Submitted:
27 June 2025
Posted:
30 June 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Data Collection and Advanced Molecular Formulation Database (AMF-DB)
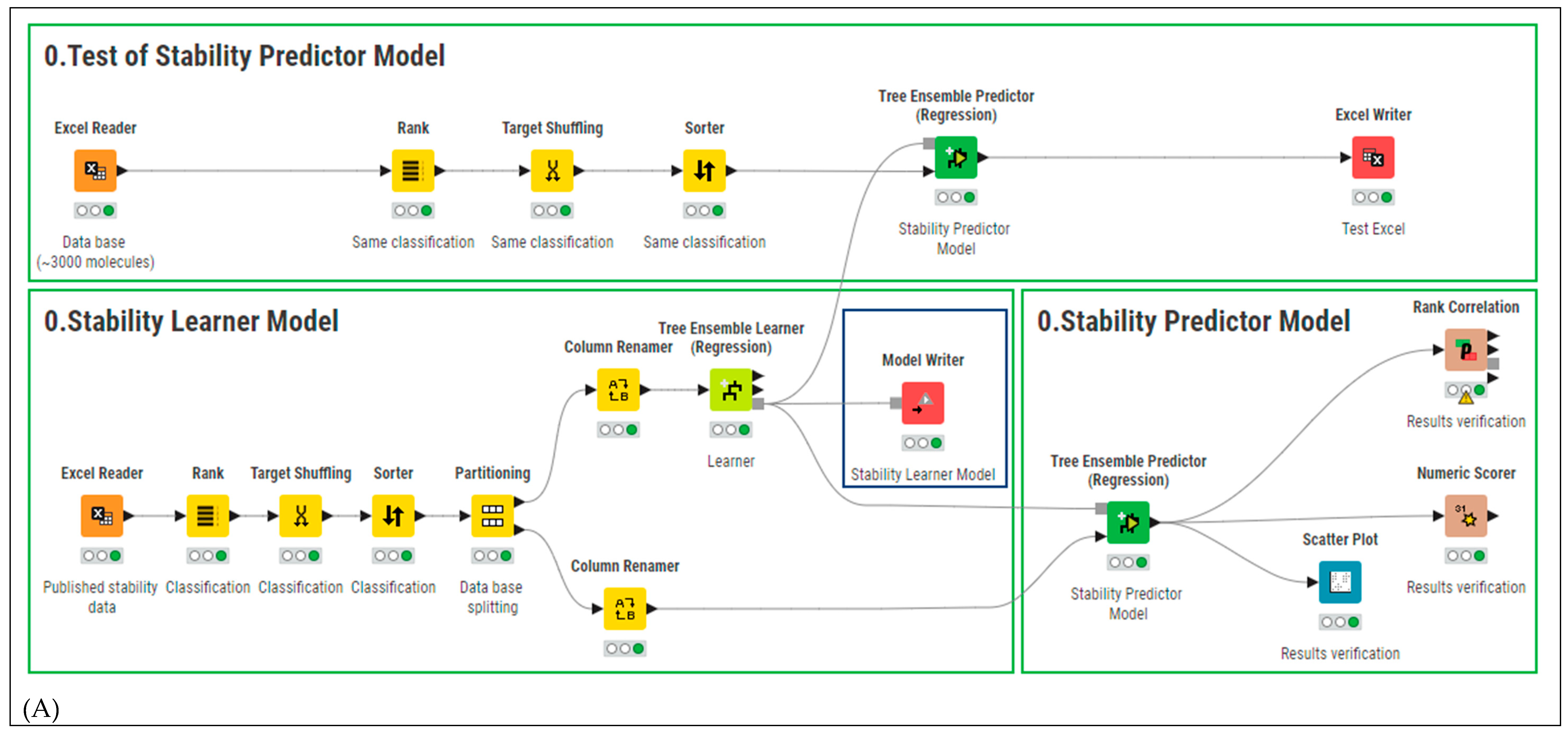
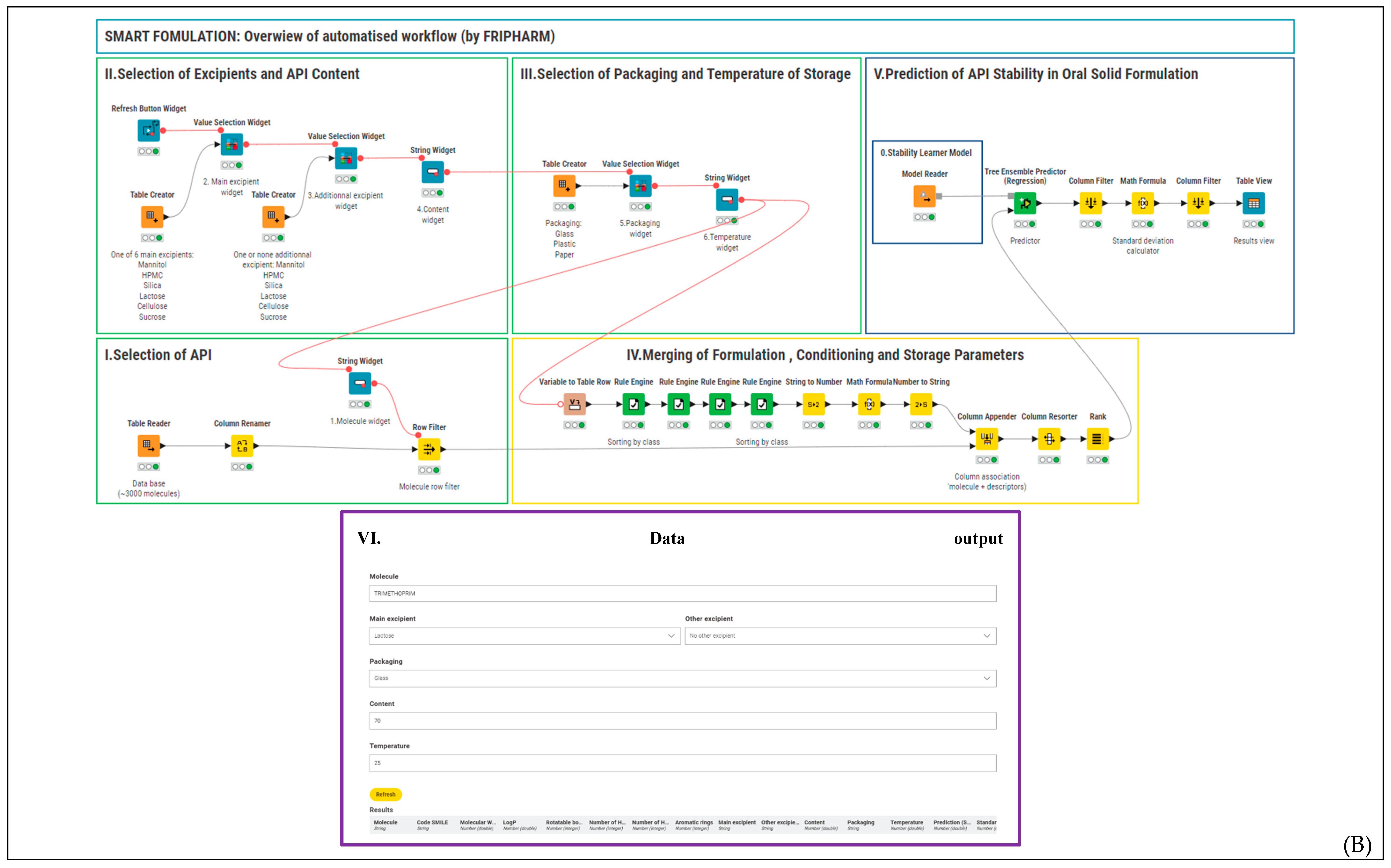
2.2. Smart Formulation Development
2.3. Model Evaluation
2.4. Model Validation and Performance Testing
2.5. Web Integration
3. Results and Discussion
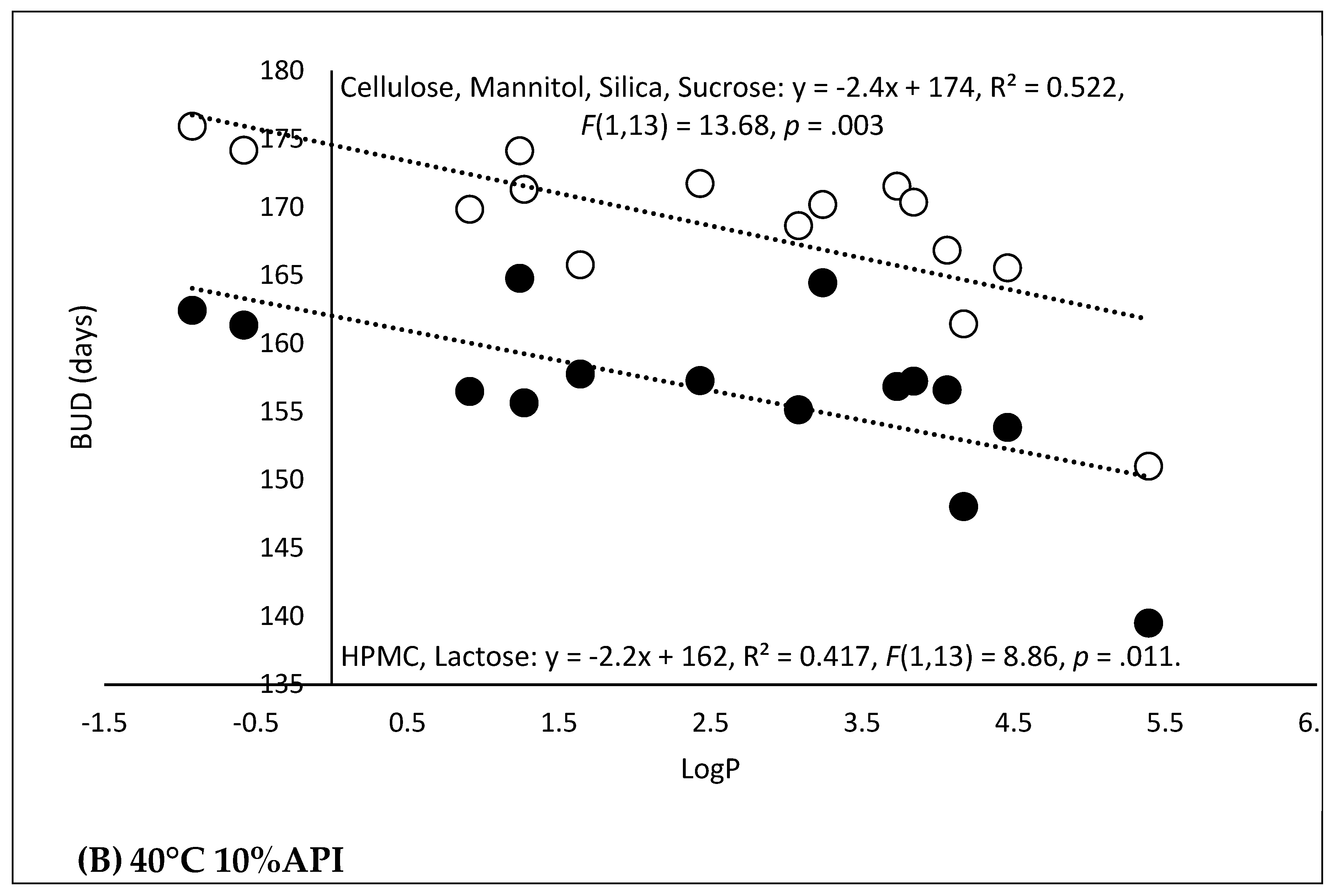
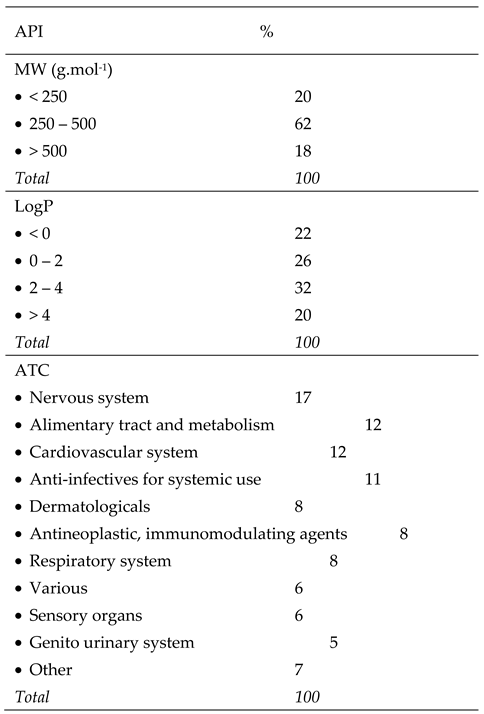
3.1. AMF-DB and Smart Formulation Development and Model Evaluation
| Metrics | Single Decision Method | Ensemble Methods | Boosting Methods | ||||||
| Decision Trees | Random Forests | Random Forest Regression |
Tree Ensembles | Tree Ensembles Regression | Gradient Boosted Trees |
Gradient Boosted Trees Regression |
|||
| R² | 0.93 | 0.758 | 0.536 | 0.491 | 0.975 | -0.447 | 0.849 | ||
| Mean Absolute Error (MAE) | 9 | 17.94 | 48.86 | 24.54 | 10.16 | 98.17 | 19.45 | ||
| Mean Squared Error (MSE) | 912.90 | 3160.86 | 6073.61 | 6661.17 | 358.16 | 18931.90 | 1972.69 | ||
| Root Mean Squared Error (RMSE) | 30.21 | 56.22 | 77.93 | 81.62 | 18.93 | 137.60 | 44.42 | ||
| Mean Signed Difference (MSD) | -1.17 | 8.34 | 20.20 | 24.54 | -0.13 | -76.46 | -7.15 | ||
| Mean Absolute Percentage Error (MAPE) | 0.05 | 0.23 | 0.59 | 0.44 | 0.064 | 0.50 | 0.16 | ||
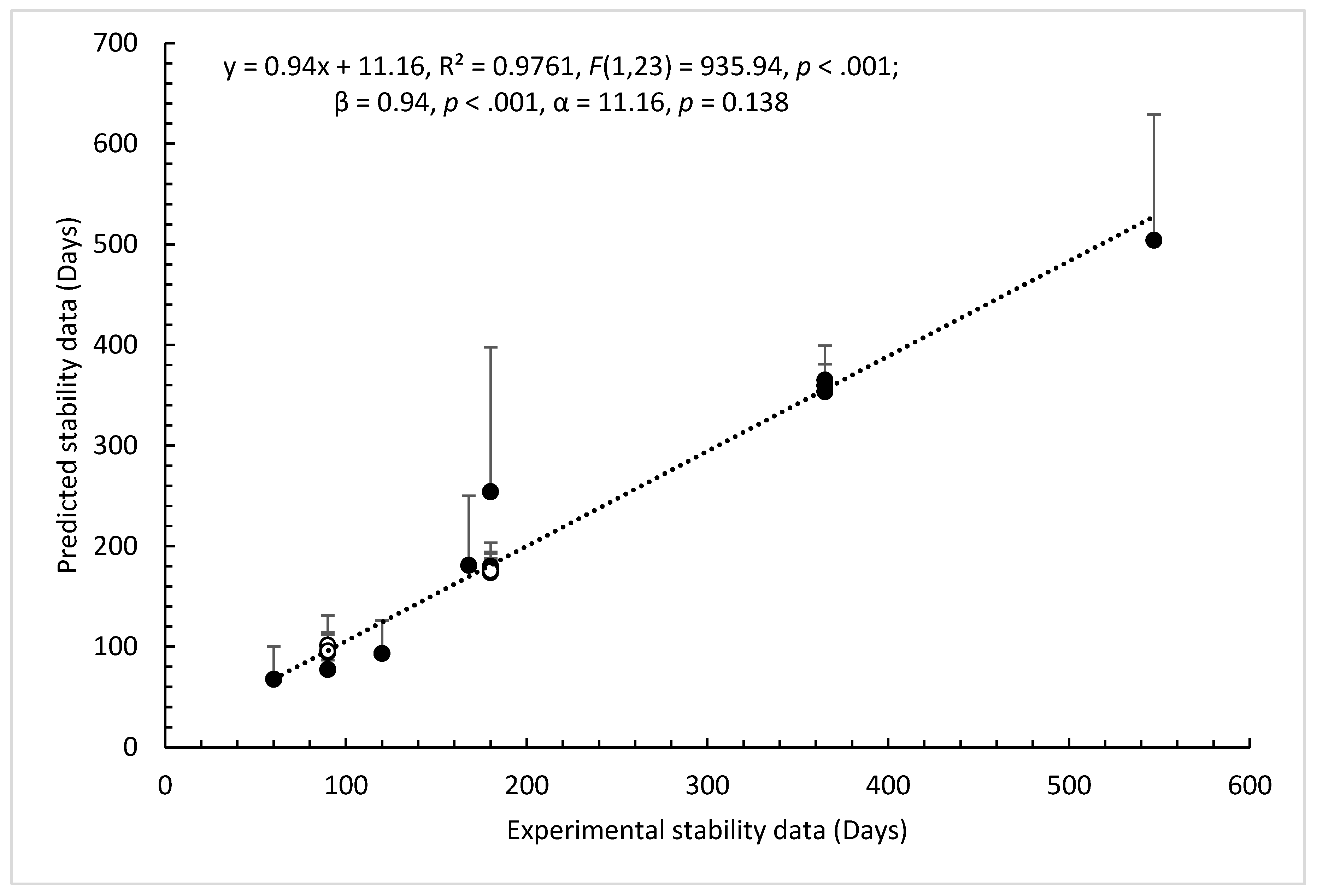
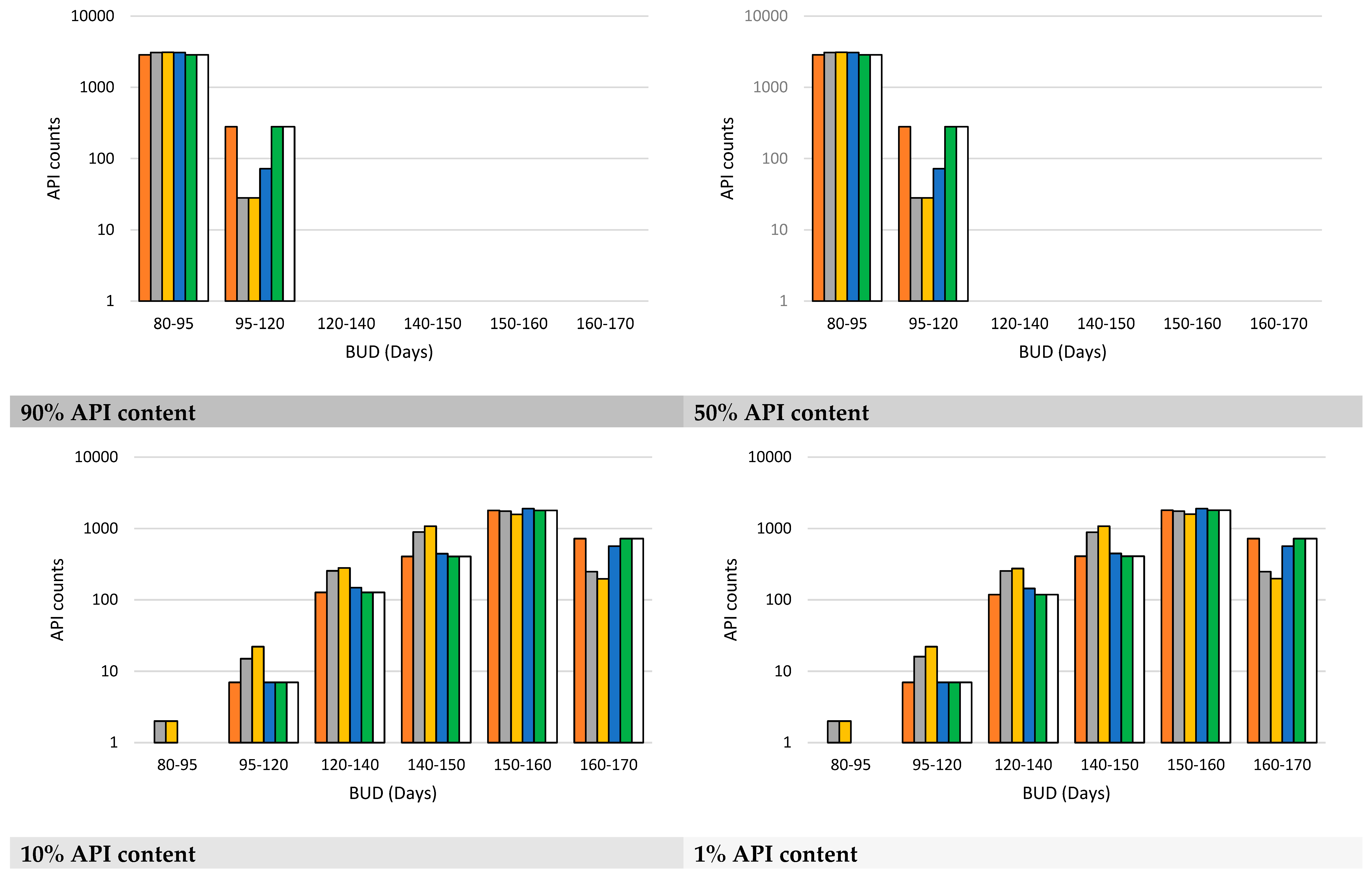
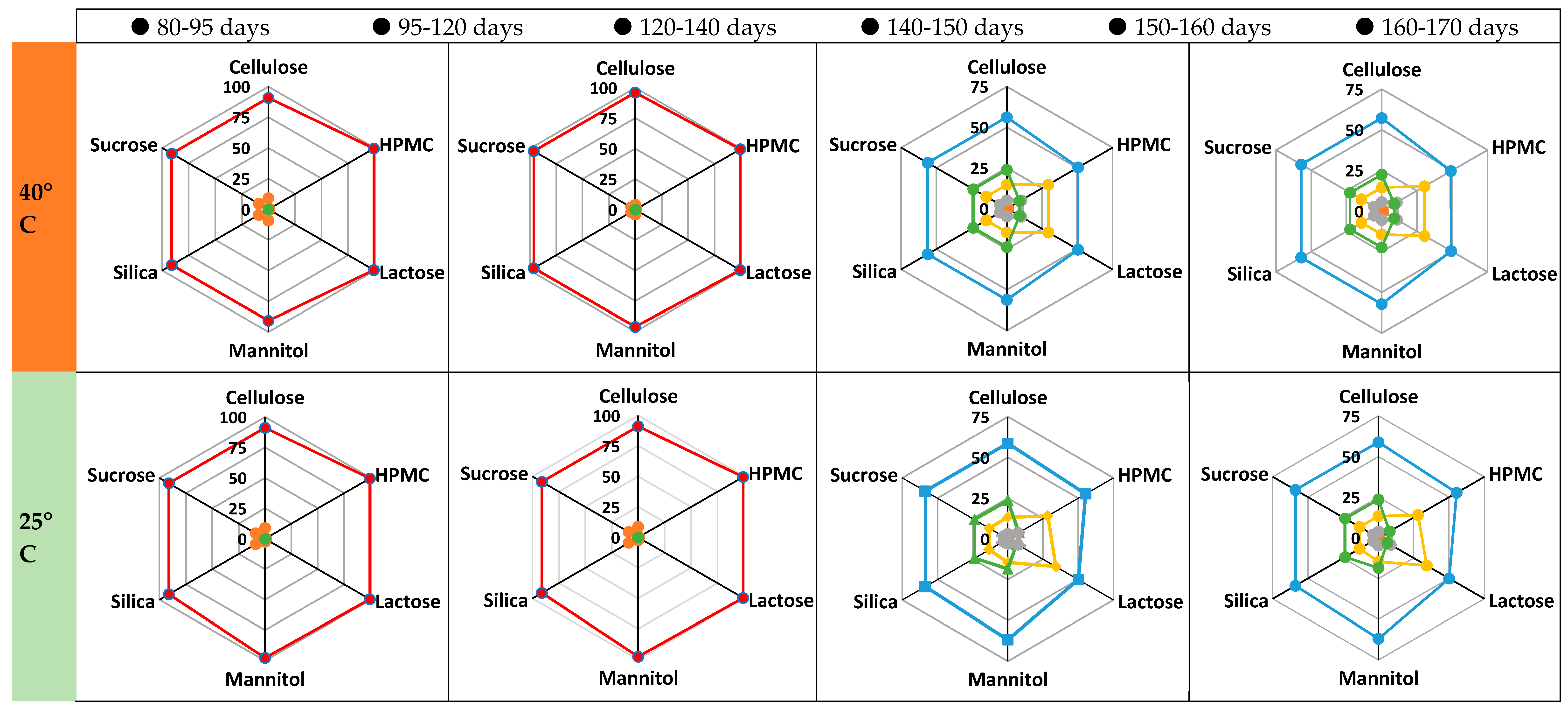
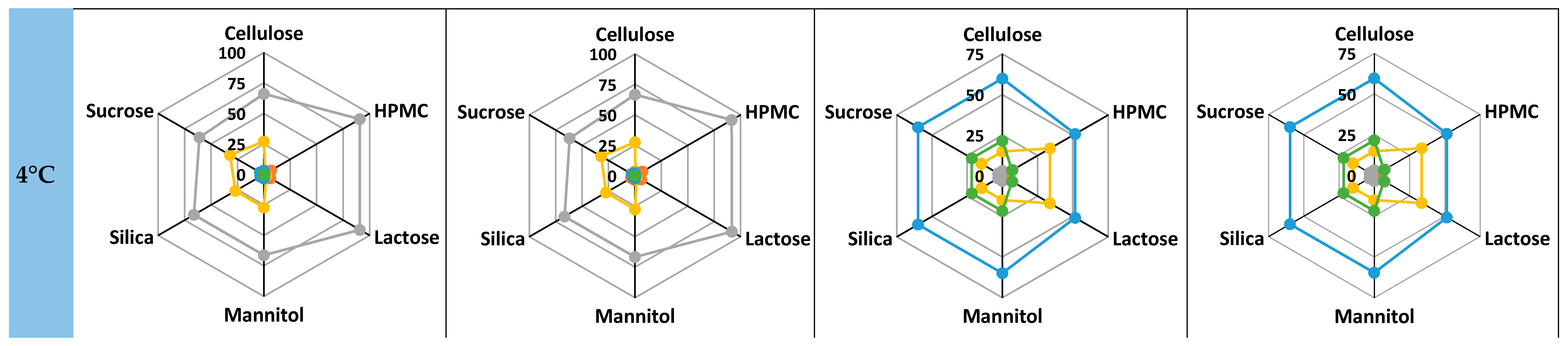
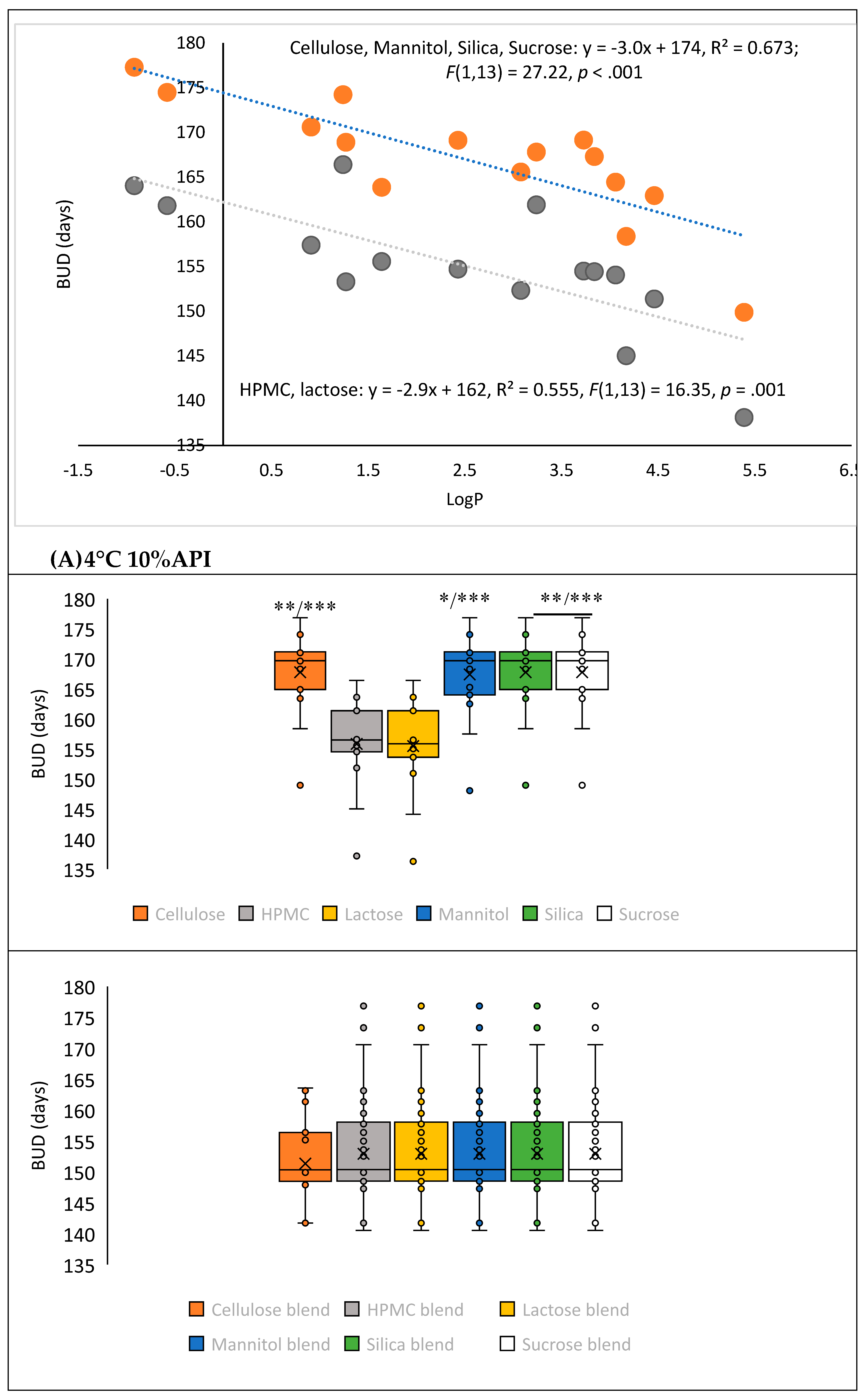
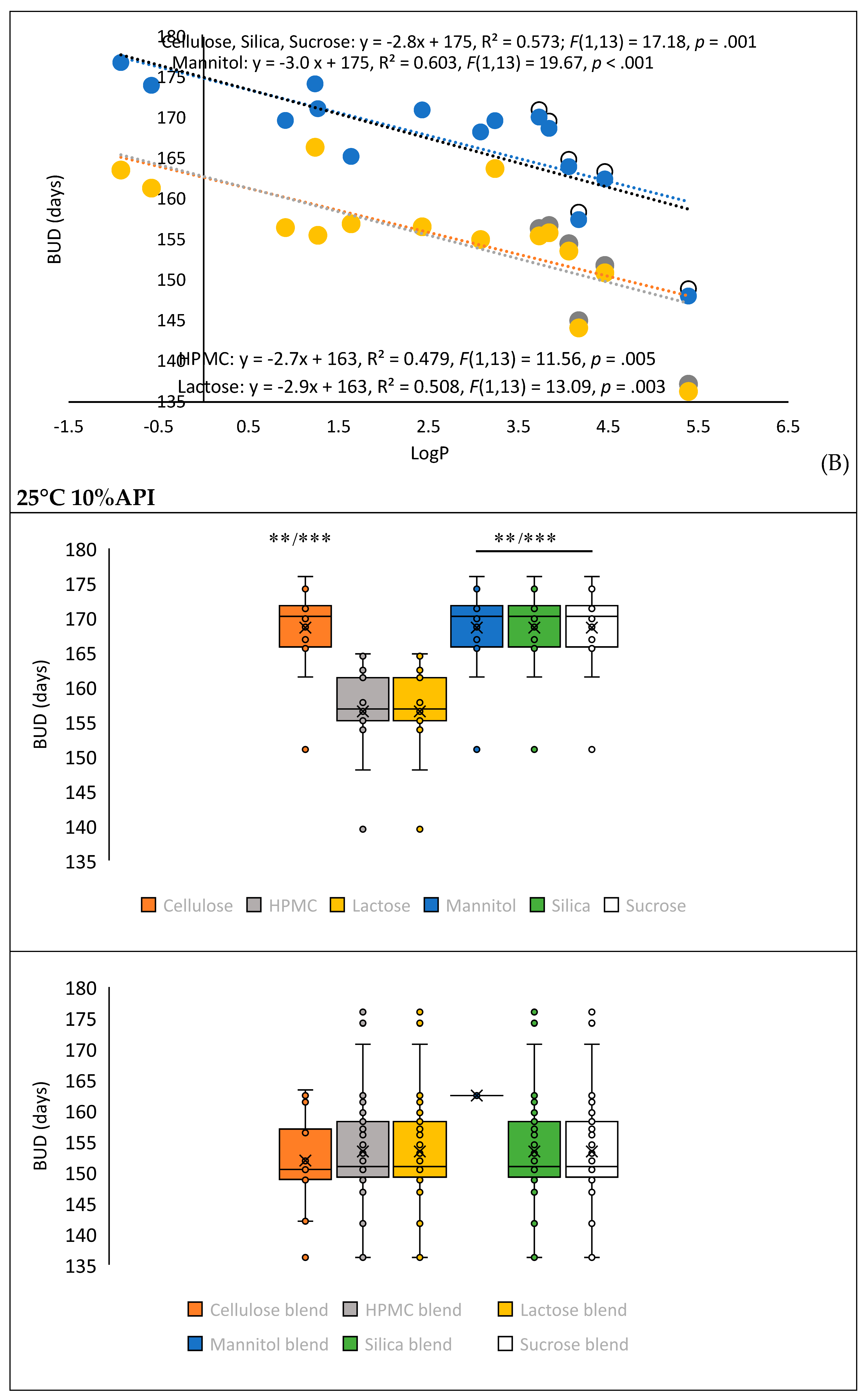
3.2. Model Validation and Performance Testing
| API | Log P | Shelf-life (Raw material) |
BUD (Preparation) | Expiration date (Specialty)‡ | Film coated tablet | ||
| 1 EXP | 2 EXP | FormulationAI | |||||
| Acetaminophen (C) | 0.91 | 4 years; 15-25°Ca |
156 – 170 days | 156 – 171 days | 180 days | 5 EXP/5 years Claradol 500 mg | No |
| Amlodipine (besylate) (C) |
1.64 | 5 years; 15-25°Ca |
157 – 165 days | 148 – 153 days | 180 days | 3 EXP/3 years Amlodipine 10 mg | No |
| Aspirin(C) | 1.24 | 3 years; 15-25°Ca |
166 – 174 days | 163 – 173 days | 180 days | 2 EXP/3 years Aspirine du Rhône 500 mg | No |
| Atorvastatin (calcium) (A) |
5.39 | 5 years; 15-25°Cb |
136 – 149 days | 134 – 141 days | 180 days | 6 EXP/2 years Atorvastatin 80 mg | Yes |
| Clarithromycin (C) | 3.24 | 3 years; 15-25° Cc |
164 – 170 days | 155 – 158 days | 180 days | 7 EXP/3 years Clarithromycine 250 mg | Yes |
| Diazepam(C) | 3.08 | 5 years; 15-25°Ca |
155 – 168 days | 149 – 154 days | 180 days | 4 EXP/3 years Diazepam 10 mg | No |
| Fluoxetine (Chlorhydrate) (C) |
1.7 | 5 years; 15-25°Cd |
144 – 158 days | 141 – 147 days | 180 days | 3 EXP/3 years Fluoxetine 20 mg | Dispersible |
| Hydrochlorothiazide (C) | - 0.58 | 5 years; 15-25°Ca |
161 – 174 days | 161 – 174 days | 180 days | 5 EXP/3 years Esidrex 25 mg | No |
| Ibuprofen(C) | 3.84 | 5 years; 15-25°Ca |
156 – 170 days | 150 days | 180 days | 11 EXP/3 years Advil 200 mg | Yes |
| Levothyroxine (sodium) (A) |
-2.3 | 2 years; 15-25°Cf |
155 – 171 days | 150 – 160 days | 180 days | 5 EXP/3 years Thyrofix 100 µg | No |
| Losartan (potassium) (C) |
4.06 | 5 years; 15-25°Cb |
154 – 165 days | 151 – 156 days | 180 days | 7 EXP/3 years Losartan 50 mg | Yes |
| Metformin (hydrochloride) (C) |
-0.92 | 5 years; 15-25°Ce |
164 – 177 days | 164 – 177 days | 180 days | 4 EXP/5 years Glucophage 500 mg | No |
| Omeprazole (C) | 2.43 | 2 years; 15-25°Ca |
157 – 171 days | 150 – 160 days | 180 days | 8 EXP/3 years Omeprazole 10 mg | Enteric film |
| Prednisolone (C) | 1.27 | 3 years; 15-25°Ca |
156 – 171 days | 148 – 157 days | 180 days | 4 EXP/3 years Prednisolone 20 mg | No |
| Simvastatin (A) | 3 years; 15-25°Ca | 151 – 163 days | 149 – 155 days | 180 days /Instable¥ |
11 EXP/2 years Simvastatine 40 mg | Yes | |
| API (API content %) |
Shelf-life (Raw material) | BUD (Preparation) | Expiration date (Specialty)‡ | ||||||||||||
| 1 EXP | 2 EXP | MTF | |||||||||||||
| Acetazolamide 250 mg (> 50%) |
3-4 years; 15-25°Cb |
Lactose G:165 Pl:167 P:162 days |
Lactose/silica G:173 Pl:175 P:170 days |
60 days Lactose/silica Hard capsules n° 2 | 4 EXP/3 years Diamox 250 mg |
||||||||||
| Cetirizine dichlorhydrate 10 mg (< 50%) |
5 years; 15-25°Ca | Lactose G:148 Pl:148 P:145 days |
Lactose/silica G:162 Pl:163 P:159 days |
60 days Lactose/silica Hard capsules n° 3 | 5 EXP/4 years Cetirizine 10 mg |
||||||||||
| Chenodesoxycholic acid 250 mg (< 50%) (> 50%) |
NA | Mannitol G:170 Pl:171 P:166 days Mannitol G:160 Pl:161 P:157 days |
Mannitol/silica G:154 Pl:155 P:150 days Mannitol/silica G:146 Pl:147 P:143 days |
180 days Mannitol/silica | NA | ||||||||||
| Cholecalciferol 100.000 U.I/g 4,0 mg (< 50%) |
NA | Lactose G:109 Pl:110 P:110 days |
- | 60 days Lactose | NA | ||||||||||
| Clindamycine 150, 300 mg Clindamycine phosphate 163.5, 327 mg (< 50%) |
2 years; 15-25°Cc |
Lactose G:164 Pl:166 P:162 days Mannitol G:171 Pl:174 P:169 days |
Lactose/silica G:170 Pl:172 P:168 days Mannitol/silica G:163 Pl:166 P:161 days |
60 days Lactose/silica Hard capsules n° 0 60 days Mannitol/silica Hard capsules n° 0 |
4 EXP/3 years Clindamycine 150, 300 mg |
||||||||||
| Diosmine 500 mg (< 50%) |
NA | Lactose G:142 Pl:145 P:144 days |
Lactose/silica G:154 Pl:157 P:155 days |
60 days Lactose/silica Hard capsules n° 000 | 7 EXP/3 years Diosmine 600 mg |
||||||||||
| Domperidone 10 mg (< 50%) |
NA | Lactose G:162 Pl:164 P:159 days |
Lactose/silica G:172 Pl:174 P:169 days |
60 days Lactose/silica Hard capsules n° 3 | 10 EXP/3 years Domperidone Arrow 10 mg |
||||||||||
| Doxycycline 50, 100 mg Doxycycline hyclate 58, 116 mg (< 50%) |
3 years; 15-25°Cc |
Lactose G:167 Pl:170 P:165 days Mannitol G:175 Pl:178 P:173 days |
Lactose/silica G:175 Pl:178 P:173 days Mannitol/silica G:167 Pl:170 P:165 days |
60 days Lactose/silica Hard capsules n° 1 60 days Mannitol/silica Hard capsules n° 1 |
10 EXP / 3 years Doxy 50 mg, 100 mg |
||||||||||
| Folic acid 0.4, 4 mg (< 50%) | NA | Mannitol G:173 Pl:176 P:171 days |
Mannitol/silica G:165 Pl:168 P:163 days |
60 days Mannitol/silica | 5 EXP/30 months 0.4 mg 5 EXP/2 years 5 mg |
||||||||||
| Fludrocortisone acetate 0.025, 0.050, 0.1 mg (< 50%) |
NA | Mannitol G:175 Pl:177 P:173 days |
Mannitol/silica G:170 Pl:173 P:168 days |
60 days Mannitol/silica Hard capsules n° 2 |
3 EXP/ 3 years Flucortac 50 µg 6 EXP/ 2 years Flucortac 0.1 mg |
||||||||||
| Furosemide 1 mg à 10 mg (< 50%) |
NA | Lactose G:152 Pl:154 P:149 days |
Lactose/silica G:165 Pl:167 P:161 days |
60 days Lactose/silica | 4 EXP/3 years Furosemide 20 mg |
||||||||||
| Hydrocortisone 10, 20 mg (< 50%) |
2-5 years; 15-25°Cd |
Mannitol G:140 Pl:141 P:136 days |
Mannitol/silica G:126 Pl:127 P:122 days |
60 days Mannitol/silica Hard capsules n° 2 |
4 EXP/3 years Hydrocortisone 10 mg |
||||||||||
| Loperamide chlorhydrate 2 mg (< 50%) |
2 years; 2-8°Ce | Lactose G:147 Pl:148 P:144 days |
Lactose/silica G:160 Pl:160 P:156 days |
60 days Lactose/silica Hard capsules n° 3 | 3 EXP/ 3 years Diaretyl 2 mg | ||||||||||
| Mebeverine chlorhydrate 135 mg (< 50%) |
NA | Lactose G:159 Pl:160 P:157 days |
Lactose/silica G:167 Pl:169 P:167 days |
60 days Lactose/silica Hard capsules n° 0 |
4 EXP/ 3 years Mebeverine 100 mg |
||||||||||
| Menadione sodium bisulfite 1 mg (< 50%) |
NA | Mannitol G:105 Pl:106 P:104 days |
Mannitol/silica G:89 Pl:90 P:87 days |
60 days Mannitol/silica | NA | ||||||||||
| Minocycline chlorhydrate dihydrate 58, 116 mg (< 50%) | NA | Mannitol G:173 Pl:174 P:169 days |
Mannitol/silica G:165 Pl:166 P:161 days |
60 days Mannitol/silica Hard capsules n° 1 |
1 excipient/ 2 years Minocyne 100 mg |
||||||||||
| Primaquine phosphate 30 mg (< 50%) |
NA | Mannitol G:160 Pl:162 P:159 days |
Mannitol/silica G:147 Pl:148 P:145 days |
180 days Mannitol/silica | 10 EXP/ 3 years Primaquine 15 mg |
||||||||||
| Pyridoxal phosphate 10 mg (< 50%) |
5 years; 15-25°Ca | Mannitol G:161 Pl:164 P:161 days |
Mannitol/silica G:149 Pl:152 P:149 days |
180 days Mannitol/silica | NA | ||||||||||
| Ranitidine 150mg Ranitidine chlorhydrate 167.5 mg (< 50%) |
36 months ;15-25°Cf | Lactose G:155 Pl:158 P:153 days |
Lactose/silica G:167 Pl:169 P:164 days |
60 days Lactose/silica Hard capsules n° 00 | 8 EXP/ 3 years Ranitine EG 150 mg |
||||||||||
| Retinol acetate 325.000 U.I/g 12,3 mg (< 50%) |
2 years; 15-25°C [43] | Lactose G:154 Pl:156 P:150 days |
- | 60 days Lactose | NA | ||||||||||
| Riboflavine 400 mg (< 50%) (> 50%) |
4 years; 15-25°Ca | Lactose G:165 Pl:166 P:161 days Lactose G:153 Pl:155 P:150 days |
Lactose/silica G:174 Pl:175 P:170 days Lactose/silica G:164 Pl:165 P:160 days |
60 days Lactose/silica | NA | ||||||||||
| Scopolamine butylbromure 10 mg (< 50%) |
NA | Lactose G:149 Pl:152 P:149 days |
Lactose/silica G:164 Pl:166 P:163 days |
60 days Lactose/silica | NA | ||||||||||
| Simvastatin 5, 20, 40 mg (< 50%) |
3 years; 15-25°Ca | Lactose G:157 Pl:157 P:153 days |
Lactose/silica G:167 Pl:167 P:164 days |
60 days Lactose/silica Hard capsules n° 2 | 11 EXP / 2 years Simvastatine Accord 10, 20,40 mg |
||||||||||
| Spironolactone 25 mg (< 50%) |
5 years; 15-25°Ca | Lactose G:162 Pl:164 P:159 days |
Lactose/silica G:172 Pl:174 P:169 days |
60 days Lactose/silica | 5 EXP/ 18 months Aldactone 25 mg |
||||||||||
| Sulpiride 50 mg (< 50%) |
NA | Lactose G:138 Pl:138 P:138 days |
Lactose/silica G:149 Pl:150 P:150 days |
60 days Lactose/silica | 4 EXP/ 2 years Dogmatil 50 mg |
||||||||||
| Triamcinolone 4 mg (< 50%) |
NA | Mannitol G:158 Pl:158 P:156 days |
Mannitol/silica G:149 Pl:149 P:147 days |
60 days Mannitol/silica Hard capsules n° 2 |
NA | ||||||||||
| Trimethoprime 50 mg (< 50%) |
NA | Lactose G:163 Pl:165 P:160 days Mannitol G:172 Pl:174 P:169 days |
Lactose/silica G:172 Pl:174 P:169 days Mannitol/silica G:163 Pl:165 P:160 days |
60 days Lactose/silica Hard capsules n° 3 60 days Mannitol/silica Hard capsules n° 3 |
5 EXP/ 3 years Delprim 300 mg |
||||||||||
| Trimethoprime 300 mg (> 50%) |
Lactose G:153 Pl:155 P:151 days Mannitol G:163 Pl:166 P:161 days |
Lactose/silica G:163 Pl:166 P:161 days Mannitol/silica G:153 Pl:155 P:151 days |
60 days Lactose/silica Hard capsules n° 1 60 days Mannitol/silica Hard capsules n° 1 |
||||||||||||
3.3. Web Integration
4. Conclusion
Declaration of Interest
Data Availability Statement
- Stabilis Database (https://www.stabilis.org): A reference platform for drug stability, used for extracting experimental BUD data.
- PubChem (https://pubchem.ncbi.nlm.nih.gov): For molecular structure and descriptor data of active pharmaceutical ingredients.
- DrugBank (https://go.drugbank.com): For physicochemical and pharmacological data on APIs.
- PubMed (https://pubmed.ncbi.nlm.nih.gov): For literature-derived stability data and formulation context.
- ChemBL(https://www.ebi.ac.uk/chembl/): For bioactivity and structural information related to APIs.
- Vidal Hoptimal® (https://www.vidal.fr/hoptimal): For pharmaceutical formulation components and hospital-use references.
Declaration of generative AI and AI-assisted technologies in the writing process.
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seghers, F.; Taylor, M.M.; Storey, A.; Dong, J.; Wi, T.C.; Wyber, R.; Ralston, K.; Nguimfack, B.D. Securing the Supply of Benzathine Penicillin: A Global Perspective on Risks and Mitigation Strategies to Prevent Future Shortages. Int. Health 2024, 16, 279–282. [CrossRef]
- European Drug Shortages Formulary Project: Approval of Framework and Procedure Documents - European Directorate for the Quality of Medicines & HealthCare - EDQM Available online: https://www.edqm.eu/en/-/european-drug-shortages-formulary-project-approval-of-framework-and-procedure-documents (accessed on 26 March 2025).
- Mian, P.; Maurer, J.M.; Touw, D.J.; Vos, M.J.; Rottier, B.L. Pharmacy Compounded Pilocarpine: An Adequate Solution to Overcome Shortage of Pilogel® Discs for Sweat Testing in Patients with Cystic Fibrosis. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2024, 23, 126–131. [CrossRef]
- Allen, L.V. PreScription: Shortages Continue--Compounding Pharmacies Fill the Gap…Again! Int. J. Pharm. Compd. 2023, 27, 180.
- Gudeman, J.; Jozwiakowski, M.; Chollet, J.; Randell, M. Potential Risks of Pharmacy Compounding. Drugs RD 2013, 13, 1–8. [CrossRef]
- Watson, C.J.; Whitledge, J.D.; Siani, A.M.; Burns, M.M. Pharmaceutical Compounding: A History, Regulatory Overview, and Systematic Review of Compounding Errors. J. Med. Toxicol. 2021, 17, 197–217. [CrossRef]
- Timko, R.J.; Crooker, P.E.M. Pharmaceutical Compounding or Pharmaceutical Manufacturing? A Regulatory Perspective. Int. J. Pharm. Compd. 2014, 18, 101–111.
- Timko, R.J. Applying Quality by Design Concepts to Pharmacy Compounding. Int. J. Pharm. Compd. 2015, 19, 453–463.
- Broughel, J. Allowing Compounding Pharmacies to Address Drug Shortages. Int. J. Pharm. Compd. 2022, 26, 100–109.
- Merienne, C.; Filali, S.; Marchand, C.; Lapras, B.; Paillet, C.; Pirot, F. Predictive Stability, Novel HPLC-MS Analysis and Semi-Automatic Compounding Process for the Emergency Implementation of a Production Line of Pancuronium in Injectable Solution. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2023, 106464. [CrossRef]
- Patel, T.; Patel, M.; Shah, U.; Patel, A.; Patel, S.; Solanki, N.; Shah, S. Comprehensive Analysis of Aspirin and Apixaban: Thedevelopment, Validation, and Forced Degradation Studies of Bulk Drugs and in-House Capsule Formulations Using the RP-HPLC Method.
- USP-NF〈795〉 Pharmaceutical Compounding—Nonsterile Preparations. [CrossRef]
- USP-NF 〈797〉 Pharmaceutical Compounding—Sterile Preparations Available online: https://online.uspnf.com/uspnf/document/1_GUID-A4CAAA8B-6F02-4AB8-8628-09E102CBD703_8_en-US?source=Search%20Results&highlight=797 (accessed on 26 March 2025).
- European Pharmacopoeia 11.4, E.D. for the Q. of M.& H. 2619. Pharmaceutical Preparations 2024.
- Huanbutta, K.; Burapapadh, K.; Kraisit, P.; Sriamornsak, P.; Ganokratanaa, T.; Suwanpitak, K.; Sangnim, T. Artificial Intelligence-Driven Pharmaceutical Industry: A Paradigm Shift in Drug Discovery, Formulation Development, Manufacturing, Quality Control, and Post-Market Surveillance. Eur. J. Pharm. Sci. 2024, 203, 106938. [CrossRef]
- Noorain; Srivastava, V.; Parveen, B.; Parveen, R. Artificial Intelligence in Drug Formulation and Development: Applications and Future Prospects. Curr. Drug Metab. 24, 622–634. [CrossRef]
- Gholap, A.D.; Uddin, M.J.; Faiyazuddin, M.; Omri, A.; Gowri, S.; Khalid, M. Advances in Artificial Intelligence for Drug Delivery and Development: A Comprehensive Review. Comput. Biol. Med. 2024, 178, 108702. [CrossRef]
- Wang, W.; Ye, Z.; Gao, H.; Ouyang, D. Computational Pharmaceutics - A New Paradigm of Drug Delivery. J. Controlled Release 2021, 338, 119–136. [CrossRef]
- Vora, L.K.; Gholap, A.D.; Jetha, K.; Thakur, R.R.S.; Solanki, H.K.; Chavda, V.P. Artificial Intelligence in Pharmaceutical Technology and Drug Delivery Design. 2023.
- Jiang, J.; Ma, X.; Ouyang, D.; Robert O Williams, I.I.I. Emerging Artificial Intelligence (AI) Technologies Used in the Development of Solid Dosage Forms. Pharmaceutics 2022, 14, 2257. [CrossRef]
- Dong, J.; Gao, H.; Ouyang, D. PharmSD: A Novel AI-Based Computational Platform for Solid Dispersion Formulation Design. Int. J. Pharm. 2021, 604, 120705. [CrossRef]
- Han, R. Predicting Physical Stability of Solid Dispersions by Machine Learning Techniques. J. Controlled Release 2019.
- Nowotka, M.; Gaulton, A.; Mendez, D.; Bento, A.; Hersey, A.; Leach, A. Using ChEMBL Web Services for Building Applications and Data Processing Workflows Relevant to Drug Discovery. Expert Opin. Drug Discov. 2017, 12, 1–11.
- Takada, N.; Ohmori, N.; Okada, T. Mining Basic Active Structures from a Large-Scale Database. J. Cheminformatics 2013, 5, 15. [CrossRef]
- Taketomo, C.K.; Chu, S.A.; Cheng, M.H.; Corpuz, R.P. Stability of Captopril in Powder Papers under Three Storage Conditions. Am. J. Hosp. Pharm. 1990, 47, 1799–1801. [CrossRef]
- Helin, M.M.; Kontra, K.M.; Naaranlahti, T.J.; Wallenius, K.J. Content Uniformity and Stability of Nifedipine in Extemporaneously Compounded Oral Powders. Am. J. Health. Syst. Pharm. 1998, 55, 1299–1301. [CrossRef]
- Rughoo, L.; Vigneron, J.; Zenier, H.; May, I.; Demoré, B. Stability Study of Amiodarone Hydrochloride in Capsules for Paediatric Patients Using a High-Performance Liquid Chromatography Method. Ann. Pharm. Fr. 2012, 70, 88–93. [CrossRef]
- Joshi, R.; Zheng, Z.; Agarwal, P.; Hatmal, M.M.; Chang, X.; Seidler, P.; Haworth, I.S. KNIME Workflows for Applications in Medicinal and Computational Chemistry. Artif. Intell. Chem. 2024, 2, 100063. [CrossRef]
- Moreira-Filho, J.T.; Ranganath, D.; Conway, M.; Schmitt, C.; Kleinstreuer, N.; Mansouri, K. Democratizing Cheminformatics: Interpretable Chemical Grouping Using an Automated KNIME Workflow. J. Cheminformatics 2024, 16, 101. [CrossRef]
- Wagner, L.A.F.; Neininger, M.P.; Hensen, J.; Zube, O.; Bertsche, T. Avoiding Incompatible Drug Pairs in Central-Venous Catheters of Patients Receiving Critical Care: An Algorithm-Based Analysis and a Staff Survey. Eur. J. Clin. Pharmacol. 2023, 79, 1081–1089. [CrossRef]
- Dong, J.; Wu, Z.; Xu, H.; Ouyang, D. FormulationAI: A Novel Web-Based Platform for Drug Formulation Design Driven by Artificial Intelligence.
- Federal Agency for Medicines and Health Products (FAMHP) Magistral Therapeutic Formulary. Https://Www.Afmps.Be/En/Authorisation/Magistral_therapeutic_formulary Available online: https://www.afmps.be/en/authorisation/magistral_therapeutic_formulary (accessed on 27 March 2025).
- Royal Dutch Pharmacists Association (KNMP) Formulary of Dutch Pharmacists (FNA). https://www.knmp.nl/over-de-knmp/producten-en-diensten/productzorg-bereiding-en-toediening/fna-boek-2013 (accessed on 27 March 2025).
- Deutscher Arzneimittel-Codex® / Neues Rezeptur-Formularium® (DAC/NRF). https://www.deutscher-apotheker-verlag.de/Deutscher-Arzneimittel-Codex-Neues-Rezeptur-Formularium-DAC-NRF/9783774100442 Available online: https://www.deutscher-apotheker-verlag.de/Deutscher-Arzneimittel-Codex-Neues-Rezeptur-Formularium-DAC-NRF/9783774100442 (accessed on 27 March 2025).
- Lehmann, H. Le Formulaire National de La Pharmacopée Française, Au Service de La Fabrication et Du Contrôle Des Préparations Officinales. Actual. Pharm. 2017, 56, 34–36. [CrossRef]
- Sheskey, P.J.; Hancock, B.C.; Moss, G.P.; Goldfarb, D.J. Handbook of Pharmaceutical Excipients – Ninth Edition; Pharm. Press.; 2020; ISBN 978-0-85711-375-7.
- Falcón-Cano, G.; Molina, C.; Cabrera-Pérez, M.Á. ADME Prediction with KNIME: In Silico Aqueous Solubility Consensus Model Based on Supervised Recursive Random Forest Approaches. ADMET DMPK 2020, 8, 251–273. [CrossRef]
- Palazzotti, D.; Fiorelli, M.; Sabatini, S.; Massari, S.; Barreca, M.L.; Astolfi, A. Q-raKtion: A Semiautomated KNIME Workflow for Bioactivity Data Points Curation. J. Chem. Inf. Model. 2022, 62, 6309–6315. [CrossRef]
- Lamrabet, N.; Hess, F.; Leidig, P.; Marx, A.; Kipping, T. Exploring 3D Printing in Drug Development: Assessing the Potential of Advanced Melt Drop Deposition Technology for Solubility Enhancement by Creation of Amorphous Solid Dispersions. Pharmaceutics 2024, 16, 1501. [CrossRef]
- Nurzyńska, K.; Booth, J.; Roberts, C.J.; McCabe, J.; Dryden, I.; Fischer, P.M. Long-Term Amorphous Drug Stability Predictions Using Easily Calculated, Predicted, and Measured Parameters. Mol. Pharm. 2015, 12, 3389–3398. [CrossRef]
- Veith, H.; Wiechert, F.; Luebbert, C.; Sadowski, G. Combining Crystalline and Polymeric Excipients in API Solid Dispersions – Opportunity or Risk? Eur. J. Pharm. Biopharm. 2021, 158, 323–335. [CrossRef]
- Szakonyi, G.; Zelkó, R. The Effect of Water on the Solid State Characteristics of Pharmaceutical Excipients: Molecular Mechanisms, Measurement Techniques, and Quality Aspects of Final Dosage Form. Int. J. Pharm. Investig. 2012, 2, 18–25. [CrossRef]
- Yang, H.; Xu, L.; Hou, L.; Xu, T.C.; Ye, S.H. Stability of Vitamin A, E, C and Thiamine during Storage of Different Powdered Enteral Formulas. Heliyon 2022, 8, e11460. [CrossRef]



| API | MW | LogP | RB | PS | HBD | HBA | AR | MC | MSC | Excipients | Cond. | Content | T | BUD | |||||||||||||
| Main | Other | (mg) | (%) | °C | (days) | ||||||||||||||||||||||
| Acetylsalicylic acid | 180.16 | 1.24 | 2 | 63.60 | 1 | 3 | 1 | 30 | 5 | Lactose | - | ND | 4 | 4 | 25 | 365 | |||||||||||
| 19 | 4 | 25 | 365 | ||||||||||||||||||||||||
| 56 | 4 | 25 | 365 | ||||||||||||||||||||||||
| 76 | 4 | 25 | 365 | ||||||||||||||||||||||||
| Alpha-tocopherol acetate | 472.74 | 10.42 | 13 | 35.53 | 0 | 3 | 1 | 53 | 9 | Lactose | - | G | 100 | 56 | 8 | 60 | |||||||||||
| 100 | 56 | 25 | 60 | ||||||||||||||||||||||||
| 4-Aminopyridine | 94.12 | -0.07 | 1 | 38.91 | 1 | 2 | 1 | 24 | 4 | Lactose | Silica | Pl | 5 | 2 | 25 | 180 | |||||||||||
| 5 | 2 | 40 | 30 | ||||||||||||||||||||||||
| 3,4-Diaminopyridine | 109.13 | -0.9 | 0 | 64.93 | 1 | 2 | 1 | 25 | 5 | Lactose | Silica | Pl | 5 | 2 | 4 | 180 | |||||||||||
| 5 | 2 | 25 | 180 | ||||||||||||||||||||||||
| Amiodarone Hydrochloride | 681.78 | 7.64 | 11 | 42.68 | 1 | 4 | 3 | 52 | 9 | Cellulose | - | ND | 5 | 2 | 25 | 30 | |||||||||||
| 20 | 10 | 25 | 30 | ||||||||||||||||||||||||
| 50 | 25 | 25 | 30 | ||||||||||||||||||||||||
| Mannitol | - | G | 10 | 4 | 25 | 365 | |||||||||||||||||||||
| 60 | 25 | 25 | 365 | ||||||||||||||||||||||||
| 100 | 50 | 25 | 365 | ||||||||||||||||||||||||
| Amoxicillin trihydrate | 365,41 | -2.31 | 4 | 132.96 | 4 | 6 | 1 | 28 | 8 | - | - | Pl | 125 | 100 | 25 | 90 | |||||||||||
| 250 | 100 | 25 | 56 | ||||||||||||||||||||||||
| 250 | 100 | 40 | 56 | ||||||||||||||||||||||||
| 500 | 100 | 25 | 90 | ||||||||||||||||||||||||
| Atenolol | 266.34 | 0.43 | 6 | 84.58 | 2 | 5 | 1 | 30 | 7 | Cellulose | - | PI | 25 | 50 | 30 | 120 | |||||||||||
| Captopril | 217.29 | 0.28 | 3 | 95.00 | 2 | 4 | 0 | 29 | 6 | Lactose | - | P | 2 | 2 | 25 | 84 | |||||||||||
| Carbidopa | 244.24 | -1.21 | 4 | 115.81 | 5 | 5 | 1 | 27 | 7 | Cellulose | - | ND | 200 | 30 | 25 | 336 | |||||||||||
| Cholecalciferol | 384.64 | 7.13 | 6 | 20.53 | 1 | 1 | 0 | 45 | 6 | Lactose | - | G | 0.025 | 0.008 | 8 | 60 | |||||||||||
| 0.025 | 0.008 | 25 | 60 | ||||||||||||||||||||||||
| Cholic acid | 408.57 | 2.48 | 4 | 97.99 | 3 | 5 | 0 | 37 | 7 | Silica | Lactose | Pl | 25 | 95 | 25 | 365 | |||||||||||
| Silica | - | Pl | 250 | 97 | 25 | 365 | |||||||||||||||||||||
| Silica | Lactose | Pl | 25 | 100 | 40 | 180 | |||||||||||||||||||||
| Silica | - | PI | 250 | 100 | 40 | 180 | |||||||||||||||||||||
| Clonidine hydrochloride | 266.56 | 2.49 | 1 | 36.42 | 2 | 3 | 1 | 33 | 5 | Cellulose | - | ND | 0.02 | 1 | 25 | 365 | |||||||||||
| API | MW | LogP | RB | PS | HBD | HBA | AR | MS | MSC | Excipients | Cond. | Content | T | BUD | |||||||||||||
| Main | Other | (mg) | (%) | °C | (days) | ||||||||||||||||||||||
| Cyclo- phosphamide |
261.09 | 0.10 | 5 | 41.57 | 1 | 2 | 0 | 29 | 5 | Lactose | - | ND | 10 | - | 4 | 70 | |||||||||||
| 25 | - | 4 | 70 | ||||||||||||||||||||||||
| Erythromycin | 733.94 | 2.60 | 7 | 193.91 | 5 | 14 | 0 | 46 | 11 | Cellulose | - | ND | 20 | 46 | 25 | 365 | |||||||||||
| Fludrocortisone acetate |
422.49 | 1.76 | 3 | 110.90 | 2 | 6 | 0 | 37 | 7 | Lactose | - | ND | 0.01 | - | 25 | 180 | |||||||||||
| Cellulose | - | ND | 0.01 | - | 25 | 180 | |||||||||||||||||||||
| Sucrose | - | ND | 0.01 | - | 25 | 180 | |||||||||||||||||||||
| Hydrocortisone | 362.47 | 1.61 | 0 | 94.00 | 3 | 5 | 1 | 35 | 6 | Lactose | - | P | 20 | 0.4 | 25 | 12 | |||||||||||
| Melatonin | 232.28 | 1.15 | 4 | 54.12 | 2 | 4 | 2 | 31 | 6 | Lactose | HPMC | G | 3 | 0.7 | 25 | 90 | |||||||||||
| Lactose | HPMC | G | 3 | 0.7 | 40 | 90 | |||||||||||||||||||||
| Cellulose | - | ND | 0.5 | 0.65 | 25 | 547 | |||||||||||||||||||||
| Cellulose | - | ND | 2 | 2.6 | 25 | 547 | |||||||||||||||||||||
| Cellulose | - | ND | 6 | 8.4 | 25 | 547 | |||||||||||||||||||||
| Lactose | - | P | 18 | 0.7 | 25 | 90 | |||||||||||||||||||||
| Lactose | - | P | 18 | 0.7 | 40 | 90 | |||||||||||||||||||||
| Lactose | - | P | 18 | 2 | -20 | 168 | |||||||||||||||||||||
| Menadione | 172.18 | 1.89 | 0 | 34.14 | 0 | 2 | 1 | 30 | 4 | Lactose | - | G | 1 | 0.5 | 8 | 60 | |||||||||||
| Lactose | - | G | 1 | 0.5 | 25 | 60 | |||||||||||||||||||||
| Midazolam Hydrochloride | 362.20 | 3.97 | 1 | 30.18 | 0 | 3 | 3 | 38 | 6 | Cellulose | - | ND | 1 | 1 | 25 | 365 | |||||||||||
| Naltrexone | 341.42 | 1.36 | 2 | 70.00 | 2 | 5 | 1 | 33 | 5 | Cellulose | - | G | 1.5 | 10 | 25 | 360 | |||||||||||
| Nifedipine | 346.34 | 2.56 | 4 | 107.00 | 1 | 6 | 1 | 37 | 7 | Lactose | - | P | 1 | 0.2 | 6 | 365 | |||||||||||
| Lactose | - | P | 1 | 0.2 | 22 | 365 | |||||||||||||||||||||
| Retinyl acetate | 328.50 | 5.14 | 6 | 26.30 | 0 | 2 | 0 | 40 | 6 | Lactose | - | G | 5.5 | 1 | 8 | 60 | |||||||||||
| Lactose | - | G | 5.5 | 1 | 25 | 60 | |||||||||||||||||||||
| Excipients | Physico-chemical properties | Functional category | Shelf-life† (15°C-25°C) |
||||||||||||
| MW | LogP | RB | PS | HBD | HBA | AR | MC | MSC | Key functional roles | Notable properties | |||||
| Cellulose (C)a | 342.30 | -5.40 | 4 | 190.00 | 8 | 11 | 0 | 22 | 10 | Adsorbent, disintegrant, binder, diluent | Hygroscopic, used in wet/dry granulation | 4 years | |||
| HPMCb (A) | 1261.40 | -2.32 | 30 | 365.00 | 8 | 30 | 0 | 50 | 20 | Dispersing, solubilizing, stabilizing, thickening, film-coating, binder | Nonionic, used in extended-release tablets and film coatings | 3 years | |||
| Lactose (C/A) | 360.31 | -5.73 | 4 | 191.00 | 9 | 12 | 0 | 22 | 12 | Binder, filler, diluent | Exists in different crystalline forms | 3 years | |||
| Mannitol (C) | 182.17 | -3.73 | 2 | 131.38 | 6 | 6 | 0 | 21 | 9 | Diluent, plasticizer | Non-hygroscopic; suitable for moisture-sensitive APIs. | 5 years | |||
| Silicac (A) |
60.84 | -0.62 | 0 | 34.10 | 2 | 0 | 0 | 23 | 4 | Adsorbent, disintegrant, thermal stabilizer | Hygroscopic; widely used in oral formulations | 5 years | |||
| Sucrose (C) | 342.3 | -4,53 | 5 | 189.55 | 8 | 11 | 0 | 40 | 10 | Binder, filler | stable at room temperature; absorbs ~1% moisture. | 5 years | |||
| Parameters | Correlation value | p- value | ||
| Molecular descriptors | ||||
| Molecule | -0,269 | 0,204 | ||
| Code SMILE | -0,163 | 0,447 | ||
| MW | 0,305 | 0,148 | ||
| MW class | 0,089 | 0,678 | ||
| LogP | 0,503 | 0,012 | ||
| LogP class | 0,502 | 0,012 | ||
| Rotatable bonds | 0,087 | 0,686 | ||
| Rotatable bonds class | 0,157 | 0,464 | ||
| Polar surface | 0,319 | 0,129 | ||
| Polar surface class | -0,153 | 0,476 | ||
| H-donor bonds | -0,183 | 0,392 | ||
| H donor bonds class | -0,271 | 0,201 | ||
| H-acceptor bonds | -0,162 | 0,449 | ||
| H acceptor bonds class | -0,128 | 0,550 | ||
| Aromatic rings | 0,357 | 0,087 | ||
| Aromatic rings class | 0,283 | 0,180 | ||
| Molecule class | 0,439 | 0,032 | ||
| Molecular structure class | -0,139 | 0,517 | ||
| Formulation descriptors | ||||
| Main excipient | 0,041 | 0,849 | ||
| Encoded excipients | 0,300 | 0,154 | ||
| Content | -0,212 | 0,321 | ||
| Content class | -0,269 | 0,203 | ||
| Conditioning and storage descriptors | ||||
| Packaging | -0,200 | 0,349 | ||
| Packaging class | 0,145 | 0,500 | ||
| Temperature | -0,336 | 0,109 | ||
| Temperature class | -0,110 | 0,608 | ||
| Storage class | -0,266 | 0,209 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





