1. Introduction
The Nasolabial folds (NLFs), alternatively known as “laugh lines” [
1,
2]are caused by a variety of factors that include the loss of deep fat or muscle contour in the midface, the reduction of hyaluronic acid as well as the degeneration of collagen and elastic fibers [
3,
4,
5]. Over time, this leads to sagging of the skin and formation of wrinkles and folds, widely known as skin aging. [
6,
7]. Skin aging is a natural process, influenced by internal and external factors, that however non-life-threatening it can be, significantly affects the quality of life [
8] . That is why minimally invasive aesthetic procedures, aiming to reverse the signs of aging, continue to progressively increase in number over time [
9,
10,
11,
12]. In the last years, more than 8.5 million nonsurgical injection procedures were performed annually worldwide, with botulinum toxin type A and hyaluronic acid filler injections being respectively the first and second most common [
13]. Hyaluronic acid (HA) is a linear anionic polysaccharide composed of repeating units of disaccharides D-glucuronic acid and N-acetyl-D-glucosamine linked together through alternating ß-1,3 and ß-1,4 glycosidic bonds [
14,
15,
16]. It is a primary component of extracellular matrix, vitreous humor, and synovial fluid of vertebrates [
16,
17]. Traditionally extracted from rooster combs, HA is now increasingly produced through microbial fermentation. Modifications of HA are relatively widespread, with cross-linking, via hydroxyl and carboxyl groups, being the most common one, that influences the prevention of rapid degradation and provides long-term treatment effects [
18].
Hyaluronic acid has been used in medicine for many years and, in its native form, has implanted in millions of individuals as a highly biocompatible filling material by exploiting both its the physio-chemical characteristics, as well as its biological properties of cellular interactions [
19,
20]. In particular, the restorative power of hyaluronic acid, the ability to promote the proliferation and migration of fibroblasts by stimulating the neo-synthesis of collagen and other constituents of the extracellular matrix has been highly valued [
21]. Multiple clinical trials have shown the effectiveness of a series of hyaluronic acids as dermal fillers in anti-wrinkle procedures and tissue regeneration [
22,
23]. The consolidated use of this molecule has produced not only a considerable number of clinical efficacy data but also significant evidence of excellent tolerability [
24]. Based on its biological characteristics, hyaluronic acid is considered a real natural filler and its intradermal injections, at the maximum concentration of 2%, do not show any acute or chronic toxicity in several animal models. HA is not immunogenic, does not induce any type of stabilization, does not interfere with the mechanisms of reproduction and is not genotoxic, while its secondary effects are defined as minimal and negligible [
25].
The HA in center of this study, Foliage Intense, is obtained through a bacterial bio-fermentation process, which does not present any risk of contamination by antigenic proteins, chemically cross-linked with BDDE (1,4-butanediol, diglyceryl ether) and stabilized with phosphated saline suspension buffer at pH 7.4 [
19,
26]. It has a lower molecular weight than the natural product, and its viscoelastic properties tend to be more viscous showing a lower dissolution rate. The formulation thus stabilized determines a long duration, while remaining biocompatible and injectable. In a comparative assessment, based in scientific literature of similar medical devices, Foliage Intense demonstrated equivalence with Restylane (produced by Q-Med), Juvéderm_Ultra (Allergen), both approved by the FDA and 20 years in the market, and Belotero Intense, Belotero Volume (Merz Aesthetics) in terms of efficacy and safety [
27,
28].
2. Objectives of the Study
2.1. Primary Objective
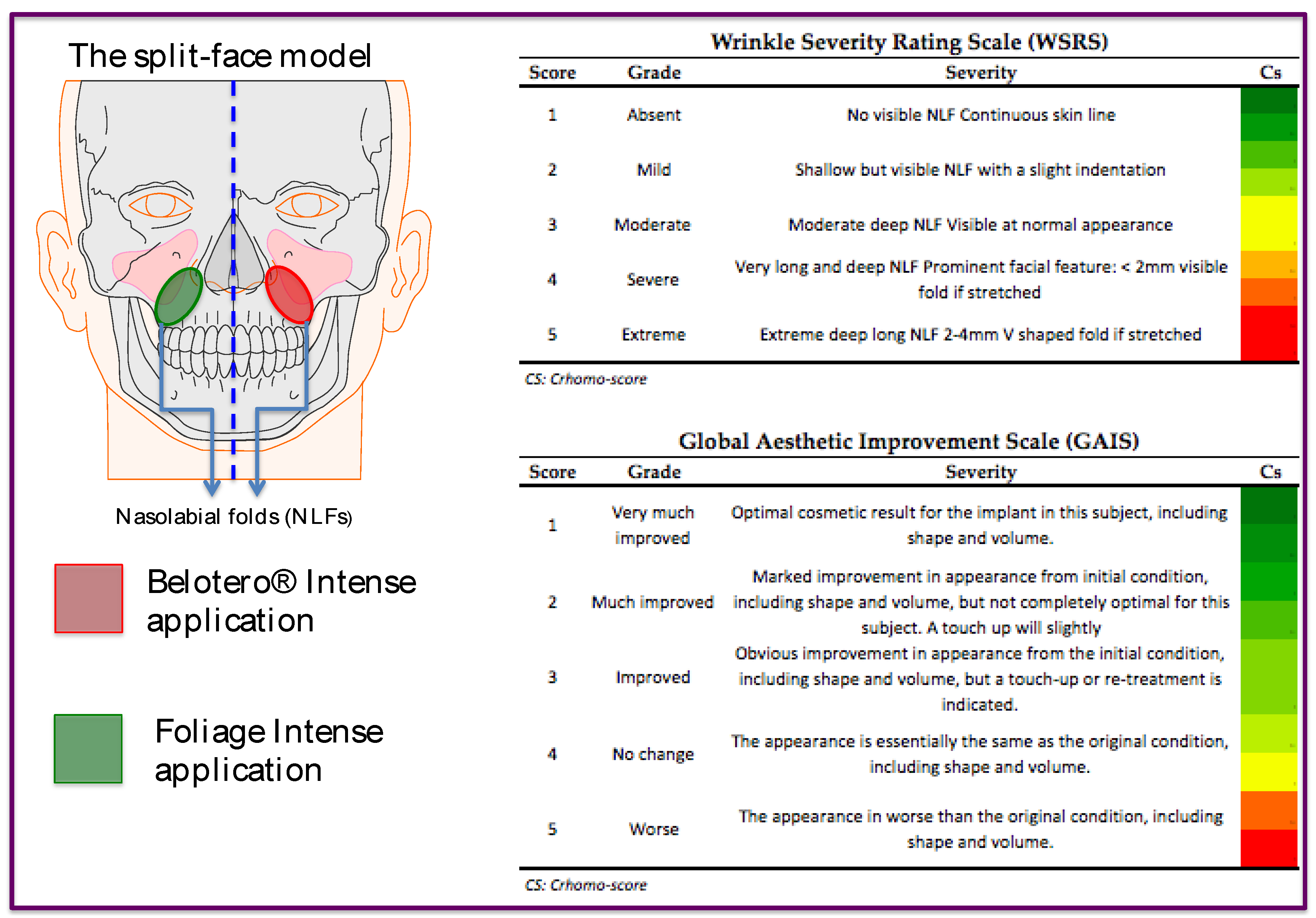
The main objective of the study is to assess the Foliage Intense performance in correcting moderate and severe nasolabial folds, in terms of efficacy by using the Wrinkle Severity Rating Scale (WSRS) [
29]. In particular, at each observation, the variations of the WSRS in the areas treated with Foliage Intense will be compared with those in the areas treated with Belotero Intense, aiming at non-inferiority of the investigational device with the comparator. The main time for testing the effectiveness will be the 6 months visit after the treatment (baseline treatment or after subsequent optional touch-up).
2.2. Secondary Objectives
The list of secondary objectives of this study is the following: 1) To evaluate the aesthetic result over time, by using subjective evaluations like the Wrinkle Severity Rating Scale (WSRS) [
30] and the Global Aesthetic Improvement Scale (GAIS) [
31] , by the subjects and Evaluating Investigator; 2) To objectively evaluate the persistence over time of the effects of the product in the dermis by ultrasound of the soft tissue; 3) To objectively evaluate the difference of volume over time by a 3D image system, VECTRA H2 (Canfield Scientific, Inc); 4) To analyze the subject’s view on all the treatment and outcomes as well as the overall satisfaction degree; 5) To assess the quality of the injection by recording the judgment of the Treating Investigator about the handling and efficacy of the product, on the treatment visits; 6) To assess the product’s tolerability during the study, for allergies or other reactions that can occur as a result of the treatments.
In addition, the assessment of the safety measures including presence and severity of injection site responses (ISRs), procedural pain assessment or any adverse events (AEs) will be carried out throughout the study.
Figure 1.
Split-face model representation and the Algorithms concerning WSRS and GAIS score evaluations.
Figure 1.
Split-face model representation and the Algorithms concerning WSRS and GAIS score evaluations.
3. Results
3.1. Summary of Populations
Ninety-five subjects were enrolled, randomized and received an injection on both treatment sides. One (1) no longer returned from visit V1, twelve (12) from visit V2, three (3) from visit V3, thirteen (13) from visit V4, twenty (20) from visit V5, fourteen (14) from visit V6 in order to have a total of sixty-three (63/95; 66.3%) withdrawn subjects.
The subjects who completed the study, therefore present at visit V7, were thirty-one (32) (33.7%), of which twenty-two (22) completed all visits (with the exception of the optional V3 visit for those who did not carry out the touch-up). Sixty- four (64) (67.4%) subjects completed visit V5, essential for the analysis of the primary endpoint. The
Table 1 reports all data concerning the population distribution among the visit.
The number of subjects included in the Safety, ITT and PP populations are presented in Table below. A total of 95 subjects were screened, randomized and treated, all (100%) analyzed for safety and efficacy with ITT population and 64 (67.4%) analyzed with PP (
Table 2).
3.2. WSRS Scroreanalyses After 6 Months of Treatments
3.2.1. PP Population
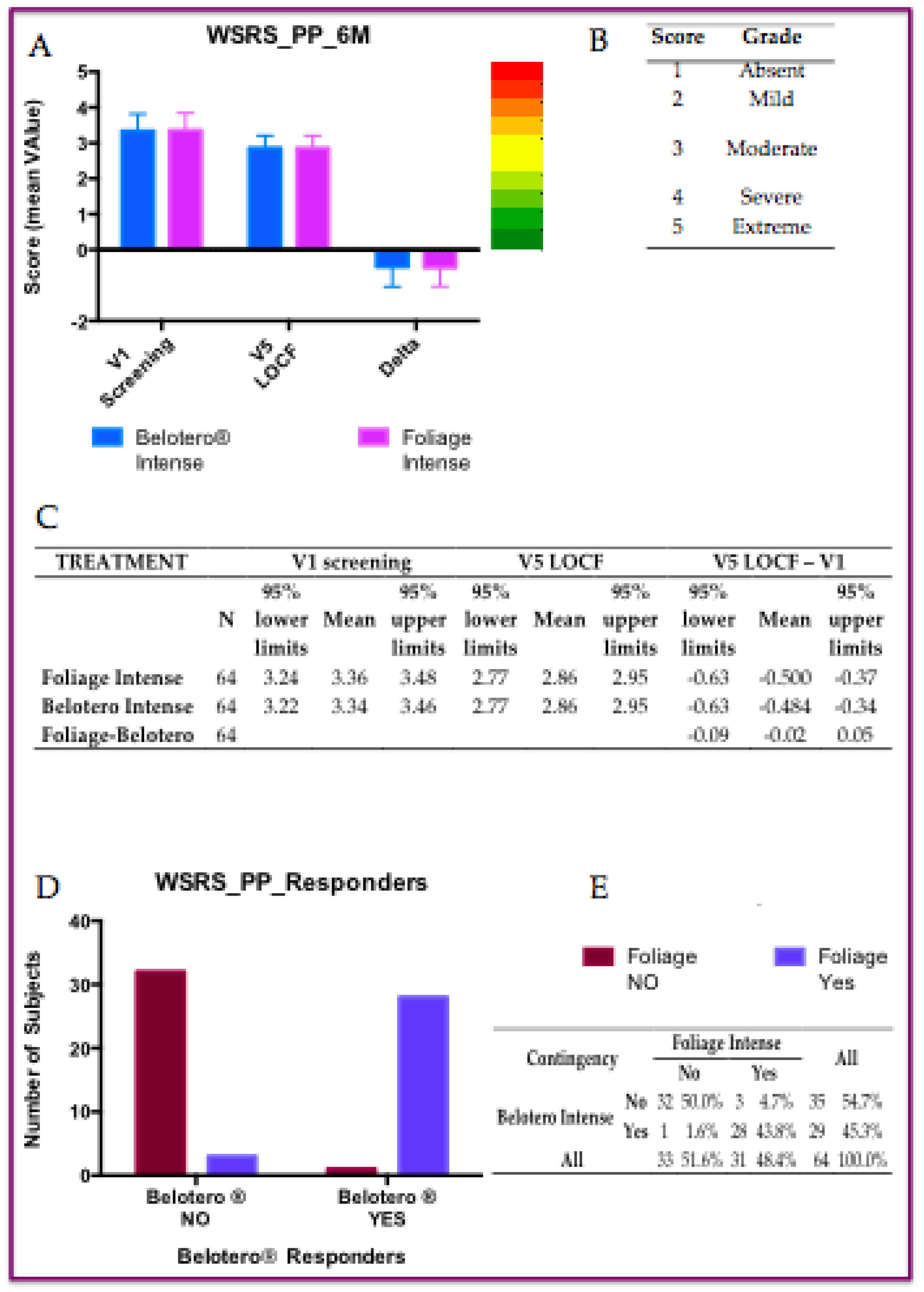
Sixty-four subjects present at both the screening and V5 visit (six months after treatment) were included in the PP analysis. In the PP analysis, the changes from baseline in WSRS scores at 6 months were -0.50±0.53 in the Foliage Intense group and -0.48±0.56 in the Belotero Intense group, both of which were highly significant using the Wilcoxon signed-rank test (p<0.001). Since the reduction in the two treatment groups is very similar, the comparison between the two treatments was statistically not significant with the Wilcoxon signed-rank test (
Figure 1).
Figure 2.
WSRS analyse in Per Protocol population (PP): A) Six months after treatments (p<0.001); B) Chromo-score and grades of WSRS parameter; C) Statistics of both tretments at V1, V5 and delta equal of V5-V1; D) Distribution of PP responders; E) Contiingency tableof responders, chi-square test: p<0.0001).
Figure 2.
WSRS analyse in Per Protocol population (PP): A) Six months after treatments (p<0.001); B) Chromo-score and grades of WSRS parameter; C) Statistics of both tretments at V1, V5 and delta equal of V5-V1; D) Distribution of PP responders; E) Contiingency tableof responders, chi-square test: p<0.0001).
3.2.2. ITT Population
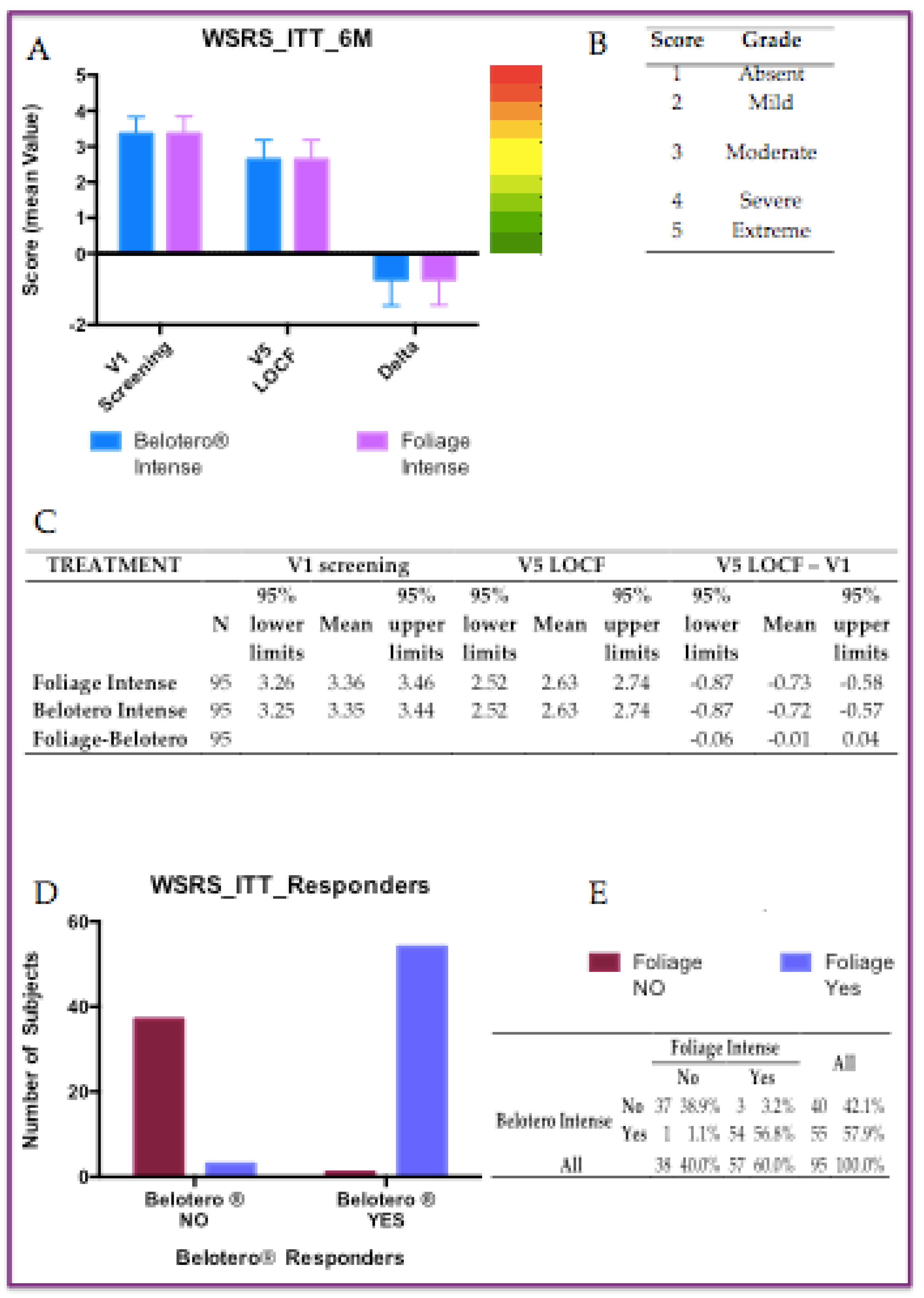
All 95 randomized subjects were included in the ITT population.
Of these 95 subjects, 31 did not return to the visit at 6 months (V5), so the WSRS score from previous visits V2, V3, or V4 was used as the WSRS score according to the LOCF procedure. In the ITT analysis, the changes from baseline in WSRS scores at 6 months were -0.73±0.72 in the Foliage Intense group and -0.72±0.74 in the Belotero Intense group, both of which were highly significant using the Wilcoxon signed-rank test (p<0.001). Since the reduction in the two treatment groups is very similar, the comparison between the two treatments was statistically not significant with the Wilcoxon signed-rank test (
Figure 2).
Figure 3.
WSRS analyse in Intention To Treat population (ITT): A) Six months after treatments (p<0.001); B) Chromo-score and grades of WSRS parameter; C) Statistics of both tretments at V1, V5 and delta equal of V5-V1; D) Distribution of ITT responders; E) Contiingency tableof responders, chi-square test: p<0.0001).
Figure 3.
WSRS analyse in Intention To Treat population (ITT): A) Six months after treatments (p<0.001); B) Chromo-score and grades of WSRS parameter; C) Statistics of both tretments at V1, V5 and delta equal of V5-V1; D) Distribution of ITT responders; E) Contiingency tableof responders, chi-square test: p<0.0001).
3.2.3. Responders Anlyses
The proportion in PP population of subjects with ≥ 1 grade improvement in WSRS from baseline at 6 months were 45.3% and 48.4%, respectively in Belotero Intense (BI) and Foliage Intense (FI) group. There were 3 Foliage Intense responders who were not Belotero Intense responders, while 1 subject was a Belotero Intense responder but not Foliage Intense. Indeed, McNemar’s test was not significant. While, the proportion of subjects in ITT population with ≥ 1 grade improvement in WSRS from baseline at 6 months were 57.9% and 60.0%, respectively in Belotero Intense (BI) and Foliage Intense (FI) group. Furthermore there were 3 Foliage Intense responders who were not Belotero Intense responders, vice versa 1 subject was a Belotero Intense responder but not Foliage Intense. Also in this case theMcNemar’s test was not significant. However, the positive differences between FI and BI ranged between 2% and 3% in favor of the FI treatment (
Figure 1E and 2E).
3.3. WSRS Scires Across Each Follow-Up Section
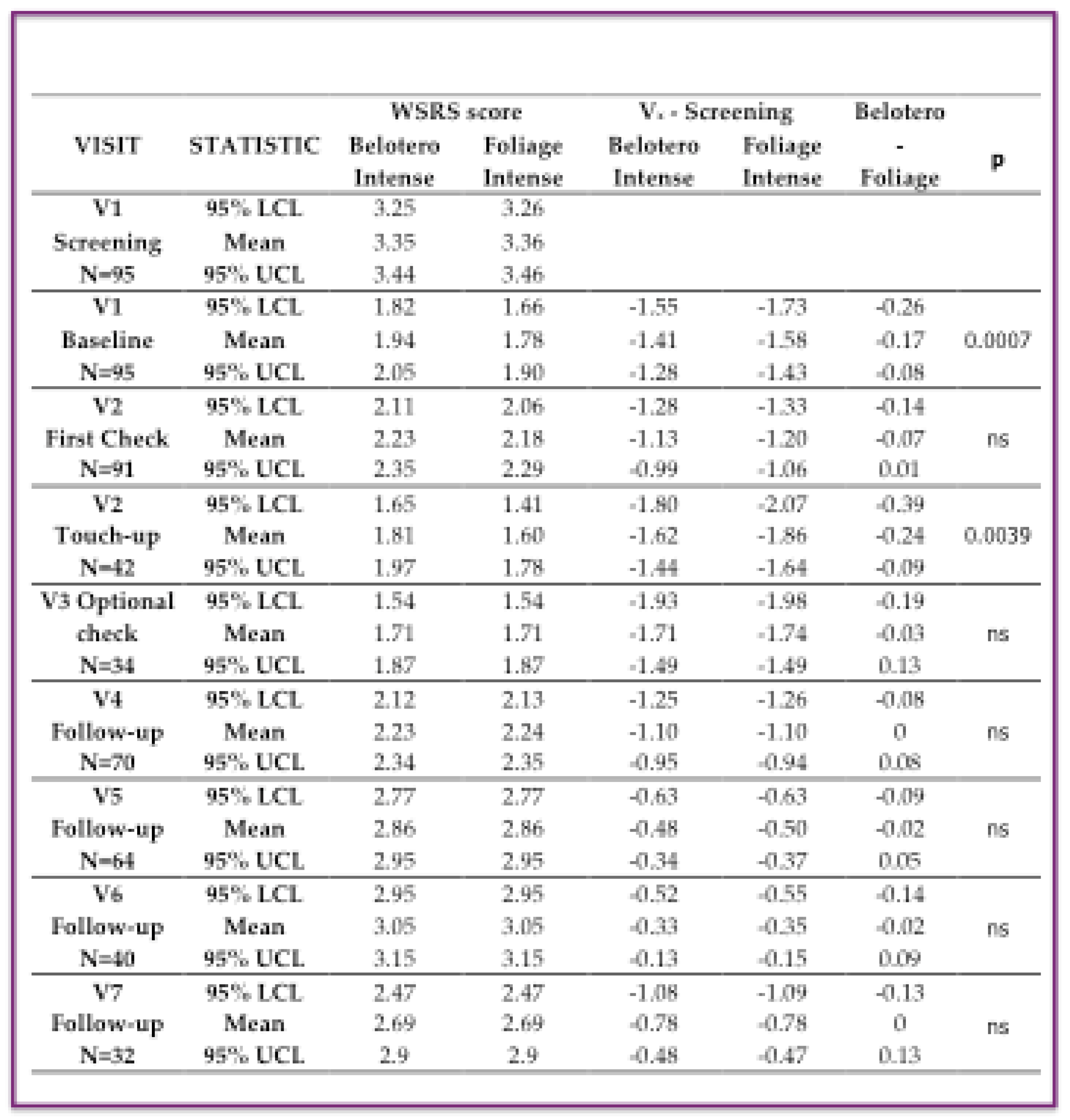
The data on the Wrinkle Severity Rating Scale (WSRS) score are listed by subjects in following summarized
Table 3, where the “WSRS score” columns show the average scores with confidence limits of the WSRS at each visit. Their differences with compared to screening visit (V
x – Screening) column are reported in the second column. the r variations from the screening visit, looking for the “Belotero - Foliage” treatment are reported in the second column. the difference between the two treatments and in the TEST column the p-value of the comparison between the two treatments. The reduction in WSRS was greater in the Foliage group compared to the one treated with Belotero immediately after the injections at baseline (-1.58 vs -1.41) and at touch-up (-1.86 vs -1.62). Differences between treatments were statistically significant with the Wilcoxon signed-rank test (p=0.0007 at V1 and 0.0039 at V2). In subsequent follow-up visits (V3-V7) this difference disappeared and the two treatment groups were almost identical (
Table 3).
3.4. Global Aesthetic Improvement Scale (GAIS): Score Analyses
All results concerning the GAIS analyses in ITT population (six different aspects) are reported in the serie of tables below (Figure 4). The significant differences were observed looking for the scores observerved in the following parameters:
- (1)
Evaluating Injector (EI, Table 4). Differences between treatments were statistically significant (p=0.0156) by the Wilcoxon signed-rank test. In the other visits this difference disappeared and the two treatment groups were almost identical.
- (2)
The mean volume difference assessed by 3D image at First Check was 0.42 (CI 0.32-0.53) cc in the Foliage Intense group and 0.35 (CI 0.26-0.44) cc., respectively. Difference between treatments revealed a statistically significant value (ANOVA p=0.0342), while, at other visits it is not (Table 7).
- (3)
The degree of satisfaction of the Injectors was better in the Foliage group compared to that treated with Belotero immediately after the baseline injection (FI 3.75 vs BI 3.56) and the touch-up visit (FI 3.71 vs BI 3.45). Differences between treatments were statistically highly significant using the Wilcoxon signed-rank test (V1 p=0.0005 and V2 p=0.0039; Table 9).
Figure 4.
GAIS analyse in ITT population (secondary end-points); Note: LCL+lover confidence level; UCL=upper confidence level. SSignificant statistica results were remarket in bold.
Figure 4.
GAIS analyse in ITT population (secondary end-points); Note: LCL+lover confidence level; UCL=upper confidence level. SSignificant statistica results were remarket in bold.
| Table 4: GAIS by Evaluating Investigator (EI) |
| Visits |
N |
Foliage Intense |
Belotero® Intense |
p |
| 95% LCL |
Mean |
95% UCL |
95% LCL |
Mean |
95% UCL |
| V2 First Check |
91 |
2.07 |
2.21 |
2.35 |
2.11 |
2.24 |
2.37 |
ns |
| V2 Touch-up |
42 |
1.34 |
1.52 |
1.71 |
1.52 |
1.69 |
1.87 |
0.0156 |
| V3 Optional check |
34 |
1.71 |
1.94 |
2.17 |
1.71 |
1.91 |
2.11 |
ns |
| V4 Follow-up |
70 |
2.29 |
2.46 |
2.62 |
2.33 |
2.49 |
2.64 |
ns |
| V5 Follow-up |
64 |
3.06 |
3.19 |
3.31 |
3.06 |
3.19 |
3.31 |
ns |
| V6 Follow-up |
40 |
3.52 |
3.68 |
3.83 |
3.52 |
3.68 |
3.83 |
ns |
| V7 Follow-up |
32 |
3.24 |
3.47 |
3.69 |
3.24 |
3.47 |
3.69 |
ns |
| Table 5: GAIS by subject |
| Visits |
N |
Foliage Intense |
Belotero® Intense |
p |
| 95% LCL |
Mean |
95% UCL |
95% LCL |
Mean |
95% UCL |
| V2 First Check |
91 |
1.89 |
2.04 |
2.20 |
1.89 |
2.04 |
2.20 |
ns |
| V2 Touch-up |
42 |
1.49 |
1.69 |
1.89 |
1.52 |
1.71 |
1.91 |
ns |
| V3 Optional check |
34 |
1.55 |
1.79 |
2.03 |
1.57 |
1.79 |
2.02 |
ns |
| V4 Follow-up |
70 |
2.28 |
2.43 |
2.58 |
2.30 |
2.44 |
2.59 |
ns |
| V5 Follow-up |
64 |
3.01 |
3.14 |
3.27 |
3.01 |
3.14 |
3.27 |
ns |
| V6 Follow-up |
40 |
3.31 |
3.48 |
3.64 |
3.31 |
3.48 |
3.64 |
ns |
| V7 Follow-up |
32 |
3.20 |
3.41 |
3.61 |
3.20 |
3.41 |
3.61 |
ns |
| Table 6: Dermal thickness in mm assessed by ultrasound |
| Visits |
N |
Foliage Intense |
Belotero® Intense |
p |
| 95% LCL |
Mean |
95% UCL |
95% LCL |
Mean |
95% UCL |
| V1 baseline |
95 |
1.13 |
1.31 |
1.49 |
1.12 |
1.30 |
1.48 |
ns |
| V2 Touch-up |
41 |
1.10 |
1.36 |
1.61 |
1.15 |
1.40 |
1.65 |
ns |
| V4 Follow-up |
62 |
0.86 |
0.94 |
1.02 |
0.87 |
0.95 |
1.03 |
ns |
| V5 Follow-up |
64 |
0.68 |
0.71 |
0.74 |
0.68 |
0.71 |
0.74 |
ns |
| V6 Follow-up |
39 |
0.64 |
0.67 |
0.69 |
0.62 |
0.65 |
0.69 |
ns |
| V7 Follow-up |
17 |
0.63 |
0.67 |
0.71 |
0.57 |
0.62 |
0.66 |
ns |
| Table 7: Volume difference (cc) |
| Visits |
N |
Foliage Intense |
Belotero® Intense |
p |
| 95% LCL |
Mean |
95% UCL |
95% LCL |
Mean |
95% UCL |
| V1 Baseline |
95 |
0.69 |
0.79 |
0.90 |
0.65 |
0.74 |
0.84 |
ns |
| V2 First Check |
91 |
0.32 |
0.42 |
0.53 |
0.26 |
0.35 |
0.44 |
0.0342 |
| V2 Touch-up |
42 |
0.47 |
0.64 |
0.82 |
0.48 |
0.66 |
0.83 |
ns |
| V3 Optional check |
34 |
0.24 |
0.42 |
0.60 |
0.20 |
0.36 |
0.53 |
ns |
| V4 Follow-up |
70 |
0.04 |
0.13 |
0.23 |
-0.03 |
0.08 |
0.19 |
ns |
| V5 Follow-up |
64 |
-0.21 |
-0.10 |
0.02 |
-0.29 |
-0.16 |
-0.02 |
ns |
| V6 Follow-up |
40 |
-0.20 |
-0.03 |
0.15 |
-0.22 |
-0.06 |
0.10 |
ns |
| V7 Follow-up |
32 |
-0.19 |
-0.02 |
0.15 |
-0.13 |
0.02 |
0.16 |
ns |
| Table 8: Subject’s overall satisfaction |
| Visits |
N |
Foliage Intense |
Belotero® Intense |
p |
| 95% LCL |
Mean |
95% UCL |
95% LCL |
Mean |
95% UCL |
| V2 First Check |
91 |
1.63 |
1.76 |
1.88 |
1.64 |
1.77 |
1.89 |
ns |
| V2 Touch-up |
42 |
1.18 |
1.36 |
1.54 |
1.20 |
1.38 |
1.56 |
ns |
| V3 Optional check |
34 |
1.18 |
1.35 |
1.52 |
1.18 |
1.35 |
1.52 |
ns |
| V4 Follow-up |
70 |
1.63 |
1.74 |
1.86 |
1.65 |
1.76 |
1.87 |
ns |
| V5 Follow-up |
64 |
2.12 |
2.25 |
2.38 |
2.15 |
2.27 |
2.39 |
ns |
| V6 Follow-up |
40 |
2.36 |
2.53 |
2.69 |
2.36 |
2.53 |
2.69 |
ns |
| V7 Follow-up |
32 |
2.20 |
2.44 |
2.68 |
2.20 |
2.44 |
2.68 |
ns |
| Table 9: Injectors’ satisfaction |
| Visits |
N |
Foliage Intense |
Belotero® Intense |
p |
| 95% LCL |
Mean |
95% UCL |
95% LCL |
Mean |
95% UCL |
| V1 Baseline |
95 |
3.64 |
3.75 |
3.85 |
3.44 |
3.56 |
3.68 |
0.0005 |
| V2 Touch-up |
42 |
3.52 |
3.71 |
3.91 |
3.22 |
3.45 |
3.68 |
0.0039 |
3.5. Safety and Tollerability
Adverse Events
In the 95 subjects treated with both Foliage Intense and Belotero Intense, no adverse events, neither serious nor non-serious, were reported.
Local tolerability
There were 5 subjects with an erythema, of which 3 with both Belotero Intense and Foliage Intense and 2 only with Belotero Intense. There were 4 subjects with redness, of which 3 with both Belotero Intense and Foliage Intense and 1 only with Belotero Intense. There were 2 subjects with pain, of which 1 with both Belotero Intense and Foliage Intense and 1 only with Belotero Intense. All local events were mild except redness in subject 01-028 of moderate intensity on both sides and redness plus pain of moderate intensity in subject 01-016 only on the side treated with Belotero.
No cases of oedema or itching. Patients with at least one local event were 7 (7.4%) treated with Belotero Intense for a total duration of 20 days and 5 (5.3%) treated with Foliage Intense for a total duration of 12 days.
4. Discussion
Tissue fillers injections remain to be one of the most commonly performed cosmetic procedures [
32,
33,
34,
35]. The one most important meta-analysis systematized and presented the available data on the aesthetic outcomes and safety of clinical trials concerning the nasolabial fold area treated with HA fillers [
23]. The authors performed a systematic review of randomized clinical trials harvesting documents in the following on lines databases: MEDLINE [
36], Science Direct [
37], EMBASE [
38], BIOSIS [
39] , SciELO [
40], Scopus [
41], Cochrane Controlled Register of Trials [
42] and Web of Science databases [
43]. The primary outcomes included aesthetic improvement measured using the Wrinkle Severity Rating Scale score [
29] and Global Aesthetic Improvement Scale [
31]. Secondary outcomes were incidence rates of complications occurring after the procedure. In this analysis (at baseline time), the pooled mean of WSRS score was 3.23 (95% CI: 3.20–3.26). One month after the procedure, the pooled WSRS score had reached 1.79 (95% CI: 1.74–1.83), after six months of treatment the WSRS was 2.02 (95% CI: 1.99–2.05) and after 12 months it was 2.46 (95% CI: 2.4–2.52). Looking at the GAIS scores, ne month after the procedure, the pooled GAIS score had reached 2.21 (95% CI: 2.14–2.28), after six months, it was 2.32 (95% CI: 2.26–2.37), and after 12 months, it was 1.27 (95% CI: 1.12–1.42). Overall, the pooled incidence of all complications was 0.58 (95% CI: 0.46–0.7). Most common included lumpiness (43%), tenderness (41%), swelling (34%) and bruising (29%) [
23]. These calculation were made comprising 50 different clinical trials, so far (
in bone fide), were considered point of reference for the results included in this clinical study. The result of this study are very close to those reported in the large meta-analysis mentioned above [
23], indeed, the trend of effectiveness could be confirmed. So far the fillers used for nasolabial fold area treatment allow achieving a satisfying and sustainable improvement.
In this study the results presented above point out to compare two different fillers. One study is still present in literature in which 21 different HA-composed fillers, were compared [
44], but any data concerning WSRS and GAIS, were compared. A comparative assessment between Foliage Intense (FI) and Belotero Intense (BI), was found previously. Therefore the primary endpoint hypothesis of this study, in which the non-inferiority of Foliage Intense (FI) in comparison with Belotero Intense (BI), was demonstrated through the WSRS scores at six months (V5). Indeed, in the PP population composed of 64 subjects, the 95% confidence limits of the difference between treatments, ranged from -0.09 to 0.05, lower than the pre-set margin of error of 0.25. This result is also consolidated by the analysis of the ITT population (95 subjects), where the 95% confidence limits of the difference between treatments were between -0.06 and 0.04. The proportion of responders (≥ 1 grade improvement in WSRS from baseline at 6 months) was 57.9% and 60.0%, in Belotero Intense and Foliage Intense group, respectively. Here, these difference were in favor of Foliage, but do not were significant according to McNemar’s test. Furthermore, Looking in the WSRS scores the analyzed data are almost identical, not only at V5, but along all other follow-up visits, while immediately after the injections the reduction in WSRS scores is greater with FI rather than with BI in a statistically significant manner (p= 0.0007 at V1 and 0.0039 at V2 with the Wilcoxon signed-rank test). On the other hand, GAIS by EI and subject scores are almost identical in the two treatment groups at all visits, except for a greater improvement in the GAIS score by EI in the Foliage intense group at visit V2 of touch-up (p=0.0156), where the two treatment groups did not differ regarding volume difference and dermal thickness.
In addition, the two groups of treatment did not differ regarding: dermal thickness assessed by ultrasound, volume difference by a 3D image system. However, they differ in a statistically significant manner (ANOVA p=0.0342) at the first check according the volume difference: 0.42 (CI 0.32-0.53) cc and 0.35 (CI 0.26-0.44) cc in the FI and BI group, respectively. Overall subject satisfaction was nearly identical for the two treatment groups at all visits, while injector satisfaction was better in the Foliage assessment with respect to in Belotero one, in comparison with the baseline injection (p=0.0005), and after touching-up (p=0.0039). Furthermore, analysis of incidence among specific complications revealed that most common are mild, transient, and reversible. They include lumpiness (43%), tenderness (41%), swelling (34%), bruising (29%), pain (28%), and redness (26%) [
13,
23,
45,
46]. More severe complications that could potentially lead to irreversible damage occur very sporadically. In the present cohort of patients, were revealed no presence of heavy and complicated adverse event associate with HA administration and the most common mild transient phenomenon was the redness, present only in 4.21% of treatments.
5. Materials and Methods
5.1. Demografic Characteristics of Patients
All demographic data concerning all patients enrolled in this study were reported in the
Table 10.
5.2. Features of the Device
In this trial, a cross linked hyaluronic resorbable acid filler named Foliage Intense 1x1ml, manufactured and distributed by Phitogen Holding S.p.A. (San Benedetto del Tronto, AP, Italy) will be compared to Belotero Intense, manufactured by ANTEIS SA (Geneva, Switzerland) in order to correct both moderate and severe nasolabial folds. The two devices belong to Class III medical devices according to Annex VIII of the Regulation (EU) 2017/745 on Medical Devices and will be used comparatively on the same subjects through a split face model. Foliage Intense (FI) 1x1mL, is a class III, intradermal, resorbable medical device (sterile, apyrogenic and physiological gel) to be used as a remedy for the correction of medium and deep skin sagging and for increasing the volume and contour of the lips. (Phitogen Holding Spa, 2020).
5.3. Comparator
Belotero Intense® (BI) an injectable resorbable implant indicated to fill deep wrinkles and folds, as well as to restore and enhance soft tissue volume (e.g., contours of the face, lip volume etc.). It is also suitable for correction of facial atrophic scars. It is a sterile, non-pyrogenic, viscoelastic, colorless, transparent cross-linked sodium hyaluronate gel of non-animal origin in a physiological phosphate buffer. Composition: The main component is cross-linked sodium hyaluronate, of non-animal origin (in a concentration of 25.5 mg/ml), which is stabilized in a phosphate buffer with pH=7 and is injected intradermally by needles of 27G½. It is stored between 2°C and 8°C.
Table 10.
Demographic data of patients.
Table 10.
Demographic data of patients.
| Variable |
Category |
Statistic |
All enrolled subjects (N=95) |
|
| |
|
|
|
|
| Gender |
Male |
n (%) |
4 (4.2%) |
|
| |
Female |
n (%) |
91 (95.8%) |
|
| |
|
|
|
|
| Skin color |
I. Pale white skin or very light or Celtic |
n (%) |
3 (3.2%) |
|
| |
II. Fair skin or European |
n (%) |
19 (20.0%) |
|
| |
III. Darker white skin or dark European |
n (%) |
68 (71.6%) |
|
| |
IV. Light brown skin |
n (%) |
5 (5.3%) |
|
| Age (years) |
|
n |
95 |
|
| |
|
Mean |
54.3 |
|
| |
|
SD |
8.09 |
|
| |
|
Minimum |
38 |
|
| |
|
Median |
53.0 |
|
| |
|
Maximum |
75 |
|
| |
|
|
|
|
| Height (cm) |
|
n |
95 |
|
| |
|
Mean |
164.5 |
|
| |
|
SD |
6.58 |
|
| |
|
Median |
165.0 |
|
| |
|
Maximum |
180 |
|
| |
|
|
|
|
| Weight (kg) |
|
n |
95 |
|
| |
|
Mean |
62.5 |
|
| |
|
SD |
9.80 |
|
| |
|
Minimum |
46 |
|
| |
|
Median |
60.0 |
|
| |
|
Maximum |
95 |
|
| Body mass index (kg/m2) |
n |
95 |
|
| |
|
Mean |
23.0 |
|
| |
|
SD |
3.07 |
|
| |
|
Minimum |
17 |
|
| |
|
Median |
22.8 |
|
| |
|
Maximum |
33 |
|
5.4. Functional Component
The main component of Foliage Intense is cross-linked hyaluronic acid. Hyaluronic acid is obtained through a bacterial bio-fermentation process, excluding any risk of contamination by antigenic proteins. It is cross-linked by the agent BDDE and stabilized with phosphate saline suspension buffer at pH 7.4. It has a molecular weight similar to the endogenous hyaluronic acid, and a concentration of 2.5% which allows a uniform distribution of the product in the dermis. Furthermore, Foliage Intense does not contain tissues or their derivatives, as the hyaluronic acid originates from bacterial fermentation and does not contain substances or derivatives of human blood. Finally, within the composition of the product it does not include, as an integral part, any substance which, if used separately, can be considered a medicinal specialty. Considering the absence of lidocaine or any other anesthetic substance in the product, the manufacturer advices to practice local anesthetic, before injection, in order to guarantee the necessary comfort to the subject.
5.5. Clinical Procedures
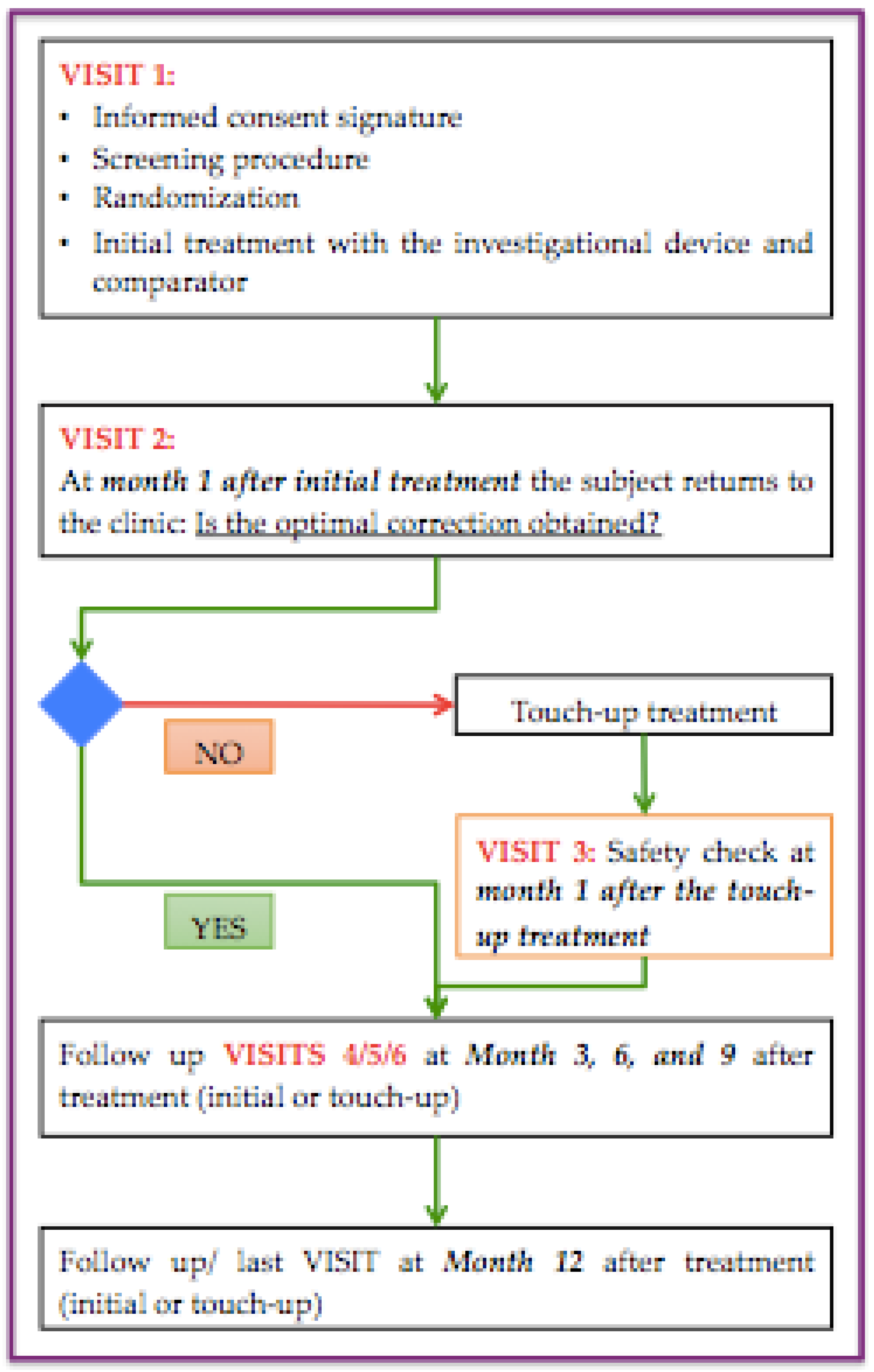
Each subject was treated with both products according to a split-face model: left and right sides of the face are treated in a randomized, observer-blinded manner (Subject and Evaluator blinded), respectively with Foliage Intense (FI) 1x1mL and with Belotero Intense (BI). In all enrolled subjects (after having signed the Informed Consent, had been screened for all inclusion/exclusion criteria, and having been randomized), the injections of the two products were performed, by the Treating Investigator, in the locations that need to be corrected according with the first baseline visit. The volume to be injected is at the discretion of the Treating Investigator depending on the width, length, and depth of the NLF, with the condition that the volume of 1.0mL. In any case, the volume of injection of both the investigating device and the comparator was equal. After 4 weeks, the subjects were return to perform the first safety check visit. When the subject does not present a complete aesthetic improvement on both folds, a secondary injection was performed (optional touch-up), if deemed necessary by the Treating Investigator, to ensure a satisfactory correction in the treatment area. The subjects will undergo the touch-up treatment, in both nasolabial folds, according to the randomization list of the initial injection. Those subjects who undergo the optional touch-up returned 30 days after the touch-up for a second safety check visit. The next follow-up visits was scheduled at 3 months after the last treatment (either initial treatment or optional touch-up). The subjects returned at month 6 after the last treatment for the evaluation of the primary efficacy endpoint. The following checks are provided at 9 months and then at 12 months after the last treatment (either initial treatment or touch-up), for the evaluation of the secondary endpoints and follow-up. Before injection, after 1, 3, 6, 9 and 12 months an ultrasound of the treated areas was performed in order to test the variation of the thickness of the soft tissues. The improvement in the Wrinkle Severity Rating Scale (WSRS) and Global Aesthetic Improvement Scale (GAIS) were assessed at each visit, in order to evaluate the changes in the nasolabial folds volume from baseline. In addition, the subjects were photographed to assess more objectively the changes over time of the NLFs, using a 3D imaging system (Vectra H2) in which calculated the differences in terms of the volume over time. The Treating Investigator gave his opinion on the quality of the injection and each subject confirmed his satisfaction on the product through a structured scale. A maximum number of seven (7) visits will be performed throughout the study, depending on whether the subjects will undergo touch-up or not, according to the scheme presented in
Figure 5.
The performance evaluation of the study treatment will be done according to criteria based in quantitative analysis and subjects/investigators satisfaction judgements as reported in the following sections. The blinded, independent expert medical professional (Evaluating Investigator) performed the observations and evaluations. Standard conditions for the treatment performance evaluations were:
hours before the study visit, subjects should avoid smoking and consumption of coffee or alcohol.
2 hours before each visit, the subjects should avoid the application of any cosmetic product on the skin test areas. All study evaluations are performed at room temperature (no temperature/humidity recording is required).
5.6. Scores
5.6.1. Wrinkle Severity Rating Scale (WSRS)
The WSRS parameter is a 5-levels assessment system, starting from 1 (absent) to 5 (extreme), of nasolabial folds. The evaluation by WSRS was performed by the Evaluating Investigator at screening, prior to treatment administration, after treatment administration and during all visits (Day DJ. et.al., 2004).
5.6.2. Global Aesthetic Improvement Scale (GAIS)
The GAIS is a 5-grade subjective test for performance analysis. The Evaluating Investigator evaluated the aesthetic improvement in the subjects of nasolabial folds using a 5-point scale: from 1 (very much improved) to 5 (worse). Aesthetic improvement by the GAIS scale was applied starting from the second visit and during all the study visits. (Thomas, J.A. and Walker, P., 2010).
5.7. Product Persistence over Time Assessment Through Ultrasound
The persistence over time of the product will be assessed by ultrasound, at the treatment visits and every follow-up visit, measuring dermal thickness before treatment and at all control visits, until month 12. The product will be considered reabsorbed when the thickness is similar to the basic measurement, i.e., the measurement before injection.
5.8. Evaluation of Subject’s Satisfaction
Subject’s satisfaction was assessed during all visity inside the study using a Numerical Rating Scale from 1 to 5 (where 1 means the subject is very much satisfied with the product and 5 the subjects is not satisfied).
5.9. Evaluation of Injection Satisfaction
Quality injection satisfaction degree was assessed at the baseline visit and on the optional touch-up visit, after treatment, using a 0-4 Numerical Rating Scale: where 0 = no satisfaction, 4 maximum satisfaction. The assessment was on the bases of handling of the product (e.g., viscosity, extrusion force, smoothness of injection, lumpiness, etc), according to the following categories: bad, sufficient, poor, good, or excellent.
5.10. Evaluation of Product Tolerability
Product’s tolerability was evaluated by a means of an electronic diary filled for two weeks after treatment by the patient and during all the visit of the study for allergies or other reactions that can occur as a result of the treatments (pain, erythema, edema, ecchymosis, pruritus, etc.), any other adverse event, even systemic, possibly elapsed during the investigation. Each reaction was associated with a score: 0: None; 1: Mild; 2: Moderate; 3: Severe.
5.11. Subject Replacement
Each subject withdrawn from the study after the treatment has taken place neither can be enrolled again in the current study nor can be replaced by other subjects with the same subject study number. Assuming 10% of dropouts, we established the recruitment a total of 95 subjects to have a minimum of 86 subjects needed to complete the study.
Figure 5.
Flow-chart representing the clinical procedure and the follow-up visit of patients.
Figure 5.
Flow-chart representing the clinical procedure and the follow-up visit of patients.
6. Conclusions
The present study and their statistical analyses data-associated, revealed no significant differences between FI and BI treatments. Both primary and secondary end-points were achieved according the adherence of protocol established at the beginning of the study. Looking in the safety of product, in the 95 subjects treated with both Foliage and Belotero, no adverse events, neither serious nor non-serious, were reported. Furthermore, local events found in the 5 subjects treated with Foliage were also found to be of equal extent and duration on the Belotero side in the split-face model. However, only two subjects had mild erythema and one had moderate redness and moderate pain only on the side treated with Belotero. Is important to remark there wasn’t one local event only on the side of Foliage Intense application. Nonetheless, looking for a recent extensive review of literature, the main result observed in several studies are very close to those observed in the present study, especially regarding the 6 months results of WSRS, GAIS and safety parameters.
Author Contributions
Conceptualization, M.R. and A.M.; methodology, M.R. and A.M..; software, A.M. and N.F.; validation, M.R.; M.M. and N.F.; formal analysis, N.F.; investigation, M.R. and A.M.; resources, M.R. and M.M..; data curation, M.M. and N.F.; writing—original draft preparation, M.R. and N.F.; writing—review and editing, M.R. and N.F.; visualization, M.R.; M.M. and N.F. supervision, M.R.; project administration, M.M.; funding acquisition, M.R. and M.M.
Funding
This research received external funding by Phitogen holding spa and the APC was funded by Phitogen holding spa”.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethical Committee for Clinical Trials of the Tuscany Region, (CEAVNO) on November, 21st 2021 (studies involving humans; Number of approval: 20743).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study, according the disposition of Ethical Committee CEAVNO.
Data Availability Statement
All data obtained from this study are available for consultation. The data controller is Phitogen holding spa.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kaminer, M.S.; Avelar, R.L.; Baumann, L.; Callender, V.; Dayan, S.H.; Green, J.B.; Grekin, S.K.; Guyon, S. Long-Term Safety and Effectiveness of Cold-Crosslinked Hyaluronic Acid Fillers: Multicenter, Randomized, Controlled, Double-Blind Study. Aesthet. Surg. J. 2025, sjaf080. [CrossRef]
- Li, Q.; Cui, H.; Tseng, F.-W.; Liu, Q.; Xue, Z.; Van Loghem, J.; Hung, K.-C.; Zhou, L.; Xie, W.; Zhao, J. The Assessment, Strategy, and Treatment Protocol: Nasolabial Fold Assessment, Strategy, and Treatment With Hyaluronic Acid Fillers in Chinese Patients. Plast. Reconstr. Surg. - Glob. Open 2025, 13, e6792. [CrossRef]
- Nilforoushzadeh, M.A.; Heidari-Kharaji, M.; Fakhim, T.; Hosseini, S.T.; Rafiee, S.; Shahverdi, M.; Najar Nobari, N. Efficacy Evaluation of Endolift Laser for Treatment of Nasolabial Folds and Marionette Lines. Skin Res. Technol. 2023, 29, e13480. [CrossRef]
- Chung, J.H.; Hanft, V.N.; Kang, S. Aging and Photoaging. J. Am. Acad. Dermatol. 2003, 49, 690–697. [CrossRef]
- Rabe, J.H.; Mamelak, A.J.; McElgunn, P.J.S.; Morison, W.L.; Sauder, D.N. Photoaging: Mechanisms and Repair. J. Am. Acad. Dermatol. 2006, 55, 1–19. [CrossRef]
- Fagien, S.; Monheit, G.; Jones, D.; Bank, D.; Sadick, N.; Nogueira, A.; Mashburn, J.H. Hyaluronic Acid Gel With (HARRL) and Without Lidocaine (HAJU) for the Treatment of Moderate-to-Severe Nasolabial Folds: A Randomized, Evaluator-Blinded, Phase III Study. Dermatol. Surg. 2018, 44, 549–556. [CrossRef]
- Monheit, G.; Beer, K.; Hardas, B.; Grimes, P.E.; Weichman, B.M.; Lin, V.; Murphy, D.K. Safety and Effectiveness of the Hyaluronic Acid Dermal Filler VYC-17.5L for Nasolabial Folds: Results of a Randomized, Controlled Study. Dermatol. Surg. 2018, 44, 670–678. [CrossRef]
- Shin, J.-W.; Kwon, S.-H.; Choi, J.-Y.; Na, J.-I.; Huh, C.-H.; Choi, H.-R.; Park, K.-C. Molecular Mechanisms of Dermal Aging and Antiaging Approaches. Int. J. Mol. Sci. 2019, 20, 2126. [CrossRef]
- Myung, Y.; Jung, C. Mini-Midface Lift Using Polydioxanone Cog Threads. Plast. Reconstr. Surg. - Glob. Open 2020, 8, e2920. [CrossRef]
- Fuente-del-Campo, A.; Lucchesi, R.; Ley, Ma.D.P.C. Bidirectional Armed Needle: A Useful Surgical Tool in Plastic Surgery: Plast. Amp Reconstr. Surg. 1997, 100, 695–698. [CrossRef]
- Yi, K.-H.; Wan, J.; Yoon, S.E.; Wong Kai Jie, I.; Kim, S.-B.; Jitaree, B. A Novel Approach for Facial Slimming Using Injectable Ascorbic Acid and Ferrous Gluconate: A Case Report. J. Craniofac. Surg. 2025. [CrossRef]
- Han, W.Y.; Kim, H.J.; Kwon, R.; Kang, S.M.; Yon, D.K. Safety and Efficacy of Poly-L-Lactic Acid Filler (Gana V vs. Sculptra) Injection for Correction of the Nasolabial Fold: A Double-Blind, Non-Inferiority, Randomized, Split-Face Controlled Trial. Aesthetic Plast. Surg. 2023, 47, 1796–1805. [CrossRef]
- Goodman, G.J.; Liew, S.; Callan, P.; Hart, S. Facial Aesthetic Injections in Clinical Practice: Pretreatment and Posttreatment Consensus Recommendations to Minimise Adverse Outcomes. Australas. J. Dermatol. 2020, 61, 217–225. [CrossRef]
- Dicker, K.T.; Gurski, L.A.; Pradhan-Bhatt, S.; Witt, R.L.; Farach-Carson, M.C.; Jia, X. Hyaluronan: A Simple Polysaccharide with Diverse Biological Functions. Acta Biomater. 2014, 10, 1558–1570. [CrossRef]
- Necas, J.; Bartosikova, L.; Brauner, P.; Kolar, J. Hyaluronic Acid (Hyaluronan): A Review. Veterinární Medicína 2008, 53, 397–411. [CrossRef]
- Rivas, F.; Erxleben, D.; Smith, I.; Rahbar, E.; DeAngelis, P.L.; Cowman, M.K.; Hall, A.R. Methods for Isolating and Analyzing Physiological Hyaluronan: A Review. Am. J. Physiol.-Cell Physiol. 2022, 322, C674–C687. [CrossRef]
- Cowman, M.K.; Lee, H.-G.; Schwertfeger, K.L.; McCarthy, J.B.; Turley, E.A. The Content and Size of Hyaluronan in Biological Fluids and Tissues. Front. Immunol. 2015, 6. [CrossRef]
- Tiwari, S.; Bahadur, P. Modified Hyaluronic Acid Based Materials for Biomedical Applications. Int. J. Biol. Macromol. 2019, 121, 556–571. [CrossRef]
- Iaconisi, G.N.; Lunetti, P.; Gallo, N.; Cappello, A.R.; Fiermonte, G.; Dolce, V.; Capobianco, L. Hyaluronic Acid: A Powerful Biomolecule with Wide-Ranging Applications—A Comprehensive Review. Int. J. Mol. Sci. 2023, 24, 10296. [CrossRef]
- Balazs, E. Hyaluronan as an Ophthalmic Viscoelastic Device. Curr. Pharm. Biotechnol. 2008, 9, 236–238. [CrossRef]
- Huynh, A.; Priefer, R. Hyaluronic Acid Applications in Ophthalmology, Rheumatology, and Dermatology. Carbohydr. Res. 2020, 489, 107950. [CrossRef]
- Bukhari, S.N.A.; Roswandi, N.L.; Waqas, M.; Habib, H.; Hussain, F.; Khan, S.; Sohail, M.; Ramli, N.A.; Thu, H.E.; Hussain, Z. Hyaluronic Acid, a Promising Skin Rejuvenating Biomedicine: A Review of Recent Updates and Pre-Clinical and Clinical Investigations on Cosmetic and Nutricosmetic Effects. Int. J. Biol. Macromol. 2018, 120, 1682–1695. [CrossRef]
- Stefura, T.; Kacprzyk, A.; Droś, J.; Krzysztofik, M.; Skomarovska, O.; Fijałkowska, M.; Koziej, M. Tissue Fillers for the Nasolabial Fold Area: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Aesthetic Plast. Surg. 2021, 45, 2300–2316. [CrossRef]
- Meçani, R.; Amiri, M.; Kadouch, J.; Sajic, D.; Lin, F.; Cheung, J.; Barrera, D.; Haroon, O.; Sil-Zavaleta, S.; Chao, Y.; et al. Combined and Hybrid Treatments of Hyaluronic Acid (HA) and Calcium Hydroxylapatite (CaHA): A Systematic Review of Mechanisms of Action, Aesthetic Effectiveness, Satisfaction, and Safety Profile. Aesthetic Plast. Surg. 2025. [CrossRef]
- El-Khalawany, M.; Fawzy, S.; Saied, A.; Al Said, M.; Amer, A.; Eassa, B. Dermal Filler Complications: A Clinicopathologic Study with a Spectrum of Histologic Reaction Patterns. Ann. Diagn. Pathol. 2015, 19, 10–15. [CrossRef]
- Mao, Z.; Shin, H.-D.; Chen, R. A Recombinant E. Coli Bioprocess for Hyaluronan Synthesis. Appl. Microbiol. Biotechnol. 2009, 84, 63–69. [CrossRef]
- Ducher, G.; Prasetyo, A.D.; Rubin, M.G.; Moretti, E.A.; Nikolis, A.; Prager, W. Hyaluronic Acid Fillers with Cohesive Polydensified Matrix for Soft-Tissue Augmentation and Rejuvenation: A Literature Review. Clin. Cosmet. Investig. Dermatol. 2016, Volume 9, 257–280. [CrossRef]
- Park, K.E.; Mehta, P.; Kherani, F.; Lee, W.W.; Woodward, J.A.; Foster, J.A.; Zhang-Nunes, S. Response of 21 Hyaluronic Acid Fillers to Recombinant Human Hyaluronidase. Plast. Reconstr. Surg. - Glob. Open 2023, 11, e5457. [CrossRef]
- Day, D.J.; Littler, C.M.; Swift, R.W.; Gottlieb, S. The Wrinkle Severity Rating Scale: A Validation Study. Am. J. Clin. Dermatol. 2004, 5, 49–52. [CrossRef]
- Day, D.J.; Littler, C.M.; Swift, R.W.; Gottlieb, S. The Wrinkle Severity Rating Scale: A Validation Study. Am. J. Clin. Dermatol. 2004, 5, 49–52. [CrossRef]
- Narins, R.S.; Brandt, F.; Leyden, J.; Lorenc, Z.P.; Rubin, M.; Smith, S. A Randomized, Double-Blind, Multicenter Comparison of the Efficacy and Tolerability of Restylane Versus Zyplast for the Correction of Nasolabial Folds. Dermatol. Surg. 2003, 29, 588–595. [CrossRef]
- Fundarò, S.P.; Salti, G.; Malgapo, D.M.H.; Innocenti, S. The Rheology and Physicochemical Characteristics of Hyaluronic Acid Fillers: Their Clinical Implications. Int. J. Mol. Sci. 2022, 23, 10518. [CrossRef]
- Murthy, R.; Roos, J.C.P.; Goldberg, R.A. Periocular Hyaluronic Acid Fillers: Applications, Implications, Complications. Curr. Opin. Ophthalmol. 2019, 30, 395–400. [CrossRef]
- Signorini, M.; Liew, S.; Sundaram, H.; De Boulle, K.L.; Goodman, G.J.; Monheit, G.; Wu, Y.; Trindade De Almeida, A.R.; Swift, A.; Vieira Braz, A. Global Aesthetics Consensus: Avoidance and Management of Complications from Hyaluronic Acid Fillers—Evidence- and Opinion-Based Review and Consensus Recommendations. Plast. Reconstr. Surg. 2016, 137, 961e–971e. [CrossRef]
- Trinh, L.N.; Gupta, A. Hyaluronic Acid Fillers for Midface Augmentation: A Systematic Review. Facial Plast. Surg. 2021, 37, 576–584. [CrossRef]
- Https://Pubmed.Ncbi.Nlm.Nih.Gov.
- Https://Www.Sciencedirect.Com.
- Https://Www.Embase.Com/Landing?Status=grey.
- Https://Www.Ebsco.Com/It-It/Prodotti/Banche-Dati-per-La-Ricerca/Biosis-Previews.
- Socialsciences.Scielo.Org.
- Https://Www.Scopus.Com/Home.Uri.
- Https://Www.Cochranelibrary.Com/Central.
- Https://Clarivate.Com/Academia-Government/Scientific-and-Academic-Research/Research-Discovery-and-Referencing/Web-of-Science/.
- Park, K.E.; Mehta, P.; Kherani, F.; Lee, W.W.; Woodward, J.A.; Foster, J.A.; Zhang-Nunes, S. Response of 21 Hyaluronic Acid Fillers to Recombinant Human Hyaluronidase. Plast. Reconstr. Surg. - Glob. Open 2023, 11, e5457. [CrossRef]
- DeLorenzi, C. Complications of Injectable Fillers, Part 2: Vascular Complications. Aesthet. Surg. J. 2014, 34, 584–600. [CrossRef]
- Callan, P.; Halstead, M.; Rogers; Goodman, G.; Liew; Muzikants; Scamp; Carlisle Efficacy and Safety of a Hyaluronic Acid Filler in Subjects Treated for Correction of Midface Volume Deficiency: A 24 Month Study. Clin. Cosmet. Investig. Dermatol. 2013, 81. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).