of inflammatory lesions
- -
Number of non-inflammatory lesions
- -
Scarring (0: none, 1: mild, 2: moderate, 3: severe)
- -
Semi-quantitative hydration measurement
- -
PBI (Patient Benefit Index): Expectation Score (Annex I)
Additionally, at T45 and T90 visits it was also assessed:
- -
Skin (facial and extra-facial) and mucosal tolerance: Rated as -2 (very poor), -1 (poor), 0 (neutral), 1 (good), 2 (very good). Specifically, erythema, xerosis, desquamation, and pruritus were assessed on a scale of 0-3.
- -
Treatment adherence: Rated as never (0), sometimes (1), always (2).
- -
Patient global improvement assessment: Rated on a scale from 0 to 3 (0: no change, 1: mild improvement, 2: moderate improvement, 3: significant improvement).
- -
Investigator global improvement assessment: Rated on a scale from 0 to 3 (0: no change, 1: mild improvement, 2: moderate improvement, 3: significant improvement).
- -
Overall patient’s satisfaction: 0: none, 1: mild, 2: moderate, 3: intense.
- -
Patient’s experience of product usage (Annex I)
2.4. Aim of the Study
The primary objective was to evaluate whether significant differences in skin sensitivity and hydration levels could be observed at 45 and 90 days of treatment, as measured by changes in skin hydratation percentage.The hypothesis was that the investigational cream (Biretix® Isorepair) would demonstrate superior efficacy compared to the standard moisturizer. Specifically a smaller reduction of skin hydration levels over time would indicate greater effectiveness of the study cream in maintaining skin barrier function and preventing dehydration.
2.5. Statistical Analysis
This was a pilot study. Therefore, no formal sample size calculation was needed. To analyze potential differences in the parameters assessed across the different visits between patients treated with Biretix isorepair® or the standard cream, a repeated measures MANOVA was performed, including one between-subjects factor (Treatment) and one within-subjects factor (Visit).
For an overall assessment of each treatment’s progression, a MANOVA considering all three visits was conducted. In cases where the interaction effect (Visit × Treatment) was statistically significant, pairwise analyses were performed between specific timepoints (T0 vs. T45 and T45 vs. T90) to determine the significance of these individual comparisons. No significant differences were observed in any parameter at the baseline visit (T0). A difference was considered statistically significant when the p-value for the hypothesis test was less than or equal to 0.05 (p ≤ 0.05). Results were described using means and standard deviations for continuous variables, while frequency tables were used for ordinal variables when appropriate. All analyses were conducted using SPSS version 25.0.
3. Results
All 20 patients who initiated the clinical experience completed the scheduled follow-up visits. No participants discontinued the treatment due to tolerability or safety issues. The stuyd population consisted of 14 women and 6 men with age ranging from 14 to 32 years, with a mean age of 20 years. All patients adhered to the prescribed oral isotretinoin regiment throughout the study. At baseline five patients (3 in the study cream group and 2 in the standard cream group) reported a previous history of sensitive skin. Product usage was comparable between groups, with an average consumption of 50ml per patient every 45 days for both the investigational and standard cream.
3.1. Evolution of Acne Severity:
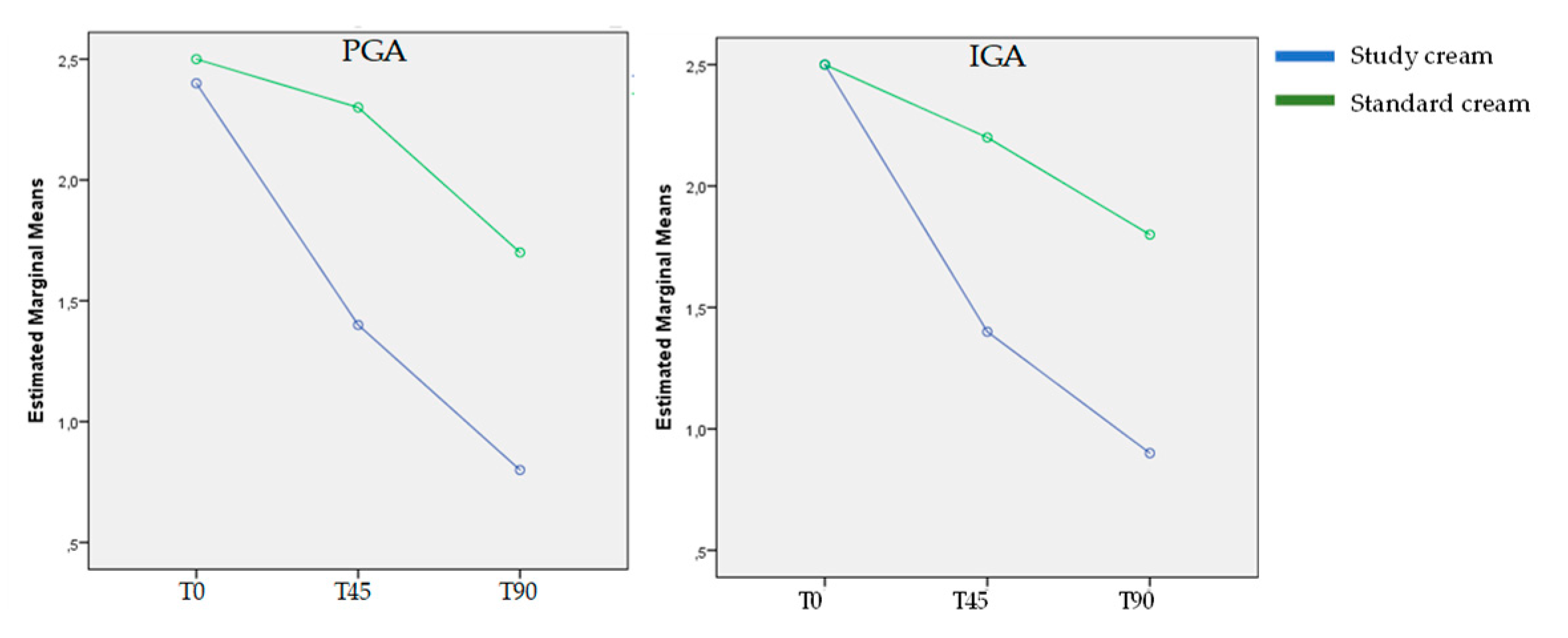
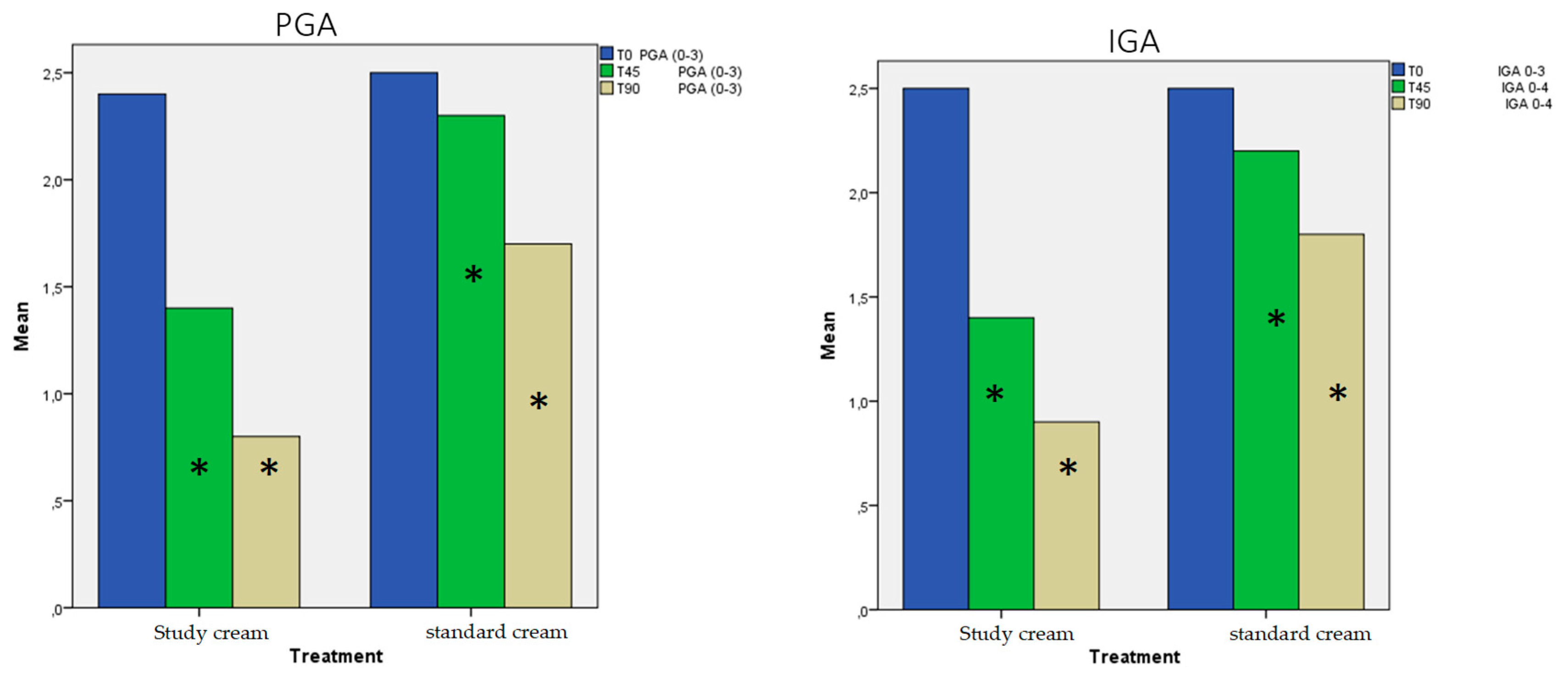
A significant reduction in acne severity was observed from day 45 (T45) onward in both groups, as assessed using the PGA and IGA scales (p<0.001). The significant interaction p-value indicates a differential progression over the study visits, with a significantly higher decline in scores observed in the group treated with the study product (p=0.001) (
Figure 1). More specifically, the PGA scores showed a significantly greater reduction in the study product, at both T45 (p = 0.026) and T90 (p < 0.001). Similarly the IGA socres favored the study product, with significant differences at T45 (p = 0.030) and T90 (p = 0.001). (
Figure 2)
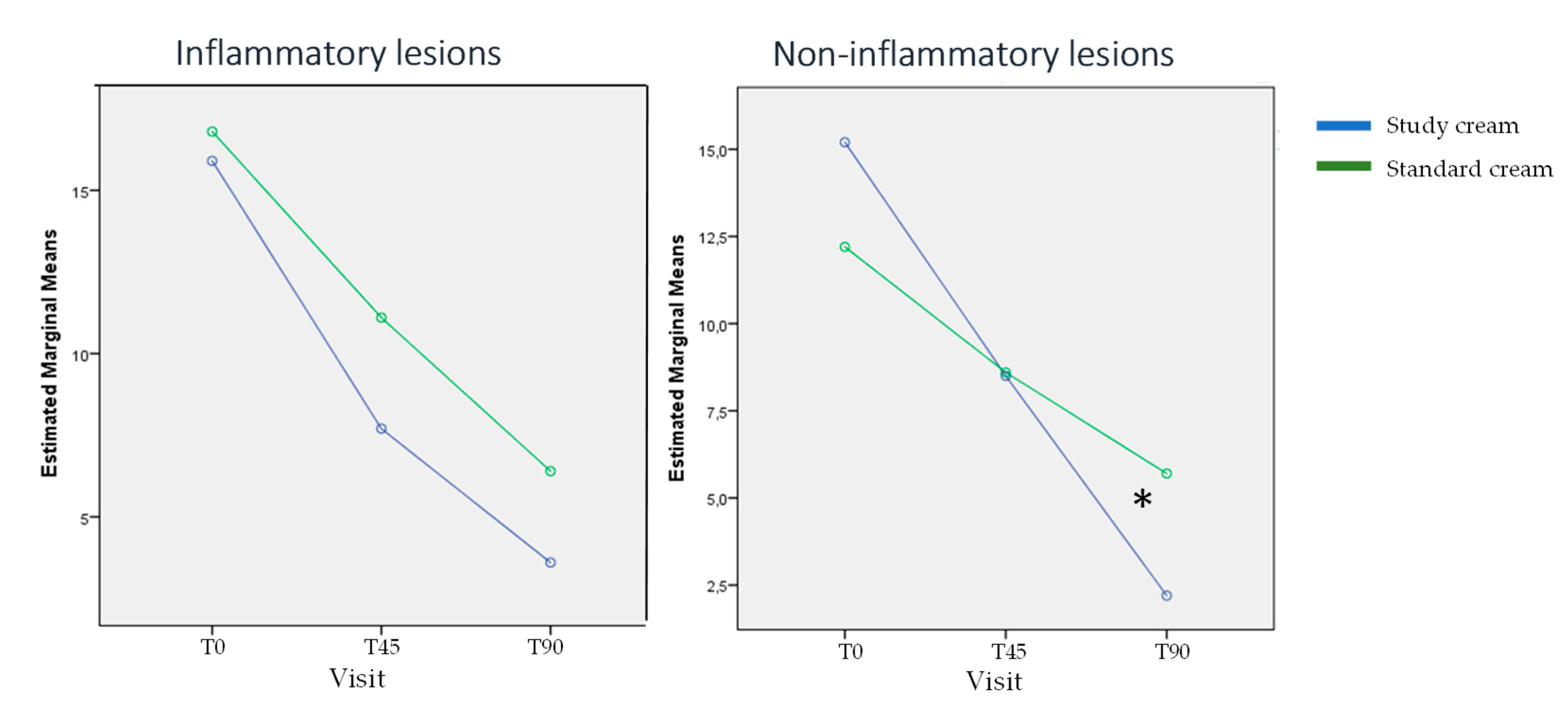
The average number of both inflammatory and non-inflammatory lesions decreased over the course of the study in both groups. While no significant differences were observed between groups for inflammatory lesions (p=0.4) the study cream group showed a significantly greater reduction in non-inflammatory lesions compared to the control group (p=0.002). (
Figure 3).
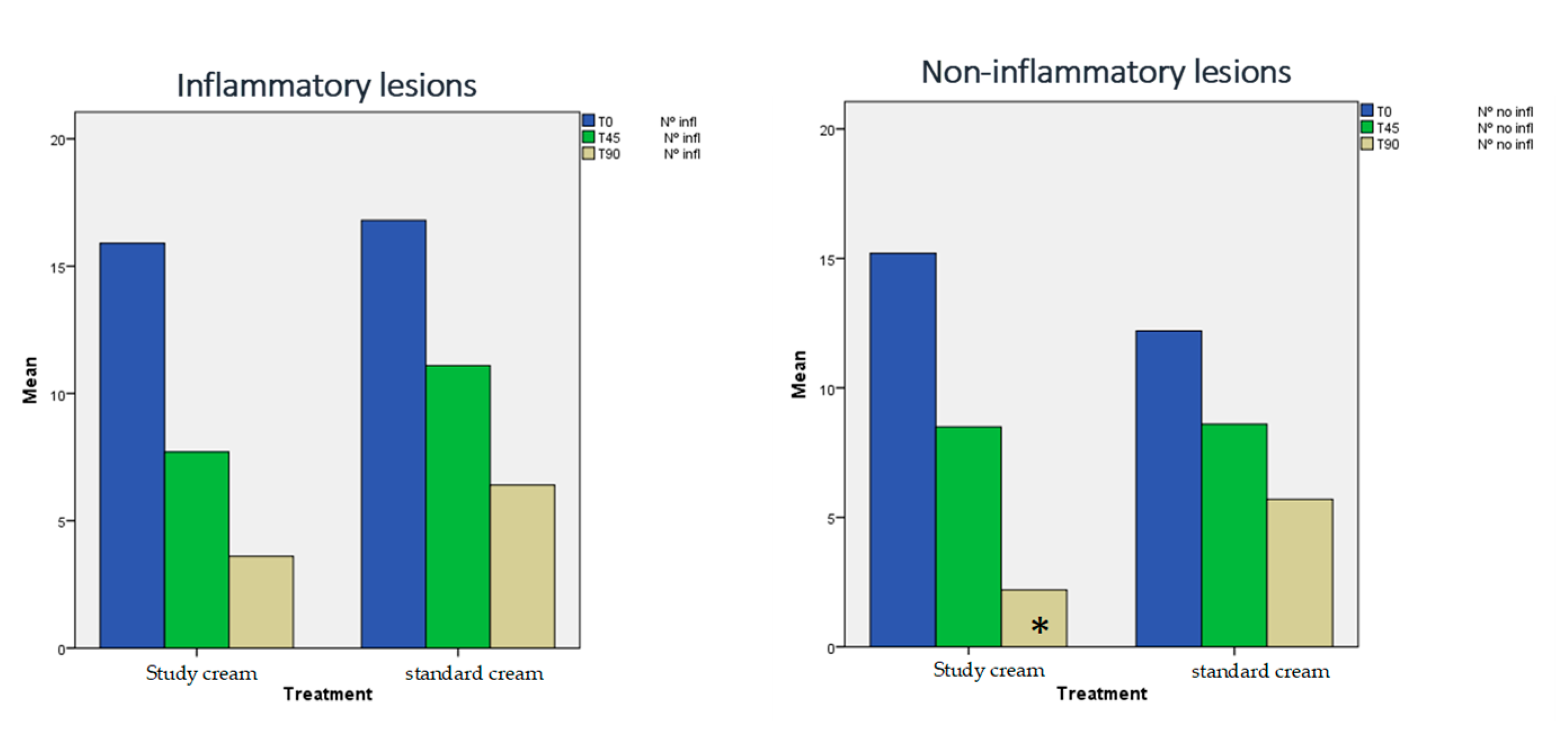
Both groups demonstrated , a significant reduction in lesion counts from T45, which was maintained in T90 (p<0.001), no statistically significant differences were found between groups for either inflammatory (p= 0,337) or non- inflammatory lesions p=0.411). However in T90 the difference in non-inflammatory lesions counts became significant in favor of the study cream (p=0.04), whereas inflammatory lesion counts remained similar between groups (p = 0.734) (
Figure 4)
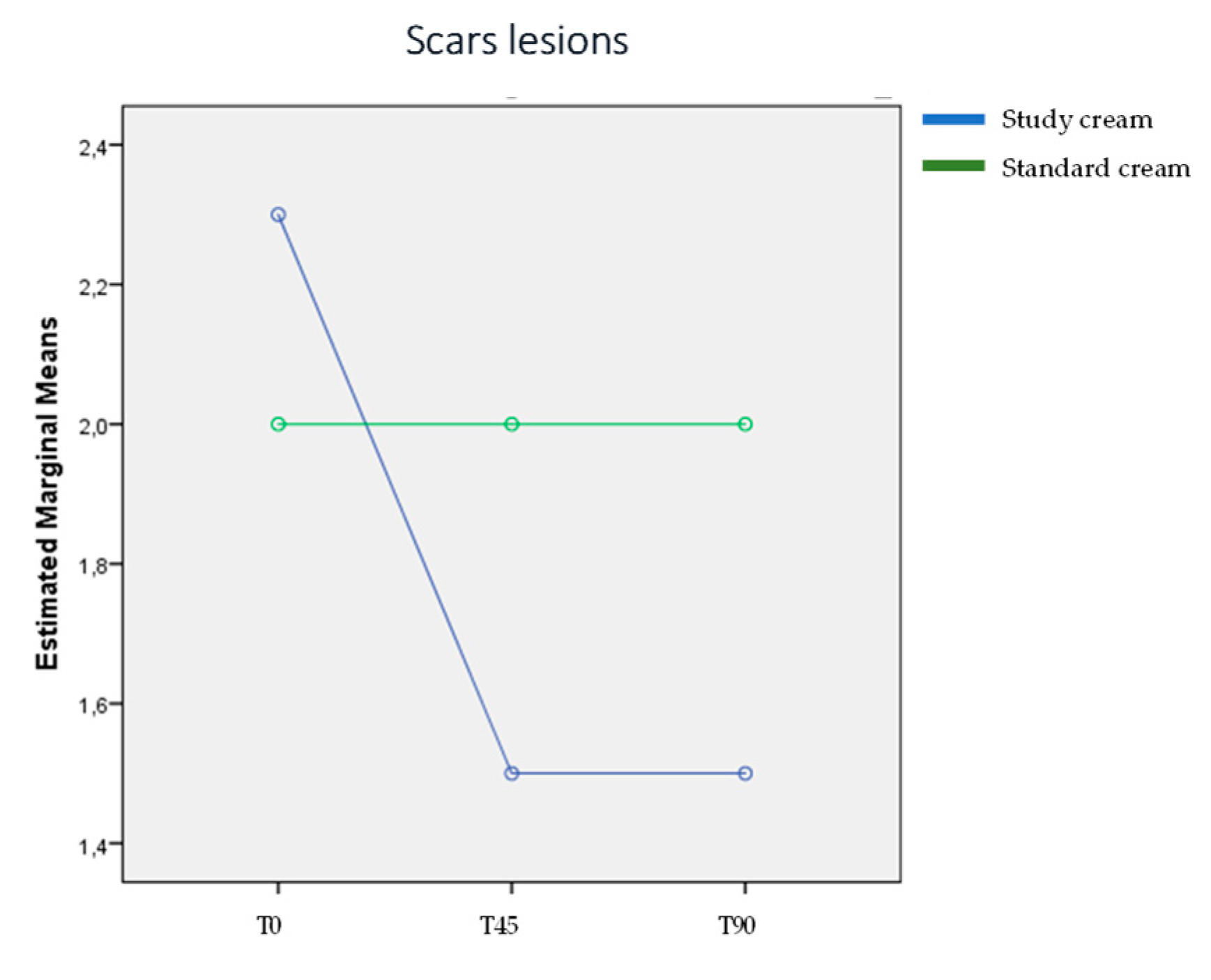
3.2. Scar Grading Evaluation:
Analysis os scar severity indicated a significantly greater reduction in scores ath T45 in the group with the study product (p=0.013) suggesting a more favorable evolution (
Figure 5). From T45 to T90 scare grading remained stable in both groups, with no further significant changes or differences between them. (p=0.131)
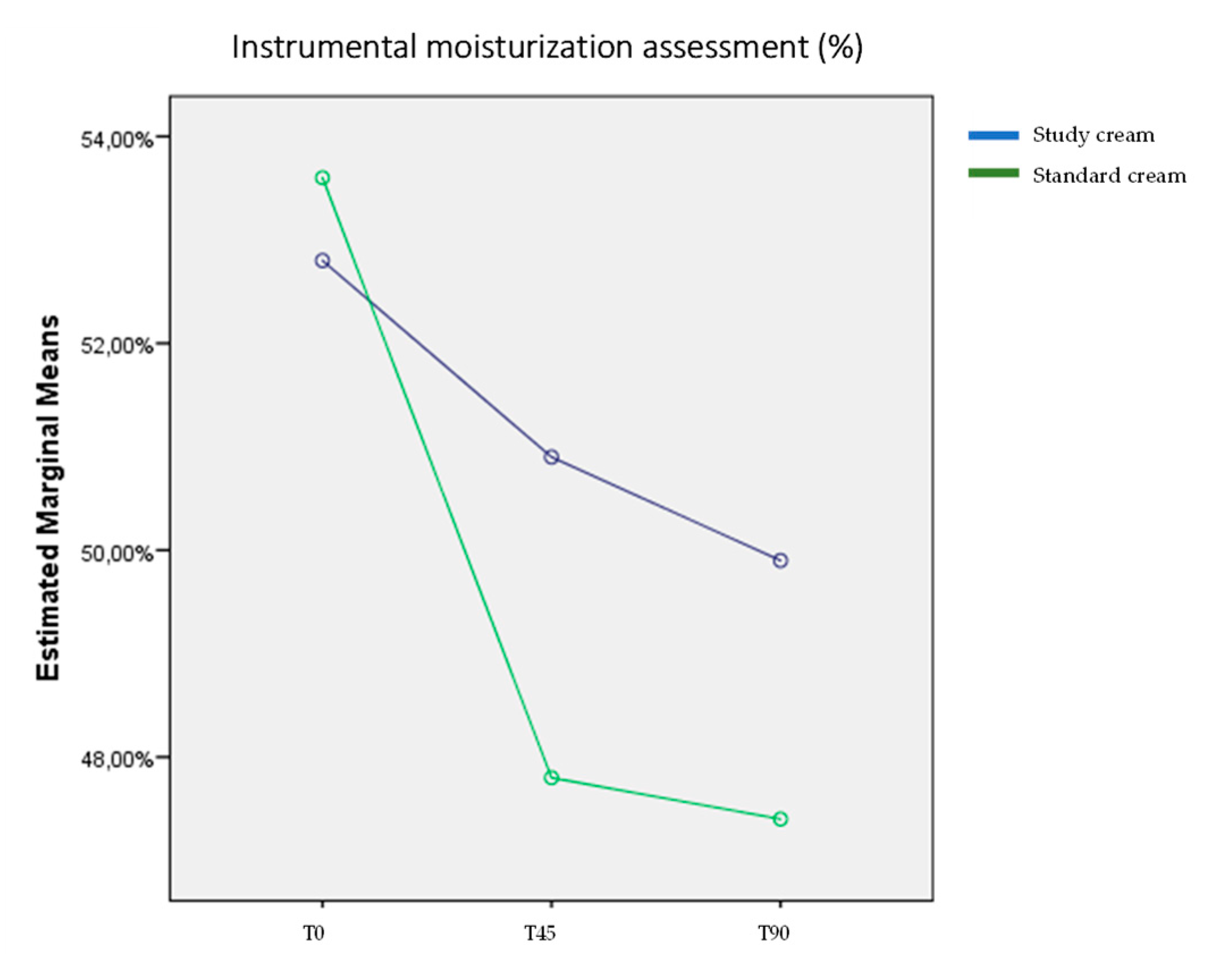
3.3. Instrumental Skin Moisturization
At baseline (T0), no significant differences were observed in skin hydration levels between groups (p=0.123). At T45, average hydration decreased from 53% to 51% in the study cream group and from 54% to 48% in the standard cream group. A multivariate analysis of variance (MANOVA) comparing T0 and T45 revealed a significantly greater reduction in hydration in the standard cream group (p = 0.009). (
Figure 6)
Between T45 and T90, hydration level declined slightly but not significantly in either groups (p=0.12). However, at T90, the study cream group maintained significantly higher hydration levels compared to the conrol group (p=0.04) indicating a sustained barrier-preserving effect. (
Figure 6)
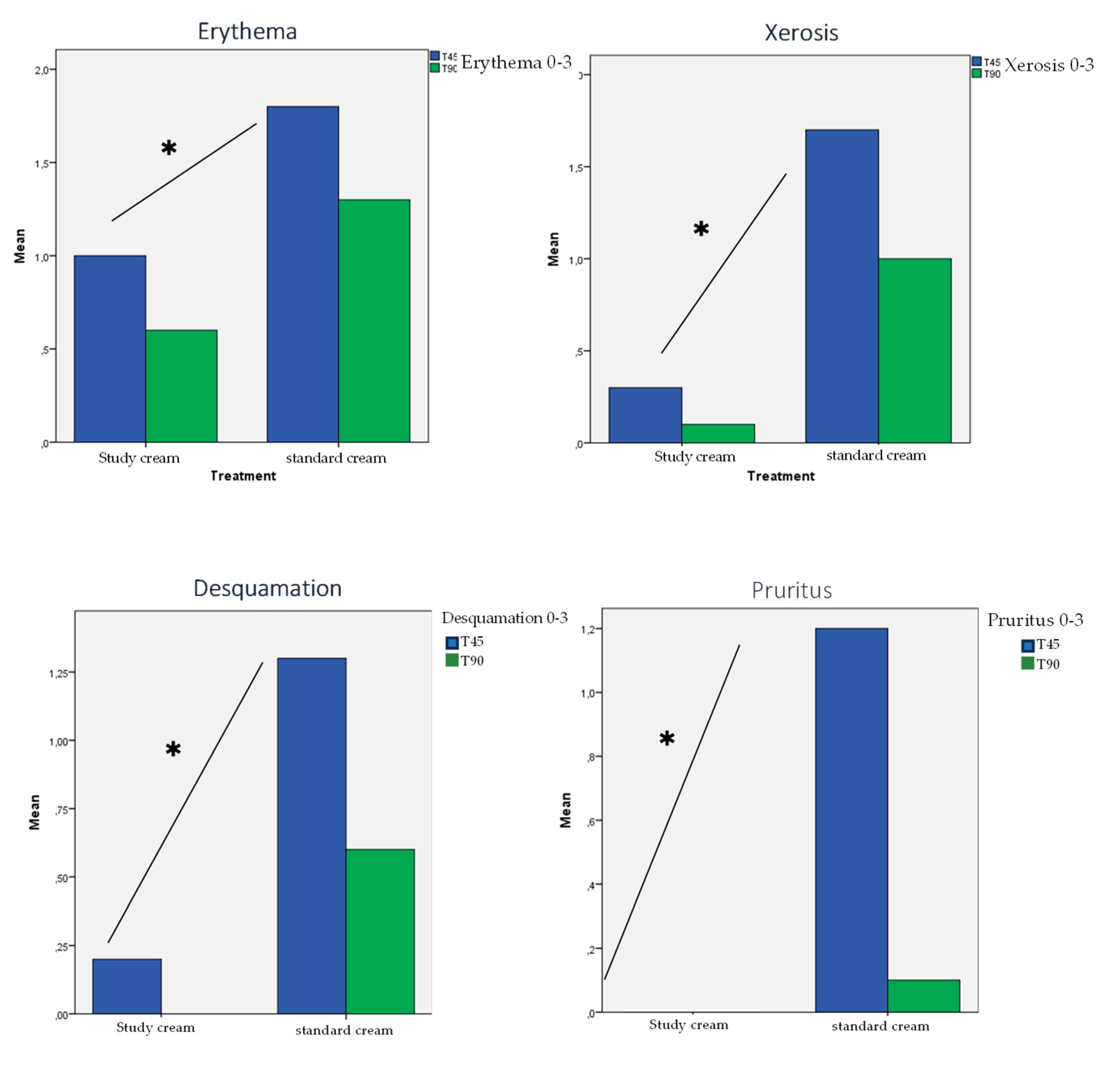
3.4. Skin Tolerance:
Skin tolerance outcomes favored the study cream, with overall tolerance rated significantly higher compared to the standard cream (p<0,001). Specific side effects such as erythema, xerosis, desquamation and pruritus improved significantly from T45 to T90 in both groups, with a significantly greater improvement in the study cream group. These findings are detailed in
Table 1 and
Figure 7. Despite differences in tolerability, therapeutic adherence was 100% in both groups, with no between-group differences (p = 1.000).
3.5. Subjective Evaluations Described by the Patient:
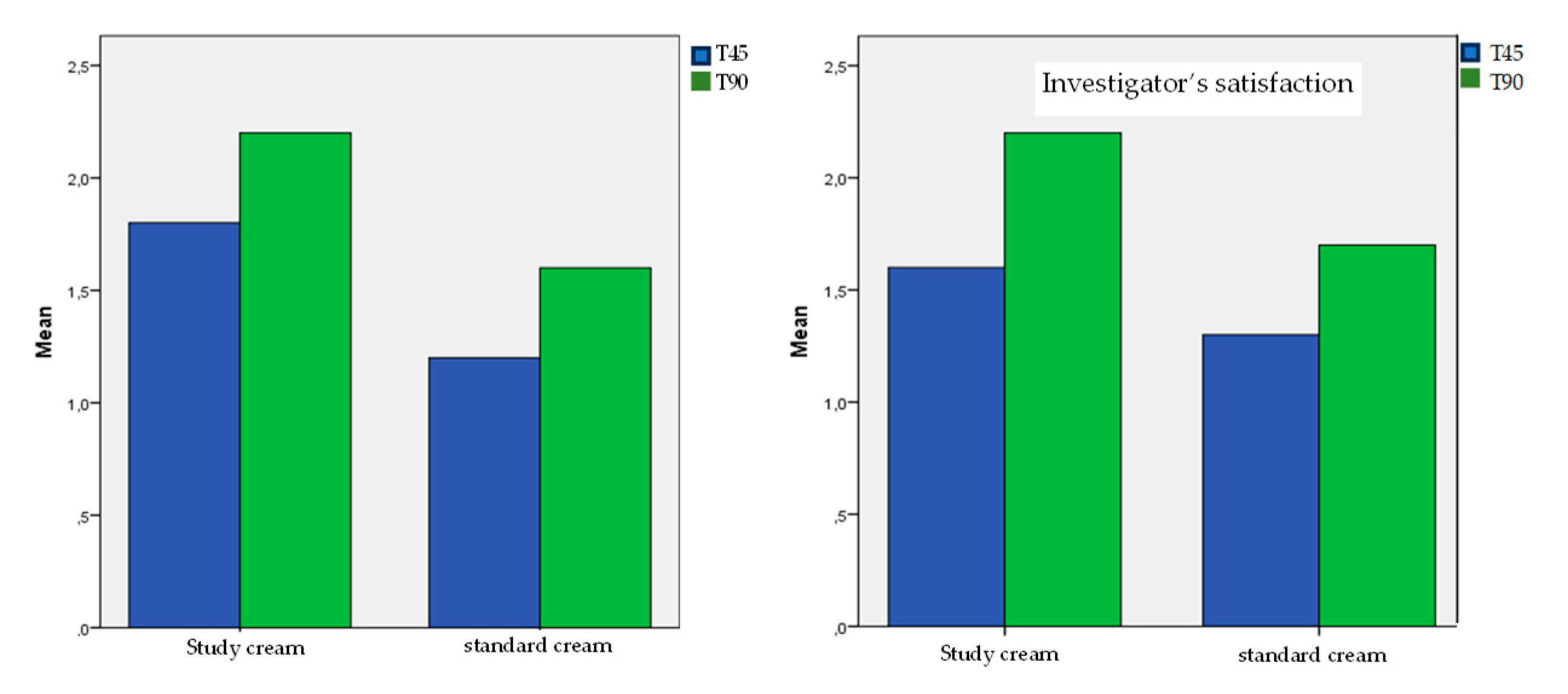
Patient in both groups reported significant improvement in overall skin condition at T45 and T90 (p=0.003). Comparing between treatments, in the Biretix isorepair group at T45 the improvement was classified as mild by 20% of the patients and moderate by 80%. In the standard group they referred no improvement in 10%, mild in 60% and moderate in 30%. In T90 these percentages reached 60% moderate and 30% intense in the test cream group vs 40% mild and 60% moderate in the standard cream group. These differences were statistically significant. (p=0.014)
Investigator-assessed improvement also showed significant improvement in both groups at T45 and T90 (p=0.002). The study cream group tended to score higher improvement ratings, with a trend toward statistical significance when compared to the control group (p=0.054). (
Figure 8)
Overall satisfaction at T45 was moderate or high in 70% of patients, and at T90, 85% achieved moderate or high satisfaction. When comparing the study cream group with the standard cream group, it was found that in T45 up to 100% referred moderate or intense satisfaction which was maintained in T90 vs only 40% reaching this grade of satisfaction in the standard cream group at T45 which elevated to 70% in T90. These differences were significant p=0.02.
As for the questionnaire about the product usage test, participants in the study cream group reported superior organoleptic and comfort characteristics (p<0.001) On the other hand, less residual or greasy sensation feeling on the skin was reported within the test cream treatment group p<0.001. Significantly enhanced treatment adherence was achieved in the study cream group compared to the standard cream group (p<0.001). (
Figure 9)
4. Discussion
Oral isotretinoin is considered the treatment of choice for severe inflammatory acne and is increasingly used in moderate or persistent mild inflammatory acne unresponsive to conventional therapies [
7].
In recent years, low-dose isotretinoin regimens have gained acceptance among dermatologists due to their favorable efficacy–tolerability balance. Piquero et al reported high efficacy rates (over 80%) in patients with moderate inflammatory acne at doses of 0.2–0.3 mg/kg/day over 12 months, followed by topical maintenance, topical therapy, particularly in individuals at risk of physical or psychological scarring. [
21] Similarly, Bettoli et al, concluded that scientific literature strongly supports that initiating treatment with a low daily dose of 0.1–0.2 mg/kg/day, or approximately 10 mg per day, followed by a gradual increase to the maximum dose tolerated by the patient, is an effective strategy to achieve favorable clinical outcomes while reducing the risk of side effects. [
1]
Despite the advantages of low doses regimens, adverse effects may still occur. Xerosis, fissures, and eczematous dermatitis remain common resulting from impaired barrier function. This disruption leads to a loss of homeostatic regulation of water balance and permeability, reduces antimicrobial defenses and compromises the antioxidant and photoprotective fuctions of the skin barrier. It aslo increases rsusceptibility to allergens and environmental stressors. [
1]
The existing literature endorses the implementation of expert-guided skincare regimens and barrier repair therapy (BRT) for individuals diagnosed with acne vulgaris (AV) who are undergoing various treatment modalities, including systemic isotretinoin therapy. [
3]. However, no well designed trials with moisturizing topical product in oral isotretinoin patients have been published.
In our study, the use of the study cream significantly reduced common cutaneous side effects—such as xerosis, erythema, desquamation and pruritus— compared to a standard cream. This benefit is likely attributable to the synergistic effect of its active ingredients: ceramides essential for preserving normal skin hydration, and hyaluronic acid, a hygroscopic compound naturally present in the stratum corneum. In addition, vitamin E and SCA® Growth Factor Technology which promotes epidermal regeneration. The combination of these ingredients provides a moisturizing effect while restoring lipid balance. There is scientific evidence demonstrating that proper restoration of the skin barrier not only improves hydration and protection against external aggressors but also exhibits antimicrobial effects. [
22] In addition, we found faster scar improvement according to results achieved in T45, which we hypothesize could be due to the improvement of erythema and inflammation due to the anti-inflammatory and moisturizing effects of the study cream; also, fibroblast stimulation induced by SCA® may play a role in accelerating scar healing.
The goal of adjuvant treatment with skin barrier repair agents is to minimize transepidermal water loss (TEWL), restore the physiological lipids profile of the stratum corneum and maintain proper hydation. Scientific literature supports the routine use of moisturizers in patients undergoing oral isotretinoin treatment, as also shows the present study. Moreover, patient education and good communication is recommended to improve treatment adherence. In addition, a detailed advice should be given about the characteristics of the skin care products patients should use. [
23] Besides therapeutic compliance, patient satisfaction also increased with the moisturizing cream use, statistically higher in the study cream group.
A systematic review of 74 articles regarding moisturization in acne patients undergoing topical and systemic treatments, found that the most recommended moisturizers typically included ingredients such as humectants, emollients, oil absorbers, and those with anti-inflammatory and/or barrier replenishing properties. Given the various adjunctive products available, decision frameworks were created for clinicians to use when selecting over-the-counter cleansers and moisturizers for acne-prone patients. Informing clinicians about skin barrier dysfunction in acne and the benefits of adjunctive skincare may help them to choose the right product(s) to complement prescription therapy. [
24]
Study limitations included the small sample sizes and the open-label design, lacking blinding designed. In view of the pilot nature of our trial the results we obtained should be considered as useful data in term of additional hypothesis generating scenarios and for an adequate sample size calculation of confirmatory trials to be conducted in the future.
5. Conclusions
In summary, the 12-week use of Biretix® Isorepair provided cream as an adjuvant to oral isotretinoine significantly improved skin hydration, reduced cutaneous side effects and contributed to greater patient satisfaction and treatment adherence.
These results support the incorporation of barrier-repairing moisturizers as a standard adjunctive measure during systemic acne therapy.
Author Contributions
“Conceptualization, M.V., M.J.G-S.,M.M.; methodology, M.V, M.H, M.M.,M.T.T-D.;.; formal analysis, M.V, M.H, M.T.T-D.; investigation, M.V, M.H, M.T.T-D.; resources, M.J.G-S.; data curation, M.T.T-D.; writing—original draft preparation, M.T.T-D.; writing—review and editing, M.V, M.H, M.T.T-D, M.J.G-S.M.M.; visualization, M.V, M.H, M.T.T-D, M.J.G-S.; supervision, M.J.G-S.; project administration, M.J.G-S.. All authors have read and agreed to the published version of the manuscript.”
Funding
This research received no external funding.
Institutional Review Board Statement
“Not applicable” as the study was a clinical experience based on daily routine with a cosmetic product.
Informed Consent Statement
Written informed consent was obtained from all subjects involved in the study
Data Availability Statement
We encourage all authors of articles published in MDPI journals to share their research data. In this section, please provide details regarding where data supporting reported results can be found, including links to publicly archived datasets analyzed or generated during the study. Where no new data were created, or where data is unavailable due to privacy or ethical restrictions, a statement is still required. Suggested Data Availability Statements are available in section “MDPI Research Data Policies” at
https://www.mdpi.com/ethics.
Acknowledgments
We acknowledge Francisca Rius for the support with the statistical analysis.
Conflicts of Interest
The authors declare that they work in Medical Affairs and in the R&D department of Cantabrialabs (Industrial Farmacéutica Cantabria SA).
Appendix A
Appendix A.1
Patient benefit index and test usage of the products
T0 (0–10)
Do you expect the product to be pleasant?
Do you expect it to have a soothing effect?
Do you expect it to provide long-lasting hydration?
Do you expect it to help with adherence to the treatment?
T45 (0–10)
Sensorial Experience
The texture of the product is pleasant.
The product spreads easily.
The product is quickly absorbed.
After absorption, the product leaves residue on the skin (white film, pilling).
The product leaves a greasy feeling after application.
The sensation on the skin after application is pleasant.
Subjective Efficacy
The product provides skin hydration.
The feeling of hydration is long-lasting.
The product helps to regenerate/repair the skin.
The product provides a feeling of comfort on the skin.
The product reduces feelings of tightness and irritation.
Tolerance
After application, a burning or stinging sensation is felt on the skin.
After application, a feeling of tightness is felt on the skin.
After application, the skin becomes red.
User Opinion
The product supports adherence to the Dercutane (oral isotretinoin) treatment.
The product has met your expectations.
Would you purchase this product?
Would you recommend this product?
T90 (0–10)
Sensorial Experience
The texture of the product is pleasant.
The product spreads easily.
The product is quickly absorbed.
After absorption, the product leaves residue on the skin (white film, pilling).
The product leaves a greasy feeling after application.
The sensation on the skin after application is pleasant.
Subjective Efficacy
The product provides skin hydration.
The feeling of hydration is long-lasting.
The product helps to regenerate/repair the skin.
The product provides a feeling of comfort on the skin.
The product reduces feelings of tightness and irritation.
Tolerance
After application, a burning or stinging sensation is felt on the skin.
After application, a feeling of tightness is felt on the skin.
After application, the skin becomes red.
User Opinion
The product supports adherence to the Dercutane (oral isotretinoin) treatment.
The product has met your expectations.
Would you purchase this product?
Would you recommend this product?
References
- Bettoli V, Guerra-Tapia A, Herane MI, Piquero-Martín J. Challenges and Solutions in Oral Isotretinoin in Acne: Reflections on 35 Years of Experience. Clin Cosmet Investig Dermatol. 2019 Dec 30;12:943-951. PMID: 32021364; PMCID: PMC6951028. [CrossRef]
- Ward A, Brogden RN, Heel RC, et al. Isotretinoin: a review of its pharmacologic properties and therapeutic efficacy in acne and other skin disorders. Drugs. 1984;28:6-37.
- Del Rosso JQ. Clinical relevance of skin barrier changes associated with the use of oral isotretinoin: the importance of barrier repair therapy in patient management. J Drugs Dermatol. 2013 Jun 1;12(6):626-31. PMID: 23839177.
- Williams ML, Elias PM. Nature of skin fragility in patients receving retinoids for systemic effect. Arch Dermatol. 1981;117(10):611-619.
- Elias PM. Epidermal effects of retinoids: supramolecular observations and clinical implications. J Am Acad Dermatol. 1986;15(4 Pt 2):797-809.
- Reyes-Hadsall S, Ju T, Keri JE. Use of Oral Supplements and Topical Adjuvants for Isotretinoin-Associated Side Effects: A Narrative Review. Skin Appendage Disord. 2024 Feb;10(1):1-9. Epub 2023 Oct 20. PMID: 38313565; PMCID: PMC10836938. [CrossRef]
- Herane MI, Fuenzalida H, Zegpi E, De Pablo C, Espadas MJ, Trullás C, Mirada A, Martin GG. Specific gel-cream as adjuvant to oral isotretinoin improved hydration and prevented TEWL increase--a double-blind, randomized, placebo-controlled study. J Cosmet Dermatol. 2009 Sep;8(3):181-5. PMID: 19735515. [CrossRef]
- Bertocchi M, Isani G, Medici F, Andreani G, Tubon Usca I, Roncada P, Forni M, Bernardini C. Anti-Inflammatory Activity of Boswellia serrata Extracts: An In Vitro Study on Porcine Aortic Endothelial Cells. Oxid Med Cell Longev. 2018 Jun 25;2018:2504305.. PMID: 30046370; PMCID: PMC6036794. [CrossRef]
- Danby SG, Andrew PV, Kay LJ, Pinnock A, Chittock J, Brown K, Williams SF, Cork MJ. Enhancement of stratum corneum lipid structure improves skin barrier function and protects against irritation in adults with dry, eczema-prone skin. Br J Dermatol. 2022 May;186(5):875-886. Epub 2022 Apr 3. PMID: 34921679; PMCID: PMC9321855. [CrossRef]
- Tempark T, Shem A, Lueangarun S. Efficacy of ceramides and niacinamide-containing moisturizer versus hydrophilic cream in combination with topical anti-acne treatment in mild to moderate acne vulgaris: A split face, double-blinded, randomized controlled trial. J Cosmet Dermatol. 2024 May;23(5):1758-1765. Epub 2024 Feb 1. PMID: 38299457. [CrossRef]
- Isoda K, Seki T, Inoue Y, et al. Efficacy of the combined use of a facial cleanser and moisturizers for the care of mild acne patients with sensitive skin. J Dermatol. 2015;42(2):181-188.
- Burke K, Clive J, Combs G, Commisso J, Keen C, Nakamura R. Effects of topical and oral vitamin E on pigmentation and skin can- cer induced by ultraviolet irradiation in Skh:2 hairless mice. Nutr Cancer. 2000;38(1):87-97.
- Keen MA, Hassan I. Vitamin E in dermatology. Indian Dermatol Online J. 2016 Jul-Aug;7(4):311-5. PMID: 27559512; PMCID: PMC4976416. [CrossRef]
- Truchuelo MT, Vitale M (2020) A cosmetic treatment based on the secretion of Cryptomphalus aspersa 40% improves the clinical results after the use of nonablative fractional laser in skin aging. J Cosmet Dermatol 19: 622-628.
- Fondevilla A, Moreno-Olmedo E, Bernal JM, Belmonte MJ, Nicolás A, López E. Prevention of radiation induced dermatitis in head and neck cancer patients using cryptomphalus aspersa secretion. Clin Transl Oncol. 2024 Sep 25. Epub ahead of print. PMID: 39322924. [CrossRef]
- Castro B, de Paz N, González S, Rodríguez-Luna A. SCA® Slows the Decline of Functional Parameters Associated with Senescence in Skin Cells. Int J Mol Sci. 2022 Jun 10;23(12):6538. PMID: 35742982; PMCID: PMC9224471. [CrossRef]
- Lim VZ, Yong AA, Tan WPM, Zhao X, Vitale M, Goh CL. Efficacy and Safety of a New Cosmeceutical Regimen Based on the Combination of Snail Secretion Filtrate and Snail Egg Extract to Improve Signs of Skin Aging. J Clin Aesthet Dermatol. 2020 Mar;13(3):31-36. Epub 2020 Mar 1. PMID: 32308795; PMCID: PMC7159309.
- Brieva A, Philips N, Tejedor R, Guerrero A, Pivel JP, et al. Molecular basis for the regenerative properties of a secretion of the mollusk Cryptomphalus aspersa. Skin Pharmacol Physiol 21: 15-22.
- Fernandez-Gonzalez P, Vitale M, Truchuelo MT. Early and maintained application of the secretion of Cryptomphalus aspersa (SCA) 40% improves cutaneous healing after ablative fractional laser in skin aging. J Cosmet Dermatol. 2021 Apr;20(4):1140-1145. Epub 2020 Sep 24. PMID: 32929855; PMCID: PMC8048427. [CrossRef]
- Del Rosso JQ. Defining criteria used to evaluate response to treatment of acne vulgaris. Cutis. 2006 Aug;78(2):117-21. PMID: 16983900.
- Piquero-Martin J, Misticone S, Piquero-Casals V, Piquero-Casals J. Topic therapy-mini isotretinoin doses vs topic therapy-systemic anti- biotics in the moderate acne patients. Ann Dermatol Venereol. 2002;129:S382.
- Park KY, Kim DH, Jeong MS, et al. Changes of antimicrobial peptides and transepidermal water loss after topical application of tacrolimus and ceramide-dominant emollient in patients with atopic dermatitis. J Korean Medi- cal Sci. 2010;25(5):766-771.
- Goh CL, Abad-Casintahan F, Aw DC, Baba R, Chan LC, Hung NT, Kulthanan K, Leong HN, Medina-Oblepias MS, Noppakun N, Sitohang IB, Sugito TL, Wong SN. South-East Asia study alliance guidelines on the management of acne vulgaris in South-East Asian patients. J Dermatol. 2015 Oct;42(10):945-53. Epub 2015 Jul 25. PMID: 26211507. [CrossRef]
- Lain E, Andriessen AE. Choosing the Right Partner: Complementing Prescription Acne Medication With Over-the-Counter Cleansers and Moisturizers. J Drugs Dermatol. 2020 Nov 1;19(11):1069-1075. PMID: 33196748. [CrossRef]
Figure 1.
A significantly higher decline in PGA (left image) and IGA (right image) scores in the study cream group, interaction p-value 0.001.
Figure 1.
A significantly higher decline in PGA (left image) and IGA (right image) scores in the study cream group, interaction p-value 0.001.
Figure 2.
Graphs show the evolution of acne severity scoring according to PGA and IGA along the study. Significant improvement was observed in both scales in both groups along the study. The (*) reflects the significant differences between the study cream group vs standard cream group.
Figure 2.
Graphs show the evolution of acne severity scoring according to PGA and IGA along the study. Significant improvement was observed in both scales in both groups along the study. The (*) reflects the significant differences between the study cream group vs standard cream group.
Figure 3.
Decrease of inflammatory (left) and non-inflammatory lesions (right) along the study. A significant greater decrease in non-inflammatory lesions was observed in the study cream group by the end of the study compared to the standard cream group. (p=0.002).
Figure 3.
Decrease of inflammatory (left) and non-inflammatory lesions (right) along the study. A significant greater decrease in non-inflammatory lesions was observed in the study cream group by the end of the study compared to the standard cream group. (p=0.002).
Figure 4.
Graphs show the evolution of inflammatory and non-inflammatory lesions along the study. Significant reduction along the time was observed in both groups without significant differences between the study cream group vs standard cream group, except for the non-inflammatory lesions in T90 (p=0.04) that were significantly lower in the study cream group.
Figure 4.
Graphs show the evolution of inflammatory and non-inflammatory lesions along the study. Significant reduction along the time was observed in both groups without significant differences between the study cream group vs standard cream group, except for the non-inflammatory lesions in T90 (p=0.04) that were significantly lower in the study cream group.
Figure 5.
Evolution of scar grading along the study, showing significant higher decrease in T45 in the Biretix group (p=0.013).
Figure 5.
Evolution of scar grading along the study, showing significant higher decrease in T45 in the Biretix group (p=0.013).
Figure 6.
The graph shows that although both groups experienced a decrease in moisturization score, the reduction was significantly greater in the group treated with the standard cream (p=0.009 in T45 and 0.04 in T90).
Figure 6.
The graph shows that although both groups experienced a decrease in moisturization score, the reduction was significantly greater in the group treated with the standard cream (p=0.009 in T45 and 0.04 in T90).
Figure 7.
Graphs showing the different parameters evaluating side-effects usually found in patients under oral isotretinoin treatment. With the complementary treatment, it is shown the significant decrease of the side effects in both groups from T45 to T90. In addition, for all evaluated parameters, the group treated with the study product showed significantly lower scores compared to the standard treatment group, indicating a favorable effect of the investigational product. (*).
Figure 7.
Graphs showing the different parameters evaluating side-effects usually found in patients under oral isotretinoin treatment. With the complementary treatment, it is shown the significant decrease of the side effects in both groups from T45 to T90. In addition, for all evaluated parameters, the group treated with the study product showed significantly lower scores compared to the standard treatment group, indicating a favorable effect of the investigational product. (*).
Figure 8.
Significant improvement was assessed both by the patient (left) and the investigator (right) along the study. * indicates the statistical differences in favor of the Biretix isorepair® group treatment.
Figure 8.
Significant improvement was assessed both by the patient (left) and the investigator (right) along the study. * indicates the statistical differences in favor of the Biretix isorepair® group treatment.
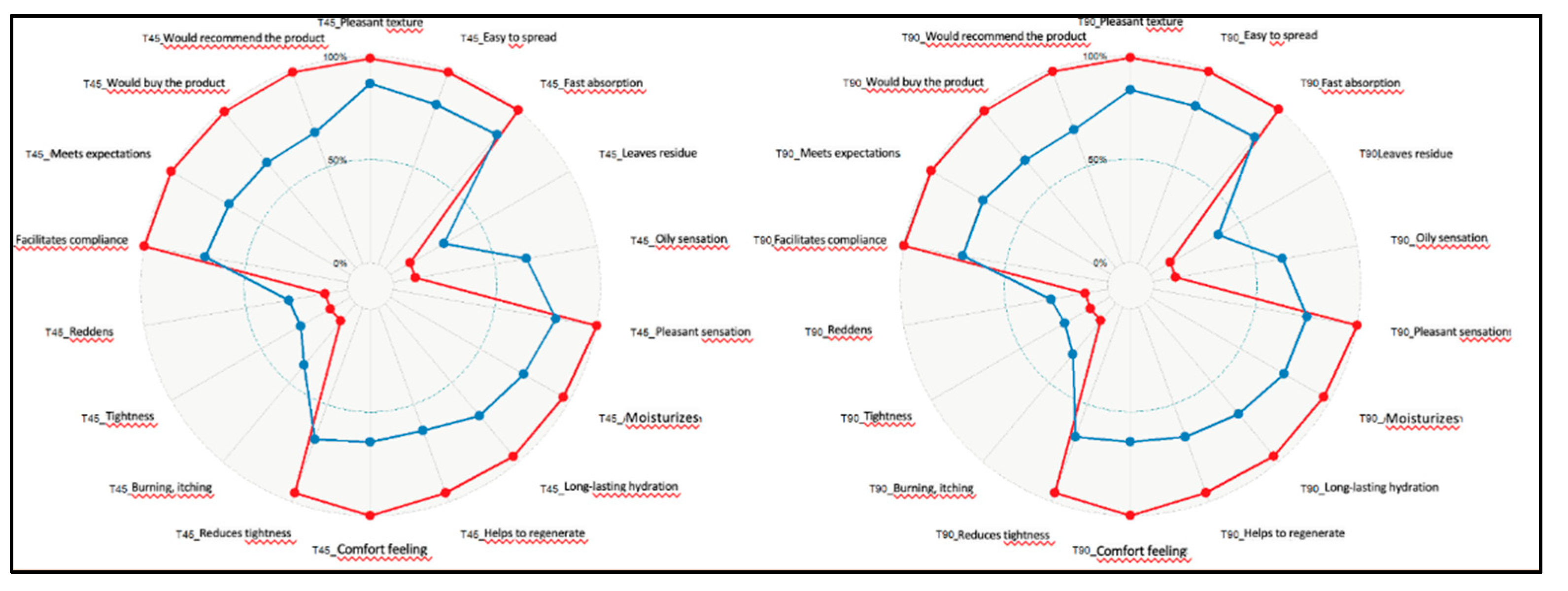
Figure 9.
Radar graph shows the better score (p<0.001) reported by patients treated with the study cream (red line) compared to the standard cream (blue line) according to the product test use.
Figure 9.
Radar graph shows the better score (p<0.001) reported by patients treated with the study cream (red line) compared to the standard cream (blue line) according to the product test use.
Table 1.
Evolution of expected adverse skin reactions in patients under oral isotretinoin treatment along the study. Significantly lower percentages were detected in the test cream group vs the standard cream group in T45 and T90.
Table 1.
Evolution of expected adverse skin reactions in patients under oral isotretinoin treatment along the study. Significantly lower percentages were detected in the test cream group vs the standard cream group in T45 and T90.
| |
T45 |
T90 |
| |
Biretix® isorepair |
standard cream |
Biretix® isorepair |
standard cream |
| Erythema. T45 vs T90 p= 0.029. SD between treatments p 0.02 |
20% moderate |
60% moderate or severe |
20% moderate |
30% moderate or severe |
Xerosis. T45 vs T90 p= 0.006.
SD between treatments p <0.001 |
only 30% mild |
70% moderate or severe |
only 10% mild |
30% moderate. |
| Desquamation. T45 vs T90 p=0.006. SD between treatments p =0.002 |
only 20% mild |
40% moderate |
None |
Only 10% severe, 30% mild |
| Pruritus .T45 vs T90 p = 0.005. SD between treatments p =0.001 |
None |
20% severe, 60% mild |
None |
10% mild |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).